Published online Sep 26, 2025. doi: 10.4252/wjsc.v17.i9.107025
Revised: May 10, 2025
Accepted: August 25, 2025
Published online: September 26, 2025
Processing time: 195 Days and 1.8 Hours
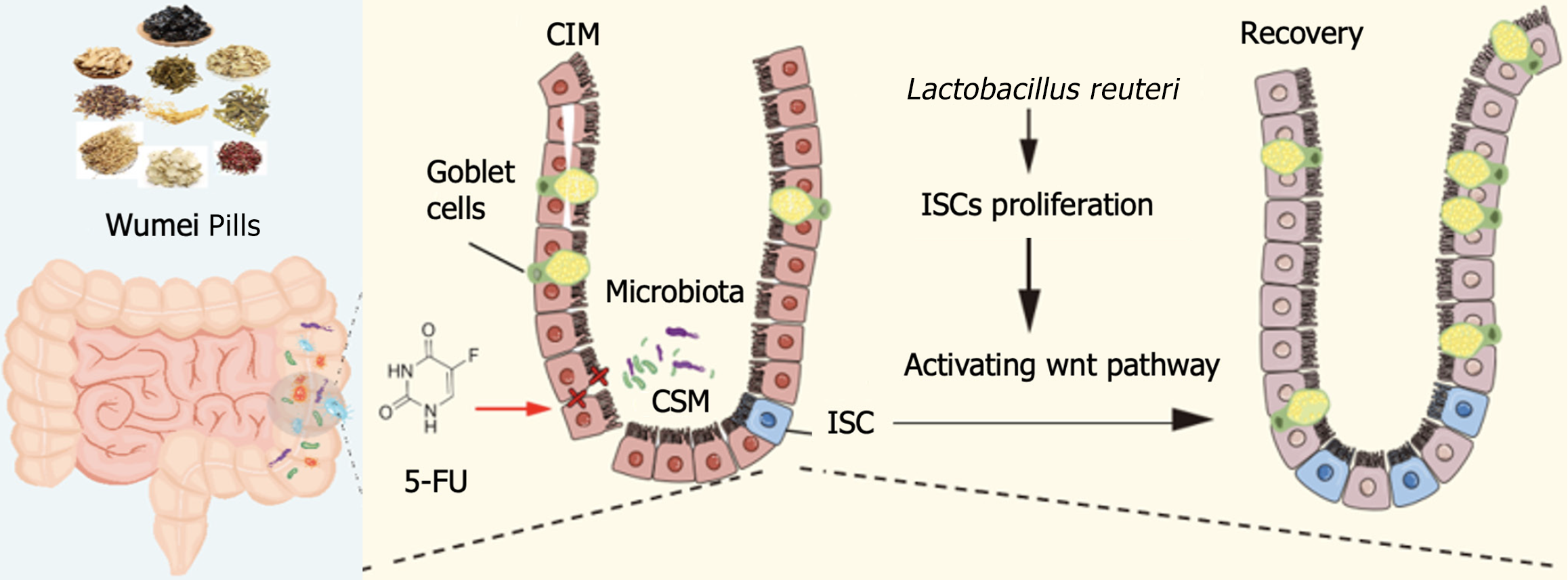
Intestinal mucositis is a severe and common complication of chemotherapy, characterized by disruption of the gut microbiota, intestinal inflammation, and epithelial barrier damage. Intestinal stem cells (ISCs) are essential for epithelial renewal and barrier maintenance, yet chemotherapy impairs ISC proliferation and function, delaying mucosal repair. We hypothesized that Wumei Pills (WMP) could protect against chemotherapy-induced intestinal mucositis by modulating gut microbiota - particularly Lactobacillus reuteri (L. reuteri) - to restore ISC activity, preserve microbial balance, reduce inflammation, and promote epithelial regene
To characterize these changes and the safety of WMP via a 5-fluorouracil (5-FU) induced intestinal mucositis mouse model.
In this study, we established a 5-FU induced intestinal mucositis mouse model, to explore the protective effect of WMP regulating L. reuteri on integrity of intestinal mucosa.
We found that intestinal flora is an important mechanism causing chemotherapy-induced intestinal mucositis, but WMP and live L. reuteri were effective in protecting the morphology of intestinal mucositis and normal proliferation of epithelial. L. reuteri colonized in the intestinal mucosa and WMP ameliorated intestinal mucosa damage caused by 5-FU treatment, including improvement of body weight, pathological change, and proliferation level, reducement proinflammatory cytokine secretion (tumor necrosis factor-α, interleukin-6) and the lipopolysaccharides concentration in serum. The repair process stimulated by both L. reuteri and WMP were also accompanied with increased leucine-rich-repeat-containing G-protein-coupled receptor 5 (+) and proliferating cell nuclear antigen of mice intestine. Furthermore, we demonstrated that WMP and L. reuteri activated the Wingless-type/β-catenin pathway to accelerate proliferation of intestinal epithelial, thus recovering damaged intestinal mucosa. However, the relieving effect of L. reuteri on intestinal mucosa was inferior to that of WMP.
Our findings indicate that WMP regulating L. reuteri protects intestinal barrier and activates intestinal epithelial proliferation, which sheds light on treatment approaches for intestinal inflammation based on ISCs with traditional Chinese medicine and probiotics L. reuteri.
Core Tip: In this study, we established a 5-fluorouracil induced intestinal mucositis mouse model, and found that intestinal flora is an important mechanism causing chemotherapy-induced intestinal mucositis. Lactobacillus reuteri colonized in the intestinal mucosa and Wumei Pills ameliorated intestinal mucosa damage caused by 5-fluorouracil treatment, including improvement of body weight, pathological change, and proliferation level, reducement proinflammatory cytokine secretion (tumor necrosis factor-α, interleukin-6) and the lipopolysaccharides concentration. The repair process stimulated were also accompanied with increased leucine-rich-repeat-containing G-protein-coupled receptor 5 (+) and proliferating cell nuclear antigen. Furthermore, Wumei Pills and Lactobacillus reuteri activated the Wingless-type/β-catenin pathway to accelerate proliferation of intestinal epithelial, thus recovering damaged intestinal mucosa.
- Citation: Lu DX, Wang ZX, Liu XM, Wu H, Ji LJ, Yan J. Wumei Pills enhance intestinal stem cell - mediated repair in chemotherapy-induced mucositis via Lactobacillus reuteri - dependent modulation of the gut microbiota. World J Stem Cells 2025; 17(9): 107025
- URL: https://www.wjgnet.com/1948-0210/full/v17/i9/107025.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v17.i9.107025
Continuously exposed to gut microbiota, the intestinal barrier is vital for mucosal homeostasis. This stability relies on effective defense mechanisms against chemical and microbial threats[1,2]. The gut lining is composed of columnar epithelium featuring glandular invaginations known as crypts. Residing at the crypt base, intestinal stem cell (ISC) centrally govern epithelial proliferation and differentiation[3,4]. A critical equilibrium between ISC self-renewal and differentiation regulates both epithelial homeostasis and tissue repair, particularly during mucosal damage or inflammation[5].
Chemotherapy-induced intestinal mucositis (CIM) is a common adverse reaction in the course of anti-tumor therapy, characterized by disruption of intestinal environmental homeostasis such as flora disturbance and intestinal mucosal barrier integrity impairment. Some scholars suggested that the key to maintaining intestinal barrier homeostasis was ISCs proliferation and differentiation[6,7]. In physiological conditions, leucine-rich-repeat-containing G-protein-coupled receptor 5 (+) (Lgr5+) ISCs located at the base of the crypt are regulated by Wingless-type (Wnt) signals and continuously proliferate and differentiate into intestinal epithelial cells to maintain intestinal barrier homeostasis[8]. When intestinal injury occurs, the activation of Wnt signaling pathway is blocked and the proliferation of ISCs is reduced[9], which leads to a decrease in the number of new intestinal epithelial cells, and eventually damage to the intestinal barrier and repeated inflammation. However, ISCs are in direct contact with intestinal flora, especially crypt-specific core microbiota, to stimulate ISCs proliferation and renewal through activation of Wnt signals, and maintain intestinal homeostasis[10]. Lactobacillus rhamnosus can regulate intestinal flora homeostasis, promote ISCs proliferation, and alleviate inflammatory bowel disease[11]. Lactobacillus plantarum can induce proliferation of adult midgut progenitor cells and intestinal tissue development[12].
“Regulating, is harmonizing” is an important rule of traditional Chinese medicine (TCM) to adjust yin-yang and maintain the homeostasis. Our research group clinically prescribed Wumei Pills (WMP), a classic prescription for co-treatment of cold and heat, for the treatment of 50 patients with chemotherapeutic intestinal catarrhal diarrhea, and found that WMP can significantly reduce the incidence of CIM diarrhea and reduce the levels of inflammatory cytokines and intestinal mucosal barrier related factors, with a total effective rate of 88%[13]. Experimental studies have also confirmed: WMP alleviates intestinal epithelial damage in CIM model mice, repairs the integrity of intestinal mucosal barrier, and alleviates diarrhea[14]. The initial mechanism of WMP is related to the decrease of intestinal permeability and the up-regulation of tight connectin zonula occludens-1 and E-cadherin expression, which can increase microbial diversity and stabilize microbial community in CIM model mice. Increase the abundance of Lactobacillus, the family of bacteria that produce short chain fatty acids, especially Lactobacillus reuteri (L. reuteri).
L. reuteri, also the main lactic acid bacteria living in the intestinal tract of animals, supports the growth and repair process of mucosal cells by enhancing the resistance of mucosal barrier, and has a multi-aspect impact on intestinal health[15,16]. Previous studies have established that WMP ameliorate CIM by modulating the intestinal flora[14], particularly through enhancing L. reuteri abundance. However, the role of the gut microbiota in CIM pathogenesis, along with the mechanistic details of how WMP and L. reuteri preserve intestinal mucosal integrity - specifically their effects on ISCs - remains unclear. Furthermore, the potential for WMP to restore intestinal barrier homeostasis via regulation of L. reuteri warrants investigation. Moreover, whether WMP regulate L. reuteri to rebuild intestinal barrier homeostasis needs to be explored.
In this study, we detected the protective effects of WMP and L. reuteri against CIM in mouse model. Our hypothesis is that WMP ameliorate CIM by modulating L. reuteri and promoting ISC regeneration, thereby repairing intestinal damage. Therefore, this study investigates the anti-inflammatory effects of WMP against CIM and elucidates the underlying mechanism involving L. reuteri-mediated ISC repair.
C57BL/6 mice (4 weeks old, male, 18 ± 2 g, specific pathogen-free) were purchased from Weitong Lihua Laboratory Animal Co., Ltd. [Production license: SYXK(Su) 2023-0077, China]. Mice were kept under specific pathogen-free conditions (12 hours light/12 hours dark cycle, 45%-55% relative humidity, and temperature of 26-28 °C) with free access to food and clean water. The study protocols were approved by the Animal Experimental Ethics Committee of Nanjing University of Chinese Medicine (Approval No. 202409A097) and carried out on the basis of the animal ethics standards of the Nanjing University of Chinese Medicine Laboratory Animal Ethics Committee. The animal certificate number is 20170005027187. All reasonable efforts were made to minimize animal suffering. The L. reuteri strain (10 mL, 108) was confirmed by 16S rDNA sequencing results (BeNa Culture Collection) and grown in de Man, Rogosa and Sharpe agar medium at 37 °C.
WMP (Wu Mei: 24 g, Xi Xin: 9 g, Dang Gui: 6 g, Fu Zi: 9 g, Hua Jiao: 6 g, Huang Bo: 9 g, Gui Zhi: 9 g, Huang Lian: 24 g, Ren Shen: 15 g, and Gan Jiang: 15 g) was purchased from the Chinese Pharmacy of Jiangsu Provincial Hospital of Traditional Chinese Medicine (Nanjing, China). 5-fluorouracil (5-FU) was obtained from Shanghai Yuanye Bio-Technology Co., Ltd. (CAS: 51-21-8, Shanghai, China). Vancomycin hydrochloride (1 g, CAS: 1404-93-9), metronidazole (25 g, CAS: 1404-93-9), neomyein sulfate (10 g, CAS: 1405-10-3) and ampicillin, sodium salt (1 g, CAS: 69-52-3) were obtained from Solarbio (Beijing, China). Antibiotic mixture (ABX) solution: Suspension with 0.5 g/L vancomycin hydrochloride, 1.0 g/L ampicillin, 1.0 g/L neomyein sulfate and 1.0 g/L metronidazole concentrations prepared with ultra-pure water.
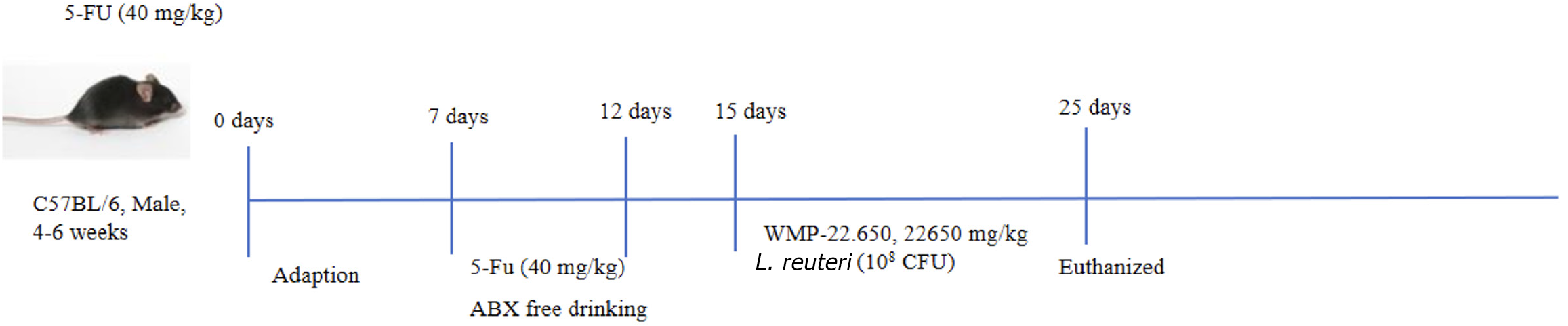
After 7 days of adaptive feeding, the C57BL/6 mice were divided randomly into 7 groups (n = 10 per group): Control group, 5-FU group (40 mg/kg; as the previous research)[14], 5-FU + ABX group, 5-FU + WMP treatment group (WMP-22.650, 22650 mg/kg; as the previous research)[14], 5-FU + L. reuteri group [108 colony-forming unit (CFU)], 5-FU + ABX + WMP treatment group, 5-FU + ABX + L. reuteri group. Except for the control mice, 5-FU was administered to the mice through intraperitoneal injection once daily for the first 5 days. The pseudo-germ-free (PGF) model was established by ABX solution. In addition, WMP and L. reuteri were intragastrically administered to the mice during the experiment for continuous 10 days. In 16th day, the mice were fasted for 12 hours, and they were anesthetized and sacrificed via cervical dislocation. The experimental process is illustrated in Figure 1.
Indicators of disease severity, including body weight, food intake, and diarrhea severity, were evaluated daily for the assessment of mucositis. Diarrhea severity was classified into five grades according to stool consistency: (1) 0: Normal; (2) 1: Slightly wet; (3) 2: Moderate wet; (4) 3: Loose; and (5) 4: Watery stool[17]. Jejunal and colonic tissues were removed quickly and douched with clean cold saline, and then the length of the colon was measured. The samples (1 cm × 1 cm) were immersed in 4% paraformaldehyde for 24 hours and embedded in paraffin for histopathological, immunohistochemical, and immunofluorescence analyses. Blood samples were collected and centrifuged at 12000 rpm for 10 minutes at 4 °C. The residual intestinal tissue samples were stored at -80 °C and used for biochemical analysis. The feces were also collected from the colon/rectum of the mice to perform a microbiota analysis.
The serum supernatants were stored at -20 °C until use for cytokine analysis. Cytokine analysis was performed on thawed samples with interleukin (IL)-1β, lipopolysaccharides (LPS) and tumor necrosis factor (TNF)-α ELISA kits (Nanjing Lapuda Biotechnology Co., Ltd, China) according to the manufacturer’s protocol.
In order to verify the colonization of L. reuteri in the intestine, mice were orally administrated with phosphate-buffered saline (PBS) (200 μL) or 108 CFU L. reuteri suspended in 200 μL PBS only once. Fresh mouse. The feces were collected before and after intragastric infusion. With the help of Fast DNA Spin Kit for Soil (FastPure Feces DNA Isolation Kit, Major Yuhu, Shanghai, China), the corresponding genomic DNA of fecal samples was extracted. Using the above genomic DNA as a template, quantitative fluorescent polymerase chain reaction amplification was carried out using L. reuteri specific primers, and values were obtained. The primes of Ler_16s are as follows (Table 1).
| Target gene | Primes | 5’-3’ |
| Ler_16s | F | GCTTTGGCTATCACTCTG |
| R | ACGCACGTTCTTCTCC |
The paraffin blocks were cut into 3.0 μm thick slices and stained with hematoxylin and eosin (H&E). The intestinal mucosal injury and inflammatory cell infiltration were observed under light microscope. The histopathological score was calculated based on previous studies[18].
The toxic effects of chemotherapy may weaken the intestinal epithelial barrier, allowing the contents of the lumen to enter the bloodstream through the paracellular space. The animals began fasting at 9 pm. on the first day and were denied water for 12 hours. On day 2, fluorescein isothiocyanate (FITC)-dextran (50 mg/mL) was administered orally according to the animal’s body weight (0.1 mL/10 g). After 3 hours, blood was removed from the orbit and serum was isolated. Serum fluorescence (480 nm excitation wavelength, 520 nm emission wavelength) was measured by enzyme-labeler, and FITC-glucan standard was used to prepare the standard curve of FITC leakage in serum.
The jejunum sections measuring 2 cm in length were collected from different groups of mice and fixed overnight with 4% paraformaldehyde, and then embedded in the optimal cutting temperature compound. Jejunum slices were permeated and incubated overnight with anti-mouse proliferating cell nuclear antigen (PCNA) antibody (1:50, Abcam, United Kingdom). ISC staining, tissue sections were fixed with paraformaldehyde for 1 hour, infiltrated with 0.4% Triton X-100 for 30 minutes, and incubated in 5% BSA for 2 hours. It was then incubated overnight with the primary antibody (anti-rabbit Lgr5 antibody, 1:100, Abcam, United Kingdom) at 4 °C. The samples were incubated at room temperature for 60 minutes with goat anti-rabbit antibodies coupled with Alexa Fluor 594 (1:250, Abcam, United Kingdom) or Alexa Fluor 488 (1:200, Abcam, United Kingdom). Fluorescence images were collected for further qualitative and quantitative analysis.
The paraffin sections were dewaxed with xylene and absolute ethanol, washed with PBS (pH = 7.4), and blocked with 5% bovine serum albumin. Then, the paraffin sections were incubated with an anti-Wnt3 antibody (1:100), anti-β-catenin antibody (1:100) overnight at -4 °C and incubated with an anti-rabbit secondary antibody the next day. The slides were stained with 3,3-diaminobenzidine and counterstained with hematoxylin. The sections were observed with a light microscope.
Results are expressed as mean ± SEM. Statistical significance, defined as P-value < 0.05, was determined using GraphPad Prism 8.0: Unpaired Student’s t-tests for differences between two groups and one-way ANOVA for differences among multiple groups.
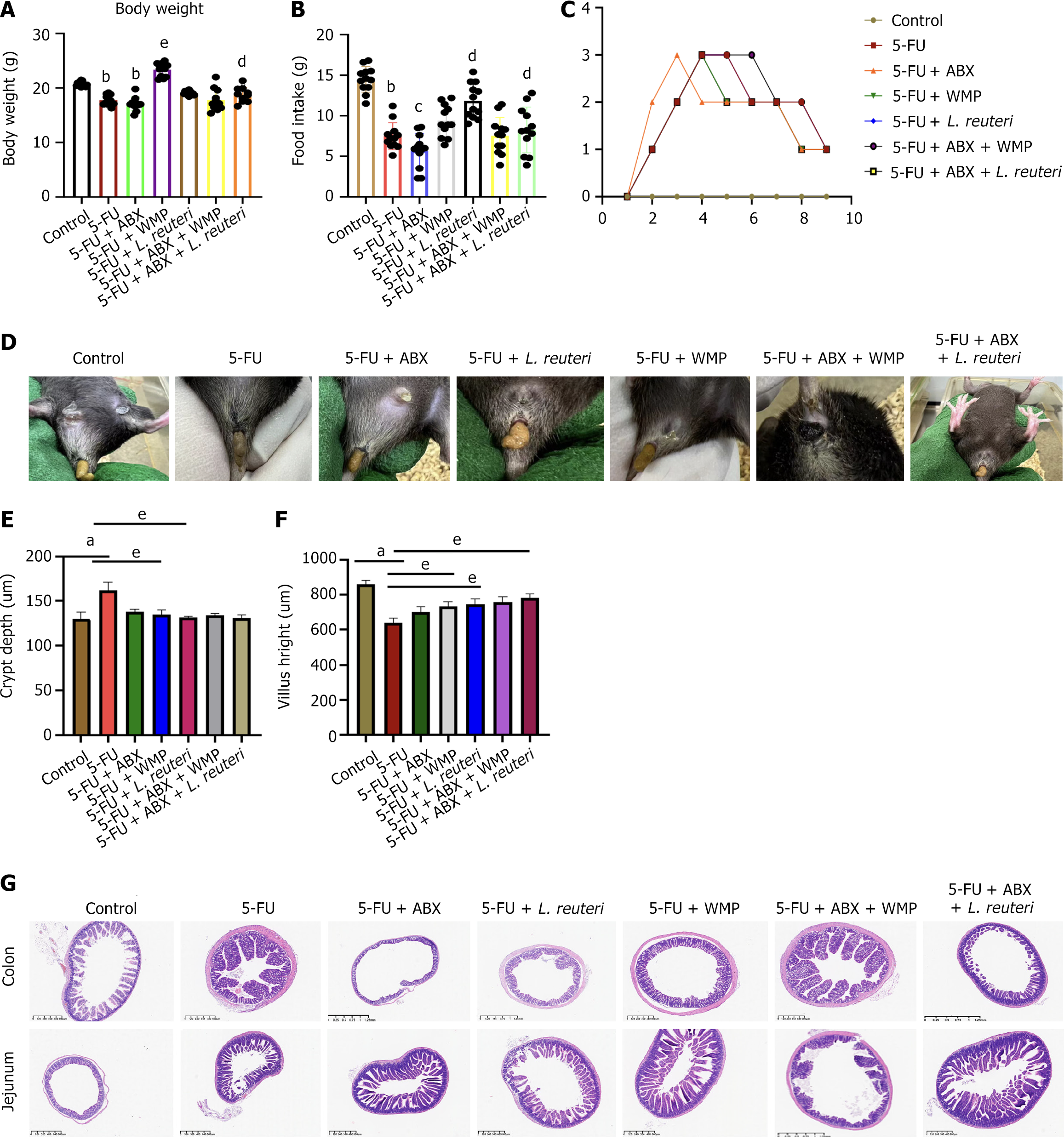
First, to determine the role of intestinal microbiota in ISC regulation, we kept 5-FU mice in PGF conditions with ABX solution for 5 consecutive days. Compared with the 5-FU group, we observed that PGF mice had less diarrhea, less intestinal permeability and less reduction in the number of crypt depth (CD) and more increase in the number of villus height (VH). The H&E staining fragment showed that 5-FU caused intestinal epithelial injury of varying degrees. The epithelial injury of jejunal tissue is the most obvious. No histological abnormalities were observed in the control group, while obvious histological changes occurred in the model mice, including inflammatory cell infiltration, large ulcers, glandular atrophy, and shortening of jejunal epithelial villi. The injury of the colonic mucosa was relatively mild, manifested as a small amount of inflammatory cell infiltration and villi shortening. The ELISA results showed that the expression of inflammatory cytokines, especially LPS increased. However, it was not obvious in PGF mice, suggesting that intestinal flora played an important role in maintaining the repair and function of ISCs (Figures 2, 3A and 3B).
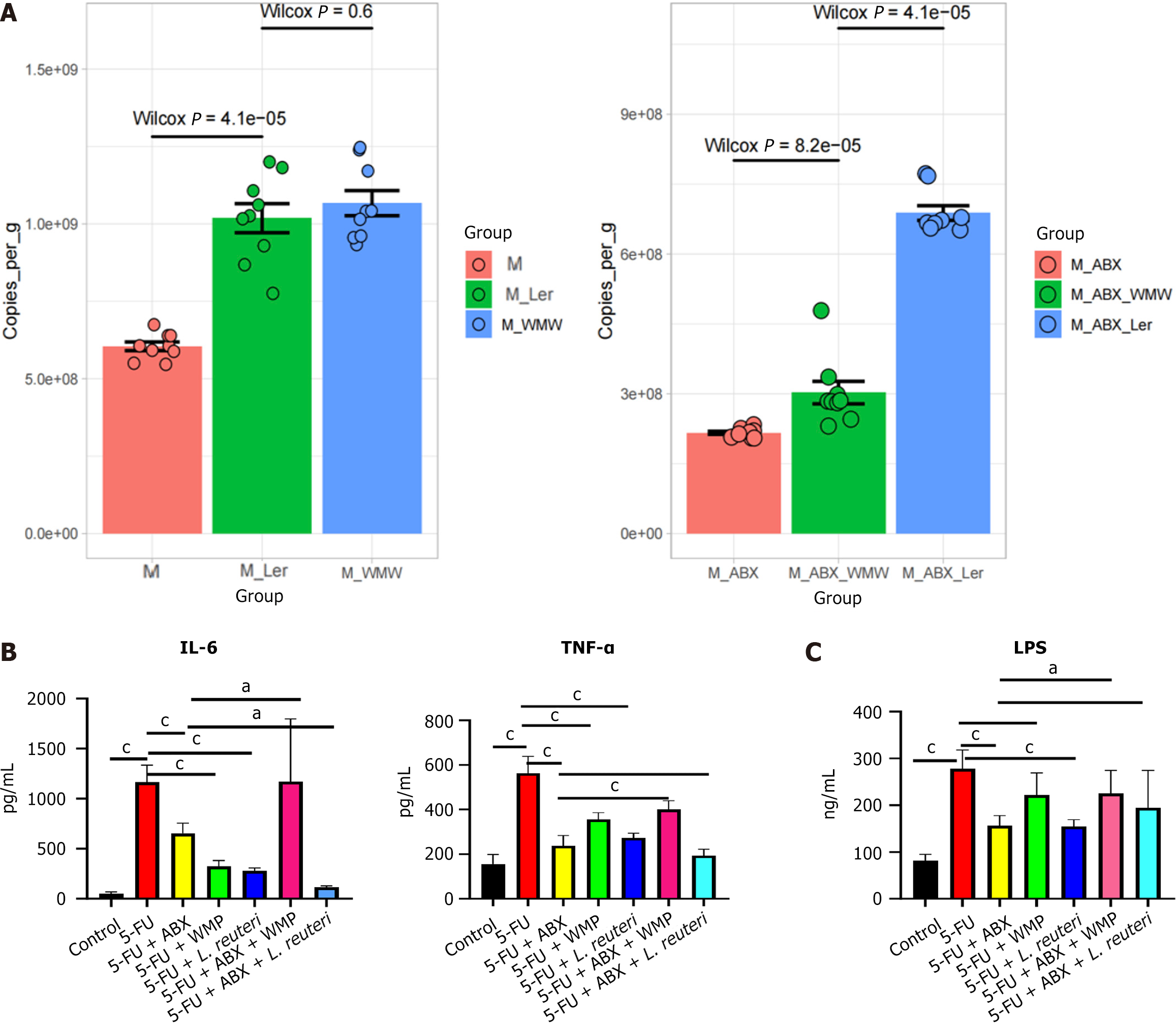
Then, to verify colonization of L. reuteri in the intestine, mice were orally administered 108 CFU/L. reuteri for 7 consecutive days. DNA testing of the bacterial flora in the stool of mice after intragastric administration confirmed that L. reuteri was indeed colonizing the intestine (Figure 3C). In addition, we also examined the effects of WMP and L. reuteri on the general condition and intestinal inflammation of mice. After the administration of WMP and L. reuteri to 5-FU mice, the body mass and food intake of the mice were increased, and diarrhea was significantly relieved. H&E and ELISA results showed that both WMP and L. reuteri could reverse the inflammatory infiltration and expression of inflammatory factors in 5-FU mice, especially in WMP group (Figures 2G, 3A and 3B). After administration of WMP and L. reuteri, PGF mice had no significant improvement on the degree of diarrhea, and the food intake and body mass of L. reuteri mice increased, but there was no statistical difference in WMP group (Figure 2A and B). H&E and ELISA results showed that WMP did not inhibit the intestinal inflammation in PGF mice, but promoted it, while the inflammatory infiltration and inflammatory factors were reversed in PGF mice given L. reuteri.
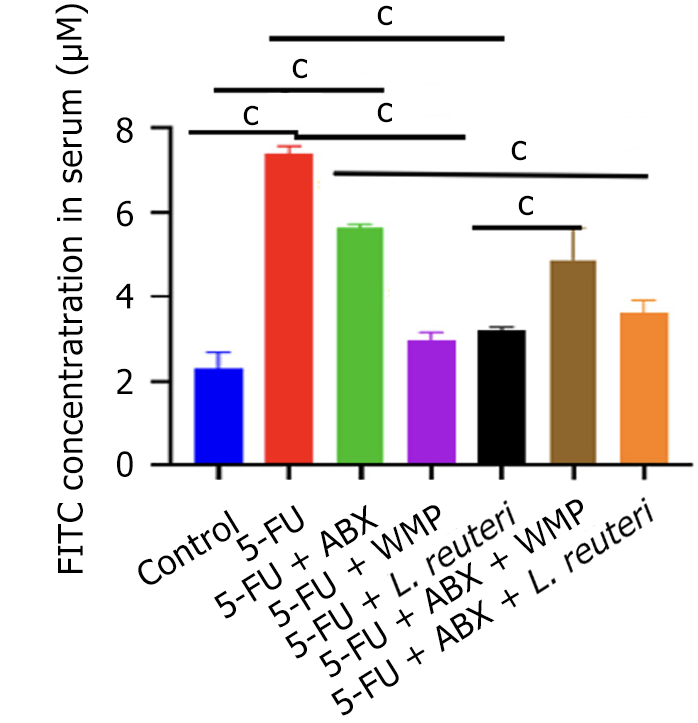
To assess the impact of WMP and L. reuteri on intestinal mucosal barrier integrity, we conducted a FITC-dextran leakage assay (Figure 4). The 5-FU group exhibited significantly greater dextran leakage compared to both the control and ABX groups (P < 0.05). However, both WMP and L. reuteri significantly reduced this leakage, as indicated by lower serum FITC-dextran concentrations, while the PGF group after WMP and L. reuteri administered had significantly higher FITC-dextran concentration than the control group. It suggests that intestinal flora plays an important role in the occurrence and development of inflammation.
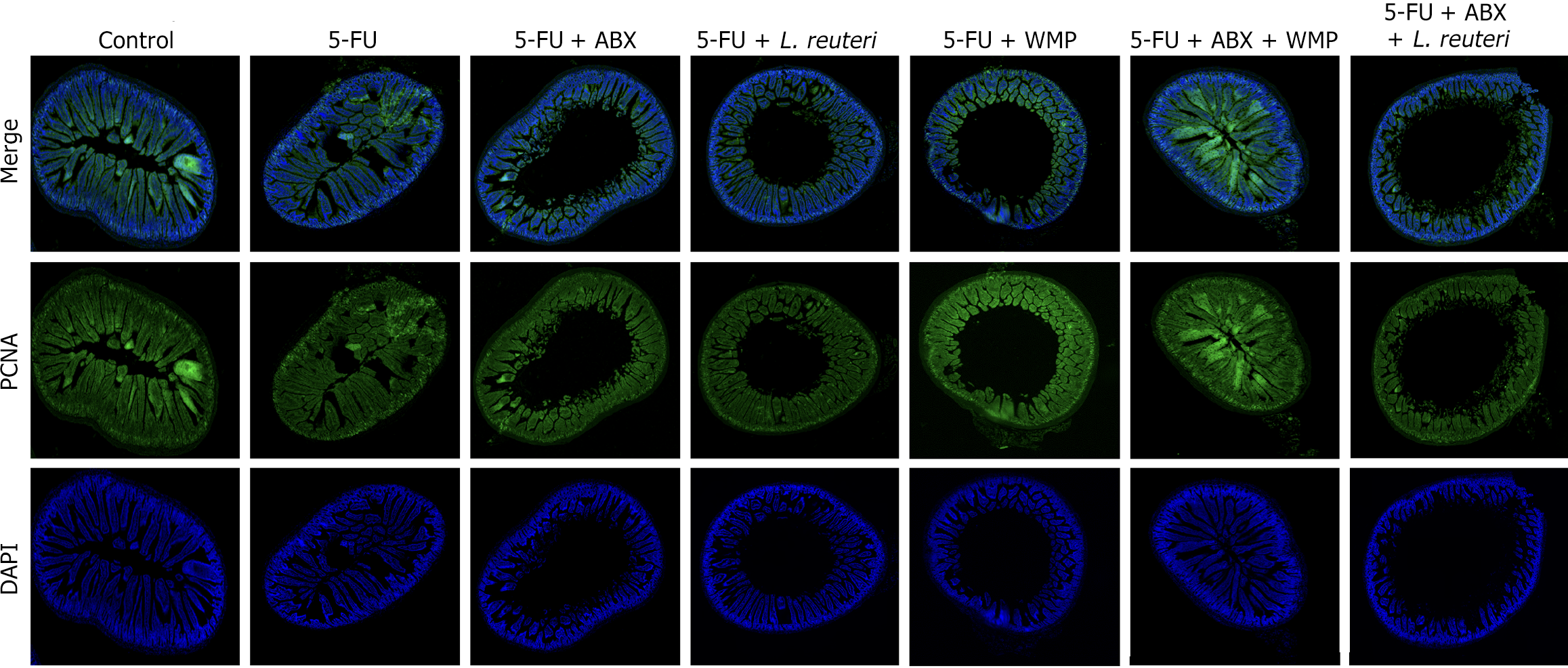
Cell proliferation and apoptosis promote intestinal epithelial cell renewal, maintaining epithelial barrier integrity. In order to further confirm the promoting effect of WMP and L. reuteri on the proliferation of intestinal epithelial cells, we detected the number and gene expression of PCNA on jejunum tissues of mice in each group. The results showed that 5-FU reduced the gene expression of PCNA in jejunum of normal mice and PGF mice. WMP and L. reuteri treatment significantly increased PCNA gene expression in jejunum of 5-FU mice and PGF mice, and PCNA expression in PGF mice after WMP administration was higher than that in 5-FU group, indicating the promoting effect of WMP on intestinal flora (Figure 5).
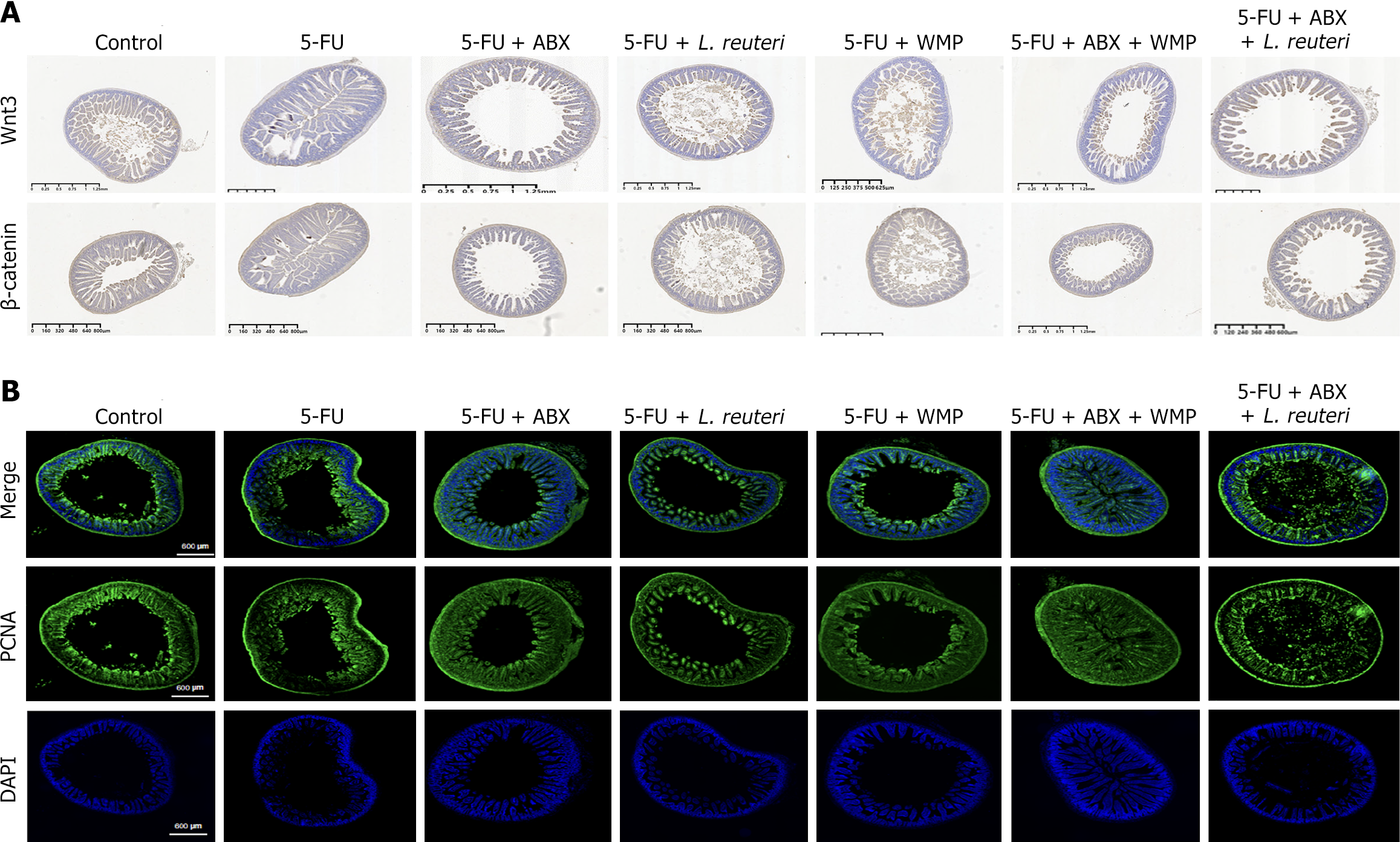
Immunohistochemistry assessed changes in the Wnt/β-catenin signaling pathway within jejunum tissue, confirming its role in regulating ISC activity and mitigating 5-FU-induced intestinal inflammation. The study found that WMP and L. reuteri treatment significantly reversed the activity of key pathway markers (Lgr5, β-catenin) and the downstream ligand Wnt3. The findings further confirm that activation of the Wnt/β-catenin pathway promotes the expansion of ISCs, helping to alleviate the damage caused by 5-FU in the gut (Figure 6 and 7).
“Regulating, is harmonizing” is an important rule of TCM to adjust yin-yang and maintain the homeostasis. Originating from the Treatise on Exogenous Febrile Disease, WMP comprises ten herbs: Wu Mei, Dang Gui, Xi Xin, Gui Zhi, Fu Zi, Huang Bo, Hua Jiao, Ren Shen, Gan Jiang, and Huang Lian. Master of TCM Zhong-Ying Zhou and our previous clinical studies both demonstrated the efficacy of WMP in treating chemotherapy-induced diarrhea/mucositis[13,19]. However, as WMP is primarily indicated for diarrhea of the upper cold and lower heat syndrome type, dosage should be flexibly adjusted based on syndrome diagnosis. In previous studies, we found that WMP can regulate intestinal flora, especially increase the abundance of L. reuteri, so as to repair the intestinal mucosal barrier and inhibit intestinal inflammation caused by 5-FU[14]. Lactobacillus, a predominant intestinal bacterium in animals, promotes gut health by strengthening the mucosal barrier and supporting cellular regeneration[20,21]. Studies have shown that L. reuteri can significantly improve the diarrhea symptoms of adults with severe irritable bowel syndrome[22]. However, as the microbiota treatment is affected by diet and emotions, the clinical effects are still inconsistent. The latest research has found that lactic acid bacteria exert anti-inflammatory effects by promoting the proliferation of ISCs, regulating the repair and renewal of intestinal epithelial cells, and maintaining the integrity of intestinal barrier function[23,24]. In this study, we found that both WMP and L. reuteri significantly reduced 5-FU-induced intestinal inflammation and enhanced the epithelial barrier in mice. This study further reveals that these beneficial effects are mediated by L. reuteri, which drives the expansion of Lgr5+ ISCs by up-regulating the activation of the Wnt/β-catenin signaling pathway, thereby repairing the integrity of the intestinal epithelial barrier and reducing inflammation.
VH and CD are key indicators to evaluate the absorption effect of nutrients in small intestine[23]. Greater villi height typically improves digestion and absorption in the small intestine. This is partly because a higher villus-crypt ratio increases the absorptive area, meaning fewer epithelial cells are needed for renewal. Consequently, more cells are available for nutrient digestion and absorption[24,25]. Our results show that 5-FU induced mice exhibit significant villi damage, characterized by disorganization, reduced number and height, and increased atrophy, mucosal collapse, and cell detachment. WMP and L. reuteri could reverse the intestinal pathological damage caused by 5-FU, and the improvement was more obvious in L. reuteri group. In addition, after supplementation with WMP and L. reuteri, VH was increased and CD was decreased in both 5-FU group and PGF mice, indicating that WMP and L. reuteri promoted intestinal development and formed a more developed intestinal structure, which was conducive to enhancing nutrient absorption.
Intestinal barrier is an important defense mechanism that maintains intestinal homeostasis by preventing pathogens, toxins and antigens from entering mucosal tissues[26,27]. Previous studies have shown that WMP can reverse the increase in intestinal permeability induced by 5-FU and increase the expression of tight junction proteins zonula occludens-1, claudin-1, and E-cadherin[14]. Our results show that WMP and L. reuteri can reduce the increase in intestinal permeability induced by 5-FU, indicating that 5-FU damages intestinal barrier function and increases the penetration of harmful substances in food or feed, while both WMP and L. reuteri can alleviate this change, and the improvement of mice in PGF group is less than that in 5-FU group. It shows that intestinal flora is involved in this process.
The production of diverse epithelial cells by ISCs is indispensable for sustaining the intestinal epithelial barrier. Concurrently, the balance between ISCs proliferation and apoptosis is vital for maintaining intestinal homeostasis[28,29]. Studies have shown that in mouse experiments, 5-FU promoted the apoptosis of ISCs, inhibited the proliferation of intestinal mucosal epithelial cells[30], and caused an immunoinflammatory cascade reaction, resulting in increased expressions of LPS, IL-6 and TNF-α. As an endogenous marker of cell replication, PCNA is an indicator of proliferative activity[31]. Our study showed that WMP and L. reuteri increased the expression of PCNA gene in jejunum, indicating that WMP and L. reuteri promoted the regeneration of intestinal epithelial cells by promoting the proliferation of intestinal epithelial cells, and ultimately improved the intestinal damage caused by 5-FU.
Previous studies have clarified that the dynamic regulation of the intestinal tight-connecting barrier by cytokines, pro-inflammatory cytokines like TNF-α, LPS, and IL-6 play key roles in immune stress responses but also disrupt intestinal barrier integrity, increasing permeability and triggering systemic inflammation[32]. Intestinal organoid experiments have confirmed that LPS can cause epithelial cell damage and stem cell apoptosis[33]. In our study, WMP and L. reuteri reversed the increase in 5-FU induced proinflammatory cytokine expression. Repair of intestinal damage requires preserving the stem-cell nature of ISCs, which can differentiate into all intestinal epithelial cell types. Residing in crypt niches within a specific Wnt microenvironment, ISCs critically protect epithelial integrity and enable sensitive responses to external stimuli[34]. During injury, activation of Wnt/β-catenin signaling enhances ISC function, whereas Bmi1+ stem cells restore the stem cell population after Lgr5+ stem cell ablation[35]. Previous studies in animals have shown that probiotics can help heal intestinal inflammation by triggering the Wnt/β-catenin signaling pathway[36]. In our study, WMP and L. reuteri significantly increased the fluorescence level of Lgr5 and the gene expression of Wnt3 and β-catenin in CIM mice.
In summary, our findings suggest that intestinal microbiota contribute to the maintenance of ISC function, and that both WMP and L. reuteri are associated with improvements in CIM-related intestinal injury and barrier function in mice. The data indicate that WMP treatment may increase L. reuteri abundance and modulate the Wnt/β-catenin signaling pathway, which could support ISC activity and epithelial renewal. While these results are consistent with a potential role for WMP in preserving mucosal integrity, further quantitative studies - particularly to confirm ISC proliferation and mechanistic specificity - are needed. These findings provide preliminary support for exploring WMP and L. reuteri as candidates for CIM mitigation, within the broader framework of TCM’s “harmonization” concept, but clinical translation will require additional validation.
| 1. | Citi S. Intestinal barriers protect against disease. Science. 2018;359:1097-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 105] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 2. | Bjarnason I, Scarpignato C, Holmgren E, Olszewski M, Rainsford KD, Lanas A. Mechanisms of Damage to the Gastrointestinal Tract From Nonsteroidal Anti-Inflammatory Drugs. Gastroenterology. 2018;154:500-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 356] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 3. | Si X, Song Z, Liu N, Jia H, Liu H, Wu Z. α-Ketoglutarate Restores Intestinal Barrier Function through Promoting Intestinal Stem Cells-Mediated Epithelial Regeneration in Colitis. J Agric Food Chem. 2022;70:13882-13892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 4. | Andersson-Rolf A, Zilbauer M, Koo BK, Clevers H. Stem Cells in Repair of Gastrointestinal Epithelia. Physiology (Bethesda). 2017;32:278-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 5. | Kou Y, Li J, Zhu Y, Liu J, Ren R, Jiang Y, Wang Y, Qiu C, Zhou J, Yang Z, Jiang T, Huang J, Ren X, Li S, Qiu C, Wei X, Yu L. Human Amniotic Epithelial Stem Cells Promote Colonic Recovery in Experimental Colitis via Exosomal MiR-23a-TNFR1-NF-κB Signaling. Adv Sci (Weinh). 2024;11:e2401429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 6. | Palikuqi B, Rispal J, Reyes EA, Vaka D, Boffelli D, Klein O. Lymphangiocrine signals are required for proper intestinal repair after cytotoxic injury. Cell Stem Cell. 2022;29:1262-1272.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 56] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 7. | Azkanaz M, Corominas-Murtra B, Ellenbroek SIJ, Bruens L, Webb AT, Laskaris D, Oost KC, Lafirenze SJA, Annusver K, Messal HA, Iqbal S, Flanagan DJ, Huels DJ, Rojas-Rodríguez F, Vizoso M, Kasper M, Sansom OJ, Snippert HJ, Liberali P, Simons BD, Katajisto P, Hannezo E, van Rheenen J. Retrograde movements determine effective stem cell numbers in the intestine. Nature. 2022;607:548-554. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 47] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 8. | Hou Q, Huang J, Ayansola H, Masatoshi H, Zhang B. Intestinal Stem Cells and Immune Cell Relationships: Potential Therapeutic Targets for Inflammatory Bowel Diseases. Front Immunol. 2020;11:623691. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 93] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 9. | Wang Y, You K, You Y, Li Q, Feng G, Ni J, Cao X, Zhang X, Wang Y, Bao W, Wang X, Chen T, Li H, Huang Y, Lyu J, Yu S, Li H, Xu S, Zeng K, Shen X. Paeoniflorin prevents aberrant proliferation and differentiation of intestinal stem cells by controlling C1q release from macrophages in chronic colitis. Pharmacol Res. 2022;182:106309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 10. | Luo H, Li M, Wang F, Yang Y, Wang Q, Zhao Y, Du F, Chen Y, Shen J, Zhao Q, Zeng J, Wang S, Chen M, Li X, Li W, Sun Y, Gu L, Wen Q, Xiao Z, Wu X. The role of intestinal stem cell within gut homeostasis: Focusing on its interplay with gut microbiota and the regulating pathways. Int J Biol Sci. 2022;18:5185-5206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 11. | Wang J, Zhao Y, Cui T, Bao H, Gao M, Cheng M, Sun Y, Lu Y, Guan J, Zhang D, Jiang Y, Huang H, Shi C, Wang J, Wang N, Hu J, Yang W, Qian H, Jiang Q, Yang G, Zeng Y, Wang C, Cao X. AhR ligands from LGG metabolites promote piglet intestinal ILC3 activation and IL-22 secretion to inhibit PEDV infection. J Virol. 2024;98:e0103924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 12. | Reedy AR, Luo L, Neish AS, Jones RM. Commensal microbiota-induced redox signaling activates proliferative signals in the intestinal stem cell microenvironment. Development. 2019;146:dev171520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 13. | Lu DX, Yan J, Sun ZG, Li H, Liu F, Wang YJ, Ge F. [Clinical Efficacy and Mechanism of Wumei Pill in the Treatment of Chemotherapy-induced Intestinal Mucositis]. Nanjing Zhongyiyao Daxue Xuebao. 2021;37:371-375. [DOI] [Full Text] |
| 14. | Lu DX, Liu F, Wu H, Liu HX, Chen BY, Yan J, Lu Y, Sun ZG. Wumei pills attenuates 5-fluorouracil-induced intestinal mucositis through Toll-like receptor 4/myeloid differentiation factor 88/nuclear factor-κB pathway and microbiota regulation. World J Gastroenterol. 2022;28:4574-4599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 15. | Luo Z, Chen A, Xie A, Liu X, Jiang S, Yu R. Limosilactobacillus reuteri in immunomodulation: molecular mechanisms and potential applications. Front Immunol. 2023;14:1228754. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 42] [Reference Citation Analysis (0)] |
| 16. | Xu Y, Ding X, Wang Y, Li D, Xie L, Liang S, Zhang Y, Li W, Fu A, Zhan X. Bacterial Metabolite Reuterin Attenuated LPS-Induced Oxidative Stress and Inflammation Response in HD11 Macrophages. Antioxidants (Basel). 2022;11:1662. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 17. | Akbarali HI, Muchhala KH, Jessup DK, Cheatham S. Chemotherapy induced gastrointestinal toxicities. Adv Cancer Res. 2022;155:131-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 123] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 18. | Kullenberg F, Peters K, Sjöblom M, Heindryckx F, Dahlgren D, Lennernäs H. Anakinra and dexamethasone treatment of idarubicin-induced mucositis and diarrhoea in rats. Basic Clin Pharmacol Toxicol. 2023;132:511-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 19. | Gong SD, Shi Y. [Experience of Professor ZHOU Zhongying Using Wumei Pill in Treating Chemotherapy Related Diarrhea of Esophageal Cancer]. Zhejiang Zhongyiyao Daxue Xuebao. 2018;42:287-289. [DOI] [Full Text] |
| 20. | Shao Y, Zhen W, Guo F, Hu Z, Zhang K, Kong L, Guo Y, Wang Z. Pretreatment with probiotics Enterococcus faecium NCIMB 11181 attenuated Salmonella Typhimurium-induced gut injury through modulating intestinal microbiome and immune responses with barrier function in broiler chickens. J Anim Sci Biotechnol. 2022;13:130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 27] [Reference Citation Analysis (0)] |
| 21. | Li M, Ding Y, Wei J, Dong Y, Wang J, Dai X, Yan J, Chu F, Zhang K, Meng F, Ma J, Zhong W, Wang B, Gao Y, Yang R, Liu X, Su X, Cao H. Gut microbiota metabolite indole-3-acetic acid maintains intestinal epithelial homeostasis through mucin sulfation. Gut Microbes. 2024;16:2377576. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 70] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 22. | Cruchet S, Hirsch S, Villa-López D, Moreno-Portillo M, Palomo JC, Abreu-Abreu AT, Abdo-Francis JM, Jiménez-Gutiérrez C, Rojano M, López-Velázquez G, Gutiérrez-Castrellón P. Limosilactobacillus reuteri DSM 17938 and ATCC PTA 6475 for the treatment of moderate to severe irritable bowel syndrome in adults: a randomized controlled trial. Front Gastroenterol. 2024;2:1296048. [DOI] [Full Text] |
| 23. | Li G, Wang X, Liu Y, Gong S, Yang Y, Wang C, Wang H, He D. Bile acids supplementation modulates lipid metabolism, intestinal function, and cecal microbiota in geese. Front Microbiol. 2023;14:1185218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 24. | La J, Raghunathan K, Silvester JA, Thiagarajah JR. ViCE: An automated and quantitative program to assess intestinal tissue morphology. J Pathol Inform. 2024;15:100397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Saadatmand N, Toghyani M, Gheisari A. Effects of dietary fiber and threonine on performance, intestinal morphology and immune responses in broiler chickens. Anim Nutr. 2019;5:248-255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 26. | Li S, Li T, Jiang Z, Hou W, Hou Q, Serrano BR, Barcenas AR, Wang Y, Zhao W. Dietary Mulberry leaf 1-deoxynijirimycin supplementation shortens villus height and improves intestinal barrier in fattening rabbits. Anim Biosci. 2024;37:2101-2112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 27. | Wang X, Xiao K, Yu C, Wang L, Liang T, Zhu H, Xu X, Liu Y. Xylooligosaccharide attenuates lipopolysaccharide-induced intestinal injury in piglets via suppressing inflammation and modulating cecal microbial communities. Anim Nutr. 2021;7:609-620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 28. | Wu H, Mu C, Xu L, Yu K, Shen L, Zhu W. Host-microbiota interaction in intestinal stem cell homeostasis. Gut Microbes. 2024;16:2353399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 20] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 29. | Baghdadi MB, Houtekamer RM, Perrin L, Rao-Bhatia A, Whelen M, Decker L, Bergert M, Pérez-Gonzàlez C, Bouras R, Gropplero G, Loe AKH, Afkhami-Poostchi A, Chen X, Huang X, Descroix S, Wrana JL, Diz-Muñoz A, Gloerich M, Ayyaz A, Matic Vignjevic D, Kim TH. PIEZO-dependent mechanosensing is essential for intestinal stem cell fate decision and maintenance. Science. 2024;386:eadj7615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 44] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 30. | Günther C, Neumann H, Neurath MF, Becker C. Apoptosis, necrosis and necroptosis: cell death regulation in the intestinal epithelium. Gut. 2013;62:1062-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 358] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 31. | Zhou JY, Lin HL, Wang Z, Zhang SW, Huang DG, Gao CQ, Yan HC, Wang XQ. Zinc L-Aspartate enhances intestinal stem cell activity to protect the integrity of the intestinal mucosa against deoxynivalenol through activation of the Wnt/β-catenin signaling pathway. Environ Pollut. 2020;262:114290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 32. | Zhao X, Ma B, Zhu H, Bai J, Liu L, Li X, Cai J, Wang B, Wang L, Pang Y, Zhang H, Yang YI, Li N, Chen M. PI3K/Akt and Wnt/β-catenin Signaling Cross-regulate NF-κB Signaling in TNF-α-induced Human Lgr5(+) Intestinal Stem Cells. Anticancer Res. 2022;42:3325-3340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 33. | Zhang Z, Zuo L, Song X, Wang L, Zhang Y, Cheng Y, Huang J, Zhao T, Yang Z, Zhang H, Li J, Zhang X, Geng Z, Wang Y, Ge S, Hu J. Arjunolic acid protects the intestinal epithelial barrier, ameliorating Crohn's disease-like colitis by restoring gut microbiota composition and inactivating TLR4 signalling. Phytomedicine. 2024;123:155223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 34. | Yun J, Hansen S, Morris O, Madden DT, Libeu CP, Kumar AJ, Wehrfritz C, Nile AH, Zhang Y, Zhou L, Liang Y, Modrusan Z, Chen MB, Overall CC, Garfield D, Campisi J, Schilling B, Hannoush RN, Jasper H. Senescent cells perturb intestinal stem cell differentiation through Ptk7 induced noncanonical Wnt and YAP signaling. Nat Commun. 2023;14:156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 44] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 35. | Ding X, Tang R, Zhao J, Xu Y, Fu A, Zhan X. Lactobacillus reuteri alleviates LPS-induced intestinal mucosal damage by stimulating the expansion of intestinal stem cells via activation of the Wnt/β-catenin signaling pathway in broilers. Poult Sci. 2024;103:104072. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 36. | Zhou JY, Huang DG, Zhu M, Gao CQ, Yan HC, Li XG, Wang XQ. Wnt/β-catenin-mediated heat exposure inhibits intestinal epithelial cell proliferation and stem cell expansion through endoplasmic reticulum stress. J Cell Physiol. 2020;235:5613-5627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/