Published online Dec 24, 2025. doi: 10.5306/wjco.v16.i12.111701
Revised: August 24, 2025
Accepted: November 14, 2025
Published online: December 24, 2025
Processing time: 169 Days and 10.5 Hours
Intrathyroidal thymic carcinoma (ITC) is a rare malignant epithelial tumour of thymic origin occurring within the thyroid. Histologically, it resembles thymic carcinoma, with squamous cell carcinoma being the most common subtype, and immunohistochemical staining typically exhibits features consistent with thymic neoplasms.
We report the case of a 68-year-old woman who presented with a left-sided neck mass of one year’s duration. And the neck lump had been gradually enlarging over the course of a year, reaching the size of a goose egg within six months. Thyroid ultrasound revealed a normally sized thyroid gland. A 3.9 cm × 3.4 cm × 2.7 cm hypoechoic lesion with irregular echogenicity was observed outside the capsule of the lower pole of the left lobe. The mass exhibited regular morphology, well-defined margins, and close adherence to the thyroid’s lower pole. Micro
Based on these findings, the patient was diagnosed as ITC with both squamous cell and small cell carcinoma components. To date, nearly 100 cases of ITC have been reported in the literature. However, no prior reports of ITC exhibiting both squamous cell and small cell carcinoma components. This case report provides information on the microscopic morphological features of ITC with both squamous cell and small cell carcinoma components, which can help pathologists to expands the understanding of the pathological spectrum of the disease.
Core Tip: Intrathyroidal thymic carcinoma (ITC) is a rare malignant epithelial tumour of thymic origin occurring within the thyroid. ITC with both squamous cell and small cell carcinoma components has not been reported to date. This case report provides information on the microscopic morphological features of ITC with both squamous cell and small cell carcinoma components, and new potential evidence for the origin of ITC. As cluster of differentiation 117+ immunophenotype is a critical clue that should prompt consideration of ITC, which requires a different diagnostic and therapeutic approach than metastatic small cell lung cancer.
- Citation: Shan BW, Yu JH, Ren T. Intrathyroidal thymic carcinoma comprising squamous cell and small cell carcinoma components: A case report. World J Clin Oncol 2025; 16(12): 111701
- URL: https://www.wjgnet.com/2218-4333/full/v16/i12/111701.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i12.111701
Intrathyroidal thymic carcinoma (ITC) is a malignant thyroid neoplasm exhibiting thymic epithelial differentiation, also known as carcinoma showing thymus-like differentiation. Although typically a low-grade tumour, it is frequently misdiagnosed as more aggressive malignancies such as squamous cell carcinoma or poorly differentiated carcinoma[1]. We present an exceptionally rare case of ITC demonstrating both squamous cell and small cell carcinoma components. As subsequent treatment and prognosis differ significantly from other thyroid malignancies, accurate diagnosis is crucial for optimal patient management.
The patient is a 68-year-old woman presented with a left-sided neck lump that had been gradually enlarging over the course of a year.
