Published online Sep 26, 2025. doi: 10.4252/wjsc.v17.i9.109715
Revised: June 17, 2025
Accepted: August 29, 2025
Published online: September 26, 2025
Processing time: 128 Days and 2.8 Hours
Current drugs primarily target inflammation control but do not reverse tissue remodeling changes for asthma. Human mesenchymal stem cells are known for their anti-inflammatory and tissue remodeling capabilities. However, limited research has explored the therapeutic impact of varying doses and frequencies of human umbilical cord blood-derived mesenchymal stem cells (HUC-MSCs) on established airway remodeling in experimental asthma.
To explore and optimize the dosage and administration frequency of HUC-MSCs in experimental models of ovalbumin (OVA)-induced asthma.
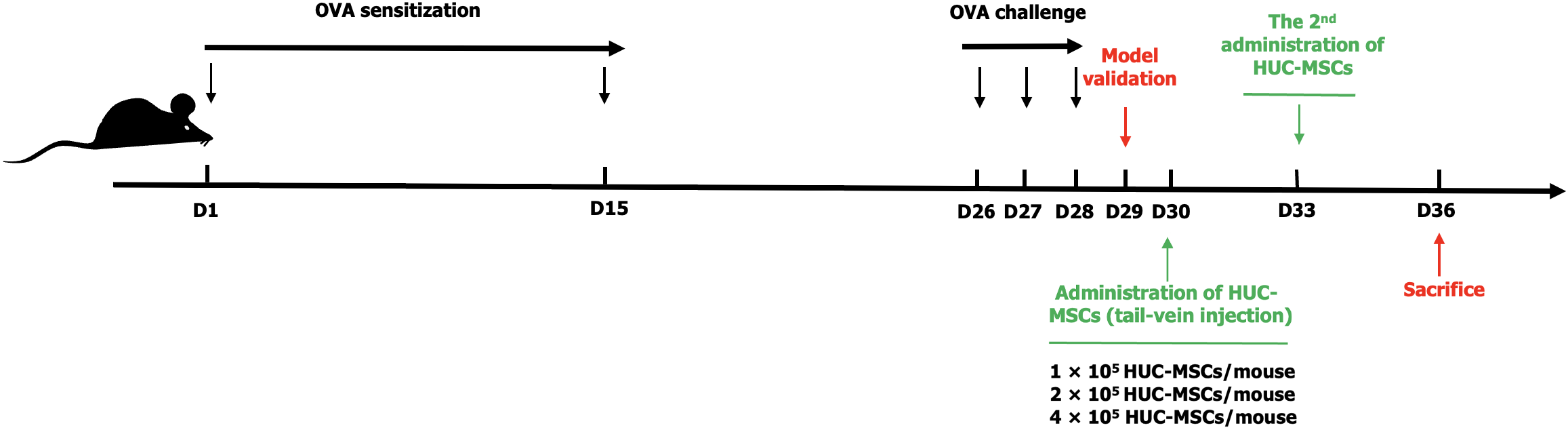
BALB/c mice underwent sensitization and were challenged using OVA. Control animals were administered a saline solution following the same protocol. HUC-MSCs were identified using flow cytometry. HUC-MSCs at incremental dosages (1 × 105, 2 × 105, 4 × 105) were injected via tail veins on day 30 (the second after the final stimulation). After comparing each group and determining the optimal dose, supplement the optimal dose twice on day 30 and day 33 (the second and fifth day after the final stimulation). Bronchoalveolar lavage fluid (BALF) and serum were harvested for analysis of concentrations of interleukin-4 (IL-4), IL-13, immunoglobulin E and interferon-gamma (IFN-γ) by enzyme-linked immunosorbent assay. Pharmacology of airways and lung functions were also evaluated to identify the optimal group.
The study shows that HUC-MSC transplantation ameliorates OVA-induced asthma by significantly reducing airway inflammation and obstruction in preclinical models. This effect is associated with decreased Th2 cytokines IL-4 and IL-13, and increased Th1 cytokine IFN-γ. The optimal dose of 2 × 105 cells/mouse was identified as the most effective in reducing local asthmatic airway inflammation and changing levels of IL-4, IL-13, and IFN-γ in serum and BALF compared to other single doses of HUC-MSC. Multiple treatments with the medium dose (2 × 105 cells) of HUC-MSCs on days 30 and 33 yield the best pathological and lung function outcomes. However, double treatments do not reduce IL-4 and IL-13 expression or enhance IFN-γ production in serum or BALF more effectively than a single medium dose.
HUC-MSCs effectively regulate pro-inflammatory mediators in serum and BALF, modulating airway remodeling and lung function. In this acute mouse asthma model, a single dosage of 2 × 105 is optimal, with more significant effects of decreasing airway obstruction requiring repeated administration.
Core Tip: This study reports, human umbilical cord blood-derived mesenchymal stem cell (HUC-MSC) therapy shows potential for treating asthma with high purity. HUC-MSC has been demonstrated to modulate airway remodeling, enhance lung function, and inhibit the inflammatory response in acute ovalbumin-induced asthma model. This study provides appropriate reference dosages to explore the possible intervention of HUC-MSC further. A single dosage of 2 × 105 cells is optimal, while double treatments with 2 × 105 cells may be optimal in reducing airway obstruction, but not for modulating airway inflammation.
