Published online Sep 26, 2025. doi: 10.4252/wjsc.v17.i9.106381
Revised: May 16, 2025
Accepted: August 4, 2025
Published online: September 26, 2025
Processing time: 187 Days and 12.8 Hours
Non-small cell lung cancer (NSCLC) is the most prevalent subtype of lung cancer, accounting for approximately 85% of all lung cancer cases and remaining a major cause of cancer-related mortality worldwide. Despite advances in diagnostic and therapeutic approaches, the incidence and mortality rates of NSCLC continue to rise, especially in low-income and middle-income countries.
To investigate the expression of cancer stem cell (CSC) markers and their rela
A retrospective analysis was conducted on the clinical data and survival follow-up information of 61 patients with stage IIIA NSCLC treated at our hospital from February 2020 to June 2022, and all cases were confirmed as primary (non-recurrent) diagnoses based on clinical and pathological records. All patients were followed up through outpatient visits or telephone interviews. The follow-up duration ranged from 6 to 51 months with a median follow-up time of 36 months. Overall survival (OS) was defined as the time from the date of pathological diagnosis to death or the last follow-up. Univariate and multivariate Cox regre
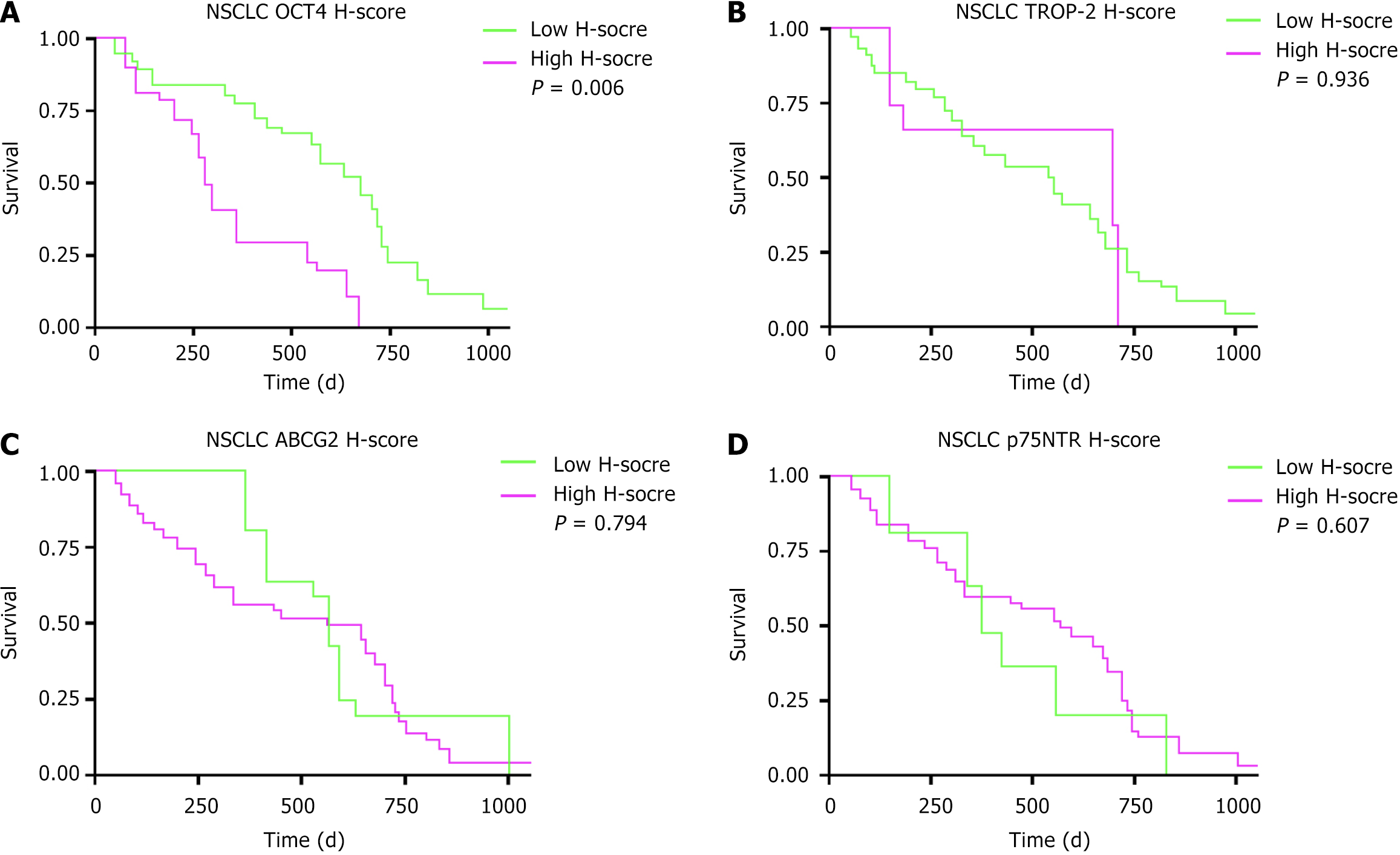
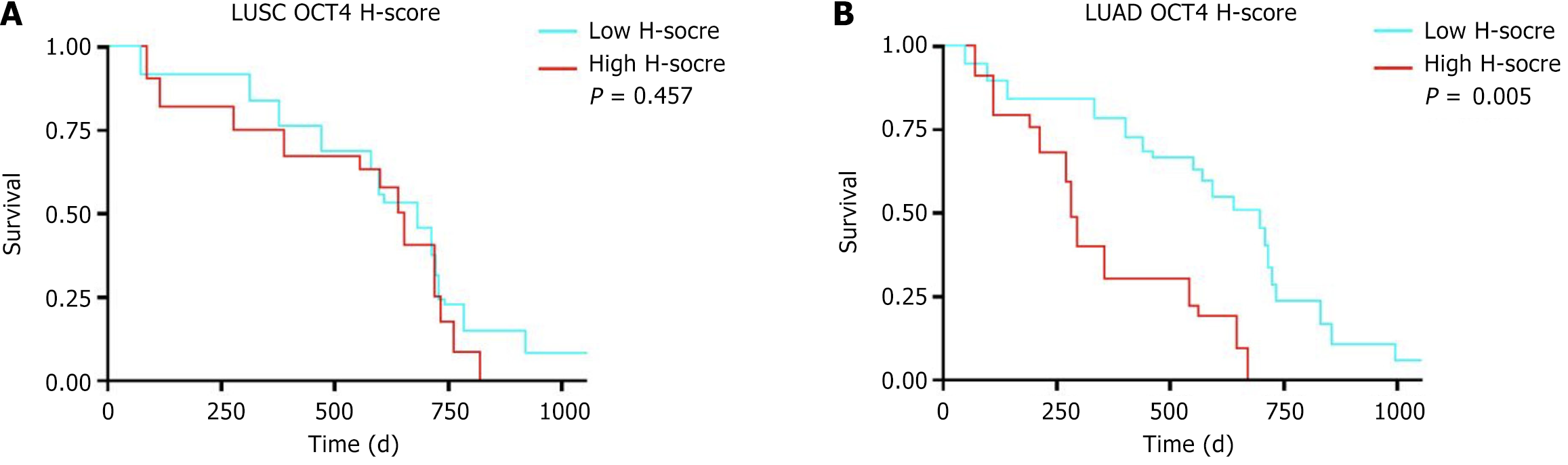
Multivariate Cox regression analysis showed that age [hazard ratio (HR) = 1.952, 95% confidence interval (CI): 1.087-2.481, P = 0.029] and micropapillary components (HR = 2.716, 95%CI: 1.259-5.837, P = 0.013) were significantly associated with OS. In NSCLC there were 21 cases with high OCT4 H-scores, 27 cases with high TROP-2 H-scores, 44 cases with high ABCG2 H-scores, and 44 cases with high p75NTR H-scores. In the survival analysis the high OCT4 expression group had a poorer prognosis (P = 0.006). Further subtype analysis revealed no statistically significant difference in OS between high and low OCT4 H-score groups in patients with lung squamous cell carcinoma (P = 0.457). However, in patients with lung adenocarcinoma high OCT4 expression had significantly poorer OS compared with those with low OCT4 expression (P = 0.005). TROP-2, ABCG2, and p75NTR did not significantly affect the prognosis. TSI was significantly associated with OS in patients with NSCLC (HR = 2.209, 95%CI: 1.238-3.681, P = 0.027).
Age and micropapillary components were related to OS in patients with stage IIIA NSCLC. High expression of OCT4 and high TSI were associated with poor prognosis.
Core Tip: In patients with stage IIIA non-small cell lung cancer, older age and the presence of micropapillary components were significantly associated with worse overall survival, indicating their potential role in risk stratification. Furthermore, high expression of octamer-binding transcription factor 4 and elevated mRNA expression-based stemness index, both markers of tumor stemness, were closely linked to poor prognosis. These factors may serve not only as important prognostic indicators but also as potential therapeutic targets, contributing to more personalized and effective treatment strategies in clinical practice.
- Citation: Lin T, Jiang SC, He XM, Xu WZ, Jin CJ, Guo YD. Expression of cancer stem cell markers and their prognostic significance in stage IIIA non-small cell lung cancer. World J Stem Cells 2025; 17(9): 106381
- URL: https://www.wjgnet.com/1948-0210/full/v17/i9/106381.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v17.i9.106381
Non-small cell lung cancer (NSCLC) is the most prevalent subtype of lung cancer, accounting for approximately 85% of all lung cancer cases and remaining a major cause of cancer-related mortality worldwide. Despite advances in diagnostic and therapeutic approaches, the incidence and mortality rates of NSCLC continue to rise, especially in low-income and middle-income countries[1]. Among its clinical stages stage IIIA NSCLC represents a particularly challenging subset characterized by involvement of ipsilateral mediastinal lymph nodes without distant metastasis[2]. Due to its heterogeneity in tumor burden, lymph node spread, and surgical resectability, the optimal treatment strategy remains controversial, and the long-term prognosis is poor with 5-year survival rates ranging between 15% and 30% depending on individual patient and tumor characteristics[3].
A growing body of evidence suggests that tumor heterogeneity and the presence of cancer stem cells (CSCs) are major contributors to therapeutic resistance, tumor recurrence, and metastasis in NSCLC[4,5]. CSCs are a subpopulation of tumor cells with stem-like properties, such as self-renewal and multi-lineage differentiation, and they are capable of driving tumor initiation, sustaining growth, and evading conventional therapies[6]. In the tumor microenvironment CSCs can remain quiescent, resist chemotherapy or radiotherapy, and later contribute to relapse, making them key targets for novel treatment strategies. Thus, exploring the biological significance of CSCs and identifying reliable CSC-associated biomarkers are crucial for improving prognostic assessment and tailoring individualized treatment plans.
Several markers have been identified to characterize CSCs in NSCLC, including octamer-binding transcription factor 4 (OCT4), trophoblast cell surface antigen-2 (TROP-2), ATP-binding cassette subfamily G member 2 (ABCG2), and p75 neurotrophin receptor (p75NTR). These markers have been implicated in stemness maintenance, drug efflux, epithelial-mesenchymal transition, and modulation of the tumor microenvironment[7-11]. The selection of these four markers in our study was based on their demonstrated relevance in NSCLC stemness features and availability of validated antibodies for immunohistochemical analysis.
For example, OCT4 is a well-known transcription factor essential for maintaining pluripotency and self-renewal in CSCs. TROP-2 is involved in cell proliferation and metastatic potential. ABCG2 mediates drug resistance through its role as an efflux transporter, and p75NTR is linked to cell survival and migration within the tumor microenvironment. While other established CSC markers such as CD133 and aldehyde dehydrogenase 1 have also been widely studied, their expression patterns and prognostic significance can vary significantly among NSCLC subtypes and stages. Furthermore, due to limitations in specimen availability and antibody validation within our retrospective cohort, these markers were not included in the current analysis but are recognized as important targets for future research.
Although numerous studies have explored the prognostic relevance of these markers in NSCLC, few have specifically focused on their expression patterns and clinical implications in patients with stage IIIA. Given the unique biological characteristics of stage IIIA NSCLC, it is imperative to examine the role of CSC markers in this subset more closely. In the present study we retrospectively analyzed tumor specimens from 61 patients with stage IIIA NSCLC to evaluate the expression levels of four CSC markers, OCT4, TROP-2, ABCG2, and p75NTR, using immunohistochemistry (IHC). We further proposed and calculated a composite tumor stemness index (TSI) by integrating the expression status of these markers with selected clinicopathological parameters. This study aimed to explore the relationship between CSC marker expression and overall survival (OS) and to assess the utility of TSI in stratifying prognosis for patients with stage IIIA NSCLC. This integrative approach will provide new insights into CSC biology and offer a potential tool for clinical decision-making in this complex disease stage.
A retrospective analysis was conducted on the clinical data and survival follow-up information of 61 patients with stage IIIA NSCLC treated at our hospital from February 2020 to June 2022, and all cases were confirmed as primary (non-recurrent) diagnoses based on clinical and pathological records. Inclusion criteria were: (1) Patients aged ≥ 18 years and < 80 years, regardless of gender; (2) Histopathologically confirmed stage IIIA NSCLC via surgical resection; (3) Complete clinical pathological data and follow-up information; (4) Tumor-node-metastasis staging evaluated according to the American Joint Committee on Cancer/tumor-node-metastasis classification system[12]; and (5) Patients and their families signed informed consent and were willing to participate in the study and accept follow-up. Exclusion criteria were: (1) Concomitant other malignant tumors; (2) Presence of severe comorbidities or other diseases affecting survival; (3) Patients with known prior treatment for lung cancer, recurrence, or distant metastasis at initial diagnosis were excluded to ensure cohort homogeneity; (4) Patients lost to follow-up for unknown reasons or with incomplete follow-up data, unable to obtain complete survival or prognostic information; and (5) Recent antitumor treatment.
Among the 61 patients 50 were male and 11 were female; the median age of the patients was 64 years with 25 patients aged ≤ 65 years and 36 patients aged ≥ 65 years. Overall, 29 cases were lung squamous cell carcinoma (LUSC), and 32 cases were lung adenocarcinoma (LUAD). All patients had completed OS endpoint follow-up with a median follow-up time of 20 months. This study was approved by the Medical Ethics Committee of the First Affiliated Hospital of Nanchang Medical College (approval number: FA240011), and the study strictly adhered to the ethical guidelines of the “Helsinki Declaration.”
IHC staining was used to detect the expression of CSC markers (OCT4, TROP-2, ABCG2, and p75NTR) in tumor tissue samples from patients with stage IIIA NSCLC. Formalin-fixed, paraffin-embedded tissues were cut into 4 μm sections, mounted on poly-L-lysine-coated slides, and baked at 60 °C for 2 h. Sections were dewaxed in xylene, rehydrated through graded ethanol, and subjected to antigen retrieval using sodium citrate buffer (pH = 6.0) in a pressure cooker at 121 °C for 2 min. After cooling endogenous peroxidase activity was blocked with 3% hydrogen peroxide for 10 min. Non-specific binding was blocked with 10% goat serum for 30 min at room temperature.
Primary antibodies against OCT4 (1:200), TROP-2 (1:100), ABCG2 (1:150), and p75NTR (1:200) (all from Abcam, United States) were applied and incubated overnight at 4 °C. After washing HRP-labeled goat anti-rabbit or anti-mouse secondary antibodies (1:500) were applied for 30 min at 37 °C. Immunoreactivity was visualized using DAB chromogen, followed by hematoxylin counterstaining. Slides were then dehydrated, cleared, and mounted using neutral resin. Positive controls (known marker-expressing tissues) and negative controls (omission of primary antibodies) were included in each staining run to ensure specificity and consistency.
In this study IHC staining results were independently evaluated by two senior pathologists who were blinded to the clinical and pathological data of the patients to ensure objectivity and consistency. In the event of discrepancies, a consensus was reached through discussion. Tumor sections were observed under a light microscope at × 200 magnification to assess marker expression. Staining intensity was scored based on an established 4-tier scale: 0 (negative, no staining); 1 (weak, light yellow); 2 (moderate, brown-yellow); and 3 (strong, dark brown), following the criteria reported by Robert et al[13]. In addition the percentage of positively stained tumor cells was estimated in representative high-power fields. The H-score was calculated using the formula: H-score = ∑(pixi) = (weak positive area percentage × 1) + (moderate positive area percentage × 2) + (strong positive area percentage × 3) with a total score range of 0-300. Based on preliminary data distribution and previous literature[14,15], an H-score < 5 was defined as low expression and ≥ 5 as high expression.
Image analysis was performed using Image-Pro Plus 6.0 (Media Cybernetics, MD, United States). All images were acquired under standardized microscope and camera settings. No tissue sections were excluded from analysis, and each sample was evaluated in at least three representative fields to reduce variability. Although we were unable to provide representative IHC images in the current version due to technical constraints, the scoring method described above has been applied in multiple peer-reviewed studies and is well validated.
All statistical analyses in this study were performed using R language (version 3.6.3). In the survival analysis univariate and multivariate Cox proportional hazards regression models were used to evaluate the association between various clinical variables and OS. The calculations for the Cox regression model were based on the coxph function from the R survival package, and the results were expressed as hazard ratios (HR) with 95% confidence intervals (CI). Kaplan-Meier survival analysis was performed to plot survival curves, and the log-rank test was used to compare survival rates bet
Univariate Cox regression analysis revealed that age, carcinoembryonic antigen, squamous cell carcinoma, neuron-specific enolase, cancer antigen-125, and micropapillary components were associated with OS. Multivariate Cox regression analysis indicated that age (HR = 1.952, 95%CI: 1.087-2.481, P = 0.029) and micropapillary component (HR = 2.716, 95%CI: 1.259-5.837, P = 0.013) were significantly associated with OS. However, other factors showed no statistically significant correlation with OS as shown in Table 1.
| Characteristic | Univariate analysis | Multivariate analysis | |||
| OS (95%CI) | HR (95%CI) | P value | HR (95%CI) | P value | |
| Gender | |||||
| Male | 614.2 (449.6-715.3) | ||||
| Female | 581.7 (336.4-828.5) | 1.122 (0.527-2.418) | 0.759 | ||
| Age (years) | |||||
| < 65 | 688.7 (581.6-814.9) | ||||
| ≥ 65 | 554.3 (274.3-682.1) | 1.903 (1.059-3.398) | 0.043 | 1.952 (1.087-2.481) | 0.029 |
| Smoking history | |||||
| Yes | 639.3 (477.5-814.9) | ||||
| No | 584.5 (335.6-711.3) | 0.981 (0.551-1.763) | 0.954 | ||
| Pathological type | |||||
| LUSC | 625.8 (451.7-763.4) | ||||
| LUAD | 597.2 (434.5-735.3) | 0.873 (0.635-1.492) | 0.897 | ||
| Tumor position | |||||
| Central | 680.7 (582.3-774.6) | ||||
| Peripheral | 587.8 (361.5-708.6) | 1.395 (0.772-2.521) | 0.264 | ||
| pT stage | |||||
| T1 | 708.4 (204.3-1212.6) | ||||
| T2 | 556.2 (361.4-715.8) | 0.918 (0.395-2.156) | 0.847 | ||
| T3 | 695.7 (589.3-802.5) | 0.931 (0.337-2.578) | 0.891 | ||
| T4 | 616.1 (450.4-782.6) | 1.163 (0.442-3.073) | 0.772 | ||
| pN stage | |||||
| N0 | 585.7 (582.6-590.3) | ||||
| N1 | 681.5 (365.7-997.4) | 0.796 (0.278-2.274) | 0.675 | ||
| N2 | 559.3 (361.2-715.8) | 0.762 (0.285-1.998) | 0.581 | ||
| CEA (ng/mL) | |||||
| ≥ 3.60 | 647.3 (582.6-715.8) | ||||
| < 3.60 | 412.7 (273.9-643.4) | 1.712 (1.008-2.572) | 0.047 | 1.256 (0.987-1.976) | 0.086 |
| SCC (ng/mL) | |||||
| ≥ 1.10 | 611.8 (336.7-714.8) | ||||
| < 1.10 | 453.6 (359.2-671.5) | 1.408 (1.002-1.981) | 0.045 | 1.119 (0.978-1.421) | 0.095 |
| NSE (ng/mL) | |||||
| ≥ 21.30 | 583.6 (551.7-711.8) | ||||
| < 21.30 | 662.7 (453.2-709.4) | 0.977 (0.935-0.999) | 0.049 | 0.982 (0.951-1.016) | 0.102 |
| CA125 (kU/L) | |||||
| ≥ 30.00 | 662.4 (478.5-724.7) | ||||
| < 30.00 | 483.5 (271.6-609.3) | 1.018 (1.004-1.052) | 0.048 | 1.072 (0.993-1.217) | 0.091 |
| Squamous | |||||
| Without | 558.9 (336.5-708.4) | ||||
| With | 636.2 (581.9-718.5) | 0.845 (0.483-1.479) | 0.558 | ||
| Acinar | |||||
| Without | 642.3 (478.6-715.4) | ||||
| With | 559.2 (361.5-714.8) | 0.985 (0.561-1.774) | 0.996 | ||
| Lepidic | |||||
| Without | 585.2 (451.6-710.7) | ||||
| With | 647.3 (582.9-715.1) | 5.976 (0.753-47.871) | 0.093 | ||
| Micropapillary | |||||
| Without | 647.5 (582.3-715.9) | ||||
| With | 303.2 (270.8-709.5) | 2.554 (1.243-5.256) | 0.013 | 2.716 (1.259-5.837) | 0.013 |
| Papillary | |||||
| Without | 647.3 (478.5-715.6) | ||||
| With | 556.7 (270.4-709.2) | 1.351 (0.732-2.495) | 0.343 | ||
| Solid | |||||
| Without | 638.5 (559.7-714.4) | ||||
| With | 381.6 (270.2-734.8) | 1.047 (0.583-1.886) | 0.892 | ||
| Chemotherapy | |||||
| Yes | 617.5 (478.6-710.7) | ||||
| No | 586.3 (314.4-725.9) | 0.991 (0.542-1.816) | 0.965 | ||
| PORT | |||||
| Yes | 648.2 (361.7-936.5) | ||||
| No | 589.4 (450.3-709.2) | 1.116 (0.403-3.107) | 0.837 | ||
In NSCLC 21 cases had high OCT4 H-scores, 27 cases had high TROP-2 H-scores, 44 cases had high ABCG2 H-scores, and 44 cases had high p75NTR H-scores as shown in Table 2. Survival analysis revealed that patients with NSCLC with high OCT4 H-scores had a worse OS compared with those with low OCT4 H-scores (P = 0.006). However, the H-scores of TROP-2, ABCG2, and p75NTR had no statistically significant impact on OS (P > 0.05) as shown in Figure 1. Further subtype analysis revealed that among 29 patients with LUSC, 13 had high OCT4 H-scores, while among 32 patients with LUAD, 8 showed high OCT4 H-scores. Survival analysis showed no statistically significant difference in OS between high and low OCT4 H-score groups in patients with LUSC (P = 0.457). However, in patients with LUAD high OCT4 expression had significantly poorer OS compared with those with low OCT4 expression (P = 0.005) as shown in Figure 2.
| Variables | NSCLC (n = 61) |
| OCT4 | |
| Low H-score | 40 (65.57) |
| High H-score | 21 (34.43) |
| TROP-2 | |
| Low H-score | 34 (55.74) |
| High H-score | 27 (44.26) |
| ABCG2 | |
| Low H-score | 17 (27.87) |
| High H-score | 44 (72.13) |
| p75NTR | |
| Low H-score | 17 (27.87) |
| High H-score | 44 (72.13) |
To comprehensively understand whether the simultaneous expression of multiple CSC-related markers was correlated with NSCLC prognosis, this study combined several indicators into a composite score called TSI. The TSI calculation method involved adding up the genes with an H-score above 5 and including age ≥ 65 years and the presence of a micropapillary component as positive indicators. The cumulative sum of these indicators formed the TSI. Using the median as a cutoff, a TSI > 3 was considered high TSI, and a TSI < 3 was considered low TSI. Survival analysis showed that patients with high TSI had a median survival of 502.6 days (95%CI: 468.3-645.8), which was worse than the 708.6 days (95%CI: 559.4-735.1) for patients with low TSI. The difference was statistically significant (HR = 2.209, 95%CI: 1.238-3.681, P = 0.027) as shown in Figure 2.
Lung cancer, particularly NSCLC, has remained one of the leading causes of cancer-related mortality globally, posing a severe threat to human health[16]. The poor prognosis of NSCLC primarily stems from the fact that patients often already have distant metastasis at the time of early diagnosis, or they experience high recurrence rates after surgery. For some patients conventional treatments such as surgery, radiotherapy, and chemotherapy have limited efficacy, leading to shorter survival times[17]. In recent years CSCs have become a hotspot in cancer research with increasing attention on their critical role in tumorigenesis, progression, and metastasis[18,19]. CSCs are not only the origin cells of tumors but are also closely related to tumor resistance, metastasis, and recurrence[20]. Therefore, investigating the expression of CSC markers in NSCLC and their relationship with patient prognosis is crucial for improving early diagnosis and precise treatment of lung cancer.
Therefore, this study systematically explored the relationship between the clinical characteristics of patients with NSCLC, CSC-related markers (OCT4, TROP-2, ABCG2, p75NTR), and patient prognosis. Our study found that patient age and the presence of micropapillary components significantly affected OS. In particular the pathological feature of micropapillary components was closely related to patient prognosis, showing poorer survival even in early stages[21]. One possible explanation is that micropapillary structures may reflect underlying invasive characteristics of the tumor, such as increased propensity for vascular or lymphatic invasion, both of which are known to correlate with higher metastatic potential and unfavorable prognosis. Although vascular and lymphatic invasion were not separately analyzed in our cohort due to incomplete pathological records in some cases, the strong prognostic association observed suggests that micropapillary morphology may serve as a surrogate marker for other aggressive histopathological features.
Future studies should include comprehensive pathological assessments, including vascular and lymphatic invasion status, to clarify the biological and clinical implications of micropapillary patterns in NSCLC progression. Therefore, in clinical practice it is recommended that more detailed and targeted follow-up strategies be established for patients with such characteristics after surgery. This will help to detect potential recurrence or metastasis in a timely manner, improving the patient’s quality of life and survival time.
OCT4 is a transcription factor closely associated with stem cell pluripotency and self-renewal capacity. Recent studies have demonstrated that OCT4 is overexpressed in various types of cancers[22,23]. Its role in tumor cells is not limited to maintaining stem cell-like properties but also involves regulating tumor invasiveness, metastatic potential, and chemoresistance. In the present study we found that high OCT4 expression was significantly associated with poor prognosis in patients with NSCLC. Further subtype analysis showed that in patients with LUAD high OCT4 H-scores had significantly worse OS compared with those with low H-scores (P = 0.005); however, no similar trend was observed in patients with LUSC. These findings are consistent with several previous studies[24-26], suggesting that OCT4 may play a differential role in the tumorigenesis and progression of different NSCLC subtypes. This also implies its potential value as an auxiliary indicator for clinical staging. The overexpression of OCT4 may promote the self-renewal and proliferation of cancer cells, thereby enhancing malignant biological behaviors and ultimately affecting patient prognosis.
Moreover, OCT4 has been linked not only to the malignancy of NSCLC but also to treatment resistance. Studies have shown that OCT4 overexpression can enhance tumor cell resistance to conventional chemotherapy agents, contributing to tumor recurrence[27]. Therefore, as a classical marker of CSCs, OCT4 not only offers new perspectives for cancer research but also provides a potential therapeutic target in clinical practice. Although the prognostic value of OCT4 in NSCLC has been partially validated, the strength of its association with prognosis varies across studies and may be attributed to differences in sample selection, treatment regimens, and other clinical variables.
In comparison to OCT4 the relationship between the expression of TROP-2, ABCG2, and p75NTR and prognosis in patients with NSCLC was more complex in this study. TROP-2 as a tumor-associated antigen is highly expressed in various tumor types and is closely related to tumor proliferation, metastasis, and chemotherapy resistance[28,29]. Although some studies suggest that high TROP-2 expression is associated with poor prognosis in patients with lung cancer, we did not find a significant correlation between TROP-2 and OS in patients with NSCLC in this study[30,31]. This discrepancy may be due to several factors, including patient cohort heterogeneity, differences in the cutoff values used to define high vs low expression, and technical variability inherent to IHC staining and interpretation. Furthermore, the biological role of TROP-2 may vary among NSCLC subtypes and stages, complicating its prognostic significance. Therefore, the clinical application of TROP-2 as a prognostic marker in NSCLC requires further large-scale and stan
ABCG2, a drug efflux pump protein, is often highly expressed in CSCs and is associated with tumor chemotherapy resistance[32,33]. In our study we did not find a significant relationship between ABCG2 expression and prognosis in patients with NSCLC. This result may be explained by the principal role of ABCG2 in mediating drug resistance and tumor recurrence rather than directly influencing tumor growth and metastasis, suggesting its effect on prognosis is more indirect. Additionally, variations in treatment regimens and patient responses might affect the observed prognostic value of ABCG2. Future research should explore the role of ABCG2 in diverse therapeutic contexts, especially targeted therapies and immunotherapies where it may have a more pronounced impact on treatment outcomes.
p75NTR, a neurotrophin receptor, is expressed in various tumors and is closely associated with tumor cell migration, invasion, and resistance[34,35]. However, we did not observe a significant correlation between p75NTR expression and OS in patients with NSCLC. Possible reasons include the heterogeneity of tumor biology across NSCLC subtypes and stages as well as the complexity of p75NTR signaling pathways, which may have context-dependent protumor or antitumor effects. Moreover, technical factors such as antibody specificity and scoring methods could influence results. Therefore, the clinical relevance of p75NTR in NSCLC prognosis requires further investigation incorporating multidimensional analysis and larger cohorts.
In this study we established a TSI composite scoring system that combines OCT4, TROP-2, ABCG2, p75NTR, and clinical characteristics of patients with NSCLC, aiming for a more comprehensive assessment of tumor stemness characteristics. We found that patients with a high TSI had worse OS, suggesting that TSI may be an independent predictor of prognosis in patients with stage IIIA NSCLC. Compared with single markers, TSI through the combined analysis of multiple stem cell markers better reflects the biological characteristics of the tumor, thus providing a more accurate prognosis assessment. Previous studies have also indicated that TSI is closely related to prognosis in various cancer types[36,37]. For example, in breast cancer and gastric cancer, high TSI scores are often associated with poor prognosis.
Our study further confirmed the application value of TSI in NSCLC and provided a new prognostic evaluation tool for clinical practice. However, the clinical application of TSI still faces several challenges. First, the accuracy and consistency of the immunohistochemical scoring method may affect the calculation of the stemness index, and standardized scoring processes are required. In this study IHC H-scores were used to semi-quantitatively assess the expression levels of OCT4, TROP-2, ABCG2, and p75NTR in tumor samples. Although the scoring was independently performed by two experienced pathologists with high interobserver consistency, we acknowledge that H-scores remain a semi-quantitative method and are subject to inherent limitations such as observer bias. To enhance the reliability of IHC-based evaluation, future studies could incorporate more quantitative validation approaches, such as assessing the correlation between H-scores and mRNA levels (via quantitative reverse transcription PCR) or protein expression levels (via western blot or proteomics data), thereby confirming whether IHC scoring accurately reflects molecular expression. Moreover, digital pathology and artificial intelligence-assisted image analysis could further standardize and refine IHC scoring in future prospective studies.
Second, the TSI scoring system still needs further refinement, especially in terms of marker selection and weight distribution. In this study the TSI was calculated by integrating three equally weighted factors: IHC H-score total > 5; age ≥ 65 years; and presence of micropapillary structures. While this composite index is practical and demonstrated prognostic utility, we acknowledge that the equal weighting lacks detailed biological rationale. These factors likely contribute to prognosis via different mechanisms and may have varying degrees of impact on tumor stemness and patient outcomes. Alternative weighting schemes or inclusion of additional clinicopathological variables could potentially improve the predictive accuracy of TSI. We have not yet performed systematic sensitivity analyses to explore different combinations or weights of these factors. Such analyses alongside validation in independent cohorts would be critical to optimize the construction of the TSI and confirm its generalizability and robustness. Future prospective studies are planned to address these points, including the application of machine learning methods to refine the index and biological investigations to elucidate the underlying mechanisms linking these factors to tumor stemness and prognosis.
Despite providing important data on CSC markers and TSI in the clinical significance of stage IIIA NSCLC, this study still had some limitations. First, as a single-center retrospective study, the sample size was relatively small, potentially leading to nonsignificant relationships between some markers and prognosis. Future large-scale multicenter prospective studies could further validate the reliability and generalizability of these results. Second, this study only analyzed four markers, OCT4, TROP-2, ABCG2, and p75NTR, while CSC characteristics go beyond these markers. With the development of molecular biology techniques, future studies can explore more CSC markers using genomics, proteomics, and other methods, optimizing TSI and enhancing its clinical predictive ability in different NSCLC subtypes and stages. Additionally, CSC markers are not only closely related to the biological characteristics of tumors but are also influenced by factors such as the immune microenvironment, tumor metabolic features, and the responsiveness to external therapies. Therefore, future research could explore the application of CSC markers in immunotherapy and targeted therapies, particularly in the context of novel treatments such as immune checkpoint inhibitors and molecular-targeted drugs in which CSC characteristics may impact treatment efficacy and resistance.
This study provided new directions for the prognosis assessment and treatment of patients with stage IIIA NSCLC. OCT4, TSI, and other markers could serve as potential biomarkers for prognosis evaluation, assisting clinicians in formulating personalized treatment plans. However, before clinical application further validation of the effectiveness and reliability of these markers in various clinical settings is required, particularly in assessing prognosis across different tumor subtypes and treatment backgrounds. Additionally, with the development of molecular targeted therapies and immunotherapies, CSC markers may become new therapeutic targets, offering patients more treatment options. In conclusion CSC markers and TSI provide valuable information for the prognosis assessment of stage IIIA NSCLC, laying a foundation for further clinical research and the development of personalized treatment strategies.
| 1. | Ding CZ, Wang GL, Jiang GQ, Wang HT, Liu YY, Zhang HL, Sun F, Wei L. [circDDX17 targets miR-223-3p/RIP3 to regulate the proliferation and apoptosis of non-small cell lung cancer cells]. Zhonghua Zhong Liu Za Zhi. 2024;46:239-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 2. | Maliarchuk K, Ganul AV, Borisyuk BO, Bororov LV, Shevchenko AI, Sovenko VM. Prospects of neoadjuvant chemoradiotherapy in patients with stage III A non-small cell lung cancer as a method of improving survival. Wiad Lek. 2022;75:2098-2102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Parisi S, Ferini G, Lillo S, Brogna A, Chillari F, Ferrantelli G, Settineri N, Santacaterina A, Platania A, Leotta S, Casablanca G, Russo A, Pontoriero A, Adamo V, Minutoli F, Bottari A, Cacciola A, Pergolizzi S. Stereotactic boost on residual disease after external-beam irradiation in clinical stage III non-small cell lung cancer: mature results of stereotactic body radiation therapy post radiation therapy (SBRTpostRT) study. Radiol Med. 2023;128:877-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (1)] |
| 4. | Chang JY, Kim JH, Kang J, Park Y, Park SJ, Cheon JH, Kim WH, Kim H, Park JJ, Kim TI. mTOR Signaling Combined with Cancer Stem Cell Markers as a Survival Predictor in Stage II Colorectal Cancer. Yonsei Med J. 2020;61:572-578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Tsuchiya H, Shiota G. Clinical and Biological Implications of Cancer Stem Cells in Hepatocellular Carcinoma. Yonago Acta Med. 2021;64:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Dai L, Zhao X, Ma L, Li X, Zuo D, Li Y, Wei B, Sui Y, Xu F. [TNF-α activates PI3K/AKT pathway to promote proliferation of SW620(Lgr5+) colon cancer stem cells]. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2020;36:33-41. [PubMed] |
| 7. | Kalantari E, Taheri T, Fata S, Abolhasani M, Mehrazma M, Madjd Z, Asgari M. Significant co-expression of putative cancer stem cell markers, EpCAM and CD166, correlates with tumor stage and invasive behavior in colorectal cancer. World J Surg Oncol. 2022;20:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 8. | Li H, Jin Y, Zhu Y, Shen B, Xu Y. Suppression of ZNF205-AS1/EGR4 positive feedback loop attenuates cisplatin resistance of non-small cell lung cancer cells via targeting miR-138-5p/OCT4 pathway. J Thorac Dis. 2024;16:296-310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 9. | Xu J, Liu J, Mei S, Zhou Q. [Research Progress and Perspectives of Antibody-drug Conjugates Targeting Trophoblast Cell Surface Antigen-2 in Advanced Non-small Cell Lung Cancer]. Zhongguo Fei Ai Za Zhi. 2024;27:763-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Sorf A, Vagiannis D, Ahmed F, Hofman J, Ceckova M. Dabrafenib inhibits ABCG2 and cytochrome P450 isoenzymes; potential implications for combination anticancer therapy. Toxicol Appl Pharmacol. 2022;434:115797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 11. | Kosuge H, Nakakido M, Nagatoishi S, Fukuda T, Bando Y, Ohnuma SI, Tsumoto K. Proteomic identification and validation of novel interactions of the putative tumor suppressor PRELP with membrane proteins including IGFI-R and p75NTR. J Biol Chem. 2021;296:100278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Sanchez DF, Fernandez-Nestosa MJ, Cañete-Portillo S, Cubilla AL. Evolving insights into penile cancer pathology and the eighth edition of the AJCC TNM staging system. Urol Oncol. 2022;40:215-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Robert ME, Rüschoff J, Jasani B, Graham RP, Badve SS, Rodriguez-Justo M, Kodach LL, Srivastava A, Wang HL, Tang LH, Troncone G, Rojo F, Van Treeck BJ, Pratt J, Shnitsa I, Kumar G, Karasarides M, Anders RA. High Interobserver Variability Among Pathologists Using Combined Positive Score to Evaluate PD-L1 Expression in Gastric, Gastroesophageal Junction, and Esophageal Adenocarcinoma. Mod Pathol. 2023;36:100154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 43] [Reference Citation Analysis (0)] |
| 14. | Park E, Park SY, Sun PL, Jin Y, Kim JE, Jheon S, Kim K, Lee CT, Kim H, Chung JH. Prognostic significance of stem cell-related marker expression and its correlation with histologic subtypes in lung adenocarcinoma. Oncotarget. 2016;7:42502-42512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 15. | Shimada Y, Saji H, Nomura M, Matsubayashi J, Yoshida K, Kakihana M, Kajiwara N, Ohira T, Ikeda N. Cancer stem cell-related marker expression in lung adenocarcinoma and relevance of histologic subtypes based on IASLC/ATS/ERS classification. Onco Targets Ther. 2013;6:1597-1604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Society of Cancer Precision of Chinese Anti-Cancer Association; Lung Cancer Expert Group of Chinese Medical Journal; Chinese Society of Clinical Oncology, Expert Committee on Non-small Cell Lung Cancer. [Chinese expert consensus on the diagnosis and treatment of advanced RET fusion-positive non-small cell lung cancer (2023 edition)]. Zhonghua Zhong Liu Za Zhi. 2023;45:991-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 17. | Oncology Society of Chinese Medical Association. [Chinese Medical Association guideline for clinical diagnosis and treatment of lung cancer (2024 edition)]. Zhonghua Zhong Liu Za Zhi. 2024;46:805-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 18. | Joshi P, Waghmare S. Molecular signaling in cancer stem cells of tongue squamous cell carcinoma: Therapeutic implications and challenges. World J Stem Cells. 2023;15:438-452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 19. | Xie WJ, Li J. Obesity and cancer stem cells: Roles in cancer initiation, progression and therapy resistance. World J Stem Cells. 2023;15:120-135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Yu Z, Sun J, Fang K, Xu J, Yang J, Chunlei D, Gong Y, Ma H. SLC2A1 boosts the resistance of non-small cell lung cancer to taxanes by stimulating the formation of EPCAM(+) cancer stem-like cells via glycolysis. Transl Oncol. 2024;49:102082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 21. | Jeon HW, Kim YD, Sim SB, Moon MH. Significant difference in recurrence according to the proportion of high grade patterns in stage IA lung adenocarcinoma. Thorac Cancer. 2021;12:1952-1958. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 22. | Zhang J, Qi MX, Li YX, Li XB, Zhang GZ, Chai YM. [Oct4 promotes the progression and radioresistance of esophageal squamous cell carcinoma by regulating epithelial-mesenchymal transition]. Zhonghua Zhong Liu Za Zhi. 2024;46:1019-1028. [PubMed] [DOI] [Full Text] |
| 23. | Guo XP, Chen YF, Chen P, Pan J, Ying PT, Zhao N, Tang YM. [Effect of Human Bone Marrow Mesenchymal Stem Cells with Ectopic High OCT4 Expression on T Lymphocyte Function]. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2023;31:1523-1530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 24. | Tyagunova EE, Drozd SF, Kalennik OV, Samoylenkova NS, Savchenko EA, Danilov GV, Pavlova GV. Prognostic model for assessing the human glioma cell malignancy grade based on MDM2, MELK, SOX2, CDK4, DR5 and OCT4 gene expression. Zh Vopr Neirokhir Im N N Burdenko. 2023;87:43-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 25. | Lu CS, Shiau AL, Su BH, Hsu TS, Wang CT, Su YC, Tsai MS, Feng YH, Tseng YL, Yen YT, Wu CL, Shieh GS. Oct4 promotes M2 macrophage polarization through upregulation of macrophage colony-stimulating factor in lung cancer. J Hematol Oncol. 2020;13:62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 105] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 26. | Upadhyay VA, Shah KA, Makwana DP, Raval AP, Shah FD, Rawal RM. Putative stemness markers octamer-binding transcription factor 4, sex-determining region Y-box 2, and NANOG in non-small cell lung carcinoma: A clinicopathological association. J Cancer Res Ther. 2020;16:804-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 27. | Liu L, Zhu H, Liao Y, Wu W, Liu L, Liu L, Wu Y, Sun F, Lin HW. Inhibition of Wnt/β-catenin pathway reverses multi-drug resistance and EMT in Oct4(+)/Nanog(+) NSCLC cells. Biomed Pharmacother. 2020;127:110225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 28. | Wang X, Qi L, Chen M, Zhang Y, Gao X, Cai Y. Feasibility study of ADCs targeting TROP-2, HER2, and CD46 in Ductal Adenocarcinoma and Intraductal Carcinoma of the prostate. World J Urol. 2024;42:404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 29. | Izci H, Punie K, Waumans L, Laenen A, Wildiers H, Verdoodt F, Desmedt C, Ardui J, Smeets A, Han SN, Nevelsteen I, Neven P, Floris G. Correlation of TROP-2 expression with clinical-pathological characteristics and outcome in triple-negative breast cancer. Sci Rep. 2022;12:22498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 30. | Belluomini L, Avancini A, Sposito M, Milella M, Rossi A, Pilotto S. Antibody-drug conjugates (ADCs) targeting TROP-2 in lung cancer. Expert Opin Biol Ther. 2023;23:1077-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 31. | Parisi C, Mahjoubi L, Gazzah A, Barlesi F. TROP-2 directed antibody-drug conjugates (ADCs): The revolution of smart drug delivery in advanced non-small cell lung cancer (NSCLC). Cancer Treat Rev. 2023;118:102572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 32. | Yousaf M, Ali M. Modulation of ABCG2 surface expression by Rab5 and Rab21 to overcome multidrug resistance in cancer cells. Xenobiotica. 2020;50:988-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 33. | Tan Y, Cao K, Ren G, Qin Z, Zhao D, Li N, Chen X, Xia Y, Lu Y. Effects of the ABCB1 and ABCG2 polymorphisms on the pharmacokinetics of afatinib in healthy Chinese volunteers. Xenobiotica. 2020;50:237-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 34. | Chen J, Wang N, Zhang H, Zhang X, Zhao L, Zhu L, Li Z, Bei C. [Lentivirus-mediated silencing of P75 neurotrophin receptor combined with nerve growth factor overexpression and transfection of bone marrow mesenchymal stem cells combined with demineralized bone matrix for heterotopic osteogenesis]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2020;34:1438-1445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 35. | Meek S, Singh-Dolt K, Sutherland L, Sharp MGF, Del-Pozo J, Walker D, Burdon T. Redundancy of p75NTR neurotrophin receptor function in development, growth and fertility in the rat. Transgenic Res. 2024;33:255-266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 36. | Wu Z, Fang Y, Wu J, Wang J, Ling Y, Liu T, Tong Q, Yao Y. Activation of Glycolysis by MCM10 Increases Stemness and Paclitaxel Resistance in Gastric Cancer Cells. Turk J Gastroenterol. 2023;34:1107-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 37. | Zheng J, Zhang YW, Pan ZF. [Dysregulation of MAD2L1/CAMK2A/PTTG1 Gene Cluster Maintains the Stemness Characteristics of Uterine Corpus Endometrial Carcinoma]. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2021;43:685-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/