Published online Oct 24, 2025. doi: 10.5306/wjco.v16.i10.110511
Revised: June 25, 2025
Accepted: September 11, 2025
Published online: October 24, 2025
Processing time: 138 Days and 16.2 Hours
Hepatocellular carcinoma (HCC) remains a leading cause of cancer-related mortality worldwide. Despite improvements in surgical techniques and systemic therapies, long-term outcomes after liver resection are limited by high recurrence rates. While adjuvant strategies have shown limited benefit, the role of neo
To assess the efficacy, feasibility, and safety of neoadjuvant immunotherapy in resectable HCC through a meta-analysis of current literature.
A systematic search was conducted across PubMed, Web of Science, EMBASE, Cochrane Library, and Scopus for studies published in the past five years evaluating neoadjuvant immunotherapy in resectable HCC. Primary endpoints included major pathological response (MPR), pathological complete response (pCR), overall response rate (ORR), resection rate, and grade 3-4 treatment-related adverse events (TRAEs). A random-effects meta-analysis was conducted using log odds ratios (ORs) and pooled event rates were calculated to provide absolute estimates of clinical endpoints.
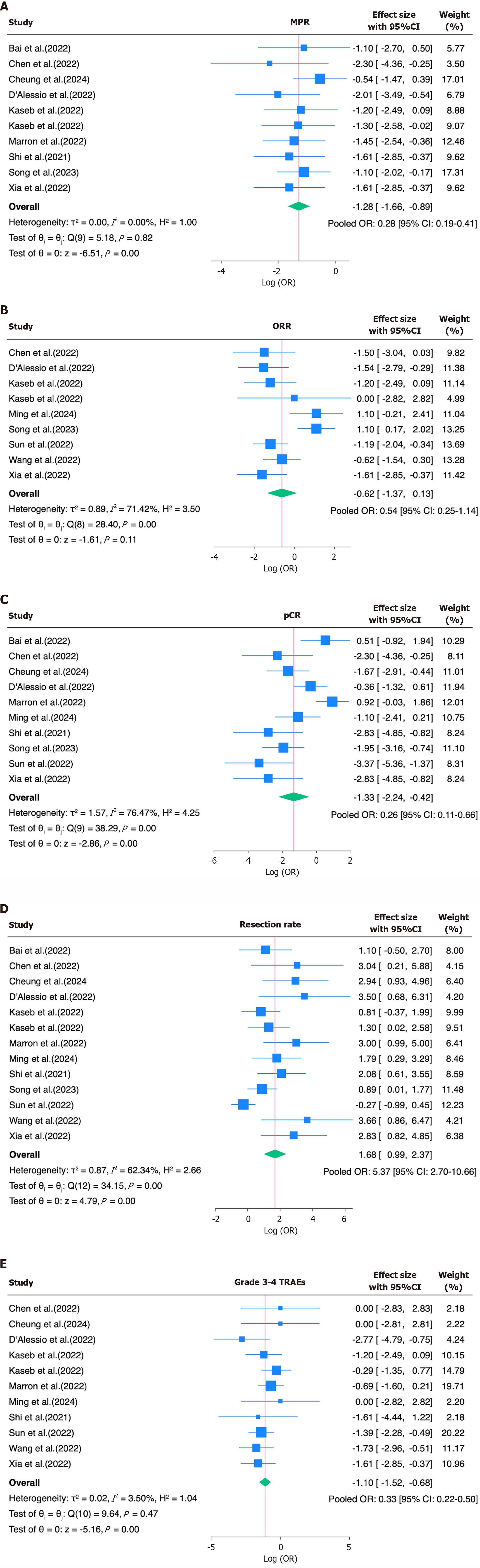
Twelve studies were included in the final analysis. The pooled ORs were 0.28 (95%CI: 0.19-0.41) for MPR, 0.54 (95%CI: 0.25-1.14) for ORR, 0.26 (95%CI: 0.11-0.66) for pCR, 5.37 (95%CI: 2.70-10.66) for resection rate, and 0.33 (95%CI: 0.22-0.50) for grade 3-4 TRAEs. Corresponding pooled event rates were 19% for MPR, 35% for ORR, 22% for pCR, 81% for resection feasibility, and 19% for severe TRAEs.
Neoadjuvant immunotherapy appears to be a feasible and safe approach in patients with resectable HCC, achi
Core Tip: This meta-analysis evaluates the efficacy and safety of neoadjuvant immunotherapy in resectable hepatocellular carcinoma (HCC). Pooled data suggest that this approach is feasible, with moderate pathological response rates and high resection feasibility, without a significant increase in severe toxicities. Although evidence is still limited, early findings support further investigation in prospective trials to define optimal treatment strategies and identify biomarkers predictive of response. Neoadjuvant immunotherapy may represent a promising strategy to improve surgical outcomes and reduce early recurrence in resectable HCC.
- Citation: Cicerone O, Oliviero B, Mantovani S, Maiocchi L, Ravetta V, Berton F, Corallo S, Vanoli A, Maestri M. Neoadjuvant immunotherapy in resectable hepatocellular carcinoma: A meta-analysis of the current evidence. World J Clin Oncol 2025; 16(10): 110511
- URL: https://www.wjgnet.com/2218-4333/full/v16/i10/110511.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i10.110511
Hepatocellular carcinoma (HCC) is the fourth leading cause of cancer-related death worldwide[1,2]. Its development is associated with several risk factors, including chronic alcohol abuse, hepatotropic viruses such as hepatitis C virus and hepatitis B virus, metabolic dysfunction-associated steatotic liver disease and metabolic dysfunction-associated steatohepatitis, and metabolic syndrome, as well as rare genetic disorders[1,3].
Over the past two decades, the therapeutic landscape of HCC has evolved significantly across all disease stages[4]. Major advances include the optimization of liver transplantation protocols, the widespread adoption of minimally invasive and robotic surgical techniques, and the development of novel systemic therapies—most notably, immunotherapy[5-7]. In addition, the implementation of liver hypertrophy-inducing strategies, such as portal vein embolization and associating liver partition and portal vein ligation for staged hepatectomy, has expanded the pool of patients eligible for curative liver resection by increasing the future liver remnant volume[8,9].
Surgical resection remains the mainstay curative option for patients with HCC[7]. However, tumor resectability depends on multiple factors, including the tumor’s size and location, the presence of macrovascular invasion, portal hypertension, liver function (e.g., the presence of cirrhosis), and the patient’s overall clinical condition[2,10]. Despite its curative intent, the long-term outcomes remain suboptimal. The five-year overall survival (OS) rates after resection is around 70%. Tumor recurrence remains a major concern, occurring in up to 50%-70% of patients within five years after surgery, with early recurrence (within two years) being particularly associated with poor prognosis[7,11,12].
While adjuvant therapies have been partially investigated, the role of neoadjuvant strategies in the management of HCC —particularly immunotherapy—has remained largely unexplored. Emerging evidence suggests the potential of neoadjuvant immunotherapy to achieve tumor downstaging in initially unresectable cases and to reduce the risk of recurrence in resectable disease[6,13-15].
Despite these promising preliminary findings, the role of neoadjuvant immunotherapy in resectable HCC remains poorly defined, with no standardized protocols or robust consensus on its clinical utility. In this context, a comprehensive meta-analysis of the available evidence is warranted to better understand the efficacy, feasibility, and safety profile of this emerging strategy. This study aims to fill this gap by synthesizing the current data on neoadjuvant immunotherapy in resectable HCC.
A systematic literature search was conducted using PubMed, Web of Science, EMBASE, Cochrane Library, and Scopus to identify studies published in the last five years (January 2021 to May 2025) that evaluated the use of neoadjuvant immunotherapy in patients with resectable HCC[16,17]. The following search string was used: “(neoadjuvant therapy) AND (hepatocellular carcinoma) AND (immunotherapy)”.
Study screening and selection were conducted using Rayyan, an online platform for managing systematic reviews. The eligible studies included clinical trials and retrospective analyses investigating neoadjuvant immunotherapy prior to liver resection in resectable HCC patients.
The primary endpoints assessed were the pathological complete response (pCR), major pathological response (MPR), overall response rate (ORR), grade 3-4 treatment-related adverse events (TRAEs) and the rate of successful resection.
The following exclusion criteria were applied: (1) Non-English language publications; (2) Case reports, editorials, and review articles; (3) Studies not involving neoadjuvant immunotherapy; (4) Studies examining unresectable HCC or other treatment settings (e.g., liver transplantation or transarterial chemoembolization); (5) Studies evaluating neo-adjuvant immunotherapy strategies incorporating cancer vaccines; and (6) Studies that did not report at least three of the five primary endpoints of interest (MPR, pCR, ORR, grade 3-4 TRAEs, resection rate).
For studies reporting single-arm binary outcomes (e.g., the pCR, MPR, ORR, or TRAEs), the log odds [ln(odds)] and corresponding standard error (SE) were calculated as follows: Ln(odds) = ln[X/(n - X)], SE = Ö1/X + [1/(n - X)]. Here, X is the number of events and n is the total number of patients. Studies with zero or full event counts were adjusted using continuity correction where necessary[18].
Meta-analyses were conducted using Stata BE 19. The pooled effect sizes were estimated using a random-effects model due to the clinical and methodological heterogeneity among the included studies. Between-study heterogeneity was assessed using Cochran’s Q-test, the I² statistic, and τ². Forest plots were generated to visually summarize the individual study estimates and their corresponding 95% confidence intervals (95%CIs). The pooled effects were computed on the log(odds) scale and subsequently back-transformed into odds ratios (ORs) for interpretability[19]. In addition to the log-odds-based analysis, the pooled proportions were calculated for each binary outcome. This method allowed for the direct estimation of the overall event rates (expressed as percentages) using a random-effects model and a direct estimate of the treatment efficacy and safety as percentages, complementing the OR analysis.
In one included study evaluating two immunotherapy regimens in separate arms, each arm was treated as an inde
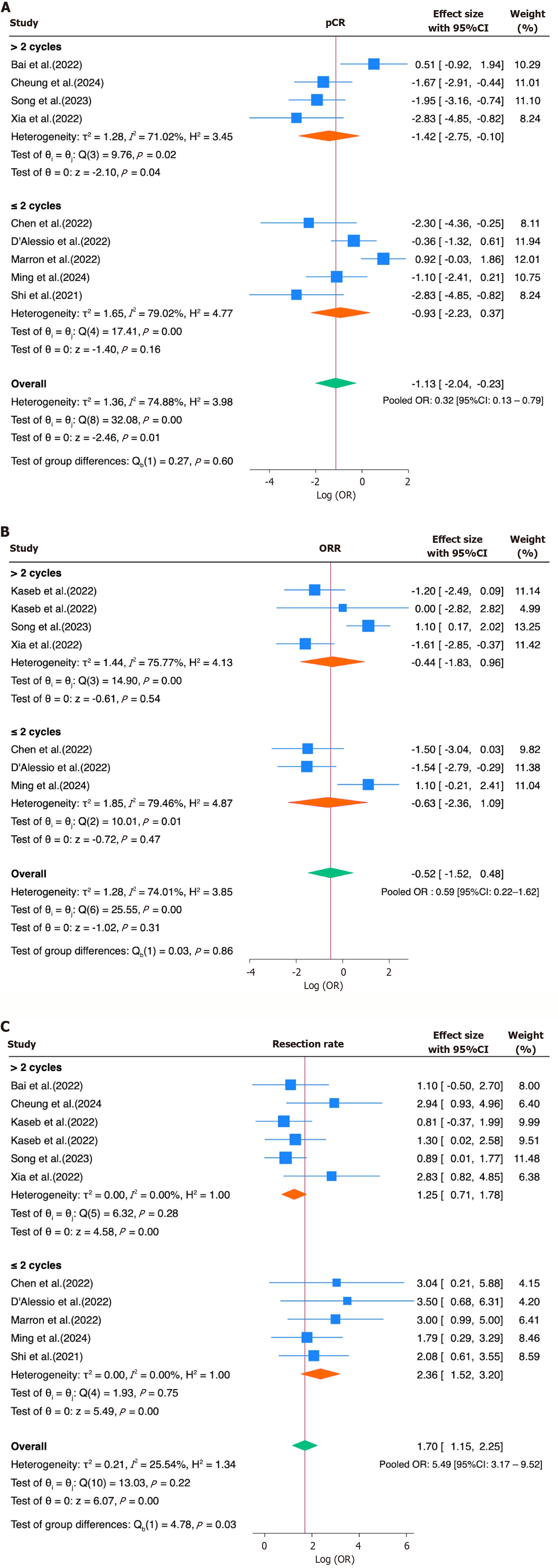
To further explore the heterogeneity in the treatment response, a subgroup analysis was conducted for the ORR, pCR, and resection rate based on the type of immune checkpoint inhibitor (ICI) regimen—monotherapy vs combination therapy. The studies were grouped accordingly, and the log ORs and their SEs were pooled using a random-effects model. For interpretability, the results were subsequently back-transformed and reported as ORs with 95%CIs. In a second subgroup analysis, the studies were also stratified based on the number of ICI cycles administered before surgery (≤ 2 cycles vs > 2 cycles). This allowed us to explore whether the treatment duration accounted for the heterogeneity in response outcomes. The log ORs and corresponding SEs were pooled within each subgroup using a random-effects model, and the differences between the subgroups were tested for heterogeneity using a Q-test.
To evaluate the potential small-study effects and publication bias, funnel plots were visually inspected for asymmetry. In addition, Egger’s regression test was performed for each primary endpoint by regressing the standardized effect sizes on their precision. A statistically significant non-zero intercept (β1) was considered indicative of potential bias, with a P value < 0.05 suggesting asymmetry.
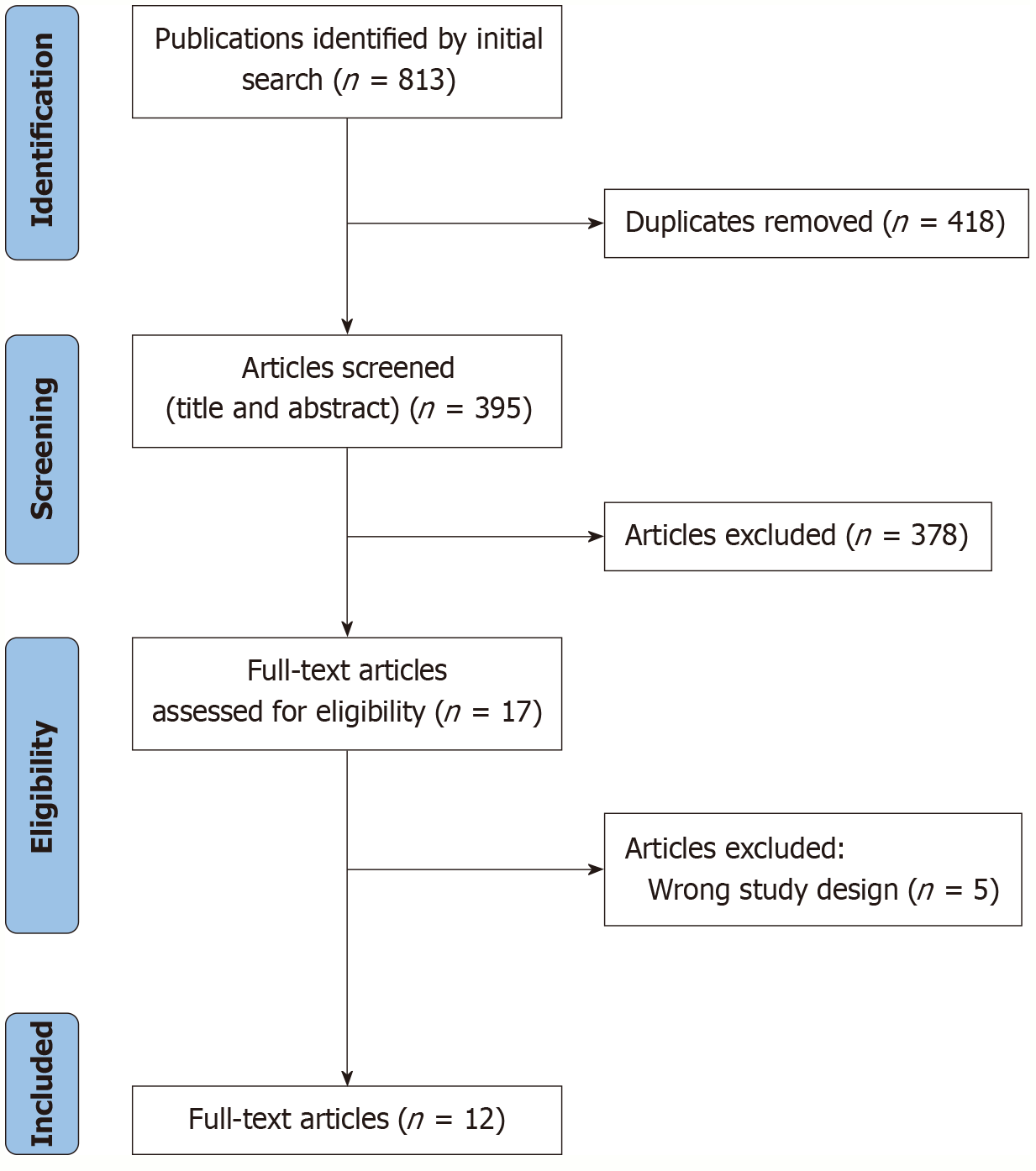
After the removal of duplicates, a total of 395 studies were screened. Following the application of inclusion and exclusion criteria, 12 studies were deemed eligible and included in the final meta-analysis[20-31] (Figure 1; Table 1). Notably, the study by Kaseb et al[23] was included as two separate entries, each corresponding to a distinct treatment arm.
| Ref. | Sample size | Study phase | Study type | Article type | Randomization | NI | Cycles of NI | ICI post-surgery |
| Bai et al[20], 2022 | 24 | 2 | Clinical trial | Conference abstract | Randomized | Camrelizumab + apatinib (n = 13) | 4 | Yes |
| Chen et al[21], 2022 | 11 | 2 | Clinical trial | Conference abstract | Non-randomized | Tislelizumab | 2 | Yes |
| Cheung et al[30], 2024 | 20 | / | Clinical trial | Full text | / | Nivolumab | 3 | / |
| D'Alessio et al[22], 2022 | 17 | 1b | Clinical trial | Conference abstract | / | Nivolumab + ipilimumab | 2 | / |
| Kaseb et al[23], 2022 | 30 | 2 | Clinical trial | Full text | Randomized | Nivolumab (n = 13) or nivolumab + ipilimumab (n = 14) | 3 | Yes |
| Marron et al[27], 2022 | 21 | 2 | Clinical trial | Full text | / | Cemiplimab | 2 | Yes |
| Ming et al[31], 2024 | 14 | 2 | Clinical trial | Conference abstract | / | Tislelizumab + lenvatinib | 2 | Yes |
| Shi et al[24], 2021 | 18 | 1b/2 | Clinical trial | Conference abstract | Randomized | Toripalib (n = 10) or toripalib + lenvatinib (n = 8) | 1 | Yes |
| Song et al[25], 2023 | 24 | 2 | Clinical trial | Conference abstract | / | Tislelizumab + lenvatinib | 4 | Yes |
| Sun et al[26], 2022 | 30 | 2 | Clinical trial | Conference abstract | / | Sintilimab + bevacizumab | N/A | / |
| Wang et al[28], 2022 | 20 | / | Retrospective study | Full text | / | Apatinib + camrelizumab | N/A | / |
| Xia et al[29], 2022 | 18 | 2 | Clinical trial | Full text | / | Camrelizumab +apatinib | 3 | Yes |
The pooled OR for achieving MPR was 0.28 (95%CI: 0.19-0.41), indicating a relatively low but consistent response rate across the studies. For the ORR, the pooled OR was 0.54 (95%CI: 0.25-1.14), indicating a modest overall response to neoadjuvant immunotherapy. Substantial heterogeneity was observed (I² = 71.4%, τ² = 0.89, P = 0.0004). The pooled OR for the pCR was 0.26 (95%CI: 0.11-0.66), suggesting limited complete response rates. Notably, the heterogeneity was high (I² = 76.5%, τ² = 1.57, P < 0.0001; Figure 2A-C). In contrast, the resection rate showed a significantly increased pooled OR of 5.37 (95%CI: 2.70-10.66), suggesting feasibility after immunotherapy. Moderate heterogeneity was present
| Endpoint | Pooled OR | 95%CI lower | 95%CI upper | I² (%) | τ² | P value (Q test) |
| MPR | 0.28 | 0.19 | 0.41 | 0.0 | 0.0 | 0.8188 |
| ORR | 0.54 | 0.25 | 1.14 | 71.4 | 0.89 | 0.0004 |
| pCR | 0.26 | 0.11 | 0.66 | 76.5 | 1.57 | < 0.0001 |
| Resection rate | 5.37 | 2.70 | 10.66 | 62.3 | 0.87 | < 0.0006 |
| Grade 3-4 TRAEs | 0.33 | 0.22 | 0.50 | 3.5 | 0.02 | 0.4725 |
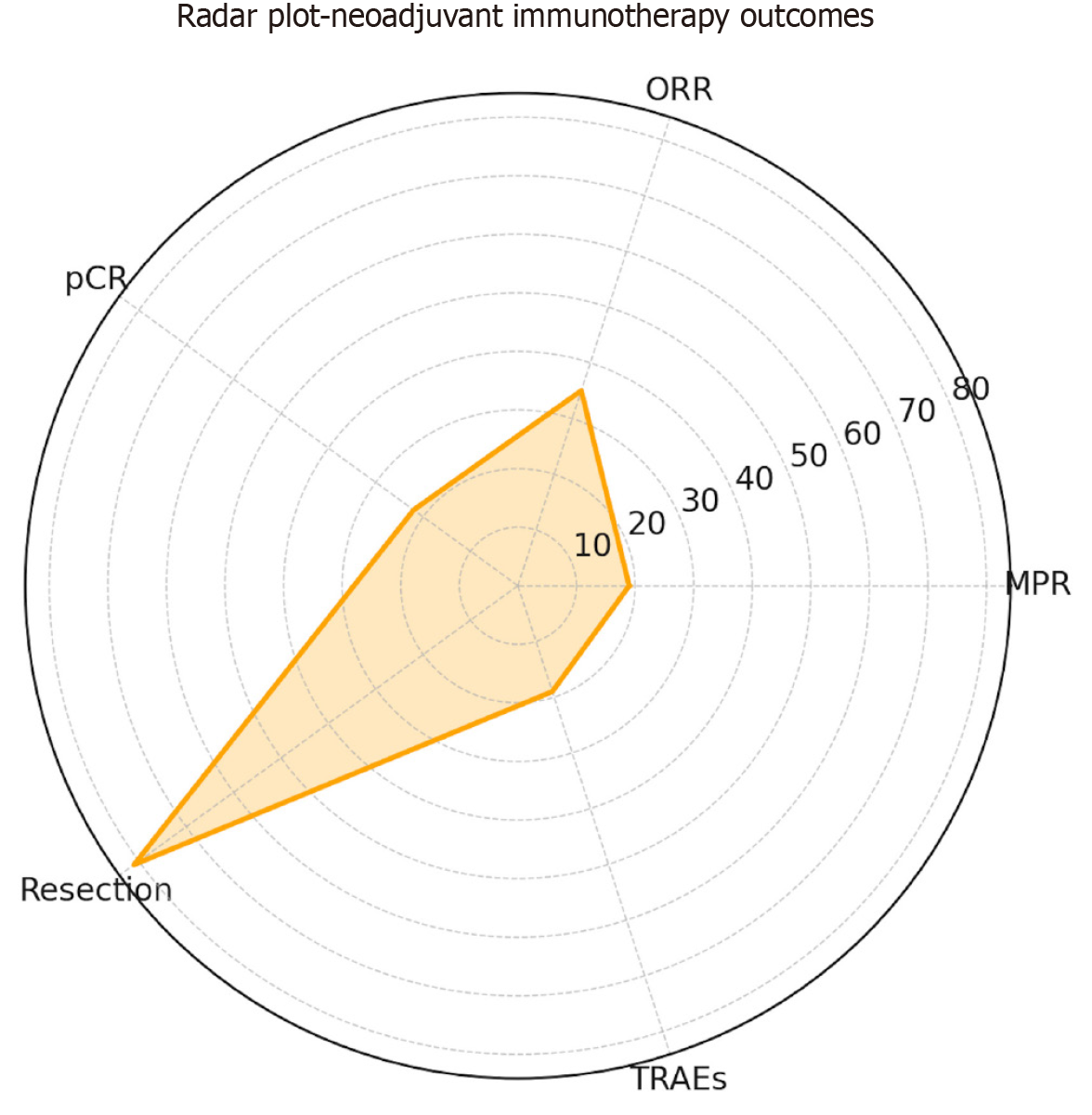
In parallel with the OR estimates, the pooled event rates were calculated to provide absolute measures of the efficacy and safety across studies, expressed as percentages, yielding estimated rates of 19% for the MPR, 35% for the ORR, 22% for the pCR, 81% for the resection feasibility, and 19% for the grade 3-4 TRAEs (Table 3; Figure 3).
| Endpoint | Pooled % | 95%CI | I² | P value |
| MPR | 19 | 13-25 | 0.0 | 0.77 |
| ORR | 35 | 18-52 | 84.1 | < 0.001 |
| pCR | 22 | 10-34 | 85.5 | < 0.001 |
| Resection rate | 81 | 72-91 | 75.9 | < 0.001 |
| Grade 3-4 TRAEs | 19 | 11-26 | 34.2 | 0.16 |
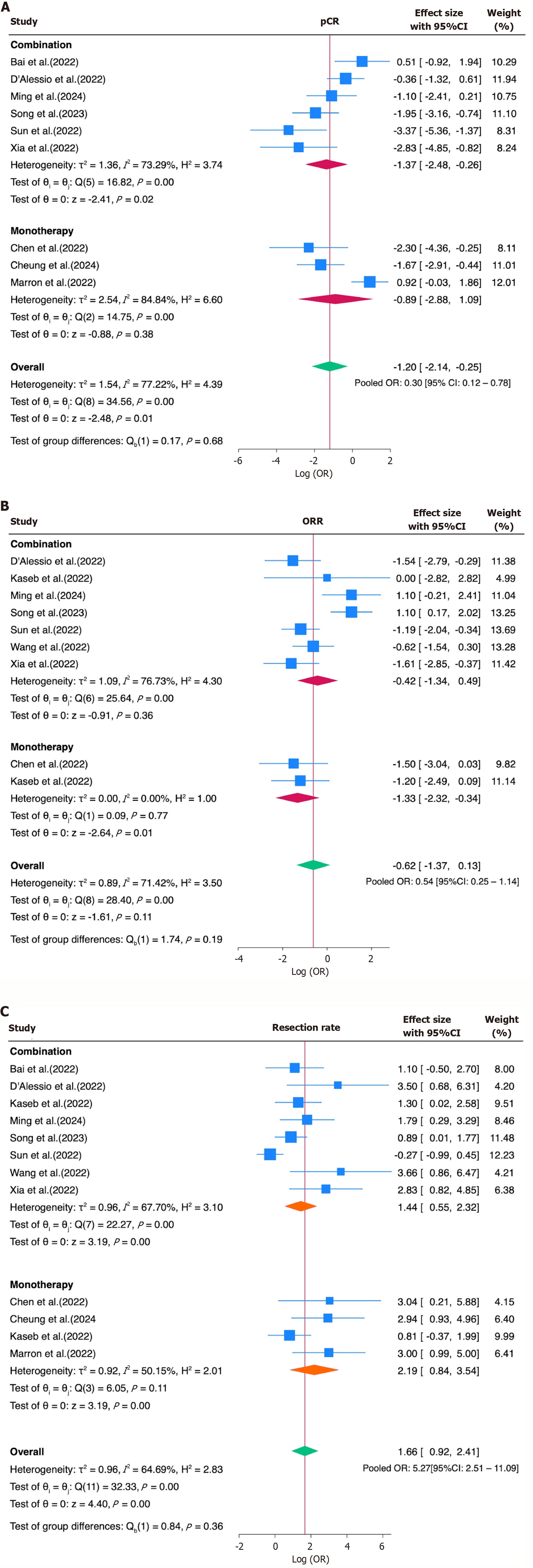
The subgroup analysis for the ORR, pCR, and resection rate based on the ICI regimen showed comparable pooled estimates for both monotherapy and combination therapy. For the ORR, the pooled OR was 0.27 (95%CI: 0.10-0.71) for monotherapy and 0.65 (95%CI: 0.26-1.63) for combination therapy. For the pCR, the pooled OR was 0.41 (95%CI: 0.06-2.97) for monotherapy and 0.25 (95%CI: 0.08-0.77) for combination therapy. Regarding the resection rate, monotherapy yielded a pooled OR of 8.94 (95%CI: 2.33-34.35), while combination therapy showed a pooled OR of 4.22 (95%CI: 1.74-10.22). Although the point estimates differed numerically between the subgroups, none of the differences reached statistical significance. These findings suggest that the type of ICI regimen alone may not fully explain the heterogeneity observed across the studies in their response and resectability outcomes (Figure 4).
An additional subgroup analysis stratified the studies based on the number of ICI cycles administered before surgery (≤ 2 vs > 2). For the ORR, the pooled OR was 0.65 (95%CI: 0.16-2.62) for > 2 cycles and 0.53 (95%CI: 0.09-2.99) for ≤ 2 cycles (P = 0.86). For the pCR, the pooled OR was 0.24 (95%CI: 0.06-0.91) for > 2 cycles and 0.39 (95%CI: 0.11-1.45) for ≤ 2 cycles (P = 0.60). Notably, the resection rate analysis showed a statistically significant difference in favor of shorter regimens: The pooled OR for ≤ 2 cycles was 10.61 (95%CI: 4.57-24.68), compared to 3.49 (95%CI: 2.04-5.95) for > 2 cycles (P for the subgroup difference = 0.03), suggesting that a shorter treatment duration may be associated with improved surgical feasibility (Figure 5).
Finally, the assessment of small-study effects using Egger’s test and funnel plots showed no significant evidence of a publication bias for the MPR (β1 = -2.36, P = 0.109), ORR (β1 = -0.07, P = 0.969), or grade 3-4 TRAEs (β1 = +0.11, P = 0.887). In contrast, statistically significant asymmetry was detected for the pCR (β1 = -5.00, P = 0.0016) and resection rate (β1 = +3.68, P < 0.0001), suggesting the presence of small-study effects or reporting bias in these outcomes (Table 4; Supplementary Figures 1-5).
| Endpoint | No. of studies | Funnel plot | β1 (Egger) | SE | P value | Interpretation |
| MPR | 10 | Symmetrical | -2.36 | 1.47 | 0.109 | No evidence of small-study effects; the funnel plot does not suggest publication bias |
| ORR | 9 | Symmetrical (2 studies lie outside 95%CI limits) | -0.07 | 1.81 | 0.969 | No indication of small-study effects; the distribution is balanced across effect sizes |
| pCR | 10 | Asymmetrical; small studies cluster to the left | -5.00 | 1.59 | 0.0016 | Significant small-study effects; suggests potential publication bias favoring negative findings |
| Resection rate | 13 | Asymmetrical; small studies cluster to the right | +3.68 | 0.68 | < 0.0001 | Strong evidence of small-study effects; indicative of possible reporting bias or selective publication of favorable outcomes |
| Grade 3-4 TRAEs | 11 | Symmetrical | +0.11 | 0.79 | 0.887 | No evidence of small-study effects; results appear robust and not influenced by study size |
This meta-analysis evaluated the efficacy and safety of neoadjuvant immunotherapy in patients with resectable HCC. The pooled results suggest that this approach is associated with a favorable pathological response without a significant increase in high-grade toxicity (Table 5). Specifically, the pooled MPR rate was 19% (OR = 0.28, 95%CI: 0.19-0.41), the ORR was 35% (OR = 0.54, 95%CI: 0.25-1.14), and the pCR rate was 22% (OR = 0.26, 95%CI: 0.11-0.66). The pooled resection rate was 81% (OR = 5.37, 95%CI: 2.70-10.66), and the incidence of grade 3-4 TRAEs was 19% (OR = 0.33, 95%CI: 0.22-0.50).
| Ref. | Neoadjuvant immunotherapy | Cycles of NI | Most common grade 3-4 TRAEs reported |
| Bai et al[20], 2022 | Camrelizumab + apatinib (n = 13) | 4 | Liver disfunction; thrombocytopenia; and hand-foot syndrome |
| Chen et al[21], 2022 | Tislelizumab | 2 | No severe events |
| Cheung et al[30], 2024 | Nivolumab | 3 | No severe events |
| D'Alessio et al[22], 2022 | Nivolumab + ipilimumab | 2 | ALT/AST elevation |
| Kaseb et al[23], 2022 | Nivolumab (n = 13) or nivolumab + ipilimumab (n = 14) | 3 | ALT/AST elevation |
| Marron et al[27], 2022 | Cemiplimab | 2 | ALT/AST elevation; increased blood creatine phosphokinase; and hypoalbuminaemia |
| Ming et al[31], 2024 | Tislelizumab + lenvatinib | 2 | No severe events |
| Shi et al[24], 2021 | Toripalib (n = 10) or toripalib + lenvatinib (n = 8) | 1 | Not specified |
| Song et al[25], 2023 | Tislelizumab + lenvatinib | 4 | No severe events |
| Sun et al[26], 2022 | Sintilimab + bevacizumab | N/A | Not specified |
| Wang et al[28], 2022 | Apatinib + camrelizumab | N/A | Biliary fistula |
| Xia et al[29], 2022 | Camrelizumab +apatinib | 3 | Rash; hypertension; liver damage; and neutropenia |
Moreover, several studies included in the analysis also reported encouraging survival outcomes: In a phase II study by Xia et al[29], using camrelizumab plus apatinib as neoadjuvant therapy led to a 1-year recurrence-free survival rate of 53.85%. Similarly, Kaseb et al[23] observed no recurrences among patients achieving an MPR after the perioperative administration of nivolumab (with or without ipilimumab) at 24 months. In addition, a retrospective analysis by Wang et al[28] demonstrated that neoadjuvant treatment with apatinib and camrelizumab significantly prolonged both the recu
Subgroup analyses based on the treatment regimen (monotherapy vs combination) were conducted to explore potential sources of heterogeneity. While numerical differences in the point estimates were observed across the subgroups for the ORR, pCR, and resection rate, none of these differences reached statistical significance. These findings suggest that the type of ICI regimen alone may not have been the primary driver of outcome variability, and other factors—such as the tumor burden, treatment duration, patient selection criteria, or surgical eligibility thresholds—may have contributed more substantially to the heterogeneity across studies. However, the small number of available studies, each evaluating different agents or combinations, limited the feasibility of performing direct comparisons between specific immunotherapeutic regimens and highlights the need for more consistent and comparable data. A secondary subgroup analysis stratified the studies according to the number of ICI cycles administered before surgery (≤ 2 vs > 2). Interestingly, while no significant differences emerged in the ORR and pCR between the cycle groups, the resection feasibility appeared to have been higher in the ≤ 2-cycle group (OR of 10.61, 95%CI: 4.57-24.68) than the > 2-cycle group (OR = 3.49, 95%CI: 2.04-5.95), with a significant subgroup difference (P = 0.03). These findings suggest that a shorter treatment duration may be sufficient to achieve tumor downsizing or immune priming, facilitating surgery without compromising its efficacy.
Furthermore, correlative immunological analyses by Kaseb et al[23] provided additional insights into the mechanisms underlying treatment responses. Patients who achieved a major pathological response exhibited a markedly higher density of tumor-infiltrating immune cells, particularly CD8+ T lymphocytes, along with an increased CD8+/Treg ratio within the tumor microenvironment. These findings suggest that enhanced intratumoral immune activation may play a critical role in mediating the response to neoadjuvant immune checkpoint blockade. Notably, none of the patients who achieved an MPR experienced disease recurrence at a median follow-up of approximately 24 months, supporting the prognostic value of a pathological response in this setting. These data collectively highlight the potential of using immu
Despite promising results, neoadjuvant immunotherapy has not yet been formally incorporated into the treatment guidelines for resectable HCC. The absence of standardized protocols regarding agent selection, the treatment duration, and the timing of surgery hinders the comparability of study outcomes and complicates the assessment of clinical efficacy. Additionally, definitions of MPR vary across studies, with different thresholds and histological criteria adopted, further limiting interpretability.
Moreover, substantial heterogeneity was observed across the studies for key endpoints such as the ORR, pCR, and resection rate, which may reflect differences in the treatment regimens, patient selection, and surgical criteria. Egger’s test indicated small-study effects suggestive of potential bias for the pCR (β1 = -5.00, P = 0.0016) and resection rate (β1 = +3.68, P < 0.0001), while no significant asymmetry was detected for the MPR, ORR, or grade 3-4 TRAEs.
A key limitation of this meta-analysis is the small number of available studies, many of which were preliminary and reported only as conference abstracts, lacking a full, peer-reviewed methodology. This underscores the early stage of clinical development in this field and highlights the need for high-quality, prospective trials to better define the role of neoadjuvant immunotherapy in the curative setting of resectable HCC.
This meta-analysis supports the feasibility and potential clinical value of neoadjuvant immunotherapy in patients with resectable HCC. While the pCR and MPR remain limited, the treatment demonstrates a favorable safety profile and high resection rates. These findings suggest that neoadjuvant immunotherapy may be a promising strategy to improve surgical outcomes and long-term disease control. However, larger, well-designed prospective trials are essential to validate these preliminary results and define standardized treatment protocols, optimal timing, and predictive bio
| 1. | McGlynn KA, Petrick JL, El-Serag HB. Epidemiology of Hepatocellular Carcinoma. Hepatology. 2021;73 Suppl 1:4-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 820] [Cited by in RCA: 1498] [Article Influence: 299.6] [Reference Citation Analysis (2)] |
| 2. | Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16:589-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2184] [Cited by in RCA: 3113] [Article Influence: 444.7] [Reference Citation Analysis (17)] |
| 3. | European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). J Hepatol. 2024;81:492-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 949] [Article Influence: 474.5] [Reference Citation Analysis (1)] |
| 4. | Vitale A, Cabibbo G, Iavarone M, Viganò L, Pinato DJ, Ponziani FR, Lai Q, Casadei-Gardini A, Celsa C, Galati G, Gambato M, Crocetti L, Renzulli M, Giannini EG, Farinati F, Trevisani F, Cillo U; HCC Special Interest Group of the Italian Association for the Study of the Liver. Personalised management of patients with hepatocellular carcinoma: a multiparametric therapeutic hierarchy concept. Lancet Oncol. 2023;24:e312-e322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 148] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 5. | Di Benedetto F, Magistri P, Di Sandro S, Sposito C, Oberkofler C, Brandon E, Samstein B, Guidetti C, Papageorgiou A, Frassoni S, Bagnardi V, Clavien PA, Citterio D, Kato T, Petrowsky H, Halazun KJ, Mazzaferro V; Robotic HPB Study Group. Safety and Efficacy of Robotic vs Open Liver Resection for Hepatocellular Carcinoma. JAMA Surg. 2023;158:46-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 103] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 6. | Akula V, Chen L, Acikgoz Y, Klein K, Yavuz BG, Cevik L, Demir T, Manne A, Sahin I, Kaseb A, Hasanov E. Neoadjuvant immune checkpoint inhibitors for hepatocellular carcinoma. NPJ Precis Oncol. 2025;9:60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 7. | Vogel A, Meyer T, Sapisochin G, Salem R, Saborowski A. Hepatocellular carcinoma. Lancet. 2022;400:1345-1362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1284] [Cited by in RCA: 1569] [Article Influence: 392.3] [Reference Citation Analysis (42)] |
| 8. | Schnitzbauer AA, Lang SA, Goessmann H, Nadalin S, Baumgart J, Farkas SA, Fichtner-Feigl S, Lorf T, Goralcyk A, Hörbelt R, Kroemer A, Loss M, Rümmele P, Scherer MN, Padberg W, Königsrainer A, Lang H, Obed A, Schlitt HJ. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann Surg. 2012;255:405-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 837] [Cited by in RCA: 957] [Article Influence: 68.4] [Reference Citation Analysis (0)] |
| 9. | Korenblik R, Heil J, Smits J, James S, Bechstein W, Bemelmans M, Binkert C, Breitenstein S, Williams M, Detry O, Dewulf M, Dili A, Grochola LF, Grote J, Heise D, Kalil J, Metrakos P, Neumann U, Pappas SG, Pennetta F, Schnitzbauer A, Tasse J, Winkens B, Olde Damink S, van der Leij C, Schadde E, van Dam RM. Rapid liver regeneration following PVE/HVE improves overall survival compared to PVE alone - A midterm analysis of the multicenter DRAGON 0 cohort. Zeitschrift für Gastroenterologie. 2024. [DOI] [Full Text] |
| 10. | Kinsey E, Lee HM. Management of Hepatocellular Carcinoma in 2024: The Multidisciplinary Paradigm in an Evolving Treatment Landscape. Cancers (Basel). 2024;16:666. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 63] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 11. | Tabrizian P, Jibara G, Shrager B, Schwartz M, Roayaie S. Recurrence of hepatocellular cancer after resection: patterns, treatments, and prognosis. Ann Surg. 2015;261:947-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 719] [Article Influence: 65.4] [Reference Citation Analysis (1)] |
| 12. | Ivanics T, Murillo Perez CF, Claasen MPAW, Patel MS, Morgenshtern G, Erdman L, Shwaartz C, Rajendran L, O'Kane GM, Hansen BE, Cleary SP, Sapisochin G. Dynamic risk profiling of HCC recurrence after curative intent liver resection. Hepatology. 2022;76:1291-1301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 13. | Koulouris A, Tsagkaris C, Spyrou V, Pappa E, Troullinou A, Nikolaou M. Hepatocellular Carcinoma: An Overview of the Changing Landscape of Treatment Options. J Hepatocell Carcinoma. 2021;8:387-401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 72] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 14. | Pinato DJ, Fessas P, Sapisochin G, Marron TU. Perspectives on the Neoadjuvant Use of Immunotherapy in Hepatocellular Carcinoma. Hepatology. 2021;74:483-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 15. | Ho WJ, Sharma G, Zhu Q, Stein-O'Brien G, Durham J, Anders R, Popovic A, Mo G, Kamel I, Weiss M, Jaffee E, Fertig EJ, Yarchoan M. Integrated immunological analysis of a successful conversion of locally advanced hepatocellular carcinoma to resectability with neoadjuvant therapy. J Immunother Cancer. 2020;8:e000932. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 16. | Schmucker CM, Blümle A, Schell LK, Schwarzer G, Oeller P, Cabrera L, von Elm E, Briel M, Meerpohl JJ; OPEN consortium. Systematic review finds that study data not published in full text articles have unclear impact on meta-analyses results in medical research. PLoS One. 2017;12:e0176210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 134] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 17. | Schmucker C, Schell LK, Portalupi S, Oeller P, Cabrera L, Bassler D, Schwarzer G, Scherer RW, Antes G, von Elm E, Meerpohl JJ; OPEN consortium. Extent of non-publication in cohorts of studies approved by research ethics committees or included in trial registries. PLoS One. 2014;9:e114023. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 161] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 18. | Higgins JPT, Li T, Deeks JJ. Choosing effect measures and computing estimates of effect. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane Handbook for Systematic Reviews of Interventions, Second Edition. Hoboken: John Wiley & Sons, 2019. [DOI] [Full Text] |
| 19. | Deeks JJ, Higgins JPT, Altman DG. Analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane Handbook for Systematic Reviews of Interventions, Second Edition. Hoboken: John Wiley & Sons, 2019. |
| 20. | Bai X, Chen Y, Zhang X, Zhang F, Liang X, Zhang C, Wang X, Lu B, Yu S, Liang T. 712P CAPT: A multicenter randomized controlled trial of perioperative versus postoperative camrelizumab plus apatinib for resectable hepatocellular carcinoma. Ann Oncol. 2022;33 Suppl 7:S868. [RCA] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 21. | Chen S, Wang Y, Xie W, Shen S, Peng S, Kuang M. 710P Neoadjuvant tislelizumab for resectable recurrent hepatocellular carcinoma: A non-randomized control, phase II trial (TALENT). Ann Oncol. 2022;33 Suppl 7:S867. [RCA] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 22. | D'alessio A, Pai M, Spalding D, Rajagopal P, Talbot T, Goldin R, Fulgenzi CA, Ward C, Yip V, Slater S, Sodergren M, Tait P, Habib NA, Thomas R, Cortellini A, Sharma R, Pinato DJJ. Preliminary results from a phase Ib study of neoadjuvant ipilimumab plus nivolumab prior to liver resection for hepatocellular carcinoma: The PRIME-HCC trial. J Clin Oncol. 2022;40:4093-4093. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 23. | Kaseb AO, Hasanov E, Cao HST, Xiao L, Vauthey JN, Lee SS, Yavuz BG, Mohamed YI, Qayyum A, Jindal S, Duan F, Basu S, Yadav SS, Nicholas C, Sun JJ, Singh Raghav KP, Rashid A, Carter K, Chun YS, Tzeng CD, Sakamuri D, Xu L, Sun R, Cristini V, Beretta L, Yao JC, Wolff RA, Allison JP, Sharma P. Perioperative nivolumab monotherapy versus nivolumab plus ipilimumab in resectable hepatocellular carcinoma: a randomised, open-label, phase 2 trial. Lancet Gastroenterol Hepatol. 2022;7:208-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 208] [Article Influence: 52.0] [Reference Citation Analysis (0)] |
| 24. | Shi YH, Ji Y, Liu WR, Pang YR, Ding ZB, Fu XT, Zhang X, Huang C, Sun YF, Zhu XD, Sun HC, Zhou J, Fan J. Abstract 486: A phase Ib/II, open-label study evaluating the efficacy and safety of Toripalimab injection (JS001) or combination with Lenvatinib as a neoadjuvant therapy for patients with resectable hepatocellular carcinoma (HCC). Cancer Res. 2021;81:486. [RCA] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 25. | Song T. A prospective, single-arm, phase II clinical study of tislelizumab in combination with lenvatinib for perioperative treatment of resectable primary hepatocellular carcinoma with high risk of recurrence. J Clin Oncol. 2023;41:e16218-e16218. [DOI] [Full Text] |
| 26. | Sun H, Zhu X, Gao Q, Qiu S, Shi Y, Wang X, Huang X, Yang X, Ji Y, Pang Y, He Y, Xu Y, Li M, Zhu J, Shen Y, Huang C, Zhou J, Fan J. 711P Sintilimab combined with bevacizumab biosimilar as a conversion therapy in potentially resectable intermediate stage hepatocellular carcinoma (HCC): A phase II trial. Ann Oncol. 2022;33 Suppl 7:S867-S868. [RCA] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 27. | Marron TU, Fiel MI, Hamon P, Fiaschi N, Kim E, Ward SC, Zhao Z, Kim J, Kennedy P, Gunasekaran G, Tabrizian P, Doroshow D, Legg M, Hammad A, Magen A, Kamphorst AO, Shareef M, Gupta NT, Deering R, Wang W, Wang F, Thanigaimani P, Mani J, Troncoso L, Tabachnikova A, Chang C, Akturk G, Buckup M, Hamel S, Ioannou G, Hennequin C, Jamal H, Brown H, Bonaccorso A, Labow D, Sarpel U, Rosenbloom T, Sung MW, Kou B, Li S, Jankovic V, James N, Hamon SC, Cheung HK, Sims JS, Miller E, Bhardwaj N, Thurston G, Lowy I, Gnjatic S, Taouli B, Schwartz ME, Merad M. Neoadjuvant cemiplimab for resectable hepatocellular carcinoma: a single-arm, open-label, phase 2 trial. Lancet Gastroenterol Hepatol. 2022;7:219-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 176] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 28. | Wang C, Li F, Lian Y, He X. Clinical Analysis of Targeted Therapy Combined with Immunotherapy for Neoadjuvant Treatment of Advanced Hepatocellular Carcinoma. Indian J Pharm Sci. 2022;84:262-266. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 29. | Xia Y, Tang W, Qian X, Li X, Cheng F, Wang K, Zhang F, Zhang C, Li D, Song J, Zhang H, Zhao J, Yao A, Wu X, Wu C, Ji G, Liu X, Zhu F, Qin L, Xiao X, Deng Z, Kong X, Li S, Yu Y, Xi W, Deng W, Qi C, Liu H, Pu L, Wang P, Wang X. Efficacy and safety of camrelizumab plus apatinib during the perioperative period in resectable hepatocellular carcinoma: a single-arm, open label, phase II clinical trial. J Immunother Cancer. 2022;10:e004656. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 157] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 30. | Cheung TT, Wai-Hung Ho D, Lyu SX, Zhang Q, Tsui YM, Ching-Yun Yu T, Man-Fong Sze K, Man-Fong Lee J, Lau VW, Yin-Lun Chu E, Hing-Yin Tsang S, She WH, Ching-Yu Leung R, Chung-Cheung Yau T, Ng IO. Multimodal Integrative Genomics and Pathology Analyses in Neoadjuvant Nivolumab Treatment for Intermediate and Locally Advanced Hepatocellular Carcinoma. Liver Cancer. 2024;13:70-88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 31. | Ming K, Xie W, Chen J, Chen S, Wang Y, Shen S, Peng S. Neo-adjuvant tislelizumab combined with lenvatinib treatment for resectable, recurrent hepatocellular carcinoma. J Clin Oncol. 2024;42:517. [DOI] [Full Text] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/