- Citation: Chen QH, Zheng JY, Zhu YQ, Zhang JY, Lin CY, Zhuang XE, Cheng J, Huang XY. Exploring the critical therapeutic window: Dose-frequency optimization of human umbilical cord mesenchymal stem cells for preclinical asthma treatment. World J Stem Cells 2025; 17(9): 109715
- URL: https://www.wjgnet.com/1948-0210/full/v17/i9/109715.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v17.i9.109715
Asthma is an inflammatory airway disease that affects all age groups, especially children[1,2]. In 2021, approximately 260 million people globally were estimated to have asthma[3]. Asthma is a complex immune disease linked to an imbalance of Th1/Th2 cells, a key factor causing airway inflammation and overreaction[4]. A variety of inflammatory cells, inflammatory mediators, and cytokines are involved in the pathogenesis of asthma, which is characterized by recurrent episodes of wheezing, chest tightness, shortness of breath, or cough[5]. Primary treatments include inhaled glucocorticoids, β2-adrenergic agonists, anticholinergics, and theophyllines[6]. Despite advances in conventional therapies, they are unable to revert to established remodeling, leading a significant proportion of patients to exhibit poor symptom control and disease progression. Mesenchymal stem cells (MSCs) are promising therapeutic candidates because of their immunomodulatory properties, including direct cell-cell interactions and paracrine factor secretion. Human umbilical cord blood-derived MSCs (HUC-MSCs) can be easily isolated and expanded through a painless, noninvasive procedure[7]. However, the optimal dosing strategies and underlying molecular mechanisms remain incompletely understood. The systematic review highlights two primary dosage regimens for HUC-MSCs in murine asthma models: A low dose of 1 × 105 cells per mouse and a high dose of 1 × 106 cells per mouse[8]. Consequently, we selected 1 × 105, 2 × 105, and 4 × 105 as doses to represent the low, medium, and high-dose study groups to explore the optimal dosage.
This study focuses on the appropriate dosage and frequency of HUC-MSCs to treat asthma. Changes in lung tissue, lung function, and inflammation markers before and after treatment were assessed. This study systematically investigated the dose-dependent effects and frequency of HUC-MSCs in an acute ovalbumin (OVA)-induced asthma model, providing critical insights for clinical translation.
The development of the mouse asthma airway remodeling model involved two stages. During the initial sensitization phase, mice received intraperitoneal injections of 100 μg OVA and 2 mg KAl(SO4)2 on days 1 and 15. During the second stimulation phase on days 26, 27 and 28, each mouse received 50 μL of a 2 mg/mL OVA challenge solution nasally under inhaled anesthesia. Control and model groups were selected for model validation 24 hours after the final stimulation (day 29) (Figure 1). Thirty BALB/c mice were allocated into five groups. The sample size was determined based on previous MSC studies and institutional guidelines. This model primarily induces acute allergic inflammation but does not adequately replicate chronic asthma features. The study was reviewed and approved by the Quanzhou Children’s Hospital Institutional Review Board [Approval No. 2022(41)].
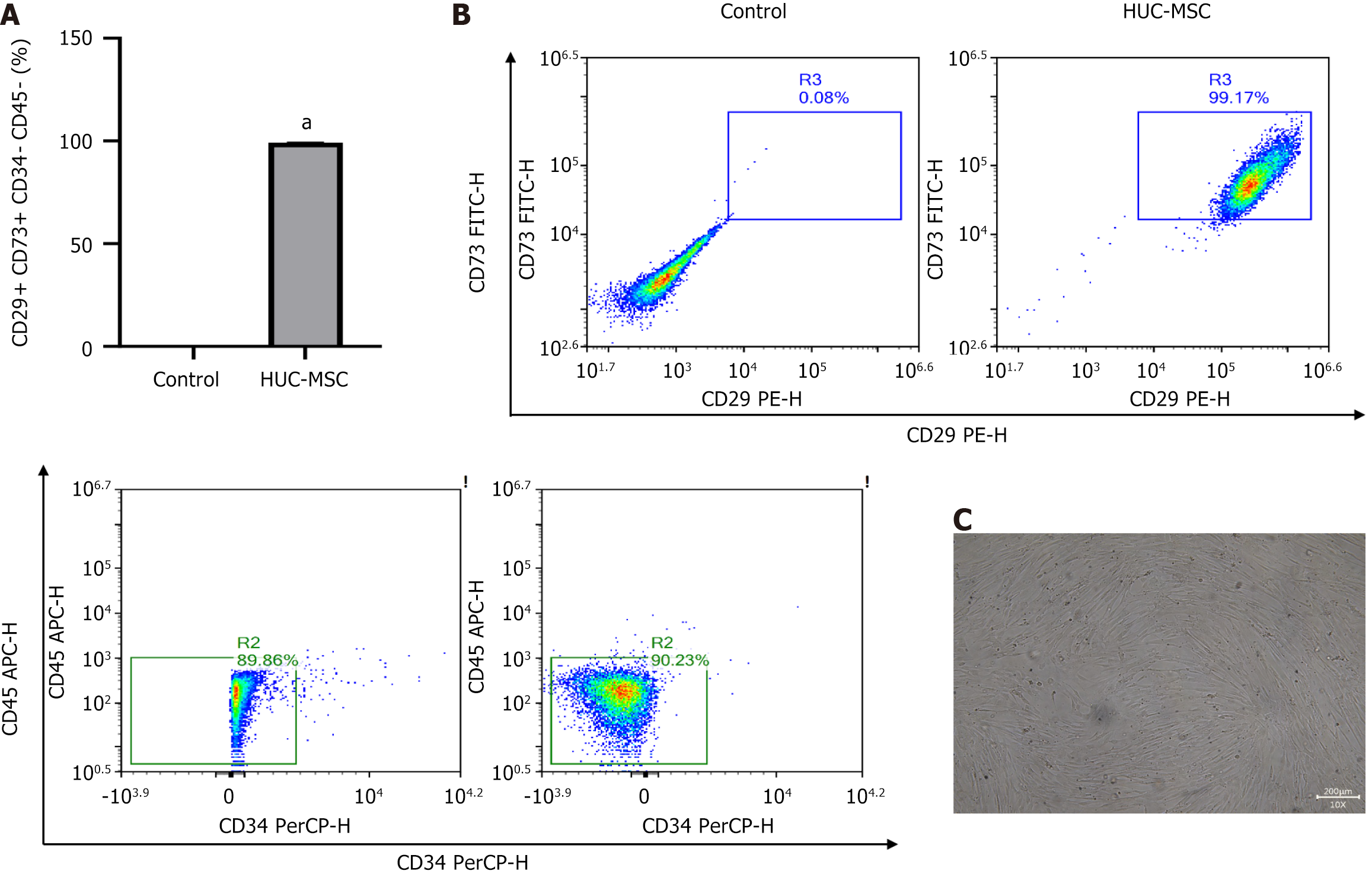
HUC-MSCs were isolated from the umbilical cord post-neonatal delivery with maternal consent and processed following standard manufacturing protocols at iCell Biosciences (Shanghai, China). HUC-MSCs (HUM-iCell-e009, iCell) were sourced from iCell Biosciences in Shanghai, China. Flow cytometry confirmed the HUC-MSCs using antibodies targeting human CD73 (FITC), CD29 (PE), CD34 (PerCP), and CD45 (APC), all sourced from Biolegend, San Diego, CA, United States. The labeled cells were analyzed using a NovoCyte™ flow cytometer (NovoCyte 2060R, Aisen Biotechnology, Hangzhou, China).
We investigated the therapeutic effect of incremental dosages and frequencies of HUC-MSCs on asthmatic airway remodeling. On the second day following model establishment (the time of single injection), HUC-MSCs of different dosages (1 × 105, 2 × 105, 4 × 105 cells/mouse) were administrated to acute OVA-induced asthmatic mouse models via tail veins. The control group was treated similarly with an equal amount of normal saline. After comparing each group to determine the optimal dose, supplementing the optimal dose on day 30 and day 33 (the second and fifth day after the final stimulation) for relevant testing and evaluation as the twice optimal dosage group (Figure 1).
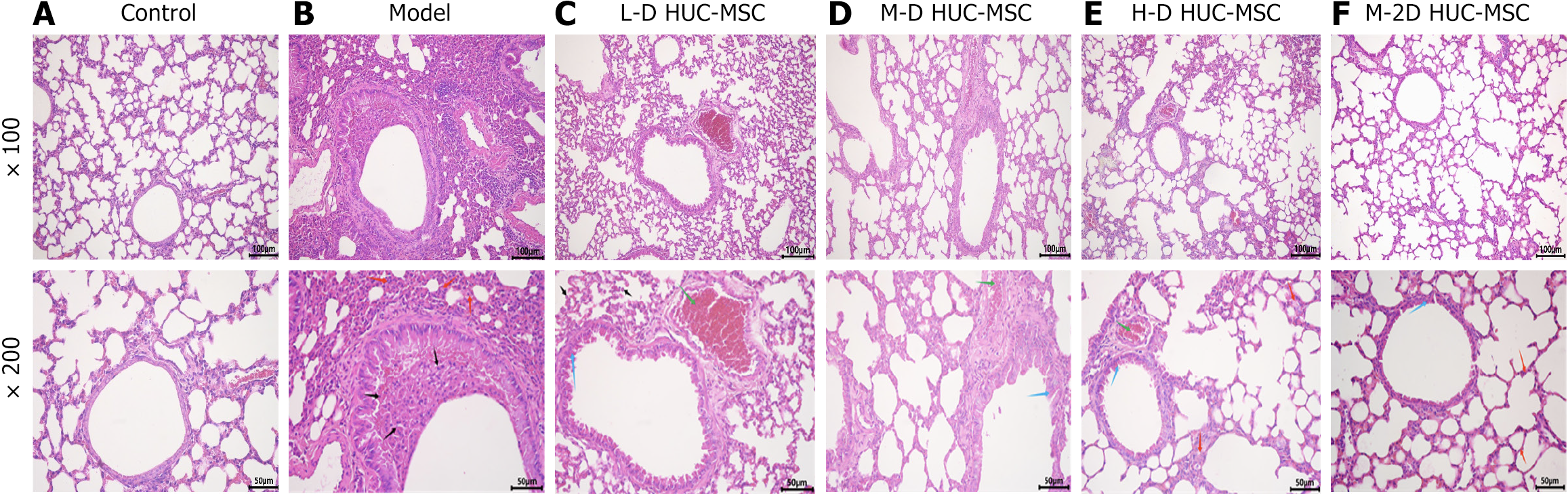
Control and model groups were selected for model validation 24 hours after the final OVA challenge (on day 29). Lung tissues from the middle zone of the left lung were collected to assess pathological changes in the lung parenchyma. Samples were fixed in 4% neutral buffered formalin, processed, embedded in paraffin, and sectioned at 3-mm intervals. On day 36, the seventh day post-HUC-MSC transplantation, hematoxylin and eosin staining identified peribronchial and perivascular inflammation.
Bronchoalveolar lavage fluid (BALF) was collected on the seventh day following HUC-MSC transplantation. BALF involved inserting a cannula into the bronchi and administering 500 μL of normal saline. BALF was retrieved using a syringe. Cytokine responses were assessed by centrifuging samples at 1000 × g for 10 minutes, followed by collecting the supernatant for analysis on the seventh day post-HUC-MSC transplantation. Serum and BALF levels of interleukin-4 (IL-4), IL-13, and interferon-gamma (IFN-γ), along with serum immunoglobulin E (IgE) levels in mice were quantified using enzyme-linked immunosorbent assay kits following the manufacturer’s protocols. Absorbance for each well was measured at 450 nm using a SuperMax3100 multifunctional microplate reader.
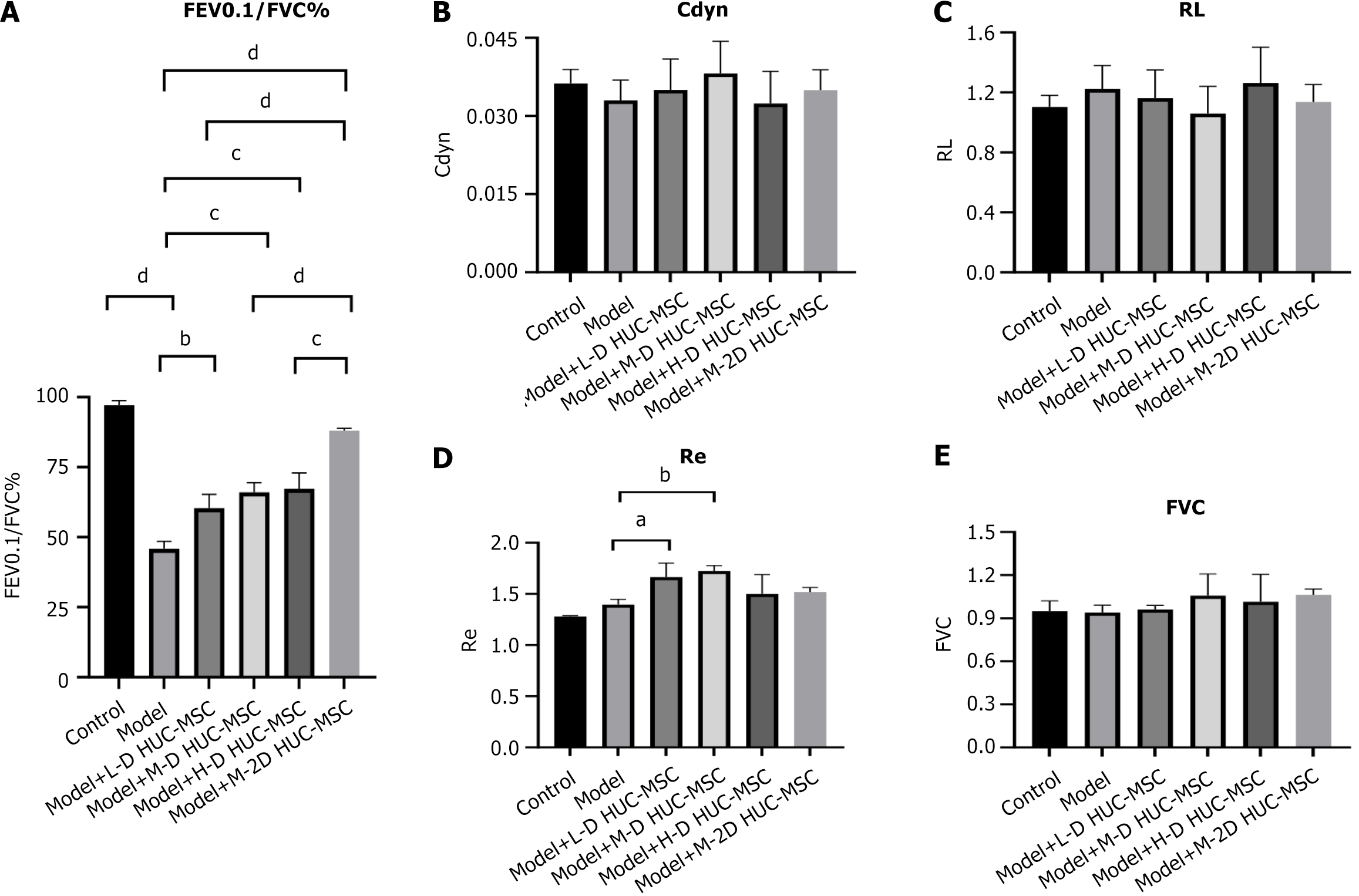
On the seventh day after HUC-MSC transplantation, mice were anesthetized with pentobarbital (100 mg/kg) and connected to a computer-controlled ventilator through a tracheal tube, as per the anires 2005 pulmonary function analysis system (Version 3.5, Bestlab, China). Pressure variations in the plethysmography chamber were recorded via the sensor-equipped port in the connecting tube. Pulmonary function was assessed using the following indices: Forced expiratory volume (FEV), forced vital capacity (FVC), FEV0.1/FVC%, respiratory system elastic resistance, dynamic lung compliance, and airway resistance.
Data were graphed and analyzed using the GraphPad Prism 10.0 statistical software package. Values are presented as means ± SEM. A t-test for independent samples was used to compare the two groups. Group differences were assessed using one-way ANOVA, followed by Tukey’s test. A P-value below 0.05 was deemed statistically significant. Bonferroni correction was applied to evaluate cytokines and lung function metrics in multiple treatment groups.
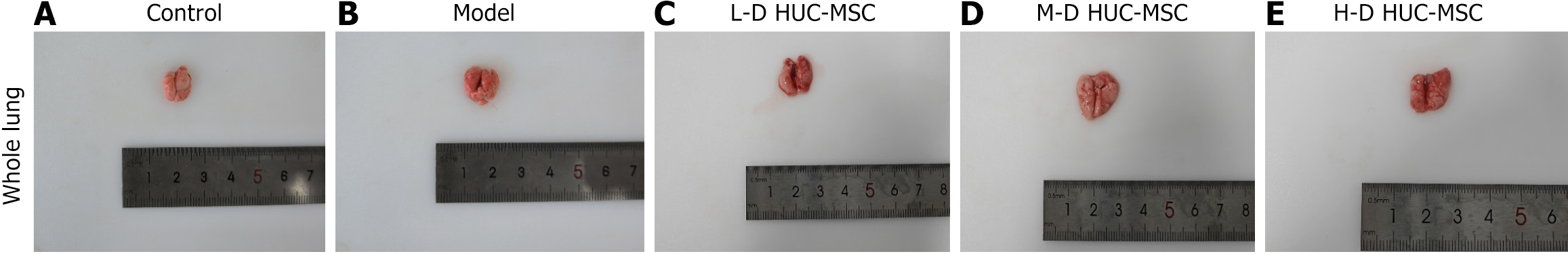
HUC-MSCs were identified using flow cytometry, showing that 99% of cells were characterized by CD73-positive, CD29-positive, CD34-negative, and CD45-negative (Figure 2), confirming the successful identification of HUC-MSCs. Following the establishment of the OVA-induced asthma model, mice received intravenous administration of HUC-MSCs at three different dosages: Low dose (1 × 105 cells), medium dose (2 × 105 cells), and high dose (4 × 105 cells). Gross examination of lung tissues revealed distinctive pathological features, with asthmatic lungs appearing dark red or purple. In contrast, lungs from mice treated with the medium dose exhibited a uniform light pink coloration, suggesting optimal therapeutic efficacy at this intermediate dosage (Figure 3).
Bioinformatical predictions indicate that HUC-MSC treatment for asthma is significantly associated with inflammation. Th2-mediated immune dysregulation plays a pivotal role in the pathogenesis of asthma. Consequently, the expression levels of inflammatory factors in serum and BALF from asthma mouse models are examined.
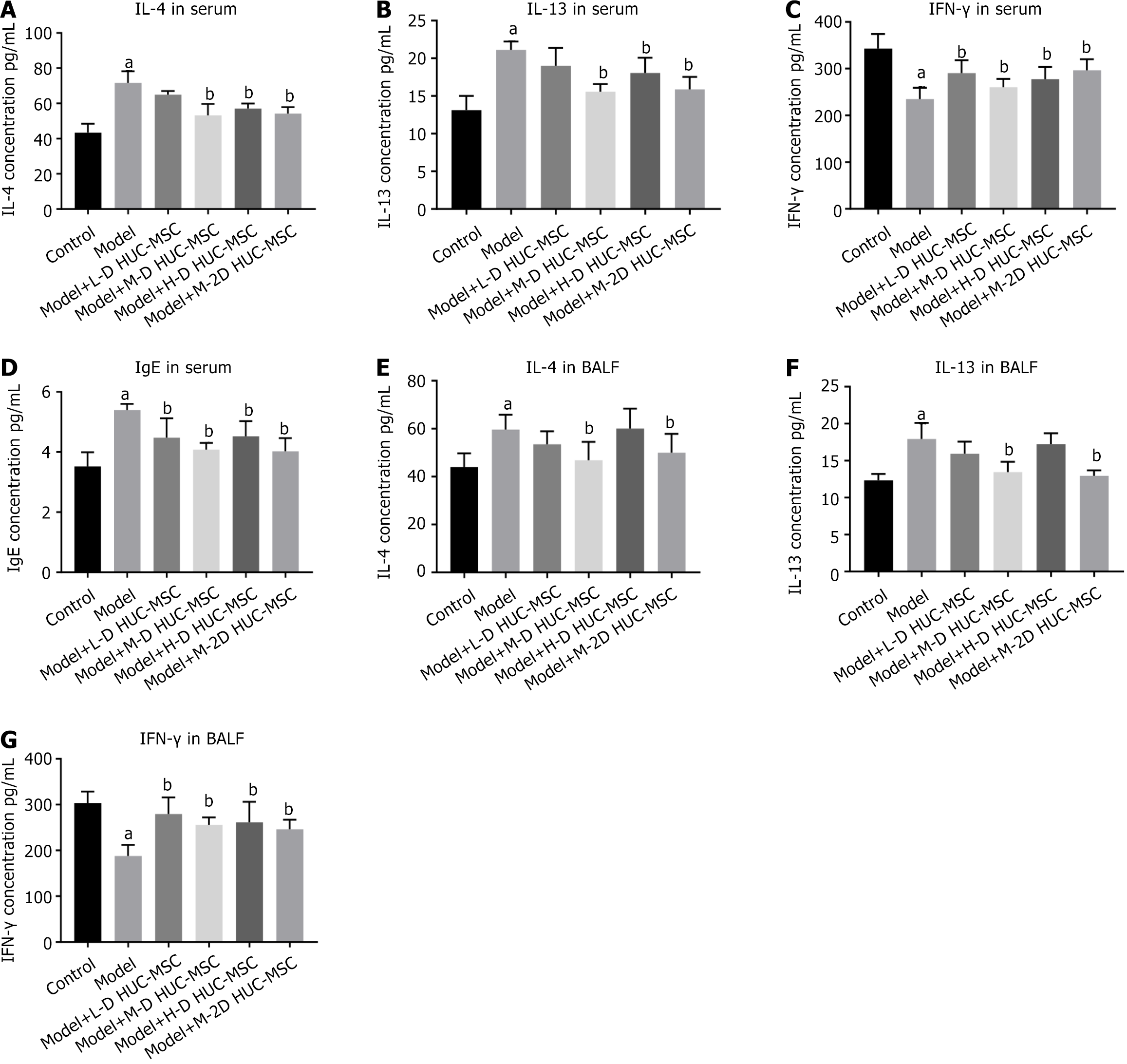
The analysis demonstrates that acute OVA-induced asthmatic mice exhibit significantly elevated serum levels of IgE, IL-4, and IL-13 (P < 0.05), with concomitant reduction in IFN-γ concentration (P < 0.05). Following HUC-MSC intervention, all treatment groups demonstrated notable immunomodulatory effects, characterized by dose-dependent reductions in IgE, IL-4, and IL-13 Levels and corresponding elevations in IFN-γ concentration. Intriguingly, the intermediate dose cohort (2 × 105 cells) exhibited superior efficacy (P < 0.05), suggesting the existence of an optimal therapeutic window where the immunomodulatory functions of HUC-MSCs achieve maximal potency.
The levels of Th2 cytokines in BLAF were then investigated to define the anti-inflammatory effects further. The asthmatic model group exhibited elevated levels of IL-4 and IL-13 in BALF, with significantly diminished IFN-γ concentration (P < 0.05), demonstrating high concordance with the serum cytokine alterations and confirming the synchronization between local and systemic immune responses. Post-HUC-MSC intervention, the inflammatory cytokine signature in BALF underwent substantial restructuring, with the medium dose group demonstrating the most pronounced downregulation of IL-4 and IL-13, accompanied by upregulation of IFN-γ (P < 0.05) (Figure 4).
The control group displayed normal lung tissue architecture and cellular morphology, without inflammatory cell infiltration. Conversely, the asthmatic model group exhibited characteristic inflammatory cell infiltration, alveolar wall thickening, and structural disruption. These pathological alterations were substantially mitigated after HUC-MSC treatment, particularly in the medium-dose group (Figure 5). Pulmonary function tests demonstrated a significant reduction in the FEV0.1/FVC ratio in the model group compared to the control group, indicating airway obstruction in the asthmatic mice. Conversely, the FEV0.1/FVC ratios in the various dosage groups significantly increased (Figure 6). Such data suggests that HUC-MSC treatment effectively ameliorates airway function in asthmatic mice.
Comparative analysis of the three dosage groups shows the best-pronounced effects with the intermediate dose (2 × 105 cells) treatment. The effect of double intravenous HUC-MSC treatment in OVA-induced asthma was then assessed. The data indicates that administering intermediate doses (2 × 105 cells) of HUC-MSCs on days 30 and 33 yields optimal outcomes in pathological changes and lung function (Figures 5 and 6A). Double hUC-MSC treatment did not surpass a single intermediate dose to inhibit IL-4 and IL-13 expression levels or enhance IFN-γ production in serum and BALF (Figure 4).
Preclinical research confirms that MSCs can reduce airway inflammation, improve oxidative stress, and enhance paracrine secretion, offering new treatment possibilities for asthma[9]. Over the past 50 years, MSCs have been the most studied for experimental cell therapy. Compared to MSCs from other sources, umbilical cord-derived MSCs exhibit enhanced immunosuppressive properties and circumvent ethical concerns, given their ability to be non-invasively harvested in substantial quantities[10]. Moreover, few studies directly compare different dosages and single vs multiple administrations of HUC-MSCs in the same asthma model. The mice were euthanized on day 36 for sample collection and analysis to evaluate acute therapeutic efficacy. This time point was selected to capture peak MSC effects, though more extended follow-up periods would be required to assess treatment durability.
HUC-MSCs utilized in this study were characterized as high-purity MSCs, with a purity exceeding 99%, illustrating the differentiation potential inherent to MSCs. MSCs can be administered intravenously, intranasally, or via the trachea[11]. Intravenous administration was undertaken in the study as it enables systemic distribution, potentially mediating effects through both direct pulmonary migration and remote immunomodulation via paracrine signaling mechanisms[12]. This comprehensive investigation into the dose-dependent effects of HUC-MSCs on cytokine expression profiles in asthmatic mice provides critical insights into the molecular foundation and dose optimization strategies for immunomodulatory therapy. HUC-MSCs effectively reestablished Th1/Th2 balance, suppressed airway inflammation, and improved pulmonary function, demonstrating significant potential as a novel biological therapeutic approach for asthma.
The current study involves xenogeneic transplantation of human MSCs into an acute animal asthma model. Human-derived MSCs have therapeutic effects in mouse asthma models due to their potent immunomodulatory properties[13]. Inflammation is considered an important molecular mechanism in asthma and can be classified into type 1 (TNF-α and IFN-γ) and type 2 responses (IL-4, IL-6, IL-8, and IL-13)[14]. In the pathogenesis of asthma, Th2-mediated immune dysregulation plays a pivotal role. Such data suggests that HUC-MSCs inhibit the development of airway inflammation by reducing serum expression of IgE and cytokines associated with inflammation, primarily IL-4 and IL-13, while increasing serum expression of IFN-γ. The simultaneous decrease in Th2 cytokines (IL-4, IL-13) and serum IgE indicates that HUC-MSCs probably influence the Th2-driven B cell activation process, rather than just stabilizing mast cells, since IL-4 and IL-13 are crucial for IgE class switching and plasma cell differentiation.
BALF directly reflects the airway microenvironment and holds substantial diagnostic value in disease progression assessment[15]. The data from this study reveals that the asthmatic model group exhibited significantly elevated levels of IL-4 and IL-13 in BALF, with diminished IFN-γ concentration. Such trends demonstrate a high concordance with the serum cytokine alterations and confirm the synchronization between local and systemic immune responses. This amelioration of the local microenvironment directly correlated with attenuated airway inflammation, providing perspectives on the therapeutic mechanisms of HUC-MSCs. Moreover, MSCs are known to secrete various paracrine factors, including transforming growth factor-β, IL-10, and prostaglandin E2, which can directly modulate airway-resident immune cells such as alveolar macrophages and dendritic cells[16]. The observed systemic and local cytokine changes suggest complex paracrine mechanisms warrant further investigation. HUC-MSCs were confirmed to alleviate bronchial inflammation in asthma model mice, further verifying their treatment potential. Histopathological examination substantiated the intrinsic connection between cytokine alterations and tissue morphological changes. Notably, pulmonary function parameters, specifically FEV0.1/FVC ratios, demonstrated high concordance with cytokine modulation patterns, confirming the tight association between immunomodulation and functional restoration.
Determining the optimal therapeutic window for novel targeted therapies is crucial in precision medicine to ensure appropriate dosing and mitigate the overutilization risk[17]. The optimal dosage of MSCs appears to be model-dependent, but most studies show significant outcomes using doses of 1 × 105 to 1 × 106 cells per mouse, particularly using the dose of 1 × 105[8]. Intriguingly, the intermediate dose (2 × 105 cells) consistently exhibited superior efficacy across multiple parameters, including serum and BALF cytokine profiles, histopathological features, and pulmonary function indices. This observation backs the hormetic dose-response theory in clinical pharmacology, showing that medium doses yield the best therapeutic outcomes, aligning with the “optimal therapeutic window” concept. In clinical practice, allergen-specific immunotherapy is employed to sustain patient tolerance and achieve desensitization upon reaching and maintaining the maximum dose. Analogously, an optimal dose via OVA induces asthma in animal models[18]. This finding holds substantial implications for clinical treatment protocols and indicates that HUC-MSCs may work through multiple mechanisms with differential contribution weights at varying dosages. Based on allometric scaling, our mouse dose of 2 × 105 cells/20 g mouse equals 107 cells/kg, exceeding the typical clinical MSC doses of (1-2) × 106 cells/kg. Factors such as MSC half-life, biodistribution, clearance rates, and tissue homing efficiency may differ considerably between mice and humans, potentially requiring dose adjustments for optimal clinical translation.
Compared with the single medium dosage group, repeating the treatment of HUC-MSC on day 36 is correlated with marked effects in improving lung function and pathological changes. However, in house dust mite-induced asthma, a single MSC dose partially decreased inflammation but did not enhance lung function or remodeling. Castro et al[19] discovered that administering multiple doses of adipose-derived MSCs (1 × 105 cells) significantly alleviated lung inflammation, remodeling, and induced immunosuppression in house dust mite-induced asthma. This observation suggests that repeated administrations of HUC-MSCs could potentially induce more pronounced pathological alterations in pulmonary tissue, which is in accordance with the current study.
Notably, a single medium HUC-MSC injection was more effective than double injections in reducing OVA-induced airway inflammation in the current murine model. This feature indicates that injection frequency is crucial in MSC therapies for asthma. While double administration improved lung function, it did not further reduce Th2 cytokines. It was probably because the immune-modulatory capacity of MSCs was saturated or there was insufficient time between doses for cumulative effects, which need temporal tracking in the future. Moreover, employing a single injection reduces the overall cost of the therapy. The marginal benefits may not justify the substantial increases in clinical complexity, costs, and patient burden associated with repeated MSC infusions.
The delivery method is vital for cell therapy success. However, intravenous infusion may cause MSCs to become lodged in pulmonary capillaries due to their larger size, potentially leading to lung changes[20]. The current study found differences in lung inflammation between single- and double-treated HUC-MSCs, likely due to cell size and treatment frequency. The average diameter of cultured HUC-MSCs ranged from 17.9 μm to 30.4 μm[21], much larger than pulmonary capillaries, making passage difficult. Cases of pulmonary thromboembolism have been associated with intravenous MSC transfer[22], inferring the existence of small lung capillaries and the highly adhesive nature of MSCs. Previous studies have also linked MSC lung trapping to severe lung damage in mice, but more research is needed for definitive conclusions[23]. However, repeated intravenous injections did not increase signs of pulmonary embolism or tissue damage, such as neutrophilic infiltration or hemorrhage, in histopathological analyses of our study (Figure 5F). However, a systematic quantitative assessment of these parameters was not performed.
There are several limitations in this study. Although HUC-MSCs exhibit immunomodulatory effects in mice, species-specific differences in cytokine profiles, immune cell interactions, and MSC homing/engraftment could influence results. Our results show statistical significance compared to the saline control group, and the magnitude of MSC-specific effects may be overestimated due to the absence of vehicle controls. Moreover, HUC-MSCs may offer benefits over conventional treatments, such as modifying disease progression, being effective in steroid-resistant cases, causing fewer long-term side effects than corticosteroids, and promoting airway regeneration. However, these benefits need to be confirmed through direct comparative research. Assessments were conducted only on day 35, limiting the ability to track MSC effects and optimal dosing kinetics over time. Moreover, MSC biodistribution or survival analysis is not conducted, so we cannot distinguish whether our observed effects result from direct MSC engraftment in pulmonary tissues or systemic paracrine effects from cells distributed elsewhere.
Future research should investigate the optimal interval between multiple administrations for maximum therapeutic effect and assess repeated administrations’ long-term safety and efficacy with frequent sampling (e.g., at day 31 and 34), and extend observations to 8 weeks post-treatment to evaluate long-term airway remodeling changes. Additionally, systematic safety evaluations should be conducted, such as organ toxicity biomarker monitoring, in-depth molecular mechanisms of MSC lung homing (e.g., chemokine receptor expression), and direct comparison between HUC-MSCs and clinical standard therapies (e.g., inhaled corticosteroids). Intravenous administration enables systemic distribution and targeting of multiple inflammatory compartments, while local delivery provides direct airway access and potentially improved efficiency. Our study represents one validated approach, but comparative studies evaluating different delivery routes would be valuable for optimizing therapy. Furthermore, incorporating additional experimental groups would introduce more variables into the comparative statistical analysis.
HUC-MSC therapy shows potential for treating asthma with high purity. HUC-MSC has been demonstrated to modulate airway remodeling, enhance lung function, and inhibit the inflammatory response. This study provides appropriate reference dosages to further explore the possible intervention of HUC-MSC. The study data indicate that double treatments with HUC-MSCs at 2 × 105 cells may be optimal in reducing airway obstruction, but not for modulating airway inflammation. Long-term follow-up, safety assessments, molecular mechanism exploration, and comparisons with standard therapies are required in the future to comprehensively evaluate the clinical translational value of HUC-MSCs for asthma treatment.
Professor Da-Chun Wang, the important author of this article, deceased March 29, 2024. The authors thank Professor Da-Chun Wang for technical support, encouragement and care during the experiment. We will always miss him.
| 1. | Malmström K, Pelkonen AS, Mäkelä MJ. Remodeling, inflammation and airway responsiveness in early childhood asthma. Curr Opin Allergy Clin Immunol. 2013;13:203-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 2. | Reddel HK, Bacharier LB, Bateman ED, Brightling CE, Brusselle GG, Buhl R, Cruz AA, Duijts L, Drazen JM, FitzGerald JM, Fleming LJ, Inoue H, Ko FW, Krishnan JA, Levy ML, Lin J, Mortimer K, Pitrez PM, Sheikh A, Yorgancioglu AA, Boulet LP. Global Initiative for Asthma Strategy 2021: Executive Summary and Rationale for Key Changes. Am J Respir Crit Care Med. 2022;205:17-35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 303] [Article Influence: 60.6] [Reference Citation Analysis (0)] |
| 3. | GBD 2021 Asthma and Allergic Diseases Collaborators. Global, regional, and national burden of asthma and atopic dermatitis, 1990-2021, and projections to 2050: a systematic analysis of the Global Burden of Disease Study 2021. Lancet Respir Med. 2025;13:425-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 50] [Reference Citation Analysis (0)] |
| 4. | Shrestha Palikhe N, Wu Y, Konrad E, Gandhi VD, Rowe BH, Vliagoftis H, Cameron L. Th2 cell markers in peripheral blood increase during an acute asthma exacerbation. Allergy. 2021;76:281-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 5. | Mandlik DS, Mandlik SK. New perspectives in bronchial asthma: pathological, immunological alterations, biological targets, and pharmacotherapy. Immunopharmacol Immunotoxicol. 2020;42:521-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 6. | Yeh JJ, Lin HC, Yang YC, Hsu CY, Kao CH. Asthma Therapies on Pulmonary Tuberculosis Pneumonia in Predominant Bronchiectasis-Asthma Combination. Front Pharmacol. 2022;13:790031. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 7. | Nagamura-Inoue T, He H. Umbilical cord-derived mesenchymal stem cells: Their advantages and potential clinical utility. World J Stem Cells. 2014;6:195-202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 258] [Cited by in RCA: 319] [Article Influence: 26.6] [Reference Citation Analysis (5)] |
| 8. | Huang S, Li Y, Zeng J, Chang N, Cheng Y, Zhen X, Zhong D, Chen R, Ma G, Wang Y. Mesenchymal Stem/Stromal Cells in Asthma Therapy: Mechanisms and Strategies for Enhancement. Cell Transplant. 2023;32:9636897231180128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 9. | Chen QH, Zheng JY, Wang DC. Asthma and stem cell therapy. World J Stem Cells. 2025;17:103599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 10. | Kim RL, Bang JY, Kim J, Mo Y, Kim Y, Lee CG, Elias JA, Kim HY, Kang HR. Mesenchymal stem cells exert their anti-asthmatic effects through macrophage modulation in a murine chronic asthma model. Sci Rep. 2022;12:9811. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 11. | Kurtz A. Mesenchymal stem cell delivery routes and fate. Int J Stem Cells. 2008;1:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 174] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 12. | Alvites R, Branquinho M, Sousa AC, Lopes B, Sousa P, Maurício AC. Mesenchymal Stem/Stromal Cells and Their Paracrine Activity-Immunomodulation Mechanisms and How to Influence the Therapeutic Potential. Pharmaceutics. 2022;14:381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 92] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 13. | Shin JW, Ryu S, Ham J, Jung K, Lee S, Chung DH, Kang HR, Kim HY. Mesenchymal Stem Cells Suppress Severe Asthma by Directly Regulating Th2 Cells and Type 2 Innate Lymphoid Cells. Mol Cells. 2021;44:580-590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 14. | Wang Y, Liu L. Immunological factors, important players in the development of asthma. BMC Immunol. 2024;25:50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 15. | Ngo LT, Rekowski MJ, Koestler DC, Yorozuya T, Saito A, Azeem I, Harrison A, Demoruelle MK, Boomer J, England BR, Wolters P, Molyneaux PL, Castro M, Lee JS, Solomon JJ, Koronuma K, Washburn MP, Matson SM. Proteomic profiling of bronchoalveolar lavage fluid uncovers protein clusters linked to survival in idiopathic forms of interstitial lung disease. ERJ Open Res. 2024;10:00192-02024. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 16. | Han Y, Yang J, Fang J, Zhou Y, Candi E, Wang J, Hua D, Shao C, Shi Y. The secretion profile of mesenchymal stem cells and potential applications in treating human diseases. Signal Transduct Target Ther. 2022;7:92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 450] [Article Influence: 112.5] [Reference Citation Analysis (0)] |
| 17. | Goldstein MJ, Peters M, Weber BL, Davis CB. Optimizing the Therapeutic Window of Targeted Drugs in Oncology: Potency-Guided First-in-Human Studies. Clin Transl Sci. 2021;14:536-543. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | Chen C, Sun N, Li Y, Jia X. A BALB/c mouse model for assessing the potential allergenicity of proteins: comparison of allergen dose, sensitization frequency, timepoint and sex. Food Chem Toxicol. 2013;62:41-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Castro LL, Kitoko JZ, Xisto DG, Olsen PC, Guedes HLM, Morales MM, Lopes-Pacheco M, Cruz FF, Rocco PRM. Multiple doses of adipose tissue-derived mesenchymal stromal cells induce immunosuppression in experimental asthma. Stem Cells Transl Med. 2020;9:250-260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 20. | Leibacher J, Henschler R. Biodistribution, migration and homing of systemically applied mesenchymal stem/stromal cells. Stem Cell Res Ther. 2016;7:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 211] [Cited by in RCA: 265] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 21. | Ge J, Guo L, Wang S, Zhang Y, Cai T, Zhao RC, Wu Y. The size of mesenchymal stem cells is a significant cause of vascular obstructions and stroke. Stem Cell Rev Rep. 2014;10:295-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 163] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 22. | Moll G, Ankrum JA, Kamhieh-Milz J, Bieback K, Ringdén O, Volk HD, Geissler S, Reinke P. Intravascular Mesenchymal Stromal/Stem Cell Therapy Product Diversification: Time for New Clinical Guidelines. Trends Mol Med. 2019;25:149-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 305] [Article Influence: 43.6] [Reference Citation Analysis (0)] |
| 23. | Hur J, Kang JY, Kim YK, Lee SY, Jeon S, Kim Y, Jung CK, Rhee CK. Evaluation of Human MSCs Treatment Frequency on Airway Inflammation in a Mouse Model of Acute Asthma. J Korean Med Sci. 2020;35:e188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |