Published online Sep 26, 2025. doi: 10.4252/wjsc.v17.i9.109662
Revised: June 22, 2025
Accepted: August 13, 2025
Published online: September 26, 2025
Processing time: 129 Days and 21.3 Hours
Osteogenesis is driven by the differentiation of osteoblasts and the mineralization of the bone matrix, with oral-derived stem cells playing a significant role in this process. Various post-translational modifications (PTMs), such as phos
Core Tip: This review clarifies how post-translational modifications (PTMs), including phosphorylation, acetylation, methylation, and lactylation, dynamically modulate the osteogenic differentiation of oral-derived stem cells (ODSCs) under inflammatory or hypoxic conditions. We highlight PTMs as essential molecular switches integrating extracellular signals with transcriptional and epigenetic mechanisms to facilitate bone regeneration. Emerging therapeutic strategies - including histone deacetylase inhibitors, extracellular vesicle-based delivery, and metabolic-epigenetic crosstalk modulation - are proposed to improve ODSCs efficacy in osteoporosis and periodontitis. This review clarifies the PTMs “regulatory code” in ODSCs, connecting mechanistic insights to clinical translation while addressing unresolved challenges in precision regenerative medicine.
- Citation: Shi ZJ, Liu W. Post-translational modifications in osteogenic differentiation of oral-derived stem cells: Mechanisms and clinical implications. World J Stem Cells 2025; 17(9): 109662
- URL: https://www.wjgnet.com/1948-0210/full/v17/i9/109662.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v17.i9.109662
Bone regeneration remains a major challenge in regenerative medicine, particularly in oral and maxillofacial reconstruction and orthopedic procedures. Stem cell-based methodologies offer promising potential due to their self-renewal ability and multilineage differentiation. Oral-derived stem cells (ODSCs) - including dental pulp stem cells (DPSCs), periodontal ligament stem cells (PDLSCs), and gingiva-derived mesenchymal stem cells (GMSCs) - exhibit significant osteogenic capacity, low immunogenicity, and easy accessibility[1].
Osteogenesis involves mesenchymal stem cell (MSC) differentiation into osteoblasts and remains regulated by transcription factors, including runt-related transcription factor 2 (RUNX2), SP7 (osterix), alkaline phosphatase (ALP), osteocalcin (OCN), and canonical signaling pathways including bone morphogenetic proteins (BMP)/transforming growth factor β (TGF-β), Wnt/β-catenin, and mitogen-activated protein kinase (MAPK)[2]. Recent advancements in omics technologies and proteomics have revealed the importance of post-translational modifications (PTMs) in regulating osteogenic protein networks[3]. These dynamic and reversible modifications, including phosphorylation, acetylation, methylation, and glycosylation, regulate the stability, localization, and protein functions and integrate extracellular signals into lineage-specific transcriptional programs[4].
Phosphorylation and acetylation affect RUNX2 nuclear translocation and transcriptional activity, while glycosylation and methylation alter chromatin accessibility and protein-protein interactions[5]. Despite these advancements, the mechanisms by which PTMs regulate osteogenic differentiation (OD) in ODSCs remain unclear. The interaction between multiple PTMs and their correlation with inflammatory, mechanical, or hypoxic environments constitutes a vital frontier in skeletal tissue engineering.
Furthermore, recent studies demonstrate that pharmacological manipulations of specific PTMs can enhance bone regeneration in diseases including osteoporosis, periodontitis, and trauma-induced bone loss[6]. A systematic under
PTMs are essential for osteogenesis because ODSCs’ differentiation potential and regenerative capacity directly affect how these modifications impact bone formation. Expanding on the above-mentioned biological assumptions, which highlight the potential of ODSCs for bone regeneration, it is essential to specify the features, differentiation profiles, and osteogenic capabilities of various ODSC subtypes. Recent studies have demonstrated that these cells do not constitute a homogeneous population but instead comprise a diverse array of MSC-like subtypes originating from specific niches within the oral cavity. These subpopulations exhibit significant phenotypic characteristics and differentiation potential heterogeneity, resulting in functional diversity in tissue regeneration contexts. ODSCs are regarded as potential candidate cells for craniofacial and alveolar bone regeneration applications due to their easy accessibility, low immunogenicity, and robust capacity for osteogenic, chondrogenic, and adipogenic differentiation[7].
DPSCs: DPSCs were first isolated by Gronthos et al[8] from adult human dental pulp and have since gained recognition for their exceptional regenerative abilities. These cells display key MSC markers, such as CD29, CD44, CD73, CD90, and CD105, but do not show hematopoietic markers like CD34 and CD45. This unique marker profile points to their potential in regenerative therapies, as they exhibit low immunogenicity, reducing the likelihood of rejection after transplantation. DPSCs are capable of differentiating into various cell types, including odontoblasts, osteoblasts, chondrocytes, and neurocytes, showcasing their broad potential for tissue regeneration. Their ability to regenerate bone-like structures has been proven in vivo when seeded into bio-ceramic scaffolds. These scaffolds provide the necessary structural support for DPSC differentiation into osteoblasts, promoting bone regeneration, especially in cases like bone deformities and fractures. Additionally, studies have shown that DPSCs’ regenerative properties extend beyond bone repair, aiding in the regeneration of other tissues, including nerves[9]. This makes DPSCs a promising candidate for various clinical applications, particularly in the treatment of dental, maxillofacial, and orthopedic conditions[9].
Stem cells from human exfoliated deciduous teeth: Stem cells from human exfoliated deciduous teeth (SHEDs) are derived from exfoliated primary teeth and have enhanced proliferative capacity and broader differentiation potential compared to DPSCs. These cells differentiate into osteoblasts for bone regeneration and exhibit the ability to induce angiogenesis, facilitating blood vessel formation. This dual capability renders SHEDs particularly valuable for pediatric applications, including tooth regeneration and socket preservation, where the regenerative potential in young patients is essential. Their enhanced proliferation rates and plasticity position SHEDs as a promising tool in regenerative medicine, especially in pediatric dental and bone healing[10].
PDLSCs: PDLSCs are located in the perivascular regions of the periodontal ligament, an essential tissue involved in maintaining the integrity of the tooth-supporting structures. These cells are essential in regenerating periodontal ligament-like tissue, cementum, and alveolar bone, which are essential for periodontal tissue repair[11]. Gene-modified PDLSCs that overexpress BMPs or anti-inflammatory regulators demonstrate improved regenerative abilities, facilitating tissue healing and diminished inflammation. This makes PDLSCs a promising candidate for periodontal regeneration, providing a therapeutic approach for periodontitis and other inflammatory dental conditions[12].
GMSCs: GMSCs are isolated from the lamina propria of gingival tissue and have gained significant attention for their remarkable capacity to withstand inflammatory insults. Unlike other ODSCs, GMSCs demonstrate higher ALP activity, which is essential for OD, and they maintain mitochondrial function, ensuring cellular energy production under stress. Furthermore, GMSCs effectively inhibit nuclear factor kappa B (NF-κB) signaling in response to lipopolysaccharide (LPS) stimulation, mitigating inflammation. These cells display potent immunomodulatory capabilities by secreting interleukin (IL)-10 and TGF-β[13], rendering them particularly valuable for tissue regeneration in inflammatory environments such as periodontitis.
Dental follicle progenitor cells: Dental follicle progenitor cells (DFPCs) are derived from the dental follicle tissues encasing unerupted teeth and have remarkable differentiation potential. These cells can differentiate into osteoblasts, cementoblasts, and periodontal fibroblasts, rendering them essential for periodontal regeneration and tooth development. In osteoinductive conditions, DFPCs significantly express key osteogenic markers, including RUNX2, ALP, and OCN, which are essential for bone formation[14]. Besides facilitating root development, DFPCs are essential in bone regeneration, especially regarding dental tissue repair and the restoration of alveolar bone structures.
The osteogenic potential of ODSCs is regulated by intrinsic transcription factors and external signals. During in vitro induction, markers, including RUNX2, osterix, ALP, OCN, and collagen type I (COL1A1), are upregulated. DPSCs and PDLSCs have demonstrated reliable expression of these markers and matrix mineralization across several osteogenic protocols[15].
GMSCs surpass DPSCs in maintaining osteogenic function during oxidative or inflammatory stress. For instance, in LPS-rich conditions mimicking periodontitis, GMSCs resist mitochondrial damage and maintain the expression of osteogenic markers, partly owing to superior antioxidant response and reduced reactive oxygen species (ROS) generation[1]. Comparative transcriptomic profiling revealed that DPSCs preferentially engage BMP2/SMAD1/5/8 signaling, while PDLSCs tend to activate TGF-β/SMAD2/3 pathways more robustly[16]. These intrinsic distinctions necessitate customized strategies in scaffold construction and biochemical induction based on cell origin.
Epigenetic modifications - including histone acetylation, methylation, crotonylation, and DNA methylation - are recognized as essential regulators of osteogenesis in ODSCs. Recent studies demonstrate that histone crotonylation (H3K18cr) in PDLSCs activates Wnt target genes, including β-catenin and lymphoid enhancer-binding factor 1 (LEF1), resulting in enhanced mineral deposition[17]. Similarly, lysine lactylation of histones reverses LPS-induced osteogenic inhibition by upregulating RUNX2 and OCN expression[1].
ODSCs have exhibited translational potential in various in vivo models. DPSCs seeded on β-tricalcium phosphate scaffolds effectively repaired calvarial lesions and exhibited osteointegration within eight weeks[18]. GMSCs embedded within bovine pericardial membranes markedly improved vascularized bone formation, presumably owing to their immune-tolerant phenotype and paracrine signaling[5]. Furthermore, gene-edited PDLSCs and DFPCs have been integrated into hydrogels or three-dimensional-printed scaffolds. The overexpression of BMP9 or RUNX2 genes markedly upregulated mineralization markers and microvessel density in grafted sites[12]. These innovations indicate a transition toward clinically relevant, cell-enhanced biomaterials. Collectively, these applications underscore the importance of matching specific ODSC subtypes to distinct regenerative contexts based on their inherent capabilities.
Table 1 presents a comparative overview based on the distinct characteristics and preclinical performance of individual ODSC populations to facilitate rational selection and translational design. It consolidates their documented osteogenic efficacy, defining cellular features and context-specific therapeutic potential. Although all ODSCs share mesenchymal origin and multilineage potential, significant variation exists in their response to inflammatory stress, proliferative kinetics, immunomodulatory profiles, and scaffold compatibility. These distinctions directly affect their application scope across various craniofacial and orthopedic regenerative settings.
| ODSC type | Osteogenic potential | Key features | Clinical application scenarios | Ref. |
| DPSCs | High | Forms bone-like structures, suitable for pulp and alveolar repair | Dental pulp regeneration, calvarial and alveolar bone defects | [8,9,18] |
| GMSCs | Very high | High ALP activity, strong anti-inflammatory and antioxidant capacity | Periodontitis and chronic inflammatory environments | [5,13] |
| PDLSCs | Moderate | Responsive to gene modification, secretes paracrine factors | Periodontal regeneration, alveolar ridge restoration | [11,12,15] |
| SHEDs | High | Fast proliferation, suitable for pediatric regenerative applications | Tooth socket preservation, pediatric bone regeneration | [10] |
| DFPCs | High | Differentiates into osteoblasts, cementoblasts; important for root development | Tooth root and periodontal tissue engineering | [14,16] |
Understanding the unique features of ODSCs establishes a foundation for investigating how essential signaling pathways, including BMP and Wnt/β-catenin, are regulated by PTMs to coordinate OD. OD is a complex and rigorously regulated biological process whereby MSCs acquire the phenotype and function of mature osteoblasts, culminating in extracellular matrix (ECM) deposition and mineralized bone formation. In ODSCs, including DPSCs, PDLSCs, SHEDs, and GMSCs, this transition is orchestrated by a multilevel network involving transcriptional regulators, intracellular signaling pathways, epigenetic mechanisms, and PTMs. Understanding these pathways is essential for utilizing ODSCs in therapeutic bone regeneration.
RUNX2 is regarded as a master transcription factor that regulates osteoblast lineage specification and facilitates the transcription of essential osteogenic genes, including COL1A1, ALP, OCN, and osterix. These indicators function synergistically to facilitate ECM maturation and mineralization. Recent studies have demonstrated that high RUNX2 expression, combined with BMP2 and osterix activity, significantly enhances osteogenic gene profiles and facilitates mineral deposition in osteogenic environments[19]. Osterix functions downstream of RUNX2 and is essential for the preosteoblast to transition into an osteoblast. Additional transcription factors, including activating transcription factor 4, distal-less homeobox 5, and β-catenin, coordinate with RUNX2 to fine-tune osteogenesis. ALP facilitates early mineral ion release, while OCN and bone sialoprotein contribute to hydroxyapatite crystallization at later stages[20].
BMP/TGF-β pathway: The BMP/TGF-β signaling pathway is essential in osteogenesis by regulating downstream transcriptional processes. BMP2 and BMP7 stimulate type I and II BMP receptors, resulting in the phosphorylation of SMAD1/5/8 proteins, which subsequently form complexes with SMAD4 and translocate to the nucleus to initiate the transcription of osteogenic regulators, particularly RUNX2. This pathway is essential for promoting MSC differentiation into osteoblasts. Conversely, TGF-β1 predominantly stimulates progenitor cell proliferation while inhibiting terminal OD through SMAD2/3 pathway activation, thereby preserving a regulatory balance between proliferation and differentiation during bone development[21].
Wnt/β-catenin pathway: The Wnt/β-catenin pathway plays a crucial role in osteogenesis by regulating osteoblast differentiation. Canonical Wnt ligands, like Wnt3a, activate frizzled and lipoprotein receptor-related protein 5/6 receptors on the cell membrane, which inhibits glycogen synthase kinase 3 beta (GSK3β). This inhibition leads to the stabilization of β-catenin, which then moves to the nucleus and works with key transcription factors like RUNX2 and T-cell factor/LEF to activate genes that drive osteoblast differentiation. On the other hand, the non-canonical Wnt ligand Wnt5a activates alternative signaling pathways, such as Ca2+/calmodulin and protein kinase C, which help fine-tune bone formation and cellular responses during OD[22].
MAPK pathway: The MAPK signaling pathway is essential in osteogenesis as it integrates mechanical and biochemical signals that affect MSC differentiation. Within the MAPK family members, extracellular signal-regulated kinases (ERK), c-Jun N-terminal kinase, and p38 kinases function uniquely in coordinating osteogenic processes. ERK activation significantly enhances COL1A1 synthesis, facilitating ECM architecture during bone formation. c-Jun N-terminal kinase contributes to cytoskeletal remodeling and focal adhesion dynamics, promoting the morphological transformation of osteoblast progenitors. The p38 MAPK pathway is essential for enhancing the phosphorylation and transcriptional activity of RUNX2, subsequently upregulating ALP and other osteogenic genes involved in matrix mineralization. These findings highlight the pathway’s multifaceted role in regulating osteoblast lineage commitment in response to mechanical stimulation[23].
Phosphatidylinositol 3-kinase/protein kinase B pathway: The phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT) signaling pathway plays a crucial role in regulating osteogenesis by integrating a variety of extracellular signals that promote osteoblast differentiation and bone formation. Upon activation, PI3K phosphorylates AKT, which then inhibits GSK3β, a negative regulator of β-catenin. This inhibition stabilizes β-catenin, allowing it to move to the nucleus and activate osteogenic gene expression. Additionally, AKT activates the mammalian target of rapamycin pathway, which boosts protein synthesis, essential for cell growth and matrix production. In MC3T3-E1 cells, ERK1/2 signaling, which acts upstream of AKT, further promotes osteoblast differentiation by enhancing matrix synthesis, demonstrating the synergy between these pathways in driving osteogenesis[24].
The cAMP response element-binding protein (CREB) integrates signals from protein kinase A (PKA), calcium, and MAPK. Upon phosphorylation, CREB binds cAMP-response elements within osteogenic gene promoters, stimulating RUNX2, ALP, and OCN expression. It additionally interacts with coactivators, including CREB binding protein (CBP)/p300, to facilitate chromatin remodeling during osteogenesis[25].
Emerging evidence indicates that CREB activity is regulated by PTMs, including palmitoylation. Particularly, S-palmitoylation of BMP receptors enhances their stability and downstream SMAD signaling. Recent findings indicate that ZDHHC9, rather than ZDHHC16, is responsible for the palmitoylation of BMP receptor 1a (BMPR1a), which enhances osteogenesis in preosteoblasts[26]. The function of these fundamental signaling cascades and transcription factors is further refined by an additional regulatory layer comprising epigenetic modifications and PTMs.
Epigenetic modifications and PTMs provide essential regulatory frameworks that influence the fundamental transcriptional regulators and signaling pathways described above. They precisely regulate gene expression and protein function during OD through dynamic and frequently interconnected mechanisms. Although these mechanisms have historically been studied independently, there is increasing evidence of their interaction in regulating osteogenesis. Both modifications collaborate to regulate the expression of osteogenic genes and the activity of transcription factors, including RUNX2.
Epigenetic modifications, including histone acetylation, methylation, and lactylation[27], affect the chromatin structure, thereby regulating DNA accessibility for transcription. However, PTMs modulate protein function, stability, subcellular localization, and protein-protein interactions, directly regulating essential osteogenic proteins and signaling pathways[28].
RUNX2, a major regulator of osteoblast development, undergoes both epigenetic modifications and PTMs. Histone acetylation and methylation at the RUNX2 promoter enhance its transcriptional activity, while PTMs such as phosphorylation and acetylation boost RUNX2 stability and nuclear retention[29]. These modifications enable the proper activation of osteogenic genes, including ALP, OCN, and COL1A1.
Consequently, epigenetic modifications and PTMs are interrelated regulatory layers that refine osteogenesis. The ability of PTMs to regulate epigenetically modified transcription factors enables precise control over OD. Understanding the interplay between these modifications will be essential for developing techniques to enhance bone regeneration and OD in stem cells.
Histone modifications: Histone modifications are crucial in regulating osteogenesis by affecting gene expression. Histone acetylation, catalyzed by histone acetyltransferases, including p300 and CBP, enhances osteogenic gene transcription, including RUNX2, by relaxing chromatin structure and making the DNA more accessible for transcription factors. Histone deacetylases (HDACs), such as HDAC4 and HDAC5, inhibit osteogenic gene expression by removing acetyl groups, resulting in chromatin condensation. Inhibiting HDACs with compounds such as valproic acid enhances mineralization in DPSCs, demonstrating the potential of epigenetic modulation to promote osteogenesis and bone formation[1].
DNA methylation: DNA methylation plays a crucial role in regulating osteogenesis by controlling the expression of osteogenic genes. During the later stages of osteoblast maturation, the promoters of genes like osterix and OCN undergo demethylation, which is linked to increased matrix mineralization. This epigenetic change helps activate these key genes that are essential for the final stages of bone formation. Research has shown that removing repressive DNA methylation marks from osteoblast-specific promoters is necessary for osteoblast differentiation and bone matrix synthesis[27]. This underscores the importance of DNA methylation in regulating osteogenesis and mineralization.
Ferroptosis: Ferroptosis, a type of cell death caused by iron and lipid ROS, has been identified as a key mechanism that inhibits OD during oxidative stress. This process involves the accumulation of lipid peroxides, which causes cellular damage and impaired function. In DPSCs, ferroptosis hinders their osteogenic potential, reducing their regenerative capacity. However, pharmacological inhibitors of ferroptosis, including ferrostatin-1, protect DPSCs, enhancing their survival and promoting OD. These findings reveal that targeting ferroptosis may improve stem cell-based bone regeneration therapy[28].
Hypoxia and hypoxia-inducible factor 1-alpha: Hypoxia is a common occurrence in various tissues during repair, leading to the stabilization of hypoxia-inducible factor 1-alpha (HIF-1α), which in turn promotes the expression of vascular endothelial growth factor (VEGF) and encourages angiogenesis. Since bone repair relies heavily on vascularization for nutrient and oxygen supply, the ability of ODSCs to adapt to low oxygen conditions provides them with a significant regenerative advantage[29]. This adaptability enhances their potential for bone tissue engineering by supporting osteogenesis and angiogenesis - both crucial processes for effective bone regeneration. The importance of HIF-1α signaling in ODSCs is particularly notable, as it enhances their functionality under hypoxic conditions.
The osteogenic network is complex; however, it forms a highly dynamic system with extensive feedback. RUNX2 functions as a signaling hub activated by SMADs (BMP), β-catenin (Wnt), and CREB (PKA/MAPK). Crosstalk examples include Wnt enhancing BMP signaling by upregulating BMP2 expression, AKT stabilizing β-catenin by inhibiting GSK3β, and CREB interacting with SMAD1 for cooperative transcription at osteogenic promoters[21].
PTMs, including phosphorylation (RUNX2-Ser319), acetylation (histone H3K9ac), and palmitoylation (BMPR1a-Cys), regulate the timing and location of these signals[30]. PTMs facilitate complex inter-pathway communication through shared molecular hubs. The acetylation of β-catenin by p300/CBP amplifies its physical interaction with SMAD1, facilitating the assembly of a transcriptional complex that co-occupies the promoters of osteogenic genes, including RUNX2. SMAD4 methylation by protein arginine methyltransferase 1 facilitates its nuclear translocation and stabilizes the β-catenin-SMAD1 complex, establishing a positive feedback loop between Wnt and BMP signaling[31]. Under hypoxic conditions, histone lactylation at H3K18 functions as a metabolic sensor that epigenetically prepares chromatin regions of both Wnt (LEF1) and BMP (BMPR2) pathway components. This facilitates HIF-1α to recruit lactylated β-catenin to hypoxia-response elements, synergistically enhancing mineral deposition in hypoxic microenvironments[32].
This multilayered regulatory structure enables ODSCs to adaptively respond to microenvironmental cues, making them optimal for personalized bone regeneration strategies. Having established the molecular signaling pathways, we now direct our focus to the specific PTMs that affect these pathways, therefore regulating the osteogenic process.
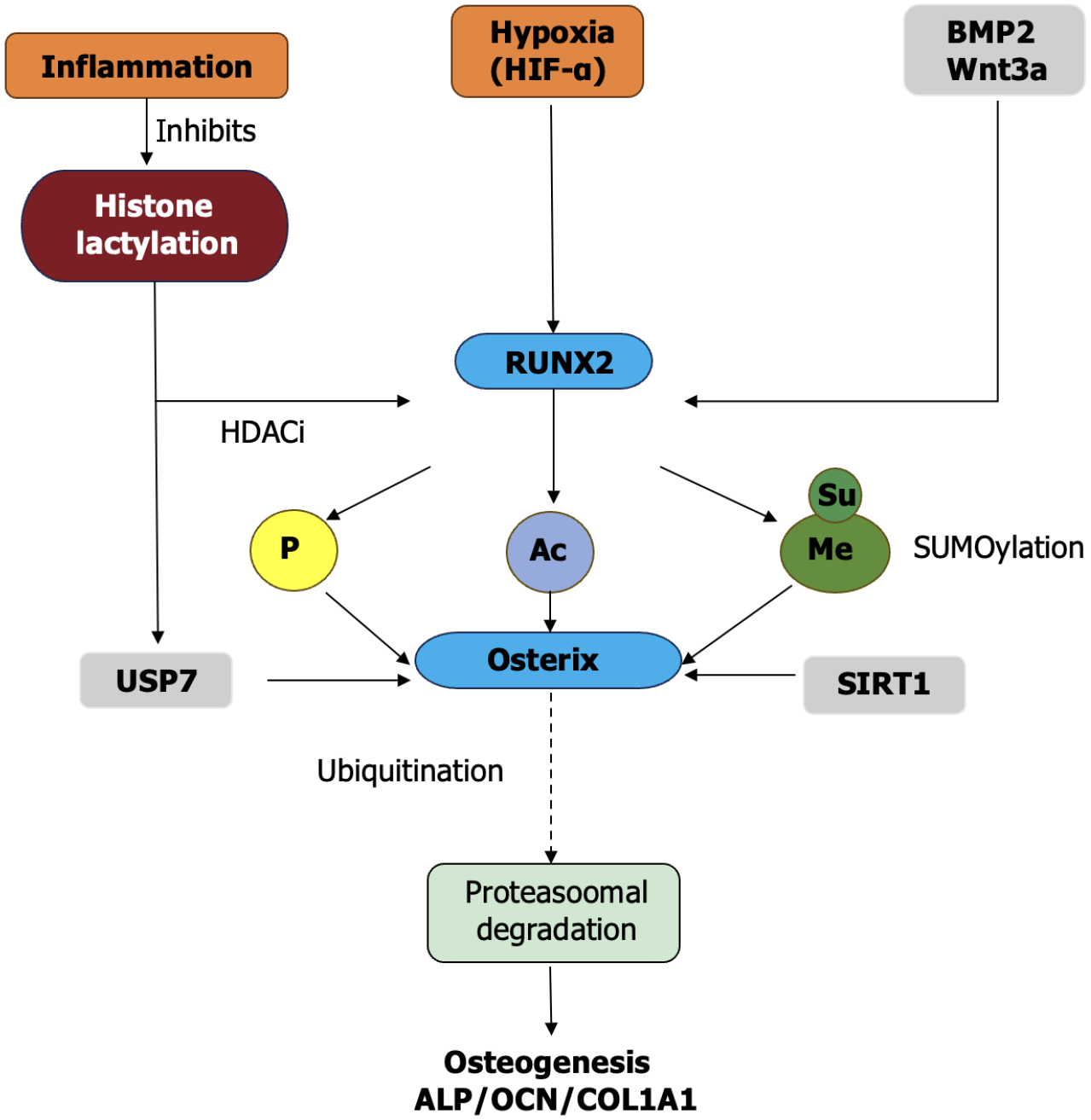
PTMs constitute the fundamental regulatory machinery that dynamically coordinates OD in ODSCs, acting as biochemical translators that transform extracellular signaling inputs into specific transcriptional outputs through rapid, reversible modifications, including phosphorylation, acetylation, methylation, glycosylation, ubiquitination, palmitoylation, lactylation, and crotonylation. These modifications function through three core mechanisms: (1) Direct protein function (activity, stability, and localization) modulation; (2) Epigenetic regulation of chromatin states; and (3) Dynamic adjustment of receptor signaling dynamics. Collectively, they allow ODSCs to interpret complex microenvironmental signals during bone regeneration while offering strategic therapeutic targets across physiological and pathological contexts[1,6,19,30]. The integration of upstream osteoinductive signals and intracellular transcriptional mechanisms is regulated by various PTMs. Figure 1 depicts this multilayered regulatory network.
Phosphorylation acts as the essential initiation switch for osteogenic signaling cascades and functions as the primary signaling pathway through which BMP2 stimulation induces sequential SMAD1/5/8 phosphorylation at C-terminal SSXS motifs by type I receptors (ALK2/3/6), facilitating complex formation with SMAD4 and nuclear translocation to commence RUNX2 transcription[19,21,23]. Concurrent MAPK signaling through ERK1/2 and p38 kinases phosphorylates RUNX2 at specific serine residues (Ser347 and Ser369 in human RUNX2), enhancing its DNA-binding affinity through conformational changes and facilitating nuclear retention by masking nuclear export signals while simultaneously providing resistance to ubiquitin-mediated proteasomal degradation[19,21]. This dual phosphorylation cascade culminates in the transcriptional activation of essential matrix genes, including COL1A1, ALP, and OCN, with CREB serving as a phosphorylation-sensitive signaling integrator. PKA-mediated phosphorylation at Ser133 or ERK-dependent modification facilitates CREB dimerization and its binding to cAMP response elements in osteogenic promoters, effectively bridging mechanical stimulation (fluid shear stress) and biochemical signaling pathways[25]. The indispensability of this mechanism is evidenced by complete mineralization failure in CREB-deficient DPSCs, where BMP2-induced ALP expression and calcium deposition are eliminated despite normal RUNX2 Levels[33]. Extensive phosphoproteomic profiling during MSC osteogenesis has identified > 400 dynamically modified targets that regulate cytoskeletal reorganization (paxillin phosphorylation at Tyr31), metabolic adaptation (AMPKα-Thr172 phosphorylation), and epigenetic modifier activity (phosphorylation of histone demethylase KDM6A at Ser1204), thereby confirming phosphorylation’s role as the master initiation switch in osteogenic commitment[34].
Complex feedback regulation occurs among phosphorylation modifications. For instance, ERK-mediated RUNX2 phosphorylation recruits phosphatases to form a dynamic equilibrium network, ensuring accurate signal output[35]. Furthermore, in response to mechanical stimuli, crosstalk transpires between the phosphorylation cascades of the integrin-FAK-Src pathway and the BMP/SMAD pathway, collectively enhancing osteogenic signals[36].
Acetylation exerts multifaceted regulation over osteogenesis through distinct mechanisms that impact chromatin accessibility and non-histone protein function. Histone acetyltransferases (P300/CBP) acetylate lysine residues on histone H3 (H3K9 and H3K27) utilizing acetyl-CoA as a donor substrate, thereby relaxing chromatin structure at osteogenic loci to facilitate transcriptional machinery access to RUNX2, osterix, and bone gamma carboxyglutamate protein promoters[37]. Simultaneously, non-histone acetylation specifically modifies RUNX2 at Lys225 inside its transactivation domain, enhancing protein stability by inhibiting ubiquitin-dependent degradation and boosting DNA-binding capacity through charge neutralization[33]. This coordinated acetylation establishes a conducive epigenetic environment that is actively antagonized by class IIa HDACs (HDAC4/5/7) and class I HDAC3, which inhibit transcription through RUNX2 deacetylation and H3K9 deacetylation at osteogenic promoters[1,37]. The therapeutic susceptibility of this balance is demonstrated by HDAC3 depletion, which results in a 3.2-fold increase in calcium deposition and ALP activity in human MSCs[38]. Conversely, HDAC inhibitors such as trichostatin A restore osteogenesis in tumor necrosis factor (TNF)-α-inflamed DPSCs through Wnt/β-catenin pathway reactivation and genome-wide histone hyperacetylation, increasing mineralization by 68% compared to controls[39].
Acetylation modifications are closely associated with cellular metabolic conditions. Acetyl-CoA, serving as the acetyl group donor, is modulated by glycolytic and TCA cycle activities. During hypoxic or energetic stress, diminished acetyl-CoA levels may lead to inadequate acetylation of essential osteogenic transcription factors (RUNX2), compromising their stability and function - a potential metabolic mechanism contributing to impaired bone repair[40].
Methylation facilitates context-specific gene regulation, while lactylation creates a direct connection between cellular metabolism and the epigenetic regulation of transcription. H3K4me3 activates RUNX2 and OCN promoters through chromatin opener recruitment, while trimethylation of H3 on lysine 27 (H3K27me3) enforces transcriptional suppression through polycomb repressive complex binding, requiring KDM6B-mediated H3K27me3 demethylation during commitment[41]. TNF-α pathologically disrupts mineralization in DPSCs by inhibiting KDM6B expression through NF-κB activation. This sustains repressive histone modification and severely hinders mineralization capacity. This dysfunction is reversible with KDM6B-targeted activators, including GSK-J4[42].
The accuracy of methylation depends on the spatiotemporal regulation of methyltransferases (SET7/9 for H3K4me) and demethylases (KDM6B). Previous studies demonstrated that hyperglycemic conditions in diabetes inhibit KDM6B expression and enhance enhancer of zeste homolog 2 (EZH2) (PRC2 catalytic subunit) activity, causing abnormal H3K27me3 accumulation at osteogenic gene promoters - an essential epigenetic mechanism impairing bone formation in diabetic osteopathy[43].
Lactylation, a glycolysis-linked PTM, creates a metabolic-epigenetic interface where lactate alters histone lysine residues through lactyl-CoA transfer. LPS-induced inflammation diminishes global H3K18 La levels in PDLSCs, which correlates with suppressed RUNX2, ALP, and COL1A1 expression, while proanthocyanidin treatment restores lactylation and osteogenic capacity through sirtuin 1 (SIRT1) activation[1]. Lactylation of non-histone proteins (transcription factors) has been identified in addition to histones. During OD, high-lactate microenvironments (inflammation or hypoxia) may dynamically modulate critical signaling molecules through lactylation, offering new insights into bone metabolic dysregulation in metabolic diseases[44]. Lactylation dynamically couples cellular metabolism with transcriptional regulation through multifaceted mechanisms: (1) Histone lactylation at H3K18 recruits bromodomain-containing protein 4 (BRD4), thereby enhancing RNA polymerase II processivity at osteogenic loci[45]; (2) Lactylation of HIF-1α stabilizes its inte
Glycosylation significantly modulates OD by regulating receptor signaling fidelity and ECM stability. N-glycosylation of key receptors (BMP and Wnt) affects ligand binding affinity and internalization dynamics, hence directly impacting downstream osteogenic pathway activation. Furthermore, proper glycosylation of ECM components is essential for cell-matrix interactions and mechanotransduction during osteogenic maturation. Pathological conditions, including inflammation or metabolic disease, can alter glycosylation patterns, thereby compromising signaling and matrix function[48].
The N-glycosylation of BMP and Wnt receptors affects their ligand-binding affinity, hence modulating the duration and intensity of signaling pathways essential for osteogenesis. Glycosylation of BMP receptors affects the receptor’s ability to bind its ligands[49], consequently regulating downstream signaling cascades essential for osteoblast differentiation. Glycosylation modifications on Wnt receptors can influence their internalization and activation, hence regulating bone formation[50].
During inflammatory or hypoxic conditions prevalent in periodontal disease and bone trauma, the glycosylation patterns of these receptors may shift, affecting receptor localization and responsiveness to signaling molecules. These changes can alter the OD potential of ODSCs, offering insight into how glycosylation modifications may influence bone regeneration in diseased or damaged tissues. In PDLSCs, appropriate ECM protein glycosylation, including fibronectin and osteopontin, is necessary for integrin-mediated adhesion and mechanotransduction, which are essential for osteogenic maturation[30].
The complexity of glycosylation lies in its structural heterogeneity and tissue specificity. Pathological conditions, including diabetes, characterized by the accumulation of advanced glycation end products, alter glycosylation patterns. Aberrant glycosylation interferes with receptor function and directly modifies ECM proteins (collagen), compromising their mechanical properties and cell-binding capacity, thus creating a “vicious cycle” that impedes regeneration[51].
Ubiquitination and palmitoylation regulate proteostasis and membrane dynamics: Ubiquitination precisely controls the temporal turnover of signaling molecules, while palmitoylation modulates receptor localization and complex formation for signal amplification. Ubiquitination offers temporal control through E3 ubiquitin ligase Smurf1-mediated polyubiquitination of RUNX2 (at Lys94/Lys148) and SMAD1 (at Lys519), directing them for 26S proteasomal degradation to significantly restrict osteoblast activity and mineralization potential[52,53]. This suppression is mitigated by BMP2-induced downregulation of Smurf1 expression and USP7-mediated stabilization of RUNX2 through direct deubiquitination. Under oxidative stress conditions in DPSCs, USP7 enhances mineralization capacity by protecting RUNX2 from ROS-induced degradation[54].
Palmitoylation dynamically tunes membrane receptor localization through ZDHHC9-mediated S-palmitoylation of BMPR1a at Cys478/Cys496, facilitating lipid raft partitioning through hydrophobic interactions and concentrating receptor complexes for amplified SMAD signaling that accelerates osteoblast maturation[26]. However, under prolonged oxidative stress, ZDHHC16 overexpression in DPSCs inhibits CREB phosphorylation at Ser133 and induces acyl-CoA synthetase long-chain family member 4-dependent ferroptosis, inhibiting mineralization through lipid peroxidation cascade activation[55].
The ubiquitin system exhibits significant substrate specificity and reversibility. Beyond Smurf1, other E3 Ligases (SCF complex members) and deubiquitinases (USP15) modulate the stability of osteogenic proteins[56]. Palmitoylation, as a reversible hydrophobic alteration, is dynamically regulated by palmitoyltransferases (ZDHHC family) and depalmitoylases (acyl-protein thioesterases 1/2), impacting receptor localization, protein-protein interactions, and signaling efficiency[57].
SUMOylation preserves SMAD4 from proteasomal degradation through SUMO2/3 conjugation at Lys507 during endoplasmic reticulum stress, sustaining BMP signaling integrity and enhancing osteogenic output under stress conditions[58]. Crotonylation synergizes with acetylation to increase transcriptional activation through elevated histone H3K9cr levels in PDLSCs. This epigenetic modification stimulates PI3K/AKT signaling through phosphatase and tensin homolog inhibition and enhances chromatin fiber compaction, thereby increasing RUNX2 and OCN expression[59]. SUMOylation specifically governs protein interactions, subcellular localization, and stress resistance, with its dysregulation associated with diseases including osteoarthritis. Crotonylation employs crotonyl-CoA as a donor, whose abundance is influenced by short-chain fatty acid metabolism. This indicates that gut microbiota-derived metabolites may remotely regulate bone metabolism through this modification[60]. PTMs collaboratively regulate signaling interactions among osteogenic pathways through precise spatiotemporal modifications. Table 2 presents the principal mechanisms of this inter-pathway regulation.
| PTM type | Molecular target | Pathway interaction | Functional consequence | Ref. |
| Acetylation | β-catenin (Lys49) | Wnt-BMP synergy | Enhanced β-catenin/SMAD1 complex stability | [33,37-39] |
| Methylation | SMAD4 (Arg37) | BMP-Wnt-PI3K integration | Facilitated β-catenin nuclear translocation | [41-43] |
| Lactylation | H3K18 histone mark | Metabolic-epigenetic coupling | Chromatin priming at osteogenic loci | [44,46,47] |
| Phosphorylation | GSK3β (Ser9) | PI3K-Wnt crosstalk | β-catenin stabilization | [35,36] |
| Ubiquitination | SMAD1 (Lys519) | BMP-TGFβ balance | Regulation of SMAD1/2/3 stoichiometry | [52,53] |
PTMs form spatially and temporally integrated networks exemplified by RUNX2 regulation: Sequential phosphorylation by ERK at Ser347 prepares it for p300-mediated acetylation at Lys225, which simultaneously inhibits ubiquitin-dependent degradation and attracts the H3K27me3 demethylase KDM6A to eliminate repressive marks at target promoters[19,37,54]. This organized cascade forms a dynamic “PTM code” that regulates transcriptional amplitude, duration, and adaptability during stem cell differentiation[61].
Therapeutic utilization of PTM networks has produced promising treatments. Small-molecule modulators, including HDAC inhibitors (valproic acid), Smurf1 antagonists (noggin), and lactylation enhancers (proanthocyanidins), demonstrate significant efficacy in rescuing impaired osteogenesis across diverse disease models[38,39,62]. The therapeutic utilization of PTM networks has produced promising treatments. Complementing pharmacological approaches, ex vivo PTM preconditioning through BMP2 + p300 co-stimulation significantly improves transplanted ODSC survival and osteogenic capacity in rat calvarial defects. Furthermore, ODSC-derived extracellular vesicles (EVs) transport functional PTM-modified cargos (phosphorylated SMADs, acetylated histones) to recipient osteoprogenitor cells, therefore activating Wnt/β-catenin and BMP pathways, which significantly enhance new bone formation in mandibular defects[6].
Despite these advances, precision medicine encounters significant difficulties. Disease-specific PTM dysregulation patterns, including H3K9 hyperacetylation at PPARγ promoters combined with RUNX2 hypophosphorylation at Ser347 in diabetic osteoporosis, demand tailored interventions[62]. Diabetic osteoporosis involves combinatorial PTM dysregulation: Hyperacetylation at PPARγ promoters induces an adipogenic shift, hypophosphorylation of RUNX2 hinders transcriptional activation, and reduced SMAD4 methylation weakens BMP-Wnt interaction. Pharmacological strategies combining HDAC inhibitors, AKT activators, and methylation cofactors exhibit synergistic bone regeneration by rectifying this multi-dimensional imbalance[47].
Future efforts must prioritize decoding cell-type-specific PTM signatures across ODSC subtypes (DPSCs vs PDLSCs) using single-cell PTMomics technologies. Concurrently, developing methods for in vivo dynamic monitoring of PTMs is imperative to resolve spatiotemporal heterogeneity and inform patient-specific therapeutic reprogramming in pathological microenvironments[63]. PTMs coordinate signaling crosstalk across osteogenic pathways with specific spatiotemporal modifications. Table 3 presents a comprehensive summary of major PTM types, their mechanisms, and therapeutic implications.
| PTM type | Mechanism of action | Key molecules/enzymes | Therapeutic implications | Ref. |
| Phosphorylation | Activates RUNX2, SMADs, CREB via ERK/p38 phosphorylation to initiate osteogenic gene transcription | ERK, p38, CREB, SMAD1/5/8 | BMP2 peptides, ERK activators promote differentiation | [19,21,23-25,35,36] |
| Acetylation | Loosens chromatin via p300/CBP; stabilizes RUNX2; HDAC inhibition enhances transcription | p300, CBP, HDACs, RUNX2 | HDAC inhibitors (TSA, VPA), resveratrol support bone formation | [1,31,35,37,38] |
| Lactylation | Restores gene expression in LPS-inflamed ODSCs; rescues RUNX2, ALP, COL1A1 | Histone H3, proanthocyanidins | Natural anti-inflammatory agents restore osteogenesis in disease states | [1,54] |
| Ubiquitination | Targets RUNX2/SMAD1 for degradation (Smurf1); USP7 reverses this to enhance osteogenesis | Smurf1, USP7, β-catenin | Proteasome or E3 Ligase inhibitors improve cell survival and differentiation | [41,42] |
| Methylation | Repressive (H3K27me3) and active (H3K4me3) methylation dynamically regulate osteogenic loci | EZH2, KDM6B, H3K27, H3K4 | EZH2 inhibitors and demethylase activators restore suppressed osteogenesis | [39,40] |
| Palmitoylation | BMPR1a palmitoylation by ZDHHC9 boosts SMAD signaling; links lipid metabolism to osteogenesis | ZDHHC9, ZDHHC16, BMPR1a, CREB | Metabolic enhancement and scaffold engineering using palmitoylation pathways | [26,43] |
| Glycosylation | Glycosylation of receptors, such as BMP and Wnt receptors, modulates ligand binding affinity and receptor internalization, influencing signaling pathways critical for osteogenesis | BMP receptors, Wnt receptors, OGT | Enhancing receptor signaling in osteogenesis; therapeutic strategies for bone regeneration in inflammatory environments | [48,50] |
Utilizing the central role of PTMs in regulating osteogenesis, focused approaches are being formulated to modulate these alterations and enhance the bone regenerative capacity of ODSCs. As understanding of the essential role of PTMs in osteogenic regulation advances, efforts have shifted from descriptive studies toward purposeful manipulation of these modifications to enhance bone regeneration. ODSCs, owing to their immunomodulatory, regenerative, and osteoinductive properties, remain an ideal model for investigating such targeted modulation. This section elaborates on current approaches - including pharmacological agents, gene modulation, scaffold engineering, and EV strategies - developed to regulate PTMs for enhanced osteogenic outcomes in ODSCs.
Pharmacological manipulations of enzymes involved in PTMs offer a viable approach to enhance osteogenesis. HDAC inhibitors, including trichostatin A, enhance histone acetylation, relax chromatin, and facilitate transcription of osteogenic genes such as RUNX2 and OCN[42]. EZH2 inhibitors diminish repressive H3K27me3 marks and restore differentiation in inflammatory environments[64]. Additionally, proteasome inhibitors stabilize β-catenin, sustaining Wnt/β-catenin signaling essential for osteoblast lineage commitment[65]. These pharmacological agents are effective tools for modulating intracellular signaling to promote bone regeneration.
Genetic manipulation offers precise and sustained control over PTM regulators. Knockdown of negative regulators such as ZDHHC16 in DPSCs enhances CREB phosphorylation, reduces oxidative damage, and upregulates COL1A1 and OCN levels[55]. Conversely, overexpression of histone demethylases, including KDM6B, mitigates chromatin repression, especially under TNF-α exposure, by reactivating osteogenic loci[66]. These genetic interventions offer cell-intrinsic strategies to reprogram epigenetic states for enhanced osteogenic fidelity.
Bioactive compounds derived from natural sources provide a safe, low-toxicity way to modulate PTMs. Proanthocyanidins, for example, can restore lysine lactylation that is inhibited by inflammation, thus rescuing osteogenic gene expression and acting as natural epigenetic regulators[1]. Resveratrol activates the SIRT1 deacetylase and enhances RUNX2-mediated transcription[67], while curcumin inhibits HDACs and reduces oxidative stress, promoting mineral deposition in SHEDs[68]. These compounds serve as nutritional epigenetic modulators and could be incorporated into scaffold coatings or used as part of systemic supplementation.
Scaffold materials influence PTMs by replicating the mechanical, chemical, and topographical features of the bone environment. Collagen-calcium phosphate scaffolds, for example, trigger SMAD phosphorylation and histone H3 acetylation in DPSCs, promoting matrix mineralization[69]. Piezoelectric scaffolds, which respond to mechanical loading, activate ERK/CREB signaling and enhance osteogenic marker expression in GMSCs[70]. These materials serve as “epigenetic instructive cues”, allowing spatial control over stem cell fate without the need for genetic or pharmacological manipulation.
EVs derived from osteoinduced ODSCs transport bioactive cargo, including phosphorylated proteins, acetylated histones, and regulatory microRNAs, which regulate target cells through paracrine signaling[71]. Their content reflects the PTM status of donor cells, allowing for preconditioning strategies to enhance therapeutic efficacy. Systems biology approaches currently allow the profiling of EV PTMs, advancing their use in personalized, cell-free regenerative therapies[61].
Combining pharmacological, genetic, and physical strategies can yield synergistic effects. For instance, HDAC inhibition combined with BMP2 enhances chromatin remodeling and SMAD signaling, producing robust matrix formation[72]. Similarly, scaffold-based delivery of epigenetic small interfering RNAs or dual agents has exhibited synergistic effects in hostile microenvironments such as inflamed bone defects[73]. These multimodal approaches aim to recapitulate the multifactorial nature of native osteogenesis.
Despite promising data, translation faces substantial challenges. The pleiotropy of PTM enzymes increases the risk of off-target consequences. Delivery systems, including hydrogel-encapsulated small interfering RNAs or EVs, require further refinement to ensure tissue-specific and prolonged effects. Furthermore, long-term safety concerns about epigenetic stability require extensive in vivo validation. Nonetheless, with the development of nanotechnology, CRISPR-based epigenetic editing, and high-resolution omics technologies, PTM-targeted modulation of ODSCs promises a new frontier in next-generation bone regeneration strategies.
Osteoimmunomodulation describes the complicated interplay between immune responses and bone regeneration. ODSCs exhibit both osteogenic and immunomodulatory properties, making them ideal candidates for bone repair in inflamed microenvironments. Recent studies demonstrated that PTMs, including phosphorylation, acetylation, methylation, ubiquitination, and lactylation, regulate this dual functionality, influencing immune signaling pathways, cytokine production, and lineage-specific gene expression[27,74].
ODSCs can modulate immune cell behavior through the secretion of TGF-β, indoleamine 2,3-dioxygenase 1, IL-10, and prostaglandin E2, promoting M2 macrophage polarization and regulatory T expansion while inhibiting T helper type 17 responses. PTM-mediated modulation of immunological and differentiation gene networks contributes to their functional adaptability under stress[74]. PTM-induced epigenetic shifts affect the regulatory phenotype of cells during inflammatory conditions, including periodontitis or pulpitis. For instance, GMSCs exposed to LPS can upregulate programmed death ligand-1 and TGF-β1 in a histone acetylation-dependent manner, modulating local immune responses[75].
In the osteoimmunomodulatory context, phosphorylation critically balances pro-regenerative and inflammatory signaling pathways. It dynamically regulates immune signal transduction, primarily through MAPK, AKT, and signal transducer and activator of transcription 3 pathways. Phosphorylated AKT enhances angiogenesis and immunosuppressive behavior in DPSCs by increasing VEGF and IL-10 secretion. Activation of the PI3K/AKT signaling in inflamed environments attenuates neutrophil recruitment and inflammatory cytokine expression[76]. However, overactivation of NF-κB through excessive phosphorylation, as observed in PDLSCs under chronic LPS exposure, leads to elevated IL-6/IL-1β levels and impaired osteogenesis[77]. Therefore, a tightly controlled phosphorylation landscape is required to maintain immuno-osteogenic balance.
Acetylation is essential in osteoimmunomodulation, simultaneously promoting bone repair genes and inhibiting inflammatory pathways. Histone acetylation increases chromatin accessibility, enabling the transcription of anti-inflammatory and osteogenic genes. HDAC inhibitors (valproic acid) restore histone H3 acetylation and inhibit pro-inflammatory cytokine release in inflamed DPSCs[78]. SIRT1 deacetylates NF-κB p65, inhibiting IL-6 and TNF-α transcription while simultaneously stabilizing RUNX2 through FOXO signaling. Resveratrol activates SIRT1 in DPSCs, enhancing osteogenesis and immunosuppression[67].
Ubiquitination offers essential temporal regulation of immunological signaling intensity and duration. The ubiquitin-proteasome system regulates the turnover of immune mediators and transcription factors. Inflammatory stimuli enhance the ubiquitination of inhibitor of kappa B, releasing NF-κB into the nucleus and promoting inflammatory gene expression. Proteasome inhibition through MG132 reduces NF-κB-mediated inflammation and restores osteogenic gene expression in stressed PDLSCs[79]. Overaccumulation of ubiquitinated nucleotide-binding oligomerization domain-, leucine-rich repeat-, and pyrin domain-containing receptor 3 inflammasome components may exacerbate IL-1β release and cause pyroptotic damage in dental tissues. Consequently, selective E3 Ligase regulation may offer a therapeutic avenue to balance immunity and regeneration.
Methylation functions as a modulator of stem cell phenotype and as an epigenetic ‘memory’ of inflammatory exposure. Chronic inflammation can imprint ODSCs epigenetically, reducing their immunomodulatory potential. Long-term TNF-α exposure lowers DPSC osteogenic capacity and inhibits IL-10 expression by increasing H3K27me3 at critical loci[80]. Inhibiting EZH2 with GSK126 restores these effects, restoring immunological resolution and OD even under inflammatory conditions[64]. This indicates that methylation regulates stem cell phenotype and serves as an immune “memory” mechanism.
Lactylation emerges as a metabolic sensor that connects cellular energy status to immunoregulatory and osteogenic processes. Lysine lactylation, derived from glycolytic metabolism, has emerged as a new PTM in immunological regulation. LPS decreases lactylation levels in PDLSCs, downregulating RUNX2 and increasing IL-1β and TNF-α expression. Supplementation with proanthocyanidins restored lactylation and reversed inflammation and osteogenic inhibition[1]. This highlights the potential of metabolic therapies to modulate PTMs and improve osteoimmunomodulatory function.
PTMs operate in an integrated network. NF-κB activity is controlled by phosphorylation (activation), acetylation (DNA binding affinity), and ubiquitination (degradation), demonstrating convergence of regulatory layers. Similarly, RUNX2 is regulated through acetylation, methylation, and ubiquitination, determining its immunomodulatory and osteogenic functions[81]. Understanding such PTM interaction is essential for developing therapies that modulate multiple axes of immune and regenerative control simultaneously.
Therapeutically targeting PTMs provides a compelling strategy for treating inflammatory bone disorders, including periodontitis, peri-implantitis, and jaw osteonecrosis. HDAC inhibitors, SIRT1 activators, and EZH2 inhibitors have all exhibited the potential to restore stem cell function in preclinical models. Translational directions include scaffold systems that locally release these agents or EV-based delivery of PTM-modified proteins[82]. Safety and reversibility remain concerns. Precision targeting using nanoparticles, aptamer-guided systems, or CRISPR epigenetic editing could help overcome these hurdles and facilitate clinical translation. Translational approaches include scaffold systems that locally administer these drugs or employ EV-based delivery of PTM-modified proteins. The following section outlines how these strategies are harnessed to repair complex oral-maxillofacial defects.
Recent advancements indicate that PTMs are essential in regulating cell interactions, biomaterials, and pathological microenvironments during oral-maxillofacial defect repair. The advancement of high-resolution single-cell transcriptomics and proteomics has increased focus on the distinct PTM signatures of specific ODSC subpopulations, including DPSCs and PDLSCs. Emerging platforms enable the reconstruction of cell-type-specific regulatory networks from single-cell omics data and are expected to enhance future profiling of PTM heterogeneity at single-cell resolution, thereby guiding the rational selection and engineering of therapeutic stem cells[83].
Among well-characterized PTMs, the ubiquitin system has significantly impacted osteogenic signaling molecules’ fate. E3 Ligases such as Smurf1 and deubiquitinases, including USP15, modulate the stability of SMADs, RUNX2, and other osteogenic regulators, impacting bone regeneration outcomes[53]. Simultaneously, palmitoylation, a reversible lipid modification catalyzed by the ZDHHC family and cleaved by acyl-protein thioesterases 1/2, has been implicated in regulating membrane localization and signal efficacy of receptors. Although direct evidence for ZDHHC9-mediated palmitoylation of BMPR1a in SHEDs is absent, studies on ZDHHC2 demonstrate its ability to regulate B-RAF and C-RAF trafficking through autophagic degradation, indicating a potentially similar function in BMP signaling cascades[57].
In pathological conditions such as diabetes, metabolic dysregulation significantly alters the levels of acetyl-CoA, crotonyl-CoA, and related cofactors, thereby impacting global and site-specific PTMs. Decreased acetylation of osteogenic transcription factors, including RUNX2, under hypoxia or energetic stress compromises their stability and osteoinductive capacity[37,84]. Gut microbiota-derived short-chain fatty acids, especially butyrate and crotonate, have been reported to modulate intracellular crotonyl-CoA levels and gene expression in distant tissues, revealing a new mechanism that connects metabolic disorders with skeletal impairment[85]. Simultaneously, advanced glycation end products concurrently modify ECM proteins, including collagen, compromising their mechanical and adhesive properties while triggering pro-inflammatory signaling through receptor for advanced glycation end products, creating a deleterious feedback loop that hinders tissue regeneration[86].
Despite significant advancements in delineating the functional roles of PTMs, a major limitation remains in the inability to visualize these modifications dynamically within living systems. Current proteomic platforms primarily offer static, population-level insights, which are inadequate for elucidating the transient and cell-specific nature of PTM signaling in vivo. Integrating real-time biosensing technologies with metabolic and microenvironmental modulation will be crucial to decoding the spatiotemporal heterogeneity of PTMs. These advances are anticipated to facilitate the development of more responsive and adaptive regenerative interventions, especially in addressing the complexities of pathological tissue repair.
PTMs have emerged as potent regulators of osteogenic and immunomodulatory behavior in ODSCs. Despite great improvements, significant challenges remain in translating PTM research into regenerative dental medicine. These include mechanistic gaps, technological limitations in PTM detection, safety concerns for clinical application, and an underdeveloped framework for precision targeting. This section explores the fundamental challenges and promising approaches for PTM-based techniques in ODSC-based bone regeneration.
One of the main conceptual challenges in PTM research is the vast combinatorial complexity and context reliance of PTM interactions. A single protein can undergo several PTMs, each of which may have contradictory impacts on function depending on the cell state or external stimuli. RUNX2, the master osteogenic transcription factor, exemplifies this complexity. It is phosphorylated to increase activity, acetylated to enhance stability, and ubiquitinated for degradation. The outcome depends on the presence of these marks and, additionally, on their sequence, stoichiometry, and cofactors. Yang et al[2] emphasized the importance of developing a multi-dimensional regulatory model of RUNX2 and related proteins, which are critical for the predictive modulation of osteogenesis. Interpreting this complex ‘PTM code’ is essential for predictive intervention.
Although mass spectrometry-based proteomics has made significant progress, detecting low-abundance and transient PTMs in stem cells remains a technical challenge. Sample preparation often leads to the loss of PTMs or the introduction of artifacts, particularly for modifications like lactylation or SUMOylation. Proteomic profiling of ODSCs often misses crucial PTM changes due to limited resolution or coverage in tandem mass spectrometry workflows[3]. To create cell-type and stage-specific PTM maps in ODSCs, better enrichment techniques, such as antibody-based PTM pull-down or metabolic labeling, are still needed. While single-cell proteomics shows promise, it is currently limited by throughput and the range of detectable PTMs. Without reliable detection methods, developing PTM-targeted therapies remains speculative.
Another challenge is the biological heterogeneity among ODSC subtypes. DPSCs, PDLSCs, SHEDs, and GMSCs exhibit different epigenetic landscapes and niche-derived stimuli. Therefore, PTM responses to external stimuli such as inflammation, hypoxia, or scaffolds remain variable. For instance, while HDAC inhibition enhances osteogenesis in DPSCs, it may delay mineralization in PDLSCs due to differences in chromatin contexts. Furthermore, Udayasuryan et al[4] emphasized how even the same PTM (H3K27 acetylation) might result in divergent gene expression outcomes depending on oxygen tension, microbial presence, or metabolic flux. Understanding these nuanced interactions is essential for developing context-aware PTM modulators.
Despite promising results in preclinical studies, applying PTM-targeting medications in vivo requires caution. Broad-spectrum inhibitors of HDACs or EZH2 may affect off-target tissues, disrupt the immune balance, or even promote tumorigenesis if misapplied. Additionally, irreversible PTM alterations could cause epigenetic instability[87]. Therefore, next-generation PTM therapeutics require spatial and temporal control. Local delivery through hydrogels, nanoparticle encapsulation, or gene-editing vectors may reduce systemic exposure, although further toxicological evaluation is essential. Furthermore, patient-specific variability - including genetic background, immunological history, and oral microbiome - will affect PTM responsiveness. Precision medicine frameworks integrating multi-omics and artificial intelligence modeling are necessary for safe, individualized applications. Innovative technologies such as nanoparticle-mediated delivery and CRISPR-based epigenetic editing offer promising avenues for achieving spatially and temporally precise PTM modulation to reduce systemic risks.
The future of PTM-based regenerative therapy lies in systems-level integration. Simultaneous profiling of the proteome, epigenome, and metabolome in ODSCs will enable the reconstruction of PTM-dependent regulatory networks. Network analysis can identify master regulators (p300, SIRT1, or general control non-depressible 5) whose modulation can affect multiple downstream PTMs. Sebastião et al[88] reported that engineered EVs can deliver pre-assembled PTM-modified proteins or regulatory RNAs, providing a cell-free, modular approach to reprogramming target cells. Synthetic biology tools may further allow in situ construction of PTM biosensors or toggle switches responsive to inflammatory signals, enabling adaptive modulation of ODSCs during healing.
While there are still significant challenges to address, the next decade is likely to see a shift from descriptive to predictive PTM modulation in oral stem cell therapy. The integration of advanced proteomics, scaffold engineering, CRISPR-based epigenetic editing, and artificial intelligence-guided target discovery will enable more precise and personalized regenerative protocols. However, successful implementation will require collaboration across stem cell biology, biomaterials, computational systems, and clinical translational sciences to fully unlock the therapeutic potential of PTMs in oral regenerative medicine.
PTMs have emerged as auxiliary regulators and central molecular switches that control the OD and immunomodulatory function of ODSCs. This review demonstrated a transition from gene-centric to modification-centric regulation of stem cell fate - where reversible, dynamic PTMs serve as integrative hubs connecting external microenvironmental cues (inflammation, hypoxia, and oxidative stress) to intracellular transcriptional and epigenetic programs and clarifies how complex PTM interaction forms a dynamic ‘regulatory language’. This language enables ODSCs to refine their osteoimmunological responses across various pathophysiological conditions.
By thoroughly examining the unique roles of various PTMs like phosphorylation, acetylation, methylation, and ubiquitination, along with emerging PTMs such as lactylation and crotonylation, we uncover their interconnected mechanisms and potential for translational applications. These PTMs offer flexibility as targets for scaffold design, small molecule intervention, and precision cell engineering. This review emphasizes that the interplay between these modifications forms a functional code - a biochemical language - that fine-tunes osteoimmunological responses in patho
Future research should aim at decoding the PTM code using high-resolution, single-cell proteomics and developing next-generation delivery systems (such as EV-based and scaffold-localized) to precisely control PTMs in both space and time in vivo. By using PTMs as adjustable switches for osteogenic commitment and immunomodulation, we can unlock a promising avenue for personalized, adaptive regenerative therapies for bone and periodontal diseases.
We would like to thank all the professionals who contributed to the discussion and elaboration of this review.
| 1. | Wu Y, Wang X, Zhang Y, Wen Z, Li Y, Zhang K, Gosar N, Li Q, Mao J, Gong S. Proanthocyanidins Ameliorate LPS-Inhibited Osteogenesis of PDLSCs by Restoring Lysine Lactylation. Int J Mol Sci. 2024;25:2947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 2. | Yang B, Qiu Y, Zhou N, Ouyang H, Ding J, Cheng B, Sun J. Application of Stem Cells in Oral Disease Therapy: Progresses and Perspectives. Front Physiol. 2017;8:197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 3. | Tsuchida S. Proteome Analysis of Molecular Events in Oral Pathogenesis and Virus: A Review with a Particular Focus on Periodontitis. Int J Mol Sci. 2020;21:5184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 4. | Udayasuryan B, Zhou Z, Ahmad RN, Sobol P, Deng C, Nguyen TTD, Kodikalla S, Morrison R, Goswami I, Slade DJ, Verbridge SS, Lu C. Fusobacterium nucleatum infection modulates the transcriptome and epigenome of HCT116 colorectal cancer cells in an oxygen-dependent manner. Commun Biol. 2024;7:551. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 31] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 5. | Pizzicannella J, Marconi GD, Pierdomenico SD, Cavalcanti MFXB, Diomede F, Trubiani O. Bovine pericardium membrane, gingival stem cells, and ascorbic acid: a novel team in regenerative medicine. Eur J Histochem. 2019;63:3064. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | Mallia A, Gianazza E, Zoanni B, Brioschi M, Barbieri SS, Banfi C. Proteomics of Extracellular Vesicles: Update on Their Composition, Biological Roles and Potential Use as Diagnostic Tools in Atherosclerotic Cardiovascular Diseases. Diagnostics (Basel). 2020;10:843. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 7. | Cabaña-Muñoz ME, Pelaz Fernández MJ, Parmigiani-Cabaña JM, Parmigiani-Izquierdo JM, Merino JJ. Adult Mesenchymal Stem Cells from Oral Cavity and Surrounding Areas: Types and Biomedical Applications. Pharmaceutics. 2023;15:2109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 8. | Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97:13625-13630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3174] [Cited by in RCA: 3433] [Article Influence: 132.0] [Reference Citation Analysis (1)] |
| 9. | Martin-Iglesias S, Milian L, Sancho-Tello M, Salvador-Clavell R, Martín de Llano JJ, Carda C, Mata M. BMP-2 Enhances Osteogenic Differentiation of Human Adipose-Derived and Dental Pulp Stem Cells in 2D and 3D In Vitro Models. Stem Cells Int. 2022;2022:4910399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 10. | Rikitake K, Kunimatsu R, Yoshimi Y, Tanimoto K. Investigation of Angiogenic Potential in CD146-Positive Stem Cells Derived from Human Exfoliated Deciduous Teeth. Int J Mol Sci. 2025;26:974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 11. | Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, Young M, Robey PG, Wang CY, Shi S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2371] [Cited by in RCA: 2558] [Article Influence: 116.3] [Reference Citation Analysis (1)] |
| 12. | Xu XY, Tian BM, Xia Y, Xia YL, Li X, Zhou H, Tan YZ, Chen FM. Exosomes derived from P2X7 receptor gene-modified cells rescue inflammation-compromised periodontal ligament stem cells from dysfunction. Stem Cells Transl Med. 2020;9:1414-1430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 13. | Shao Y, Du Y, Chen Z, Xiang L, Tu S, Feng Y, Hou Y, Kou X, Ai H. Mesenchymal stem cell-mediated adipogenic transformation: a key driver of oral squamous cell carcinoma progression. Stem Cell Res Ther. 2025;16:12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 14. | Bi F, Xiong J, Han X, Yang C, Li X, Chen G, Guo W, Tian W. Dental follicle cells show potential for treating Parkinson's disease through dopaminergic-neuronogenic differentiation. Hum Cell. 2022;35:1708-1721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Mao CY, Wang YG, Zhang X, Zheng XY, Tang TT, Lu EY. Double-edged-sword effect of IL-1β on the osteogenesis of periodontal ligament stem cells via crosstalk between the NF-κB, MAPK and BMP/Smad signaling pathways. Cell Death Dis. 2016;7:e2296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 95] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 16. | Melms H, Herrmann M, Förstner K, Bharti R, Schneider D, Mentrup B, Rudert M, Schlagenhauf U, Jakob F, Graser S. Novel molecular cues for dental defects in hypophosphatasia. Exp Cell Res. 2020;392:112026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Zhu YW, Wei YW, Ma JY, Chen W, Shen Z, Qiu J. Bioactive deproteinized bovine bone mineral based on self-assembled albumin nanoparticles promoted bone regeneration via activation of Wnt/β-catenin pathway. Mater Today Bio. 2025;32:101730. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 18. | Entezami S, Sam MR. The role of mesenchymal stem cells-derived from oral and teeth in regenerative and reconstructive medicine. Tissue Cell. 2025;93:102766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 19. | Zhang Q, Song Q, Li Z, Wu X, Chen Y, Lin H. Targeting fibroblasts in pathological bone formation: mechanisms and treatments. Front Cell Dev Biol. 2025;13:1612950. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 20. | Zhu S, Chen W, Masson A, Li YP. Cell signaling and transcriptional regulation of osteoblast lineage commitment, differentiation, bone formation, and homeostasis. Cell Discov. 2024;10:71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 185] [Reference Citation Analysis (0)] |
| 21. | Wang K, Zhou M, Zhang Y, Jin Y, Xue Y, Mao D, Rui Y. Fibromodulin facilitates the osteogenic effect of Masquelet's induced membrane by inhibiting the TGF-β/SMAD signaling pathway. Biomater Sci. 2024;12:1898-1913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 22. | Hu L, Chen W, Qian A, Li YP. Wnt/β-catenin signaling components and mechanisms in bone formation, homeostasis, and disease. Bone Res. 2024;12:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 154] [Reference Citation Analysis (0)] |
| 23. | Chen X, Xie W, Zhang M, Shi Y, Xu S, Cheng H, Wu L, Pathak JL, Zheng Z. The Emerging Role of Non-Coding RNAs in Osteogenic Differentiation of Human Bone Marrow Mesenchymal Stem Cells. Front Cell Dev Biol. 2022;10:903278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 24. | Yin X, Wei Y, Qin H, Zhao J, Chen Y, Yao S, Li N, Xiong A, Wang D, Zhang P, Liu P, Zeng H, Chen Y. Oxygen tension regulating hydrogels for vascularization and osteogenesis via sequential activation of HIF-1α and ERK1/2 signaling pathways in bone regeneration. Biomater Adv. 2024;161:213893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 25. | Ning Q, Li M, Liao Z, Chen E, Liu H, Liang Y, Chen Y, Li Y, Huang L. LncRNA MRF targeting FSHR inhibits the osteogenic differentiation of BMSCs and bone defect repair through the regulation of the cAMP-PKA-CREB signaling pathway. Stem Cell Res Ther. 2025;16:200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 26. | Bai Y, Wu Z, Leary SC, Fang C, Yu M, Genth H, Xie Y, Shi J, Xiang J. Focal Adhesion Kinase Alleviates Simulated Microgravity-Induced Inhibition of Osteoblast Differentiation by Activating Transcriptional Wnt/β-Catenin-BMP2-COL1 and Metabolic SIRT1-PGC-1α-CPT1A Pathways. Int J Mol Sci. 2025;26:1669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 27. | Zhao L, Qi H, Lv H, Liu W, Zhang R, Yang A. Lactylation in health and disease: physiological or pathological? Theranostics. 2025;15:1787-1821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 23] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 28. | Yin H, Yue H, Wang M, Zhang T, Zhao YT, Liu H, Wang J, Zheng H, Xue C. Preparation of Novel Sea Cucumber Intestinal Peptides to Promote Tibial Fracture Healing in Mice by Inducing Differentiation of Hypertrophic Chondrocytes to the Osteoblast Lineage. Mol Nutr Food Res. 2024;68:e2300344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 29. | Yalaev B, Tyurin A, Akhiiarova K, Khusainova R. Hypomethylation of the RUNX2 Gene Is a New Potential Biomarker of Primary Osteoporosis in Men and Women. Int J Mol Sci. 2024;25:7312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 30. | Singh M, Singh P, Singh B, Sharma K, Kumar N, Singh D, Mastana S. Molecular Signaling Pathways and MicroRNAs in Bone Remodeling: A Narrative Review. Diseases. 2024;12:252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 31. | Zhang L, Zhu K, Xu J, Chen X, Sheng C, Zhang D, Yang Y, Sun L, Zhao H, Wang X, Tao B, Zhou L, Liu J. Acetyltransferases CBP/p300 Control Transcriptional Switch of β-Catenin and Stat1 Promoting Osteoblast Differentiation. J Bone Miner Res. 2023;38:1885-1899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 32. | Dong F, Yin H, Zheng Z. Hypoxia-Inducible Factor-1α Regulates BNIP3-Dependent Mitophagy and Mediates Metabolic Reprogramming Through Histone Lysine Lactylation Modification to Affect Glioma Proliferation and Invasion. J Biochem Mol Toxicol. 2025;39:e70069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 33. | Chen M, Cui Y, Li H, Luan J, Zhou X, Han J. Icariin Promotes the Osteogenic Action of BMP2 by Activating the cAMP Signaling Pathway. Molecules. 2019;24:3875. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 34. | Lo T, Tsai CF, Shih YR, Wang YT, Lu SC, Sung TY, Hsu WL, Chen YJ, Lee OK. Phosphoproteomic analysis of human mesenchymal stromal cells during osteogenic differentiation. J Proteome Res. 2012;11:586-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 35. | Görlitz S, Brauer E, Günther R, Duda GN, Knaus P, Petersen A. Temporal regulation of BMP2 growth factor signaling in response to mechanical loading is linked to cytoskeletal and focal adhesion remodeling. Commun Biol. 2024;7:1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 36. | Liu DD, Zhang CY, Liu Y, Li J, Wang YX, Zheng SG. RUNX2 Regulates Osteoblast Differentiation via the BMP4 Signaling Pathway. J Dent Res. 2022;101:1227-1237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 37. | Saranya I, Akshaya RL, Gomathi K, Mohanapriya R, He Z, Partridge NC, Selvamurugan N. Circ_ST6GAL1-mediated competing endogenous RNA network regulates TGF-β1-stimulated matrix Metalloproteinase-13 expression via Runx2 acetylation in osteoblasts. Noncoding RNA Res. 2024;9:153-164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 38. | Huang Y, Zheng Y, Jia L, Li W. Long Noncoding RNA H19 Promotes Osteoblast Differentiation Via TGF-β1/Smad3/HDAC Signaling Pathway by Deriving miR-675. Stem Cells. 2015;33:3481-3492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 255] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 39. | Li Q, Liu F, Dang R, Feng C, Xiao R, Hua Y, Wang W, Jia Z, Liu D. Epigenetic modifier trichostatin A enhanced osteogenic differentiation of mesenchymal stem cells by inhibiting NF-κB (p65) DNA binding and promoted periodontal repair in rats. J Cell Physiol. 2020;235:9691-9701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 40. | Zhou X, Tian X, Chen J, Li Y, Lv N, Liu H, Liu T, Yang H, Chen X, Xu Y, He F. Youthful Stem Cell Microenvironments: Rejuvenating Aged Bone Repair Through Mitochondrial Homeostasis Remodeling. Adv Sci (Weinh). 2025;12:e2409644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 41. | Adithya SP, Balagangadharan K, Selvamurugan N. Epigenetic modifications of histones during osteoblast differentiation. Biochim Biophys Acta Gene Regul Mech. 2022;1865:194780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 42. | Yu B, Wang R, Luo H, Yang D, Wang S, Yu Y, Okamura H, Qiu L. Histone Demethylase Jmjd3 Regulates the Osteogenic Differentiation and Cytokine Expressions of Periodontal Ligament Cells. Acta Med Okayama. 2022;76:281-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 43. | Lai L, Juntilla DL, Del C Gomez-Alonso M, Grallert H, Thorand B, Farzeen A, Rathmann W, Winkelmann J, Prokisch H, Gieger C, Herder C, Peters A, Waldenberger M. Longitudinal association between DNA methylation and type 2 diabetes: findings from the KORA F4/FF4 study. Cardiovasc Diabetol. 2025;24:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 44. | Shi P, Ma Y, Zhang S. Non-histone lactylation: unveiling its functional significance. Front Cell Dev Biol. 2025;13:1535611. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 45. | Yang Y, Luo N, Gong Z, Zhou W, Ku Y, Chen Y. Lactate and lysine lactylation of histone regulate transcription in cancer. Heliyon. 2024;10:e38426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 46. | Luo Y, Yang Z, Yu Y, Zhang P. HIF1α lactylation enhances KIAA1199 transcription to promote angiogenesis and vasculogenic mimicry in prostate cancer. Int J Biol Macromol. 2022;222:2225-2243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 163] [Reference Citation Analysis (0)] |
| 47. | Gilglioni EH, Bansal M, St-Pierre-Wijckmans W, Talamantes S, Kasarinaite A, Hay DC, Gurzov EN. Therapeutic potential of stem cell-derived somatic cells to treat metabolic dysfunction-associated steatotic liver disease and diabetes. Obes Rev. 2025;26:e13899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 48. | Di Gregorio J, Di Giuseppe L, Terreri S, Rossi M, Battafarano G, Pagliarosi O, Flati V, Del Fattore A. Protein Stability Regulation in Osteosarcoma: The Ubiquitin-like Modifications and Glycosylation as Mediators of Tumor Growth and as Targets for Therapy. Cells. 2024;13:537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 49. | Wang D, Li Q, Xie C. The role and mechanism of protein posttranslational modification in vascular calcification (Review). Exp Ther Med. 2024;28:419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 50. | Iwamoto R, Koide M, Udagawa N, Kobayashi Y. Positive and Negative Regulators of Sclerostin Expression. Int J Mol Sci. 2022;23:4895. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 51. | Liu Y, Ho C, Wen D, Sun J, Liu Y, Li Q, Zhang Y, Gao Y. PKM2-mediated collagen XVII expression is critical for wound repair. JCI Insight. 2025;10:e184457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 52. | Zhang Y, Zhang J, Lesani P, Lu Z, Zreiqat H. Osteopontin Rejuvenates Senescent Adipose-Derived Stem Cells and Restores their Bone Tissue Regenerative Function. Stem Cell Rev Rep. 2024;20:1106-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 53. | He H, Wang L, Xian B, Xia Y. Regulatory Roles of E3 Ubiquitin Ligases and Deubiquitinases in Bone. Biomolecules. 2025;15:679. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 54. | Li K, Huang K, Lu Q, Geng W, Jiang D, Guo A. TRIM16 mitigates impaired osteogenic differentiation and antioxidant response in D-galactose-induced senescent osteoblasts. Eur J Pharmacol. 2024;979:176849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 55. | Liu W, Yu W, Zhou L, Ling D, Xu Y, He F. Inhibition of ZDHHC16 promoted osteogenic differentiation and reduced ferroptosis of dental pulp stem cells by CREB. BMC Oral Health. 2024;24:388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 56. | Zhang HR, Wang YH, Xiao ZP, Yang G, Xu YR, Huang ZT, Wang WZ, He F. E3 ubiquitin ligases: key regulators of osteogenesis and potential therapeutic targets for bone disorders. Front Cell Dev Biol. 2024;12:1447093. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 57. | Jayaraman S, Kochiss A, Alcalay TL, Del Rivero Morfin PJ, Ben-Johny M. Engineered depalmitoylases enable selective manipulation of protein localization and function. Nat Commun. 2025;16:3514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 58. | Wu C, Zhu X, Dai Q, Chu Z, Yang S, Dong Z. SUMOylation of SMAD4 by PIAS1 in Conjunction with Vimentin Upregulation Promotes Migration Potential in Non-Small Cell Lung Cancer. Front Biosci (Landmark Ed). 2023;28:192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 59. | Han R, Dang R, Liu F, Nie S, Tao S, Xing L, Yang T, Hu M, Liu D. Protein Crotonylation Promotes Osteogenic Differentiation of Periodontal Ligament Stem Cells via the PI3K-AKT Pathway. Stem Cells. 2024;42:650-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 60. | Pan H, Yang S, Kulyar MF, Ma H, Li K, Zhang L, Mo Q, Li J. Lactobacillus fermentum 016 Alleviates Mice Colitis by Modulating Oxidative Stress, Gut Microbiota, and Microbial Metabolism. Nutrients. 2025;17:452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 61. | Ma B, Trieu TJ, Cheng J, Zhou S, Tang Q, Xie J, Liu JL, Zhao K, Habib SJ, Chen X. Differential Histone Distribution Patterns in Induced Asymmetrically Dividing Mouse Embryonic Stem Cells. Cell Rep. 2020;32:108003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 62. | Dong Y, Chen Y, Ma G, Cao H. The role of E3 ubiquitin ligases in bone homeostasis and related diseases. Acta Pharm Sin B. 2023;13:3963-3987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 63. | Chen J, Chen J, Chen J, Lu R, Liu Z, Zhang Y, Zhang C. Pretreated exosomes by electrical stimulation accelerate bone regeneration. Bioact Mater. 2025;51:383-398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 64. | Sun S, Jiang Y, Chen K, Chen X, Chai J, Sun H. Impact of DLX3/SAHH axis on osteogenic differentiation of BMSCs in alveolar bone. J Oral Biosci. 2025;67:100658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 65. | Zhang H, Li X, Liu J, Lin X, Pei L, Boyce BF, Xing L. Proteasome inhibition-enhanced fracture repair is associated with increased mesenchymal progenitor cells in mice. PLoS One. 2022;17:e0263839. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 66. | Ohguchi H, Harada T, Sagawa M, Kikuchi S, Tai YT, Richardson PG, Hideshima T, Anderson KC. KDM6B modulates MAPK pathway mediating multiple myeloma cell growth and survival. Leukemia. 2017;31:2661-2669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 69] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 67. | Huang Y, Lu J, Zhan L, Wang M, Shi R, Yuan X, Gao X, Liu X, Zang J, Liu W, Yao X. Resveratrol-induced Sirt1 phosphorylation by LKB1 mediates mitochondrial metabolism. J Biol Chem. 2021;297:100929. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 68. | Sathyabhama M, Priya Dharshini LC, Karthikeyan A, Kalaiselvi S, Min T. The Credible Role of Curcumin in Oxidative Stress-Mediated Mitochondrial Dysfunction in Mammals. Biomolecules. 2022;12:1405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 64] [Reference Citation Analysis (0)] |
| 69. | Sequeira DB, Diogo P, Gomes BPFA, Peça J, Santos JMM. Scaffolds for Dentin-Pulp Complex Regeneration. Medicina (Kaunas). 2023;60:7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 70. | Han J, Li Z, Du J, Zhang Q, Ge S, Liu H, Ma B. Natural collagen scaffold with intrinsic piezoelectricity for enhanced bone regeneration. Mater Today Bio. 2025;31:101532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 71. | Han P, Johnson N, Abdal-Hay A, Moran CS, Salomon C, Ivanovski S. Effects of periodontal cells-derived extracellular vesicles on mesenchymal stromal cell function. J Periodontal Res. 2023;58:1188-1200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 72. | Cai J, Deng Y, Min Z, Li C, Zhao Z, Yi J, Jing D. Unlocking the Epigenetic Symphony: Histone Acetylation Orchestration in Bone Remodeling and Diseases. Stem Cell Rev Rep. 2025;21:291-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 73. | Guo J, Wang F, Hu Y, Luo Y, Wei Y, Xu K, Zhang H, Liu H, Bo L, Lv S, Sheng S, Zhuang X, Zhang T, Xu C, Chen X, Su J. Exosome-based bone-targeting drug delivery alleviates impaired osteoblastic bone formation and bone loss in inflammatory bowel diseases. Cell Rep Med. 2023;4:100881. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 90] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 74. | Lin Y, Qiu T, Wei G, Que Y, Wang W, Kong Y, Xie T, Chen X. Role of Histone Post-Translational Modifications in Inflammatory Diseases. Front Immunol. 2022;13:852272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 58] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 75. | Chen J, Shi X, Deng Y, Dang J, Liu Y, Zhao J, Liang R, Zeng D, Wu W, Xiong Y, Yuan J, Chen Y, Wang J, Lin W, Chen X, Huang W, Olsen N, Pan Y, Fu Q, Zheng SG. miRNA-148a-containing GMSC-derived EVs modulate Treg/Th17 balance via IKKB/NF-κB pathway and treat a rheumatoid arthritis model. JCI Insight. 2024;9:e177841. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 28] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 76. | Li J, Wang Z, Wang J, Guo Q, Fu Y, Dai Z, Wang M, Bai Y, Liu X, Cooper PR, Wu J, He W. Amphiregulin regulates odontogenic differentiation of dental pulp stem cells by activation of mitogen-activated protein kinase and the phosphatidylinositol 3-kinase signaling pathways. Stem Cell Res Ther. 2022;13:304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 77. | Liu X, Zhao W, Peng Y, Liu N, Liu Q. The relationship between MAPK signaling pathways and osteogenic differentiation of periodontal ligament stem cells: a literature review. PeerJ. 2025;13:e19193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 78. | Luo Z, Wang Z, He X, Liu N, Liu B, Sun L, Wang J, Ma F, Duncan H, He W, Cooper P. Effects of histone deacetylase inhibitors on regenerative cell responses in human dental pulp cells. Int Endod J. 2018;51:767-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 79. | Ye S, Tan C, Yang X, Wang J, Li Q, Xu L, Wang Z, Mao J, Wang J, Cheng K, Chen A, Zhou P, Li S. Transcriptome Analysis of Retinoic Acid-Inducible Gene I Overexpression Reveals the Potential Genes for Autophagy-Related Negative Regulation. Cells. 2022;11:2009. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 80. | Carrasco ME, Thaler R, Nardocci G, Dudakovic A, van Wijnen AJ. Inhibition of Ezh2 redistributes bivalent domains within transcriptional regulators associated with WNT and Hedgehog pathways in osteoblasts. J Biol Chem. 2023;299:105155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 81. | Luo W, Zhang G, Wang Z, Wu Y, Xiong Y. Ubiquitin-specific proteases: Vital regulatory molecules in bone and bone-related diseases. Int Immunopharmacol. 2023;118:110075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 82. | Wang Z, Wen S, Zhong M, Yang Z, Xiong W, Zhang K, Yang S, Li H, Guo S. Epigenetics: Novel crucial approach for osteogenesis of mesenchymal stem cells. J Tissue Eng. 2023;14:20417314231175364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 83. | Kaur J, Adhikari M, Sabol HM, Anloague A, Khan S, Kurihara N, Diaz-delCastillo M, Andreasen CM, Barnes CL, Stambough JB, Palmieri M, Reyes-Castro O, Zarrer J, Taipaleenmäki H, Ambrogini E, Almeida M, O'Brien CA, Nookaew I, Delgado-Calle J. Single-Cell Transcriptomic Analysis Identifies Senescent Osteocytes That Trigger Bone Destruction in Breast Cancer Metastasis. Cancer Res. 2024;84:3936-3952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 84. | Wang T, Bai M, Geng W, Adeli M, Ye L, Cheng C. Bioinspired artificial antioxidases for efficient redox homeostasis and maxillofacial bone regeneration. Nat Commun. 2025;16:856. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 33] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 85. | Nshanian M, Gruber JJ, Geller BS, Chleilat F, Lancaster SM, White SM, Alexandrova L, Camarillo JM, Kelleher NL, Zhao Y, Snyder MP. Short-chain fatty acid metabolites propionate and butyrate are unique epigenetic regulatory elements linking diet, metabolism and gene expression. Nat Metab. 2025;7:196-211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 55] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 86. | Kamml J, Ke CY, Acevedo C, Kammer DS. The influence of AGEs and enzymatic cross-links on the mechanical properties of collagen fibrils. J Mech Behav Biomed Mater. 2023;143:105870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 87. | Zhao Y, He J, Qiu T, Zhang H, Liao L, Su X. Epigenetic therapy targeting bone marrow mesenchymal stem cells for age-related bone diseases. Stem Cell Res Ther. 2022;13:201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 88. | Sebastião MJ, Serra M, Gomes-Alves P, Alves PM. Stem cells characterization: OMICS reinforcing analytics. Curr Opin Biotechnol. 2021;71:175-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |