Published online Sep 26, 2025. doi: 10.4252/wjsc.v17.i9.106282
Revised: March 27, 2025
Accepted: September 5, 2025
Published online: September 26, 2025
Processing time: 209 Days and 20.8 Hours
Anterior cruciate ligament reconstruction (ACLR) is the dominant clinical modality for the treatment of anterior cruciate ligament injuries. The success of ACLR is largely dependent on tendon-bone healing, and stem cell biotherapies are often used to facilitate this process. Histone lactylation modifications are involved in the regulation of various diseases. Lactate dehydrogenase A (LDHA) has been shown to play an important role in exosomes.
To explore the regulation of tendon-bone healing after ACLR by LDHA in exosomes derived from bone marrow mesenchymal stem cells (BMSC-Exos).
BMSC-Exos and LDHA were characterized and analyzed by transmission electron microscopy, qNano, immunofluorescence and western blotting assay. The corresponding low expression cell lines were obtained using RNA interference trans
The spherical nanosized BMSC-Exos could be uptaken by CSPCs. LDHA was highly expressed in BMSC-Exos, which could infiltrate into the bone tissue of ACLR rats and promoted the generation of new bone tissue, as well as significantly increased the regeneration of tendon and fibrocartilage. Co-incubation of CSPCs with high-expressing LDHA BMSC-Exos increased the secretion of lactate content from CSPCs, cell viability, and the expression of markers related to cell proliferation and differentiation, including collagen II, SOX9, and aggrecan; LDHA in BMSC-Exos upregulated BMP7 through histone H3K18 lactate modification; high LDHA expression reversed the knockdown of BMP7, further increasing the proliferation and differentiation of CSPCs, thereby inducing cartilage formation.
LDHA in BMSC-Exos promotes BMP7 expression via H3K18 lactylation modification, which further promotes tendon-bone healing after ACLR.
Core Tip: This study reveals that lactate dehydrogenase A (LDHA) in exosomes from bone marrow mesenchymal stem cells significantly enhances tendon-bone healing after anterior cruciate ligament reconstruction. LDHA promotes chondrocyte stem cell proliferation and differentiation by increasing lactate secretion and upregulating bone morphogenetic protein 7 via histone H3K18 lactylation. High LDHA expression boosts cartilage and fibrocartilage regeneration, accelerates new bone formation, and reverses bone morphogenetic protein 7 knockdown effects. These findings highlight LDHA-rich exosomes from bone marrow mesenchymal stem cells as a promising therapeutic strategy for improving anterior cruciate ligament reconstruction outcomes.
- Citation: Zhang T, Huang Q, Gan KF. Bone marrow mesenchymal stem cell-derived exosomal lactate dehydrogenase A promotes tendon-bone healing via histone lactylation-mediated cartilage regeneration. World J Stem Cells 2025; 17(9): 106282
- URL: https://www.wjgnet.com/1948-0210/full/v17/i9/106282.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v17.i9.106282
Anterior cruciate ligament (ACL) injury is the most common knee sports injury, and its incidence has been steadily increasing in recent years. In the last decade, arthroscopic ACL reconstruction (ACLR) of the knee has been recognized as an effective treatment for ACL rupture[1]. However, regardless of surgical or conservative treatment, more than half of the patients need 1-2 years to return to normal sports, and even a small number of patients are unable to return to sports after ACLR[2]. In addition, a high recurrent tear rate of 11.7% after surgical intervention has been observed in clinical practice, which may be due to graft remodeling failure or poor tendon-bone healing during the graft healing process after ACLR. Studies have shown that the tendon-bone junction is usually replaced by fibrous scar tissue after ACLR, resulting in weakened biomechanics of the tendon-bone junction, which ultimately leads to ACLR failure[3]. Therefore, strategies such as altering the surgical approach and the shape and angle of the bone tunnel, platelet-rich plasma, cytokines, stem cell therapy, periosteal coverage, biomaterials, and physical interventions have been proposed to promote tendon-bone healing after ACLR[4]. However, none of these strategies has been shown to be effective in the clinical setting[5].
Emerging evidence suggests that metabolic-epigenetic crosstalk may play a pivotal role in tissue repair. Among these mechanisms, histone lactylation - a novel post-translational modification driven by lactate - has recently been implicated in regulating cellular plasticity and repair processes. Histone lactylation modifies lysine residues on histones through lactate-derived lactyl groups, directly linking cellular metabolism to epigenetic gene regulation. While initially discovered in immune modulation and tumor microenvironments, its potential role in musculoskeletal repair remains unexplored.
The discovery and investigation of cartilage-derived stem cells or cartilage-derived stem/progenitor cells have created a new path for the treatment of cartilage lesions from different perspectives[6]. When normal articular cartilage encounters trauma, chondrogenic stem cells from the cell surface layer can migrate to the injured area and spontaneously repair damaged cartilage[7]. Recent studies have shown that abundant stem cells in cartilage fragments play an important role in promoting the formation of tendon-bone healing, and pulverized cartilage fragments as a source of cells have been utilized for cartilage repair[8,9]. Zhang et al[10] demonstrated that cartilage fragments were effective in preventing the widening of the femoral tunnel after ACLR and promoting the tendon-bone integration process. However, further exploration is still needed due to the unclear mechanism of action of cartilage fragments in promoting cartilage regeneration.
Bone marrow mesenchymal stem cells (BMSCs) are pluripotent stem cells with the potential to differentiate directionally into tissues such as bone, cartilage, tendon, muscle, etc. under specific induction conditions. Autologous BMSCs can not only be used to rebuild the structure and function of tissues and organs, but also avoid the immune rejection caused by allogeneic transplantation[11]. Studies have demonstrated that BMSCs promote and enhance the tendon-bone healing interface[12]. Ouyang et al[13] demonstrated that BMSCs effectively promoted the production of collagen fibers and formed a large amount of fibrocartilage at the tendon bone healing interface, which further confirmed the ability of BMSCs to promote the healing of the tendon-bone interface. In addition, exosomes derived from BMSCs (BMSC-Exos) have biological properties similar to those of MSCs, which have also been shown to enhance cartilage formation and tendon-bone healing, further leading to effective treatment of cartilage defects and gyratory sleeve tears[14-16].
Notably, the metabolic activity of BMSC-Exos may extend beyond traditional paracrine signaling. Lactate dehydrogenase A (LDHA), a key enzyme in lactate metabolism, is enriched in BMSC-Exos and regulates lactate production - a substrate for histone lactylation[17,18]. Researchers at Northwestern University found that LDHA regulates metabolic reprogramming and tumor-macrophage symbiosis in glioblastoma[19]. Macrophage-derived extracellular vesicles promote glioblastoma cell glycolysis, growth and survival[19]. This raises the intriguing possibility that BMSC-Exos could similarly modulate cartilage fragment metabolism through LDHA-driven lactate synthesis, potentially influencing histone lactylation patterns during tendon-bone healing[20]. However, the role of histone lactylation in cartilage fragmentation after ACL injury and reconstruction is unknown. Therefore, here, we aimed to investigate the mechanism by which LDHA in BMSC-Exos regulates cartilage fragmentation in tendon-bone healing after ACLR to provide some help for tendon-bone healing treatment strategies after ACLR.
Extraction of chondrocyte stem cells: Knee cartilage from 2-week-old rats was extracted and digested with collagenase II, primary chondrocytes were obtained. The cells were then re-suspended and inoculated on plates coated with 10 μg/mL fibronectin, and cultured followed by cultured in an incubator at 37 °C with 5% CO2 with the addition of low sugar Dulbecco’s modified Eagle’s medium (DMEM)/F12 1:1 medium, 10% (v/v) foetal bovine serum (FBS), and 1% penicillin/streptomycin. The medium was changed once every 2 days. 12 days later, chondrocyte stem cell (CSPC) colonies were formed.
Extraction of BMSCs: 4-week-old Sprague-Dawley (SD) rats were euthanized, and the bone marrow was extracted from the femur and tibia, blown, filtered, and centrifuged to obtain BMSCs, which were then cultured in an incubator at 37 °C, 5% CO2 with the addition of DMEM medium, 10% FBS, and 1% penicillin/streptomycin. For the isolation of BMSC-Exos, BMSCs cells were ultracentrifuged, the supernatant was taken and filtered through 0.8 μm Poretics PCTE, followed by continued ultrahigh-speed centrifugation for concentration, and the obtained exosomes were resuspended using phosphate buffered saline (PBS) for subsequent experimental studies. For the identification of BMSC-Exos, transmission electron microscopy was used to observe the morphological features of BMSC-Exos. qNano analysis was used to determine the size distribution of BMSC-Exos.
CSPCs viability assay was performed by Cell Counting Kit-8 (Beyotime, China). First, CSPCs with or without bone morphogenetic protein 7 small interfering RNA (BMP7-siRNA) were inoculated into a 96-well plate and then incubated with BMSC-Exos with or without LDHA-siRNA overnight. For lactate rescue experiments, cells were co-treated with 5-20 mmol/L sodium lactate (Sigma, MA, United States) or 10 mmol/L FX11 (LDHA inhibitor). Then the cell supernatant was aspirated and 10 μL of cell counting kit 8 solution was added, followed by continued incubation for 4 hours. Finally, the absorbance at 450 nm was measured under an enzyme meter.
For the establishment of siRNA knockdown cell lines, cells were transfected with LDHA-siRNA, BMP7-siRNA LDHB-siRNA (to exclude LDH isoform interference) or only siRNA (siNC group) using the LipofectamineTM RNAiMAX reagent (Invitrogen, CA, United States) according to the manufacturer’s instructions.
All rats (8-week-old SD rats, male, 250-300 g) were purchased. The study was approved by the Affiliated Lihuili Hospital of Ningbo University Medical Ethics Committee for Laboratory Animals and complied with the Guidelines for the Management and Use of Laboratory Animals. All animals were housed in a specific pathogen-free environment for one week before subsequent experimental studies. For the construction of the ACLR model, rats were anesthetized using sodium pentobarbital (intraperitoneal injection, 40 mg/kg), and then the shoulder joint was incised, the trapezius muscle was pulled back, and the unilateral ACL was excised. Four weeks later, the autologous tendon was grafted and fixed into the bone tunnel on the femoral and tibial sides using surgical sutures. After surgery, the incision was sutured, and the rats were allowed to move and eat freely. A total of 36 rats were randomly divided into 3 groups (n = 12/group): The control group, the BMSC-Exos group, and the BMSC-Exos shLDHA group. All rats were injected with 50 μL PBS into the joint cavity of the rats for control group after ACLR. For the BMSC-Exos and BMSC-Exos shLDHA group, equal volumes of BMSC-Exos and BMSC-Exos shLDHA were administered after the operation, respectively.
Cellular protein samples of different groups were extracted using RIPA lysate and protein concentration was determined by BCA kit, followed by sodium-dodecyl sulfate gel electrophoresis, and then polyvinylidene fluoride membrane was used for membrane transfer. The polyvinylidene fluoride membrane was placed in goat serum for sealing, and then the corresponding primary antibody, HRP-labeled goat anti-rabbit or goat anti-mouse secondary antibody were added for incubation, respectively. Finally, the ECL luminescent solution was evenly added to the membrane and the target bands were imaged using a chemiluminescence instrument. Primary antibodies included: LDHA (1:1000, Abcam ab52488, United Kingdom); H3K18 La (1:500, PTM Bio PTM-1401, China); BMP7 (1:1000, Santa Cruz sc-514365, TX, United States); p-Smad1/5/8 (1:1000, Cell Signaling 13820, MA, United States); CD63 (1:1000, System Biosciences EXOAB-CD63A-1, CA, United States); CD81 (1:1000, System Biosciences EXOAB-CD81A-1. CA, United States); calnexin (1:1000, negative control, Abcam ab22595, United Kingdom).
The micro-computed tomography (micro-CT) system was used to analyze and evaluate new bone formation in tendon terminals of ACLR rats after injection of BMSC-Exos with high or low expression of LDHA. The parameters of micro-CT were 11.5 μm voxel size, 45 KVp, 100 μA, and 231 ms exposure time/view. Then we selected a cylindrical region of interest with a diameter of 1.2 mm and a height of 2 mm (bone trabeculae within the humeral head near the point of tendon attachment within approximately 1 mm and proximal to the growth plate) to calculate bone mineral density.
CSPCs were cultured in 24-well plates and then co-incubated with BMSC-Exos knocking down LDHA or silencing BMP7 for 24 hours, respectively. And then the supernatant medium was collected and centrifuged to separate the cell debris and floating cells, following the secreted lactic acid in the medium was measured using a 1:20 dilution of L-lactate assay kit I. DMEM in 10% FBS was used as a control to subtract the influence of background.
Trizol reagent was used to extract and isolate total RNA from differently treated BMSC-Exos and CSPCs. Then, cDNA was synthesized by reverse transcription of mRNA using SuperScript III reverse transcriptase and Oligo(dT)18 primer. Next, a SYBR Green amplification kit was utilized to analysis the cDNA by quantitative polymerase chain reaction (PCR) in a PCR detection system. All experiments were repeated three times.
The enrichment of H3K18 Lactylationon the BMP7 promoter was determined using the chromatin immunoprecipitation assay kit according to the manufacturer’s protocol. Briefly, CSPCS was fixed, lysed and sonicated into chromatin with an average size of 500 bp, and then the chromatin was spun at 4 °C for 8 hours with anti-H3K18 La antibody (PTM Bio, China) or immunoglobulin G control. Quantitative PCR primers flanking the BMP7 promoter Smad-binding element were designed (forward: 5’-GCGAGCTCGGTACCCGG-3’; reverse: 5’-TCCCCCGGGCTGCAGGA-3’). The immunoprecipitated DNA was then purified with RNAase A and proteinase K, respectively, and the purified DNA was evaluated with specific primers and the level of BMP7 was detected by PCR.
For the BMP7 promoter activity assay, CSPCs were seeded in 6-well plates and cultured for 24 hours before transfection. The cells were then transfected with the BMP7 promoter-luciferase reporter (pGL3-BMP7-Smad-binding element) and Smad1/5/8 dominant-negative plasmid (Addgene #31737, MA, United States) using Lipofectamine 2000. After 48 hours of incubation, cells were lysed, and firefly/Renilla luciferase activity was measured using the Dual-Luciferase® Reporter Assay System (Promega, WI, United States) to assess BMP7 promoter activity.
Firstly, lip-loving fluorescent PKH dye (PKH-26) was used to label BMSC-Exos, and then labeled BMSC-Exos were incubated with CSPCs for 24 hours. Then after fixation with paraformaldehyde and washing with PBS, the nuclei were labeled with 4’,6-diamidino-2-phenylindole (1:1000, Solarbio, China). Finally, the internalization of BMSC-Exos was analyzed by a confocal fluorescence microscope.
The samples were decalcified and dehydrated, embedded in paraffin wax to make paraffin sections, which were then cut into 5-μm sections on a microtome, followed by hematoxylin and eosin (H&E) staining using H&E staining solutions, and Safranin O-Fast green (SOFG) staining using Senna-Fast Green staining solution SOFG, respectively. Staining was performed according to the procedure provided by the reagent vendor. For H&E staining, a scoring system was used to assess regeneration of tendons for CSPCs, vascular distribution, and collagen fiber orientation. For SOFG staining, ImageJ software was applied to quantify the total area of regenerated fibrocartilage.
Reagents and instruments: Nano-flow cytometer, CFDA-SE fluorescent probe, anti-CD63-FITC antibody, anti-CD81-APC antibody, sterile PBS, ultracentrifuge (100000 × g), 0.22 μm filter, 1.5 mL EP tube, low-protein binding tubes, flow cytometry analysis software (FlowJo).
BMSC-Exos sample preparation: SD rat BMSCs were cultured in exosome-depleted FBS for 48 hours. The culture medium was collected and centrifuged at 300 × g for 10 minutes to remove cells, followed by centrifugation at 2000 × g for 20 minutes to remove apoptotic cell debris, at 10000 × g for 30 minutes to remove large extracellular vesicles, and finally at 100000 × g for 120 minutes to pellet the exosomes. The sample was filtered through a 0.22 μm filter and resuspended in PBS, then stored at -80 °C. Exosome concentration was measured using nanoparticle tracking analysis or a BCA kit, ensuring a concentration of approximately 1 × 1012 particles/mL.
Nano-flow cytometry detection: 100 μL of BMSC-Exos sample was taken and mixed with 5 μL CD63-FITC and 5 μL CD81-APC antibodies, then incubated at 4 °C in the dark for 30 minutes. After incubation, the sample was washed twice with 400 μL PBS (centrifuged at 100000 × g for 70 minutes), and resuspended in 200 μL PBS. The nano-flow cytometer was preheated and calibrated according to the manufacturer’s instructions. The laser channels were set (FITC: 488 nm, APC: 635 nm), and the particle detection mode was configured. PBS was used as the background signal for gating adjustments. Single-particle detection mode was run to ensure particles in the 50-200 nm range were detected. 10000-50000 particle events were collected, and a dual-fluorescence gating strategy was applied to calculate the proportion of CD63+/CD81+ double-positive particles. Data were analyzed using FlowJo software, and scatter plots and histograms were generated to output the analysis results.
Data in this study were obtained from five parallel experiments and presented as mean ± SD. The Graphpad and SPSS 22.0 software were used to analyze the data. Comparisons between two groups of data were analyzed by unpaired t-tests. Statistical differences between multiple groups of data were analyzed by one-way/two-way analysis of variance (ANOVA). Tukey’s post hoc test was used to test for differences between groups, aP value < 0.05 was considered significant. Sample size determination, experimental repetition, and statistical power analysis.
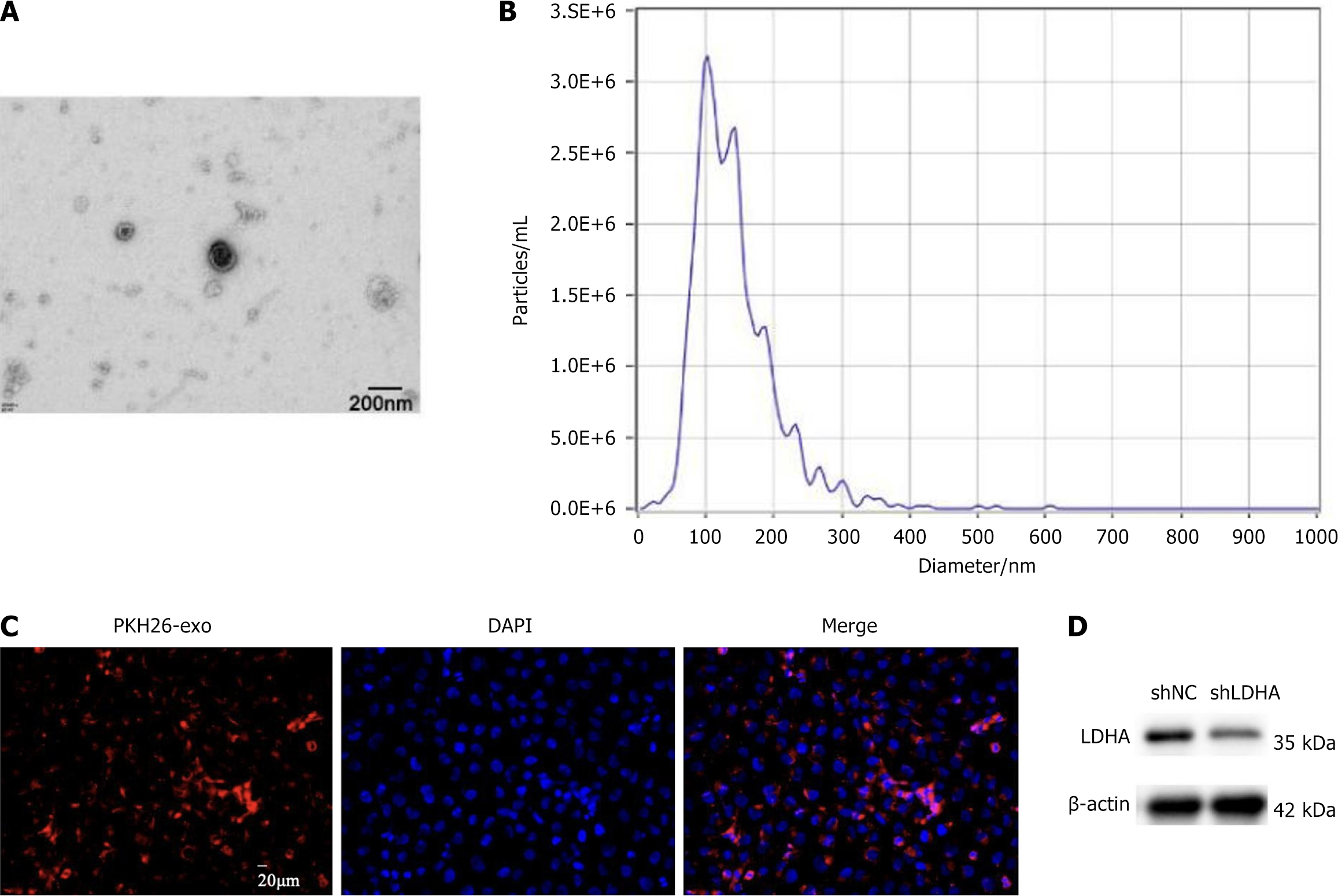
Morphological analysis of BMSC-Exos by transmission electron microscopy showed that BMSC-Exos presented rounded particles, and qNano analysis indicated that its size was approximately about 100 nm (Figure 1A and B). To further confirm the successful isolation of BMSC-Exos, we analyzed the expression of exosome surface markers CD81, CD63, and tumor susceptibility gene 101, while calnexin was used as a negative control. Western blotting analysis indicated that these exosome markers were highly enriched in BMSC-Exos, whereas calnexin was nearly undetectable. Nanoflow cytometry further demonstrated that 89.7% of BMSC-Exos were CD63+/CD81+ double-positive particles (Supplementary Figure 1). Next, the infiltration of BMSC-Exos in CSPCs was analyzed, and immunofluorescence images showed that CSPCs could swallow BMSC-Exos completely (Figure 1C). Bone marrow MSC exosomes extracted from SD rats were transfected to knock down LDHA, and BMSC-Exos transfected with blank short hairpin RNA were used as control. The expression level of LDHA protein was analyzed by western blotting assay, and LDHA was highly expressed in BMSC-Exos as seen in Figure 1D.
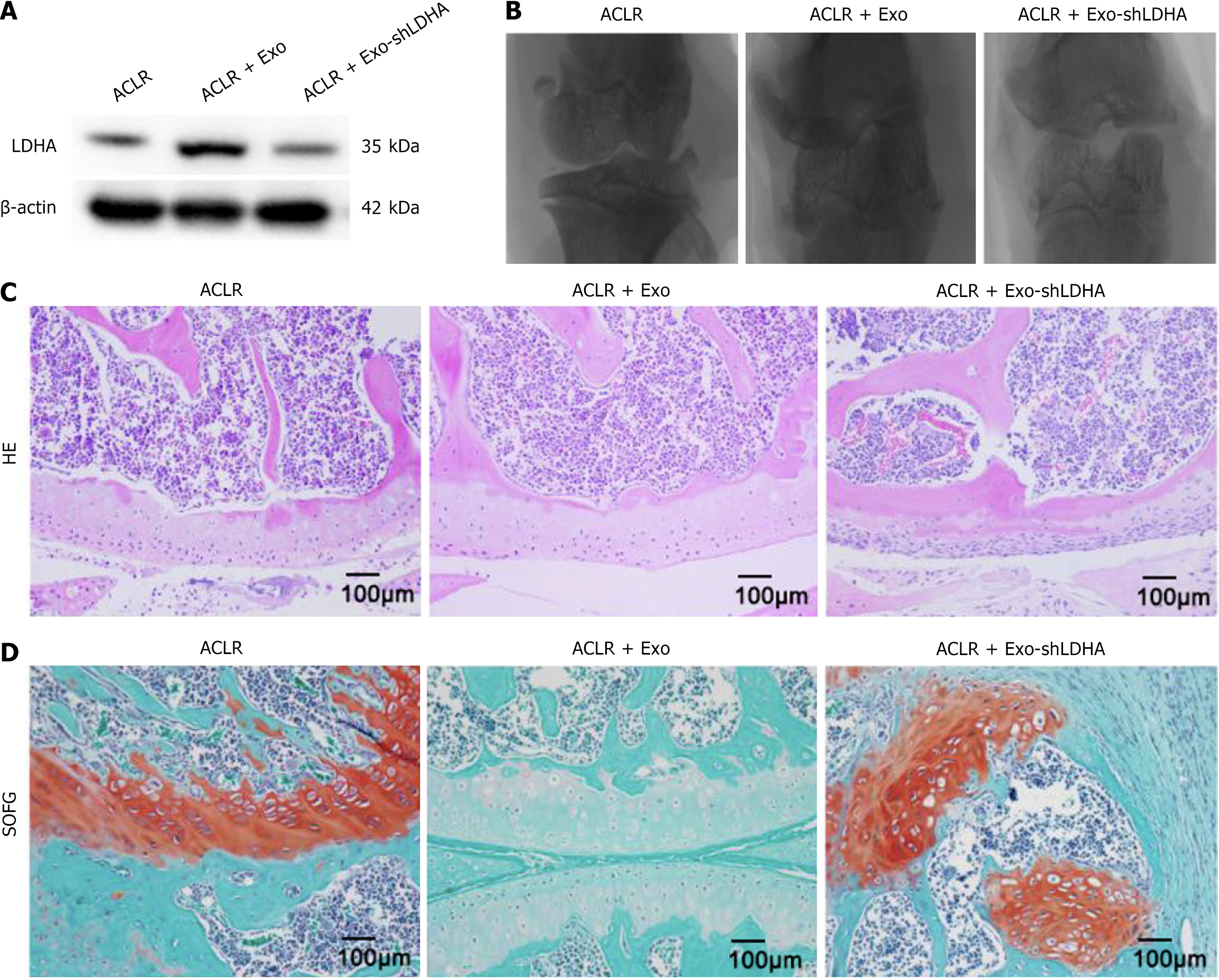
Then the in vivo study was performed, the ACLR rat model was randomized into 3 groups and treated with PBS and BMSC-Exos injections: ACLR, ACLR + BMSC-Exos, ACLR + BMSC-Exos shLDHA. Next, the LDHA expression levels of the different groups were analyzed by western blotting, and as can be seen in Figure 2A, BMSC-Exos successfully infiltrated into the bone tissues of ACLR rats and the LDHA protein was highly expressed. Subsequently, micro-CT images showed (Figure 2B) that the BMSC-Exos group produced more neoplastic bone tissue at the fracture site, and macroscopically there was no difference in the amount of regenerated bone between the ACLR group and the LDHA knockdown group. Quantitative analysis revealed that BMSC-Exos significantly increased bone mineral density by 2.6-fold vs PBS control (0.31 ± 0.04 g/cm3vs 0.15 ± 0.03 g/cm3, cP < 0.001), with a 1.9-fold elevation in bone volume fraction (35.6% ± 4.5% vs 18.7% ± 3.2%, cP < 0.001). Histological evaluation of ACLR tissues in each group was performed at 8 weeks postoperatively, and H&E staining showed that obvious tendon-bone interface and disorganized fibrovascular tissues were visible in the BMSC-Exos group (Figure 2C). SOFG staining showed that the relative width of the interface between the host bone and the graft was also significantly reduced and fibrocartilage regeneration was more pronounced compared with the control group, suggesting better tendon-bone healing (Figure 2D). These results suggest that LDHA from BMSC-Exos plays an important role in promoting new bone formation at the site of ACLR tendon bone healing (Table 1).
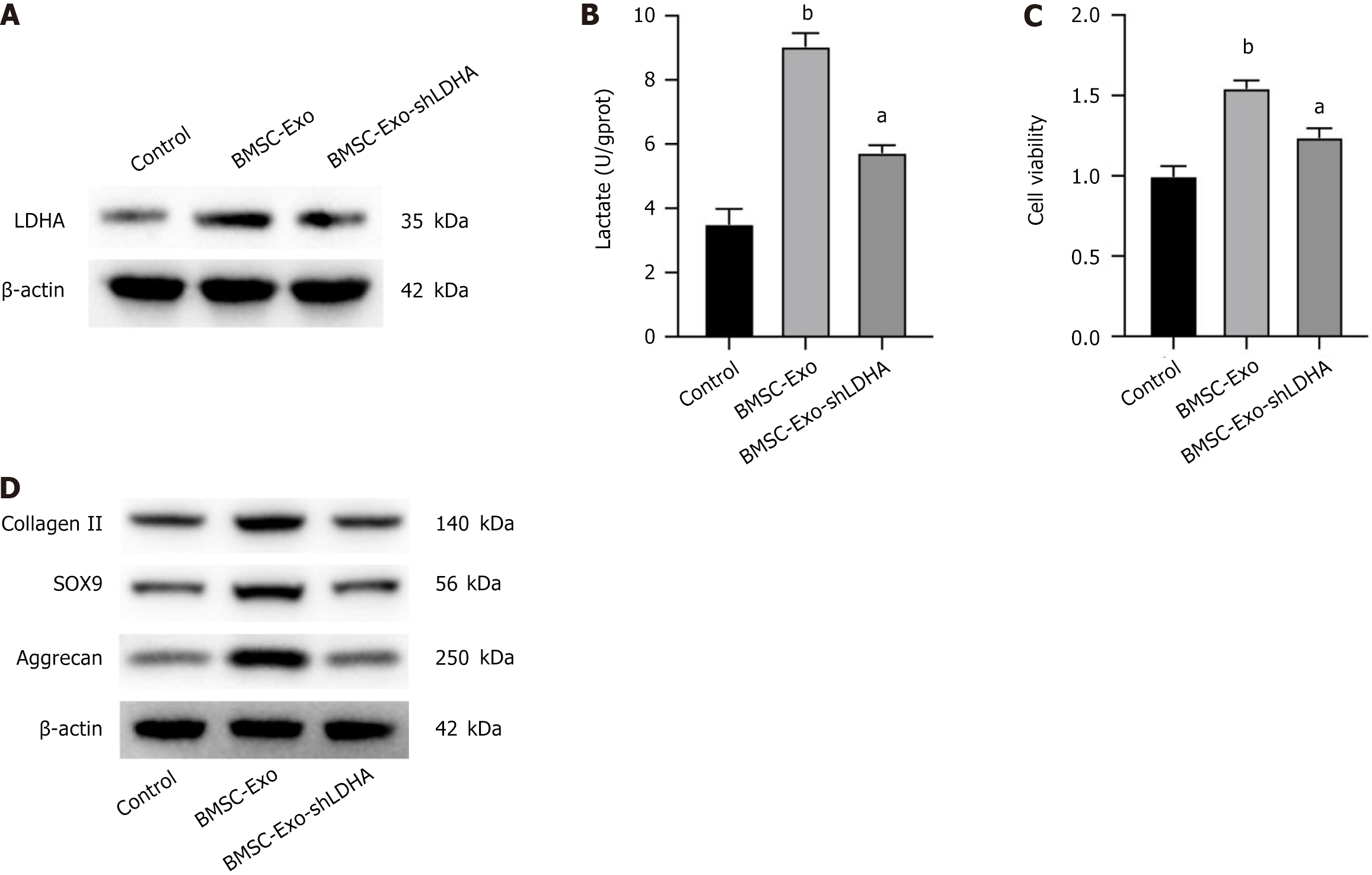
CSPCs isolated from rat knee cartilage fragments were co-incubated with BMSC-Exos with and without knockdown of LDHA, respectively, and then protein expression levels of LDHA in CSPCs were analyzed by western blotting. CSPCs treated with BMSC-Exos showed elevated level of LDHA expression (Figure 3A). LDHA is required to maintain glycolysis and ATP production by regenerating NAD+ to form NADH, and lactate is the final byproduct of this reaction. We then determined the lactate content in cell supernatants, and BMSC-Exos treatment significantly increased the amount of lactate secreted by the cells, whereas knockdown of LDHA reversed this effect, suggesting that BMSC-Exos LDHA works in CSPCs (Figure 3B). Cell activity analysis showed that BMSC-Exos treatment significantly enhanced the proliferation of CSPCs (Figure 3C), and the expression of cartilage-related genes including collagen II, SOX9 and aggrecan proteins were all significantly increased (Figure 3D), which was abolished by knocking down LDHA. The above data revealed that LDHA in BMSC-Exos played an important role in promoting the proliferation and differentiation of CSPCs (Table 2).
| Assay | Sample size (n) | Independent repeats | Power analysis (α = 0.05, β = 0.2) |
| In vivo ACLR model | 12/group | 3 | Effect size = 1.8, power = 0.85 |
| CCK-8 | 6 wells/group | 5 | Effect size = 1.5, power = 0.91 |
| qPCR | 3 replicates | 5 | Effect size = 1.2, power = 0.88 |
| WB | 3 replicates | 5 | Effect size = 1.3, power = 0.89 |
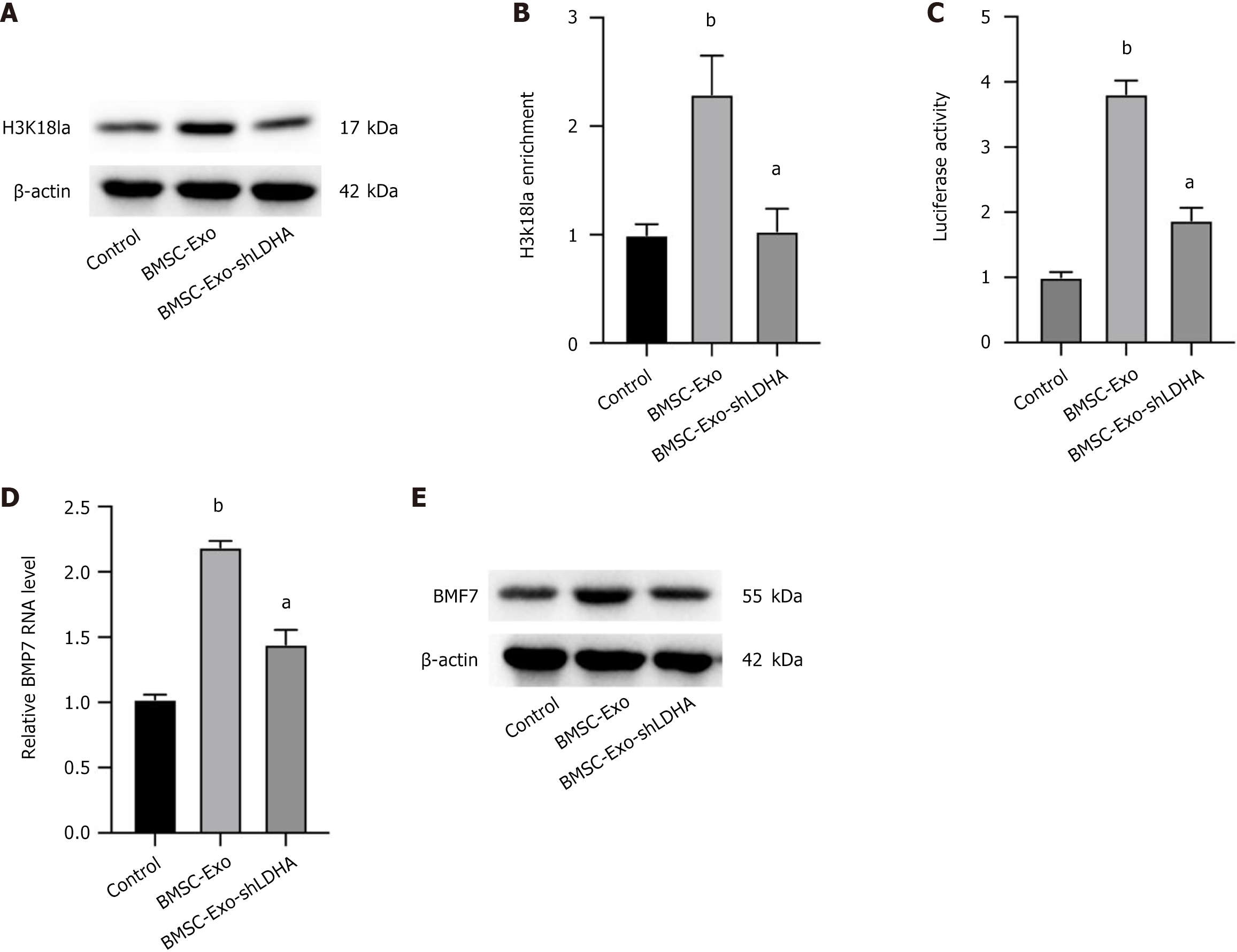
To explore the potential mechanism by which LDHA regulates tendon-bone healing, we analyzed the protein expression levels of the H3K18 Lactylation modification in CSPCs. As shown in Figure 4A, LDHA up-regulated the level of the H3K18 Lactylation modification in CSPCs, and the knockdown of LDHA was able to reverse this effect. As shown in Figure 4A, LDHA up-regulated the level of the H3K18 Lactylation modification in CSPCs, and the knockdown of LDHA was able to reverse this effect. To further validate whether lactate directly influences H3K18 Lactylation, we treated CSPCs with different concentrations of lactate (5 mmol/L, 10 mmol/L, 20 mmol/L). A dose-dependent increase in H3K18 Lactylation was observed (Supplementary Figure 2A). In addition, inhibition of LDHA using FX11 significantly reduced lactate production and subsequently decreased H3K18 Lactylation levels (Supplementary Figure 2B). Furthermore, supplementation with exogenous lactate partially rescued H3K18 Lactylation in LDHA-knockdown BMSC-Exos (Supplementary Figure 2C). The binding of H3K18 La to the BMP7 promoter was further analyzed by chromatin immunoprecipitation-quantitative PCR, and the results showed that knockdown of LDHA significantly suppressed the enrichment of H3K18 La at the BMP7 promoter (Figure 4B). Analysis of BMP7 promoter activity showed that LDHA played an important role in up-regulating BMP7, and conversely, knockdown of LDHA showed no difference from the control (Figure 4C). Consistently, quantitative PCR and western blotting analyses also showed that BMP7 expression levels were significantly enhanced in the presence of BMSC-Exos, and knockdown of LDHA reversed this trend (Figure 4D and E). Findings suggested that BMSC-Exos LDHA can epigenetically upregulate BMP7 expression in vitro. Additionally, to determine whether LDHA is the sole contributor to H3K18 Lactylation, we further examined the effects of LDHB knockdown and dichloroacetate, an inhibitor of pyruvate metabolism. LDHA, but not LDHB, significantly reduced H3K18 Lactylation levels (Supplementary Figure 3A and B). Additionally, treatment with dichloroacetate did not significantly alter BMP7 expression in CSPCs treated with BMSC-Exos (Supplementary Figure 3C), further supporting the specific role of LDHA in this modification.
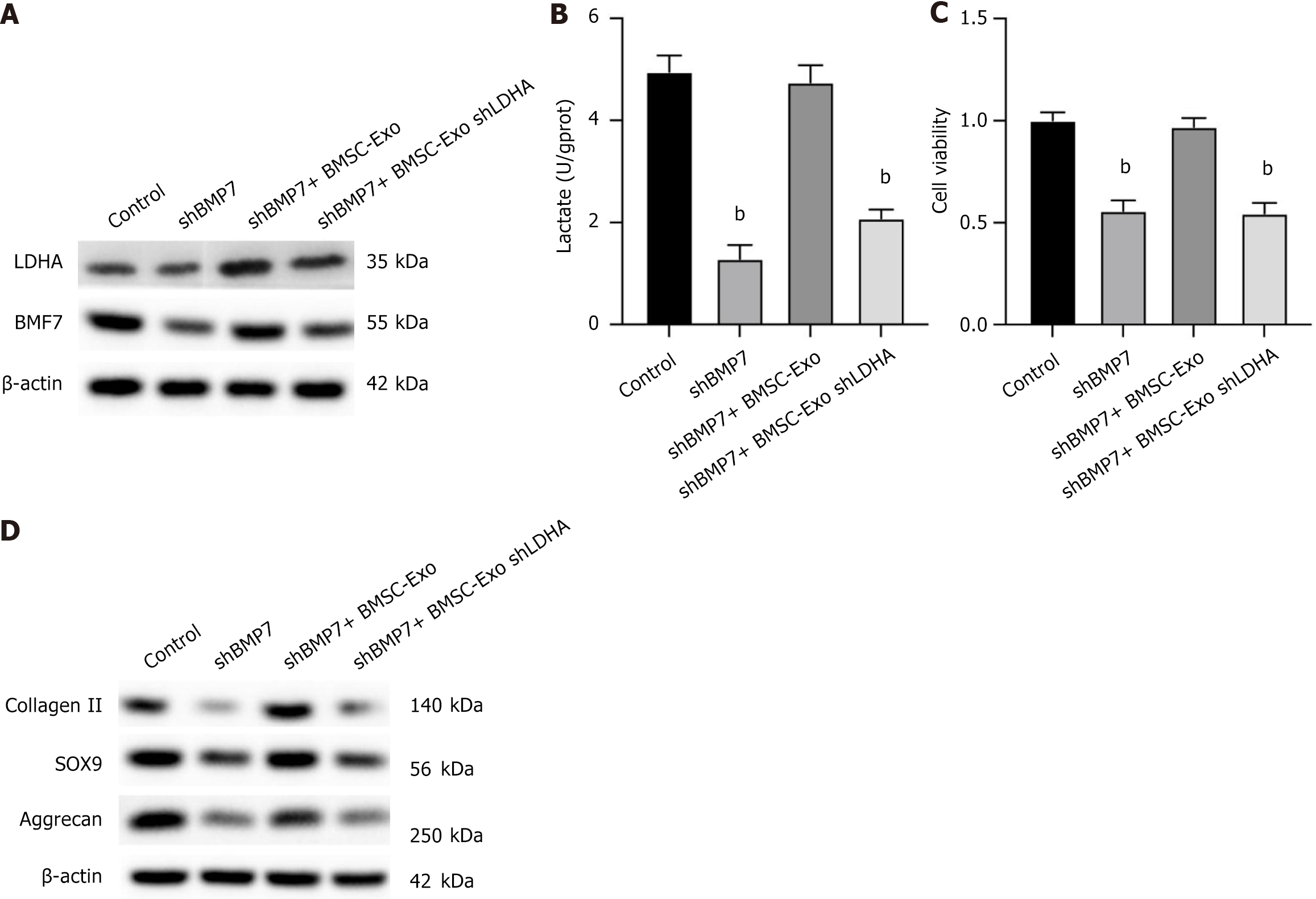
CSPCs were transfected with si-BMP7 to knock down their expression for further investigate the role of LDHA on cartilage formation. CSPCs with and without transfection of BMP7-short hairpin RNA were co-cultured with BMSC-Exos with and without knockdown of LDHA in chondrogenic medium, respectively. As shown in Figure 5A, knockdown of BMP7 downregulated the protein level of BMP7 but not the LDHA, and treatment with BMSC-Exos recovered the expression of BMP7, along with elevated level of LDHA, yet knockdown of LDHA abolished the effects of exosomes (Figure 5A). Next, analysis of the extracellular lactate content showed that knockdown of BMP7 reduced the amount of lactate secreted by the cells, treatment with BMSCs-Exo recovered the production of lactate, which was reversed by LDHA silencing (Figure 5B). Similarly, analysis of cell viability showed a consistent trend (Figure 5C). In addition, western blotting results showed that protein expression levels of collagen II, SOX9, and aggrecan were significantly downregulated upon LDHA knockdown in the BMSC-Exos-treated BMP7 silencing cells (Figure 5D). The above findings suggest that LDHA in BMSC-Exos plays a pivotal part in inducing cartilage formation by upregulating BMP7. The above findings suggest that LDHA in BMSC-Exos plays a pivotal part in inducing cartilage formation by upregulating BMP7. To further confirm the role of BMP7 in LDHA-mediated CSPCs proliferation, we knocked down BMP7 in CSPCs and analyzed key cartilage markers. BMP7 silencing significantly decreased CSPCs proliferation and reduced the expression of cartilage-related proteins, including collagen II, SOX9, and aggrecan, even in the presence of BMSC-Exos (Supplementary Figure 4). These findings confirm that BMP7 acts as a key downstream effector in LDHA-induced cartilage differentiation.
ACL is one of the critical stabilizing structures of the knee joint, and ACL rupture is a common locomotor system injury. With the continuous improvement of clinical research, ACLR is the preferred treatment modality for ACL rupture[21], which can anatomically restore the ACL. However, the biomechanical properties of the scar tissue formed during the tendon-bone healing process are very limited, making the healed tendon susceptible to re-injury and leading to re-rupture or tearing of the ACL in the knee after surgery[22]. Therefore, researchers currently seek biologic therapies to enhance the tendon-bone healing interface to assess whether good surgical outcomes can be achieved after ACLR[23]. Scholars have studied the factors affecting tendon-bone healing in ACL that mainly including growth factor therapy, stem cell therapy, genetic engineering techniques, autologous tissues, biomaterials, and pharmacological interventions, and have achieved considerable progress[24-26]. At present, we do not have a comprehensive and in-depth scientific understanding of these biological therapeutic measures, and their mechanisms of action and metabolites have not been clearly explained and illustrated, which need to be further explored.
Stem cells, with their unlimited self-dividing ability and multiple differentiation potentials, play a role in the tendon-bone healing process. A large number of studies have demonstrated that stem cells have an obvious enhancement and promotion effect on tendon-bone healing. Li et al[27] showed that BMSC-Exos could promote the polarization of M1 macrophages to M2 macrophages through miR-23a-3p, attenuate the early inflammatory response at the tendon-bone interface, and promote early healing after ACLR. Huang et al[15] demonstrated that BMSC-Exos could promote angiogenesis through the vascular endothelial growth factor and Hippo signaling pathways while inhibiting inflammation to promote tendon-bone healing after rotator cuff reconstruction in rats. Cartilage fragments contain a large number of chondrocytes and CSPC, which highly express β-catenin and calreticulin to promote cartilage proliferation and formation, and further promote chondrocyte adhesion and signaling during the process of cartilage formation. Numerous studies have shown that cartilage fragments have been induced for cartilage repair, which is further confirmed by the fact that BMSC-Exos/chondrofragmentation complexes can prevent the enlargement of bone tunnels and promote tendon-bone healing after ACLR via the BMP7/Smad5 signaling axis[5]. It has also been shown that LDHA is a promising target for cancer therapy, and high LDHA levels are strongly associated with tumor size and poor prognosis in patients with gastric cancer, non-small cell lung cancer, and brain tumors[28,29]. In addition, Arra et al[30] demonstrated that LDHA in chondrocytes is a potential therapeutic target for osteoarthritis. This prompted us to further explore the deeper mechanism of LDHA in BMSC-Exos on tendon-bone healing after ACLR.
In the present study, we confirmed the high expression of LDHA in BMSC-Exos. The proliferation of chondrocytes migrating from cartilage debris was clearly observed in CSPCs by western blotting analysis as well as cell counting kit 8 assay, enhancing the expression of collagen-II, SOX9 and aggrecan, accompanied by an increase in lactate content. Furthermore, in the in vivo ACLR model, micro-CT showed that more new bone tissues were formed in the tunnel, and knockdown of LDHA rather reduced these indicators, which was further supported by immunohistochemical staining results. Previous studies reported that the expression of BMP7 is continuously upregulated during cartilage formation at the tendon-bone interface formation, which promotes stem cell differentiation wield osteoblasts and fibrocartilage[31,32]. In the present study, we found that the expression of histone H3K18 Lactylation modification and its binding at the BMP7 promoter, as well as the activity and expression of the BMP7 promoter were increased, which prompted us to silence BMP7 expression and found that the presence of LDHA was necessary for BMP7 expression as well as chondrocyte proliferation. All the results indicated that LDHA in BMSC-Exos may enhance BMP7 expression through histone H3K18 Lactonization, further promoting the differentiation of cartilage fragments into cartilage.
Our findings hold significant translational potential for ACLR. First, BMSC-Exos could be developed as an intra-articular injectable therapeutic agent to augment tendon-bone healing. The exosomal LDHA-lactylation-BMP7 axis identified here provides a druggable target: Localized delivery of LDHA-enriched exosomes (e.g., via hydrogel carriers or scaffold coatings) may accelerate fibrocartilage formation at the graft interface. Second, small-molecule LDHA activators (e.g., N-acetylcysteine) could be repurposed to amplify endogenous lactate production, synergizing with exosome therapy. To advance toward clinical application, the following steps are critical: (1) Preclinical validation in large animals: Testing BMSC-Exos in porcine or ovine ACLR models to assess scalability and dosing regimens; (2) Safety profiling: Long-term evaluation of systemic lactate levels and off-target epigenetic effects; and (3) Clinical trial design: Phase I trials could focus on autologous BMSC-Exos injections combined with standard ACLR surgery, monitoring outcomes via magnetic resonance imaging-based fibrocartilage volume and second-look arthroscopy.
However, there are still many limitations here, such as the fact that the effect of LDHA in BMSC-Exos on cartilage debris was only validated in CSPCs and SD rats. While our study included a PBS-injected control group to account for potential effects of intra-articular injection procedures, the absence of an untreated ACLR group (no injection) limits our ability to fully distinguish between the mechanical impact of PBS injection and the natural healing process. Future studies should incorporate both untreated and vehicle control groups to dissect injection-related confounding factors. In the future, a large number of clinical trials are still needed to confirm the mechanism study of biological intervention in tendon-bone healing, so as to provide help and guidance for clinical treatment, accelerate the rapid recovery of patients and improve their quality of life.
In conclusion, our results suggest that BMSC-Exos can significantly promote tendon-bone healing, which is mainly achieved by the high expression of LDHA in BMSC-Exos up-regulating the expression of BMP7 through histone H3K18 Lactylation modification. These findings pave the way for developing LDHA-targeted exosome therapies to improve ACLR outcomes, with a clear roadmap for preclinical optimization and clinical translation.
| 1. | Diermeier TA, Rothrauff BB, Engebretsen L, Lynch A, Svantesson E, Hamrin Senorski EA, Meredith SJ, Rauer T, Ayeni OR, Paterno M, Xerogeanes JW, Fu FH, Karlsson J, Musahl V; Panther Symposium ACL Treatment Consensus Group. Treatment after ACL injury: Panther Symposium ACL Treatment Consensus Group. Br J Sports Med. 2021;55:14-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 82] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 2. | van Meer BL, Waarsing JH, van Eijsden WA, Meuffels DE, van Arkel ER, Verhaar JA, Bierma-Zeinstra SM, Reijman M. Bone mineral density changes in the knee following anterior cruciate ligament rupture. Osteoarthritis Cartilage. 2014;22:154-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 3. | de Andrade ALL, Sardeli AV, Garcia TA, Livani B, Belangero WD. PRP does not improve the objective outcomes of anterior cruciate ligament reconstruction: a systematic review and meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2021;29:3049-3058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Hao ZC, Wang SZ, Zhang XJ, Lu J. Stem cell therapy: a promising biological strategy for tendon-bone healing after anterior cruciate ligament reconstruction. Cell Prolif. 2016;49:154-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 5. | Zhang C, Jiang C, Jin J, Lei P, Cai Y, Wang Y. Cartilage fragments combined with BMSCs-Derived exosomes can promote tendon-bone healing after ACL reconstruction. Mater Today Bio. 2023;23:100819. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 6. | Barbero A, Ploegert S, Heberer M, Martin I. Plasticity of clonal populations of dedifferentiated adult human articular chondrocytes. Arthritis Rheum. 2003;48:1315-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 286] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 7. | Seol D, McCabe DJ, Choe H, Zheng H, Yu Y, Jang K, Walter MW, Lehman AD, Ding L, Buckwalter JA, Martin JA. Chondrogenic progenitor cells respond to cartilage injury. Arthritis Rheum. 2012;64:3626-3637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 177] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 8. | Marmotti A, Bonasia DE, Bruzzone M, Rossi R, Castoldi F, Collo G, Realmuto C, Tarella C, Peretti GM. Human cartilage fragments in a composite scaffold for single-stage cartilage repair: an in vitro study of the chondrocyte migration and the influence of TGF-β1 and G-CSF. Knee Surg Sports Traumatol Arthrosc. 2013;21:1819-1833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 9. | Jiang Y, Cai Y, Zhang W, Yin Z, Hu C, Tong T, Lu P, Zhang S, Neculai D, Tuan RS, Ouyang HW. Human Cartilage-Derived Progenitor Cells From Committed Chondrocytes for Efficient Cartilage Repair and Regeneration. Stem Cells Transl Med. 2016;5:733-744. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 140] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 10. | Zhang C, Pan J, Chen JD, Zhang YJ, Gu PC, Lin XJ, Cai YZ. The Effect of Cartilage Fragments on Femoral Tunnel Widening After Anterior Cruciate Ligament Reconstruction: A Prospective Randomized Controlled Study. Arthroscopy. 2018;34:2218-2227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Čamernik K, Zupan J. Complete Assessment of Multilineage Differentiation Potential of Human Skeletal Muscle-Derived Mesenchymal Stem/Stromal Cells. Methods Mol Biol. 2019;2045:131-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Han L, Liu H, Fu H, Hu Y, Fang W, Liu J. Exosome-delivered BMP-2 and polyaspartic acid promotes tendon bone healing in rotator cuff tear via Smad/RUNX2 signaling pathway. Bioengineered. 2022;13:1459-1475. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 13. | Ouyang HW, Goh JC, Lee EH. Use of bone marrow stromal cells for tendon graft-to-bone healing: histological and immunohistochemical studies in a rabbit model. Am J Sports Med. 2004;32:321-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 131] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 14. | Harrell CR, Jovicic N, Djonov V, Volarevic V. Therapeutic Use of Mesenchymal Stem Cell-Derived Exosomes: From Basic Science to Clinics. Pharmaceutics. 2020;12:474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 92] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 15. | Huang Y, He B, Wang L, Yuan B, Shu H, Zhang F, Sun L. Bone marrow mesenchymal stem cell-derived exosomes promote rotator cuff tendon-bone healing by promoting angiogenesis and regulating M1 macrophages in rats. Stem Cell Res Ther. 2020;11:496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 152] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 16. | Yao X, Li C. Lactate dehydrogenase A mediated histone lactylation induced the pyroptosis through targeting HMGB1. Metab Brain Dis. 2023;38:1543-1553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 51] [Reference Citation Analysis (0)] |
| 17. | Gan X, Hu J, Pang Q, Yan R, Bao Y, Liu Y, Song J, Wang Z, Sun W, Huang F, Cai C, Wang L. LDHA-mediated M2-type macrophage polarization via tumor-derived exosomal EPHA2 promotes renal cell carcinoma progression. Mol Carcinog. 2024;63:1486-1499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 18. | Wang N, Wang W, Wang X, Mang G, Chen J, Yan X, Tong Z, Yang Q, Wang M, Chen L, Sun P, Yang Y, Cui J, Yang M, Zhang Y, Wang D, Wu J, Zhang M, Yu B. Histone Lactylation Boosts Reparative Gene Activation Post-Myocardial Infarction. Circ Res. 2022;131:893-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 339] [Article Influence: 84.8] [Reference Citation Analysis (0)] |
| 19. | Khan F, Lin Y, Ali H, Pang L, Dunterman M, Hsu WH, Frenis K, Grant Rowe R, Wainwright DA, McCortney K, Billingham LK, Miska J, Horbinski C, Lesniak MS, Chen P. Lactate dehydrogenase A regulates tumor-macrophage symbiosis to promote glioblastoma progression. Nat Commun. 2024;15:1987. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 77] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 20. | Dai M, Wang L, Yang J, Chen J, Dou X, Chen R, Ge Y, Lin Y. LDHA as a regulator of T cell fate and its mechanisms in disease. Biomed Pharmacother. 2023;158:114164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 21. | Marieswaran M, Jain I, Garg B, Sharma V, Kalyanasundaram D. A Review on Biomechanics of Anterior Cruciate Ligament and Materials for Reconstruction. Appl Bionics Biomech. 2018;2018:4657824. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 93] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 22. | Matsumoto T, Takayama K, Hayashi S, Niikura T, Matsushita T, Kuroda R. Therapeutic potential of vascular stem cells for anterior cruciate ligament reconstruction. Ann Transl Med. 2019;7:S286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 23. | Bi F, Shi Z, Jiang S, Guo P, Yan S. Intermittently administered parathyroid hormone [1-34] promotes tendon-bone healing in a rat model. Int J Mol Sci. 2014;15:17366-17379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Liu Y, Wang L, Li S, Zhang T, Chen C, Hu J, Sun D, Lu H. Mechanical stimulation improves rotator cuff tendon-bone healing via activating IL-4/JAK/STAT signaling pathway mediated macrophage M2 polarization. J Orthop Translat. 2022;37:78-88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 58] [Reference Citation Analysis (0)] |
| 25. | Geng R, Lin Y, Ji M, Chang Q, Li Z, Xu L, Zhang W, Lu J. MFG-E8 promotes tendon-bone healing by regualting macrophage efferocytosis and M2 polarization after anterior cruciate ligament reconstruction. J Orthop Translat. 2022;34:11-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 26. | Chen W, Sun Y, Gu X, Cai J, Liu X, Zhang X, Chen J, Hao Y, Chen S. Conditioned medium of human bone marrow-derived stem cells promotes tendon-bone healing of the rotator cuff in a rat model. Biomaterials. 2021;271:120714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 90] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 27. | Li Z, Li Q, Tong K, Zhu J, Wang H, Chen B, Chen L. BMSC-derived exosomes promote tendon-bone healing after anterior cruciate ligament reconstruction by regulating M1/M2 macrophage polarization in rats. Stem Cell Res Ther. 2022;13:295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 92] [Reference Citation Analysis (38)] |
| 28. | Valvona CJ, Fillmore HL, Nunn PB, Pilkington GJ. The Regulation and Function of Lactate Dehydrogenase A: Therapeutic Potential in Brain Tumor. Brain Pathol. 2016;26:3-17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 255] [Cited by in RCA: 412] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 29. | Wu H, Wang Y, Ying M, Jin C, Li J, Hu X. Lactate dehydrogenases amplify reactive oxygen species in cancer cells in response to oxidative stimuli. Signal Transduct Target Ther. 2021;6:242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 30. | Arra M, Swarnkar G, Ke K, Otero JE, Ying J, Duan X, Maruyama T, Rai MF, O'Keefe RJ, Mbalaviele G, Shen J, Abu-Amer Y. LDHA-mediated ROS generation in chondrocytes is a potential therapeutic target for osteoarthritis. Nat Commun. 2020;11:3427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 293] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 31. | Klatte-Schulz F, Pauly S, Scheibel M, Greiner S, Gerhardt C, Hartwig J, Schmidmaier G, Wildemann B. Characteristics and stimulation potential with BMP-2 and BMP-7 of tenocyte-like cells isolated from the rotator cuff of female donors. PLoS One. 2013;8:e67209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 32. | Chubinskaya S, Hurtig M, Rueger DC. OP-1/BMP-7 in cartilage repair. Int Orthop. 2007;31:773-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 149] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/