Published online Oct 24, 2025. doi: 10.5306/wjco.v16.i10.110466
Revised: July 3, 2025
Accepted: September 23, 2025
Published online: October 24, 2025
Processing time: 139 Days and 20.3 Hours
The γδT cells are an emerging class of immune effectors with potent antitumor activity, bridging innate and adaptive immunity. Their unique ability to recognise stress-induced ligands independently of major histocompatibility complex re
Core Tip: Vδ2 γδT cells hold promise for cancer immunotherapy due to their major histocompatibility complex-independent recognition of phosphoantigens and relative ease of their large scale GMP-compliant expansion in vitro. This mini-review outlines current strategies to optimize Vδ2 T cell expansion, highlighting the roles of stimuli, cytokine combinations, and culture conditions. Emerging insights on direct phosphoantigen stimulation and Toll-like receptor-based co-stimulation are also discussed, offering new avenues to enhance γδT cell yield and functionality for therapeutic use.
- Citation: Lehman N, Zarobkiewicz M. Expansion strategies for Vδ2 γδT cells in cancer immunotherapy: Activation, cytokines, and culture conditions. World J Clin Oncol 2025; 16(10): 110466
- URL: https://www.wjgnet.com/2218-4333/full/v16/i10/110466.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i10.110466
Human T cells consist of two major subsets-αβT (90%-95% of total T cells) and γδT (usually up to 5% of total T cells). Human γδT cells are further subdivided into Vδ1, Vδ2, Vδ3, Vδ4, and Vδ5 based on the variable fragment of TCRδ they use[1]. Vδ3-Vδ5 are poorly understood due to several factors, most notably the lack of available antibodies and their scarcity in peripheral blood. Vδ1 cells can be found in substantial numbers in peripheral blood, but are mostly limited to epithelial surfaces[2]. Vδ2 is the dominant subset in peripheral blood, where it usually accounts for 70%-90% of total γδT cells except for newborns and young children, in whom Vδ1 dominates. Both Vδ1 and Vδ2 are naturally cytotoxic against cancerous and viral-infected cells. γδT cells recognise antigens mostly in a major histocompatibility complex-unrestricted manner, thus even allogeneic transfers of γδT cells are immunologically safe[3]. γδT recognise targets via a plethora of cytotoxicity receptors e.g. natural killer group 2 D (NKG2D), DNAX accessory molecule 1 (DNAM-1), natural killer (NK) p30 and NKp44[1,3].
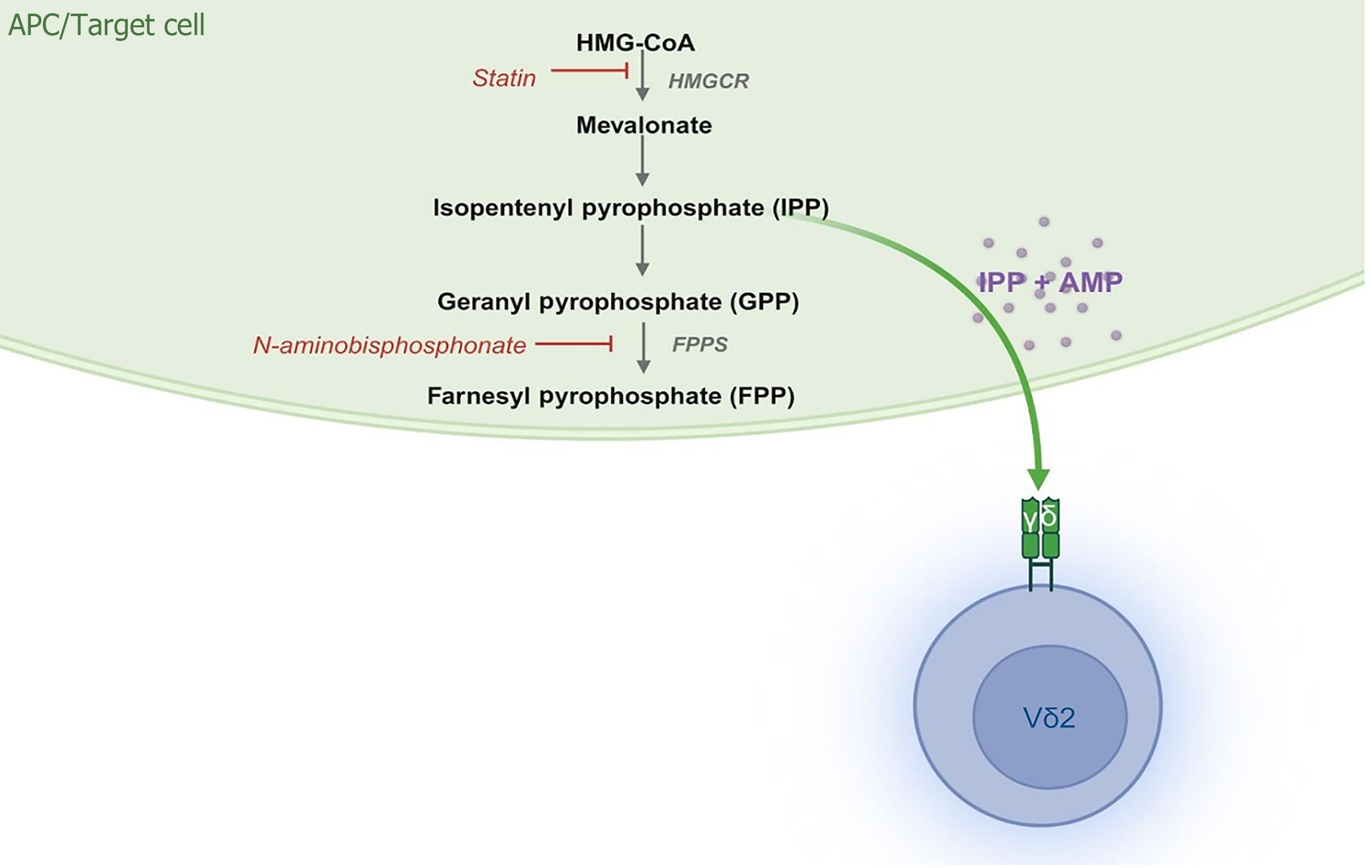
Vδ2 cells recognise phosphoantigens in a butyrophilin-dependent manner[3]. Phosphoantigens can be of both bacterial and eukaryotic origins. The (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate (HMBPP), a most potent phosphoantigen, is of bacterial origin, while its eukaryotic equivalent isopentenyl pyrophosphate (IPP) is considerably weaker. IPP is commonly accumulated in various tumours due to disruptions in the mevalonate pathway[4]. This can be artificially obtained in in vitro cultures by the addition of N-aminobisphosphonates, most commonly zoledronate. N-aminobisphosphonates are thus used to activate and induce the proliferation of Vδ2 from peripheral blood mononuclear cells (PBMCs), where they disrupt the mevalonate pathway of monocytes and induce the accumulation of IPP therein. This results in a culture of approximately 95%-99% pure Vδ2 cells after 2 weeks[5]. Such products were previously tested for immunotherapy[6].
γδT-based immunotherapy of tumours is a promising avenue that requires substantial fine-tuning. Current in vitro expansion approaches result in products that are safe even in the case of allogeneic γδT[7]. So far, the clinical studies have yielded unsatisfactory results, mostly partial remission or stable disease, with complete remission only in isolated cases[3]. Nevertheless, current products have severely limited efficacy[8]. Thus, a strong need for optimization exists.
In the current paper, we aim to discuss different approaches to Vδ2 expansion protocols with a final immunotherapeutic aim. Thus, we focus on protocols that may be GMP-compliant and enable good scaling.
The traditional approach to γδT (Vδ2) in vitro expansion is a zoledronate-based stimulation of PBMCs with interleukin (IL)-2 added every 2-3 days[5]. Under those settings, external IL-2 is crucial for the proliferation and survival of Vδ2 cells. IL-2 belongs to the common gamma-chain cytokine family[9]. Thus, some efforts were made to test whether IL-2 could be substituted by other family members: (1) IL-4; (2) IL-7; (3) IL-9; (4) IL-15; and (5) IL-21[10]. IL-4 seems to have a negative impact on γδT cytotoxic potential, while the effect of IL-9 is almost unexplored[10]. IL-9 is only known to activate isolated human γδT cells[11]. Interestingly, IL-33, a member of the IL-1 family, is also capable of sustaining γδT proliferation in vitro, although with inferior results to the common gamma-chain cytokines[12,13]. This effect is dependent on conventional T helper (Th) cells, which increased IL-2 production in response to IL-33; at the same time, CD25 was increased on γδT cells. Finally, the addition of IL-33 to IL-2 significantly increased the final yield[13].
The combination of IL-2 and IL-15 significantly improves γδT proliferation in response to zoledronate[14-17]. IL-15 alone promotes the proliferation of γδT cells stimulated with phosphoantigens stronger than IL-2. Moreover, it promotes Th1-related program and improves their cytotoxicity[16,18]. Addition of IL-15 to the standard IL-2 and zoledronate-based expansion protocol does not affect the CD16, CD56, NKG2D, DNAM-1, programmed cell death protein 1 (PD-1), or natural cytotoxicity receptors expression, but it increases the production and release of granzyme B, granulysin, interferon-gamma (IFN-γ), and perforin[18,19]. Interestingly, the addition of IL-15 also increases the Bcl-2 expression and lowers the expression of caspases 3 and 8[20]. IL-15 with zoledronate promotes a shift from late effector memory towards early central memory phenotype in comparison to IL-2 and zoledronate[21]. Moreover, the addition of IL-15 and/or IL-21 facilitates expansion of γδT cells from poorly responding donors[22]. IL-21 seems to have no direct effect on γδT cytotoxic potential, but it promotes IL-10 production and expression of CD73 on γδT cells, thus promoting their regulatory phenotype[22,23]. On the contrary, Thedrez et al[24] reported a significantly increased cytotoxicity of γδT cells cultured with IL-21, suggesting that the exact effect may be target-dependent. Similarly, the addition of IL-21 promoted cytotoxicity of γδT cells against some hepatocellular carcinoma cell lines, but not others[25]. Additionally, IL-21 is known for the selective upregulation of chemokine C-C motif ligand (CCL) 13 in expanded γδT cells[26]. CCL13 is an important chemoattractant for T cells, monocytes and eosinophils[27]. Therefore, an increased expression of CCL13 in γδT may further promote their in vivo immunotherapeutic potential by increasing the immune-cell infiltration of the tumour. Further studies with the assessment of transcriptional changes by RNAseq could potentially explain those discrepancies observed for IL-21.
While IL-2 or other common-gamma-chain cytokines are needed to sustain the proliferation, other cytokines may be added to promote certain phenotypes. Preincubation with IL-12 seems to promote CD56 expression and cytotoxic potential of γδT cells[28]. CD56+ γδT cells have superior cytotoxic potential and higher CD16 expression compared to CD56- ones[29]. The addition of IL-12 also promotes a Th1-like program in γδT cells[30]. Finally, IL-12, along with IL-2 at the beginning of zoledronate-induced expansion, seems to further facilitate proliferation and boost cytotoxic potential[31]. IL-12, along with HMBPP, promotes the similar expansion of Vδ2 cells as HMBPP + IL-2, but significantly increases T-bet expression[32]. Similarly, IL-12 together with IL-15 synergistically increases IFN-γ production by Vδ2 cells[33]. IL-12-dependent increase in IFN-γ production by conventional CD8+ T cells is mediated by modulation of downstream T-cell receptor (TCR) signalling[34]. Finally, IL-12 promotes INF-γ and granzyme B production and cytotoxicity of γδT cells when added alone to zoledronate lines at the end of the expansion phase, but induces significant apoptosis when applied together with IL-18[35]. IL-18, along with IL-2 and zoledronate, provides better proliferation than IL-2 and zoledronate alone[36,37]. Moreover, IL-18 promotes higher production of IFN-γ and tumor necrosis factor (TNF)[37]. IL-18 also restores γδT reactivity in human immunodeficiency virus patients and increases their cytotoxic potential[38]. IL-12, along with IL-18, without any TCR stimulation, seems to significantly improve γδT cytotoxicity[39,40] and increase the expression of granzyme B, IFN-γ, and NKG2D on γδT cells[40]. Moreover, the enhanced expression of CD56 upon IL-18 supplementation was noted[41]. On the other hand, a mixture of IL-12 and IL-18 promotes higher T-cell immunoglobulin and mucin domain-3 expression on γδT cells, which may hamper their cytotoxic potential against cancer[42]. The 24-48 hours preincubation of γδT cells with either IL-15 or IL-18 significantly activates γδT cells and increases their cytotoxic potential[17]. Additionally, IL-18 supplemented along with geranylgeranyl pyrophosphate seems to protect γδT cells from zoledronate toxicity[41]. So far, both IL-12 and IL-18 seem to be promising candidates to further increase the cytotoxic potential of γδT cells, but this should be cautiously explored as they may also increase the checkpoint expression.
Transforming growth factor-beta (TGF-β) is another cytokine that was assessed as the co-stimulation factor for γδT cells. TGF-β seems to promote cytotoxic potential and adhesion molecule expression in IL-2 and zoledronate expanded γδT[43,44]. On the other hand, a mixture of IL-2, IL-15, and TGF-β potently promotes the expansion of FoxP3+ regulatory γδT cells[45]. Moreover, according to Capietto et al[46], 48 hours preincubation of Vδ2 cells with TGF-β lowers their cytotoxic potential. While some studies claim positive effects, others state the opposite; thus, the impact of TGF-β is uncertain. As a short term preincubation of TGF-β seems to increase γδT cytotoxic potential, the effect seems to be time-dependent, but additional studies are need to fully comprehend this phenomenon.
Other cytokines received severely limited attention. TNF-α, but not IFN-γ, is required for efficient γδT proliferation and IFN-γ production after phosphoantigen stimulation, but TNF-α in this setting is released by stimulated PBMCs[47]. IL-7, a less-known common-gamma-chain cytokine, to a limited degree promotes IL-17 production by human γδT cells[48]. Interesting results were noted for IL-23 and IL-27. IL-27 increases IFN-γ and perforin production by γδT cells and their cytotoxic potential[49]. Recent reports suggest that IL-23, along with phosphoantigen, may promote γδT expansion and increase their cytotoxic potential[50]. Cytokines like IL-23 and IL-27, to some degree, also TNF-α seem to be interesting, but further studies are needed to better understand their function.
Overall, the cytokine milieu during expansion is a key factor influencing both the expansion rate and determining the final phenotype. The optimal cytokine environment likely depends on the intended therapeutic target-different phenotypes may be desirable when manufacturing γδT cells for glioblastoma compared to leukemia. Although significant knowledge has already been gained, further studies are needed to fully understand the impact of specific cytokines on γδT cell phenotype. Major findings are summarized in Table 1[5,11,13,18-24,26,31,33,35-37,41,43,45,46,49,51-58].
| Cytokine | Impact of cytokine on Vδ2 T expansion | Concentration | Ref. | |
| Common gamma chain | ||||
| IL-2 | + | Supports robust γδT cell proliferation and enhances cell viability during extended in vitro culture. Drives the cytotoxic phenotype, induces CD107a, IFN-γ, and TNF-α expression in a dose-dependent manner, indicating enhanced degranulation and effector function. Upregulation of early activation markers, such as CD69 and CD25, as well as molecules involved in adhesion and cytotoxicity (LFA-1, ICAM-1, NKG2D, CD94). It promotes a proinflammatory effector phenotype (CD27-, CD28-) and CXCL10, but also an increased expression of KLRG1, 2B4. Upregulate expression of Th1-type cytokines and effector molecules, including IFN-γ, TNF-α, GM-CSF, CCL3, IL-5, and IL-13 | 100-1000 IU/mL | Kondo et al[5], Nada et al[21], Peters et al[51], Mariani et al[52], Li and David Pauza[53] |
| IL-4 | + | Enhances the proliferation, however, weaker than IL-2 and IL-15. Upregulation of early activation markers, e.g. CD69, and LFA-1, ICAM-1, NKG2D, CD94, CD27, CD269 | 10 ng/mL | Vermijlen et al[26] |
| IL-9 | +/- | Enhances the expression of IL-9R mRNA. Upregulates IL-17 and IFN-γ expression | 2 ng/mL | Guggino et al[11] |
| IL-15 | + | Promotes γδT cell proliferation and viability, even at low concentrations. Improves survival by increasing Bcl-2 expression and downregulating apoptotic mediators such as caspases 3 and 8, thereby supporting long-term maintenance of γδT cells in culture. Enhances cytotoxic potential by promoting the production and secretion of granzyme B, perforin, and granulysin. Increases the proportion of CD56+ and CD16+ γδT cells. Induces early activation markers, including CD25 and CD69. Favours the development of an early/central memory phenotype (CD27+, CD28+/-) and, when combined with IL-2, further enhances the expression of CCR9, HLA-DR, CD86, and CD70. Notably, while IL-15 does not significantly modulate the surface levels of CD16, CD56, NKG2D, DNAM-1, PD-1, or other natural cytotoxicity receptors, it functionally boosts downstream effector pathways. IL-15-stimulated γδT cells exhibit robust IFN-γ secretion, reportedly higher than in traditional IL-2-based protocols, while lacking expression and secretion of IL-10, IL-13, and IL-17, suggesting a Th1-skewed, non-regulatory profile | 2-40 IU/mL | Aehnlich et al[18], Dunne et al[19], Sawaisorn et al[20], Nada et al[21], Burnham et al[22], Vermijlen et al[26], Peters et al[43], Chen and Freedman[54], Tyler et al[55], Izumi et al[56], Guo et al[57] |
| IL-21 | +/- | Does not support long-term proliferation of γδT cells following phosphoantigen activation. However, it can enhance expansion in “non-expanders”, i.e., donors who respond poorly to conventional protocols. Enhances cytotoxic potential by upregulating CD107a expression and promoting degranulation. Increases expression of key effector molecules, including granzyme A, granzyme B, and perforin. IL-21 induces early activation markers (CD25, CD69) and upregulates surface molecules involved in adhesion and cytotoxicity, such as CD56, LFA-1, ICAM-1, NKG2D, CD94, and CXCL10. Favours an effector memory phenotype and upregulates regulatory and exhaustion-associated molecules (CD244, CD152, KLRG1). Selectively induces expression of CD73 and overexpression of CXCL13. Promotes a proinflammatory Th1-skewed response, though weaker than IL-2. It stimulates secretion of IFN-γ almost exclusively, but also induces IL-8 and IL-10 production. | 10-30 ng/mL | Burnham et al[22], Barjon et al[23], Thedrez et al[24], Vermijlen et al[26] |
| Others | ||||
| IL-7 | +/- | Enhances expanded γδT cells viability. No effect on the induction of naive CD4+ Vγ9/Vδ2 T cells proliferation. Does not induce HLA-DR expression. | 20-50 ng/mL | Tyler et al[55] |
| IL-12 | +/- | The effects of IL-12 on proliferation are inconsistent. Some studies report limited impact on cell expansion when IL-12 is used alone. However, in specific contexts, particularly when TNF-α is present, IL-12 may support expansion without the need for IL-2. May have dual effects on viability. When combined with IL-18, it induces apoptosis via the c-Jun N-terminal kinase signalling pathway. Alone, IL-12 appears less cytotoxic. IL-12 skews the phenotype toward both central and effector memory. When added to already expanded γδT cells, upregulates granzyme B and perforin expression. It also promotes secretion of perforin, indicating heightened degranulation and effector activity. In combination with IL-18, IL-12 upregulates inhibitory and exhaustion-associated markers such as CTLA-4, PD-1, T cell immunoglobulin and mucin-domain-containing-3, and Lymphocyte activation gene-3. Induces robust production of proinflammatory mediators, including CCL4, GM-CSF, IL-1Ra, IL-12 (autocrine loop), IL-13, TNF-α, IFN-γ, and IFN-α | 10-25 ng/mL | Assy et al[31], García et al[33], Song et al[35] |
| IL-18 | +/- | Promotes expansion of γδT cells by mitigating zoledronate-induced toxicity, indirectly supporting proliferation. Drives the differentiation toward an effector memory phenotype. Enhances cell survival by increasing anti-apoptotic signalling pathways. Upregulates expression of protein kinase B, Bcl-2, phosphorylated I kappa B, and cellular Fas-associated death domain-like interleukin-1 beta-converting enzyme-like inhibitory protein, while downregulating caspase 3 Levels. The effects on cytotoxicity are somewhat contradictory. While some data report downregulation of granzyme B and IFN-γ at the expression level, IL-18 also promotes the generation of CD56brightCD11c+ γδT cells with high cytotoxic cytokine release, co-expressing CD25, CD122, NKG2D, and HLA-DR. Enhances secretion of IFN-γ, TNF-α, and GM-CSF, while not inducing IL-12 production | 10-200 ng/mL | Song et al[35], Sugie et al[36], Li et al[37], Nussbaumer et al[41], Tsuda et al[58] |
| IL-23 | +/- | No conclusive data indicate a direct effect of IL-23 on γδT cell proliferation. Enhances the expression of IL-23R mRNA. Induces elevated IL-17 and IFN-γ expression. Combined with CD26 Ligation, promotes the generation of CD26-negative cytotoxic γδT cells. Enhances CD94, CD226, and granzyme B expression. However, the effect is noted only on CD26-positive cells | 10-50 ng/mL | Guggino et al[11], Capietto et al[46] |
| IL-27 | + | No conclusive data indicate a direct effect of IL-27 on γδT cell proliferation. Enhances cytotoxicity specifically in resting γδT cells, increasing the expression of granzyme A, granzyme B, and perforin. These effects are not observed in already activated or expanded γδT cells. Upregulates CD62 L, suggesting a shift toward a central memory phenotype. It does not affect the expression of CD16 or NKG2A. In activated γδT cells, IL-27 does not affect the secretion of IL-2, IL-4, IL-17A, IFN-γ, IL-10, IL-1β, or TNF-α. In contrast, in resting γδT cells, IL-27 enhances IL-17A secretion. Additionally, it leads to downregulation of Th2-type cytokines | 100 ng/mL | Morandi et al[49] |
| IL-33 | + | Promote in vivo proliferation in dose dose-dependent manner, requiring CD4 T lymphocytes. Exhibit an effector memory phenotype (CD27-, CD45RA-). Elevated cytotoxic potential has been observed, characterised by increased granzyme B, perforin, and CD107a expression. Upregulation of CCR5, CXCR3, NKG2D, CD161, and TNF-related apoptosis-inducing ligand. It does not affect the expression of CD16, CD28, or NKG2S. Moreover, KIR2DL-1, KIR2DL-2, and KIR2DL-3 are not expressed. Promotes secretion of Th1-type cytokines. | 100-1000 ng/mL | Duault et al[13] |
| TGF-β | +/- | Shows contradictory effects on γδT cell expansion. In some settings, it supports robust proliferation of purified Vδ2 T cells. However, when added to already expanded γδT cells, it may inhibit proliferation and delay maturation. Promotes differentiation toward central memory (CD27+, CD45RA-) and effector memory (CD27-, CD45RA+) phenotypes. It also supports the emergence of regulatory-like FoxP3+ γδT cells, especially when combined with IL-15. The impact of TGF-β on cytotoxicity is context-dependent. In some studies, it enhances cytotoxic potential and conjugate formation with tumor cells, associated with CD103 upregulation. However, downregulation of CD107a, absence of intracellular granzyme B, perforin, and IFN-γ, and reduced NKG2D expression have also been observed. Upregulates CCR4, CCR7, CXCR3, CXCR4, LFA-1, CD56, and CD103, while downregulating CCR6, CCR10, CD107a, and NKG2D. It also induces expression of FoxP3, CD25, CD69, CTLA-4, HLA-DR, and CD45RA in certain conditions, particularly in combination with IL-15. Promotes secretion of IFN-γ, TNF-α, IL-9, and granzyme B. However, granzyme A expression is reduced, and perforin levels remain comparable to the standard IL-2 + zoledronate protocol | 1.7-10 ng/mL | Peters et al[43], Casetti et al[45], Capietto et al[46] |
| IFN-γ | 0 | No specific data on proliferation effects have been reported | 1-100 ng/mL | Vermijlen et al[26] |
While the cytokine milieu remains a key determinant of γδT cell expansion, it is not the only extrinsic factor influencing this process. Equally important is the choice of culture media, which can substantially affect the magnitude and phenotype of expanded γδT cells. Media composition, including nutrient content, buffering capacity, and serum supplementation, may synergise with cytokine signalling or, in some cases, impose limiting conditions that skew the expansion outcome. According to Peters et al[51], different media yield significantly different expansion folds, with RPMI-1640 + 10% foetal bovine serum (FBS) having the best results. Usually, zoledronate and direct phosphoantigens (HMBPP) yield similar expansion rates, but this turned out to differ in serum-free complete media[51]. RPMI-1640 supplemented with 10% human AB plasma provides good expansion, although not compared directly, it seems to be similar to that usually observed with 10% FBS[59].
Serum-free media are especially interesting from the translational perspective-while a wide selection of those is available, only some have been tested. According to Sutton et al[60], serum-free media are far less effective for γδT expansion than RPMI-1640 with FBS. On the other hand, Zhou et al[61] reported better proliferation in those same serum-free media than in RPMI with FBS. AIM-V gives similar yields to RPMI-1640, while much higher (up to 5-fold) yields were observed for OpTimizer[62]. However, OpTimizer showed approximately 12% of NK cells in the final product and thus had approximately 10% lower purity than the remaining two[62]. At the same time, γδT cells from OpTimizer had a 2-fold higher expression of CD56 (almost 50%), suggesting higher cytotoxic potential[62].
In addition to cytokines and media, the specific stimulation protocol employed plays a prominent role in shaping γδT cell growth. Among these, zoledronate-based and, more broadly, N-aminobisphosphonate-driven protocols are currently the most prevalent due to their simplicity and efficacy. However, alternative strategies are increasingly being explored. The majority of those are based on direct phosphoantigen stimulation-see Table 2[63-67] for the overview of available phosphoantigens and Table 3[68-71] for the comparison of different N-aminobisphosphonates.
| Abbrev name | Full name | Relative strength | Source | Stability in aqueous solution | Ref. |
| IPP | Isopentenyl pyrophosphate | Approximately 1000 × weaker than HMBPP | Eucaryotic cells | Stable | Latha et al[63], Vantourout et al[64] |
| HMBPP | (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate | Most potent | Prokaryotic cells | Not very stable | Kilcollins et al[65] |
| BrHPP | Bromohydrin pyrophosphate | Stronger than isopentenyl pyrophosphate, weaker than HMBPP | Synthetic | Stable | Sicard et al[66] |
| MEP | Monoethyl phosphate | Unknown | Prokaryotic cells | Stable | Tanaka et al[67] |
| Name | Food and Drug Administration approval | Water solubility | Relative potency1 | Ref. |
| Zoledronate | Yes | 4.17 mg/mL | 10000 × | Kondo et al[68], Ruggiero et al[69] |
| Pamidronate | Yes | 50 mg/mL | 100 × | Ruggiero et al[69], Kunzmann et al[70] |
| Alendronate | Yes | 20 mg/mL | 1000 × | Ruggiero et al[69] |
| Ibandronate | Yes | 72 mg/mL | 5000 × | Ruggiero et al[69] |
| Risedronate | Yes | 11 mg/mL | 5000 × | Ruggiero et al[69] |
| Minodronate | No | 20 mg/mL2 | 10000 × | Ohishi and Matsuyama[71] |
| Neridronate | No | 5 mg/mL2 | 100 × | Ruggiero et al[69] |
| Olpadronate | No | 50 mg/mL2 | 1000 × | Ruggiero et al[69] |
In theory, any N-aminobisphosphonate can be used to stimulate γδT expansion from PBMCs. In practice, they differ in their potency of farnesyl pyrophosphate synthase (FPPS) inhibition-the IC50 for inhibition is inversely correlated to the expected proliferation response[72]. As mentioned, zoledronate is the most commonly used one and also one of the most potent FPPS inhibitors[62,72]. Titration experiments suggest an optimal concentration of approximately 1-3 μmol/L, both lower and higher doses give inferior results[73,74]. A novel compound, tetrakis-pivaloyloxymethyl 2-(thiazole-2-ylamino)ethylidene-1,1-bisphosphonate, showed very promising results, with far better response than that to zoledronate[75]. Pamidronate, on the other hand, produces unsatisfactory outcome, usually comparable to direct IPP stimulation[76]. Neridronate showed reasonable expansion results, but it was directly compared to zoledronate at suboptimal doses (5-10-fold lower than the optimal)[77]. Alendronate shows expansion similar to that induced by direct phosphoantigen stimulation with bromohydrin pyrophosphate (BrHPP)[78]. To date, there is no direct comparison of various n-aminobisphosphonates at optimal concentration. Thus, it is not possible to draw a definitive conclusion, but it appears that zoledronate is the optimal choice after all.
Different phosphoantigens were tested for γδT expansion in vitro. HMBPP is one of the most potent and widely utilised phosphoantigens; it promotes significant proliferation of γδT cells and induces the Th1-like program[65]. Monoethyl phosphate, similarly to other, better-known phosphoantigens, induces the proliferation of Vδ2 cells, comparable to that induced by Mycobacterium tuberculosis lysates[67]. A good proliferative response can also be achieved with BrHPP, a synthetic phosphoantigen with a moderate activation strength[79,80]. IPP, the only eukaryotic phosphoantigen, can also be used successfully for γδT expansion in vitro with an optimal concentration of approximately 2 μg/mL and IL-2 at around 100 IU/mL. The doses of IL-2 higher than 1000 IU/mL yielded significantly lower final cell counts after 2 weeks, despite initially showing a much better growth rate, an effect that was most likely related to overactivation and activation-induced cell death[81]. Nevertheless, IPP yielded cultures with approximately 50% purity, far inferior to the zoledronate and HMBPP. Interestingly, Nada et al[21] observed better proliferation of Vδ2 cells in response to HMBPP than to zoledronate. While this is in contrast to our own observations (unpublished), this may be due to the use of frozen PBMCs. Zoledronate proliferation requires functional myeloid cells[82,83], whereas freezing may significantly impact their viability.
Stimulation with zoledronate at concentrations exceeding 10 μM significantly decreases the Vδ2 proliferative response after prolonged exposure; however, doses of up to 100 μM can be used if PBMCs are washed with a clear medium after approximately 4 hours[21]. Interestingly, this pulse treatment resulted in an overall similar purity but a higher cell count after 14 days. Notably, the pulse treatment resulted in better degranulation and higher expression of perforin[21]. As recently suggested, blockage of the mevalonate pathway by zoledronate also affects human γδT cells, substantially impacting their cytotoxic potential (Figure 1)[84]. Pulse treatment may be a solution to overcome this impairment.
Finally, apart from the classical approach discussed in the current paper, one can also try conventional T cell expansion protocols that may require prior γδT cell purification or a protocol based on artificial antigen-presenting cells[85,86]. While those approaches may be important, they are not within the scope of the current review.
Beyond the foundational cytokine, media, and stimulation components, further optimisation of in vitro expansion can be achieved by introducing additional co-stimulatory signals. In our laboratory, we have tested various bacterial lysates with some positive results (unpublished data). Interestingly, this improvement may be mediated via Toll-like receptor (TLR). Indeed, TLR agonists [resiquimod (TLR7/8), flagellin from Salmonella typhimurium (TLR5), multiple primary lung adenocarcinomas (TLR4), lipopolysaccharide (TLR4), CL429 (TLR2 and nucleotide-binding oligomerization domain 2), PAM3CSK4 (TLR1/2)] improve the proliferative response of Vδ2 after PBMC stimulation with IPP[87]. Resiquimod itself provoked only a very limited proliferative response[87]. Similarly, a better proliferation was observed when resiquimod was added to the anti-TCRγδ antibody[87]. Moreover, Vδ2 cells obtained by IPP + resiquimod stimulation have lower PD-1 expression while at the same time having better degranulation response and higher expression of IFN-γ, TNF-α, and Granzyme B, when compared to both IPP and zoledronate[87]. Moreover, those cells were approximately twice as cytotoxic against melanoma cells, both in vitro and in vivo, in the orthotopic mouse model[87]. Interestingly, resiquimod, when added along with zoledronate, tended to decrease proliferation[87,88]. Similarly, polyinosinic-polycytidylic acid (TLR3 agonist) significantly improves γδT proliferation in response to IPP. This effect is mediated by dendritic cells and is dependent on IFN-α[89]. This also suggests that some of those results could be mimicked by additional IFN-α added to the culture.
Despite these various technical refinements, it is important to acknowledge that expansion outcomes remain highly donor-dependent. Substantial inter-individual variability in γδT cell responsiveness is a well-documented phenomenon[22,87], which may be influenced by prior antigen exposures or intrinsic functional states. This variability represents a key challenge in standardising expansion protocols. Nevertheless, the underlying cause remains largely a mystery. Burnham et al[22] attempted to categorise the donors into expanders and non-expanders, observing that non-expanders tended to have a more sedentary lifestyle. Apart from that, they also analysed the expression of chemokine receptors, without noticing any differences. Interestingly, the addition of IL-21 or IL-21 and IL-15 significantly narrowed the proliferative gap between expanders and non-expanders[22]. Interestingly, a high neutrophil-to-lymphocyte ratio predicts poor expansion, at least in cancer patients, where it may be related to granulocyte myeloid-derived suppressor cells[90]. However, if zoledronate-preatreated dendritic cells are used as a stimulus, then proper proliferation is restored, suggesting that, indeed, the problem is not with γδT per se[90]. On the other hand, Nicol et al[59] observed significant differences in γδT cells before expansion; non-expanders had higher γδT cells with a phenotype of CD45RA+ effector memory and naive T cells, while good responders were skewed towards the effector memory and central memory phenotypes before expansion. Some predictive value also lies in the initial Vδ2 percentage and the age of donors, with better results for young individuals with higher Vδ2 count[91].
The ratio of non-expanders rises in multiple cancers, especially in haematological ones, where over 50% of patients, for chronic lymphocytic leukaemia even over 80%, should be classified as non-responders in contrast to approximately 10% of healthy individuals[92]. This highlights the impact of immunosuppressive microenvironment, possibly also on the dysregulation of butyrophilin, as we have observed in chronic lymphocytic leukaemia patients[93]. Indeed, Tomogane et al[94] noted a modest correlation between expansion efficiency and BTN3A1 expression in monocytes. Our own, yet unpublished, data do not support this finding-we have not observed any differences in proliferation between patients with low or high expression of BTN3A, either in monocytes or T cells.
Apart from in vitro expansion, there were also attempts to stimulate it in vivo in cancer patients. Unfortunately, no proliferation in vivo in response to pamidronate and IL-2 was observed in a cohort of haematological cancer patients. Those patients who responded to that treatment showed a clear proliferative response in vitro as well. Still, only minimal clinical efficacy was noted[92]. Similarly, no significant in vivo proliferation or satisfactory clinical outcome was observed in renal cell carcinoma and prostate cancer patients treated with zoledronate and IL-2[95,96]. Wilhelm et al[97] tried an adoptive transfer of haploidentical αβ-depleted PBMCs to haematological patients after induction chemotherapy. After the transfer, patients were given IL-2 and zoledronate; a notable proliferation of γδT cells was observed, as well as short-term clinical response[97]. While some efficacy was noted, the results of in vivo attempts are rather discouraging, possibly due to the highly immunosuppressive environment in cancer patients.
Apart from the classical, most widely used, expansion protocol, based on zoledronate and IL-2, there are several alternatives. Despite numerous studies published to date, it is challenging to identify the most effective protocol. Recent data regarding the negative impact of mevalonate blockade on γδT cells adds another layer of complexity; it seems viable to further test direct phosphoantigen stimulation instead of the N-aminobisphosphonate-based one. In parallel, future research should aim to identify predictive biomarkers of γδT cell expandability, which could enable pre-selection of optimal donors and tailoring of expansion strategies. Immunophenotypic features, such as baseline expression of activation or exhaustion markers, as well as metabolic or transcriptomic profiles, may serve as promising candidates and should be systematically investigated.
| 1. | Zarobkiewicz MK, Bojarska-Junak AA. The Mysterious Actor-γδ T Lymphocytes in Chronic Lymphocytic Leukaemia (CLL). Cells. 2022;11:661. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | Pang DJ, Neves JF, Sumaria N, Pennington DJ. Understanding the complexity of γδ T-cell subsets in mouse and human. Immunology. 2012;136:283-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 154] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 3. | Kabelitz D, Serrano R, Kouakanou L, Peters C, Kalyan S. Cancer immunotherapy with γδ T cells: many paths ahead of us. Cell Mol Immunol. 2020;17:925-939. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 204] [Cited by in RCA: 228] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 4. | Kabelitz D, Lettau M, Janssen O. Immunosurveillance by human γδ T lymphocytes: the emerging role of butyrophilins. F1000Res. 2017;6:F1000 Faculty Rev-F1000 Faculty 782. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Kondo M, Izumi T, Fujieda N, Kondo A, Morishita T, Matsushita H, Kakimi K. Expansion of human peripheral blood γδ T cells using zoledronate. J Vis Exp. 2011;3182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 6. | Deniger DC, Moyes JS, Cooper LJ. Clinical applications of gamma delta T cells with multivalent immunity. Front Immunol. 2014;5:636. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 97] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 7. | Xu Y, Xiang Z, Alnaggar M, Kouakanou L, Li J, He J, Yang J, Hu Y, Chen Y, Lin L, Hao J, Li J, Chen J, Li M, Wu Q, Peters C, Zhou Q, Li J, Liang Y, Wang X, Han B, Ma M, Kabelitz D, Xu K, Tu W, Wu Y, Yin Z. Allogeneic Vγ9Vδ2 T-cell immunotherapy exhibits promising clinical safety and prolongs the survival of patients with late-stage lung or liver cancer. Cell Mol Immunol. 2021;18:427-439. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 195] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 8. | Pauza CD, Liou ML, Lahusen T, Xiao L, Lapidus RG, Cairo C, Li H. Gamma Delta T Cell Therapy for Cancer: It Is Good to be Local. Front Immunol. 2018;9:1305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 82] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 9. | Wolfarth AA, Dhar S, Goon JB, Ezeanya UI, Ferrando-Martínez S, Lee BH. Advancements of Common Gamma-Chain Family Cytokines in Cancer Immunotherapy. Immune Netw. 2022;22:e5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Van Acker HH, Campillo-Davo D, Roex G, Versteven M, Smits EL, Van Tendeloo VF. The role of the common gamma-chain family cytokines in γδ T cell-based anti-cancer immunotherapy. Cytokine Growth Factor Rev. 2018;41:54-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Guggino G, Ciccia F, Di Liberto D, Lo Pizzo M, Ruscitti P, Cipriani P, Ferrante A, Sireci G, Dieli F, Fourniè JJ, Giacomelli R, Triolo G. Interleukin (IL)-9/IL-9R axis drives γδ T cells activation in psoriatic arthritis patients. Clin Exp Immunol. 2016;186:277-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 12. | Cayrol C, Girard JP. Interleukin-33 (IL-33): A critical review of its biology and the mechanisms involved in its release as a potent extracellular cytokine. Cytokine. 2022;156:155891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 197] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 13. | Duault C, Franchini DM, Familliades J, Cayrol C, Roga S, Girard JP, Fournié JJ, Poupot M. TCRVγ9 γδ T Cell Response to IL-33: A CD4 T Cell-Dependent Mechanism. J Immunol. 2016;196:493-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Khan MWA, Otaibi AA, Sherwani S, Alshammari EM, Al-Zahrani SA, Khan WA, Alsukaibi AKD, Alouffi S, Khan SN. Optimization of methods for peripheral blood mononuclear cells isolation and expansion of human gamma delta T cells. Bioinformation. 2021;17:460-469. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Khan MW, Curbishley SM, Chen HC, Thomas AD, Pircher H, Mavilio D, Steven NM, Eberl M, Moser B. Expanded Human Blood-Derived γδT Cells Display Potent Antigen-Presentation Functions. Front Immunol. 2014;5:344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 16. | Van Acker HH, Anguille S, Willemen Y, Van den Bergh JM, Berneman ZN, Lion E, Smits EL, Van Tendeloo VF. Interleukin-15 enhances the proliferation, stimulatory phenotype, and antitumor effector functions of human gamma delta T cells. J Hematol Oncol. 2016;9:101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 100] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 17. | Field KR, Wragg KM, Lee WS, Rigau M, Uldrich AP, Kent SJ, Juno JA. Cytokines enhance human Vγ9Vδ2 T-cell TCR-dependent and TCR-independent effector functions. Eur J Immunol. 2023;53:e2250220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 18. | Aehnlich P, Carnaz Simões AM, Skadborg SK, Holmen Olofsson G, Thor Straten P. Expansion With IL-15 Increases Cytotoxicity of Vγ9Vδ2 T Cells and Is Associated With Higher Levels of Cytotoxic Molecules and T-bet. Front Immunol. 2020;11:1868. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 19. | Dunne MR, Mangan BA, Madrigal-Estebas L, Doherty DG. Preferential Th1 cytokine profile of phosphoantigen-stimulated human Vγ9Vδ2 T cells. Mediators Inflamm. 2010;2010:704941. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Sawaisorn P, Gaballa A, Saimuang K, Leepiyasakulchai C, Lertjuthaporn S, Hongeng S, Uhlin M, Jangpatarapongsa K. Human Vγ9Vδ2 T cell expansion and their cytotoxic responses against cholangiocarcinoma. Sci Rep. 2024;14:1291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 21. | Nada MH, Wang H, Workalemahu G, Tanaka Y, Morita CT. Enhancing adoptive cancer immunotherapy with Vγ2Vδ2 T cells through pulse zoledronate stimulation. J Immunother Cancer. 2017;5:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 22. | Burnham RE, Zoine JT, Story JY, Garimalla SN, Gibson G, Rae A, Williams E, Bixby L, Archer D, Doering CB, Spencer HT. Characterization of Donor Variability for γδ T Cell ex vivo Expansion and Development of an Allogeneic γδ T Cell Immunotherapy. Front Med (Lausanne). 2020;7:588453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 23. | Barjon C, Michaud HA, Fages A, Dejou C, Zampieri A, They L, Gennetier A, Sanchez F, Gros L, Eliaou JF, Bonnefoy N, Lafont V. IL-21 promotes the development of a CD73-positive Vγ9Vδ2 T cell regulatory population. Oncoimmunology. 2017;7:e1379642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 24. | Thedrez A, Harly C, Morice A, Salot S, Bonneville M, Scotet E. IL-21-mediated potentiation of antitumor cytolytic and proinflammatory responses of human V gamma 9V delta 2 T cells for adoptive immunotherapy. J Immunol. 2009;182:3423-3431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 59] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 25. | Jiang H, Yang Z, Song Z, Green M, Song H, Shao Q. γδ T cells in hepatocellular carcinoma patients present cytotoxic activity but are reduced in potency due to IL-2 and IL-21 pathways. Int Immunopharmacol. 2019;70:167-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 26. | Vermijlen D, Ellis P, Langford C, Klein A, Engel R, Willimann K, Jomaa H, Hayday AC, Eberl M. Distinct cytokine-driven responses of activated blood gammadelta T cells: insights into unconventional T cell pleiotropy. J Immunol. 2007;178:4304-4314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 119] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 27. | Li L, Dai F, Wang L, Sun Y, Mei L, Ran Y, Ye F. CCL13 and human diseases. Front Immunol. 2023;14:1176639. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 36] [Reference Citation Analysis (0)] |
| 28. | Satoh M, Seki S, Hashimoto W, Ogasawara K, Kobayashi T, Kumagai K, Matsuno S, Takeda K. Cytotoxic gammadelta or alphabeta T cells with a natural killer cell marker, CD56, induced from human peripheral blood lymphocytes by a combination of IL-12 and IL-2. J Immunol. 1996;157:3886-3892. [PubMed] |
| 29. | Alexander AA, Maniar A, Cummings JS, Hebbeler AM, Schulze DH, Gastman BR, Pauza CD, Strome SE, Chapoval AI. Isopentenyl pyrophosphate-activated CD56+ {gamma}{delta} T lymphocytes display potent antitumor activity toward human squamous cell carcinoma. Clin Cancer Res. 2008;14:4232-4240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 140] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 30. | Ness-Schwickerath KJ, Jin C, Morita CT. Cytokine requirements for the differentiation and expansion of IL-17A- and IL-22-producing human Vgamma2Vdelta2 T cells. J Immunol. 2010;184:7268-7280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 168] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 31. | Assy L, Khalil SM, Attia M, Salem ML. IL-12 conditioning of peripheral blood mononuclear cells from breast cancer patients promotes the zoledronate-induced expansion of γδ T cells in vitro and enhances their cytotoxic activity and cytokine production. Int Immunopharmacol. 2023;114:109402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 32. | Yang R, Yao L, Shen L, Sha W, Modlin RL, Shen H, Chen ZW. IL-12 Expands and Differentiates Human Vγ2Vδ2 T Effector Cells Producing Antimicrobial Cytokines and Inhibiting Intracellular Mycobacterial Growth. Front Immunol. 2019;10:913. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 33. | García VE, Jullien D, Song M, Uyemura K, Shuai K, Morita CT, Modlin RL. IL-15 enhances the response of human gamma delta T cells to nonpeptide [correction of nonpetide] microbial antigens. J Immunol. 1998;160:4322-4329. [PubMed] |
| 34. | Vacaflores A, Freedman SN, Chapman NM, Houtman JC. Pretreatment of activated human CD8 T cells with IL-12 leads to enhanced TCR-induced signaling and cytokine production. Mol Immunol. 2017;81:1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 35. | Song Y, Teo HY, Liu Y, Zhang X, Chen J, Zhang Y, Liu H. Reviving human γδT cells from apoptosis induced by IL-12/18 via p-JNK inhibition. J Leukoc Biol. 2022;112:1701-1716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 36. | Sugie T, Murata-Hirai K, Iwasaki M, Morita CT, Li W, Okamura H, Minato N, Toi M, Tanaka Y. Zoledronic acid-induced expansion of γδ T cells from early-stage breast cancer patients: effect of IL-18 on helper NK cells. Cancer Immunol Immunother. 2013;62:677-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 37. | Li W, Kubo S, Okuda A, Yamamoto H, Ueda H, Tanaka T, Nakamura H, Yamanishi H, Terada N, Okamura H. Effect of IL-18 on expansion of gammadelta T cells stimulated by zoledronate and IL-2. J Immunother. 2010;33:287-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 38. | Murday AS, Chaudhry S, Pauza CD. Interleukin-18 activates Vγ9Vδ2(+) T cells from HIV-positive individuals: recovering the response to phosphoantigen. Immunology. 2017;151:385-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 39. | Schilbach K, Welker C, Krickeberg N, Kaißer C, Schleicher S, Hashimoto H. In the Absence of a TCR Signal IL-2/IL-12/18-Stimulated γδ T Cells Demonstrate Potent Anti-Tumoral Function Through Direct Killing and Senescence Induction in Cancer Cells. Cancers (Basel). 2020;12:130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 40. | Domae E, Hirai Y, Ikeo T, Goda S, Shimizu Y. Cytokine-mediated activation of human ex vivo-expanded Vγ9Vδ2 T cells. Oncotarget. 2017;8:45928-45942. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 41. | Nussbaumer O, Gruenbacher G, Gander H, Komuczki J, Rahm A, Thurnher M. Essential requirements of zoledronate-induced cytokine and γδ T cell proliferative responses. J Immunol. 2013;191:1346-1355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 42. | Schofield L, Ioannidis LJ, Karl S, Robinson LJ, Tan QY, Poole DP, Betuela I, Hill DL, Siba PM, Hansen DS, Mueller I, Eriksson EM. Synergistic effect of IL-12 and IL-18 induces TIM3 regulation of γδ T cell function and decreases the risk of clinical malaria in children living in Papua New Guinea. BMC Med. 2017;15:114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 43. | Peters C, Meyer A, Kouakanou L, Feder J, Schricker T, Lettau M, Janssen O, Wesch D, Kabelitz D. TGF-β enhances the cytotoxic activity of Vδ2 T cells. Oncoimmunology. 2019;8:e1522471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 44. | Beatson RE, Parente-Pereira AC, Halim L, Cozzetto D, Hull C, Whilding LM, Martinez O, Taylor CA, Obajdin J, Luu Hoang KN, Draper B, Iqbal A, Hardiman T, Zabinski T, Man F, de Rosales RTM, Xie J, Aswad F, Achkova D, Joseph CR, Ciprut S, Adami A, Roider HG, Hess-Stumpp H, Győrffy B, Quist J, Grigoriadis A, Sommer A, Tutt ANJ, Davies DM, Maher J. TGF-β1 potentiates Vγ9Vδ2 T cell adoptive immunotherapy of cancer. Cell Rep Med. 2021;2:100473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 45. | Casetti R, Agrati C, Wallace M, Sacchi A, Martini F, Martino A, Rinaldi A, Malkovsky M. Cutting edge: TGF-beta1 and IL-15 Induce FOXP3+ gammadelta regulatory T cells in the presence of antigen stimulation. J Immunol. 2009;183:3574-3577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 145] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 46. | Capietto AH, Martinet L, Cendron D, Fruchon S, Pont F, Fournié JJ. Phosphoantigens overcome human TCRVgamma9+ gammadelta Cell immunosuppression by TGF-beta: relevance for cancer immunotherapy. J Immunol. 2010;184:6680-6687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 47. | Li H, Luo K, Pauza CD. TNF-alpha is a positive regulatory factor for human Vgamma2 Vdelta2 T cells. J Immunol. 2008;181:7131-7137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 48. | Michel ML, Pang DJ, Haque SF, Potocnik AJ, Pennington DJ, Hayday AC. Interleukin 7 (IL-7) selectively promotes mouse and human IL-17-producing γδ cells. Proc Natl Acad Sci U S A. 2012;109:17549-17554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 184] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 49. | Morandi F, Prigione I, Airoldi I. Human TCRγδ+ T cells represent a novel target for IL-27 activity. Eur J Immunol. 2012;42:1547-1552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 50. | Wragg KM, Tan HX, Kristensen AB, Nguyen-Robertson CV, Kelleher AD, Parsons MS, Wheatley AK, Berzins SP, Pellicci DG, Kent SJ, Juno JA. High CD26 and Low CD94 Expression Identifies an IL-23 Responsive Vδ2(+) T Cell Subset with a MAIT Cell-like Transcriptional Profile. Cell Rep. 2020;31:107773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 51. | Peters C, Kouakanou L, Oberg HH, Wesch D, Kabelitz D. In vitro expansion of Vγ9Vδ2 T cells for immunotherapy. Methods Enzymol. 2020;631:223-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 52. | Mariani S, Muraro M, Pantaleoni F, Fiore F, Nuschak B, Peola S, Foglietta M, Palumbo A, Coscia M, Castella B, Bruno B, Bertieri R, Boano L, Boccadoro M, Massaia M. Effector gammadelta T cells and tumor cells as immune targets of zoledronic acid in multiple myeloma. Leukemia. 2005;19:664-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 107] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 53. | Li H, David Pauza C. Interplay of T-cell receptor and interleukin-2 signalling in Vγ2Vδ2 T-cell cytotoxicity. Immunology. 2011;132:96-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 54. | Chen Z, Freedman MS. Correlation of specialized CD16(+) gammadelta T cells with disease course and severity in multiple sclerosis. J Neuroimmunol. 2008;194:147-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 55. | Tyler CJ, McCarthy NE, Lindsay JO, Stagg AJ, Moser B, Eberl M. Antigen-Presenting Human γδ T Cells Promote Intestinal CD4(+) T Cell Expression of IL-22 and Mucosal Release of Calprotectin. J Immunol. 2017;198:3417-3425. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 56. | Izumi T, Kondo M, Takahashi T, Fujieda N, Kondo A, Tamura N, Murakawa T, Nakajima J, Matsushita H, Kakimi K. Ex vivo characterization of γδ T-cell repertoire in patients after adoptive transfer of Vγ9Vδ2 T cells expressing the interleukin-2 receptor β-chain and the common γ-chain. Cytotherapy. 2013;15:481-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 57. | Guo BL, Hollmig KA, Lopez RD. Down-regulation of IL-2 receptor alpha (CD25) characterizes human gammadelta-T cells rendered resistant to apoptosis after CD2 engagement in the presence of IL-12. Cancer Immunol Immunother. 2002;50:625-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 58. | Tsuda J, Li W, Yamanishi H, Yamamoto H, Okuda A, Kubo S, Ma Z, Terada N, Tanaka Y, Okamura H. Involvement of CD56brightCD11c+ cells in IL-18-mediated expansion of human γδ T cells. J Immunol. 2011;186:2003-2012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 59. | Nicol AJ, Tokuyama H, Mattarollo SR, Hagi T, Suzuki K, Yokokawa K, Nieda M. Clinical evaluation of autologous gamma delta T cell-based immunotherapy for metastatic solid tumours. Br J Cancer. 2011;105:778-786. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 209] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 60. | Sutton K, Dasgupta A, David M, Doering C, Spencer HT. 402. Bioengineering of Peripheral Blood Derived Gamma Delta T Cells in a Serum-Free Expansion Medium. Mol Ther. 2016;24:S159. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 61. | Zhou JH, Kang N, Cui LX, He W. [Selection of culture media for the mass production of gamma delta T cells used in adoptive immunotherapy]. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2011;33:644-648. [PubMed] |
| 62. | Sato K, Kondo M, Sakuta K, Hosoi A, Noji S, Sugiura M, Yoshida Y, Kakimi K. Impact of culture medium on the expansion of T cells for immunotherapy. Cytotherapy. 2009;11:936-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 63. | Latha TS, Reddy MC, Durbaka PV, Rachamallu A, Pallu R, Lomada D. γδ T Cell-Mediated Immune Responses in Disease and Therapy. Front Immunol. 2014;5:571. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 64. | Vantourout P, Mookerjee-Basu J, Rolland C, Pont F, Martin H, Davrinche C, Martinez LO, Perret B, Collet X, Périgaud C, Peyrottes S, Champagne E. Specific requirements for Vgamma9Vdelta2 T cell stimulation by a natural adenylated phosphoantigen. J Immunol. 2009;183:3848-3857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 65. | Kilcollins AM, Li J, Hsiao CH, Wiemer AJ. HMBPP Analog Prodrugs Bypass Energy-Dependent Uptake To Promote Efficient BTN3A1-Mediated Malignant Cell Lysis by Vγ9Vδ2 T Lymphocyte Effectors. J Immunol. 2016;197:419-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 66. | Sicard H, Ingoure S, Luciani B, Serraz C, Fournié JJ, Bonneville M, Tiollier J, Romagné F. In vivo immunomanipulation of V gamma 9V delta 2 T cells with a synthetic phosphoantigen in a preclinical nonhuman primate model. J Immunol. 2005;175:5471-5480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 166] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 67. | Tanaka Y, Sano S, Nieves E, De Libero G, Rosa D, Modlin RL, Brenner MB, Bloom BR, Morita CT. Nonpeptide ligands for human gamma delta T cells. Proc Natl Acad Sci U S A. 1994;91:8175-8179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 298] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 68. | Kondo M, Sakuta K, Noguchi A, Ariyoshi N, Sato K, Sato S, Sato K, Hosoi A, Nakajima J, Yoshida Y, Shiraishi K, Nakagawa K, Kakimi K. Zoledronate facilitates large-scale ex vivo expansion of functional gammadelta T cells from cancer patients for use in adoptive immunotherapy. Cytotherapy. 2008;10:842-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 138] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 69. | Ruggiero A, Triarico S, Romano A, Maurizi P, Attina G, Mastrangelo S. Bisphosphonates: From Pharmacology to Treatment. Biomed Pharmacol J. 2023;16:221-229. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 70. | Kunzmann V, Bauer E, Feurle J, Weissinger F, Tony HP, Wilhelm M. Stimulation of gammadelta T cells by aminobisphosphonates and induction of antiplasma cell activity in multiple myeloma. Blood. 2000;96:384-392. [PubMed] |
| 71. | Ohishi T, Matsuyama Y. Minodronate for the treatment of osteoporosis. Ther Clin Risk Manag. 2018;14:729-739. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 72. | Benzaïd I, Mönkkönen H, Bonnelye E, Mönkkönen J, Clézardin P. In vivo phosphoantigen levels in bisphosphonate-treated human breast tumors trigger Vγ9Vδ2 T-cell antitumor cytotoxicity through ICAM-1 engagement. Clin Cancer Res. 2012;18:6249-6259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 73. | Sato K, Kimura S, Segawa H, Yokota A, Matsumoto S, Kuroda J, Nogawa M, Yuasa T, Kiyono Y, Wada H, Maekawa T. Cytotoxic effects of gammadelta T cells expanded ex vivo by a third generation bisphosphonate for cancer immunotherapy. Int J Cancer. 2005;116:94-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 110] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 74. | Hironaka K, Okawaki M, Yamamura M, Yamaguchi Y. Generation of γδT cells. Biotherapy. 2008;22:303-308. |
| 75. | Okuno D, Sugiura Y, Sakamoto N, Tagod MSO, Iwasaki M, Noda S, Tamura A, Senju H, Umeyama Y, Yamaguchi H, Suematsu M, Morita CT, Tanaka Y, Mukae H. Comparison of a Novel Bisphosphonate Prodrug and Zoledronic Acid in the Induction of Cytotoxicity in Human Vγ2Vδ2 T Cells. Front Immunol. 2020;11:1405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 76. | Xi Y, Miao T, Wan L, Wang Y, Feng T, Gong T, Li M. [Amplification efficency and optimization of culture conditions of γδ T cells in peripheral blood by different phosphate compounds]. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2014;30:868-871. [PubMed] |
| 77. | Ferlazzo V, Sferrazza C, Caccamo N, Di Fede G, Di Lorenzo G, D'Asaro M, Meraviglia S, Dieli F, Rini G, Salerno A. In vitro effects of aminobisphosphonates on Vgamma9Vdelta2 T cell activation and differentiation. Int J Immunopathol Pharmacol. 2006;19:309-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 78. | Kabelitz D, Wesch D, Pitters E, Zöller M. Characterization of tumor reactivity of human V gamma 9V delta 2 gamma delta T cells in vitro and in SCID mice in vivo. J Immunol. 2004;173:6767-6776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 136] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 79. | Bennouna J, Bompas E, Neidhardt EM, Rolland F, Philip I, Galéa C, Salot S, Saiagh S, Audrain M, Rimbert M, Lafaye-de Micheaux S, Tiollier J, Négrier S. Phase-I study of Innacell gammadelta, an autologous cell-therapy product highly enriched in gamma9delta2 T lymphocytes, in combination with IL-2, in patients with metastatic renal cell carcinoma. Cancer Immunol Immunother. 2008;57:1599-1609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 205] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 80. | Salot S, Laplace C, Saïagh S, Bercegeay S, Tenaud I, Cassidanius A, Romagne F, Dreno B, Tiollier J. Large scale expansion of gamma 9 delta 2 T lymphocytes: Innacell gamma delta cell therapy product. J Immunol Methods. 2007;326:63-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 81. | Wang RN, Wen Q, He WT, Yang JH, Zhou CY, Xiong WJ, Ma L. Optimized protocols for γδ T cell expansion and lentiviral transduction. Mol Med Rep. 2019;19:1471-1480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 82. | Roelofs AJ, Jauhiainen M, Mönkkönen H, Rogers MJ, Mönkkönen J, Thompson K. Peripheral blood monocytes are responsible for gammadelta T cell activation induced by zoledronic acid through accumulation of IPP/DMAPP. Br J Haematol. 2009;144:245-250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 202] [Cited by in RCA: 224] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 83. | Fowler DW, Copier J, Dalgleish AG, Bodman-Smith MD. Zoledronic acid causes γδ T cells to target monocytes and down-modulate inflammatory homing. Immunology. 2014;143:539-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 84. | Suen TK, Al B, Ulas T, Reusch N, Bahrar H, Bekkering S, Bhat J, Kabelitz D, Schultze JL, van de Veerdonk FL, van Lennep JR, Riksen NP, Joosten LAB, Netea MG, Placek K. Human γδ T Cell Function Is Impaired Upon Mevalonate Pathway Inhibition. Immunology. 2025;175:300-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 85. | Boucher JC, Yu B, Li G, Shrestha B, Sallman D, Landin AM, Cox C, Karyampudi K, Anasetti C, Davila ML, Bejanyan N. Large Scale Ex Vivo Expansion of γδ T cells Using Artificial Antigen-presenting Cells. J Immunother. 2023;46:5-13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 86. | Verkerk T, Pappot AT, Jorritsma T, King LA, Duurland MC, Spaapen RM, van Ham SM. Isolation and expansion of pure and functional γδ T cells. Front Immunol. 2024;15:1336870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 87. | Wang H, Chen H, Liu S, Zhang J, Lu H, Somasundaram R, Choi R, Zhang G, Ou L, Scholler J, Tian S, Dong L, Yeye G, Huang L, Connelly T, Li L, Huang A, Mitchell TC, Fan Y, June CH, Mills GB, Guo W, Herlyn M, Xu X. Costimulation of γδTCR and TLR7/8 promotes Vδ2 T-cell antitumor activity by modulating mTOR pathway and APC function. J Immunother Cancer. 2021;9:e003339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 88. | Serrano R, Wesch D, Kabelitz D. Activation of Human γδ T Cells: Modulation by Toll-Like Receptor 8 Ligands and Role of Monocytes. Cells. 2020;9:713. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 89. | Kunzmann V, Kretzschmar E, Herrmann T, Wilhelm M. Polyinosinic-polycytidylic acid-mediated stimulation of human gammadelta T cells via CD11c dendritic cell-derived type I interferons. Immunology. 2004;112:369-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 56] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 90. | Cabillic F, Toutirais O, Lavoué V, de La Pintière CT, Daniel P, Rioux-Leclerc N, Turlin B, Mönkkönen H, Mönkkönen J, Boudjema K, Catros V, Bouet-Toussaint F. Aminobisphosphonate-pretreated dendritic cells trigger successful Vgamma9Vdelta2 T cell amplification for immunotherapy in advanced cancer patients. Cancer Immunol Immunother. 2010;59:1611-1619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 91. | Alfaguter I, Lanier C, Mohamed A, Walter S, Kalra M. 226 An effective donor screening program for manufacturing of allogeneic γδ T cell products. J Immunother Cancer. 2024;12. [DOI] [Full Text] |
| 92. | Wilhelm M, Kunzmann V, Eckstein S, Reimer P, Weissinger F, Ruediger T, Tony HP. Gammadelta T cells for immune therapy of patients with lymphoid malignancies. Blood. 2003;102:200-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 449] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 93. | Lehman N, Bojarska-junak A, Zarobkiewicz M. Butyrophilin Downregulation in Chronic Lymphocytic Leukaemia: An Important Barrier to γδ T Cell-Mediated Cytotoxicity. Biocell. 2025;49:1085-1099. [DOI] [Full Text] |
| 94. | Tomogane M, Omura M, Sano Y, Shimizu D, Toda Y, Hosogi S, Kimura S, Ashihara E. Expression level of BTN3A1 on the surface of CD14(+) monocytes is a potential predictor of γδ T cell expansion efficiency. Biochem Biophys Res Commun. 2022;588:47-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 95. | Lang JM, Kaikobad MR, Wallace M, Staab MJ, Horvath DL, Wilding G, Liu G, Eickhoff JC, McNeel DG, Malkovsky M. Pilot trial of interleukin-2 and zoledronic acid to augment γδ T cells as treatment for patients with refractory renal cell carcinoma. Cancer Immunol Immunother. 2011;60:1447-1460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 118] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 96. | Dieli F, Vermijlen D, Fulfaro F, Caccamo N, Meraviglia S, Cicero G, Roberts A, Buccheri S, D'Asaro M, Gebbia N, Salerno A, Eberl M, Hayday AC. Targeting human {gamma}delta} T cells with zoledronate and interleukin-2 for immunotherapy of hormone-refractory prostate cancer. Cancer Res. 2007;67:7450-7457. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 408] [Cited by in RCA: 413] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 97. | Wilhelm M, Smetak M, Schaefer-Eckart K, Kimmel B, Birkmann J, Einsele H, Kunzmann V. Successful adoptive transfer and in vivo expansion of haploidentical γδ T cells. J Transl Med. 2014;12:45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 126] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/