Published online Sep 26, 2025. doi: 10.4252/wjsc.v17.i9.105896
Revised: April 7, 2025
Accepted: August 4, 2025
Published online: September 26, 2025
Processing time: 226 Days and 19.6 Hours
Inflammatory bowel disease (IBD) is a chronic, progressive inflammatory condition of the intestine. Mesenchymal stem cell (MSC) therapy for IBD has made significant progress in recent years. To better exploit the therapeutic poten
To evaluate the therapeutic efficacy of QUR-pretreated hUCMSCs.
We induced colitis in a mouse model using a 2,4,6-trinitrobenzenesulfonic acid solution. Intraperitoneal injection of QUR-pretreated hUCMSCs significantly improved clinical and pathological manifestations of colitis compared to the model group. Interestingly, the therapeutic effect was superior to that of untre
Our study demonstrated that QUR pretreatment of hUCMSCs significantly enhanced their immune-regulatory capacity. This approach effectively mitigated colonic inflammation in a mouse colitis model by modulating the IL-10/Janus kinase/STAT signaling pathway.
These findings suggest that QUR pretreatment acts synergistically to augment the inherent anti-inflammatory and immune-regulatory properties of hUCMSCs, resulting in enhanced therapeutic efficacy for IBD treatment.
Core Tip: Quercetin (QUR) pretreatment enhanced the viability of human umbilical cord-derived mesenchymal stem cells (hUCMSCs), which may improve their immune regulatory ability. Intraperitoneal injection of QUR-hUCMSCs effectively alleviated inflammation in 2,4,6-trinitrobenzenesulfonic acid mice, and the therapeutic effect was superior to untreated hUCMSCs. QUR-hUCMSC treatment significantly enhanced interleukin 10 (IL-10) expression in 2,4,6-trinitrobenzenesulfonic acid mice. QUR-hUCMSCs inhibited inflammatory bowel disease inflammation by regulating the IL-10/Janus kinase/signal transducer and activator of transcription signaling pathway.
- Citation: Shi MY, Liu L, Yang FY. Quercetin pretreated umbilical cord mesenchymal stem cells alleviate inflammatory bowel disease via IL-10 /Janus kinase 2/STAT3 signaling. World J Stem Cells 2025; 17(9): 105896
- URL: https://www.wjgnet.com/1948-0210/full/v17/i9/105896.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v17.i9.105896
Inflammatory bowel disease (IBD), a chronic and relapsing intestinal disorder encompassing Crohn’s disease (CD) and ulcerative colitis (UC), is characterized by complex pathophysiology with potential contributions from genetic, environmental, microbial, and immunological factors[1]. Persistent inflammation in patients with IBD drives progressive intestinal injury and complications. Moreover, a higher accumulation burden of inflammation has been linked to increased risk of colorectal neoplasia, with colorectal cancer emerging as the leading cause of mortality in this patient population[2]. The epidemiology of IBD has undergone significant changes, with a sharp increasing incidence in developing countries, and more recently, in newly industrialized nations[3]. The epidemiology data suggest an increasing disparity in IBD prevalence, as evidenced by the rise in the unequal slope index from 17.1 [95% confidence interval (CI): 12.4-21.7] in 1990 to 25.2 (95%CI: 20.1-30.2) in 2019[4]. An epidemiological survey in China revealed a rise in IBD prevalence of 2.53% between 1990 and 2019, while the mortality rate encouragingly decreased by 3.62%. Age is a significant factor influencing both the incidence and mortality of IBD. The disease primarily affects young adults, with mortality risk increasing with age. These findings highlight the ongoing global challenge presented by the burden of IBD[5,6].
Currently, several treatment options are available for IBD, including aminosalicylic acids, corticosteroids, and immu
Mesenchymal stem cells (MSCs) are present in nearly all tissues and possess the remarkable ability to differentiate into various cell types, including osteoblasts, adipocytes, and chondrocytes. This unique property contributes to tissue regeneration and homeostasis[9]. MSCs are increasingly being explored as a therapeutic strategy for autoimmune diseases such as IBD, and rheumatoid arthritis (RA), demonstrating promise in treating chronic inflammatory conditions. Their immunomodulatory effects involve regulating the phenotype and function of both innate (macrophages, dendritic cells, neutrophils, eosinophils, basophils) and adaptive (lymphocytes) immune cells. MSCs achieve this modulation through paracrine secretion (release of soluble factors) or direct cell contact[10]. Thus, MSC therapy holds immense promise in regenerative medicine. However, a significant challenge lies in the potential loss of its biological function after prolonged in vitro isolation and expansion. Furthermore, animal studies highlight the relatively low recruitment and persistence of MSCs in vivo[11]. To address these limitations and enhance MSC function both in vitro and in vivo, several key strategies have emerged, with pretreatment before the application being a particularly prominent approach[12].
Quercetin (QUR) is a natural flavonoid found in various vegetables, fruits, and herbs. It exhibits a broad spectrum of pharmacological effects, including anti-inflammatory, antioxidant, anti-allergic, antitumor, and immune regulation. Its potent anti-inflammatory properties are primarily exerted through the inhibition of cytokines production[13]. For instance, QUR attenuates TNF-α-induced intercellular adhesion molecule 1 and matrix metalloprotease-9 (MMP9) expression in human retinal pigment epithelial cells (ARPE-19 cells) via the mitogen-activated protein kinase kinase 1/2-extracellular regulated kinase 1/2 and protein kinase C delta-c-Jun N-terminal kinase 1/2-c-Jun and nuclear factor kappa B (NF-κB) pathways[14]. Experiments have shown that this compound significantly inhibits the growth of the fungus Aspergillus fumigatus in a concentration-dependent manner. Furthermore, QUR downregulates the expression of inflammatory mediators [Toll-like receptor 4 (TLR4), TLR2, interleukin 1 beta (IL-1β), TNF-α, and high mobility group box 1] in both in vitro and in vivo model[15]. QUR has also demonstrated anti-inflammatory properties in the RA model, reducing inflammation and downregulating key inflammatory mediators such as IL-1β, IL-6, IL-17, TNF-α, and MMP through immune cell modulation[16]. Consequently, this compound exhibits a broad spectrum of biological activities by targeting various pathways. However, its low solubility and poor bioavailability limit its therapeutic potential, thereby researchers are actively exploring methods to improve QUR utilization[17].
IL-10 is a critical anti-inflammatory cytokine secreted by a variety of immune cells from both the adaptive and innate branches. Aberrant IL-10/IL-10 receptor signaling is strongly linked to IBD development[18], IL-10 exerts its anti-inflammatory effects through two main mechanisms: Suppressing the production of pro-inflammatory mediators, and promoting the production of anti-inflammatory factors, including TNF-α receptors and IL-1 receptor antagonist. Additionally, IL-10 downregulates the expression of major histocompatibility complex class II molecules [both constitutive and those induced by interferon gamma (IFN-g)], as well as co-stimulatory molecule cluster of differentiation 86 (CD86) and adhesion molecule CD58[19]. Despite normal lymphocyte development and antibody response, IL-10-deficient mice exhibit a range of pathological effects, including growth retardation, anemia, and chronic enterocolitis. This phenotype is further exacerbated in IL-10 knockout mice, which display lymphocyte and myeloid structural changes, elevated serum amyloid A levels, altered responses to inflammatory and autoimmune stimuli, an increased risk of colorectal cancer, and spontaneous development of chronic enterocolitis[20]. IL-10 knockout mice spontaneously develop colitis under specific pathogen-free conditions. Infections or drugs can further exacerbate this condition, making these mice a highly relevant and widely used model for CD[21]. Studies have shown that increasing IL-10 Levels can be beneficial in various animal models of colitis. For instance, inhibiting cyclin-dependent kinase 8 while simultaneously upregulating IL-10 abundance in activated myeloid dendritic cells, has been demonstrated as an effective therapeutic strategy for IBD[22].
The Janus kinase (JAK)/signal transducer and activator of transcription (STAT) pathway plays a critical role in maintaining inflammatory homeostasis. Hyperactivation of STAT3 Leads to uncontrolled inflammatory responses. Upon phosphorylation, STAT3 translocates to the nucleus and bind to target genes, including IL-10[23]. Our study investigated the role of IL-10 as a target gene in QUR-pretreated human umbilical cord-derived MSCs (hUCMSCs) therapy for colitis in mice using RNA-sequencing (RNA-seq) analysis. We observed a significant upregulation of IL-10 expression in treated colonic tissue, suggesting its potential role in inhibiting the JAK2/STAT3 signaling pathway. The JAK/STAT signaling pathway plays a protective role in the intestine by regulating intestinal mucosal barrier function and inflammatory responses induced by dextran sodium sulfate and 2,4,6-trinitrobenzenesulfonic acid (TNBS)[24,25]. We assume that pretreatment of hUCMSCs with QUR can enhance their immunomodulatory effects. Based on this, we propose that QUR pretreatment may enhance the therapeutic effects of hUCMSCs on IBD by combining anti-inflammatory and immune regulatory mechanisms. We injected QUR-pretreated umbilical cord MSCs into TNBS-induced colitis mice to test the therapeutic effects. At the same time, we investigated whether QUR pretreatment of hUCMSC mediates these effects by upregulating the expression of anti-inflammatory cytokine IL-10 and downregulating the expression of pro-inflammatory cytokine IL-6. In addition, we determined whether hUCMSCs can inhibit the phosphorylation of JAK2 and STAT3, thereby affecting the upregulation of the inhibitory protein, suppressor of cytokine signaling 3 (SOCS3), in the JAK/STAT pathway.
After obtaining approval from the ethics committee of Yangtze University (Hubei, China) and written informed consent from the pregnant woman, umbilical cord tissue was obtained from a full-term newborn. Under sterile conditions at the affiliated hospital, the tissue was rinsed with phosphate-buffered saline (PBS), and the umbilical veins and arteries were removed and then cut into small 1-2 mm2 pieces before being placed in a 10 cm culture dish. The tissue was placed in a culture dish containing Alpha Minimal Essential Medium complete culture medium supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin solution. Then the culture was incubated at 37 °C with 5% CO2 after 4 hours, allowing for tissue adherence, and approximately 10 mL fresh complete culture medium was added to continue cultivation. The culture medium was changed every 3-4 days. After approximately 10 days, hUCMSCs migrated outward from the tissue block. When a sufficient number of cells migrated from the tissue pieces, the tissue pieces were removed. When cell density reached 80%-90%, the cells were passaged. HUCMSCs from the 3rd to 6th passages were used in this work. This study was conducted following the ethical principles outlined in the Declaration of Helsinki (1989).
Phenotypic identification of hUCMSCs (1 × 106 cells/tube) was performed using flow cytometry (FACS Melody; BD Biosciences, CA, United States) with CD29-APC, CD44-FITC, CD90 (Thy1)-PE, CD105-FITC, CD34-FITC, CD45-FITC antibodies (BioLegend, San Diego, CA, United States) to identify cell surface markers. FlowJo software (BD Biosciences) was used to analyze and plot the data.
HUCMSCs possess the ability to differentiate into osteoblasts, adipocytes, and chondrocytes under specific culture conditions. To induce differentiation, hUCMSCs were seeded into 6-well culture plates (2 × 105/well), and allowed to adhere for 1-2 days until the density reached 70%-80%. Then the culture medium was replaced with either osteogenic or adipogenic medium to promote differentiation toward osteoblasts or adipocytes, respectively. The medium was changed every 2-3 days to maintain optimal conditions. After 14 and 21 days of cultivation, alizarin and Oil Red O staining techniques were performed to evaluate osteogenesis and adipogenesis, respectively.
To assess the effect of QUR (high-performance liquid chromatography ≥ 98%, Alpha Beijing Biotechnology Co., Ltd., Beijing, China) on hUCMSC viability, we employed the Cell Counting Kit-8 (CCK-8) (Biosharp, Anhui, China) assay. HUCMSCs were seeded into 96-well plates (4 × 103 cells/well) and treated with QUR for 24 or 48 hours. Following incubation, 10 μL CCK-8 solution was added to each well, and the cells continue incubating for 1-4 hours. Cell viability was determined by measuring the optical density (OD) value at 450 nm using a microplate reader (Multikan FC; Thermo Fisher Scientific, Carlsbad, CA, United States). For gene expression analysis, hUCMSCs were pretreated with 10 μmol/L QUR for 24 hours. Then cells were collected, and RNA levels were quantified using reverse transcription-quantitative polymerase chain reaction (RT-qPCR) (Takara, Shiga, Japan).
Male C57BL/6J mice (6-8 weeks, 18 ± 22 g) were obtained from Sanxia Animal Center (No. SYXK(E)2022-0012; Yichang, Hubei, China). The mice were housed in a specific pathogen-free environment with ad libitum access to food and water. All animal experiments were approved by the ethics committee of Yangtze University (Hubei, China) and conducted following relevant guidelines. The work has been reported in line with ARRIVE guidelines 2.0. The mice were randomly divided into five groups: Control, TNBS, TNBS + hUCMSCs, TNBS + QUR-hUCMSCs, and QUR (100 mg/kg administered by gavage). For presensitization, mice received 1% TNBS solution applied topically to their shaved shoulders and backs. On day 7, mice were anesthetized with 1% pentobarbital. Then a catheter was inserted into the colon through the anus to a depth of approximately 5 cm. A total of 100 μL 2.5% TNBS solution was subsequently injected, and the mice were inverted for 5 minutes[26]. Twelve hours after TNBS administration, mice in the treatment groups received an intraperitoneal injection of hUCMSCs or QUR-hUCMSCs resuspended in PBS. The weight, survival rate, and fecal characteristics of all mice were monitored daily. Disease severity was evaluated using the Disease Activity Index (DAI) (Table 1). On the 3rd and 7th days post-TNBS administration, mice were euthanized, and serum and colon tissue samples were collected for further analyses.
| Score | Weight loss (%) | Stool consistency | Blood |
| 0 | None | Normal | Negative Hemocult |
| 1 | 1%-5% | Soft but still formed | Negative Hemocult |
| 2 | 5%-10% | Soft | Positive Hemocult |
| 3 | 10%-15% | Very soft; wet | Blood traces in stool visible |
| 4 | > 15% | Watery diarrhea | Gross rectal bleeding |
Colon tissues were stained with hematoxylin and eosin (H&E) to evaluate the degree of inflammation and tissue damage. Images were captured using a light microscope (Olympus, Tokyo, Japan) and, subsequently, used to estimate the Histological Activity Index (Table 2).
| Score | Organizational changes |
| 0 | No inflammatory changes |
| 1 | Mild inflammation with scattered infiltrating mononuclear cells (1-2 Lesions) |
| 2 | Moderate inflammation with multiple lesions visible |
| 3 | Significant inflammatory changes, increased vascular density, and thickening of intestinal wall |
| 4 | Severe inflammatory changes, lymphocyte transmural infiltration, and decreased goblet cells |
Mouse serum samples were incubated with each reagent provided in a myeloperoxidase (MPO) assay kit (Nanjing Jiancheng Bioengineering Inc., Jiangsu, China) according to the manufacturer’s instructions. After incubation, 200 μL of the reaction mixture was transferred to a 96-well plate. The MPO activity in the samples leads to the oxidation of the substrate o-dianisidine, producing a yellow-colored compound. Then the absorbance of this product is measured at 460 nm to assess the extent of hydrogen peroxide reduction and the number of white blood cells. Finally, the OD was normalized to each serum sample.
Colon tissue homogenates were used for RNA-seq. Total RNA was isolated from the homogenates using TRIzol reagent (Invitrogen, Carlsbad, CA, United States) following the manufacturer’s protocol. RNA-seq library construction was performed using the Illumina TruSeqTM RNA Sample Prep Kit. The quality and quantity of the isolated RNA were assessed using the Agilent Bioanalyzer system (Agilent Technologies, Santa Clara, CA, United States). RNA integrity was confirmed by a 28S:16S ribosomal RNA ratio exceeding 1.5, while a 260/280 absorbance ratio between 1.8 and 2.2 indicated the purity. Following RNA library preparation, the samples were sequenced on the Illumina NovaSeq 6000 platform (LC Sciences, Houston, TX, United States) using a standard sequencing protocol. The quality of the raw sequencing data was assessed using fastx_toolkit_0.0.14 to generate high-quality, “clean” reads. These clean reads were then aligned to the mouse reference genome using Hisat2 software. Transcript assembling was subsequently performed using StringTie software. Finally, transcript abundance was quantified using RSEM with the Fragments Per Kilobase of transcript per Million mapped reads method to generate read counts. DESeq2 software was employed to analyze the read counts and generate a differential gene expression profile. Genes with a statistically significant fold change (|log2 fold change| ≥ 1) and adjusted P-value (P ≤ 0.05) were considered differentially expressed. Functional enrichment analysis of these differentially expressed genes (DEGs) was performed using the Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) databases to identify significantly enriched pathways. Additionally, protein-protein interaction (PPI) analysis of the DEGs was conducted using the String database to explore potential interaction networks.
Total RNA was isolated from colin tissue homogenates using TRizol reagent (Invitrogen) following the manufacturer’s protocol. Then the RNA concentration was quantified using a spectrophotometer (NanoPhotometer® N60; Implen, Munich, Germany). Subsequently, 1 μg total RNA from each sample was reverse transcribed into cDNA using the PrimeScript™ RT Reagent Kit (Takara). Real-time quantitative PCR (qPCR) was performed using SYBR Green Reagent (Thermo Fisher Scientific) on an ABI 7500 Real-Time PCR Instrument (Applied Biosystems, Carlsbad, CA, United States). Primer sequences are summarized in Table 3. Each reaction was performed in triplicate to ensure technical reproducibility. Relative expression levels of target genes were normalized to the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase.
| Gene | Forward primer | Reverse primer |
| GAPDH | GGCAAATTCAACGGCACAGTCAAG | TCGCTCCTGGAAGATGGTGATGG |
| IL-10 | GGTTGCCAAGCCTTATCGGA | CTTCTCACCCAGGGAATTCA |
| JAK2 | TTGTGGTATTACGCCTGTGTATC | ATGCCTGGTTGACTCGTCTAT |
| STAT3 | CACCTTGGATTGAGAGTCAAGAC | AGGAATCGGCTATATTGCTGGT |
Colon tissues were lysed using RIPA lysis buffer to extract proteins. Then the protein concentration was determined using the BCA protein assay. Then extracted proteins were resuspended in sodium dodecyl sulfate (SDS) loading buffer, denature at 100 °C for 10 minutes, and loaded (30 μg per lane) onto a 10% SDS-polyacrylamide gel electrophoresis and transferred onto polyvinylidene fluoride membranes (Amersham Biosciences, Piscataway, NJ, United States). The membranes were blocked in 5% defatted milk powder to prevent nonspecific binding and then incubated with primary antibodies overnight at 4 °C. The following day, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies for 1 hour at room temperature. After incubation, they were developed with ECL reagent (MeilunBio, Liaoning, China) and imaged using the ChemiDoc MP Imaging System (Bio-Rad, Hercules, CA, United States). β-actin served as the internal loading control (antibody details in Table 4).
| Antibody | Cat. No. | Company | Dilution |
| β-actin | Bs-0061R | Bioss | 1:1000 |
| IL-10 | WL03088 | Wanleibio | 1:1000 |
| IL-6 | 21865-1-AP | Proteintech | 1:1000 |
| JAK2 | ab108596 | Abcam | 1:1000 |
| P-JAK2 | ab32101 | Abcam | 1:1000 |
| STAT3 | 10253-2-AP | Proteintech | 1:2000 |
| p-STAT3 (Ser727) | WLP2412 | Wanleibio | 1:1000 |
| SOCS3 | 66797-1-Ig | Proteintech | 1:1500 |
All data are presented as the mean ± SEM. Statistical analyses were performed using GraphPad Prism 8.0.1 software (La Jolla, CA, United States). One-way analysis of variance was used for statistical comparisons between groups. Statistical significance was set at P < 0.05.
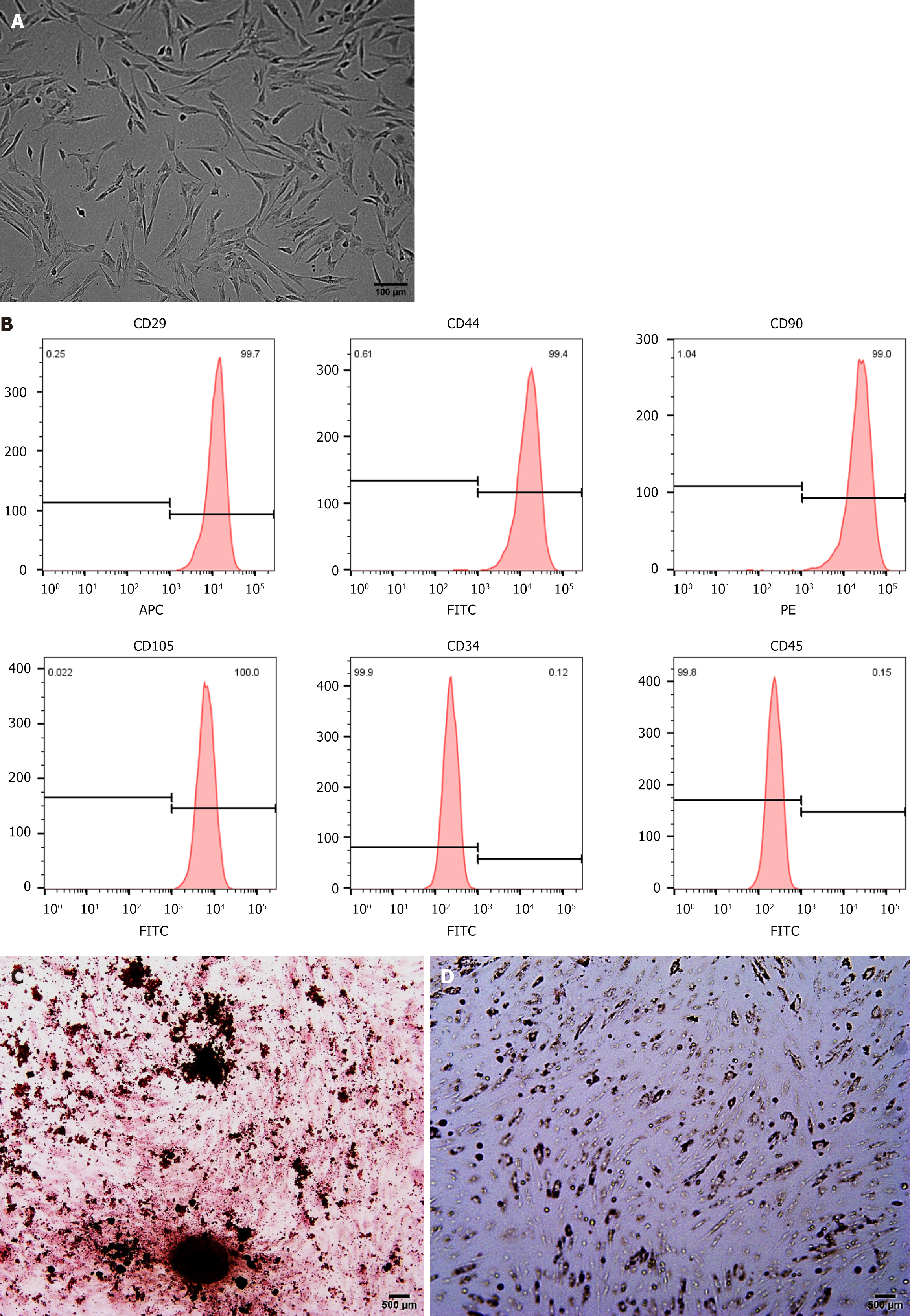
After approximately 10 days in primary cell culture, spindle-shaped, fibroblast-like hUCMSCs emerged around the tissue block. Following primary cell collection, routine passage was performed (Figure 1A). Flow cytometry analysis of hUCMSCs at passage 3 (P3) revealed a characteristic MSC immunophenotype. Specifically, CD29, CD44, CD90, and CD105 were highly expressed (> 98% positive), whereas CD34 and CD45 were negative (< 1% positive) (Figure 1B). HUCMSCs possess the ability to differentiate into osteoblasts and adipocytes under specific culture conditions. This differentiation potential was demonstrated by the formation of calcium nodules and deposition (Figure 1C), after alizarin red staining following 14 days of osteogenic induction. Similarly, Oil Red O staining revealed the presence of lipid droplets with a strong refractive index after 21 days of adipogenic induction (Figure 1D). These findings confirm that the hUCMSCs isolated and cultured in this experiment met the established identification criteria for MSCs.
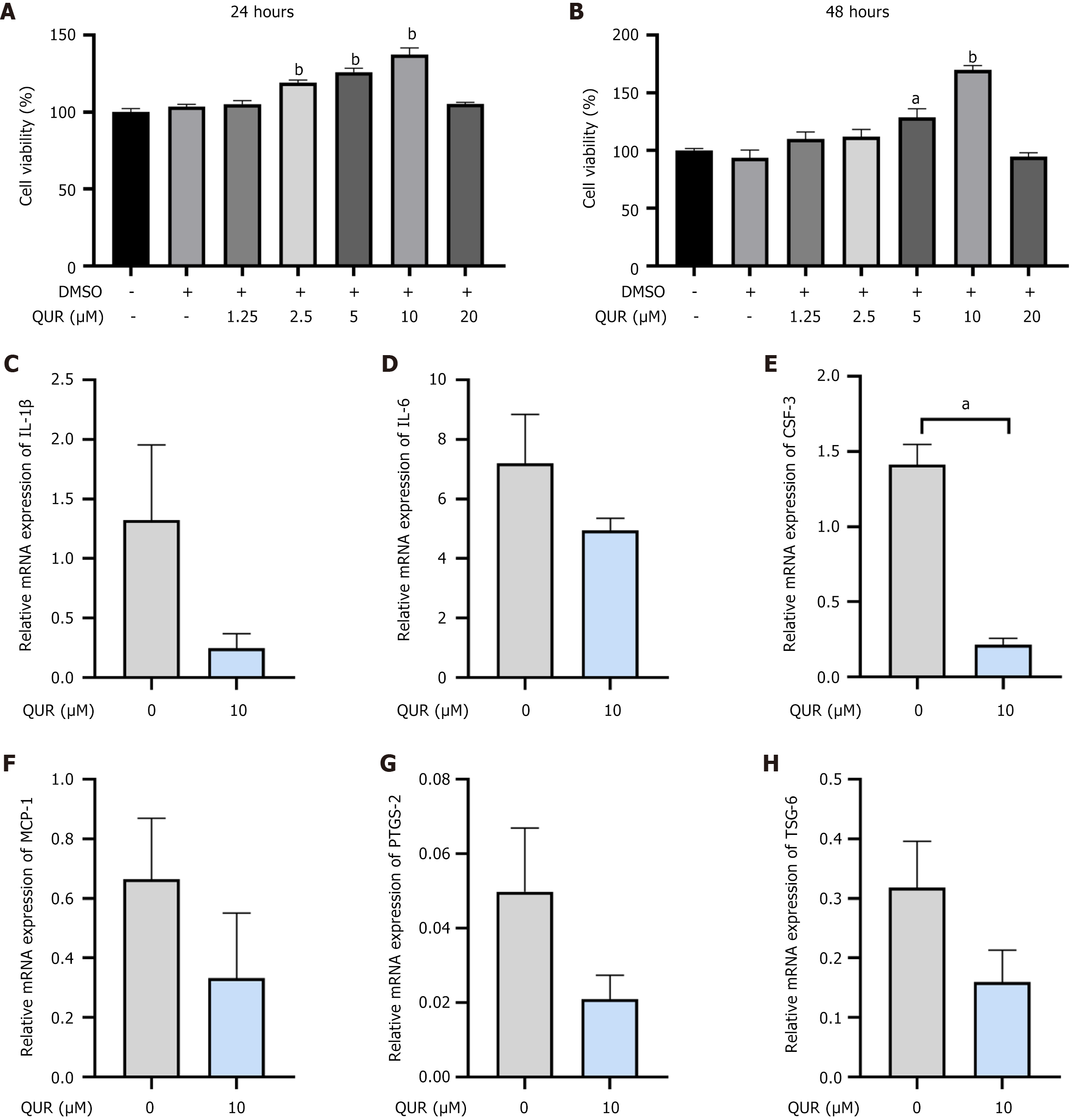
Following pretreatment with different concentrations of QUR for 24 or 48 hours, the CCK-8 assays revealed a dose-dependent increase in hUCMSC viability. The highest viability was observed at a pretreatment concentration of 10 μΜ QUR. However, cytotoxicity was observed at a high concentration of 20 μΜ (Figure 2A and B). Interestingly, QUR pretreatment did not comprise immunosuppressive function. This function was evidenced by the ability of hUCMSCs to downregulate the mRNA expression of pro-inflammatory mediators such as IL-1β, IL-6, colony-stimulating factor 6, monocyte chemoattractant protein-1, prostaglandin-endoperoxide synthase-2, and TNF-α-stimulated gene/protein-6 (Figure 2C-H). Therefore, hUCMSCs pretreated with 10 μM QUR for 24 hours were designated as QUR-hUCMSCs and used for subsequent experiments.
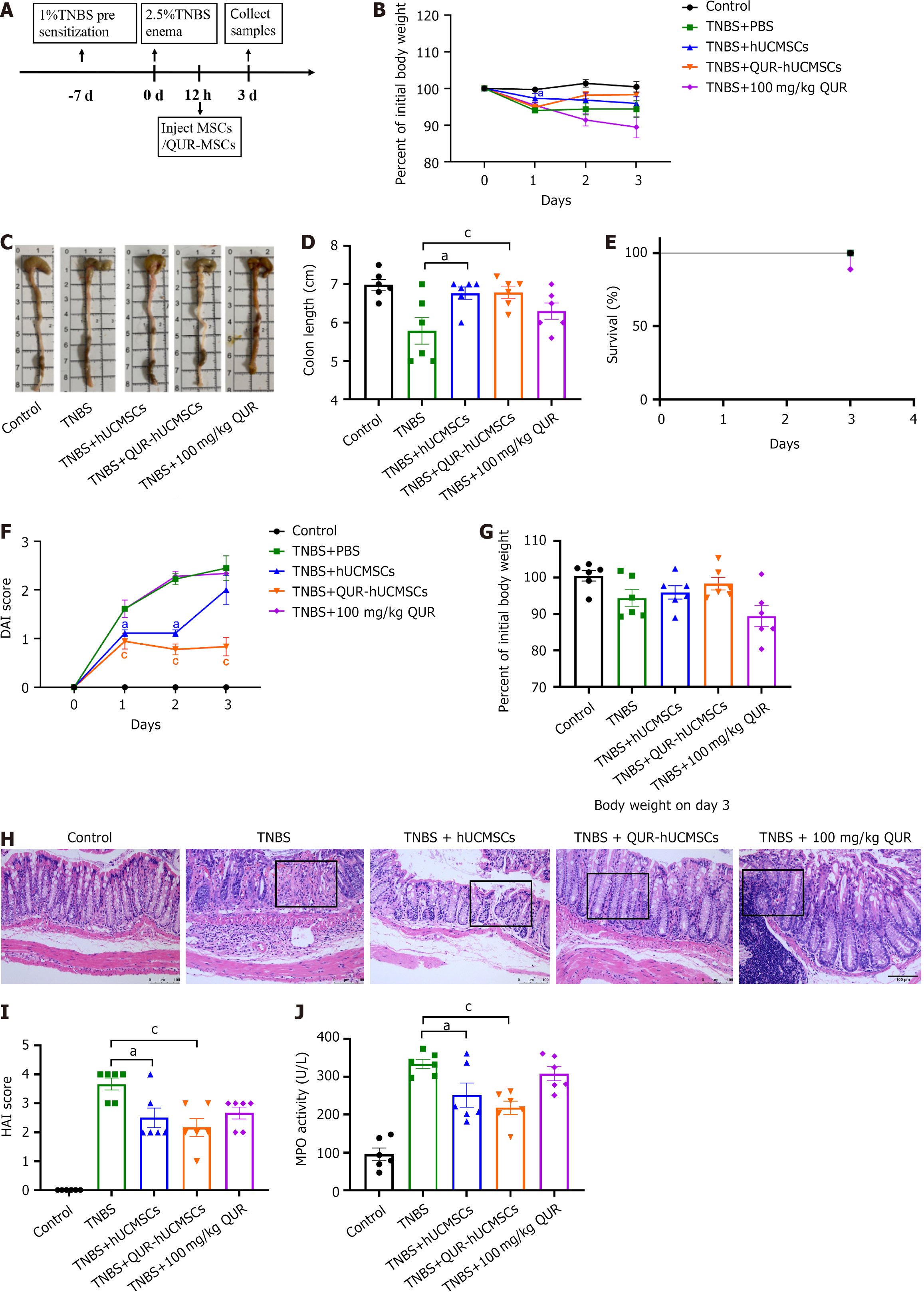
As established by previously published research, our TNBS-induced mouse colitis model successfully replicates the acute phase of the disease (Figure 3A). Compared to the control group, mice with TNBS-induced colitis displayed weight loss, colon shortening, and a significant increase in the DAI score (Figure 3B-D). Additionally, these mice exhibited clinical signs of disease activity, including diarrhea, bloody stools, and lethargy. Interestingly, the survival rate in the QUR gavage group was 80%, while all mice in the other groups survived the experiment (Figure 3E). While oral QUR administration did not alleviate colitis symptoms, likely due to its low bioavailability, both hUCMSC and QUR-hUCMSC treatments significantly improved disease outcomes compared to the TNBS model group[27]. Mice in the treatment groups exhibited reduced weight loss, a significant decrease in DAI score, and improved weight recovery by day 3. Colon shortening, a hallmark feature of colitis, was observed in the TNBS group. However, treatment with hUCMSCs or QUR-hUCMSCs significantly mitigated intestinal shortening (Figure 3F and G). We measured the length of colon tissue and observed colon shortening in the TNBS group. The hUCMSC and QUR-hUCMSC treatment groups significantly improved intestinal shortening (Figure 3C and D). H&E staining revealed severe intestinal inflammation in the TNBS group, characterized by thickening of the colon wall, crypt disarray, depletion of goblet cells, infiltration of immune cells within the epithelium (intraepithelial lymphocytes), and occasional ulcerative lesions. By contrast, mice treated with hUCMSCs or QUR-hUCMSCs displayed a marked improvement in colonic architecture. These improvements included preserved crypt structure, increased numbers of goblet cells, and reduced infiltration of inflammatory cells (Figure 3H). These findings suggest that both hUCMSC and QUR-hUCMSC treatments effectively ameliorated intestinal damage in the TNBS model of colitis. Histopathological analysis using the HAI scoring system revealed a significant reduction in inflammation scores in the hUCMSC and QUR-hUCMSC treatment groups compared to the TNBS group (Figure 3I). Compared to the hUCMSC group, mice treated with QUR-hUCMSCs exhibited a more pronounced therapeutic effect, as evidenced by reduced weight loss, lower DAI scores, and a sustained improvement throughout the acute phase. Histopathological analysis with H&E staining revealed a more preserved and regular crypt structure in the QUR-hUCMSC group, reflected by a lower HAI score. Furthermore, MPO activity, a marker of neutrophil infiltration, was significantly lower in the hUCMSC and QUR-hUCMSC treatment groups compared to the TNBS group (Figure 3J). These findings suggest that pretreatment with QUR significantly enhances the therapeutic efficacy of hUCMSCs in this model of colitis.
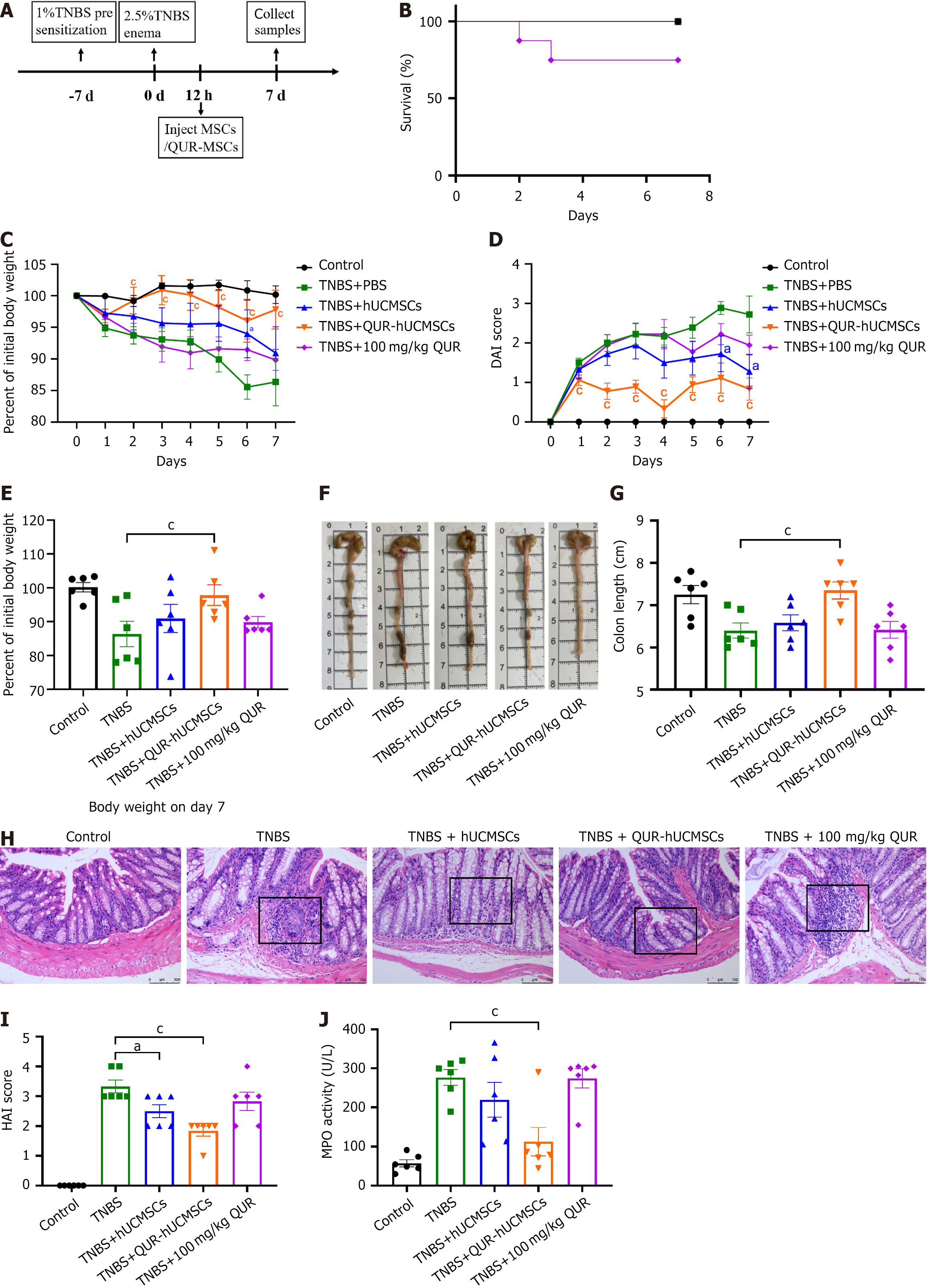
To investigate the long-term effects of QUR pretreatment on hUCMSC therapy, mice underwent a recovery period (Figure 4A). Interestingly, all groups except the QUR oral gavage group exhibited a 100% survival rate throughout the recovery period (Figure 4B). Mice treated with hUCMSCs and QUR-hUCMSCs displayed reduced weight loss and decreased DAI scores compared to the TNBS model group during recovery (Figure 4C and D). However, on day 7, both treatment groups exhibited a noticeable increase in weight loss, with the QUR-hUCMSC group showing a more pronounced effect (Figure 4E). Subsequently, the general health of the colon was assessed, including colon length evaluation. Mice in the TNBS model group continued to exhibit signs of colonic inflammation during recovery, with persistent local congestion and swelling. By contrast, the colon length in the hUCMSC and QUR-hUCMSC groups recovered to near-normal levels, and the overall colonic appearance appeared normal (Figure 4F and G). Histological analysis using H&E staining confirmed ongoing colitis in the TNBS group, characterized by inflammatory cell infiltration within the colonic tissue. However, the hUCMSC and QUR-hUCMSC groups displayed minimal to no inflammatory infiltration, suggesting successful resolution of inflammation (Figure 4H). Histological analysis using the HAI scoring system revealed a significant reduction in inflammation scores in both the hUCMSC and QUR-hUCMSC treatment groups compared to the TNBS group. Interestingly, the QUR-hUCMSC group displayed more pronounced reduction in the HAI score (Figure 4I). MPO activity was decreased in the hUCMSC group, but this reduction did not reach statistical significance. By contrast, QUR-hUCMSC treatment significantly reduced MPO activity in TNBS mice (Figure 4J). These findings collectively demonstrate that both hUCMSC and QUR-hUCMSC treatments effectively alleviated colitis in the TNBS model, with QUR-hUCMSC pretreatment further enhancing therapeutic efficacy.
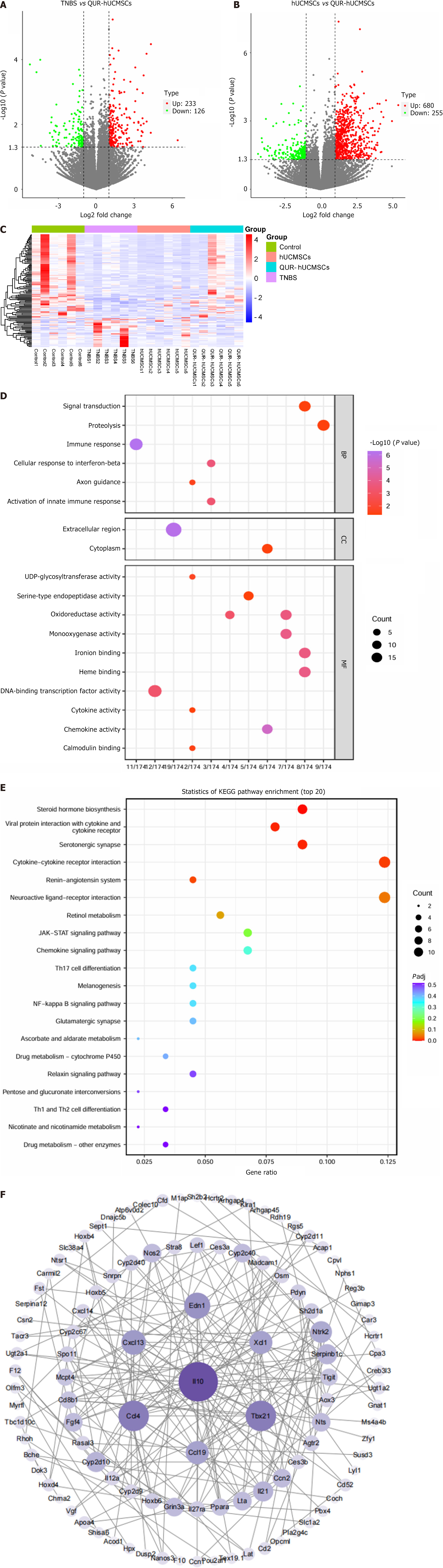
To gain further insights into the mechanisms underlying the therapeutic effects, further RNA-seq studies were conducted on colon tissue from mice on day 3, a time point coinciding with the peak of the inflammatory response. Sequencing reads were mapped to the mouse genome, and genes were identified as differentially expressed based on a threshold of adjusted P-value (P < 0.05) and absolute fold change greater than 1 (|fold changes| > 1). RNA-seq analysis revealed that QUR pretreatment of hUCMSCs significantly impacted the colonic transcriptome profile in TNBS mice. Compared to the TNBS model group, 359 genes were DEGs upon QUR-hUCMSC treatment, with 233 upregulated and 126 downregulated (Figure 5A). Compared to the TNBS model group, transcriptomic response was observed upon the hUCMSC group, with a total of 935 DEGs (680 upregulated and 255 downregulated). These findings suggest that QUR pretreatment modulates the expression of a specific range of genes in hUCMSCs, potentially influencing their therapeutic efficacy. Further analyses are required to identify the most relevant DEGs and therapeutic targets (Figure 5B).
To further explore the transcriptome profiles, hierarchical clustering analysis was performed on the DEGs identified by RNA-seq of colonic tissue (Figure 5C). This analysis revealed a distinct gene expression pattern between the TNBS group and the QUR-hUCMSC treatment group, suggesting a significant impact of QUR-hUCMSC treatment on genes dysregulated by TNBS-induced colitis. GO and KEGG pathway analyses were subsequently employed to functionally annotate the DEGs. GO enrichment analysis of DEGs revealed enrichment in functional categories related to cellular components, molecular functions, and biological processes. These categories included cytokines, chemokines, and immune response pathways (Figure 5D). Similarly, KEGG pathway analysis identified significant enrichment in pathways associated with immunity and inflammation, including the JAK/STAT signaling pathway, T helper type 17 (Th17) signaling pathway, Th1/Th2 signaling pathway, and NF-κB signaling pathway (Figure 5E).
PPI network analysis is a valuable tool for exploring functional relationships between genes and their encoded proteins. To gain further insights into the potential mechanisms underlying the observed therapeutic effects, we constructed a PPI network using Cytoscape software and thus analyzed interactions among the DEGs identified by RNA-seq. Several genes, including IL-10, C-C motif ligand 4 (CCL4), CCL19, T-box transcription factor 21, CCL13, XCL1, and endothelin 1, exhibited high average interaction values (≥ 10), suggesting their potential roles as key regulators in the therapeutic response. IL-10, a critical anti-inflammatory cytokine factor downregulated in TNBS-induced colitis, was significantly upregulated by QUR-hUCMSC treatment, suggesting its potential role as a therapeutic target for IBD (Figure 5F).
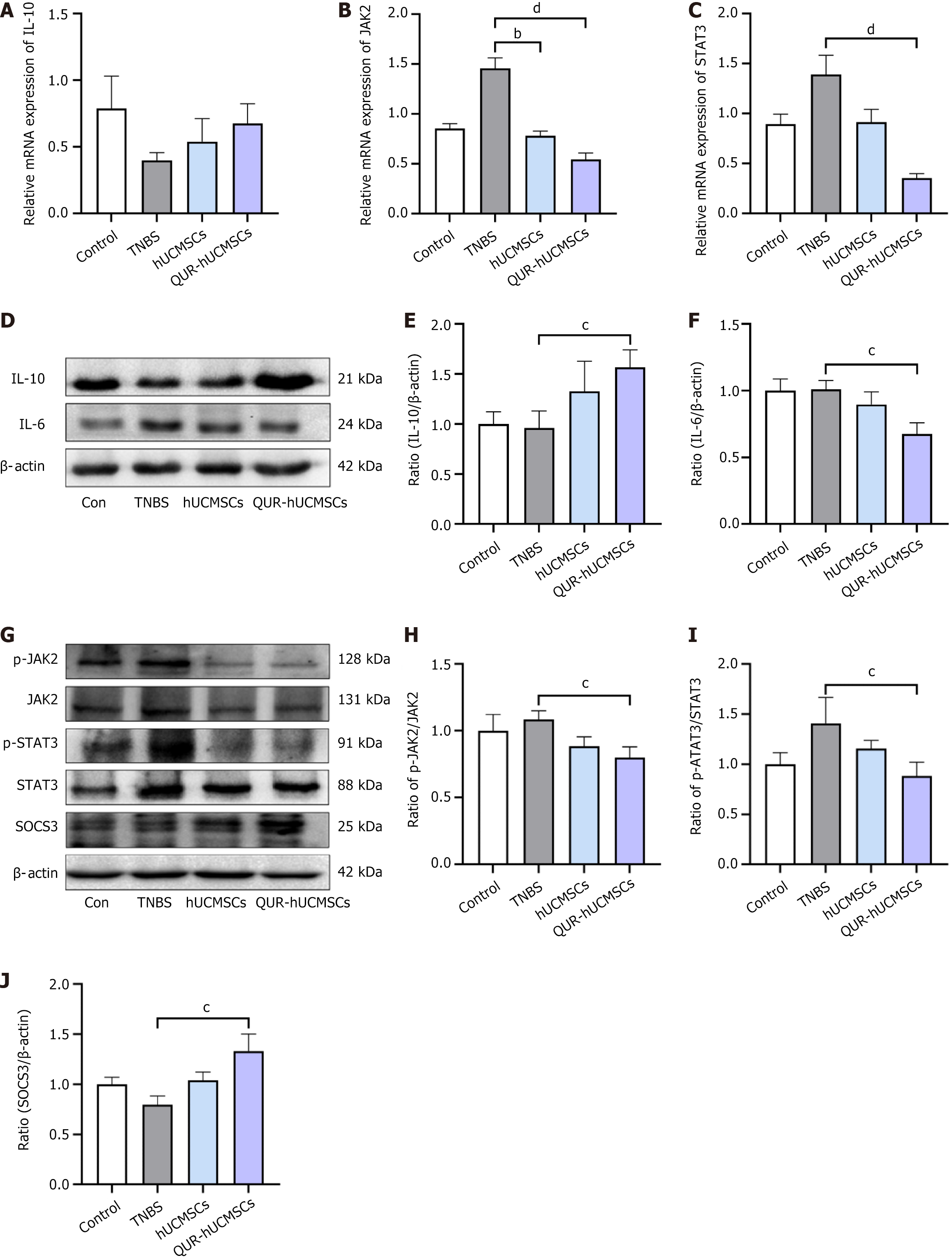
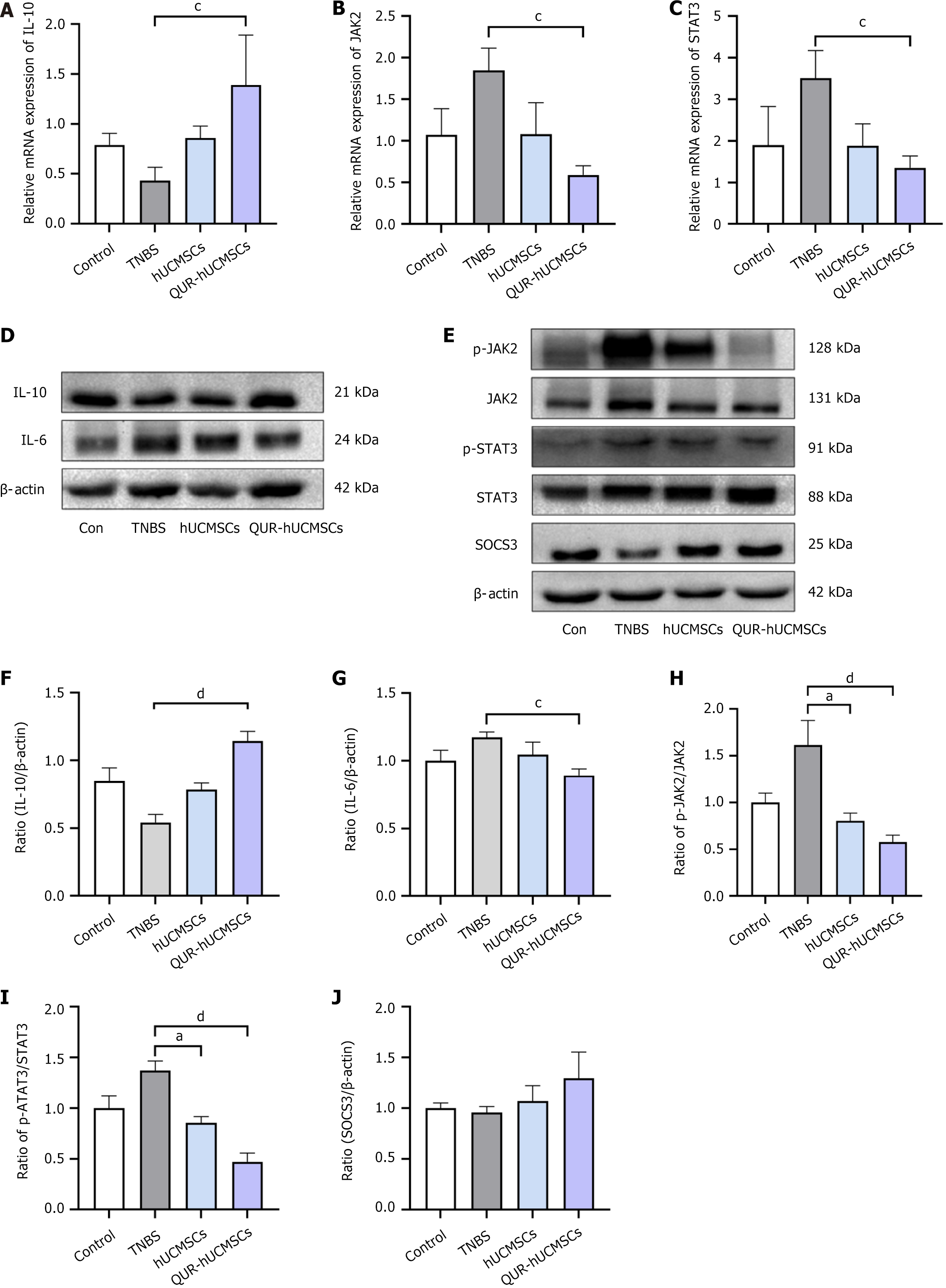
RNA-seq analysis suggested that the therapeutic efficacy of QUR-hUCMSCs in TNBS mice may be mediated by the modulation of immunosuppressive pathways. To validate these findings, studies needed to be focused on the effects of QUR-hUCMSCs on the expression and production of genes and proteins involved in this pathway. Interestingly, RNA-seq data revealed upregulation of JAK2 and STAT3 in TNBS-induced colitis mice compared to the normal control group. QUR-hUCMSC treatment significantly modulated the JAK/STAT signaling pathway in TNBS mice. Compared to the TNBS group, IL-10 mRNA expression was upregulated (Figure 6A), while JAK2 and STAT3 mRNA levels were significantly reduced (Figure 6B and C). At the protein level, the TNBS group exhibited increased phosphorylation of JAK2 and STAT3, whereas QUR-hUCMSC treatment significantly increased IL-10 protein expression (Figure 6D and E), decreased IL-6 Levels (Figure 6D and F), and potentially inhibited phosphorylated JAK2 (p-JAK2) and p-STAT3, respectively (Figure 6G-I). Furthermore, QUR-hUCMSC treatment significantly enhanced the expression of SOCS3, a downstream negative regulator of this pathway (Figure 6G and J). Consistent with the findings in the acute phase, analysis of the recovery period revealed similar modulations of the JAK/STAT pathway in IBD mice (Figure 7). The hUCMSC group also exhibited upregulation of IL-10 and SOCS3 protein expression, alongside downregulation of IL-6, p-JAK2, and p-STAT3. However, these changes in the hUCMS group did not reach statistical significance. Compared to hUCMSCs, QUR-hUCMSC pretreatment exerted a significantly more pronounced therapeutic effect.
IBD is a chronic gastrointestinal condition requiring lifelong treatment. Current treatment options, including immu
To enhance the therapeutic potential of MSCs, researchers have explored various pre-conditioning strategies, including manipulating the physical environment, utilizing drug treatments, incorporating biological factors, and employing gene transfection techniques. These pre-processing methods have demonstrated improved therapeutic effects in diverse disease models, encompassing myocardial infarction, brain injury, colitis, and graft-vs-host disease[12,29]. For instance, studies have shown that pretreatment with irisin enhances cardiac homing and effectively protects against ischemic heart injury in MSCs. This finding suggests that irisin pre-conditioning could be a valuable tool to optimize intravenous delivery of MSCs for the treatment of ischemic heart disease[30]. In another study, pretreatment with Tongxinluo, a traditional Chinese medicine, improves its therapeutic effects in cardiac repair. Mechanistically, this approach targets the miR-146a-5p/IL-1 receptor associated kinase 1/NF-κB pathway, promoting cell survival and exerting anti-inflammatory effects[31]. In a separate study, QUR pretreatment was shown to enhance the radiosensitivity of cancer colon cells by targeting the Notch-1 signaling pathway. This combination therapy resulted in significantly greater anti-cancer effects compared to QUR alone or ionizing radiation therapy, highlighting its promise as a novel treatment strategy for colon cancer. While QUR demonstrates promise in enhancing intestinal barrier function, its therapeutic efficacy for IBD is limited by its poor oral bioavailability[32].
This study investigated the potential mechanism by which QUR-hUCMSCs alleviate colitis using a TNBS-induced mouse model. TNBS mice exhibited significant weight loss and developed characteristic symptoms including loss of appetite, mental fatigue, bloody stools, and diarrhea. This study investigated the potential mechanism of QUR-hUCMSCs in alleviating colitis using a TNBS-induced mouse model. TNBS mice showed significant weight loss and exhibited characteristic symptoms including loss of appetite, mental fatigue, bloody stools, and diarrhea. Interestingly, compared to the TNBS group, both the hUCMSC group and QUR-hUCMSC treatment resulted in a significant decrease in DAI scores. However, in terms of weight loss, rectal bleeding, diarrhea, and other manifestations, QUR pretreatment was more effective than untreated hUCMSCs. This strategy aims to regulate various biological activities and pharmacological properties of QUR, thereby enhancing the anti-inflammatory and immune regulatory capabilities of MSCs, ultimately significantly improving the therapeutic effect of IBD. In our mouse model, TNBS administration successfully induced intestinal inflammation, with a significant increase in IL-6 expression and a significant decrease in IL-10 and SOCS3 Levels in colon tissue. By contrast, treatment with QUR-hUCMSCs significantly upregulated the expression of IL-10 and SOCS3, while downregulating IL-6 Levels. These findings suggest that QUR-hUCMSC therapy may have the potential to improve TNBS-induced colitis injury. We observed that the therapeutic effect of QUR-hUCMSCs persisted beyond the acute phase of colitis, even in the recovery phase of IBD mouse models. This strategy aimed to modulate the various biological activities and pharmacological properties of QUR, thereby enhancing the anti-inflammatory and immunomodulatory capacity of MSCs and ultimately leading to a significantly improved therapeutic effect in the context of IBD. In our mouse model, TNBS administration successfully induced intestinal inflammation, as evidenced by significantly increased IL-6 expression and decreased levels of IL-10 and SOCS3 in colon tissue. Conversely, treatment with QUR-hUCMSCs significantly upregulated the expression of IL-10 and SOCS3, while downregulating IL-6 Levels. These findings suggest that QUR-hUCMSCs therapy may potentially ameliorate TNBS-induced colitis damage. We observed that the therapeutic effect of QUR-hUCMSCs persisted beyond the acute phase of colitis, demonstrating efficacy even in the recovery phase of the IBD mouse model.
IL-10, a key target gene, promotes the transcription of SOCS3 through sequential activation of JAK2 and STAT3, a pathway critical for its anti-inflammatory function[33,34]. It is well-established that the JAK2/STAT3 pathway plays a crucial role in regulating immune responses. It can be activated by cytokines such as IL-6 (pro-inflammatory) or IL-10 (anti-inflammatory). Upon activation, STAT proteins also induce the expression of numerous genes involved in cell proliferation, function, and survival. Interestingly, STAT proteins also induce the expression of SOCS genes, which act as a negative feedback loop in the pathway. SOCS3, a well-known member of the SOCS family, inhibits JAK/STAT3 signaling by preventing the interaction between JAK kinases and their receptors[35]. This action ultimately leads to the inhibition of JAK and STAT3 phosphorylation. In IBD, elevated expression of SOCS3 may lead to suppression of STAT3 activity in both mice and human patients. Conversely, persistent phosphorylation of STAT3 in IBD can promote IL-6 expression within macrophages, ultimately contributing to colon tissue damage.
The JAK/STAT3 signaling pathway is a well-established contributor to the development of IBD[36]. Interestingly, the IL-10/JAK/STAT3/SOCS3 pathway has been involved in both RA and IBD. IL-10 can suppress inflammation by downregulating the phosphorylation of JAK2 and STAT3. Studies suggest that the IL-10/STAT3 pathway is involved in the anti-inflammatory effects of certain medications. For instance, in a model of chronic Chagas disease, metronidazole (an antiparasitic drug) increased IL-10 expression, supporting its potential anti-inflammatory action through this pathway[35,37,38]. To evaluate the in vitro regulation of macrophage polarization by biologic disease-modifying antirheumatic drugs targeting RA pro-inflammatory cytokines, Degboé et al[38] showed that p-STAT3 was eliminated in the presence of IL-10, indicating that anti-TNF drugs downregulated surface markers and cytokines related to the inflammatory phenotype in macrophages, and it is beneficial for phagocytosis and negative feedback of inflammation, supporting inflammation resolution through the IL-10/STAT3 pathway. Previous studies investigating the effects of curcumin on TNBS-induced colitis demonstrated reduced colon mucosal damage after 7 days of treatment. Furthermore, curcumin treatment influenced the phosphorylation of three members within the JAK/STAT/SOCS signaling pathway and increased expression of its downstream proteins. Interestingly, there was a decrease in the pro-inflammatory cytokines granulocyte-macrophage-colony-stimulating factor, IL-12p70, IL-15, and IL-23, while the anti-inflammatory cytokines IL-4, IL-10, and IFN-γ were upregulated[39]. Our findings in the QUR-hUCMSC treatment group are consistent with the aforementioned research on MSCs. Here, we observed a significant increase in IL-10 and SOCS3 expression within colon tissue, alongside a decrease in IL-6 expression and reduced p-JAK2 and p-STAT3 Levels. These results collectively suggest that the IL-10/IL-6/JAK2/STAT3/SOCS3 signaling pathway plays a critical role in the therapeutic effects of QUR-hUCMSCs on colitis.
Related clinical studies have shown that circulating MSCs can be successfully used as carriers for delivering antitumor drugs after being incorporated into the tumor matrix. By utilizing the ability of MSCs and expressing the suicide gene thymidine kinase of the herpes simplex virus through engineered bone marrow-derived MSCs, a combination of cell and gene therapy has been developed. This is the world’s first clinical trial to use genetically modified MSCs in humans[40]. However, the conversion of current preprocessing strategies is still in its early stages and requires more preclinical research. Pretreatment of MSCs with hypoxia, inflammatory factors, three-dimensional culture, engineering methods, and drug stimulation or a combination of the above methods before application is a new strategy to enhance the immunomodulatory effect of MSCs in local or systemic immune responses. Numerous experiments have shown that it is possible to improve the regulation of innate and adaptive immune responses. Our study demonstrates that QUR pretreatment can significantly enhance the therapeutic efficacy of MSCs in treating IBD. This approach has the potential to reduce reliance on traditional immunosuppressants, offering a safer and more effective treatment strategy for IBD. The findings provide valuable insights for improving the therapeutic potential of MSCs and raise novel possibilities for clinical application in IBD treatment. However, further research is necessary to elucidate the complete molecular mechanism by which QUR-pretreated hUCMSCs to exert their therapeutic effects on IBD and potentially other immune-related diseases. Similarly, the various methods and combinations used in MSC pretreatment need to be optimized, and new technologies need to be developed to better characterize and standardize them. We believe that this strategy may receive further research in the future and may be applied to individuals with immune system related diseases.
Our study demonstrates that QUR pretreatment significantly enhances the anti-inflammatory and immunosuppressive properties of MSCs. This approach effectively alleviated intestinal symptoms in a TNBS-induced colitis model. Mechanistically, we found that IL-10 plays a critical role in the therapeutic effects of QUR-hUCMSCs, acting through the JAK2/STAT3 signaling pathway.
We are grateful to the platform of the Department of Medical of Yangtze University for their support.
| 1. | Agrawal M, Allin KH, Petralia F, Colombel JF, Jess T. Multiomics to elucidate inflammatory bowel disease risk factors and pathways. Nat Rev Gastroenterol Hepatol. 2022;19:399-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 144] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 2. | Plevris N, Lees CW. Disease Monitoring in Inflammatory Bowel Disease: Evolving Principles and Possibilities. Gastroenterology. 2022;162:1456-1475.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 116] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 3. | Agrawal M, Jess T. Implications of the changing epidemiology of inflammatory bowel disease in a changing world. United European Gastroenterol J. 2022;10:1113-1120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 129] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 4. | Cao F, He YS, Wang Y, Zha CK, Lu JM, Tao LM, Jiang ZX, Pan HF. Global burden and cross-country inequalities in autoimmune diseases from 1990 to 2019. Autoimmun Rev. 2023;22:103326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 152] [Reference Citation Analysis (0)] |
| 5. | Zhang Y, Liu J, Han X, Jiang H, Zhang L, Hu J, Shi L, Li J. Long-term trends in the burden of inflammatory bowel disease in China over three decades: A joinpoint regression and age-period-cohort analysis based on GBD 2019. Front Public Health. 2022;10:994619. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 101] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 6. | Kaplan GG, Windsor JW. The four epidemiological stages in the global evolution of inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2021;18:56-66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 187] [Cited by in RCA: 916] [Article Influence: 183.2] [Reference Citation Analysis (0)] |
| 7. | Luo H, Cao G, Luo C, Tan D, Vong CT, Xu Y, Wang S, Lu H, Wang Y, Jing W. Emerging pharmacotherapy for inflammatory bowel diseases. Pharmacol Res. 2022;178:106146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 74] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 8. | Cassinotti A, Passamonti F, Segato S. Cell therapy in inflammatory bowel disease. Pharmacol Res. 2021;163:105247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 9. | Wu X, Jiang J, Gu Z, Zhang J, Chen Y, Liu X. Mesenchymal stromal cell therapies: immunomodulatory properties and clinical progress. Stem Cell Res Ther. 2020;11:345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 213] [Cited by in RCA: 225] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 10. | Zhuang WZ, Lin YH, Su LJ, Wu MS, Jeng HY, Chang HC, Huang YH, Ling TY. Mesenchymal stem/stromal cell-based therapy: mechanism, systemic safety and biodistribution for precision clinical applications. J Biomed Sci. 2021;28:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 191] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 11. | Hu C, Wu Z, Li L. Pre-treatments enhance the therapeutic effects of mesenchymal stem cells in liver diseases. J Cell Mol Med. 2020;24:40-49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 68] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 12. | Su Y, Xu C, Cheng W, Zhao Y, Sui L, Zhao Y. Pretreated Mesenchymal Stem Cells and Their Secretome: Enhanced Immunotherapeutic Strategies. Int J Mol Sci. 2023;24:1277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 42] [Reference Citation Analysis (0)] |
| 13. | Shen P, Lin W, Deng X, Ba X, Han L, Chen Z, Qin K, Huang Y, Tu S. Potential Implications of Quercetin in Autoimmune Diseases. Front Immunol. 2021;12:689044. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 150] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 14. | Cheng SC, Wu YH, Huang WC, Pang JS, Huang TH, Cheng CY. Anti-inflammatory property of quercetin through downregulation of ICAM-1 and MMP-9 in TNF-α-activated retinal pigment epithelial cells. Cytokine. 2019;116:48-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 15. | Yin J, Peng X, Lin J, Zhang Y, Zhang J, Gao H, Tian X, Zhang R, Zhao G. Quercetin amelioratesAspergillus fumigatuskeratitis by inhibiting fungal growth, toll-like receptors and inflammatory cytokines. Int Immunopharmacol. 2021;93:107435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 16. | Costa ACF, de Sousa LM, Dos Santos Alves JM, Goes P, Pereira KMA, Alves APNN, Vale ML, Gondim DV. Anti-inflammatory and Hepatoprotective Effects of Quercetin in an Experimental Model of Rheumatoid Arthritis. Inflammation. 2021;44:2033-2043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 17. | Alizadeh SR, Ebrahimzadeh MA. Quercetin derivatives: Drug design, development, and biological activities, a review. Eur J Med Chem. 2022;229:114068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 153] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 18. | Koelink PJ, Bloemendaal FM, Li B, Westera L, Vogels EWM, van Roest M, Gloudemans AK, van 't Wout AB, Korf H, Vermeire S, Te Velde AA, Ponsioen CY, D'Haens GR, Verbeek JS, Geiger TL, Wildenberg ME, van den Brink GR. Anti-TNF therapy in IBD exerts its therapeutic effect through macrophage IL-10 signalling. Gut. 2020;69:1053-1063. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 177] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 19. | Saraiva M, Vieira P, O'Garra A. Biology and therapeutic potential of interleukin-10. J Exp Med. 2020;217:e20190418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 743] [Article Influence: 123.8] [Reference Citation Analysis (0)] |
| 20. | Jung KJ, Lee GW, Park CH, Lee TJ, Kim JY, Sung EG, Kim SY, Jang BI, Song IH. Mesenchymal Stem Cells Decrease Oxidative Stress in the Bowels of Interleukin-10 Knockout Mice. Gut Liver. 2020;14:100-107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 21. | Zhao J, Wang H, Yang H, Zhou Y, Tang L. Autophagy induction by rapamycin ameliorates experimental colitis and improves intestinal epithelial barrier function in IL-10 knockout mice. Int Immunopharmacol. 2020;81:105977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | Yan Y, Xing C, Xiao Y, Shen X, Zhang Z, He C, Shi JB, Liu M, Liu X. Discovery and Anti-Inflammatory Activity Evaluation of a Novel CDK8 Inhibitor through Upregulation of IL-10 for the Treatment of Inflammatory Bowel Disease In Vivo. J Med Chem. 2022;65:7334-7362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 23. | Wang J, Wang J, Hong W, Zhang L, Song L, Shi Q, Shao Y, Hao G, Fang C, Qiu Y, Yang L, Yang Z, Wang J, Cao J, Yang B, He Q, Weng Q. Optineurin modulates the maturation of dendritic cells to regulate autoimmunity through JAK2-STAT3 signaling. Nat Commun. 2021;12:6198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 24. | Zong X, Cheng Y, Xiao X, Fu J, Wang F, Lu Z, Wang Y, Jin M. Protective effects of sulfated polysaccharide from Enterobacter cloacae Z0206 against DSS-induced intestinal injury via DNA methylation. Int J Biol Macromol. 2021;183:861-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Cheng Y, Li J, Wang L, Wu X, Li Y, Xu M, Li Q, Huang J, Zhao T, Yang Z, Zhang H, Zuo L, Zhang X, Geng Z, Wang Y, Song X, Zhang J. Eriocalyxin B ameliorated Crohn's disease-like colitis by restricting M1 macrophage polarization through JAK2/STAT1 signalling. Eur J Pharmacol. 2023;954:175876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 26. | Katsandegwaza B, Horsnell W, Smith K. Inflammatory Bowel Disease: A Review of Pre-Clinical Murine Models of Human Disease. Int J Mol Sci. 2022;23:9344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 127] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 27. | Jing S, Chen H, Liu E, Zhang M, Zeng F, Shen H, Fang Y, Muhitdinov B, Huang Y. Oral pectin/oligochitosan microspheres for colon-specific controlled release of quercetin to treat inflammatory bowel disease. Carbohydr Polym. 2023;316:121025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 28. | Saadh MJ, Mikhailova MV, Rasoolzadegan S, Falaki M, Akhavanfar R, Gonzáles JLA, Rigi A, Kiasari BA. Therapeutic potential of mesenchymal stem/stromal cells (MSCs)-based cell therapy for inflammatory bowel diseases (IBD) therapy. Eur J Med Res. 2023;28:47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 43] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 29. | Sun L, Wang J, Wang Q, He Z, Sun T, Yao Y, Wang W, Shen P. Pretreatment of umbilical cord derived MSCs with IFN-γ and TNF-α enhances the tumor-suppressive effect on acute myeloid leukemia. Biochem Pharmacol. 2022;199:115007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 30. | Yan W, Chen Y, Guo Y, Xia Y, Li C, Du Y, Lin C, Xu X, Qi T, Fan M, Zhang F, Hu G, Gao E, Liu R, Hai C, Tao L. Irisin Promotes Cardiac Homing of Intravenously Delivered MSCs and Protects against Ischemic Heart Injury. Adv Sci (Weinh). 2022;9:e2103697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 31. | Xiong Y, Tang R, Xu J, Jiang W, Gong Z, Zhang L, Ning Y, Huang P, Xu J, Chen G, Li X, Hu M, Xu J, Wu C, Jin C, Li X, Qian H, Yang Y. Tongxinluo-pretreated mesenchymal stem cells facilitate cardiac repair via exosomal transfer of miR-146a-5p targeting IRAK1/NF-κB p65 pathway. Stem Cell Res Ther. 2022;13:289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 71] [Reference Citation Analysis (0)] |
| 32. | Fan J, Li BR, Zhang Q, Zhao XH, Wang L. Pretreatment of IEC-6 cells with quercetin and myricetin resists the indomethacin-induced barrier dysfunction via attenuating the calcium-mediated JNK/Src activation. Food Chem Toxicol. 2021;147:111896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 33. | Arafa EA, Mohamed WR, Zaher DM, Omar HA. Gliclazide attenuates acetic acid-induced colitis via the modulation of PPARγ, NF-κB and MAPK signaling pathways. Toxicol Appl Pharmacol. 2020;391:114919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 34. | Yan S, Zhang C, Ji X, Wu G, Huang X, Zhang Y, Zhang Y. MSC-ACE2 Ameliorates Streptococcus uberis-Induced Inflammatory Injury in Mammary Epithelial Cells by Upregulating the IL-10/STAT3/SOCS3 Pathway. Front Immunol. 2022;13:870780. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 35. | Shafiey SI, Ahmed KA, Abo-Saif AA, Abo-Youssef AM, Mohamed WR. Galantamine mitigates testicular injury and disturbed spermatogenesis in adjuvant arthritic rats via modulating apoptosis, inflammatory signals, and IL-6/JAK/STAT3/SOCS3 signaling. Inflammopharmacology. 2024;32:405-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 36. | Gao Y, Zhao H, Wang P, Wang J, Zou L. The roles of SOCS3 and STAT3 in bacterial infection and inflammatory diseases. Scand J Immunol. 2018;88:e12727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 104] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 37. | Cevey ÁC, Penas FN, Alba Soto CD, Mirkin GA, Goren NB. IL-10/STAT3/SOCS3 Axis Is Involved in the Anti-inflammatory Effect of Benznidazole. Front Immunol. 2019;10:1267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 68] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 38. | Degboé Y, Rauwel B, Baron M, Boyer JF, Ruyssen-Witrand A, Constantin A, Davignon JL. Polarization of Rheumatoid Macrophages by TNF Targeting Through an IL-10/STAT3 Mechanism. Front Immunol. 2019;10:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 150] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 39. | Zhao HM, Xu R, Huang XY, Cheng SM, Huang MF, Yue HY, Wang X, Zou Y, Lu AP, Liu DY. Curcumin Suppressed Activation of Dendritic Cells via JAK/STAT/SOCS Signal in Mice with Experimental Colitis. Front Pharmacol. 2016;7:455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 85] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 40. | Niess H, von Einem JC, Thomas MN, Michl M, Angele MK, Huss R, Günther C, Nelson PJ, Bruns CJ, Heinemann V. Treatment of advanced gastrointestinal tumors with genetically modified autologous mesenchymal stromal cells (TREAT-ME1): study protocol of a phase I/II clinical trial. BMC Cancer. 2015;15:237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/