Published online Sep 26, 2025. doi: 10.4252/wjsc.v17.i9.109330
Revised: June 18, 2025
Accepted: August 20, 2025
Published online: September 26, 2025
Processing time: 139 Days and 1.4 Hours
The therapeutic potential of induced pluripotent stem cells (iPSCs) for Parkinson’s disease (PD) has been demonstrated. Exercise can also modulate metabolism to improve motor dysfunction in PD patients.
To investigate the therapeutic effect of exercise combined with iPSCs in a PD mouse model and explore the underlying mechanisms.
In this study, we included 10 normal mice and 40 PD model mice, which were divided into five groups: The control group (n = 10), the sedentary PD group (St group, n = 10), the exercise PD group (E group, n = 10), the iPSC-treated PD group (T group, n = 10), and the combined exercise and iPSC-treated PD group (ET group, n = 10). The T and ET groups received cell injection therapy, while the E and ET groups underwent an 8-week exercise intervention. After the intervention, behavioral tests were performed on mice from all groups. Serum levels of epinephrine (EPI) and nerve growth factor were measured, and the expression of Wnt1, Lmx1a, and other factors related to the Wnt signaling pathway in the midbrain of mice were assessed.
The motor ability of the T group was higher than that of the St group, but the difference was not significant. However, the protein and gene expression levels of Wnt1, Lmx1a, Neurog2, and TH in the T group were significantly higher than those in the St group (P < 0.01). Compared with the T group, the motor ability of the E group was significantly enhanced (P < 0.01), and the gene expression level of Wnt1 in the midbrain of the E group was significantly higher than that of the T group (P < 0.05). The levels of EPI and nerve growth factor were increased in both the E and ET groups. Exercise can improve motor dysfunction in PD, increase EPI levels, and elevate Wnt1 levels. However, western blot results revealed no significant change in the TH level of the E group, which may be because exercise does not cause a noticeable change in the number of neurons. Compared with the St group, both the E and ET groups showed improved motor function (P < 0.01). The results showed that compared with the St group, the protein and gene expression levels of Wnt1, Lmx1a, and Neurog2 were significantly increased in the E, T, and ET groups (P < 0.05). Compared with the T and E groups, the protein and gene expression levels of Wnt1, Lmx1a, and Neurog2 were significantly increased in the ET group (P < 0.05).
Exercise increases EPI levels, activates the Wnt signaling pathway through β2 receptors, enhances the Wnt1-Lmx1a regulatory loop, and promotes the differentiation of iPSCs into dopaminergic neurons, thereby increasing the number of neurons.
Core Tip: This study explores the combined therapeutic effect of exercise and induced pluripotent stem cells (iPSCs) in a Parkinson’s disease mouse model. Exercise alone enhances motor function and activates the Wnt signaling pathway, increasing adrenaline and Wnt1 levels. Combining exercise with iPSC treatment further boosts the Wnt1-Lmx1a regulatory loop, promoting iPSC differentiation into dopaminergic neurons and significantly improving motor function.
- Citation: Jiang X, Lu ZM, Wang QL, Luo Y, Zhang J. Exercise combined with induced pluripotent stem cells enhances the Wnt1-Lmx1a loop in the midbrain of Parkinsonian mice to alleviate Parkinsonian symptoms. World J Stem Cells 2025; 17(9): 109330
- URL: https://www.wjgnet.com/1948-0210/full/v17/i9/109330.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v17.i9.109330
Parkinson’s disease (PD), the second most common neurodegenerative disorder after Alzheimer’s disease, affects over 6 million people globally, with rising prevalence among individuals aged > 65 years, though earlier onset is increasingly reported[1]. PD is characterized by nigrostriatal dopaminergic (DA) neuron loss and α-synuclein aggregation, leading to motor (e.g., bradykinesia, rigidity) and non-motor symptoms (e.g., depression, autonomic dysfunction)[2,3]. Currently, the treatment of PD mainly relies on pharmacological and surgical therapies. However, surgery is expensive with uncertain long-term efficacy, while drug therapy carries significant side effects, imposing substantial burdens on patients and society. Thus, developing safe and effective therapies for PD and other neurodegenerative disease (ND) is urgently needed.
The study by Bonde-Jensen et al[4] indicates that physical activity has a beneficial effect on improving PD. Exercise has demonstrated neuroprotective effects in PD and other central nervous system disorders, improving motor/cognitive function by enhancing neurogenesis, angiogenesis, and antioxidant defenses. These factors activate specific receptors and downstream pathways (e.g., phosphatidylinositol 3-kinase/protein kinase B), modulating nuclear transcription factors to protect DA neurons and enhance their survival/regeneration[5-7]. Consequently, exercise alleviates PD motor symptoms and serves as valuable adjunctive therapy.
Meanwhile, induced pluripotent stem cells (iPSCs) have emerged as a promising therapy for PD, with clinical trials confirming the safety and efficacy of iPSC-derived DA progenitors[8-10]. Research shows midbrain DA neurons derive from the neurogenic floor plate through Wnt/Shh signaling[11,12]. Current differentiation protocols utilize activators of these pathways[13-15], particularly targeting the critical Wnt1-Lmx1a and Shh-Foxa2 regulatory loops[16,17]. Morphogens or small molecules activating these pathways are essential for efficient midbrain DA neuron generation. The Wnt1-Lmx1a autoregulatory loop, observed during both embryonic development and stem cell differentiation, appears pivotal for iPSC-to-neuron conversion. Studies have shown that exercise and stem cell intervention treatments have certain effects in treating PD[18].
Current PD medications slow progression but cause significant side effects. While iPSC therapy shows promise and exercise improves motor function, their combined effects on DA neuron differentiation remain unknown. This study evaluated combined exercise and iPSC therapy in a PD mouse model, revealing synergistic benefits and mechanistic insights that support clinical translation of this dual approach.
The PD mouse models and normal mice used in this study were purchased from Wuhan Yiklon Technology Co., Ltd. [No. SYXK (Hubei) 2023-0135], at 8 weeks of age. The construction protocol was as follows: Specific pathogen-free-grade Balb/c mice (6-8 weeks old, male), weighing approximately 20-22 g. Before the start of the experiment, all mice were acclimatized for 1 week. All animals were housed in a specific pathogen-free environment with a temperature range of 20-26 °C, relative humidity of 40%-70%, and a light cycle of 12 hours light/12 hours dark. The mice were weighed and intraperitoneally injected with a dose of 30 mg/kg (dissolved in 0.9% saline) for 5 consecutive days. The control group was maintained under normal breeding conditions.
The rotarod test: The rotarod test was conducted using a rotarod apparatus (IITC Life Science, CA, United States) to evaluate motor coordination and balance in animals. The experimental protocol was as follows: The rotating rod was programmed to increase linearly in speed from 5 rpm to 30 rpm over a 300-second period. Each animal underwent three trials on the apparatus, with an inter-trial interval of at least 30 minutes. The average latency to fall from the rod was recorded as the primary outcome measure.
The open-field test: The experiment was conducted in a standard open-field box, which measured 500 mm × 500 mm × 300 mm. The inner walls of the box were black, and the floor was evenly divided into 16 small squares of 4 × 4 each. A camera was installed above the open-field box to record the behavior of the mice. Before the experiment began, the mice were placed in the open-field box to acclimate to the environment for 5 minutes. Subsequently, the mice were positioned in the central square of the box, and the camera and timer were activated to record the behavior of the mice within the open-field box. Each mouse underwent three tests, and the average value was taken as the result. After each test, the open-field box was cleaned with 75% alcohol to eliminate the olfactory traces of the mice and to prevent any influence on subsequent experiments. All experiments were carried out in a quiet laboratory environment with controlled temperature around 20 °C and sufficient lighting.
TH immunohistochemistry analysis: Mice were deeply anesthetized with sodium pentobarbital (100 mg/kg) and perfused transcardially with 200 mL of cold phosphate buffered saline (PBS), followed by 200 mL of 4% paraformaldehyde dissolved in PBS. The brains were removed and fixed. After fixation, the brain tissues were dehydrated and embedded in paraffin to prepare paraffin blocks. The trimmed paraffin blocks were then sectioned on a microtome to a thickness of 4 μm, targeting the substantia nigra region for immunohistochemical analysis. The sections for TH immunohistochemistry were first blocked in 70% methanol containing 3% hydrogen peroxide for 7 minutes. The sections were then washed three times in PBS for 5 minutes each. Subsequently, the sections were incubated overnight at 4 °C with rabbit anti-TH antibody and 10% normal horse serum. After several rinses in PBS, the sections were incubated for 1 hour with biotinylated donkey anti-rabbit immunoglobulin G, followed by incubation with avidin-biotin-peroxidase complex for 30 minutes. The sections were then stained with 3,3’-diaminobenzidine and hydrogen peroxide, mounted on albumin-coated slides, and coverslipped.
The mice with successful modeling were randomly grouped. Using the G-power software, based on a statistical power of 80%, the minimum sample size required for each group was calculated to be 8 mice. Considering the possible withdrawal, 10 mice were set in each group, totaling 40 mice. Each cage contains 5 animals, fed with the standard diet (purchased from Xiecheng Pharmaceutical Bioengineering Co., LTD., batch number: 20190622). The animals were randomly divided into 4 groups (n = 10/group) using GraphPad Prism 9.0 computer randomization software: Sedentary PD group (St group, n = 10), iPSC-treated group (T group, n = 10), exercise group (E group, n = 10), and the combined exercise and iPSC-treated group (ET group, n = 10). The iPSCs were purchased from Qingqi Biotechnology Development Co., Ltd (Shanghai) and had undergone 7 passages prior to use. The mice were maintained under a natural light cycle, with the breeding environment temperature kept at 23 ± 3 °C and humidity at 55% ± 2%, and they had free access to ultrapure water[19]. Therapeutic protocol: IPSCs were administered to the T and ET groups via tail vein injection. Exercise protocol: Mice in the E and ET groups were placed on a treadmill for forced running. After a 1-week acclimation period, during which the treadmill speed was initially set at 6 m/minute for the first 3 days and then increased by 3 m/minute daily until reaching a final speed of 12 m/minute, the formal exercise regimen was implemented: 12 m/minute for 30 minutes per day, 5 days per week, for a total of 8 weeks[20]. Running and behavioral tests were conducted during the dark phase. Tissue collection: At the end of the intervention, mice were euthanized by intraperitoneal injection of 200 mg/mL sodium pentobarbital. Whole brains and serum samples were collected and stored at -80 °C for subsequent analyses.
Serum levels of epinephrine (EPI) (Andy Genett, E-20288, 1:1000) and nerve growth factor (NGF) (Andy Genet, SP13742, 1:1000) were measured using commercially available enzyme-linked immunosorbent assay kits according to the manufacturer’s instructions. Briefly, serum samples from each group were equilibrated at room temperature for 30 minutes and then loaded onto pre-coated plates alongside standard curve dilutions. After 90 minutes of incubation at
Total RNA was extracted from snap-frozen brain tissues (immediately stored in liquid nitrogen after dissection) using the TRIzol reagent (Invitrogen, CA, United States), followed by DNase I treatment to eliminate genomic DNA contamination. RNA quality and concentration were verified by Nanodrop spectrophotometry (A260/A280 ratio > 1.8) and agarose gel electrophoresis. First-strand cDNA was synthesized from 1 μg total RNA using the PrimeScript RT reagent kit (Takara, RR037A, Japan) with oligo(dT) primers, according to the manufacturer’s protocol. Quantitative polymerase chain reaction (qPCR) was performed using PerfectStart Green qPCR SuperMix (TransGen Biotech, AQ601, China) on a QuantStudio 5 system (Applied Biosystems, CA, United States) with the following cycling conditions: 94 °C for 30 seconds; 40 cycles of 94 °C for 5 seconds and 60 °C for 30 seconds. Reactions (20 μL) contained 10 ng cDNA, 0.2 μM primers (Table 1), and SYBR Green master mix. All samples were run in technical triplicates alongside no-template controls. Data were analyzed by the 2-ΔΔCt method with GAPDH normalization. Data were analyzed by the 2-ΔΔCt method with GAPDH normalization as follows: The threshold cycle (Ct) values for each target gene and the reference gene (GAPDH) were obtained from the qPCR instrument. The ΔCt for each sample was calculated by subtracting the Ct value of GAPDH from the Ct value of the target gene: ΔCt = Ct (target gene) - Ct (GAPDH). The ΔΔCt was then calculated by subtracting the average ΔCt of the control group from the ΔCt of each experimental sample: ΔΔCt = ΔCt (experimental sample) - ΔCt (control group average). The relative expression level (fold change) of the target gene was determined using the formula: Fold change = 2-ΔΔCt. Melting curve analysis confirmed amplification specificity, and amplification efficiencies (90%-110%) were validated by standard curves.
| Gene | F | R |
| Wnt1 | 5’-CTGGTGTCACCAAGGGTGTG-3’ | 5’-GCACCTCGTTGTAGAGGCTG-3’ |
| TH | 5’-CCAGCAACCTCACCTACACC-3’ | 5’-GCTCCAGGTCTCGTTGATCC-3’ |
| Neurog2 | 5’-CAGCCAGTACCCGATTGAAG-3’ | 5’-GCTGTAGTCGGTGGTGTTCC-3’ |
| Lmx1a | 5’-GCTGTAGTCGGTGGTGTTCC-3’ | 5’-TGGTGTTGGGTTGATGTTGA-3’ |
| GAPDH | 5’-TGAAGCAGGCATCTGAGGG-3’ | 5’-CGAAGGTGGAAGAGTGGGAG-3’ |
For protein expression analysis, brain tissues were rapidly collected from pentobarbital-anesthetized (200 mg/kg) PD model mice (both exercised and sedentary groups). Striatal tissues were obtained using 2-mm Harris Uni-Core punches (TED PELLA, INC., CA, United States), homogenized in lysis buffer, and centrifuged twice (15000 rpm, 10 minutes, 4 °C) to obtain cytosolic fractions. Proteins were denatured (6 × Laemmli buffer, 100 °C, 5 minutes), separated by 7.5% sodium-dodecyl sulfate gel electrophoresis, and transferred to Hybond-P polyvinylidene fluoride membranes. Membranes were probed overnight at 4 °C with primary antibodies against Wnt1 (Abcam ab15251, 1:1000, United Kingdom), TH (Millipore MAB318, 1:2000, MA, United States), Lmx1a (Santa Cruz sc-515576, 1:500, CA, United States), Otx2 (R&D Systems MAB1979, 1:1000, MN, United States), Neurog2 (Cell Signaling 12943S, 1:800, MA, United States), and β-actin (Sigma A5441, 1:5000; loading control, MO, United States), followed by incubation with HRP-conjugated secondary antibodies (1:5000, 1 hour, RT). Signals were developed using ECL and quantified with ImageJ (band intensity = density × area), with all values normalized to β-actin expression for statistical analysis. All experiments were performed with three independent biological replicates, and all values were normalized to β-actin expression for statistical analysis. β-actin was used as an internal loading control to normalize protein loading variations, and all target protein values were normalized to β-actin expression for statistical analysis.
The processed samples were dewaxed and baked at a high temperature of 60-65 °C, then immediately placed in xylene for 5-10 minutes to melt the paraffin. The samples were removed and transferred to absolute ethanol for tissue hydration (100% ethanol for 10 minutes, followed by 95%, 90%, 80%, and 70% ethanol each for 5 minutes), then washed with water for 3-5 minutes. Sections were subjected to microwave antigen retrieval using citrate buffer for 10 minutes, followed by cooling to room temperature. Sections were covered with goat serum and incubated at 37 °C for 50-60 minutes. Excess goat serum was blotted with filter paper, and primary antibodies including rabbit anti-mouse OCT4 (Abcam, EPR17929, 1:1000, United Kingdom) and DAPI (Invitrogen, PA5-16291, 1:250, CA, United States) were added. Incubation was performed overnight at 4 °C. For negative controls, 0.01 M PBS or rabbit serum replaced the primary antibody, with other steps unchanged. After removing the wet box, sections were equilibrated at room temperature for 15 minutes, washed three times with PBS for 5 minutes each, and incubated with biotinylated goat anti-rabbit immunoglobulin G secondary antibody (SolelyBio, ZB-0311, 1:1000, Shanghai, China) as a working mixture in a 37 °C wet box for 50-60 minutes. Slides were returned to room temperature for 15 minutes, washed three times with PBS for 5 minutes each. Finally, DAPI staining solution was applied to the slides, incubated in the dark for 10 minutes, and mounted with a coverslip. Here, blue fluorescence from DAPI labels cell nuclei, and green fluorescence from OCT4 marks iPSC. For data analysis, ImageJ software was used: Images were first split into single-channel images for measurement. Steps included clicking “Change Channel”, adjusting thresholds, selecting regions of interest, choosing an appropriate threshold algorithm, setting measurement parameters, and performing detection.
Data were analyzed using SPSS 26.0 software and are presented as mean ± SEM. For comparisons between two groups, independent samples t-tests were used, with P < 0.05 indicating significance and P < 0.01 indicating highly significant differences. For comparisons among three or more groups, one-way analysis of variance (ANOVA) was used, with P < 0.05 indicating significance and P < 0.01 indicating highly significant differences. Behavioral analysis was performed using two-way ANOVA, with P < 0.05 indicating significance and P < 0.01 indicating highly significant differences.
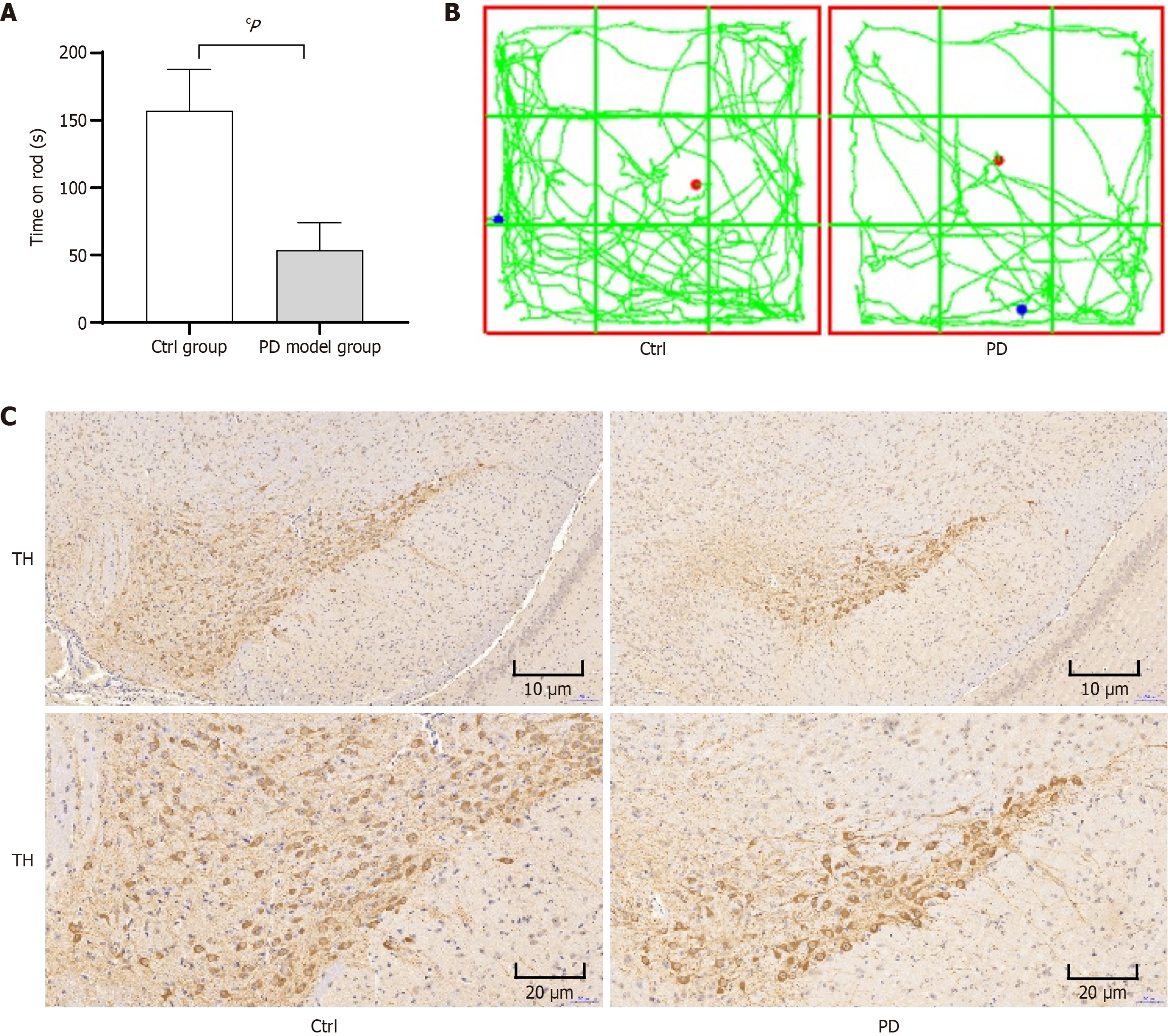
Compared with the control group, the PD model mice showed a statistically significant decrease in rotarod latency (P < 0.01) (Figure 1A). The open field test results demonstrated that the PD group mice exhibited significantly shorter movement trajectory lengths compared to the control group (Figure 1B). The results showed significant changes in pathological sections of the PD model mice, control group shows dense TH+ DA fibers and PD model group exhibits dramatic TH+ fiber loss (Figure 1C).
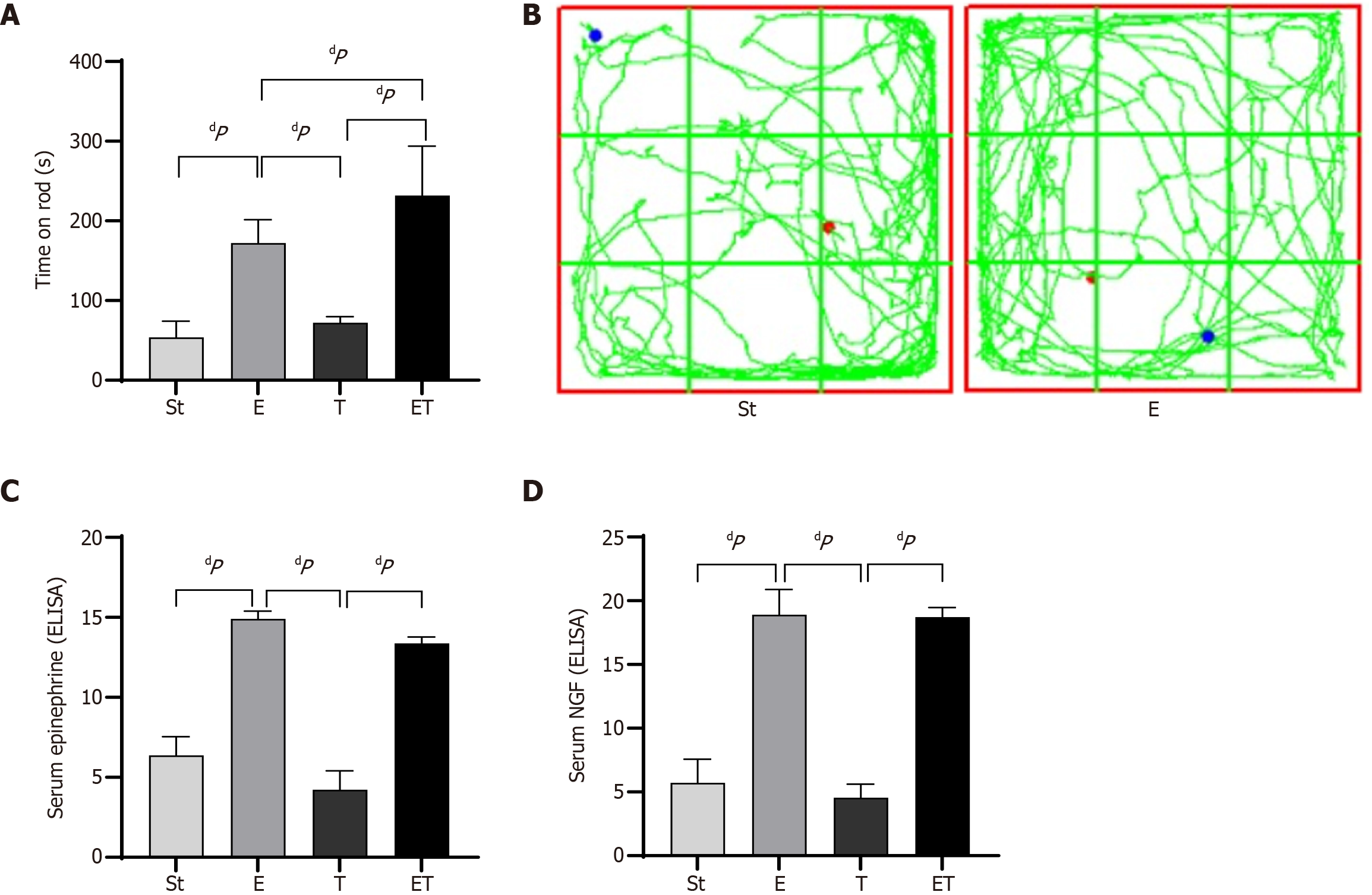
Compared with the St group, the E, T, and ET groups all exhibited prolonged latency times. The differences in prolonged times between the E and ET groups and the St group were highly statistically significant (P < 0.01). Compared with the T group, both the E and ET groups showed prolonged times, with highly statistically significant differences (P < 0.01). Compared with the E group, the ET group exhibited a prolonged time, with a statistically significant difference (P < 0.05) (Figure 2A). The open field test results demonstrated that the E group mice exhibited significantly longer movement trajectory lengths compared to the control group (Figure 2B).
Enzyme-linked immunosorbent assays were performed to measure the levels of EPI and NGF in the serum of mice from each group. The results showed that the protein expression levels of EPI in the serum of the E and ET groups were significantly higher than those in the St and T groups (P < 0.01). Similarly, the protein expression levels of NGF in the serum of the E and ET groups were significantly higher than those in the St and T groups (P < 0.01) (Figure 2C and D).
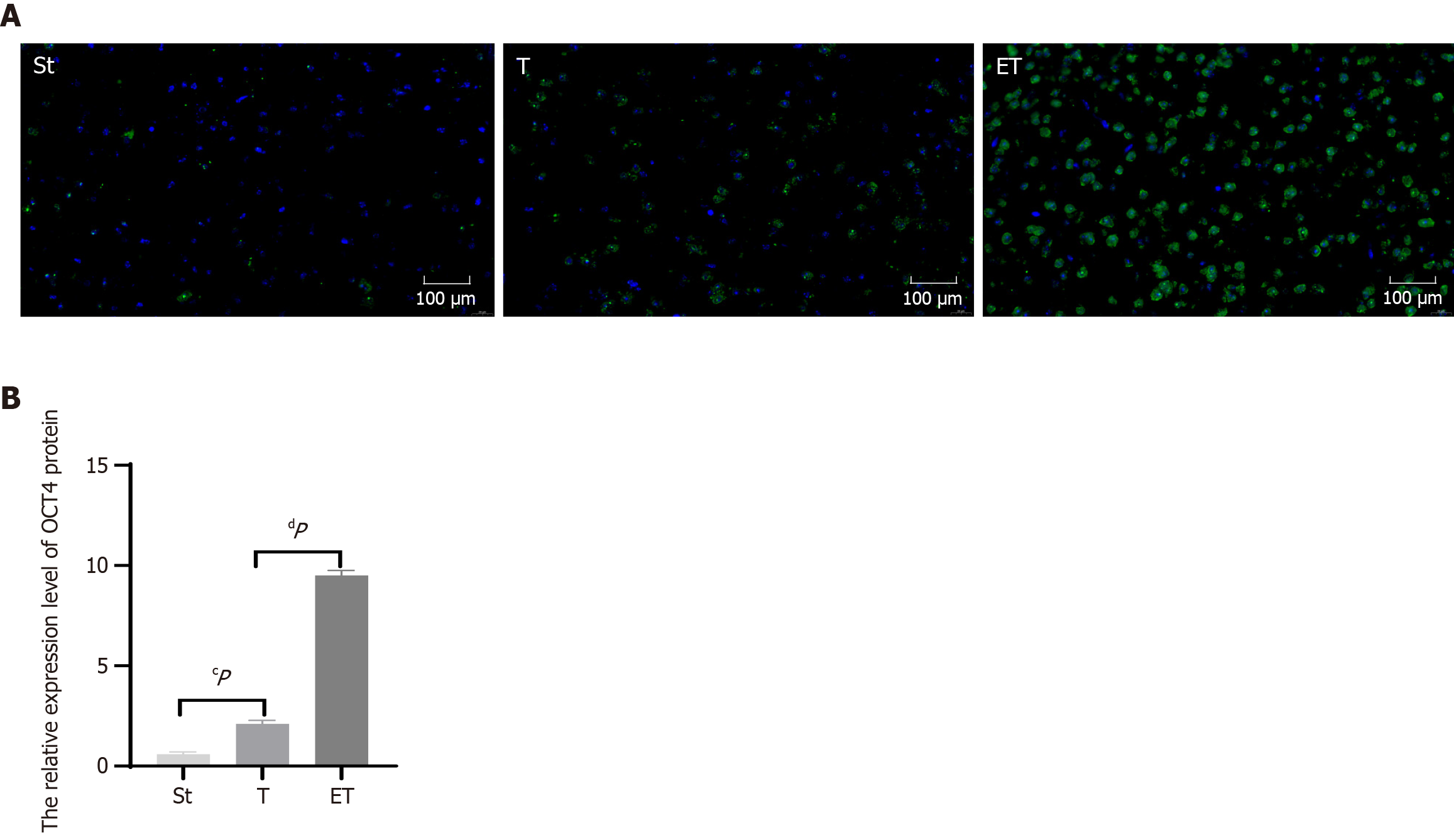
The results of immunofluorescence staining of mouse brain sections showed that compared with the St group, the number of iPSCs in the T group was significantly increased, and the relative expression of OCT4 was significantly different (P < 0.05). In addition, compared with the T group, the ET group had significantly more iPSCs, and the relative expression of OCT4 was very significantly different (P < 0.01). These results indicate that iPSCs can successfully enter the mouse brain tissue through tail vein injection. Furthermore, compared with iPSC treatment alone, exercise combined with iPSC treatment can further increase the number of iPSCs entering the brain tissue, thereby exerting a more significant intervention effect (Figure 3).
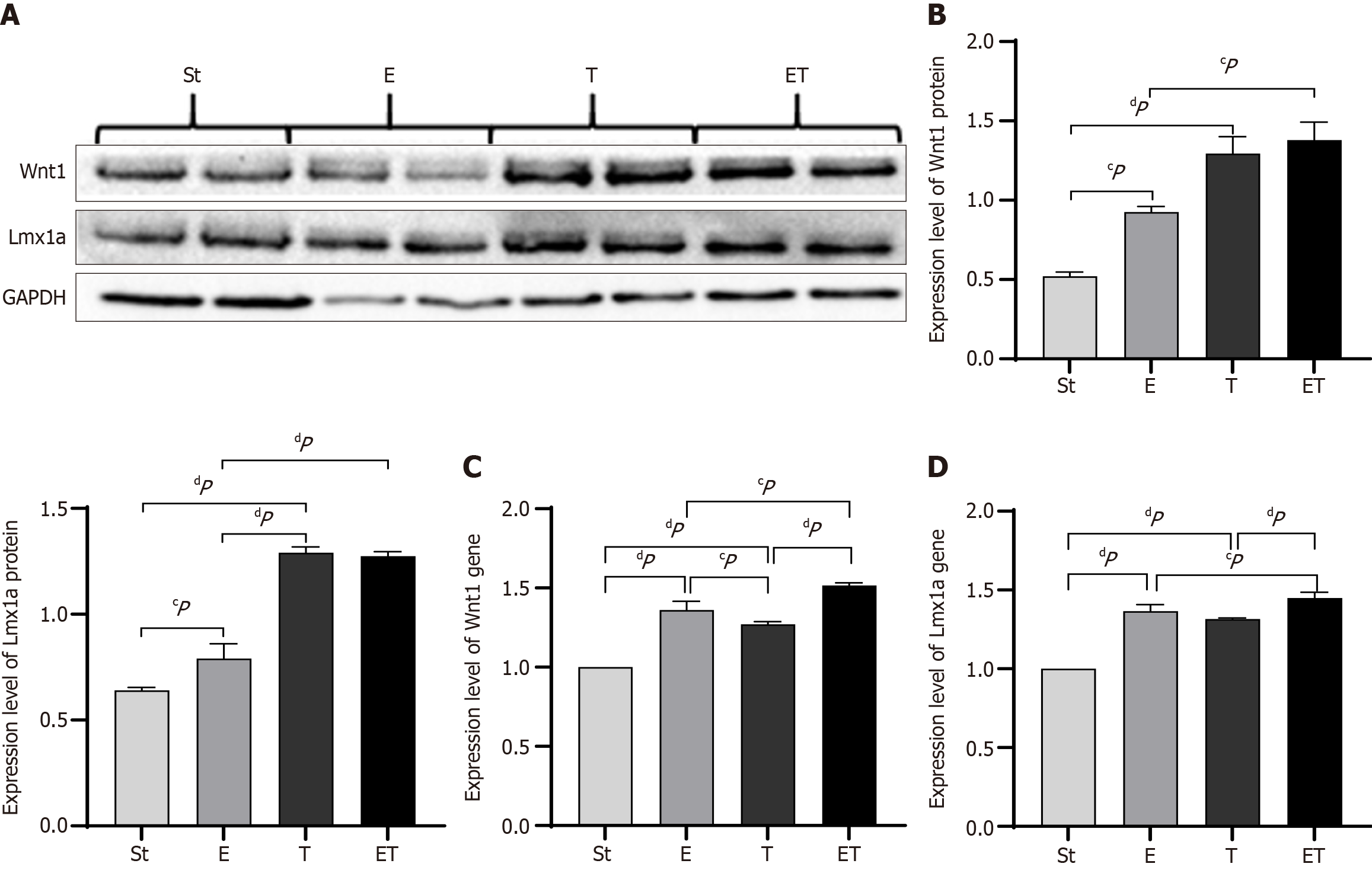
Western blot analysis was performed to detect the expression of Wnt1 and Lmx1a in the midbrain of mice from each group. The results showed that, compared with the St group, the protein expression of Wnt1 and Lmx1a in the E group was significantly increased, while in the T and ET groups, the increase was extremely significant (P < 0.01). Compared with the E group, the protein expression level of Wnt1 in the ET group was significantly increased (P < 0.05), and the protein expression level of Lmx1a was extremely significantly increased (P < 0.01). The expression level of Lmx1a in the T group was extremely significantly higher than that in the E group (P < 0.01) (Figure 4A and B).
Reverse transcription polymerase chain reaction (RT-PCR) was used to detect the gene expression of Wnt1 in the midbrain of mice from each group. The results showed that the gene expression of Wnt1 in the E, T, and ET groups was extremely significantly higher than that in the St group (P < 0.01). The expression in the E group was significantly higher than that in the T group (P < 0.05), and the expression in the ET group was significantly higher than that in the E and T groups (P < 0.05) (Figure 4C).
RT-PCR was also performed to detect the gene expression of Lmx1a in the midbrain of mice from each group. The results showed that, compared with the St group, the gene expression of Lmx1a in the E, T, and ET groups was extremely significantly increased (P < 0.01). The expression in the ET group was higher than that in the E and T groups, with a significant increase compared with the E group (P < 0.05) and an extremely significant increase compared with the T group (P < 0.01) (Figure 4D).
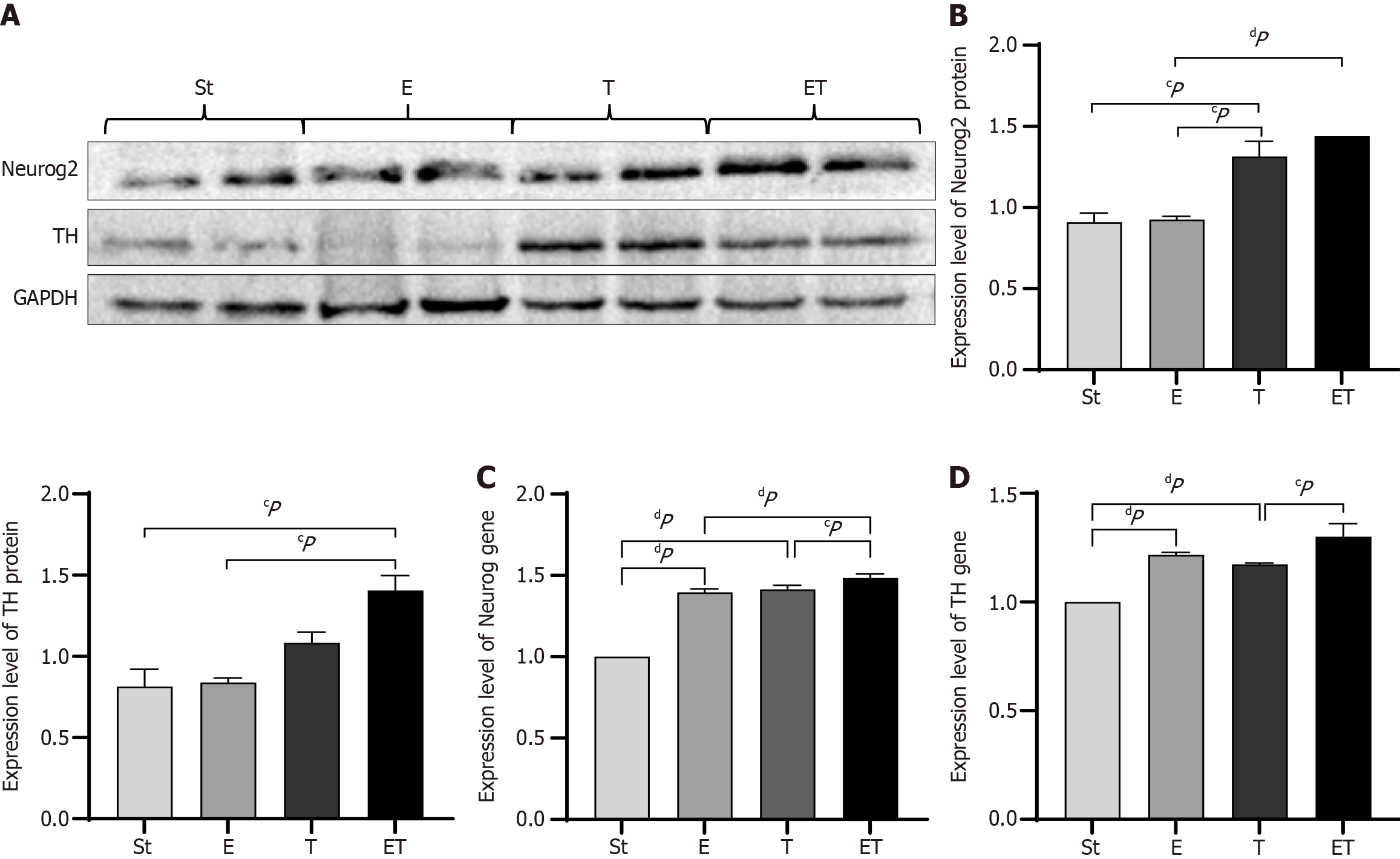
Western blotting was used to examine the expression of Neurog2 and TH in the midbrain of mice from each group. The expression of TH protein in group ET was significantly higher than that in group St and group E (P < 0.05), while there was no statistical difference between group E and group St, the lack of significant change in TH levels in the E group may be related to the following factors: First, exercise intervention may regulate TH activity through post-translational modifications (such as phosphorylation) rather than the total protein amount; second, the sample size of this experiment may limit statistical power. The protein expression level of Neurog2 was significantly increased in the T group (P < 0.05) and markedly elevated in the ET group (P < 0.01). Additionally, the protein expression level of TH in the ET group was significantly higher than that in the St and E groups (P < 0.05) (Figure 5A and B).
RT-PCR was employed to assess the gene expression of Neurog2 in the midbrain of mice from each group. The results indicated that the gene expression of Neurog2 was significantly higher in the E, T, and ET groups than in the St group (P < 0.01). The expression in the ET group was greater than that in the E and T groups, with a significant increase compared with the T group (P < 0.05) and a marked elevation compared with the E group (P < 0.01) (Figure 5C). RT-PCR was also used to evaluate the gene expression of TH in the midbrain of mice from each group. The results showed that, compared with the St group, the gene expression of TH was significantly higher in the E, T, and ET groups (P < 0.01). The gene expression in the ET group was significantly higher than that in the T group (P < 0.05) (Figure 5D).
PD is an incurable, progressive neurological disorder that can lead to debilitating motor and non-motor consequences[21]. Currently, no medications are available to prevent the onset or progression of PD, and symptomatic treatments have limited efficacy in certain domains while also producing side effects. Therefore, it is crucial to identify interventions that can prevent, slow, halt, or mitigate the disease. Exercise is safe and is the cornerstone of PD rehabilitation, but exercise may have more fundamental benefits that can change clinical practice[22].
Exercise improves motor symptoms in PD: Research has indicated that a higher degree of physical activity is linked to a reduced likelihood of developing PD[23], and it can increase the postural stability of patients with PD and improve movement deficiencies[24,25]. The finding that aligns with the results from our experimental behavioral tests, where the group engaged in exercise significantly outperformed the sedentary group. Thus, regular and potentially lifelong exercise can lower the risk of PD or at least postpone its diagnosis. Similar observations have been made in multiple sclerosis and Alzheimer’s disease, suggesting that exercise and physical activity offer broader protection against NDs. However, while some evidence suggests that medications like ibuprofen and calcium channel blockers may be associated with a lower risk of PD, the current evidence remains conflicting[26-28]. As a result, no specific medication has been definitively proven to reduce the risk of developing PD.
Studies on the effects of exercise on factors related to PD: NDs are marked by the progressive degeneration of neurons, leading to disability[29]. Neurotrophic factors such as glial cell line-derived neurotrophic factor, NGF, and brain-derived neurotrophic factor are vital for the survival, maintenance, and regeneration of specific neuronal populations in the adult brain[30,31]. Our study found that exercise increases NGF expression levels, which is consistent with previous animal studies showing that exercise induces neuroprotection and neuroregeneration through various molecular processes, including the upregulation of neurotrophic factors like brain-derived neurotrophic factor, insulin-like growth factor-1[32], and NGF, as well as a reduction in destructive free radicals in the hippocampus[33]. Exercise can influence key biomarkers related to the pathology of PD, such as the expression of α-synuclein, and can also slow down the occurrence of inflammatory responses[34]. These processes enhance neuronal survival, increase dopamine levels, and strengthen neural networks in the basal ganglia, cortex, thalamus, cerebellum, and brainstem, ultimately improving motor and/or cognitive performance.
Lmx1a is a key determinant for the generation of DA neurons[35,36]. When ectopically expressed in embryonic stem cells, Lmx1a significantly enhances the efficiency of DA neuron production[37]. It plays a central role in DA neuron generation and may be crucial for their transdifferentiation[38]. In mice with Lmx1a/b gene knockout, signs of degeneration first appeared in the SNpc at P20, with abnormally swollen terminals (referred to as “spheroids”) in midbrain DA neurons before cell death, leading to gradual loss of midbrain DA neurons and DA depletion in the striatum[39]. Lmx1a (transcription factors or LIM-homeodomain proteins), expressed in early DA progenitors, induces Msx1, which suppresses alternative cell fates by repressing the Nkx6.1 gene and induces neurogenesis by activating the proneural gene Ngn2[35]. The significant increase in Lmx1a expression levels after exercise in our study suggests that exercise may exert its effects through this pathway.
Exploration of the therapeutic mechanisms of exercise in PD: NDs may involve dysregulated Wnt signaling, which is critical for hippocampal neurogenesis, axonal guidance, and synaptic plasticity[36,40]. DA neuron induction relies on Wnt/Shh pathways, with a key Wnt1-Lmx1a autoregulatory loop regulating Otx2 (via β-catenin) and Nurr1/Pitx3 (via Lmx1a)[12]. Our data show exercise elevates EPI, potentially activating β-adrenergic receptors and frizzled 1/2 chimeric receptors, thereby triggering Wnt/β-catenin signaling. Exercise factors (e.g., myokines) and extracellular vesicle-mediated transport (confirmed in our prior studies[41,42]) may facilitate this process. Notably, while Wnt-1 and Lmx1a increased, TH protein levels were unaltered, possibly due to exercise recovery effects[43].
In this study, the combined intervention of exercise and iPSCs in Parkinson’s mice resulted in significant improvements in motor symptoms and changes in factors related to DA neurons. The levels of Wnt1, Lmx1a, Neurog2, and TH were significantly elevated in the combined therapy group[44]. Exercise alone did not significantly increase TH levels, suggesting that it may not directly generate pluripotent stem cells to transform into DA neurons. However, the injection of iPSCs via the tail vein compensated for this deficiency, promoting the transformation and increasing the number of DA neurons[14].
While it is plausible that exercise could increase EPI levels, which in turn activates β-adrenergic receptors and stimulate an increase in Wnt1 Levels, this hypothesis is not directly supported by the data[45]. The transport of Wnt1 to the brain via extracellular vesicles and its subsequent effects on the Wnt1-Lmx1a autoregulatory loop and Neurog2 Levels are speculative and require further investigation. Future studies should focus on directly measuring EPI levels and the transport of Wnt1 to confirm these mechanisms.
This research has the following limitations. First, although we attempted to detect the survival and differentiation of iPSCs in the brain using various methods, we were unable to obtain sufficient evidence. Second, this study primarily focused on the Wnt1-Lmx1a signaling pathway. However, we recognize that other biological mechanisms may also have contributed to the therapeutic effects observed with the combined treatment of exercise and iPSCs. These potential contributing factors may include: (1) The regulatory effects of exercise on neuroinflammatory processes; (2) The enhancement of mitochondrial bioenergetics; (3) The optimization of communication between glial cells and neurons; and (4) The induction of complementary neuroprotective signaling cascades. Future research will need to systematically evaluate these mechanisms to fully elucidate their underlying therapeutic mechanisms.
The combined intervention of exercise and iPSCs shows significantly greater therapeutic effects on PD than either exercise or cell therapy alone, improving both motor and non-motor symptoms of PD. Exercise also promotes the differentiation of iPSCs into DA neurons. In PD, abnormal changes in glial cells lead to neuronal death, and the Wnt signaling pathway plays a key role in neurogenesis and neuronal regulation. This study hypothesizes that the possible mechanism involves exercise increasing EPI levels, which activates β2 receptors and chimeric receptors of frizzled 1 or frizzled 2, thereby activating the Wnt signaling pathway, enhancing the Wnt1-Lmx1a regulatory loop, and promoting the differentiation of iPSCs into DA neurons, ultimately increasing the number of neurons.
| 1. | Pringsheim T, Jette N, Frolkis A, Steeves TD. The prevalence of Parkinson's disease: a systematic review and meta-analysis. Mov Disord. 2014;29:1583-1590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1663] [Cited by in RCA: 1438] [Article Influence: 119.8] [Reference Citation Analysis (0)] |
| 2. | Zigmond MJ, Cameron JL, Leak RK, Mirnics K, Russell VA, Smeyne RJ, Smith AD. Triggering endogenous neuroprotective processes through exercise in models of dopamine deficiency. Parkinsonism Relat Disord. 2009;15 Suppl 3:S42-S45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 81] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 3. | Zhang S, Yan M, Jiang X, Liu Y, Ma W, Ding L, Lu Z, Luo Y, Tian X, Wang Q. Oligodendrocyte-astrocyte crosstalk in Parkinson's disease mediates neuronal ferroptosis via the FGF signaling pathway. NPJ Parkinsons Dis. 2025;11:136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 4. | Bonde-Jensen F, Dalgas U, Langeskov-Christensen M. Are physical activity levels, cardiorespiratory fitness and peak power associated with Parkinson's disease severity? J Neurol Sci. 2024;460:122996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Ang ET, Gomez-Pinilla F. Potential therapeutic effects of exercise to the brain. Curr Med Chem. 2007;14:2564-2571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 96] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 6. | Tillerson JL, Caudle WM, Reverón ME, Miller GW. Exercise induces behavioral recovery and attenuates neurochemical deficits in rodent models of Parkinson's disease. Neuroscience. 2003;119:899-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 285] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 7. | Swain RA, Harris AB, Wiener EC, Dutka MV, Morris HD, Theien BE, Konda S, Engberg K, Lauterbur PC, Greenough WT. Prolonged exercise induces angiogenesis and increases cerebral blood volume in primary motor cortex of the rat. Neuroscience. 2003;117:1037-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 430] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 8. | Kirkeby A, Nelander J, Hoban DB, Rogelius N, Bjartmarz H; Novo Nordisk Cell Therapy R&D, Storm P, Fiorenzano A, Adler AF, Vale S, Mudannayake J, Zhang Y, Cardoso T, Mattsson B, Landau AM, Glud AN, Sørensen JC, Lillethorup TP, Lowdell M, Carvalho C, Bain O, van Vliet T, Lindvall O, Björklund A, Harry B, Cutting E, Widner H, Paul G, Barker RA, Parmar M. Preclinical quality, safety, and efficacy of a human embryonic stem cell-derived product for the treatment of Parkinson's disease, STEM-PD. Cell Stem Cell. 2023;30:1299-1314.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 79] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 9. | Kim MS, Ra EA, Kweon SH, Seo BA, Ko HS, Oh Y, Lee G. Advanced human iPSC-based preclinical model for Parkinson's disease with optogenetic alpha-synuclein aggregation. Cell Stem Cell. 2023;30:973-986.e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 41] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 10. | Okano H, Morimoto S. iPSC-based disease modeling and drug discovery in cardinal neurodegenerative disorders. Cell Stem Cell. 2022;29:189-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 119] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 11. | Joksimovic M, Yun BA, Kittappa R, Anderegg AM, Chang WW, Taketo MM, McKay RD, Awatramani RB. Wnt antagonism of Shh facilitates midbrain floor plate neurogenesis. Nat Neurosci. 2009;12:125-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 169] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 12. | Chung S, Leung A, Han BS, Chang MY, Moon JI, Kim CH, Hong S, Pruszak J, Isacson O, Kim KS. Wnt1-lmx1a forms a novel autoregulatory loop and controls midbrain dopaminergic differentiation synergistically with the SHH-FoxA2 pathway. Cell Stem Cell. 2009;5:646-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 164] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 13. | Sonntag KC, Song B, Lee N, Jung JH, Cha Y, Leblanc P, Neff C, Kong SW, Carter BS, Schweitzer J, Kim KS. Pluripotent stem cell-based therapy for Parkinson's disease: Current status and future prospects. Prog Neurobiol. 2018;168:1-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 97] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 14. | Arenas E, Denham M, Villaescusa JC. How to make a midbrain dopaminergic neuron. Development. 2015;142:1918-1936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 210] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 15. | Tao Y, Zhang SC. Neural Subtype Specification from Human Pluripotent Stem Cells. Cell Stem Cell. 2016;19:573-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 224] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 16. | Joksimovic M, Anderegg A, Roy A, Campochiaro L, Yun B, Kittappa R, McKay R, Awatramani R. Spatiotemporally separable Shh domains in the midbrain define distinct dopaminergic progenitor pools. Proc Natl Acad Sci U S A. 2009;106:19185-19190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 98] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 17. | Kittappa R, Chang WW, Awatramani RB, McKay RD. The foxa2 gene controls the birth and spontaneous degeneration of dopamine neurons in old age. PLoS Biol. 2007;5:e325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 188] [Cited by in RCA: 195] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 18. | Berlet R, Galang Cabantan DA, Gonzales-Portillo D, Borlongan CV. Enriched Environment and Exercise Enhance Stem Cell Therapy for Stroke, Parkinson's Disease, and Huntington's Disease. Front Cell Dev Biol. 2022;10:798826. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 19. | Lee JM, Kim TW, Park SS, Han JH, Shin MS, Lim BV, Kim SH, Baek SS, Cho YS, Kim KH. Treadmill Exercise Improves Motor Function by Suppressing Purkinje Cell Loss in Parkinson Disease Rats. Int Neurourol J. 2018;22:S147-S155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 20. | Tuon T, Valvassori SS, Lopes-Borges J, Luciano T, Trom CB, Silva LA, Quevedo J, Souza CT, Lira FS, Pinho RA. Physical training exerts neuroprotective effects in the regulation of neurochemical factors in an animal model of Parkinson's disease. Neuroscience. 2012;227:305-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 104] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 21. | Ma W, Geng Y, Liu Y, Pan H, Wang Q, Zhang Y, Wang L. The mechanisms of white matter injury and immune system crosstalk in promoting the progression of Parkinson's disease: a narrative review. Front Aging Neurosci. 2024;16:1345918. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 22. | Langeskov-Christensen M, Franzén E, Grøndahl Hvid L, Dalgas U. Exercise as medicine in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2024;95:1077-1088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 33] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 23. | Portugal B, Artaud F, Degaey I, Roze E, Fournier A, Severi G, Canonico M, Proust-Lima C, Elbaz A. Association of Physical Activity and Parkinson Disease in Women: Long-term Follow-up of the E3N Cohort Study. Neurology. 2023;101:e386-e398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 24. | Martiš P, Bzdúšková D, Košutzká Z, Slobodová L, Straka I, Marček Malenovská K, Mytiai O, Tirpáková V, Konrády P, Litváková V, Turi Nagy M, Urbančík Z, Valkovič P, Ukropec J, Ukropcová B, Kimijanová J. Supervised aerobic-strength exercise reduces postural sway and improves dual-task gait in Parkinson's disease. Sci Rep. 2025;15:20643. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 25. | Xu MM, Hu DT, Zhang Q, Liu XG, Li ZW, Lu LM. [Exercise preconditioning alleviates motor deficits in MPTP-induced Parkinsonian mice by improving mitochondrial function]. Sheng Li Xue Bao. 2025;77:419-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 26. | Belvisi D, Pellicciari R, Fabbrini G, Tinazzi M, Berardelli A, Defazio G. Modifiable risk and protective factors in disease development, progression and clinical subtypes of Parkinson's disease: What do prospective studies suggest? Neurobiol Dis. 2020;134:104671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 27. | Ascherio A, Schwarzschild MA. The epidemiology of Parkinson's disease: risk factors and prevention. Lancet Neurol. 2016;15:1257-1272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 824] [Cited by in RCA: 1307] [Article Influence: 130.7] [Reference Citation Analysis (0)] |
| 28. | Ren L, Yi J, Yang J, Li P, Cheng X, Mao P. Nonsteroidal anti-inflammatory drugs use and risk of Parkinson disease: A dose-response meta-analysis. Medicine (Baltimore). 2018;97:e12172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 29. | Dugger BN, Dickson DW. Pathology of Neurodegenerative Diseases. Cold Spring Harb Perspect Biol. 2017;9:a028035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 436] [Cited by in RCA: 1096] [Article Influence: 121.8] [Reference Citation Analysis (0)] |
| 30. | Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR, Mailey E, Vieira VJ, Martin SA, Pence BD, Woods JA, McAuley E, Kramer AF. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108:3017-3022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2601] [Cited by in RCA: 3085] [Article Influence: 205.7] [Reference Citation Analysis (6)] |
| 31. | Coelho FG, Vital TM, Stein AM, Arantes FJ, Rueda AV, Camarini R, Teodorov E, Santos-Galduróz RF. Acute aerobic exercise increases brain-derived neurotrophic factor levels in elderly with Alzheimer's disease. J Alzheimers Dis. 2014;39:401-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 144] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 32. | Maass A, Düzel S, Brigadski T, Goerke M, Becke A, Sobieray U, Neumann K, Lövdén M, Lindenberger U, Bäckman L, Braun-Dullaeus R, Ahrens D, Heinze HJ, Müller NG, Lessmann V, Sendtner M, Düzel E. Relationships of peripheral IGF-1, VEGF and BDNF levels to exercise-related changes in memory, hippocampal perfusion and volumes in older adults. Neuroimage. 2016;131:142-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 257] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 33. | Ionescu-Tucker A, Cotman CW. Emerging roles of oxidative stress in brain aging and Alzheimer's disease. Neurobiol Aging. 2021;107:86-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 460] [Article Influence: 92.0] [Reference Citation Analysis (0)] |
| 34. | Luthra NS, Mehta N, Munoz MJ, Fantuzzi G, Lamotte G, Haus JM, McFarland NR, Tansey MG, Gonzalez-Latapi P, Caraveo G, Kang UJ, Corcos DM. Aerobic exercise-induced changes in fluid biomarkers in Parkinson's disease. NPJ Parkinsons Dis. 2025;11:190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 35. | Andersson E, Tryggvason U, Deng Q, Friling S, Alekseenko Z, Robert B, Perlmann T, Ericson J. Identification of intrinsic determinants of midbrain dopamine neurons. Cell. 2006;124:393-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 439] [Cited by in RCA: 457] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 36. | Smidt MP, Asbreuk CH, Cox JJ, Chen H, Johnson RL, Burbach JP. A second independent pathway for development of mesencephalic dopaminergic neurons requires Lmx1b. Nat Neurosci. 2000;3:337-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 307] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 37. | Friling S, Andersson E, Thompson LH, Jönsson ME, Hebsgaard JB, Nanou E, Alekseenko Z, Marklund U, Kjellander S, Volakakis N, Hovatta O, El Manira A, Björklund A, Perlmann T, Ericson J. Efficient production of mesencephalic dopamine neurons by Lmx1a expression in embryonic stem cells. Proc Natl Acad Sci U S A. 2009;106:7613-7618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 165] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 38. | Wu J, Sheng C, Liu Z, Jia W, Wang B, Li M, Fu L, Ren Z, An J, Sang L, Song G, Wu Y, Xu Y, Wang S, Chen Z, Zhou Q, Zhang YA. Lmx1a enhances the effect of iNSCs in a PD model. Stem Cell Res. 2015;14:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 39. | Doucet-Beaupré H, Gilbert C, Profes MS, Chabrat A, Pacelli C, Giguère N, Rioux V, Charest J, Deng Q, Laguna A, Ericson J, Perlmann T, Ang SL, Cicchetti F, Parent M, Trudeau LE, Lévesque M. Lmx1a and Lmx1b regulate mitochondrial functions and survival of adult midbrain dopaminergic neurons. Proc Natl Acad Sci U S A. 2016;113:E4387-E4396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 40. | Zhang Y, Wang R, Chen R, Wang L. [Changes of Wnt/β-catenin signaling pathway in the hippocampus caused by prenatal stress induce depression- and anxiety-like behaviors in rats]. Nan Fang Yi Ke Da Xue Xue Bao. 2019;39:222-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 41. | Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Archer F, Atkin-Smith GK, Ayre DC, Bach JM, Bachurski D, Baharvand H, Balaj L, Baldacchino S, Bauer NN, Baxter AA, Bebawy M, Beckham C, Bedina Zavec A, Benmoussa A, Berardi AC, Bergese P, Bielska E, Blenkiron C, Bobis-Wozowicz S, Boilard E, Boireau W, Bongiovanni A, Borràs FE, Bosch S, Boulanger CM, Breakefield X, Breglio AM, Brennan MÁ, Brigstock DR, Brisson A, Broekman ML, Bromberg JF, Bryl-Górecka P, Buch S, Buck AH, Burger D, Busatto S, Buschmann D, Bussolati B, Buzás EI, Byrd JB, Camussi G, Carter DR, Caruso S, Chamley LW, Chang YT, Chen C, Chen S, Cheng L, Chin AR, Clayton A, Clerici SP, Cocks A, Cocucci E, Coffey RJ, Cordeiro-da-Silva A, Couch Y, Coumans FA, Coyle B, Crescitelli R, Criado MF, D'Souza-Schorey C, Das S, Datta Chaudhuri A, de Candia P, De Santana EF, De Wever O, Del Portillo HA, Demaret T, Deville S, Devitt A, Dhondt B, Di Vizio D, Dieterich LC, Dolo V, Dominguez Rubio AP, Dominici M, Dourado MR, Driedonks TA, Duarte FV, Duncan HM, Eichenberger RM, Ekström K, El Andaloussi S, Elie-Caille C, Erdbrügger U, Falcón-Pérez JM, Fatima F, Fish JE, Flores-Bellver M, Försönits A, Frelet-Barrand A, Fricke F, Fuhrmann G, Gabrielsson S, Gámez-Valero A, Gardiner C, Gärtner K, Gaudin R, Gho YS, Giebel B, Gilbert C, Gimona M, Giusti I, Goberdhan DC, Görgens A, Gorski SM, Greening DW, Gross JC, Gualerzi A, Gupta GN, Gustafson D, Handberg A, Haraszti RA, Harrison P, Hegyesi H, Hendrix A, Hill AF, Hochberg FH, Hoffmann KF, Holder B, Holthofer H, Hosseinkhani B, Hu G, Huang Y, Huber V, Hunt S, Ibrahim AG, Ikezu T, Inal JM, Isin M, Ivanova A, Jackson HK, Jacobsen S, Jay SM, Jayachandran M, Jenster G, Jiang L, Johnson SM, Jones JC, Jong A, Jovanovic-Talisman T, Jung S, Kalluri R, Kano SI, Kaur S, Kawamura Y, Keller ET, Khamari D, Khomyakova E, Khvorova A, Kierulf P, Kim KP, Kislinger T, Klingeborn M, Klinke DJ 2nd, Kornek M, Kosanović MM, Kovács ÁF, Krämer-Albers EM, Krasemann S, Krause M, Kurochkin IV, Kusuma GD, Kuypers S, Laitinen S, Langevin SM, Languino LR, Lannigan J, Lässer C, Laurent LC, Lavieu G, Lázaro-Ibáñez E, Le Lay S, Lee MS, Lee YXF, Lemos DS, Lenassi M, Leszczynska A, Li IT, Liao K, Libregts SF, Ligeti E, Lim R, Lim SK, Linē A, Linnemannstöns K, Llorente A, Lombard CA, Lorenowicz MJ, Lörincz ÁM, Lötvall J, Lovett J, Lowry MC, Loyer X, Lu Q, Lukomska B, Lunavat TR, Maas SL, Malhi H, Marcilla A, Mariani J, Mariscal J, Martens-Uzunova ES, Martin-Jaular L, Martinez MC, Martins VR, Mathieu M, Mathivanan S, Maugeri M, McGinnis LK, McVey MJ, Meckes DG Jr, Meehan KL, Mertens I, Minciacchi VR, Möller A, Møller Jørgensen M, Morales-Kastresana A, Morhayim J, Mullier F, Muraca M, Musante L, Mussack V, Muth DC, Myburgh KH, Najrana T, Nawaz M, Nazarenko I, Nejsum P, Neri C, Neri T, Nieuwland R, Nimrichter L, Nolan JP, Nolte-'t Hoen EN, Noren Hooten N, O'Driscoll L, O'Grady T, O'Loghlen A, Ochiya T, Olivier M, Ortiz A, Ortiz LA, Osteikoetxea X, Østergaard O, Ostrowski M, Park J, Pegtel DM, Peinado H, Perut F, Pfaffl MW, Phinney DG, Pieters BC, Pink RC, Pisetsky DS, Pogge von Strandmann E, Polakovicova I, Poon IK, Powell BH, Prada I, Pulliam L, Quesenberry P, Radeghieri A, Raffai RL, Raimondo S, Rak J, Ramirez MI, Raposo G, Rayyan MS, Regev-Rudzki N, Ricklefs FL, Robbins PD, Roberts DD, Rodrigues SC, Rohde E, Rome S, Rouschop KM, Rughetti A, Russell AE, Saá P, Sahoo S, Salas-Huenuleo E, Sánchez C, Saugstad JA, Saul MJ, Schiffelers RM, Schneider R, Schøyen TH, Scott A, Shahaj E, Sharma S, Shatnyeva O, Shekari F, Shelke GV, Shetty AK, Shiba K, Siljander PR, Silva AM, Skowronek A, Snyder OL 2nd, Soares RP, Sódar BW, Soekmadji C, Sotillo J, Stahl PD, Stoorvogel W, Stott SL, Strasser EF, Swift S, Tahara H, Tewari M, Timms K, Tiwari S, Tixeira R, Tkach M, Toh WS, Tomasini R, Torrecilhas AC, Tosar JP, Toxavidis V, Urbanelli L, Vader P, van Balkom BW, van der Grein SG, Van Deun J, van Herwijnen MJ, Van Keuren-Jensen K, van Niel G, van Royen ME, van Wijnen AJ, Vasconcelos MH, Vechetti IJ Jr, Veit TD, Vella LJ, Velot É, Verweij FJ, Vestad B, Viñas JL, Visnovitz T, Vukman KV, Wahlgren J, Watson DC, Wauben MH, Weaver A, Webber JP, Weber V, Wehman AM, Weiss DJ, Welsh JA, Wendt S, Wheelock AM, Wiener Z, Witte L, Wolfram J, Xagorari A, Xander P, Xu J, Yan X, Yáñez-Mó M, Yin H, Yuana Y, Zappulli V, Zarubova J, Žėkas V, Zhang JY, Zhao Z, Zheng L, Zheutlin AR, Zickler AM, Zimmermann P, Zivkovic AM, Zocco D, Zuba-Surma EK. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7:1535750. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6453] [Cited by in RCA: 8213] [Article Influence: 1026.6] [Reference Citation Analysis (1)] |
| 42. | van Niel G, D'Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19:213-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3060] [Cited by in RCA: 6065] [Article Influence: 758.1] [Reference Citation Analysis (3)] |
| 43. | Kasanga EA, Soto I, Centner A, McManus R, Shifflet MK, Navarrete W, Han Y, Lisk J, Wheeler K, Mhatre-Winters I, Richardson JR, Bishop C, Nejtek VA, Salvatore MF. Moderate intensity aerobic exercise in 6-OHDA-lesioned rats alleviates established motor deficits and reduces neurofilament light and glial fibrillary acidic protein serum levels without increased striatal dopamine or tyrosine hydroxylase protein. bioRxiv. 2023;2023.07.11.548638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 44. | Joksimovic M, Awatramani R. Wnt/β-catenin signaling in midbrain dopaminergic neuron specification and neurogenesis. J Mol Cell Biol. 2014;6:27-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 45. | Ozair MZ, Noggle S, Warmflash A, Krzyspiak JE, Brivanlou AH. SMAD7 directly converts human embryonic stem cells to telencephalic fate by a default mechanism. Stem Cells. 2013;31:35-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |