Published online Sep 26, 2025. doi: 10.4252/wjsc.v17.i9.107689
Revised: April 25, 2025
Accepted: August 8, 2025
Published online: September 26, 2025
Processing time: 180 Days and 16.2 Hours
Neural crest-derived mesenchymal stem cells (NC-MSCs) represent a unique population with remarkable regenerative potential, owing to their embryonic origin and exceptional differentiation capacity. These cells demonstrate superior performance in neural and craniofacial tissue regeneration compared to conventional mesenchymal stem cells, with dental stem cells emerging as particularly promising candidates for clinical applications in periodontics and endodontics. Despite their therapeutic promise, adult NC-MSCs face significant challenges including donor site limitations, cellular heterogeneity, and scalability issues. Recent advances in pluripotent stem cell offer potential solutions through the generation of NC-MSCs in vitro, though safety concerns regarding tumorigenicity and long-term stability remain to be addressed through comprehensive preclinical studies. This review provides a comprehensive analysis of NC-MSC biology, highlighting their developmental origins, molecular characteristics, and current applications in regenerative medicine. We critically evaluate existing challenges and future directions, emphasizing the need for standardized protocols, improved characterization methods, and rigorous preclinical evaluation to facilitate clinical translation and therapeutic implementation.
Core Tip: Recent studies have revealed the distribution and functions of neural crest (NC)-derived mesenchymal stem cells (MSCs) in various tissues, with regenerative therapies based on these cells showing positive results in research and clinical practice. Future research focuses on optimizing therapeutic strategies (timing, dosage), expanding clinical applications, and addressing current challenges. Several approaches aim to solve issues like insufficient cell numbers, heterogeneity, and trauma. Notably, MSCs differentiated from pluripotent stem cells via NC pathways show promise as a stable NC-derived MSC source, potentially overcoming current limitations in clinical applications.
- Citation: He YL, Chen SJ, Xia DS, Song WP. Neural crest-derived mesenchymal stem cells: Fates and perspectives. World J Stem Cells 2025; 17(9): 107689
- URL: https://www.wjgnet.com/1948-0210/full/v17/i9/107689.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v17.i9.107689
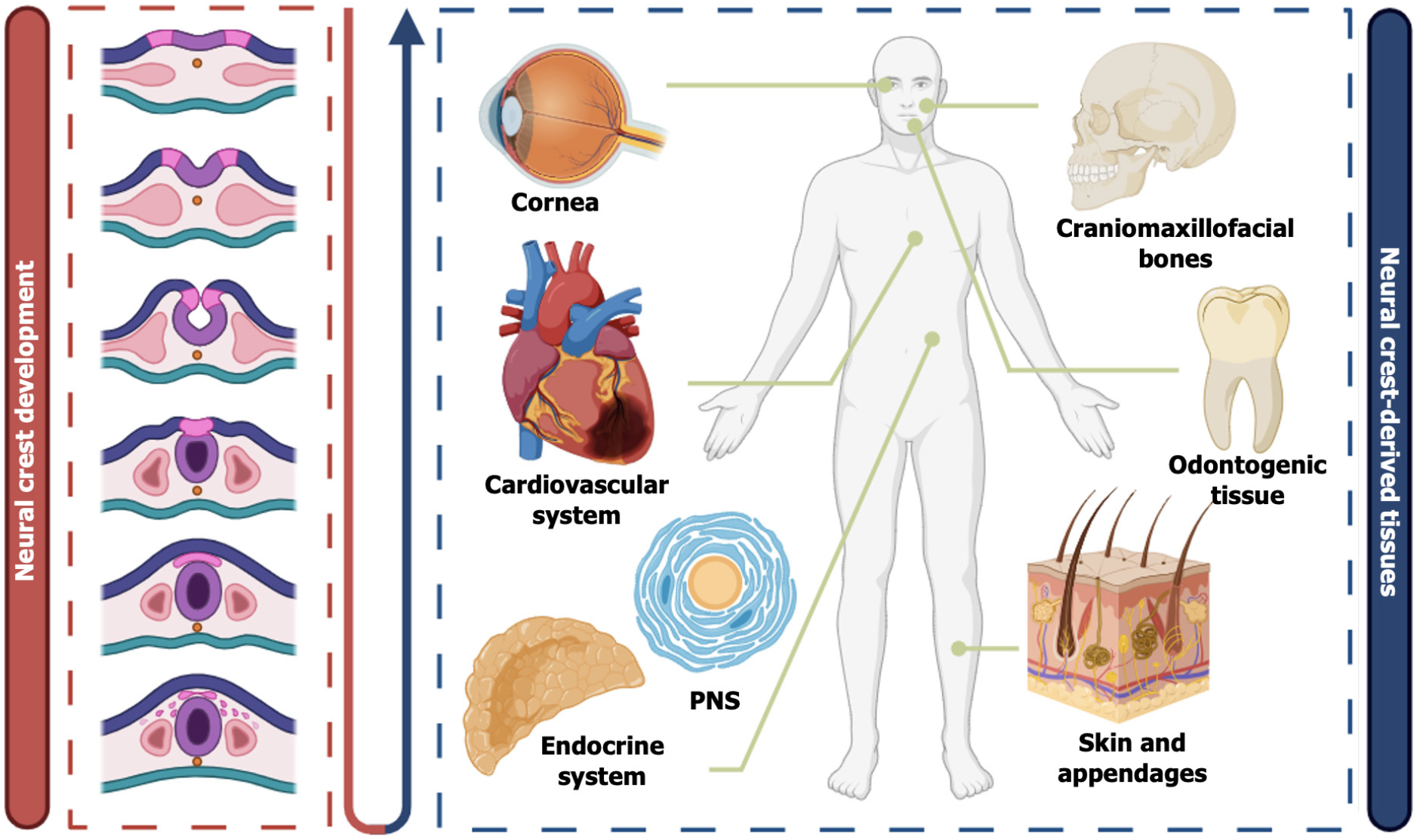
The neural crest (NC) is a highly migratory cell population derived from the neuroectoderm during embryonic development, capable of acquiring diverse cell fates[1-3]. NC cells (NCCs) undergo epithelial-mesenchymal transition (EMT), migrate to various tissues throughout the body, and differentiate into multiple cell types, including neurons, Schwann cells, and melanocytes, as well as specific mesenchymal derivatives such as craniofacial osteoblasts, chondrocytes, dental mesenchymal cells, and peripheral nerve-associated mesenchymal cells[4-6]. The mesenchymal stem cell (MSC)-like populations derived from the NC are collectively referred to as NC-derived MSCs (NC-MSCs).
In recent years, NC-MSCs have garnered increasing attention in the field of regenerative medicine due to their remarkable differentiation potential and regenerative capacity. In particular, studies have demonstrated the unique advantages of dental stem cells (DSCs), craniofacial bone stem cells, and peripheral nerve-associated MSCs in osteogenesis, neuroregeneration, and immunomodulation[7-10]. For example, dental pulp stem cells (DPSCs) have been extensively studied for their roles in bone tissue repair and neuroprotection, while Schwann cell-like cells derived from peripheral nerves have been shown to effectively promote peripheral nerve regeneration[11-14]. However, similar to conventional mesoderm-derived MSCs, such as bone marrow-derived MSCs (BMSCs) and adipose-derived MSCs (ADSCs), NC-MSCs still face several challenges, including limited cell sources, high heterogeneity, and the invasive nature of their extraction procedures[7,15-18].
To overcome these limitations, pluripotent stem cells (PSCs) have emerged as a promising alternative source for NC-MSCs. PSCs, primarily including embryonic stem cells (ESCs) and induced PSCs (iPSCs), possess self-renewal capacity and multilineage differentiation potential[19-23]. In recent years, various methods have been developed to differentiate PSCs into NC-MSCs and explore their applications in tissue engineering and cell therapy[24-26]. Moreover, the advancement of extended PSCs (EPSCs) has provided a novel candidate cell source for NC-MSC research due to their enhanced genetic and epigenetic stability and their ability to circumvent ethical and regulatory concerns[27-29]. Despite rapid progress in PSCs-derived NC-MSCs research, significant challenges remain in clinical translation. A critical concern is the risk of teratoma formation, as residual undifferentiated PSCs may lead to tumor-like overgrowth post-transplantation[30]. Therefore, developing efficient strategies to eliminate residual PSCs and ensuring the safety of PSC-derived NC-MSCs remain key challenges in the field.
This review comprehensively discusses the developmental origins, cellular properties, and clinical applications of NC-MSCs. First, we provide an overview of NC-MSC development, migration pathways, and differentiation characteristics, systematically summarizing their functional roles across different tissues. Second, we review the latest clinical research progress on NC-MSCs, including DSCs and other NC-MSCs in the treatment of oral diseases, neurological disorders, and other systemic conditions. Subsequently, we highlight PSCs-derived NC-MSCs, detailing their directed differentiation strategies and evaluating their potential in overcoming the limitations of adult NC-MSCs. Finally, we discuss the safety concerns associated with PSC-derived NC-MSCs and propose future research directions. Through this review, we aim to provide a theoretical foundation for NC-MSC applications in regenerative medicine and cell therapy, facilitating their transition toward clinical applications.
The NC originates from the region at the edge of the neural plate, situated between the neural plate and the adjacent non-neural ectoderm. The NC is a group of progenitor cells that plays a crucial role in the embryonic development of vertebrates[31,32]. During embryogenesis, NCCs undergo EMT, subsequently migrating extensively to various regions of the embryo[33,34] (Figure 1). These multipotent cells can differentiate into a variety of cell types, including chondrocytes, osteocytes, melanocytes, neurons of the peripheral nervous system (PNS), Schwann cells, chromaffin cells, and cardiomyocytes, among others, contributing to the formation of multiple tissues and organs in the embryo (Figure 1)[35,36]. However, the definition of their stem cell properties was not clearly established until 1992, when the research by Stemple and Anderson[37] provided clarity. They first proposed the concept of “neural crest stem cells” and, through in vitro experiments, confirmed that NCCs derived from mice not only possess multipotent differentiation ability but also exhibit self-renewal characteristics, which are core features of stem cells[37]. Although these adult stem cells originate from the ectoderm, under the regulation of specific signaling pathways and epigenetic memory, they have the capacity to differentiate into both ectodermal and mesodermal cells[38], opening new therapeutic targets for regenerative medicine. The following section will systematically summarize different types of MSCs derived from the NC, categorizing and discussing their developmental origins, migration paths, and related molecular markers based on tissue origin differences.
DSCs are the most extensively studied subgroup of NC-MSCs, receiving considerable attention due to their accessibility, high proliferation potential, and multipotent differentiation capabilities[39]. These cells play a crucial role in the formation of teeth and their surrounding periodontal tissues. Among DSCs, DPSCs, stem cells from human exfoliated deciduous teeth (SHED), and periodontal ligament stem cells (PDLSCs) are all types of NC-derived stem cells. These cells express MSC markers such as Stro-1, CD146, CD106, CD90, CD73, CD29, and CD13, and consistently test negative for hematopoietic stem cell markers such as CD34, CD45, CD14, and CD19, as well as class II major histocompatibility complex antigen[40].
These DSCs originate from the cranial NC (CNC), acquiring a mesenchymal fate as they migrate to the first pharyngeal arch and differentiate along various developmental programs, ultimately contributing to the formation of craniofacial structures, including teeth[41,42]. During the bud stage of tooth development, a special homogeneous population of cells emerges within the condensed CNC-derived mesenchyme. These cells are marked as Tfap2b+/Lhx6+/Pax9+, and this NCC cell population is considered to be progenitor cells that aid in tooth development[43]. Cells at this stage provide the fundamental cell source for subsequent tooth tissue formation and further differentiate into specific lineages of the dental papilla and dental sac. The dental papilla cell lineage is marked by genes such as Crym, Egr3, and fibroblast growth factor 3 (Fgf3), while the dental sac lineage is marked by Epha3, Foxf1, and Fxyd7[43]. As tooth development progresses, dental mesenchymal cells undergo partitioning and differentiate into various structures and cell types. The dental mesenchymal cells in the dental papilla region gradually differentiate into dental pulp tissue and odontoblasts that secrete dentin, while the dental sac region surrounding the papilla differentiates into periodontal-related structures, including cementum, periodontal ligament (PDL), and part of the alveolar bone[44].
Specifically, DPSCs migrate from the CNC to the first pharyngeal arch, where they gather to form the dental papilla during the cap stage of tooth development[39]. These cells are then enveloped by the enamel organ, and during the bell stage, they differentiate into odontoblasts, ultimately forming the dental pulp mesenchyme[45]. Apical papilla stem cells (SCAP) are primarily located in the apical papilla region of immature permanent teeth. After tooth eruption, NCCs continue to migrate to the apical region and interact with Hertwig’s epithelial root sheath, jointly promoting root development[46]. Due to SCAP’s high telomerase activity, these cells have strong proliferative potential, providing an important cellular source for root formation. Their self-renewal ability also ensures their vitality during the process of root growth[47]. The biological behavior of SCAP, including differentiation, maturation, and mineralization, is finely regulated by various signaling pathways such as transforming growth factor (TGF)-β, WNT, and mitogen-activated protein kinases, ensuring a continuous supply of odontoblasts and promoting proper root development[48].
Moreover, NCCs also migrate to the dental follicle and gradually differentiate into mesenchymal cell populations that give rise to the PDL[49]. PDLSCs play a crucial role in the formation and repair of periodontal tissues. Their differentiation is tightly regulated by insulin-like growth factor and the transcription factor Foxp4. Studies have shown that although insulin-like growth factor and Foxp4 do not significantly affect root development, they do result in a marked increase in PDL area and suppress PDL differentiation[43]. PDLSCs are capable of differentiating into osteoblasts and fibroblasts and contribute to the maintenance of PDL function, thereby enhancing the stability and regeneration of periodontal tissues[50].
Dental follicle stem cells (DFSCs), the only cell type obtained during the early stages of tooth development prior to eruption, also originate from the CNC. These cells are derived from ectomesenchyme and form a loose connective tissue[51]. During the bud stage, the dental follicle begins to form and surrounds the dental papilla and enamel organ. By the cap stage of tooth development, it migrates to the dental follicle region and encloses the entire tooth germ[51]. As development progresses, DFSCs gradually differentiate into key components such as cementum, PDL, and alveolar bone[52].
In conclusion, NC-MSCs regulate the lineage commitment of DSCs with spatial and temporal specificity through orchestrated migration and differentiation programs. This process involves coordinated expression of multiple genes and a dynamic balance of microenvironmental signaling networks, forming a crucial theoretical foundation for both tooth development and regenerative medicine research.
Unlike mesoderm-derived MSCs from long bones, craniofacial BMSCs (C-BMSCs) possess distinct NC origins and undergo intramembranous ossification during development, rather than endochondral ossification[53,54]. In the early stages of embryonic development, NCCs migrate along specific pathways from the neural tube to the branchial arch regions of the head and neck. In the anterior region, cranial NCCs contribute to the formation of the frontonasal skeleton; in more posterior regions, they populate the pharyngeal arches, where they give rise to the jawbones, middle ear, and various cartilaginous and skeletal structures of the neck[55-57].
Using transgenic mice (Wnt1-Cre/R26R) to trace NCCs, researchers have discovered that the interface between the frontal and parietal bones is formed through the combined contributions of NCCs and mesodermal cells[8]. Specifically, NCCs predominantly give rise to the frontal bone, while the parietal bone is derived from the mesoderm. Cranial NCCs originate mainly from the forebrain and midbrain regions. Diencephalic and anterior mesencephalic NCCs migrate to the primordia of the frontal, nasal, and parietal bones, while posterior mesencephalic NCCs contribute to the formation of the temporal bones[56]. These cranial NCCs express markers such as Sox9, Pax3, and Runx2[57]. After migrating to cranial regions, they retain the ability to differentiate into multiple cell types of the skeletal system, particularly osteoblasts and chondrocytes, playing essential roles in the formation and remodeling of craniofacial bones.
The development of the maxilla also begins with the migration and differentiation of NCCs. During the early stages of embryogenesis, NCCs aggregate in the frontonasal region and migrate into the frontonasal prominence and the first branchial arch. As development progresses, they contribute to the formation of the maxilla and its associated structures[56]. Mandibular development, on the other hand, is initiated by the formation of Meckel’s cartilage. NCCs migrate into the mandibular arch, where they intermingle with non-NC-derived cells to establish the initial ossification centers of the mandible[56,58]. NC markers such as SNAIL2, TWIST, SOX9, and SOX10 have also been identified in maxillary and mandibular BMSCs cultured in various media[59]. These cells possess the ability to differentiate into osteoblasts and chondrocytes and play critical roles in the development and repair of jawbones[60].
Among NC-MSCs, corneal stromal stem cells (CSSCs) are one of the most representative types[61-64]. CSSCs have the primary ability to differentiate into corneal epithelial cells, which are responsible for maintaining the corneal stroma[65]. In chick embryos, for instance, corneal development begins around embryonic day 3 (E3), when the optic cup and lens induce the surface ectoderm to synthesize collagen fibers, forming a primary acellular stroma[66,67]. By E4, mesenchymal NCCs begin to migrate into this stroma and form the corneal endothelium near the lens. A second wave of NCCs later enters to further develop the primary stroma[66,68]. Within the stroma, NC-derived mesenchymal cells start differentiating into keratocytes around E6, synthesizing and secreting types I, V, and VI collagen, along with proteoglycans, essential for maintaining corneal transparency and mechanical properties[69-71]. CSSCs express a variety of specific genes, including adult stem cell markers such as ABCG2, BMi1, CD166, cKIT, and Notch1, as well as PAX6 and Six2, which are involved in early corneal development[72]. In addition to exhibiting adult stem cell characteristics, CSSCs also demonstrate immune privilege. Studies have shown that injecting human CSSCs into the corneal stroma of mice does not trigger a T cell-mediated immune rejection[73]. This immune privilege makes CSSCs a promising therapeutic tool in the field of bioengineering.
As multipotent embryonic progenitors, NCCs are the developmental origin of all neurons and glial cells in the PNS[74]. Their development involves three key stages: First, following the establishment of the central nervous system primordium, NCCs delaminate from the dorsal neural tube through EMT[75-77]. This process is tightly regulated by bone morphogentic protein (BMP) and Wnt signaling pathways. BMP promotes NC migration by modulating the cell cycle and inducing EMT, while Wnt signaling activates cyclin D1 to drive the G1/S transition - a crucial step for NC lineage stratification[78,79]. As migration and differentiation proceed, NCCs give rise to various PNS cell types, including neurons and glia. In this context, NC-MSCs are considered vital for PNS development and regeneration.
Peripheral nerve-associated NC-MSCs, such as Schwann cell precursors (SCPs), express surface markers like CD271, CD90, and CD106[80,81]. These MSCs not only differentiate into Schwann cells but also secrete neurotrophic factors that support axonal regeneration[14]. Another example is the hair follicle-derived NC MSCs (HFNCSCs), which are multipotent stem cells located in the bulge region of hair follicles. During early embryogenesis, these cells migrate from the neural tube along the dorsolateral pathway, passing through the dermis into the epidermis and ultimately settling in the follicular bulge[82]. While the epithelial components of hair follicles are typically derived from surface ectoderm, the presence of NC-derived cells in the bulge region suggests the existence of a distinct population retaining NC characteristics[82]. HFNCSCs express CD271, Nestin, Sox10, and other markers associated with immature NCCs[83,84]. Due to their similarity to BMSCs and their ability to differentiate into myogenic, osteogenic, chondrogenic, and adipogenic lineages, HFNCSCs represent a promising source for cell-based therapies[85].
NC-MSCs exhibit distinct biological properties compared to mesoderm-derived MSCs. Notably, NC-MSCs demonstrate a higher proliferative capacity, indicating a stronger potential for self-renewal. This proliferative advantage is likely related to the retention of certain NC traits during development[86,87]. Moreover, NC-MSCs continue to express key multipotency markers such as Sox10 and Nestin even after birth, and they retain the ability to differentiate into multiple cell types. These markers are rarely expressed in mesodermal MSCs, further highlighting the unique identity of NC-MSCs[88]. In terms of differentiation potential, NC-MSCs display remarkable versatility under appropriate in vitro and in vivo conditions. They are capable of differentiating into osteoblasts, odontoblasts, and various neural cell types, with particularly strong capacity for generating neurons and glial cells[38,46,89,90].
Given the significant differences between NC-MSCs and mesoderm-derived MSCs (such as those from bone marrow or adipose tissue) in developmental origin, molecular profile, and functional characteristics, comparative studies between these cell types are essential. Such research will help elucidate the unique regenerative advantages of NC-MSCs in specific tissues and provide a theoretical foundation for selecting optimal stem cell sources in precision regenerative medicine.
DSCs originate from the NC, and their developmental origin distinguishes them significantly from mesoderm-derived MSCs such as BMSCs and ADSCs. These differences in developmental origin lead to notable disparities in their biological properties. While both cell types share basic MSC characteristics, such as self-renewal, multipotent differentiation, and immune modulation, they exhibit critical differences in these aspects[91,92].
Firstly, DSCs demonstrate a significantly higher proliferation rate compared to mesoderm-derived MSCs. Gronthos et al[86] found that DPSCs isolated from a single tooth exhibited higher proliferative potential. The colony-forming efficiency of DPSCs (22-70 colony forming units-fibroblast per 104 cells) was substantially greater than that of BMSCs (only 2.4-3.1 colony forming units-fibroblast per 104 cells). SHED showed an even higher proliferation rate than both DPSCs and BMSCs, with the proliferation ranking as follows: SHED > DPSC > BMSC[93-95]. This difference might be attributed to the early expression of ESC markers in SHED, such as octamer-binding transcription factor 4 (Oct4) and Nanog[94,95].
In terms of differentiation potential, DSCs display a stronger capacity for differentiating into neural cells[96]. This unique differentiation ability is likely linked to the selective retention of early NC developmental characteristics by NCCs[97-99]. Pagella et al[100] used microfluidic organ-on-a-chip technology to compare the effects of DPSCs and BMSCs on neural ganglia and found that DPSCs, after co-culture, induced neurons to express higher levels of neurotrophic factors and form more extensive axonal networks than BMSCs, suggesting the stronger neuroguiding capability of DPSCs. Furthermore, DPSCs have been shown to differentiate into functional neurons in appropriate microenvironments. When cultured in neurogenic media, DPSCs form bipolar and stellate neuron-like phenotypes and are capable of generating sodium currents consistent with functional neurons[101]. SHED also exhibits excellent neurogenic differentiation potential, and upon in vivo transplantation, SHED not only induced bone formation but also survived in the mouse brain and expressed neural markers[102]. Similarly, De Berdt et al[103] assessed the neurorepair potential of SCAP using a rat spinal cord hemi-section model, showing that SCAP implantation significantly reduced local inflammation, improved motor function, and did not induce chronic pain, demonstrating promising neurotherapeutic potential. Therefore, DSCs may outperform other tissue-derived MSCs in promoting myelination of the central nervous system and repairing neural injuries[101].
In addition to their neural potential, DSCs also exhibit stronger osteogenic and odontogenic differentiation capabilities[104-106]. Couble et al[107] cultured human dental pulp cells in Eagle’s basal medium with β-glycerophosphate and observed that the dental pulp cells exhibited typical cellular extensions and polarization, forming a spatial structure similar to odontoblasts, thereby demonstrating odontogenic differentiation potential. Compared to mesoderm-derived MSCs, DSCs show significantly higher alkaline phosphatase activity following osteogenic induction, a result further confirmed by alizarin red staining[108]. Park et al[105] applied decalcified bone matrix and fibrin scaffolds, evaluated the osteogenic capabilities of MSCs isolated from skin, bone marrow, and dental follicle, and found that DFSCs demonstrated stronger osteogenic potential, with higher expression of osteocalcin and calcium deposition. This suggests that DFSCs could serve as a potential alternative source of autologous bone tissue engineering cells[105]. It is important to note that NC-MSCs may have certain limitations in adipogenic differentiation[109,110]. DPSCs show slower adipogenic differentiation compared to ADSCs, with fewer lipid vesicles formed and weaker expression of adipogenic-related genes[111].
In terms of chondrogenic differentiation, several studies have compared the differentiation abilities of DSCs and BMSCs. Both DPSCs and BMSCs show positive staining and expression of cartilage-related genes such as collagen II during chondrogenic induction[112,113]. However, unlike mesoderm-derived MSCs, DPSCs tend to form fibrous cartilage rather than hyaline cartilage during culture, while BMSCs under certain conditions are more likely to form hyaline cartilage[114]. This suggests that the NC origin of DSCs might limit their ability to form hyaline cartilage, but they could potentially serve as a source for the regeneration of fibrous cartilage in joints[114].
From the perspective of angiogenic differentiation, DSCs show strong vascularization capabilities. In a study on bone organ vascularization, it was noted that DPSCs formed a reticular structure earlier (on day 3, compared to day 7 in BMSCs) with longer branching lengths and higher expression of angiogenesis-related markers such as vascular endothelial growth factor A and C-X-C motif ligand 1. In contrast, BMSCs from long bones exhibited almost no significant angiogenic potential under the same conditions, and no vascular-like structures were formed even after 20 days[115].
Beyond their multipotent differentiation capabilities, MSCs influence the immune system through direct cell-to-cell contact and/or intercellular communication, thereby exerting immunomodulatory effects[116]. Compared to mesoderm-derived MSCs, NC-MSCs exhibit stronger anti-inflammatory potential. Studies have shown that NC-derived gingival MSCs are more effective than mesoderm-derived gingival MSCs in ameliorating inflammation-related diseases. Specifically, NC-derived gingival MSCs alleviate symptoms of colitis in mice by inducing T-cell apoptosis[117,118]. Furthermore, Yamaguchi et al[119] found that the conditioned medium from SHED significantly reduced myocardial injury in ischemia-reperfusion mice and decreased local inflammatory factors such as tumor necrosis factor-α and interleukin-1β. The SHED-conditioned medium also demonstrated a significantly stronger anti-apoptotic effect on cardiomyocytes compared to BMSC-conditioned medium and ADSC-conditioned medium[119]. These immunomodulatory advantages offer new strategies for cell-based therapies in treating inflammation-related diseases.
Craniofacial bones can be divided into neurocranium (including the calvaria and cranial base) and viscerocranium (including the jawbones and other derivatives of the pharyngeal arches). From the perspective of bone development, the formation of the neurocranium fully depends on NCCs, while the viscerocranium is formed by both mesodermal cells and NCCs[53,120]. Other skeletal structures related to the craniofacial region are believed to originate from the mesoderm[121]. Compared to mesoderm-derived MSCs from long bones, C-BMSCs exhibit similar or even stronger osteogenic and angiogenic abilities, making them potentially more suitable for tissue engineering applications[8].
Recent studies have shown that BMSCs derived from craniofacial bones proliferate faster and exhibit stronger osteogenic potential compared to BMSCs from other anatomical sites[122]. For instance, Yamaza et al[123] isolated BMSCs from mouse mandibles and found that they exhibited greater proliferative potential, faster proliferation rates, and higher colony-forming efficiency compared to BMSCs derived from long bones. In another study comparing osteogenic potential, mandible BMSCs from the same SD rats were able to form more mineralized calcium nodules and significantly upregulate osteogenesis-related genes such as RUNX2 and osteocalcin during culture[124]. Moreover, several studies have also demonstrated the strong osteogenic capacity of NC-derived BMSCs in vivo[125,126]. Aghaloo et al[125] performed subcutaneous implantation experiments in nude mice and found that mandible BMSCs formed significantly larger bone nodules with mineralized bone content three times higher than that of long bone BMSCs. Similar results were further validated in implant-associated bone defect models[126]. However, mandible-derived BMSCs exhibited lower potential for chondrogenesis and adipogenesis compared to iliac bone BMSCs, which might explain the rare occurrence of cartilaginous healing at mandibular fracture sites[127].
The vascular system plays a crucial role in bone tissue repair, as angiogenesis is vital for normal bone development and fracture healing[128]. BMSCs have been shown to promote angiogenesis in the bone microenvironment, enhancing vascular endothelial growth factor expression[129,130]. A study comparing C-BMSCs, DPSCs, and SCAPs for their ability to form capillary-like networks when co-cultured with human umbilical vein endothelial cells in gelatin methacrylate hydrogels found that C-BMSCs exhibited higher levels of pericyte marker expression (such as neural-glial antigen 2 and alpha-smooth muscle actin), showing greater potential for pericyte differentiation[131]. Moreover, C-BMSCs were more suitable for building mature vascular networks compared to DPSCs or SCAPs[131]. Based on these properties, C-BMSCs may be more suitable for craniofacial bone defect repair, such as alveolar ridge augmentation, as they accelerate bone integration of implants, compared to long bone-derived BMSCs.
Additionally, C-BMSCs possess similar immunomodulatory potential to long bone-derived BMSCs, including the ability to inhibit monocyte activation, promote macrophage phagocytic activity, and suppress T-cell proliferation and activation[132]. As C-BMSCs can be obtained with relatively low-invasive methods, they may offer an advantage over long bone-derived BMSCs in clinical applications. However, there is still a need to verify the heterogeneity among different sources of MSCs in larger clinical trials.
Peripheral nerve-associated NC-MSCs and MSCs both have significant research value in tissue repair and regenerative medicine. However, comparative studies between the two are limited. SCPs, which originate from the NC, exhibit broad developmental potential and can differentiate into Schwann cells, neurons, and myofibroblasts[133,134]. In contrast, mesoderm-derived MSCs primarily differentiate into osteoblasts, chondrocytes, and adipocytes. While mesoderm-derived MSCs can differentiate into Schwann cell-like cells under specific stimulation by growth factors, this typically requires induced differentiation[135].
Studies have shown that ADSCs have the potential to differentiate into neural cells[136]. However, previous research has indicated that the neurorepair effect of ADSCs in clinical applications often does not meet expectations[137,138]. In comparison, NC-MSCs, due to their unique NC origin, may be more prone to differentiating into neurons or supporting cells such as Schwann cells. For example, De La Garza-Castro et al[9] compared the repair effects of SCPs and BMSCs in a rat spinal cord injury model and found that SCPs outperformed BMSCs in inflammation regulation, myelination remodeling, and motor function recovery. This suggests that NCCs may have better adaptability in the local neuroregenerative microenvironment. Further research showed that chitosan scaffolds loaded with skin-derived SCPs significantly enhanced peripheral nerve regeneration by upregulating repair-related genes (such as nerve growth factor, brain-derived neurotrophic factor) and secreting neurotrophic factors[10].
Regarding immune modulation, both peripheral nerve stem cells and MSCs exhibit good immunomodulatory functions. Studies have shown that both can influence the polarization of macrophages[8]. Skin-derived SCPs, during nerve injury repair, can increase the expression of interleukin-6, promote macrophage activation, and enhance their phagocytosis of myelin debris, thus facilitating sciatic nerve repair in rats[139]. In addition, MSCs (e.g., BMSCs and ADSCs) in co-culture conditions promote the polarization of macrophages toward the M2 phenotype, suppress inflammation, and reduce tumor necrosis factor-α secretion[140]. Based on these findings, SCPs may have greater potential for treating neuroinflammatory diseases due to their NC origin. Although MSCs are relatively easy to obtain and have wide clinical application prospects, their immunomodulatory effects may vary depending on their source[141]. Therefore, further research is needed to verify the specific mechanisms and clinical effects of MSCs from different sources in immune modulation.
In 2022, our research group conducted a comprehensive review of the clinical applications of DSCs, summarizing findings from thirty published clinical studies and twenty-eight registered clinical trials [ClinicalTrials.gov and the International Clinical Trials Registry Platform (ICTRP)][7]. These studies explored the potential use of DSCs (including DPSCs, PDLSCs, gingival MSCs, and SHEDs) in treating various conditions, including oral diseases (such as pulp necrosis, irreversible pulpitis, apical periodontitis, periodontitis, alveolar bone/jaw defects, and cleft lip and palate), neurological disorders (such as Huntington’s disease, acute ischemic stroke, and chronic post-stroke disabilities), coronavirus disease 2019, and other diseases or conditions (such as diabetes, liver cirrhosis, hair loss, wrinkles, and erectile dysfunction)[7]. Given recent advancements in clinical therapies based on DSCs over the past three years, this section will first supplement our previous summary with updated information. Additionally, the clinical applications of other NC-MSCs beyond DSCs were collected and analyzed to provide a comprehensive overview of the current landscape of NC-MSC clinical applications.
Although DSCs have demonstrated immense potential in regenerative medicine, no new clinical studies based on DSCs have been published since 2022 (as per a MEDLINE search). This may be attributed to the extended follow-up periods and significant research investment required for clinical studies. However, during this period, several new clinical trials have been registered to explore the therapeutic potential of DSCs in various diseases (Tables 1 and 2). According to ClinicalTrials.gov, four newly registered clinical trials focus on the treatment of Huntington’s disease, periodontitis, pulp necrosis, and apical periodontitis (Table 1). In addition, studies registered in another platform are primarily investigating the therapeutic potential of DSCs in type 1 diabetes, Hodgkin and non-Hodgkin lymphoma, wrinkles, and pulp necrosis (Table 2).
| Registration ID | Status | Diseases | Study design | Cell source | Administration route | Number of patients | Interventions | Follow-up period | Phase | Outcomes | Ref. | |
| Test group | Control group | |||||||||||
| NCT06097780 | Not yet recruiting | Huntington’s disease | Randomized; parallel assignment; single-blind (outcomes assessor) | Dental pulp | Intravenous administrations | 120 | Intravenous human DPSCs administrations | Intravenous placebo administrations | 1 year | Phase 3 | ||
| NCT05924373 | Recruiting | Periodontitis | Randomized; parallel assignment; double (participant investigator) | Dental pulp | Local injection at periodontal defect site | 204 | Single-dose group (DPSCs, 1.0 × 107); two-dose group (low-dose, DPSCs, 1.0 × 106); two-dose group (high-dose, DPSCs, 1.0 × 107) | Normal saline | 90, 180, 360, 720 days | Phase 2 | ||
| NCT05728346 | Not yet recruiting | Pulp necroses | Nonrandomized; single group assignment; open label | Allogeneic deciduous pulp | Transplantation after the preparation and disinfection of the root canal of the affected teeth | 30 | SHED mixed with hyaluronic acid polymers | None | 1, 3, 6, 12, 18, 24 months | Not applicable | ||
| NCT06043453 | Recruiting | Apical periodontitis; trauma | Observational; multicenter blinded | Remaining dental pulp and apical papilla | Attraction or transplantation after root canal disinfection | With apical periodontitis | Without apical periodontitis | 1, 2, 3 years | Not applicable | |||
| NCT04983225 | Recruiting to active, not recruiting | Periodontitis | Randomized; parallel assignment; double-blind (participant, investigator) | Dental pulp | Injecting into the periodontal defect site | 36 | DPSCs (1 × 106)/site; DPSCs (5 × 106)/site; DPSCs [(3-4) × 107]/three or four sites; DPSCs (1 × 107)/site; DPSCs (2 × 107)/two sites | Saline solution | 90, 180, 360, 720 days | Phase 1 | ||
| NCT02523651 | Unknown | Periodontitis | Randomized; parallel assignment; triple-blind (participant, investigator, outcomes assessor) | Allogeneic dental pulp | Injecting into the periodontal defect site | 40 | DPSCs (1 × 106) | Saline solution | 1 year | Phase 1/2 | ||
| NCT03386877 | Completed | Periodontitis | Randomized; parallel assignment; triple-blind (participant, investigator, outcomes assessor) | Autologous dental pulp | Delivering into intrabony defect via minimally invasive surgical technique | 29 | Micrografts of DPSCs + collagen sponge | Collagen sponge | 6, 12 months | Not applicable | ||
| NCT01082822 | Unknown | Periodontitis | Nonrandomized; parallel assignment; open label | Periodontal ligament | Implanted into bone defect sites via surgical approach | 80 | PDLSCs sheet fragment + DBBM (Bio-oss); PDLSCs sheet pellets + DBBM (Bio-oss); DBBM (Bio-oss) | Sham comparator | 4, 12, 24 weeks; 1 year | Phase 1/2 | ||
| NCT03638154 | Completed | Periodontitis | Randomized; parallel assignment; double-blind (care provider, outcomes assessor) | Gingival | Implanted into bone defect sites via surgical approach | 20 | GFs + GMSCs + β-TCP | β-TCP | 1, 3, 7, 14 days; 6 months | Not applicable | ||
| NCT03137979 | Unknown | Periodontitis | Randomized; parallel assignment | Gingival | Implanted into bone defect sites via surgical approach | 30 | GMSCs + collagen scaffolds; collagen scaffolds | Open flap debridement | 1, 3, 6 months | Phase1/2 | ||
| NCT01357785 | Unknown | Periodontitis | Randomized; parallel assignment; open label | Autologous periodontal ligament | 35 | None | 3-12 months | Phase1 | Chen et al[174] | |||
| NCT04641533 | Completed | Post-extraction sockets | Split-mouth; randomized; crossover assignment; double-blind (investigator, outcomes assessor) | Dental pulp | Placing into the extraction socket | 13 | DPSCs + L-PRF | L-PRF | 7 days; 6 months | Not applicable | Cubuk et al[175] | |
| NCT02731586 | Unknown | Edentulous alveolar ridge | Single group assignment; open label | Allogeneic dental pulp | Introducing dental pulp-derived mesenchymal stem cells during placement of dental implants | 10 | Dental pulp-derived MSCs | None | 3 months | Early phase 1 | ||
| NCT03766217 | Completed | Cleft lip and palate | Randomized; parallel assignment; single-blind (outcomes assessor) | Autologous deciduous pulp | Placed into the alveolar defect via surgical approach | 62 | SHED + hydroxyapatite-collagen sponge | Iliac crest autogenous bone graft | 15 days; 3, 6, 12 months | Phase 3 | Tanikawa et al[176], Pinheiro et al[177] | |
| NCT01932164 | Completed; has results | Cleft lip and palate | Single group assignment; open label | Autologous deciduous pulp | Maxillary alveolar graft by tissue engineering | 5 | SHED + hydroxyapatite-collagen sponge | None | 3, 6 months | Not applicable | Percentage of bone filling at 6 months postoperatively: 89.5% | Tanikawa et al[176] |
| NCT04130100 | Unknown | Knee osteoarthritis | Randomized; parallel assignment; open label | Dental pulp | Intraarticular injection | 60 | Low dose of DPSCs; high dose of DPSCs | Sodium hyaluronate | 12 months | Early phase 1 | ||
| NCT01814436 | Unknown | Dental pulp necrosis | Single group assignment; open label | Autologous deciduous pulp | 80 | Scaffold-free SHED-derived pellet | None | 3-12 months | Not applicable | |||
| NCT03957655 | Unknown | Liver cirrhosis | Randomized; parallel assignment; single-blind (outcomes assessor) | Autologous deciduous pulp | Peripheral vein infusion | 40 | SHED (1 × 106 cells/kg body weight) | Standard medication for viral hepatitis and cirrhosis | 4, 8, 12, 16, 24 weeks | Early phase 1 | ||
| NCT03912480 | Unknown | Type 1 diabetes | Single group assignment; open label | Deciduous pulp | Intravenous drip | 24 | SHED (0.11I U/kg body weight) + insulin + oral hypoglycemic drugs | None | 1, 2, 6 weeks; 2, 3, 6, 9, 12 months | Early phase 1 | ||
| NCT04608838 | Completed | Acute ischemic stroke | Randomized; parallel assignment; quadruple-blind (participant, care provider, investigator, outcomes assessor) | Allogeneic dental pulp | Intravenously infusion | 79 | DPSCs (JTR-161, 1 × 108 cells); DPSCs (JTR-161, 3 × 108 cells) | Placebo | 91, 366 days | Phase 1/2 | Suda et al[178] | |
| NCT02728115 | Active, not recruiting to unknown | Nonrandomized; parallel assignment; open label | Allogeneic deciduous pulp | Intravenous administration | 6 | SHED (Cellavita HD, 1 × 106 cells); SHED (Cellavita HD, 2 × 106 cells) | None | 1, 4 years | Phase 1 | |||
| NCT04219241 | Active, not recruiting to unknown | Huntington’s disease | Single group assignment; open label | Allogeneic deciduous pulp | Intravenous administration | 35 | SHED (Cellavita HD, 2 × 106 cells) | None | 1, 2 years | Phase 2/3 | ||
| NCT03252535 | Completed | Huntington’s disease | Randomized; parallel assignment; triple-blind (participant, investigator, outcomes assessor) | Allogeneic deciduous pulp | Intravenous administration | 35 | SHED (Cellavita HD, 1 × 106 cells); SHED (Cellavita HD, 2 × 106 cells) | Physiological solution without cells | Monthly for 14 months | Phase 2 | Wenceslau et al[179] | |
| NCT04336254 | Recruiting to unknown | COVID-19 | Randomized; parallel assignment; triple-blind (participant, investigator, outcomes assessor) | Allogeneic dental pulp | Intravenous injection | 20 | DPSCs (3 × 107 cells) | Saline | 28 days | Phase 1/2 | Ye et al[180] | |
| NCT04302519 | Unknown | COVID-19 | Single group assignment; open label | Dental pulp | Intravenous injection | 24 | DPSCs (1 × 107 cells/kg body weight) | None | 3, 7, 14, 28, 360 days | Early phase 1 | ||
| Registration ID | Status | Diseases | Study design | Cell Source | Administration route | Number of patients | Interventions | Follow-up period | Phase | Outcomes | Ref. | |
| Test group | Control group | |||||||||||
| IRCT20140911019125N12 | Pending | Children with type 1 diabetes | Not randomized; no placebo; not blinded | Deciduous pulp | Intravenous injection | 10 | SHEDs (3 × 106/kg, twice, 3 months apart) | None | 3, 6, 12 months | Not applicable | ||
| RCT20140911019125N10 | Pending | Hodgkin and non-Hodgkin lymphoma | Not randomized; no placebo; not blinded | Deciduous pulp | Intravenous injection | 30 | SHEDs (1 × 106/kg, 4 hours before the injection of hematopoietic stem cells); SHEDs (1 × 106/kg, on day 0, + 2, + 4, + 6 after hematopoietic stem cell transplantation); no SHEDs injection | None | Daily until discharge from the hospital | Not applicable | ||
| JPRN-UMIN000049760 | Complete: Follow-up complete | Wrinkles | Randomized; parallel assignment; single-blind | Dental pulp | 12 | All-in-one gel containing immortalized human DPSCs-CM and various useful ingredients | None | 4 weeks | Not applicable | |||
| CTRI/2024/10/075535 | Not yet recruiting | Necrosis of pulp | Randomized; parallel assignment; double-blind | Deciduous pulp | Transplantation | 30 | Scaffold of fibrin glue loaded with E-nanoHA seeded with SHED | Blood clot scaffold with MTA | 1, 3, 6, 12, 18, 24 months | Phase 3 | ||
| JPRN-UMIN000042791 | Complete: Follow-up complete | Periodontitis | Randomized; parallel assignment; single-blind (participants) | Deciduous pulp | Gargle | 30 | Mouthwash containing SHED culture supernatant | Mouthwash without SHED culture supernatant | 1 month | Not applicable | ||
| ChiCTR2100051466 | Recruiting | Periodontitis | Randomized; parallel assignment; open label | Bilateral multipoint injection on a single tooth | 96 | DPSCs (1 × 107 cells) for once; DPSCs (1 × 107 cells) for twice | Saline | 90, 180, 360 days | Phage 0 | |||
| ChiCTR2100049178 | Pending | Periodontitis | Randomized; parallel assignment; double-blind | Dental pulp | Local injection | 36 | DPSCs (1 × 106 cells) for single injection; DPSCs (5 × 106 cells) for single injection; DPSCs (1 × 107 cells) for single injection; DPSCs (1 × 107 cells) for single injection in 2 Locations; DPSCs (1 × 107 cells) for single injection in 3-4 Locations | None | Phage 1 | |||
| ISRCTN13093912 | Completed | Periodontitis | Randomized; parallel assignment; single-blind (patients and examiners) | Dental pulp | Implanted into bone defect sites via surgical approach | 20 | DPSCs (1 × 107 cells) + hydroxyapatite-collagen scaffold | Hydroxyapatite-collagen scaffold | 1, 2, 4, 12, 24, 36 weeks; 12, 24, 36, 48, 60 months | Not applicable | Sánchez et al[181] | |
| JPRN-UMIN000045926 | Complete: Follow-up complete | Wrinkles | Randomized; parallel assignment; single-blind (outcomes assessor) | Dental pulp | 12 | All-in-one gel containing immortalized DPSCs-CM solution and various beauty ingredients | No treatment | 4 weeks | Not applicable | |||
| JPRN-UMIN000043528 | Complete: Follow-up complete | Wrinkles | Randomized; parallel assignment; single-blind (outcomes assessor) | Dental pulp | 12 | All-in-one gel containing immortalized DPSC-CM solution and the latest peptide raw materials | No treatment | 4 weeks | Not applicable | |||
| JPRN-UMIN000045897 | Complete: Follow-up continuing → Complete: Follow-up complete | Hair loss | Nonrandomized;parallel assignment; open label | Deciduous pulp | Injection | 22 | SHED-CM; after SHED-CM injection, one dose of micrografts (Rigenera) followed by another SHED-CM injection; SHED-CM injection after one dose of micrografts (Rigenera) | None | 6 months | Not applicable | ||
Combining our previous summary[7], a total of 25 clinical trials have been registered in ClinicalTrials.gov to evaluate the potential of DSCs in treating periodontitis (32%, 8/25), post-extraction sockets (4.8%, 1/25), edentulous alveolar ridge (4%, 1/25), cleft lip and palate (8%, 2/25), knee osteoarthritis (4%, 1/25), dental pulp necrosis (8%, 2/25), apical periodontitis (4%, 1/25), liver cirrhosis (4%, 1/25), type 1 diabetes (4%, 1/25), acute ischemic stroke (4%, 1/25), Huntington’s disease (16%, 4/25), and coronavirus disease 2019 (8%, 2/25) (Table 1). Beyond those registered in ClinicalTrials.gov, 11 additional clinical trials related to DSCs have been recorded in the ICTRP, investigating periodontitis (36.4%, 4/11), necrosis of pulp (9.1%, 1/11), wrinkles (27.3%, 3/11), type 1 diabetes (9.1%, 1/11), Hodgkin and non-Hodgkin lymphoma (9.1%, 1/11), and hair loss (9.1%, 1/11) (Table 2).
Additionally, the statuses of several clinical trials have changed (Tables 1 and 2). In ClinicalTrials.gov, a clinical trial investigating the use of DPSCs for periodontitis (NCT04983225) has transitioned from “Recruiting” to “Active, not recruiting”. In addition, other trials (NCT04336254, NCT04219241, and NCT02728115) have changed to “Unknown” status at different trial stages. In ICTRP, the status of a registered clinical study using SHED-conditioned medium for hair loss has shifted from “Complete: Follow-up continuing” to “Complete: Follow-up complete”. Given the rapid updates in registered information and trial statuses of DSC-related studies, regularly reviewing and discussing these developments will benefit the fundamental research, clinical practice, and translational application of DSCs.
In 2018, a study by Redondo et al[142] (registration ID: NCT03070275) demonstrated that cross-linked serum scaffold combined with autologous alveolar bone MSCs significantly enhanced bone density in maxillary cystic bone defects, suggesting its potential as an alternative therapy for maxillary defects and other bone loss conditions (Table 3). Two additional clinical studies utilized autologous alveolar bone MSCs in combination with collagen scaffolds enriched with autologous fibrin/platelet lysate to investigate their therapeutic effects on chronic periodontitis[143,144] (Table 3). These two studies, sharing the same registration ID (NCT02449005) and grouping strategy, respectively examined bone defect tissue repair and inflammatory factor changes in gingival crevicular fluid. While the MSCs + scaffold group did not show significant clinical improvement in bone defect healing compared to the control group (minimal access flap surgery), it exhibited a biochemical pattern favoring inflammatory control and tissue regeneration over 12 months.
| Ref. | Registration ID | Conditions/diseases | Study design | Cell source | Administration route | Interventions | Follow-up period | Outcomes | |
| Test group | Control group | ||||||||
| Redondo et al[142] | EudraCT 2010-024246-30; NCT01389661 | Maxillary bone cysts | Nonrandomized | Autologous alveolar bone | Surgical implantation | BioMax scaffold + MSCs (n = 9) | Contralateral control area of spongy alveolar bone without treatment | 3-4, 6-8 months | Bone density (↑) |
| Apatzidou et al[143] | NCT02449005 | Intrabony defects and periodontitis | Randomized; single-blind | Autologous alveolar bone | Transplanted into the osseous defect | MAF + Biocomplex (containing BMSCs, n = 9) | MAF + aFPL (n = 10); MAF (n = 8) | Baseline; 6 weeks; 6, 9, 12 months | TNF-α, RANKL levels in GCF gradually decreased, MMP levels were higher than baseline (6-9 months), and RANKL/OPG ratio was lower than baseline (9-12 months) |
| Apatzidou et al[144] | NCT02449005 | Intrabony defects and periodontitis | Randomized; single-blind | Autologous alveolar bone | Transplanted into the osseous defect | MAF + Biocomplex (containing BMSCs, n = 9) | MAF + aFPL (n = 10); MAF (n = 8) | Baseline; 6 weeks; 6, 9, 12 months | Significant clinical improvements (attachment gain and PPD) but no between-group differences |
Beyond the two registered studies mentioned above (NCT03070275, NCT02449005), two additional phase 1/2 registered trials (NCT03070275, NCT06700655) are investigating the potential of autologous alveolar bone MSCs in implant therapy and autologous CSSCs in the treatment of limbal stem cell deficiency (Table 4). Compared to DSCs, the clinical research and applications of other NC-MSCs (excluding DSCs) are still in their early stages, likely due to challenges in cell isolation and a relatively weaker foundation in basic research.
| Registration ID | Status | Diseases | Study design | Cell source | Administration route | Number of patients | Interventions | Follow-up period | Phase | Outcomes | Ref. | |
| Test group | Control group | |||||||||||
| NCT03070275 | Completed | Implant therapy | Randomized; parallel assignment; single-blind (outcomes assessor) | Alveolar bone | Gently stabilized over the implant platform head partially covering the buccal bone | 20 | Biocomplex (aBM-MSCs/fibrin glue/collagen fleece) | No use of adjunctive grafting materials | 4 months | Phage 1/2 | ||
| NCT02449005 | Completed | Chronic periodontitis | Randomized; parallel assignment; quadruple-blind (participant care, provider, investigator, outcomes assessor) | Autologous alveolar bone | Transplanted into the osseous defect | 60 | MAF + Biocomplex (containing BMSCs, n = 9) | MAF + aFPL (n = 10); MAF (n = 8) | Baseline; 6 weeks; 6, 9, 12 months | Phage 1/2 | Apatzidou et al[143], Apatzidou et al[144] | |
| NCT01389661 | Completed | Maxillary cyst bone loss of substance | Single group assignment; open label | Autologous alveolar bone | Surgical implantation | 11 | BioMax scaffold + MSCs | Contralateral control area of spongy alveolar bone without treatment | 0, 2 weeks; 2, 6 months | Phage 1/2 | Redondo et al[142] | |
| NCT06700655 | Not yet recruiting | Limbal stem cells deficiency | Randomized; parallel assignment; single-blind (outcomes assessor) | Autologous stromal opacities | Cell transplantation | 60 | Cornea limbal stem cell; cornea limbal stem cell combined corneal stromal stem cell | Corneal transplantation | 32, 48, 108 days; 6 months; 1, 2 years | Phage 1/2 | ||
The NC-MSCs have demonstrated promising preclinical and clinical therapeutic potential. However, similar to other adult MSCs, NC-MSCs face several challenges that limit their broader application, including invasive procedures leading to donor site damage, cellular heterogeneity, and restricted cell availability[7,15-18]. In recent years, the rapid advancements in PSC research have provided a potential solution to these limitations.
PSCs refer to a unique class of cells with self-renewal capabilities and the ability to differentiate into nearly all cell types, primarily including ESCs and iPSCs[19-22]. ESCs are derived from early-stage embryos, whereas iPSCs are generated by reprogramming somatic cells (e.g., skin fibroblasts) through the introduction of specific transcription factors such as Oct4, Sox2, Kruppel-like factor 4, and c-Myc[145]. PSCs are characterized by the expression of pluripotency markers (e.g., Oct4, Nanog, and stage-specific embryonic antigen-4), indefinite proliferation in an undifferentiated state, and the capacity to give rise to cells of the three germ layers (ectoderm, mesoderm, and endoderm)[146,147]. These properties make PSCs an attractive source for generating homogeneous MSC populations with therapeutic potential in a controlled in vitro environment.
Several strategies have been developed to induce NCC differentiation from PSCs. Early methods relied on fluorescence-activated cell sorting (FACS) to isolate NCCs from mixed neural precursor cell (NPC) populations[148-150]. In 2005, Pomp et al[151] demonstrated that co-culturing ESCs with the mouse stromal cell line PA6 promoted the differentiation of ESCs into peripheral sensory and sympathetic neurons. Interestingly, prior to neuronal differentiation, PA6 cells first induced the formation of NCC-like cells, which aligns with the developmental origins of peripheral sensory and sympathetic neurons from NCCs in vivo[151]. Using a similar induction approach and FACS, Jiang et al[149] isolated a p75-positive cell population with NCC-like phenotypic and functional characteristics, which exhibited multilineage differentiation potential into peripheral neurons, glial cells, and myofibroblasts in vitro and in vivo. Despite modifications in FACS selection markers (e.g., frizzled-3+cadherin-11 and p75+HNK1), the efficiency and stability of NCC isolation from NPCs remained low[148,150]. Since NCCs typically constitute only a small subset of NPCs, sorting-based strategies suffer from inconsistency. Additionally, variations in NPC induction protocols (e.g., co-culture with stromal cells, neurosphere formation, and small molecule-based induction) introduce differences in cellular composition and proportions, further complicating NCC isolation and downstream applications[152].
To overcome these challenges, researchers have developed adherent culture protocols that simulate in vivo NC development, enabling the direct differentiation of NCCs from PSCs. Canonical Wnt signaling plays a critical role in NCC fate determination during vertebrate embryogenesis[153-155]. By activating Wnt signaling while inhibiting the activin A/Nodal pathway, several studies successfully directed PSCs toward NCC-like cells (p75+HNK1+AP2+), with minimal contamination from Pax6+ neural progenitors[156-158]. However, inhibition of the activin A/Nodal pathway does not appear to be strictly necessary for NCC differentiation. Two studies reported that transient activation of Wnt signaling (for two days) followed by withdrawal for three days was sufficient to induce NCC-like cells expressing SOX10, PAX7, PAX3, MSX1, and TFAP2A, while the addition of the activin A/Nodal inhibitor SB431542 had a negative impact on differentiation[159,160].
Building on these strategies, several studies successfully established protocols for generating MSCs from PSCs via a NC intermediate. In 2014, Fukuta et al[24] reported a two-step differentiation strategy in which PSCs were first induced into NCCs and subsequently differentiated into MSCs. In the first stage, NCC formation was promoted by inhibiting TGF-β signaling (SB431542) and glycogen synthase kinase 3β (CHIR99021), while in the second stage, alpha minimum essential medium supplemented with 10% fetal bovine serum facilitated NCC-to-MSC transition[24]. The derived MSCs expressed typical MSC markers (CD44, CD73, and CD105) but were negative for hematopoietic marker CD45 and exhibited trilineage differentiation potential (osteogenesis, chondrogenesis, and adipogenesis)[24]. Another study incorporated FGF2 into the NCC induction protocol, which further enhanced NCC differentiation efficiency[25]. Additionally, a commercial serum-free MSC medium (CTS StemPro MSC SFM) was successfully employed to induce NCC-to-MSC differentiation in vitro[25]. Compared to MSCs derived from trophoblast co-culture, NCC-derived MSCs exhibited higher purity and superior osteogenic differentiation potential, and their transcriptional profiles more closely resembled adult ADSCs, BMSCs, and umbilical cord MSCs[25]. These findings highlight the importance of lineage-specific differentiation strategies for generating MSCs with enhanced therapeutic potential. In another study, Ouchi et al[26] demonstrated that NC-like cells expressing low affinity nerve growth factor receptor and THY-1 could be induced using a combination of epidermal growth factor, FGF2, Gem21 neuroplex, GlutaMAX, and N2, and these NC-like cells retained robust differentiation potential into mesenchymal and NC lineages[26].
Despite being a major PSC subtype, ESCs pose ethical concerns due to the destruction of blastocysts during derivation, prompting ethical debates among researchers and regulatory bodies[161,162]. iPSCs offer an alternative, but their epigenetic instability may contribute to differentiation bias, raising concerns regarding their clinical applications[163-165]. Recently discovered EPSCs, while also reprogrammed from somatic cells, have demonstrated superior genetic and epigenetic stability compared to ESCs[27-29]. The advantages of EPSCs suggest that they may serve as a more suitable starting point for directed differentiation compared to ESCs and iPSCs. Although there are currently no reports of EPSC-to-MSC differentiation, future studies focusing on MSC generation from EPSCs via a NC intermediate may address current challenges in MSC-based therapies.
On the other hand, while PSC-derived cells exhibit immense therapeutic potential, concerns regarding teratoma formation limit their widespread application[19,166-168]. A major advantage of PSCs is their unlimited proliferative capacity, which enables the large-scale production of human cells for therapeutic purposes. However, this same proliferative ability also increases the risk of tumorigenicity if residual undifferentiated PSCs persist post-transplantation. Even a small number of residual PSCs can potentially form teratomas. For example, a 2022 case report documented the formation of an immature teratoma following the injection of PSC-derived pancreatic β-cells[30]. Therefore, effective detection and removal of residual PSCs from PSC-derived MSC populations are essential for improving the safety of PSC-based therapies. Developing specific clearance strategies for residual PSCs in NC-MSCs could enhance the feasibility of PSC-based MSC therapy and facilitate its clinical translation.
Additionally, NC-MSCs exhibit significant diversity and widespread distribution, with notable differences among various types of NC-MSCs as well as between NC-MSCs and other MSC populations. These differences may be attributed to variations in signaling regulation during MSC development. Although some studies have successfully induced the differentiation of PSCs into MSCs via the NC lineage in vitro[24-26], the discrepancies between PSC-derived NC-MSCs, adult NC-MSCs, and other MSCs remain incompletely understood. Further exploration of the developmental processes of different adult NC-MSCs will facilitate the directed differentiation of distinct NC-MSC subtypes in vitro. In conclusion, PSC-derived NC-MSCs represent a promising supplement and potential alternative to adult NC-MSCs, offering a viable solution to the current limitations in the clinical application of both adult NC-MSCs and MSCs in general.
Despite significant advances in the study of the developmental origin, molecular characteristics, and therapeutic applications of NC-MSCs in recent years, their clinical translation still faces numerous challenges, necessitating further exploration and optimization.
Firstly, heterogeneity and lineage specificity remain central issues in NC-MSC research. Although NC-MSCs originate from a common source (the NC), distinct subpopulations exhibit significant functional differences depending on their tissue of origin[33,169-171]. Therefore, further application of lineage-tracing techniques, single-cell transcriptomic sequencing, and epigenetic analyses is required to delineate the characteristics of different NC-MSC subtypes, thereby refining directed differentiation strategies and enhancing their therapeutic efficacy in specific disease models.
Secondly, expanding clinical applications and optimizing transplantation strategies are critical for the clinical translation of NC-MSCs. Current clinical studies primarily focus on the use of DSCs in periodontal and pulp regeneration, while research on their potential in neurological and systemic diseases remains limited. Moreover, improving the survival rate and integration of transplanted cells remains a major challenge. Future studies should explore scaffold-based delivery, in situ differentiation, and immune-compatible cell sources to optimize the clinical efficacy of NC-MSC transplantation.
In addition to addressing the heterogeneity of NC-MSCs and optimizing transplantation strategies, further investigation into the interactions and potential synergistic effects of key signaling pathways such as Wnt, Notch, TGF-β, FGF, BMP, and Hippo is also of great significance. These signaling pathways play a coordinated role in the development and function of NC-MSCs and exhibit different interaction patterns in various physiological environments. This may provide key insights for discovering new therapeutic targets and enhancing regenerative treatment strategies.
Finally, establishing standardized quality control systems and regulatory frameworks is crucial for the clinical application of NC-MSCs[172,173]. Moving forward, it is essential to develop uniform cell manufacturing standards, activity assessment protocols, and long-term safety evaluation systems to meet regulatory requirements and facilitate the commercialization of NC-MSC-based therapies.
NC-MSCs have demonstrated significant potential in regenerative medicine, particularly in craniofacial reconstruction, neural repair, and immunomodulation. However, their clinical application remains limited due to challenges such as restricted cell sources, heterogeneity, and invasive isolation procedures. Recent clinical trials have explored NC-MSCs, particularly DSCs, for treating periodontal diseases, pulp regeneration, and neural disorders, yet large-scale and long-term clinical data are still lacking. PSCs offer an alternative source for generating NC-MSCs, with EPSCs providing greater genetic stability and differentiation capacity. While PSC-derived NC-MSCs hold promise, safety concerns, including teratoma formation, require further investigation. Future research should refine differentiation strategies, optimize transplantation safety, and expand clinical studies to accelerate the translation of NC-MSCs into effective cell-based therapies.
| 1. | Roth DM, Bayona F, Baddam P, Graf D. Craniofacial Development: Neural Crest in Molecular Embryology. Head Neck Pathol. 2021;15:1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 2. | Soto J, Ding X, Wang A, Li S. Neural crest-like stem cells for tissue regeneration. Stem Cells Transl Med. 2021;10:681-693. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 3. | Szabó A, Mayor R. Mechanisms of Neural Crest Migration. Annu Rev Genet. 2018;52:43-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 135] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 4. | Leathers TA, Rogers CD. Time to go: neural crest cell epithelial-to-mesenchymal transition. Development. 2022;149:dev200712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 5. | Antonaci M, Wheeler GN. MicroRNAs in neural crest development and neurocristopathies. Biochem Soc Trans. 2022;50:965-974. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 6. | Bosch-Queralt M, Fledrich R, Stassart RM. Schwann cell functions in peripheral nerve development and repair. Neurobiol Dis. 2023;176:105952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 130] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 7. | Song WP, Jin LY, Zhu MD, Wang H, Xia DS. Clinical trials using dental stem cells: 2022 update. World J Stem Cells. 2023;15:31-51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (3)] |
| 8. | Song W, Bo X, Ma X, Hou K, Li D, Geng W, Zeng J. Craniomaxillofacial derived bone marrow mesenchymal stem/stromal cells (BMSCs) for craniomaxillofacial bone tissue engineering: A literature review. J Stomatol Oral Maxillofac Surg. 2022;123:e650-e659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | de la Garza-Castro O, Martínez-Rodríguez HG, Sánchez-González SG, Vidal-Torres O, Arreola-Romero A, de la Garza-Pineda O, Ancer-Arellano AG, Guzmán-López S, Elizondo-Omaña RE. Schwann Cell Precursor Transplant in a Rat Spinal Cord Injury Model. Rev Invest Clin. 2018;70:88-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Cong M, Wu X, Zhu L, Gu G, Ding F, Li G, Shi H. Anisotropic microtopography surface of chitosan scaffold regulating skin precursor-derived Schwann cells towards repair phenotype promotes neural regeneration. Regen Biomater. 2024;11:rbae005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 11. | Bar JK, Lis-Nawara A, Grelewski PG. Dental Pulp Stem Cell-Derived Secretome and Its Regenerative Potential. Int J Mol Sci. 2021;22:12018. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 83] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 12. | Tsutsui TW. Dental Pulp Stem Cells: Advances to Applications. Stem Cells Cloning. 2020;13:33-42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 97] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 13. | Wang X, Luo E, Li Y, Hu J. Schwann-like mesenchymal stem cells within vein graft facilitate facial nerve regeneration and remyelination. Brain Res. 2011;1383:71-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 14. | Lee DC, Chen JH, Hsu TY, Chang LH, Chang H, Chi YH, Chiu IM. Neural stem cells promote nerve regeneration through IL12-induced Schwann cell differentiation. Mol Cell Neurosci. 2017;79:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 15. | Musiał-Wysocka A, Kot M, Majka M. The Pros and Cons of Mesenchymal Stem Cell-Based Therapies. Cell Transplant. 2019;28:801-812. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 160] [Cited by in RCA: 383] [Article Influence: 54.7] [Reference Citation Analysis (0)] |
| 16. | McLeod CM, Mauck RL. On the origin and impact of mesenchymal stem cell heterogeneity: new insights and emerging tools for single cell analysis. Eur Cell Mater. 2017;34:217-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 150] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 17. | Li J, Wu Z, Zhao L, Liu Y, Su Y, Gong X, Liu F, Zhang L. The heterogeneity of mesenchymal stem cells: an important issue to be addressed in cell therapy. Stem Cell Res Ther. 2023;14:381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 100] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 18. | Fossett E, Khan WS. Optimising human mesenchymal stem cell numbers for clinical application: a literature review. Stem Cells Int. 2012;2012:465259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 19. | Yamanaka S. Pluripotent Stem Cell-Based Cell Therapy-Promise and Challenges. Cell Stem Cell. 2020;27:523-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 812] [Article Influence: 162.4] [Reference Citation Analysis (0)] |
| 20. | Takayama K, Yamanaka S. Pluripotent stem cell-based therapies and their path to the clinic. Stem Cell Reports. 2023;18:1547-1548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 21. | Ogi DA, Jin S. Transcriptome-Powered Pluripotent Stem Cell Differentiation for Regenerative Medicine. Cells. 2023;12:1442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 22. | Liang G, Zhang Y. Embryonic stem cell and induced pluripotent stem cell: an epigenetic perspective. Cell Res. 2013;23:49-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 167] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 23. | Wang F, Kong J, Cui YY, Liu P, Wen JY. Is Human-induced Pluripotent Stem Cell the Best Optimal? Chin Med J (Engl). 2018;131:852-856. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Fukuta M, Nakai Y, Kirino K, Nakagawa M, Sekiguchi K, Nagata S, Matsumoto Y, Yamamoto T, Umeda K, Heike T, Okumura N, Koizumi N, Sato T, Nakahata T, Saito M, Otsuka T, Kinoshita S, Ueno M, Ikeya M, Toguchida J. Derivation of mesenchymal stromal cells from pluripotent stem cells through a neural crest lineage using small molecule compounds with defined media. PLoS One. 2014;9:e112291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 144] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 25. | Winston T, Song Y, Shi H, Yang J, Alsudais M, Kontaridis MI, Wu Y, Gaborski TR, Meng Q, Cooney RN, Ma Z. Lineage-Specific Mesenchymal Stromal Cells Derived from Human iPSCs Showed Distinct Patterns in Transcriptomic Profile and Extracellular Vesicle Production. Adv Sci (Weinh). 2024;11:e2308975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 26. | Ouchi T, Morikawa S, Shibata S, Fukuda K, Okuno H, Fujimura T, Kuroda T, Ohyama M, Akamatsu W, Nakagawa T, Okano H. LNGFR(+)THY-1(+) human pluripotent stem cell-derived neural crest-like cells have the potential to develop into mesenchymal stem cells. Differentiation. 2016;92:270-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Dong X, Guo R, Ji T, Zhang J, Xu J, Li Y, Sheng Y, Wang Y, Fang K, Wen Y, Liu B, Hu G, Deng H, Yao H. YY1 safeguard multidimensional epigenetic landscape associated with extended pluripotency. Nucleic Acids Res. 2022;50:12019-12038. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 28. | Du Y, Wang T, Xu J, Zhao C, Li H, Fu Y, Xu Y, Xie L, Zhao J, Yang W, Yin M, Wen J, Deng H. Efficient derivation of extended pluripotent stem cells from NOD-scid Il2rg(-/-) mice. Protein Cell. 2019;10:31-42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Mazini L, Rochette L, Admou B, Amal S, Malka G. Hopes and Limits of Adipose-Derived Stem Cells (ADSCs) and Mesenchymal Stem Cells (MSCs) in Wound Healing. Int J Mol Sci. 2020;21:1306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 239] [Cited by in RCA: 356] [Article Influence: 59.3] [Reference Citation Analysis (7)] |
| 30. | Han L, He H, Yang Y, Meng Q, Ye F, Chen G, Zhang J. Distinctive Clinical and Pathologic Features of Immature Teratomas Arising from Induced Pluripotent Stem Cell-Derived Beta Cell Injection in a Diabetes Patient. Stem Cells Dev. 2022;31:97-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 43] [Reference Citation Analysis (0)] |
| 31. | Bronner ME, LeDouarin NM. Development and evolution of the neural crest: an overview. Dev Biol. 2012;366:2-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 296] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 32. | Rothstein M, Simoes-Costa M. On the evolutionary origins and regionalization of the neural crest. Semin Cell Dev Biol. 2023;138:28-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 33. | Soldatov R, Kaucka M, Kastriti ME, Petersen J, Chontorotzea T, Englmaier L, Akkuratova N, Yang Y, Häring M, Dyachuk V, Bock C, Farlik M, Piacentino ML, Boismoreau F, Hilscher MM, Yokota C, Qian X, Nilsson M, Bronner ME, Croci L, Hsiao WY, Guertin DA, Brunet JF, Consalez GG, Ernfors P, Fried K, Kharchenko PV, Adameyko I. Spatiotemporal structure of cell fate decisions in murine neural crest. Science. 2019;364:eaas9536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 238] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 34. | Sauka-Spengler T, Bronner-Fraser M. A gene regulatory network orchestrates neural crest formation. Nat Rev Mol Cell Biol. 2008;9:557-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 533] [Cited by in RCA: 560] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 35. | Simões-Costa M, Bronner ME. Insights into neural crest development and evolution from genomic analysis. Genome Res. 2013;23:1069-1080. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 100] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 36. | Simoes-Costa M, Bronner ME. Reprogramming of avian neural crest axial identity and cell fate. Science. 2016;352:1570-1573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 122] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 37. | Stemple DL, Anderson DJ. Isolation of a stem cell for neurons and glia from the mammalian neural crest. Cell. 1992;71:973-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 619] [Cited by in RCA: 611] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 38. | Kaltschmidt B, Kaltschmidt C, Widera D. Adult craniofacial stem cells: sources and relation to the neural crest. Stem Cell Rev Rep. 2012;8:658-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 39. | Xaymardan M. Orofacial Stem Cells for Cell-Based Therapies of Local and Systemic Diseases. In: Sasaki K, Suzuki O, Takahashi N. Interface Oral Health Science. Singapore: Springer, 2016. [DOI] [Full Text] |
| 40. | Chen Y, Huang H, Li G, Yu J, Fang F, Qiu W. Dental-derived mesenchymal stem cell sheets: a prospective tissue engineering for regenerative medicine. Stem Cell Res Ther. 2022;13:38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 41. | Cobourne MT, Sharpe PT. Tooth and jaw: molecular mechanisms of patterning in the first branchial arch. Arch Oral Biol. 2003;48:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 194] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 42. | Yuan Y, Loh YE, Han X, Feng J, Ho TV, He J, Jing J, Groff K, Wu A, Chai Y. Spatiotemporal cellular movement and fate decisions during first pharyngeal arch morphogenesis. Sci Adv. 2020;6:eabb0119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 43. | Jing J, Feng J, Yuan Y, Guo T, Lei J, Pei F, Ho TV, Chai Y. Spatiotemporal single-cell regulatory atlas reveals neural crest lineage diversification and cellular function during tooth morphogenesis. Nat Commun. 2022;13:4803. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 70] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 44. | Krivanek J, Adameyko I, Fried K. Heterogeneity and Developmental Connections between Cell Types Inhabiting Teeth. Front Physiol. 2017;8:376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 45. | Thesleff I, Tummers M. Tooth organogenesis and regeneration. 2009 Jan 31. In: StemBook [Internet]. Cambridge (MA): Harvard Stem Cell Institute; 2008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 46. | Rao P, Jing J, Fan Y, Zhou C. Spatiotemporal cellular dynamics and molecular regulation of tooth root ontogeny. Int J Oral Sci. 2023;15:50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 47. | Sonoyama W, Liu Y, Fang D, Yamaza T, Seo BM, Zhang C, Liu H, Gronthos S, Wang CY, Wang S, Shi S. Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS One. 2006;1:e79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 835] [Cited by in RCA: 918] [Article Influence: 45.9] [Reference Citation Analysis (1)] |
| 48. | Diao S, Lin X, Wang L, Dong R, Du J, Yang D, Fan Z. Analysis of gene expression profiles between apical papilla tissues, stem cells from apical papilla and cell sheet to identify the key modulators in MSCs niche. Cell Prolif. 2017;50:e12337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 49. | Tomokiyo A, Wada N, Maeda H. Periodontal Ligament Stem Cells: Regenerative Potency in Periodontium. Stem Cells Dev. 2019;28:974-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 216] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 50. | Wen S, Zheng X, Yin W, Liu Y, Wang R, Zhao Y, Liu Z, Li C, Zeng J, Rong M. Dental stem cell dynamics in periodontal ligament regeneration: from mechanism to application. Stem Cell Res Ther. 2024;15:389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 20] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 51. | Yang C, Du XY, Luo W. Clinical application prospects and transformation value of dental follicle stem cells in oral and neurological diseases. World J Stem Cells. 2023;15:136-149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 52. | Ten Cate AR. The development of the periodontium--a largely ectomesenchymally derived unit. Periodontol 2000. 1997;13:9-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 118] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 53. | Chai Y, Maxson RE Jr. Recent advances in craniofacial morphogenesis. Dev Dyn. 2006;235:2353-2375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 471] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 54. | Percival CJ, Richtsmeier JT. Angiogenesis and intramembranous osteogenesis. Dev Dyn. 2013;242:909-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 184] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 55. | Noden DM. The role of the neural crest in patterning of avian cranial skeletal, connective, and muscle tissues. Dev Biol. 1983;96:144-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 675] [Cited by in RCA: 627] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 56. | Chai Y, Jiang X, Ito Y, Bringas P Jr, Han J, Rowitch DH, Soriano P, McMahon AP, Sucov HM. Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development. 2000;127:1671-1679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 962] [Cited by in RCA: 987] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 57. | Santagati F, Rijli FM. Cranial neural crest and the building of the vertebrate head. Nat Rev Neurosci. 2003;4:806-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 341] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 58. | Shen Z, Zhang R, Chen X, Yang G, Si Y, Yan T, Chen S, Cheng B, Wu X, Chen D, Zhang D, Xiao G, Zhu JK, Wang S. An atlas of early human mandibular endochondral and osteogenic paracrine signaling regions of Meckel's cartilage. Proc Natl Acad Sci U S A. 2025;122:e2420466122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 59. | Suehiro F, Ishii M, Asahina I, Murata H, Nishimura M. Low-serum culture with novel medium promotes maxillary/mandibular bone marrow stromal cell proliferation and osteogenic differentiation ability. Clin Oral Investig. 2017;21:2709-2719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 60. | Zhang P, Men J, Fu Y, Shan T, Ye J, Wu Y, Tao Z, Liu L, Jiang H. Contribution of SATB2 to the stronger osteogenic potential of bone marrow stromal cells from craniofacial bones. Cell Tissue Res. 2012;350:425-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 61. | Venkatakrishnan J, Saeed Y, Kao WW. Trends in using mesenchymal stromal/stem cells (MSCs) in treating corneal diseases. Ocul Surf. 2022;26:255-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 62. | Mohan RR, Kempuraj D, D'Souza S, Ghosh A. Corneal stromal repair and regeneration. Prog Retin Eye Res. 2022;91:101090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 130] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 63. | Liu XN, Mi SL, Chen Y, Wang Y. Corneal stromal mesenchymal stem cells: reconstructing a bioactive cornea and repairing the corneal limbus and stromal microenvironment. Int J Ophthalmol. 2021;14:448-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 64. | Ghoubay D, Borderie M, Grieve K, Martos R, Bocheux R, Nguyen TM, Callard P, Chédotal A, Borderie VM. Corneal stromal stem cells restore transparency after N(2) injury in mice. Stem Cells Transl Med. 2020;9:917-935. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 65. | Volatier T, Cursiefen C, Notara M. Current Advances in Corneal Stromal Stem Cell Biology and Therapeutic Applications. Cells. 2024;13:163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 66. | Hay ED, Revel JP. Fine structure of the developing avian cornea. Monogr Dev Biol. 1969;1:1-144. [PubMed] |
| 67. | Fitch JM, Mentzer A, Mayne R, Linsenmayer TF. Acquisition of type IX collagen by the developing avian primary corneal stroma and vitreous. Dev Biol. 1988;128:396-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 63] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 68. | Hay ED. Development of the vertebrate cornea. Int Rev Cytol. 1980;63:263-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 252] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 69. | Funderburgh JL, Caterson B, Conrad GW. Keratan sulfate proteoglycan during embryonic development of the chicken cornea. Dev Biol. 1986;116:267-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 75] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 70. | Hart GW. Biosynthesis of glycosaminolgycans during corneal development. J Biol Chem. 1976;251:6513-6521. [PubMed] |
| 71. | Linsenmayer TF, Gibney E, Fitch JM. Embryonic avian cornea contains layers of collagen with greater than average stability. J Cell Biol. 1986;103:1587-1593. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 72. | Pinnamaneni N, Funderburgh JL. Concise review: Stem cells in the corneal stroma. Stem Cells. 2012;30:1059-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 157] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 73. | Du Y, Carlson EC, Funderburgh ML, Birk DE, Pearlman E, Guo N, Kao WW, Funderburgh JL. Stem cell therapy restores transparency to defective murine corneas. Stem Cells. 2009;27:1635-1642. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 185] [Cited by in RCA: 166] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 74. | Ventriglia S, Kalcheim C. From neural tube to spinal cord: The dynamic journey of the dorsal neuroepithelium. Dev Biol. 2024;511:26-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 75. | García-Castro M, Bronner-Fraser M. Induction and differentiation of the neural crest. Curr Opin Cell Biol. 1999;11:695-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 76. | Mayor R, Theveneau E. The neural crest. Development. 2013;140:2247-2251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 240] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 77. | Kalcheim C. Epithelial-Mesenchymal Transitions during Neural Crest and Somite Development. J Clin Med. 2015;5:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 78. | Burstyn-Cohen T, Stanleigh J, Sela-Donenfeld D, Kalcheim C. Canonical Wnt activity regulates trunk neural crest delamination linking BMP/noggin signaling with G1/S transition. Development. 2004;131:5327-5339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 149] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 79. | Shoval I, Ludwig A, Kalcheim C. Antagonistic roles of full-length N-cadherin and its soluble BMP cleavage product in neural crest delamination. Development. 2007;134:491-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 172] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 80. | Yu H, Kumar SM, Kossenkov AV, Showe L, Xu X. Stem cells with neural crest characteristics derived from the bulge region of cultured human hair follicles. J Invest Dermatol. 2010;130:1227-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 82] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 81. | Stierli S, Sommer L. Schwann cell precursors: a hub of neural crest development. EMBO J. 2022;41:e111955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 82. | Sieber-Blum M, Grim M. The adult hair follicle: cradle for pluripotent neural crest stem cells. Birth Defects Res C Embryo Today. 2004;72:162-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 104] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 83. | Jing J, Xu P, Xu JL, Ding YX, Yang XS, Jin XQ, Zhou LJ, Chen YH, Wu XJ, Lu ZF. Expression and localization of Sox10 during hair follicle morphogenesis and induced hair cycle. Int J Med Sci. 2021;18:3498-3505. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 84. | Li H, Masieri FF, Schneider M, Bartella A, Gaus S, Hahnel S, Zimmerer R, Sack U, Maksimovic-Ivanic D, Mijatovic S, Simon JC, Lethaus B, Savkovic V. The Middle Part of the Plucked Hair Follicle Outer Root Sheath Is Identified as an Area Rich in Lineage-Specific Stem Cell Markers. Biomolecules. 2021;11:154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 85. | Peterson A, Nair LS. Hair Follicle Stem Cells for Tissue Regeneration. Tissue Eng Part B Rev. 2022;28:695-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 86. | Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97:13625-13630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3174] [Cited by in RCA: 3436] [Article Influence: 132.2] [Reference Citation Analysis (1)] |
| 87. | Kaukua N, Shahidi MK, Konstantinidou C, Dyachuk V, Kaucka M, Furlan A, An Z, Wang L, Hultman I, Ahrlund-Richter L, Blom H, Brismar H, Lopes NA, Pachnis V, Suter U, Clevers H, Thesleff I, Sharpe P, Ernfors P, Fried K, Adameyko I. Glial origin of mesenchymal stem cells in a tooth model system. Nature. 2014;513:551-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 334] [Article Influence: 27.8] [Reference Citation Analysis (1)] |
| 88. | Isern J, García-García A, Martín AM, Arranz L, Martín-Pérez D, Torroja C, Sánchez-Cabo F, Méndez-Ferrer S. The neural crest is a source of mesenchymal stem cells with specialized hematopoietic stem cell niche function. Elife. 2014;3:e03696. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 242] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 89. | d'Aquino R, Graziano A, Sampaolesi M, Laino G, Pirozzi G, De Rosa A, Papaccio G. Human postnatal dental pulp cells co-differentiate into osteoblasts and endotheliocytes: a pivotal synergy leading to adult bone tissue formation. Cell Death Differ. 2007;14:1162-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 350] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 90. | Arthur A, Rychkov G, Shi S, Koblar SA, Gronthos S. Adult human dental pulp stem cells differentiate toward functionally active neurons under appropriate environmental cues. Stem Cells. 2008;26:1787-1795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 411] [Cited by in RCA: 435] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 91. | Liu J, Yu F, Sun Y, Jiang B, Zhang W, Yang J, Xu GT, Liang A, Liu S. Concise reviews: Characteristics and potential applications of human dental tissue-derived mesenchymal stem cells. Stem Cells. 2015;33:627-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 263] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 92. | Andrukhov O, Behm C, Blufstein A, Rausch-Fan X. Immunomodulatory properties of dental tissue-derived mesenchymal stem cells: Implication in disease and tissue regeneration. World J Stem Cells. 2019;11:604-617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 144] [Article Influence: 20.6] [Reference Citation Analysis (5)] |
| 93. | Huang GT, Gronthos S, Shi S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. J Dent Res. 2009;88:792-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1184] [Cited by in RCA: 1365] [Article Influence: 80.3] [Reference Citation Analysis (0)] |
| 94. | Kerkis I, Kerkis A, Dozortsev D, Stukart-Parsons GC, Gomes Massironi SM, Pereira LV, Caplan AI, Cerruti HF. Isolation and characterization of a population of immature dental pulp stem cells expressing OCT-4 and other embryonic stem cell markers. Cells Tissues Organs. 2006;184:105-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 311] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 95. | Lignell A, Kerosuo L, Streichan SJ, Cai L, Bronner ME. Identification of a neural crest stem cell niche by Spatial Genomic Analysis. Nat Commun. 2017;8:1830. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 85] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 96. | He P, Zhang Q, Motiwala FI, Shanti RM, Chang BM, Le AD. Potential application of dental stem cells in regenerative reconstruction of oral and maxillofacial tissues: a narrative review. Front Oral Maxillofac Med. 2022;4:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 97. | Buitrago-Delgado E, Nordin K, Rao A, Geary L, LaBonne C. NEURODEVELOPMENT. Shared regulatory programs suggest retention of blastula-stage potential in neural crest cells. Science. 2015;348:1332-1335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 138] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 98. | Yoshida S, Shimmura S, Nagoshi N, Fukuda K, Matsuzaki Y, Okano H, Tsubota K. Isolation of multipotent neural crest-derived stem cells from the adult mouse cornea. Stem Cells. 2006;24:2714-2722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 143] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 99. | Marynka-Kalmani K, Treves S, Yafee M, Rachima H, Gafni Y, Cohen MA, Pitaru S. The lamina propria of adult human oral mucosa harbors a novel stem cell population. Stem Cells. 2010;28:984-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 128] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 100. | Pagella P, Miran S, Neto E, Martin I, Lamghari M, Mitsiadis TA. Human dental pulp stem cells exhibit enhanced properties in comparison to human bone marrow stem cells on neurites outgrowth. FASEB J. 2020;34:5499-5511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 101. | Wang D, Wang Y, Tian W, Pan J. Advances of tooth-derived stem cells in neural diseases treatments and nerve tissue regeneration. Cell Prolif. 2019;52:e12572. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (1)] |
| 102. | Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, Shi S. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A. 2003;100:5807-5812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1922] [Cited by in RCA: 2016] [Article Influence: 87.7] [Reference Citation Analysis (1)] |
| 103. | De Berdt P, Vanacker J, Ucakar B, Elens L, Diogenes A, Leprince JG, Deumens R, des Rieux A. Dental Apical Papilla as Therapy for Spinal Cord Injury. J Dent Res. 2015;94:1575-1581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 104. | Kadar K, Kiraly M, Porcsalmy B, Molnar B, Racz GZ, Blazsek J, Kallo K, Szabo EL, Gera I, Gerber G, Varga G. Differentiation potential of stem cells from human dental origin - promise for tissue engineering. J Physiol Pharmacol. 2009;60 Suppl 7:167-175. [PubMed] |
| 105. | Park BW, Kang EJ, Byun JH, Son MG, Kim HJ, Hah YS, Kim TH, Mohana Kumar B, Ock SA, Rho GJ. In vitro and in vivo osteogenesis of human mesenchymal stem cells derived from skin, bone marrow and dental follicle tissues. Differentiation. 2012;83:249-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 106. | Nakajima K, Kunimatsu R, Ando K, Ando T, Hayashi Y, Kihara T, Hiraki T, Tsuka Y, Abe T, Kaku M, Nikawa H, Takata T, Tanne K, Tanimoto K. Comparison of the bone regeneration ability between stem cells from human exfoliated deciduous teeth, human dental pulp stem cells and human bone marrow mesenchymal stem cells. Biochem Biophys Res Commun. 2018;497:876-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 84] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 107. | Couble ML, Farges JC, Bleicher F, Perrat-Mabillon B, Boudeulle M, Magloire H. Odontoblast differentiation of human dental pulp cells in explant cultures. Calcif Tissue Int. 2000;66:129-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 199] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 108. | Mucientes A, Herranz E, Moro E, González-Corchón A, Peña-Soria MJ, Abasolo L, Rodriguez-Rodriguez L, Lamas JR, Fernández-Gutiérrez B. Influence of Mesenchymal Stem Cell Sources on Their Regenerative Capacities on Different Surfaces. Cells. 2021;10:481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 109. | Kim SH, Bang SH, Park SA, Kang SY, Park KD, Oh IU, Yoo SH, Kim H, Kim CH, Baek SY. Character comparison of abdomen-derived and eyelid-derived mesenchymal stem cells. Cell Prolif. 2013;46:291-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 110. | Kozlowska U, Krawczenko A, Futoma K, Jurek T, Rorat M, Patrzalek D, Klimczak A. Similarities and differences between mesenchymal stem/progenitor cells derived from various human tissues. World J Stem Cells. 2019;11:347-374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 133] [Article Influence: 19.0] [Reference Citation Analysis (1)] |
| 111. | Jin Q, Yuan K, Lin W, Niu C, Ma R, Huang Z. Comparative characterization of mesenchymal stem cells from human dental pulp and adipose tissue for bone regeneration potential. Artif Cells Nanomed Biotechnol. 2019;47:1577-1584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 65] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 112. | Zhang C, Lian X, Zhu M, Hu M, Xia D, Jin L, Yu R, Li J. Histone Demethylase KDM6B Promotes Chondrogenic Differentiation Potential of Stem Cells from the Apical Papilla via HES1. Cells Tissues Organs. 2025;214:315-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 113. | Lee YC, Chan YH, Hsieh SC, Lew WZ, Feng SW. Comparing the Osteogenic Potentials and Bone Regeneration Capacities of Bone Marrow and Dental Pulp Mesenchymal Stem Cells in a Rabbit Calvarial Bone Defect Model. Int J Mol Sci. 2019;20:5015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 159] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 114. | Longoni A, Utomo L, van Hooijdonk IE, Bittermann GK, Vetter VC, Kruijt Spanjer EC, Ross J, Rosenberg AJ, Gawlitta D. The chondrogenic differentiation potential of dental pulp stem cells. Eur Cell Mater. 2020;39:121-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 115. | Li A, Sasaki JI, Abe GL, Katata C, Sakai H, Imazato S. Vascularization of a Bone Organoid Using Dental Pulp Stem Cells. Stem Cells Int. 2023;2023:5367887. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 116. | Okić-Đorđević I, Obradović H, Kukolj T, Petrović A, Mojsilović S, Bugarski D, Jauković A. Dental mesenchymal stromal/stem cells in different microenvironments- implications in regenerative therapy. World J Stem Cells. 2021;13:1863-1880. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 117. | Xu X, Chen C, Akiyama K, Chai Y, Le AD, Wang Z, Shi S. Gingivae contain neural-crest- and mesoderm-derived mesenchymal stem cells. J Dent Res. 2013;92:825-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 131] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 118. | Kim D, Lee AE, Xu Q, Zhang Q, Le AD. Gingiva-Derived Mesenchymal Stem Cells: Potential Application in Tissue Engineering and Regenerative Medicine - A Comprehensive Review. Front Immunol. 2021;12:667221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 108] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 119. | Yamaguchi S, Shibata R, Yamamoto N, Nishikawa M, Hibi H, Tanigawa T, Ueda M, Murohara T, Yamamoto A. Dental pulp-derived stem cell conditioned medium reduces cardiac injury following ischemia-reperfusion. Sci Rep. 2015;5:16295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 99] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 120. | Bhattacherjee V, Mukhopadhyay P, Singh S, Johnson C, Philipose JT, Warner CP, Greene RM, Pisano MM. Neural crest and mesoderm lineage-dependent gene expression in orofacial development. Differentiation. 2007;75:463-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 121. | Leucht P, Kim JB, Amasha R, James AW, Girod S, Helms JA. Embryonic origin and Hox status determine progenitor cell fate during adult bone regeneration. Development. 2008;135:2845-2854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 247] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 122. | Jeyaraman M, Verma T, Jeyaraman N, Patro BP, Nallakumarasamy A, Khanna M. Is mandible derived mesenchymal stromal cells superior in proliferation and regeneration to long bone-derived mesenchymal stromal cells? World J Methodol. 2023;13:10-17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (3)] |
| 123. | Yamaza T, Ren G, Akiyama K, Chen C, Shi Y, Shi S. Mouse mandible contains distinctive mesenchymal stem cells. J Dent Res. 2011;90:317-324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 91] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 124. | Li C, Wang F, Zhang R, Qiao P, Liu H. Comparison of Proliferation and Osteogenic Differentiation Potential of Rat Mandibular and Femoral Bone Marrow Mesenchymal Stem Cells In Vitro. Stem Cells Dev. 2020;29:728-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 125. | Aghaloo TL, Chaichanasakul T, Bezouglaia O, Kang B, Franco R, Dry SM, Atti E, Tetradis S. Osteogenic potential of mandibular vs. long-bone marrow stromal cells. J Dent Res. 2010;89:1293-1298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 125] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 126. | Wang F, Zhou Y, Zhou J, Xu M, Zheng W, Huang W, Zhou W, Shen Y, Zhao K, Wu Y, Zou D. Comparison of Intraoral Bone Regeneration with Iliac and Alveolar BMSCs. J Dent Res. 2018;97:1229-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 127. | Matsubara T, Suardita K, Ishii M, Sugiyama M, Igarashi A, Oda R, Nishimura M, Saito M, Nakagawa K, Yamanaka K, Miyazaki K, Shimizu M, Bhawal UK, Tsuji K, Nakamura K, Kato Y. Alveolar bone marrow as a cell source for regenerative medicine: differences between alveolar and iliac bone marrow stromal cells. J Bone Miner Res. 2005;20:399-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 236] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 128. | Park BW, Hah YS, Kim DR, Kim JR, Byun JH. Vascular endothelial growth factor expression in cultured periosteal-derived cells. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105:554-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 129. | Chen D, Zhang X, He Y, Lu J, Shen H, Jiang Y, Zhang C, Zeng B. Co-culturing mesenchymal stem cells from bone marrow and periosteum enhances osteogenesis and neovascularization of tissue-engineered bone. J Tissue Eng Regen Med. 2012;6:822-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 130. | Tang YL, Zhao Q, Zhang YC, Cheng L, Liu M, Shi J, Yang YZ, Pan C, Ge J, Phillips MI. Autologous mesenchymal stem cell transplantation induce VEGF and neovascularization in ischemic myocardium. Regul Pept. 2004;117:3-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 292] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 131. | Parthiban SP, He W, Monteiro N, Athirasala A, França CM, Bertassoni LE. Engineering pericyte-supported microvascular capillaries in cell-laden hydrogels using stem cells from the bone marrow, dental pulp and dental apical papilla. Sci Rep. 2020;10:21579. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 132. | Cao C, Tarlé S, Kaigler D. Characterization of the immunomodulatory properties of alveolar bone-derived mesenchymal stem cells. Stem Cell Res Ther. 2020;11:102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 133. | Solovieva T, Bronner M. Schwann cell precursors: Where they come from and where they go. Cells Dev. 2021;166:203686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 134. | Kastriti ME, Faure L, Von Ahsen D, Bouderlique TG, Boström J, Solovieva T, Jackson C, Bronner M, Meijer D, Hadjab S, Lallemend F, Erickson A, Kaucka M, Dyachuk V, Perlmann T, Lahti L, Krivanek J, Brunet JF, Fried K, Adameyko I. Schwann cell precursors represent a neural crest-like state with biased multipotency. EMBO J. 2022;41:e108780. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 80] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 135. | Wakao S, Matsuse D, Dezawa M. Mesenchymal stem cells as a source of Schwann cells: their anticipated use in peripheral nerve regeneration. Cells Tissues Organs. 2014;200:31-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 136. | Han C, Zhang L, Song L, Liu Y, Zou W, Piao H, Liu J. Human adipose-derived mesenchymal stem cells: a better cell source for nervous system regeneration. Chin Med J (Engl). 2014;127:329-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 137. | Li M, Li G, Lei H, Guan R, Yang B, Gao Z, Hui Y, Chen F, Xin Z. Therapeutic Potential of Adipose-derived Stem Cell-based Microtissues in a Rat Model of Stress Urinary Incontinence. Urology. 2016;97:277.e1-277.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 138. | Cui L, Meng Q, Wen J, Gao Z, Yan Z, Tian Y, Xu P, He X, Yu H, Lian P. A Functional Comparison of Treatment of Intrinsic Sphincter Deficiency with Muscle-Derived and Adipose Tissue-Derived Stem Cells. IUBMB Life. 2018;70:976-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 139. | Stratton JA, Shah PT, Kumar R, Stykel MG, Shapira Y, Grochmal J, Guo GF, Biernaskie J, Midha R. The immunomodulatory properties of adult skin-derived precursor Schwann cells: implications for peripheral nerve injury therapy. Eur J Neurosci. 2016;43:365-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 140. | Gao F, Chiu SM, Motan DA, Zhang Z, Chen L, Ji HL, Tse HF, Fu QL, Lian Q. Mesenchymal stem cells and immunomodulation: current status and future prospects. Cell Death Dis. 2016;7:e2062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 611] [Cited by in RCA: 853] [Article Influence: 85.3] [Reference Citation Analysis (17)] |
| 141. | Wang Z, Huang M, Zhang Y, Jiang X, Xu L. Comparison of Biological Properties and Clinical Application of Mesenchymal Stem Cells from the Mesoderm and Ectoderm. Stem Cells Int. 2023;2023:4547875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 142. | Redondo LM, García V, Peral B, Verrier A, Becerra J, Sánchez A, García-Sancho J. Repair of maxillary cystic bone defects with mesenchymal stem cells seeded on a cross-linked serum scaffold. J Craniomaxillofac Surg. 2018;46:222-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 143. | Apatzidou DA, Iliopoulos JM, Konstantinidis A, Verma M, Hardy P, Lappin DF, Nile CJ. Inflammatory and bone remodelling related biomarkers following periodontal transplantation of the tissue engineered biocomplex. Clin Oral Investig. 2024;28:361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 144. | Apatzidou DA, Bakopoulou AA, Kouzi-Koliakou K, Karagiannis V, Konstantinidis A. A tissue-engineered biocomplex for periodontal reconstruction. A proof-of-principle randomized clinical study. J Clin Periodontol. 2021;48:1111-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 145. | Zhang T, Qian C, Song M, Tang Y, Zhou Y, Dong G, Shen Q, Chen W, Wang A, Shen S, Zhao Y, Lu Y. Application Prospect of Induced Pluripotent Stem Cells in Organoids and Cell Therapy. Int J Mol Sci. 2024;25:2680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 146. | Cerneckis J, Cai H, Shi Y. Induced pluripotent stem cells (iPSCs): molecular mechanisms of induction and applications. Signal Transduct Target Ther. 2024;9:112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 174] [Article Influence: 87.0] [Reference Citation Analysis (0)] |
| 147. | Ye L, Swingen C, Zhang J. Induced pluripotent stem cells and their potential for basic and clinical sciences. Curr Cardiol Rev. 2013;9:63-72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 148. | Lee G, Chambers SM, Tomishima MJ, Studer L. Derivation of neural crest cells from human pluripotent stem cells. Nat Protoc. 2010;5:688-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 251] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 149. | Jiang X, Gwye Y, McKeown SJ, Bronner-Fraser M, Lutzko C, Lawlor ER. Isolation and characterization of neural crest stem cells derived from in vitro-differentiated human embryonic stem cells. Stem Cells Dev. 2009;18:1059-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 113] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 150. | Zhou Y, Snead ML. Derivation of cranial neural crest-like cells from human embryonic stem cells. Biochem Biophys Res Commun. 2008;376:542-547. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 151. | Pomp O, Brokhman I, Ben-Dor I, Reubinoff B, Goldstein RS. Generation of peripheral sensory and sympathetic neurons and neural crest cells from human embryonic stem cells. Stem Cells. 2005;23:923-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 140] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 152. | Srinivasan A, Toh YC. Human Pluripotent Stem Cell-Derived Neural Crest Cells for Tissue Regeneration and Disease Modeling. Front Mol Neurosci. 2019;12:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 153. | Wilson SI, Rydström A, Trimborn T, Willert K, Nusse R, Jessell TM, Edlund T. The status of Wnt signalling regulates neural and epidermal fates in the chick embryo. Nature. 2001;411:325-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 214] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 154. | Patthey C, Edlund T, Gunhaga L. Wnt-regulated temporal control of BMP exposure directs the choice between neural plate border and epidermal fate. Development. 2009;136:73-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 89] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 155. | García-Castro MI, Marcelle C, Bronner-Fraser M. Ectodermal Wnt function as a neural crest inducer. Science. 2002;297:848-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 209] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 156. | Menendez L, Kulik MJ, Page AT, Park SS, Lauderdale JD, Cunningham ML, Dalton S. Directed differentiation of human pluripotent cells to neural crest stem cells. Nat Protoc. 2013;8:203-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 155] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 157. | Menendez L, Yatskievych TA, Antin PB, Dalton S. Wnt signaling and a Smad pathway blockade direct the differentiation of human pluripotent stem cells to multipotent neural crest cells. Proc Natl Acad Sci U S A. 2011;108:19240-19245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 211] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 158. | Mica Y, Lee G, Chambers SM, Tomishima MJ, Studer L. Modeling neural crest induction, melanocyte specification, and disease-related pigmentation defects in hESCs and patient-specific iPSCs. Cell Rep. 2013;3:1140-1152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 202] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 159. | Gomez GA, Prasad MS, Sandhu N, Shelar PB, Leung AW, García-Castro MI. Human neural crest induction by temporal modulation of WNT activation. Dev Biol. 2019;449:99-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 160. | Leung AW, Murdoch B, Salem AF, Prasad MS, Gomez GA, García-Castro MI. WNT/β-catenin signaling mediates human neural crest induction via a pre-neural border intermediate. Development. 2016;143:398-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 116] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 161. | Hug K, Hermerén G. Do we still need human embryonic stem cells for stem cell-based therapies? Epistemic and ethical aspects. Stem Cell Rev Rep. 2011;7:761-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 162. | Cox JL, Rizzino A. Induced pluripotent stem cells: what lies beyond the paradigm shift. Exp Biol Med (Maywood). 2010;235:148-158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 163. | Poetsch MS, Strano A, Guan K. Human Induced Pluripotent Stem Cells: From Cell Origin, Genomic Stability, and Epigenetic Memory to Translational Medicine. Stem Cells. 2022;40:546-555. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 82] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 164. | Kim K, Zhao R, Doi A, Ng K, Unternaehrer J, Cahan P, Huo H, Loh YH, Aryee MJ, Lensch MW, Li H, Collins JJ, Feinberg AP, Daley GQ. Donor cell type can influence the epigenome and differentiation potential of human induced pluripotent stem cells. Nat Biotechnol. 2011;29:1117-1119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 432] [Cited by in RCA: 476] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 165. | Kim K, Doi A, Wen B, Ng K, Zhao R, Cahan P, Kim J, Aryee MJ, Ji H, Ehrlich LI, Yabuuchi A, Takeuchi A, Cunniff KC, Hongguang H, McKinney-Freeman S, Naveiras O, Yoon TJ, Irizarry RA, Jung N, Seita J, Hanna J, Murakami P, Jaenisch R, Weissleder R, Orkin SH, Weissman IL, Feinberg AP, Daley GQ. Epigenetic memory in induced pluripotent stem cells. Nature. 2010;467:285-290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1842] [Cited by in RCA: 1720] [Article Influence: 107.5] [Reference Citation Analysis (13)] |
| 166. | Heslop JA, Hammond TG, Santeramo I, Tort Piella A, Hopp I, Zhou J, Baty R, Graziano EI, Proto Marco B, Caron A, Sköld P, Andrews PW, Baxter MA, Hay DC, Hamdam J, Sharpe ME, Patel S, Jones DR, Reinhardt J, Danen EH, Ben-David U, Stacey G, Björquist P, Piner J, Mills J, Rowe C, Pellegrini G, Sethu S, Antoine DJ, Cross MJ, Murray P, Williams DP, Kitteringham NR, Goldring CE, Park BK. Concise review: workshop review: understanding and assessing the risks of stem cell-based therapies. Stem Cells Transl Med. 2015;4:389-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 91] [Article Influence: 8.3] [Reference Citation Analysis (16)] |
| 167. | Fu X, Xu Y. Challenges to the clinical application of pluripotent stem cells: towards genomic and functional stability. Genome Med. 2012;4:55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 168. | Deng J, Zhang Y, Xie Y, Zhang L, Tang P. Cell Transplantation for Spinal Cord Injury: Tumorigenicity of Induced Pluripotent Stem Cell-Derived Neural Stem/Progenitor Cells. Stem Cells Int. 2018;2018:5653787. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 169. | La Noce M, Mele L, Tirino V, Paino F, De Rosa A, Naddeo P, Papagerakis P, Papaccio G, Desiderio V. Neural crest stem cell population in craniomaxillofacial development and tissue repair. Eur Cell Mater. 2014;28:348-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 170. | Liu JA, Cheung M. Neural crest stem cells and their potential therapeutic applications. Dev Biol. 2016;419:199-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 171. | Pi HJ, Huang B, Yuan Q, Jing JJ. Neural regulation of mesenchymal stem cells in craniofacial bone: development, homeostasis and repair. Front Physiol. 2024;15:1423539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 172. | Ducret M, Fabre H, Degoul O, Atzeni G, McGuckin C, Forraz N, Alliot-Licht B, Mallein-Gerin F, Perrier-Groult E, Farges JC. Manufacturing of dental pulp cell-based products from human third molars: current strategies and future investigations. Front Physiol. 2015;6:213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 173. | Dieterle MP, Gross T, Steinberg T, Tomakidi P, Becker K, Vach K, Kremer K, Proksch S. Characterization of a Stemness-Optimized Purification Method for Human Dental-Pulp Stem Cells: An Approach to Standardization. Cells. 2022;11:3204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 174. | Chen FM, Gao LN, Tian BM, Zhang XY, Zhang YJ, Dong GY, Lu H, Chu Q, Xu J, Yu Y, Wu RX, Yin Y, Shi S, Jin Y. Treatment of periodontal intrabony defects using autologous periodontal ligament stem cells: a randomized clinical trial. Stem Cell Res Ther. 2016;7:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 160] [Cited by in RCA: 254] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 175. | Cubuk S, Oduncuoglu BF, Alaaddinoglu EE. The effect of dental pulp stem cells and L-PRF when placed into the extraction sockets of impacted mandibular third molars on the periodontal status of adjacent second molars: a split-mouth, randomized, controlled clinical trial. Oral Maxillofac Surg. 2023;27:59-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (2)] |
| 176. | Tanikawa DYS, Pinheiro CCG, Almeida MCA, Oliveira CRGCM, Coudry RA, Rocha DL, Bueno DF. Deciduous Dental Pulp Stem Cells for Maxillary Alveolar Reconstruction in Cleft Lip and Palate Patients. Stem Cells Int. 2020;2020:6234167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 177. | Pinheiro CCG, Leyendecker Junior A, Tanikawa DYS, Ferreira JRM, Jarrahy R, Bueno DF. Is There a Noninvasive Source of MSCs Isolated with GMP Methods with Better Osteogenic Potential? Stem Cells Int. 2019;2019:7951696. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 178. | Suda S, Nito C, Ihara M, Iguchi Y, Urabe T, Matsumaru Y, Sakai N, Kimura K; J- REPAIR trial group. Randomised placebo-controlled multicentre trial to evaluate the efficacy and safety of JTR-161, allogeneic human dental pulp stem cells, in patients with Acute Ischaemic stRoke (J-REPAIR). BMJ Open. 2022;12:e054269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 179. | Wenceslau CV, de Souza DM, Mambelli-Lisboa NC, Ynoue LH, Araldi RP, da Silva JM, Pagani E, Haddad MS, Kerkis I. Restoration of BDNF, DARPP32, and D2R Expression Following Intravenous Infusion of Human Immature Dental Pulp Stem Cells in Huntington's Disease 3-NP Rat Model. Cells. 2022;11:1664. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 180. | Ye Q, Wang H, Xia X, Zhou C, Liu Z, Xia ZE, Zhang Z, Zhao Y, Yehenala J, Wang S, Zhou G, Hu K, Wu B, Wu CT, Wang S, He Y. Safety and efficacy assessment of allogeneic human dental pulp stem cells to treat patients with severe COVID-19: structured summary of a study protocol for a randomized controlled trial (Phase I / II). Trials. 2020;21:520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 181. | Sánchez N, Fierravanti L, Núñez J, Vignoletti F, González-Zamora M, Santamaría S, Suárez-Sancho S, Fernández-Santos ME, Figuero E, Herrera D, García-Sanz JA, Sanz M. Periodontal regeneration using a xenogeneic bone substitute seeded with autologous periodontal ligament-derived mesenchymal stem cells: A 12-month quasi-randomized controlled pilot clinical trial. J Clin Periodontol. 2020;47:1391-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/