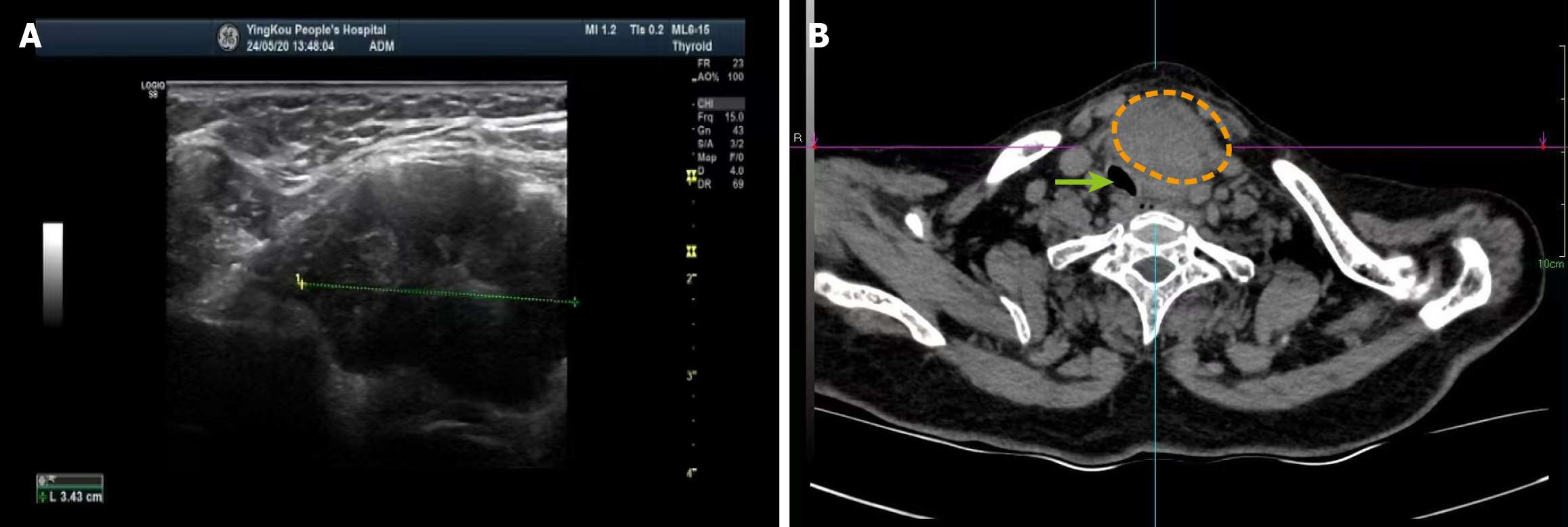
A 68-year-old woman presented with a left-sided neck lump that had been gradually enlarging over the course of a year, reaching the size of a goose egg within six months. The mass was associated with tenderness and dyspnoea, prompting her hospital admission in September 2024. Thyroid ultrasound revealed a normally sized thyroid gland. A 3.9 cm × 3.4 cm × 2.7 cm hypoechoic lesion with irregular echogenicity was observed outside the capsule of the lower pole of the left lobe. The mass exhibited regular morphology, well-defined margins, and close adherence to the thyroid’s lower pole. A sparse peripheral colour flow signal was detected, but no significantly enlarged lymph nodes were identified. Computed tomography imaging demonstrated a neck mass causing tracheal deviation due to compression.
The patient had been previously healthy.
No obvious abnormalities were found in the personal and family history.
The patient presented with a left neck mass accompanied by tenderness and respiratory difficulty.
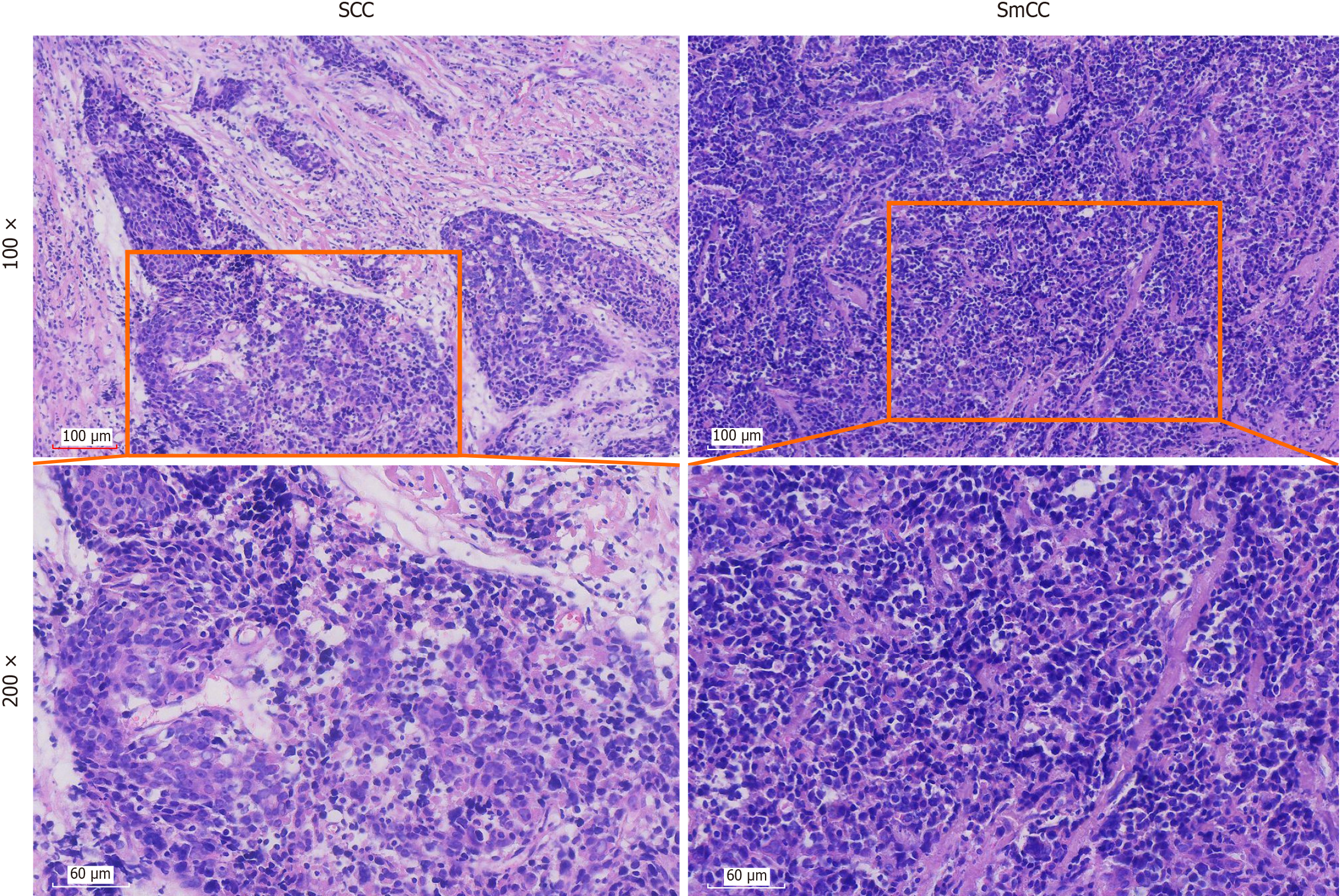
Microscopically, the tumour comprises two distinct components: Squamous cell carcinoma and small cell carcinoma. And the tumor was composed of approximately 60% poorly differentiated squamous cell carcinoma and 40% small cell carcinoma components. In the squamous cell carcinoma regions, tumour cells are arranged in variably sized nests or cords, with well-demarcated borders against the surrounding stroma. The fibrous septa separating tumour cell nests are broad and exhibit extensive hyaline degeneration. These tumour cells display: Large size and obvious atypia, vesicular or hyperchromatic nuclei, prominent nucleoli, eosinophilic cytoplasm, and visible mitotic figure. The tumour is poorly differentiated, lacking keratin pearls and distinct intercellular bridge. Lymphocytic infiltration is observed between tumour cells nests. In small cell carcinoma regions, tumour cells are densely packed in sheets with poorly defined cellular borders. These cells exhibit: Small size with scant cytoplasm (appearing almost “bare-nuclei-like”), irregular nuclear contours, uniform nuclear chromatin, inconspicuous nucleoli (Figure 1).
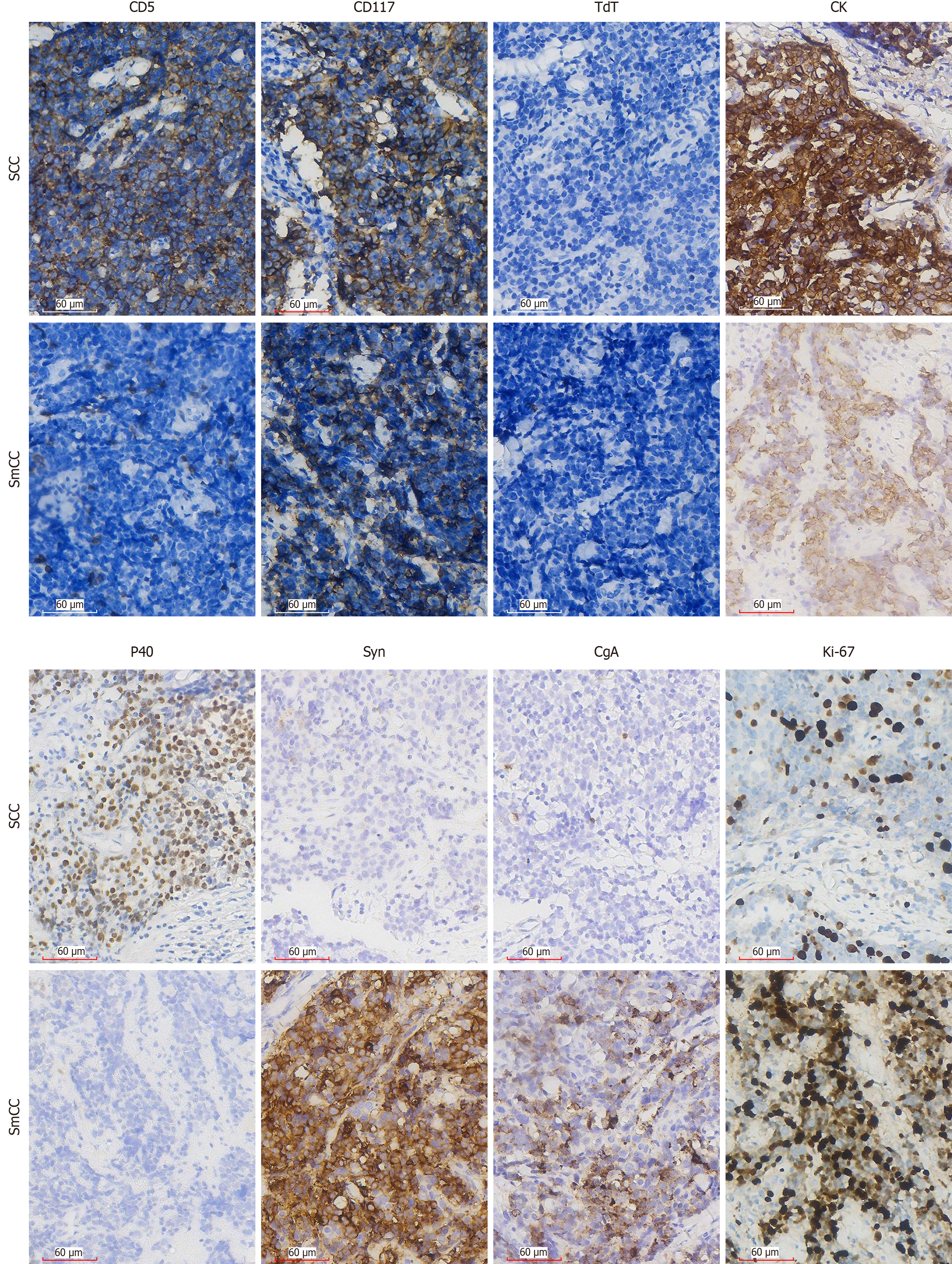
Immunohistochemical staining demonstrated distinct marker expression patterns between the two tumour components. In the squamous cell carcinoma region, the tumour cells exhibited positivity for cytokeratin, p40, cluster of differentiation (CD) 5, and CD117, while showing negative results for chromogranin A (CgA), synaptophysin (Syn), and deoxynucleotidyl transferase. The Ki-67 proliferation index in this region was 30%. In contrast, the small cell carcinoma region displayed positive immunoreactivity for cytokeratin CgA, Syn, and CD117, with a higher Ki-67 proliferation index of 60%. This component was negative for CD5, p40, and deoxynucleotidyl transferase (Figure 2).
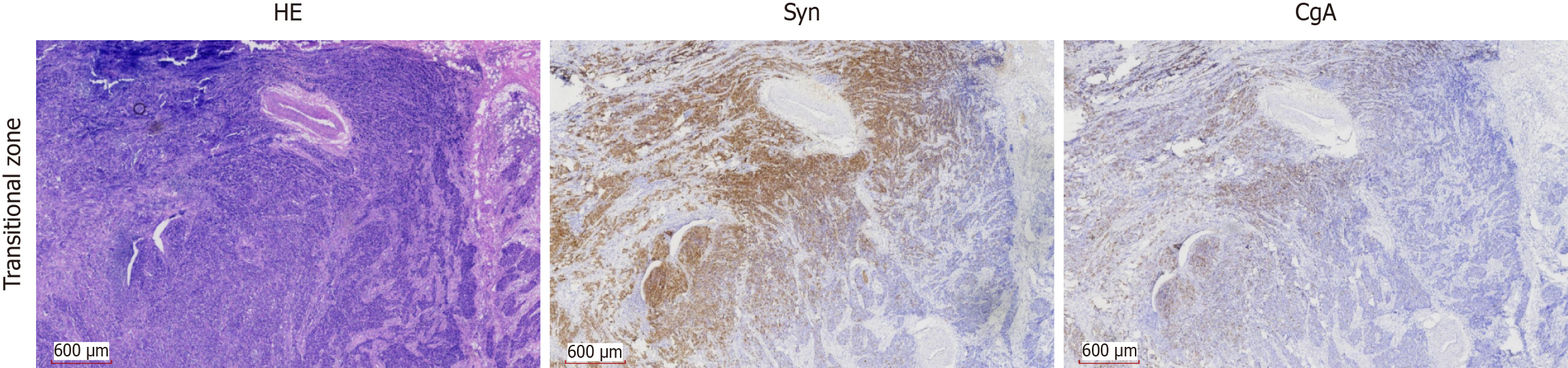
Histological examination revealed a morphological continuum between the two components, with nests of squamous cell carcinoma gradually transitioning into sheets of small cell carcinoma. This gradual shift was further supported immunohistochemically, where a parallel gradation in the expression of CgA and Syn was observed across the transitional zone (Figure 3).
Thyroid ultrasound revealed a normally sized thyroid gland. A 3.9 cm × 3.4 cm × 2.7 cm hypoechoic lesion with irregular echogenicity was observed outside the capsule of the lower pole of the left lobe. The mass exhibited regular morphology, well-defined margins, and close adherence to the thyroid’s lower pole. A sparse peripheral colour flow signal was detected, but no significantly enlarged lymph nodes were identified. Computed tomography imaging demonstrated a neck mass causing tracheal deviation due to compression (Figure 4).
Based on these findings, the patient was diagnosed as ITC with both squamous cell and small cell carcinoma components.
Following definitive diagnosis, the patient underwent four cycles of chemotherapy as adjuvant therapy to prevent tumour recurrence.
Eight months after the patient’s surgery, we had a follow-up and the patient had completed four cycles of chemotherapy with no recurrence.
ITC is a rare malignant epithelial tumour demonstrating thymic epithelial differentiation. Previously termed carcinoma showing thymic-like differentiation[2], this entity was first described by Miyauchi et al[3] in 1985. ITC represents approximately 0.15% of all thyroid malignancies, with a slight female predominance (male-to-female ratio = 1:1.22). The tumour typically presents in middle-aged individuals, with a mean age at diagnosis of 48.8 years[4,5].
This tumour most frequently arises in the lower pole of the thyroid gland, though rare cases may occur in the surrounding soft tissues. It typically manifests as a slow-growing, firm, and fixed neck mass. Some patients may experience dyspnoea, dysphagia or hoarseness secondary to tumour compression[1]. In the present case, the patient presented with a left neck mass accompanied by tenderness and respiratory difficulty. The diagnosis of ITC remains challenging due to its rarity. Initially, this case was suspected to represent a poorly differentiated thyroid squamous cell carcinoma with neuroendocrine differentiation. Following referral to our institution, the final diagnosis was established as ITC with both squamous cell and small cell carcinoma components. Notably, cases exhibiting both morphological components are exceptionally rare. While previous reports have documented neuroendocrine differentiation in ITC (evidenced by positivity for markers such as Syn and CgA), the coexistence of ITC with a neuroendocrine carcinoma component demonstrating small cell morphology has not, to our knowledge, been previously described in the literature[6,7].
ITC presents as a well- circumscribed mass that exhibits expansive growth and local invasion into adjacent thyroid tissue or extrathyroidal soft tissues. Histologically, ITC resembles thymic epithelial tumours, particularly type B3 thymoma or well-differentiated thymic carcinoma. The tumours are organised in nests or islands, bordered by dense fibrous septa, and are frequently accompanied by lymphocytic infiltration within the tumour nests or stroma. The tumour cells are polygonal, demonstrating cytological atypia, prominent nucleoli, low mitotic activity, and a low Ki-67 proliferation index. Focal areas often exhibit varying degrees of squamous differentiation, with Hassall’s corpuscles observed in rare cases[1,8]. In this case, the squamous cell carcinoma component displays characteristic ITC features morphologically: Tumour cells are compartmentalised into islands by abundant stromal tissue, with associated lymphocytic infiltration, and the tumour islands are interspersed with delicate blood vessels. The tumour cells are large, with eosinophilic cytoplasm, oval vesicular nuclei, and conspicuous nucleoli. Mitotic figures are infrequent, and the Ki-67 proliferation index is 30%.
Previous studies have demonstrated that CD5 and CD117 exhibit high specificity for diagnosing ITC, although rare cases may show negativity for either marker[9]. Ito et al[10] reported a CD5 positivity rate of 82% in ITC cases, necessitating supplementary markers for differential diagnosis. In addition to CD5 and CD117, tumour cells typically express p63, polyclonal paired box gene 8, high-molecular-weight cytokeratin, carcinoembryonic antigen, glucose transporter 1, epidermal growth factor receptor, calretinin, p53, B-cell lymphoma 2 and myeloid cell leukemia 1, while remaining negative for thyroglobulin, thyroid transcription factor 1 (TTF-1) and calcitonin[8]. To confirm the diagnosis, immunohistochemical staining was performed on the tumour tissue. The results revealed positivity for CD5, CD117, cytokeratin, p40 in the squamous cell carcinoma component, alongside negativity for deoxynucleotidyl transferase, CgA, Syn. In this case, the histological and immunohistochemical profile of the squamous cell carcinoma component aligned with previous descriptions. Notably, this case exhibited not only the typical features of squamous cell carcinoma but also characteristics suggestive of small cell carcinoma. Although Snover et al[11] reported three cases of mixed small cell and undifferentiated squamous cell carcinoma arising in the mediastinal thymus, the occurrence of mixed squamous cell carcinoma and small cell carcinoma within an intrathyroidal thymic tumour likely represents the first reported instance. Thymic small cell carcinoma originating in the mediastinum demonstrates microscopic features similar to small cell carcinoma of other organs. The tumour cells are approximately three times larger than resting lymphocytes and exhibit a high nuclear-to-cytoplasmic ratio. Their nuclei display an oval morphology with finely granular chromatin and inconspicuous nucleoli. Most thymic small cell carcinomas show positive immunoreactivity for cytokeratin, Syn and CgA, typically demonstrating a high Ki-67 proliferation index of 80%-100%[12]. In the present case, the small cell carcinoma component exhibited identical characteristics to mediastinal thymic small cell carcinoma. This finding expands the pathological understanding of this disease entity and may provide novel insights into its histogenesis.
Current evidence suggests that ITC may originate from ectopic thymic tissue or branchial pouch remnants, potentially arising from the fourth/fifth branchial pouch (ultimobranchial body)[13,14]. The higher incidence of ITC in Asian populations implies that genetic, ethnic, and environmental factors may contribute to its pathogenesis. Given the extreme rarity of small cell carcinoma arising in intrathyroidal ectopic thymus, its precise origin remains unclear. We propose two potential origins: Either development from ectopic thymic tissue or metastasis from other organs, particularly the lungs. However, since small cell carcinomas from different sites demonstrate remarkably similar morphological and immunohistochemical profiles, differentiation requires careful correlation with clinical findings and imaging studies. Notably, TTF-1 immunohistochemical staining cannot reliably distinguish between these possibilities, as thymic small cell carcinoma may also show TTF-1 positivity[12]. In the present case, the tumour’s CD117 positivity, combined with the absence of pulmonary masses on imaging, supports an origin from intrathyroidal ectopic thymic tissue rather than metastatic disease. About the histogenesis - arises from neuroendocrine cells within the ectopic thymic tissue or results from transdifferentiation of the ITC - we favor the explanation of transdifferentiation, given the morphological and immunohistochemical features. The morphological presentation in this case demonstrated gradual transitional areas where squamous cell carcinoma progressively transitioned into small cell carcinoma, with no well-defined border between the two tumor components. Therefore, the dual differentiation observed within this tumor can be explained by clonal evolution.
The prognosis for ITC, which is typically characterized by squamous cell carcinoma, is generally good. According to previous studies, patients who underwent total thyroidectomy combined with cervical lymphadenectomy had a 14% local recurrence rate, a 5-year survival rate of 90%, and a 10-year survival rate of 82%[15]. The prognosis for small cell carcinoma of the thymus is poor, with a reported 5-year survival rate of 0% and a median survival of only 14 months[12]. In comparison, the 5-year survival rate for small cell lung cancer ranges from 12% to 30%[16]. Due to the lack of reporting, it is remains unclear that ITC containing squamous cell and small cell carcinoma affects the overall prognosis. Based on the prognostic profiles of both thymic and pulmonary small cell carcinomas. Eight months after the patient’s surgery, we had a follow-up and the patient had completed four cycles of chemotherapy with no recurrence. We hypothesize that the prognosis of this mixed-variant ITC may fall somewhere between these two entities.
The primary limitation of this report is the lack of positron emission tomography/computed tomography imaging to systematically exclude metastatic disease. While the clinical and immunohistochemical profile (including CD117 expression) is highly suggestive of a primary ITC, we cannot definitively rule out metastatic without imaging examination. Therefore, we propose that future cases of this rare entity should undergo both positron emission tomography/computed tomography for accurate staging and next-generation sequencing to better elucidate its molecular pathogenesis.
In conclusion, ITC represents an exceptionally rare tumour entity for which accurate diagnosis remains challenging. Due to its scarcity, our current understanding of this malignancy remains incomplete. We present a novel case of ITC exhibiting combined histological features of small cell carcinoma and poorly differentiated squamous cell carcinoma. This report aims to contribute to the expanding knowledge of this disease entity and may facilitate future diagnostic recognition.
| 1. | International Agency for Research on Cancer. WHO Classification of Tumours of Endocrine Organs. 2017. [cited 3 July 2025]. Available from: https://publications.iarc.fr/Book-And-Report-Series/Who-Classification-Of-Tumours/WHO-Classification-Of-Tumours-Of-Endocrine-Organs-2017. |
| 2. | Wong EHC, Tetter N, Tzankov A, Muller L. CASTLE tumor of the parotid: First documented case, literature review, and genetic analysis of the cancer. Head Neck. 2018;40:E1-E4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 3. | Miyauchi A, Kuma K, Matsuzuka F, Matsubayashi S, Kobayashi A, Tamai H, Katayama S. Intrathyroidal epithelial thymoma: an entity distinct from squamous cell carcinoma of the thyroid. World J Surg. 1985;9:128-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 145] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 4. | Liang J, Huang M, Huang H, Li L, Luo H, Mao W, Gao S, Xu H. Intrathyroidal Thymic Carcinoma: A Retrospective Case Series Study. Ear Nose Throat J. 2023;102:584-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 5. | Ge W, Yao YZ, Chen G, Ding YT. Clinical analysis of 82 cases of carcinoma showing thymus-like differentiation of the thyroid. Oncol Lett. 2016;11:1321-1326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | Kakudo K, Bai Y, Ozaki T, Homma K, Ito Y, Miyauchi A. Intrathyroid epithelial thymoma (ITET) and carcinoma showing thymus-like differentiation (CASTLE): CD5-positive neoplasms mimicking squamous cell carcinoma of the thyroid. Histol Histopathol. 2013;28:543-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 7. | Kunc M, Kamieniecki A, Walczak G, Nowicki T, Wasąg B, Mikaszewski B, Stodulski D, Biernat W. Intrasalivary Thymic Carcinoma: A Case Report and Literature Review. Head Neck Pathol. 2022;16:857-864. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Cameselle-Teijeiro JM, Eloy C, Sobrinho-Simões M. Pitfalls in Challenging Thyroid Tumors: Emphasis on Differential Diagnosis and Ancillary Biomarkers. Endocr Pathol. 2020;31:197-217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 9. | Pham HT, Nguyen HP, Van Nguyen C, Van Dao T, Nguyen AV, Le UT. Intra thyroid thymic carcinoma: A case report and literature review. Int J Surg Case Rep. 2024;119:109762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Ito Y, Miyauchi A, Nakamura Y, Miya A, Kobayashi K, Kakudo K. Clinicopathologic significance of intrathyroidal epithelial thymoma/carcinoma showing thymus-like differentiation: a collaborative study with Member Institutes of The Japanese Society of Thyroid Surgery. Am J Clin Pathol. 2007;127:230-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 88] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 11. | Snover DC, Levine GD, Rosai J. Thymic carcinoma. Five distinctive histological variants. Am J Surg Pathol. 1982;6:451-470. [PubMed] |
| 12. | Barone PD, Zhang C. Neuroendocrine neoplasms of the thymus. Front Immunol. 2024;15:1465775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 13. | Chan JK, Rosai J. Tumors of the neck showing thymic or related branchial pouch differentiation: a unifying concept. Hum Pathol. 1991;22:349-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 229] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 14. | Reimann JD, Dorfman DM, Nosé V. Carcinoma showing thymus-like differentiation of the thyroid (CASTLE): a comparative study: evidence of thymic differentiation and solid cell nest origin. Am J Surg Pathol. 2006;30:994-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 73] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 15. | Vajihinejad M, Ataei A, Pashmchi M, Aledavoud A, Zand V, Broomand MA, Mohammadi M, Reshkuiyeh NZ. Coexistence of intrathyroid thymic carcinoma and papillary thyroid carcinoma: a case report and literature review. Front Oncol. 2024;14:1394020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 16. | Kim SY, Park HS, Chiang AC. Small Cell Lung Cancer: A Review. JAMA. 2025;333:1906-1917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 56] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/