Published online Sep 26, 2025. doi: 10.4252/wjsc.v17.i9.110663
Revised: July 22, 2025
Accepted: September 4, 2025
Published online: September 26, 2025
Processing time: 102 Days and 19.9 Hours
Acute cerebral infarction (ACI), a leading cause of death and disability, causes brain ischemia due to vessel blockage. Current time-limited interventions, such as clot removal, often fail to restore full function. Neurorestoration is vital, but complicated. Vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) promote angiogenesis and neuroprotection. Stem cell therapy has potential to promote neurorestoration. Specifically, neural stem cells (NSC) reconstruct neural tissue, while mesenchymal stem cells (MSCs) provide support and secrete beneficial factors. Combining NSCs and MSCs in stem cell therapy may synergistically enhance ACI recovery, potentially via the regulation of VEGF and bFGF. However, the mechanisms underlying this combined approach remain unclear.
To investigate the therapeutic effect of combined NSC and MSC transplantation on neurological recovery and bFGF/VEGF expression in ACI patients.
This study enrolled 156 patients with ACI treated from June 2022 to June 2023. Patients were randomly assigned to two groups: The control group (n = 78) received conventional drug therapy, while the observation group (n = 78) received conventional therapy and combined NSC and MSC transplantation. The following outcomes were compared between groups: National Institutes of Health Stroke Scale (NIHSS) score, Barthel index, cerebral perfusion and diffusion on magnetic resonance imaging, serum bFGF and VEGF levels, clinical efficacy, and adverse events.
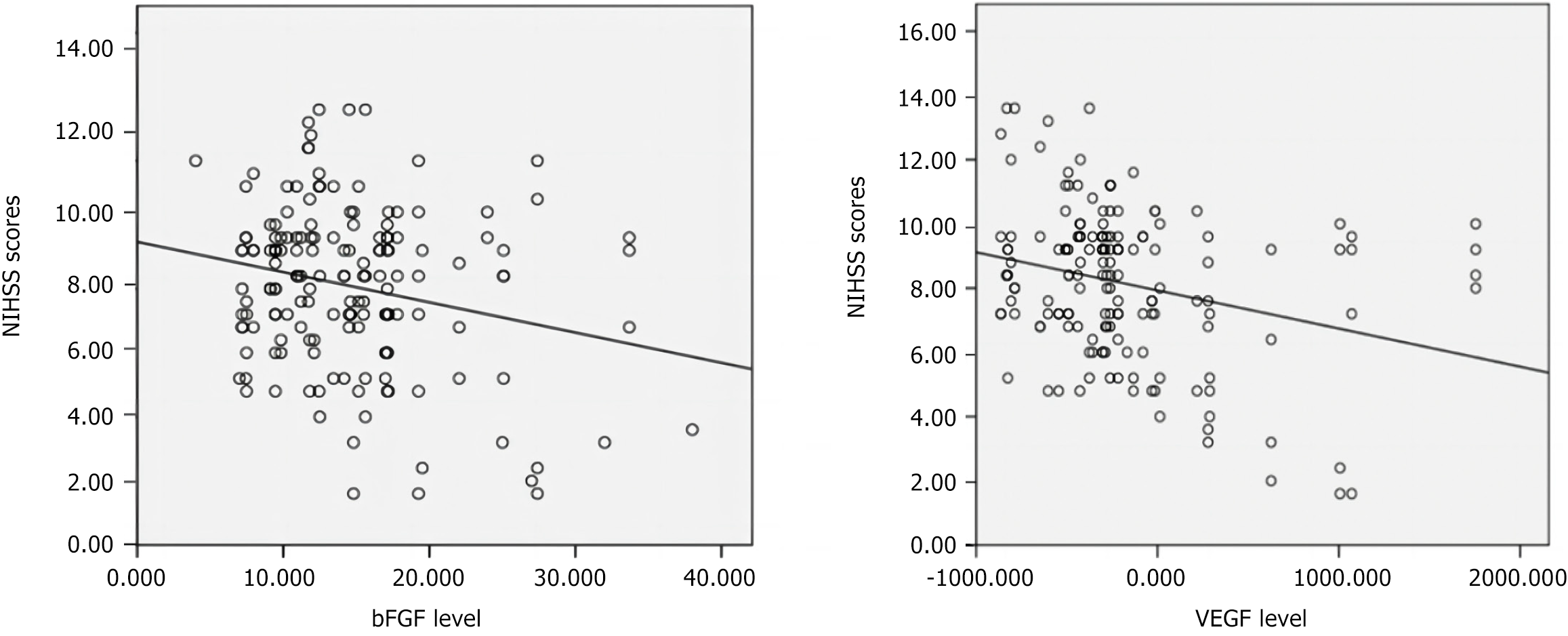
Serum VEGF and bFGF levels negatively correlated with NIHSS scores in patients with ACI (r = -0.388, r = -0.239; P < 0.05). The observation group (NSC and MSC) showed a significantly higher clinical efficacy of treatment than the controls (85.9% vs 69.2%; P < 0.05). Both groups showed improved cerebral perfusion, increased Barthel index, and decreased NIHSS scores post-treatment (P < 0.05), with significantly greater improvements in the observation group. Serum VEGF and bFGF levels increased significantly in both groups (P < 0.05), but were higher in the observation group. Adverse events in the observation group (transient fever: 4 cases; agitation: 1 case; headache: 2 cases) were mild and resolved with symptomatic treatment. Six-month follow-up revealed no abnormalities in magnetic resonance imaging, electrocardiogram, or blood tests.
NSC-MSC combination therapy enhances neurological function and cerebral perfusion in patients with ACI by upregulating VEGF and bFGF expression, demonstrating favorable clinical efficacy and safety.
Core Tip: Combination therapy with neural stem cell (NSC) and mesenchymal stem cell (MSC) promotes neurological recovery in patients with acute cerebral infarction (ACI) by modulating vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) expression, thereby enhancing cerebral perfusion, daily living capacity, and neurological function scores. This study validated the efficacy and safety of NSC/MSC stem cell combination therapy, and systematically elucidated the mechanistic roles of VEGF and bFGF in the NSC-MSC-mediated treatment of ACI. These findings further demonstrate the central regulatory roles of VEGF and bFGF in angiogenesis and neural repair, thereby advancing the application of precision medicine in ACI treatment, and providing a robust theoretical foundation and future directions for clinical translation.
- Citation: Yang T, Yu H, Han D, Xie Z. Combined mesenchymal and neural stem cell therapy enhances neurological recovery in cerebral infarction. World J Stem Cells 2025; 17(9): 110663
- URL: https://www.wjgnet.com/1948-0210/full/v17/i9/110663.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v17.i9.110663
Acute cerebral infarction (ACI) is one of the leading causes of death and long-term disability in adults worldwide. This condition is characterized by high morbidity, disability, and mortality, and has a complex pathogenesis involving localized ischemia, hypoxia, and neuronal damage following cerebrovascular occlusion[1]. Conventional therapies, such as thrombolysis and mechanical thrombectomy, partially restore cerebral blood flow; however, their narrow therapeutic time window and incomplete neurological recovery in certain patients underscore the urgent need for novel therapeutic strategies[2]. Neurorestoration remains a critical goal in ACI treatment. However, the post-infarction pathological microenvironment, which is characterized by inflammation, oxidative stress, and apoptosis, severely limits its efficacy. Emerging evidence highlights the dual roles of vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) in angiogenesis and neuroprotection. These factors enhance neovascularization, ameliorate ischemia, suppress neuronal apoptosis, and promote axonal regeneration[3,4]. Therapeutic applications of stem cells include reshaping treatment paradigms for neurodegenerative diseases, which marks an obvious evolutionary step in neurological medicine. Neural stem cells (NSCs) with self-renewal and multipotent differentiation capabilities can regenerate neurons, astrocytes, and oligodendrocytes, and repair damaged neural networks[5]. However, posttransplant survival and functional integration are hindered by hostile microenvironmental conditions. Mesenchymal stem cells (MSCs), owing to their accessibility, immunomodulatory properties, and robust secretion of trophic factors, MSCs have been proposed as synergistic partners to enhance NSC-based therapies[6]. MSCs activate the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT) pathway (through the release of paracrine VEGF), inhibit neuronal apoptosis, and promote angiogenesis. NSC secretion of bFGF drives neural differentiation via the mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) pathway. The combination of VEGF and bFGF may improve blood perfusion and nerve regeneration by synergistically upregulating VEGF/bFGF. The combined application of NSCs and MSCs may leverage their complementary roles in differentiation, paracrine signaling, and immunomodulation to achieve synergistic therapeutic effects in ACI. Although previous studies have explored the individual contributions of NSCs and MSCs in ACI treatment, the mechanisms underlying their combined therapy, particularly through VEGF and bFGF regulation, remain poorly understood. This study aimed to investigate the therapeutic mechanisms of NSC-MSC co-transplantation in ACI by focusing on the modulation of VEGF and bFGF expression, thereby providing critical insights into clinical translation.
A total of 156 patients with ACI admitted to Affiliated Hospital of Jiangnan University between June 2022 and June 2023 were enrolled and randomly allocated to either the observation group (78 patients) or the control group (78 patients). The control group comprised 40 males and 38 females, aged 35-77 years (57.5 ± 6.5 years), with a disease duration of 24.5 ± 9.3 hours and body mass index (BMI) of 23.1 ± 2.9 kg/m2. The observation group included 44 males and 34 females, aged 37-74 years (59.0 ± 7.8 years), with a disease duration of 23.9 ± 8.2 hours and BMI of 22.9 ± 2.7 kg/m2. The baseline characteristics (sex, age, disease duration, BMI, education level, and marital status) were not significantly different between the groups (P > 0.05; Table 1). Ethical clearance for this research was granted by the Institutional Review Board, and all study participants or their statutory guardians provided documented consent prior to enrollment.
| Groups | Observation group | Control group | Statistical value | P value |
| N | 78 | 78 | ||
| Gender | 0.413 | 0.521 | ||
| Male | 44 | 40 | ||
| Female | 34 | 38 | ||
| Age (years) | 59.0 ± 7.8 | 57.5 ± 6.5 | 1.148 | 0.253 |
| BMI (kg/m2) | 22.9 ± 2.7 | 23.1 ± 2.9 | 0.444 | 0.657 |
| Disease duration (hour) | 23.9 ± 8.2 | 24.5 ± 9.3 | 0.439 | 0.661 |
| Education level | 1.000 | 0.606 | ||
| Junior high school and below | 28 | 31 | ||
| High school and technical secondary school | 38 | 32 | ||
| College degree or above | 12 | 15 | ||
| Marital status | 3.794 | 0.150 | ||
| Unmarried | 0 | 3 | ||
| Married | 71 | 65 | ||
| Bereavement | 7 | 10 |
The inclusion criteria were as follows: (1) Fulfilling the diagnostic requirements for acute ischemic stroke as per the 2023 edition of the National Clinical Practice Guidelines developed by the Neurology Branch of the Chinese Medical Association[7]; (2) Patient younger than 80 years old with first-episode cerebral infarction; (3) Cerebral infarction lesions were confirmed by magnetic resonance imaging (MRI) or computed tomography; (4) The time from onset to admission was less than 72 hours; and (5) The patient had good treatment compliance, voluntarily participated in the study, fully understood the purpose, process, potential risks, and benefits of the study, signed the informed consent, and promised to participate in the follow-up on time.
The exclusion criteria were as follows: (1) History of cerebral hemorrhage or intracranial space-occupying lesions; (2) Death from hemorrhagic infarction; (3) Immune system diseases; (4) Hematological diseases; (5) Malignant tumors; (6) Severe liver function damage; and (7) History of thrombolytic therapy.
All study participants underwent conventional therapeutic protocols for acute ischemic cerebrovascular events, as stipulated in the 2023 edition of China’s National Clinical Practice Guidelines developed by the Stroke Expert Panel. These include blood pressure control, intracranial pressure reduction, blood glucose management, lipid-lowering therapy, vascular protection, neuroprotection, body temperature control, antiplatelet therapy, anticoagulation therapy, infection prevention, nutritional support, and rehabilitation therapy. The control group received only the standard treatment, whereas the observation group received NSCs combined with MSCs based on the standard treatment. At the time of transplantation, treatment was initiated in the acute phase (within 7 days after onset), and consolidation treatment was administered in the subacute phase (7-30 days after onset). For NSCs treatment (Cellonis Biotechnologies Co. Ltd., Beijing, China), NSCs differentiated from embryonic stem cells were injected intrathecally once a week, 5 × 106/time, through a lumbar puncture, and NSCs were injected into the cerebrospinal fluid to promote widespread distribution in the central nervous system. MSCs (Cellonis Biotechnologies Co. Ltd., Beijing, China) were derived from umbilical cord MSCs, and high-activity MSCs were selected after umbilical cord blood culture, amplification, and passage in vitro. After detection of surface markers (such as CD73+, CD90+, CD105+, CD34-, and CD45-) and functional tests (such as the ability to secrete cytokines), MSCs were injected into the cerebrospinal fluid via lumbar puncture to directly affect the central nervous system by intrathecal injection once a week, 1 × 107/time. NSCs and MSCs were treated at different time intervals. NSCs were treated in the first week and MSCs in the second week. The course of treatment was four weeks, with a total of three courses of treatment. The interval between treatment courses was 3 months. Immunomodulatory drugs were administered to prevent transplant rejection. Vital signs and brain images were closely monitored and complications were prevented and controlled. All patients were followed up for 6 months and regularly reviewed and evaluated every 3 months, including head imaging (MRI or computed tomography), electrocardiography, blood biochemistry, and routine blood tests.
To assess neurological impairment trajectories, the standardized National Institutes of Health Stroke Scale (NIHSS)[8] protocol was implemented for baseline and outcome measurements in stroke patients undergoing clinical intervention. The scale assesses the level of consciousness, language, visual field, motor, sensory, ataxia, and other nervous system functions with 11 items and scores ranging from 0 to 42 points, with numerical values directly correlated with the severity of neurological dysfunction. Neurological function was evaluated using the Barthel index (BI)[9] before and after treatment. The BI includes 10 basic daily activities, and each item is assigned a different score according to the patient’s independence in completing the task and the degree of auxiliary needs, with a total score of 0-100 points. Higher scores indicate stronger self-care abilities. Before and after treatment, the cerebral blood perfusion status was evaluated using brain magnetic resonance perfusion imaging and diffusion imaging to reflect hemodynamic changes in the cerebral ischemic area. A gadolinium-based contrast agent (dose: 0.1 mmol/kg) was rapidly injected intravenously at a rate of approximately 3-5 mL/second, and 20 mL of normal saline was injected immediately after injection. Fast gradient-recalled echo sequence scanning was used to continuously collect T2-weighted signal changes and cerebral blood flow data in the region of interest during the scanning process.
The clinical efficacy of treatment was evaluated based on the NIHSS score and the changes in clinical symptoms[10], and were defined as follows. Recovery: After treatment, the NIHSS score was reduced by more than 90%, the patient’s neurological function completely recovered without neurological function defects, and he could take care of himself without any sequelae. Markedly effective: After treatment, the NIHSS score was reduced by 60%-90%, and the neurological function defect of the patient recovered with mild residual symptoms, but daily life was not affected. Effective: After treatment, NIHSS score was reduced by 20%-60%, and neurological function improved; however, some functional defects or symptoms persisted. Patients may require assistance with activities of daily living. Ineffective: After treatment, NIHSS score decreased by < 20%, did not change, or even worsened, the neurological function defect of the patient did not improve, and the ability to live was still limited. Total effective rate = (recovered + markedly effective + effective) cases/total cases × 100%.
Pre- and post-treatment venous samples (5 mL) were obtained under fasting conditions and immediately anticoagulated with an EDTA solution. The plasma supernatant was isolated via ultracentrifugation at 15000 rpm for 15 minutes, followed by cryopreservation at -80 °C until analysis. VEGF/bFGF concentrations were quantified using commercial enzyme-linked immunosorbent assay kits processed on a Thermo Scientific Multiskan FC Microplate Photometer following the manufacturer’s protocols.
The related adverse reactions of both groups were analyzed.
Data were analyzed using SPSS 24.0. Continuous variables are expressed as (t-test); categorical variables as counts (%) (χ2 test). Pearson’s correlation analysis was used to analyze VEGF/bFGF-NIHSS relationship. Statistical significance was set at P < 0.05.
The correlation between serum VEGF/bFGF expression and NIHSS score is presented in Figure 1. Pearson correlation analysis revealed obvious negative correlations between serum VEGF/bFGF levels and NIHSS scores in patients with ACI (r = -0.388, r = -0.239, P < 0.05), indicating that higher VEGF and bFGF expression was associated with milder neurological deficits. As shown in Table 2, the observation group exhibited a significantly higher total effective rate than to control (85.9% vs 69.2%, P < 0.05). As shown in Table 3, no obvious differences in baseline cerebral perfusion, BI, or NIHSS scores were observed between the two groups (P > 0.05). After post-treatment, both groups showed improvements: Cerebral perfusion and BI scores increased (P < 0.05), whereas NIHSS scores decreased (P < 0.05). Notably, the observation group demonstrated superior outcomes compared to the control group, with higher cerebral perfusion and BI scores, and lower NIHSS scores (P < 0.05). As shown in Table 4, baseline serum VEGF and bFGF levels were comparable between the two groups (P > 0.05). After treatment, the two groups exhibited significant increases in bFGF and VEGF levels (P < 0.05), with the observation group showing markedly higher levels than control (P < 0.05). In the observation group, four patients experienced transient fever (temperature < 38 °C, duration ≤ 3 days) following stem cell transplantation. One patient exhibited agitation, and two reported headaches, all of which resolved with symptomatic treatment. No abnormalities on cranial MRI, electrocardiography, or blood biochemical indices were detected in either group during the 6-month follow-up period.
| Group | Control group | Observation group | χ2 | P value |
| N | 78 | 78 | ||
| Clinical efficacy | 6.225 | 0.013 | ||
| Recovered | 12 (15.4) | 6 (7.7) | ||
| Markedly effective | 18 (23.1) | 13 (16.6) | ||
| Effective | 37 (47.4) | 35 (44.9) | ||
| Ineffective | 11 (14.1) | 24 (30.8) | ||
| Efficiency | 67 (85.95) | 54 (69.2) |
| Group | Control group | Observation group | t | P value |
| N | 78 | 78 | ||
| Cerebral perfusion volume (%) | ||||
| Before | 45.1 ± 11.3 | 47.9 ± 12.4 | 1.469 | 0.144 |
| After | 52.0 ± 10.4 | 61.5 ± 16.3 | 4.341 | 0.000a |
| Barthel index | ||||
| Before | 39.2 ± 8.4 | 41.0 ± 7.1 | 1.443 | 0.151 |
| After | 58.1 ± 12.3 | 66.7 ± 10.1 | 4.758 | 0.000a |
| NIHSS scores | ||||
| Before | 10.8 ± 2.9 | 10.2 ± 3.2 | 1.151 | 0.252 |
| After | 7.1 ± 0.9 | 6.5 ± 1.3 | 3.237 | 0.001a |
ACI is defined as an ischemic brain injury caused by a sudden interruption or reduction in the blood supply to the brain. It has high disability and mortality rates and is a global medical problem. Its main pathological features include vascular endothelial dysfunction, neuronal death, and limited neural network remodeling. Currently, the conventional treatment for ACI mainly involves vascular recanalization and brain protection; however, there are still many limitations in promoting the recovery of neural function.
In recent years, as a new biological therapy strategy, stem cell therapy has been widely applied in the research of a variety of nervous system diseases. Among these, NSCs, which can directly differentiate into neurons and glial cells, are considered an ideal cell source for nerve repair and show great application prospects for repairing nerve injury and remodeling neural networks. Xiong et al[11] found that neurons derived from human stem cells can repair damaged circuits and restore neural functions. MSCs provide neuroprotection through the secretion of growth factors and immunomodulation. Ye et al[12] performed in vitro cell experiments and confirmed that MSC could repair sciatic nerve injury by upregulating c-Jun and glial cell line-derived neurotrophic factor expression. The combination of NSCs and MSCs can improve the cerebral ischemic microenvironment, promote angiogenesis, reduce inflammatory reactions and neural network repair, and enhance therapeutic effects via synergistic mechanisms. VEGF and bFGF are key angiogenic and neuroprotective factors. During cerebral infarction repair, VEGF can improve the microenvironment of the ischemic area by promoting angiogenesis and increasing cerebral blood flow perfusion, whereas bFGF plays an important role in neuronal survival, axonal regeneration, and recovery of neural function. However, systematic research on the expression levels of VEGF and bFGF is lacking, especially in the treatment of ACI in stem cells. This study aimed to investigate the therapeutic efficacy of combined NSC and MSC transplantation in patients with ACI through cellular therapy techniques. By analyzing the regulatory effects on serum expression levels of VEGF and bFGF, we systematically evaluated clinical outcome measures, including NIHSS scores, BI, and cerebral perfusion changes, which further elucidated the underlying mechanisms of this combinatorial therapy and provided a theoretical foundation for optimizing treatment strategies for ACI. These findings will contribute to the development of evidence-based approaches for neurorestorative interventions in stroke management.
MSCs have a strong paracrine ability due to their ability to secrete a variety of trophic and immunomodulatory factors, among which VEGF (an important pro-angiogenic factor) and bFGF are both important. Therefore, the combination of NSCs and MSCs can more effectively regulate the expression of key factors (VEGF and bFGF) in the ischemic microenvironment through synergistic or additive effects, thereby promoting angiogenesis, neuroprotection and functional recovery. In this study, our results showed that the levels of serum VEGF and bFGF in patients with ACI were negatively correlated with NIHSS score, indicating that VEGF and bFGF play important roles in improving neurological deficits. VEGF improves cerebral perfusion by promoting angiogenesis and blood supply in ischemic areas, whereas bFGF plays a key role in protecting injured neurons, promoting neuronal regeneration, and regulating neural network repair. Patients in the observation group received NSCs and MSCs combined treatment based on the conventional comprehensive treatment. The results showed that the effectiveness rate in the observation group was higher than that in the control group, and the improvement in cerebral blood flow perfusion, nerve function defects, and daily living ability in the observation group after treatment was better than that in the control group. After treatment, the levels of VEGF and bFGF in the two groups were obviously increased, and the combined treatment with NSCs and MSCs had a more obvious effect in promoting the expression of VEGF and bFGF related factors, suggesting that NSCs combined with MSCs treatment obviously increased the expression levels of VEGF and bFGF in patients with ACI, increased the blood perfusion of infarct lesions, was conducive to the repair of nerve injury, and improved the clinical efficacy. This analysis showed that the paracrine effects of MSCs and NSCs were a key factor in improving the levels of VEGF and bFGF. They not only directly secrete bFGF but also indirectly regulate the secretion of bFGF by other cells to promote tissue repair and regeneration[13,14]. MSCs can promote angiogenesis, release large amounts of VEGF through a paracrine mechanism, and improve blood supply to the ischemic area. Hypoxia-inducible factor-1 alpha pathway is activated under hypoxic conditions to enhance the transcription and expression of VEGF and promote angiogenesis in ischemic areas[15]. After transplantation, NSCs differentiate into neurons and astrocytes, providing structural support for tissue repair. Simultaneously, pro-angiogenic and nutritional factors secreted by NSCs can accelerate the secretion of VEGF by enhancing the survival and function of MSCs. Gu et al[16] pointed out that NSCs enhanced the expression of bFGF through the MAPK/ERK pathway, which was consistent with a previous analysis. Synergy between stem cells: VEGF and bFGF secreted by MSCs provide a good environment for NSCs’ survival and differentiation and enhance the survival rate of NSCs in the brain tissue. VEGF can improve local ischemia by activating the proliferation and migration of endothelial cells and promoting vascular remodeling in infarcted areas. An improved blood supply provides more oxygen and nutrients to the hypoxic nerve cells and accelerates their functional recovery. Simultaneously, the upregulation of VEGF expression can activate the PI3K/AKT signaling pathway, inhibit neuronal apoptosis, and enhance the tolerance of brain tissue to ischemic injury[17,18]. Moreover, the increase of VEGF release can increase the permeability of blood-brain barrier, and moderately increase the permeability of blood-brain barrier in the short term, which is conducive to the elimination of metabolic waste and anti-inflammatory cell infiltration, and create a good environment for nerve repair. bFGF enhances neuronal proliferation and differentiation by activating the MAPK and ERK pathways and promoting neural network reconstruction[19]. bFGF cooperates with VEGF to stimulate the proliferation of vascular smooth muscle and endothelial cells, further improving local blood perfusion. bFGF can reduce secondary injury by reducing the release of pro-inflammatory factors, inhibiting the inflammatory response in the infarct area, reducing the expression of pro-inflammatory factors in the brain tissue, improving the microenvironment, and reducing nerve cell apoptosis[20]. bFGF can promote the differentiation of glial cells and neurons, maintain the survival and functional stability of glial cells, prevent overactivation in the pathological state, reduce damage to neurons, and accelerate the reconstruction of neural networks. During the follow-up of adverse reactions of the patients during treatment, four patients in the observation group had transient fever, one patient had irritability, and two had headaches, which were relieved after symptomatic treatment. No serious adverse reactions were observed, indicating that the combination of NSCs and MSCs is safe. In addition, during the follow-up of 6 months, no abnormalities were found in the brain MRI, electrocardiogram, or blood biochemical indices of the two groups, further verifying the long-term safety of stem cell therapy.
This study has several limitations. First, the sample size was small and the number of patients included was limited, which may have influence the statistical reliability and universal applicability of the results. Second, this study primarily observed short-term improvements in neurological function, and did not evaluate the impact of stem cell therapy on the long-term prognosis of patients. However, the expression levels of VEGF and bFGF were clearly upregulated, and their specific pathways and relative contributions to combination therapy require further investigation. In the future, we will continue to verify the activation of different molecular pathways (such as PI3K/AKT and MAPK/ERK) at the cellular level to better analyze the VEGF/bFGF pathway and employ a combined treatment strategy of NSCs and MSCs, which failed to clearly distinguish the respective relative contributions of NSCs and MSCs in promoting the upregulation of VEGF/bFGF expression. Future studies are required to design more elaborate experiments (such as separate transplantation groups and conditioned medium studies) to decipher the specific role of each cell type in patients with ACI, stroke severity (NIHSS baseline score), and infarct location (e.g., cortex vs deep), these factors may significantly affect the therapeutic effect. Subgroup analyses based on these key variables were not performed in this study, and future studies with larger samples should allow stratified analyses to determine the efficacy of combined stem cell therapy in different patient subgroups. Furthermore, NSCs need to be induced by embryonic stem cells, and MSCs need to be expanded in vitro, which involves strict quality control standards. The operational complexity of multiple intrathecal transplantations (3 courses) may influence their large-scale promotion. In the future, it will be necessary to optimize the stem cell mass production process, explore a single drug administration strategy, and conduct a health-economic evaluation. In conclusion, NSCs combined with MSCs can improve neurological function and cerebral blood flow perfusion in patients with ACI by promoting the expression of VEGF and bFGF and improving clinical efficacy with good safety.
This study showed that NSCs combined with MSCs promoted the recovery of neurological function in patients with ACI by regulating the expression of VEGF and bFGF. However, to further verify its efficacy and safety, future studies should expand the sample size, extend the follow-up time, and explore the specific mechanisms through animal experiments and molecular biology. This study provides a solid scientific basis for the clinical applications of stem cell therapy.
| 1. | Li C, Xu BF, Zhang M, Song YM, Liu R. Severe Thrombocytopenia with Acute Cerebral Infarction: A Case Report and Literature Review. Niger J Clin Pract. 2023;26:1040-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 2. | Huo X, Ma G, Tong X, Zhang X, Pan Y, Nguyen TN, Yuan G, Han H, Chen W, Wei M, Zhang J, Zhou Z, Yao X, Wang G, Song W, Cai X, Nan G, Li D, Wang AY, Ling W, Cai C, Wen C, Wang E, Zhang L, Jiang C, Liu Y, Liao G, Chen X, Li T, Liu S, Li J, Gao F, Ma N, Mo D, Song L, Sun X, Li X, Deng Y, Luo G, Lv M, He H, Liu A, Zhang J, Mu S, Liu L, Jing J, Nie X, Ding Z, Du W, Zhao X, Yang P, Liu L, Wang Y, Liebeskind DS, Pereira VM, Ren Z, Wang Y, Miao Z; ANGEL-ASPECT Investigators. Trial of Endovascular Therapy for Acute Ischemic Stroke with Large Infarct. N Engl J Med. 2023;388:1272-1283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 490] [Cited by in RCA: 607] [Article Influence: 202.3] [Reference Citation Analysis (0)] |
| 3. | Denninger JK, Miller LN, Walters AE, Hosawi M, Sebring G, Rieskamp JD, Ding T, Rindani R, Chen KS, Senthilvelan S, Volk A, Zhao F, Askwith C, Kirby ED. Neural stem and progenitor cells support and protect adult hippocampal function via vascular endothelial growth factor secretion. bioRxiv. 2023;2023.04.24.537801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Meng XT, Du YS, Dong ZY, Wang GQ, Dong B, Guan XW, Yuan YZ, Pan H, Wang F. Combination of electrical stimulation and bFGF synergistically promote neuronal differentiation of neural stem cells and neurite extension to construct 3D engineered neural tissue. J Neural Eng. 2020;17:056048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Fernandez-Muñoz B, Garcia-Delgado AB, Arribas-Arribas B, Sanchez-Pernaute R. Human Neural Stem Cells for Cell-Based Medicinal Products. Cells. 2021;10:2377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 6. | Mishra VK, Shih HH, Parveen F, Lenzen D, Ito E, Chan TF, Ke LY. Identifying the Therapeutic Significance of Mesenchymal Stem Cells. Cells. 2020;9:1145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 111] [Article Influence: 18.5] [Reference Citation Analysis (1)] |
| 7. | Liu L, Li Z, Zhou H, Duan W, Huo X, Xu W, Li S, Nie X, Liu H, Liu J, Sun D, Wei Y, Zhang G, Yuan W, Zheng L, Liu J, Wang D, Miao Z, Wang Y. Chinese Stroke Association guidelines for clinical management of ischaemic cerebrovascular diseases: executive summary and 2023 update. Stroke Vasc Neurol. 2023;8:e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 64] [Reference Citation Analysis (0)] |
| 8. | Kwah LK, Diong J. National Institutes of Health Stroke Scale (NIHSS). J Physiother. 2014;60:61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 481] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 9. | Aminalroaya R, Mirzadeh FS, Heidari K, Alizadeh-Khoei M, Sharifi F, Effatpanah M, Angooti-Oshnari L, Fadaee S, Saghebi H, Hormozi S. The Validation Study of Both the Modified Barthel and Barthel Index, and Their Comparison Based on Rasch Analysis in the Hospitalized Acute Stroke Elderly. Int J Aging Hum Dev. 2021;93:864-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Hwang J, Lee MJ, Chung JW, Bang OY, Kim GM, Chung CS, Lee KH. NIHSS sub-item scores predict collateral flow in acute middle cerebral artery infarction. Interv Neuroradiol. 2018;24:678-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Xiong M, Tao Y, Gao Q, Feng B, Yan W, Zhou Y, Kotsonis TA, Yuan T, You Z, Wu Z, Xi J, Haberman A, Graham J, Block J, Zhou W, Chen Y, Zhang SC. Human Stem Cell-Derived Neurons Repair Circuits and Restore Neural Function. Cell Stem Cell. 2021;28:112-126.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 126] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 12. | Ye F, Li H, Qiao G, Chen F, Tao H, Ji A, Hu Y. Platelet-rich plasma gel in combination with Schwann cells for repair of sciatic nerve injury. Neural Regen Res. 2012;7:2286-2292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 13. | Li H, Gan X, Pan L, Zhang Y, Hu X, Wang Z. EGF/bFGF promotes survival, migration and differentiation into neurons of GFP-labeled rhesus monkey neural stem cells xenografted into the rat brain. Biochem Biophys Res Commun. 2022;620:76-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 14. | Huang L, Sun X, Wang L, Pei G, Wang Y, Zhang Q, Liang Z, Wang D, Fu C, He C, Wei Q. Enhanced effect of combining bone marrow mesenchymal stem cells (BMMSCs) and pulsed electromagnetic fields (PEMF) to promote recovery after spinal cord injury in mice. MedComm (2020). 2022;3:e160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Ni H, Li J, Zheng J, Zhou B. Cardamonin attenuates cerebral ischemia/reperfusion injury by activating the HIF-1α/VEGFA pathway. Phytother Res. 2022;36:1736-1747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 16. | Gu Y, Xue C, Zhu J, Sun H, Ding F, Cao Z, Gu X. Basic fibroblast growth factor (bFGF) facilitates differentiation of adult dorsal root ganglia-derived neural stem cells toward Schwann cells by binding to FGFR-1 through MAPK/ERK activation. J Mol Neurosci. 2014;52:538-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Dai W, Yang H, Xu B, He T, Liu L, Ma X, Ma J, Yang G, Si R, Pei X, Du X, Fu X. Human umbilical cord-derived mesenchymal stem cells (hUC-MSCs) alleviate excessive autophagy of ovarian granular cells through VEGFA/PI3K/AKT/mTOR pathway in premature ovarian failure rat model. J Ovarian Res. 2023;16:198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 18. | Wang HJ, Ran HF, Yin Y, Xu XG, Jiang BX, Yu SQ, Chen YJ, Ren HJ, Feng S, Zhang JF, Chen Y, Xue Q, Xu XY. Catalpol improves impaired neurovascular unit in ischemic stroke rats via enhancing VEGF-PI3K/AKT and VEGF-MEK1/2/ERK1/2 signaling. Acta Pharmacol Sin. 2022;43:1670-1685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 86] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 19. | Chang Y, Dagat LA, Yusuf A, Zahriya Y, Staputyte K, Worley E, Holt A, Canuteson N, Messieha V, Halila K. Regulation of bFGF-induced effects on rat aortic smooth muscle cells by β(3)-adrenergic receptors. Curr Res Pharmacol Drug Discov. 2022;3:100094. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 20. | Chakrabarti S, Mazumder B, Rajkonwar J, Pathak MP, Patowary P, Chattopadhyay P. bFGF and collagen matrix hydrogel attenuates burn wound inflammation through activation of ERK and TRK pathway. Sci Rep. 2021;11:3357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |