Published online Oct 26, 2025. doi: 10.4252/wjsc.v17.i10.111241
Revised: August 13, 2025
Accepted: September 22, 2025
Published online: October 26, 2025
Processing time: 121 Days and 11 Hours
Mesenchymal stem cells (MSCs) are known for their ability to differentiate into various cell lineages, including osteoblasts (bone-forming cells), and for their significant paracrine effects. Among their secreted products, exosomes have gained considerable attention as nanoscale carriers of bioactive molecules such as non-coding RNAs (ncRNAs). These ncRNAs, including microRNAs, long ncRNAs, and circular ncRNAs, are critical regulators of gene expression and cellular functions. Moreover, MSC-derived exosomes not only offer advantages such as targeted delivery, reduced immunogenicity, and protection of cargo material, but also carry ncRNAs that have therapeutic and diagnostic potential in bone-related disorders. Emerging evidence has highlighted the role of MSC-derived exosomal ncRNAs in osteogenesis, bone remodeling, and intercellular signaling in the bone microenvironment. This review consolidates recent research on the role of MSC-derived exosomal ncRNAs in maintaining bone homeostasis and bone-related disorders via various signaling pathways and epigenetic modifications. Furthermore, we explore the therapeutic potential of MSC-derived exosomal ncRNAs as biomarkers and therapeutic targets. This comprehensive review offers key insights into the regulatory roles of MSC-derived exosomal ncRNAs in bone biology and their clinical significance in bone-related diseases.
Core Tip: Mesenchymal stem cell-derived exosomal non-coding RNAs, including microRNAs, long non-coding RNAs, and circular RNAs, play vital roles in regulating bone development, remodeling, and the pathogenesis of bone-related disorders. This review provides insights into their emerging roles in bone biology and disease, highlighting their therapeutic potential and underlying molecular mechanisms.
- Citation: Chidambaram D, Subashini V, Nanthanalaxmi M, Selvamurugan N. Role of mesenchymal stem cell-derived exosomal non-coding RNAs in bone and bone-related disorders. World J Stem Cells 2025; 17(10): 111241
- URL: https://www.wjgnet.com/1948-0210/full/v17/i10/111241.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v17.i10.111241
Bone is a mineralized connective tissue comprised of several cell types, including osteoblasts, osteoclasts, and osteocytes. It serves as a mineral reservoir and houses bone marrow for blood cell production[1]. Bone-derived defects impact bone mineralization and mechanical robustness, including bone degenerative joint defects, lack of bone mass and bone mineral density, or overgrowth of bone-forming osteoblasts[2,3]. In recent years, mesenchymal stem cells (MSCs) have shown tremendous potential as biomedicines for treating bone disorders. MSCs are adult stem cells with intrinsic self-renewal and multipotent capacity that enable multilineage differentiation, making them versatile for regenerative applications[4]. MSCs play a vital role in regulating tissue homeostasis and facilitating tissue regeneration[5]. They are particularly useful in cell-based therapy and tissue engineering applications due to their differentiation capacity, ability to repair damaged environments, migration to damaged tissue, paracrine signaling effects, and immunomodulatory properties[6].
A meta-analysis of MSC-based therapies over the past 15 years has validated their safety, with no risk of serious adverse effects. However, significant concerns remain, such as oncogene activation, immune rejection, premature differentiation, reduced immune compatibility, instability, and destruction of genetic material[7]. For instance, MSC activation before intra-articular administration alleviates osteoarthritis (OA) through enhanced gait activity and chondroprotection. However, minor phenotypic characteristics of MSCs that may affect reproducibility due to variability within MSC populations has also been noted[8]. In another study involving intra-articular MSC injection for knee OA, ethical limitations precluded cartilage biopsy, limiting the assessment of the underlying mechanisms of pain relief[9]. To address these limitations, MSC-derived exosomes have gained attention as cell-free alternatives that offer similar therapeutic benefits while avoiding the risks associated with live-cell therapies.
Exosomes are nanoscale membrane-bound extracellular vesicles that carry bioactive molecules such as DNA, RNA, proteins, and lipids of the cells as part of their normal physiology or acquired abnormalities[10]. Their size typically ranges from 30-150 nm in diameter[11,12]. MSC-derived exosomes actively participate in physiological processes, including intercellular communication, cellular development, apoptosis, modulation of immune responses, tissue regeneration, metabolic regulation, drug delivery vehicles, biomarkers, and targets[13-15]. In the context of bone remodeling, exosomes ensure a proper balance between osteoblast-mediated bone formation and osteoclast-mediated bone resorption[16]. Their ability to directly target damaged tissues and deliver bioactive molecules enhances their therapeutic potential, making them powerful tools in regenerative medicine.
Given their broad therapeutic potential, recent research has focused on non-coding RNAs (ncRNAs), which do not encode proteins but regulate gene function, within MSC-derived exosomes, particularly in bone-related applications. Exosomes originate from multivesicular bodies that fuse with the plasma membrane to release bioactive molecules, including ncRNAs. These ncRNAs are a major part of the human genome, where they regulate protein-coding genes by altering various cellular activities such as transcription, translation, and post-translational modifications in osteogenic cells[17]. ncRNAs are categorized based on their nucleotide size: Small ncRNAs, such as microRNA (miRNA) and small interfering RNA are < 200 nucleotides, whereas long ncRNA (lncRNA), such as linear and circular RNA (circRNA), are > 200 nucleotides[18]. These ncRNAs mediate intercellular communication and regulate various physiological functions. In the context of bone health, they control gene expression, influence osteoblasts and osteoclasts, and help manage bone-related issues. Additionally, they are potential therapeutic targets for early diagnosis. However, dysregulation of ncRNAs can lead to serious bone disorders[17]. For example, miR-101 targets F-box and WD repeat domain-containing 7, leading to activation of the hypoxia-inducible factor 1-alpha/forkhead box protein P3 axis and osteoblast differentiation, and circ_0008542 facilitates osteoclast-induced bone resorption through increased N6-methyladenosine methylation[16]. Various delivery methods have been explored to harness the therapeutic potential of exosomal ncRNAs, including systemic administration, localized delivery, and targeted delivery methods[19,20]. For example, exosomes loaded with the lncRNA maternally expressed gene 3 and engineered with a cyclic Arg-Gly-Asp peptide could more efficiently target tumor cells, facilitate therapeutic agent release, and enhance antitumor effects in osteosarcoma (OS)[21]. Another efficient approach for delivering exosomal ncRNAs involves the use of scaffold-based systems. Bone marrow MSC (BMSC)-derived exosomes loaded with miR-26a, when integrated into a hydrogel scaffold and implanted at a defect site, enhanced bone regeneration. In this setup, exosomes promoted angiogenesis (new blood vessel formation), miR-26a supported osteogenesis (bone formation), and the hydrogel facilitated site-specific release[22]. Similarly, umbilical MSC-derived exosomes encapsulated in hyaluronic acid hydrogel and combined with customized nanohydroxyapatite/poly-ε-caprolactone scaffolds demonstrated enhanced bone regeneration in vivo. Exosomal miR-21 facilitated angiogenesis by inhibiting the NOTCH1/delta like canonical Notch ligand 4 pathway and inducing the expression of vascular endothelial growth factor A and hypoxia inducible factor 1 subunit alpha. Furthermore, this scaffold-based methodology allows prolonged and sustained exosome release[23].
Several studies have demonstrated that exosomes serve as effective vehicles for ncRNA transport. When sourced from MSCs, these exosomes offer a promising alternative for overcoming challenges associated with direct MSC-based therapies. This finding supports the hypothesis that MSC-derived exosomal ncRNAs are a novel and effective strategy for treating various bone-related disorders. We performed a comprehensive literature search using databases like Google Scholar, PubMed, and ResearchGate. The keywords used to find research articles were “MSC-derived exosomes”, “alleviates osteoarthritis”, “osteoporosis (OP)”, “OS”, “bone related disorder”, “ncRNA”, and “clinical trials”. To ensure a comprehensive and up-to-date review, studies primarily spanning from 2021 to 2025 were selected, along with a few studies from 2017 to 2020. This review highlights the physiological significance of MSC-derived exosomal ncRNAs, their diverse regulatory roles and signaling pathways in maintaining bone homeostasis (the balance between bone formation and bone resorption), and their potential as therapeutic targets in bone-related disorders.
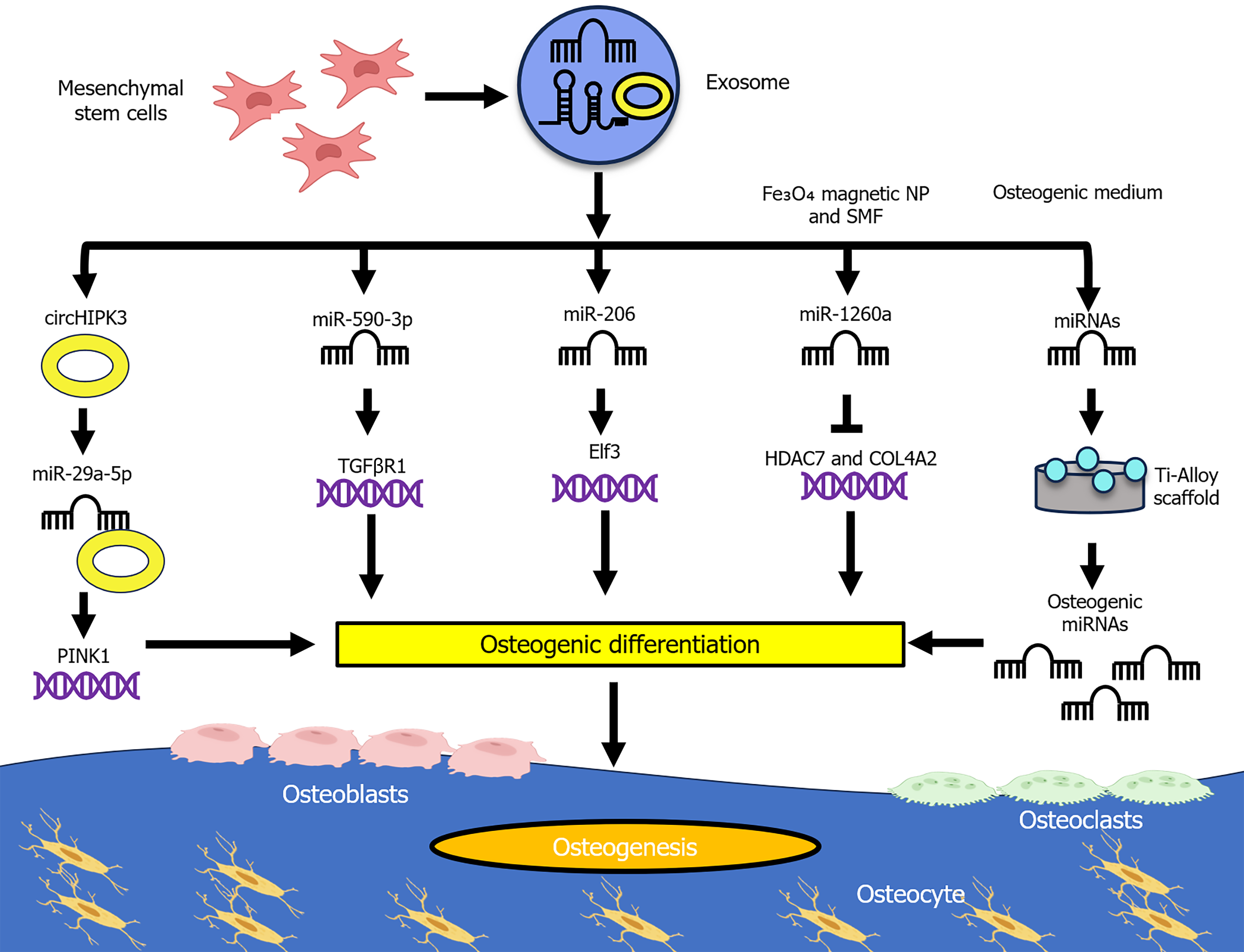
Bone undergoes continuous remodeling, wherein osteoblasts function as bone-forming cells and osteoclasts serve as bone-resorbing cells. Together, these cells contribute to maintaining bone homeostasis[24]. Osteogenesis is a crucial physiological process responsible for bone formation, regeneration, and maintenance of bone homeostasis, frequently associated with angiogenesis to facilitate bone tissue development and repair. Recent studies have emphasized the role of MSC-derived exosomal ncRNAs in promoting bone regeneration (Figure 1). For instance, BMSC-derived exosomal miR-590-3p targets transforming growth factor beta receptor 1, thereby promoting osteoblast differentiation and enhancing osteogenesis[25]. Similarly, BMSC-derived exosomal circHIPK3 promotes osteogenic differentiation of MC3T3-E1 cells by sponging miR-29a-5p, thereby upregulating phosphatase and tensin homolog induced putative kinase 1 expression, enhancing mitophagy and contributing to pro-osteogenic effects[26]. In parallel, BMSC-derived exosomal miR-206 targets E74-like factor 3 and decreases its expression, promoting osteoblast proliferation and differentiation while inhibiting osteoblast apoptosis[27].
The osteogenic potential of MSC-derived exosomal ncRNAs can be further enhanced through innovative conditioning strategies. For example, BMSC-derived exosomes preconditioned with Fe3O4 magnetic nanoparticles under a static magnetic field contain miR-1260a, which inhibits histone deacetylase 7 and collagen type IV alpha 2 chain, exhibiting enhanced osteogenesis and angiogenesis compared with BMSC-derived exosomes and phosphate buffered saline (control)[28]. Furthermore, advanced therapeutic strategies combine scaffold-based approaches with exosome coatings to improve bone regeneration efficacy. Specifically, exosomes derived from human MSCs pre-differentiated in osteogenic medium, when coated onto a cell-free titanium alloy scaffold, can induce osteogenesis by upregulating osteogenic miRNAs (hsa-miR-146a-5p, hsa-miR-503-5p, hsa-miR-483-3p, and hsa-miR-129-5p) and downregulating anti-osteogenic miRNAs (hsa-miR-32-5p, hsa-miR-133a-3p, and hsa-miR-204-5p) by activating the phosphoinositide 3-kinase/protein kinase B and mitogen-activated protein kinase (MAPK) signaling pathways[29].
In addition to their role in regeneration, exosomal ncRNAs have been implicated in the regulation of bone-related diseases by restoring disrupted homeostasis and mitigating disease progression. For instance, BMSC-derived exosomal miR-150-3p promotes osteoblast proliferation and differentiation while inhibiting apoptosis, thereby alleviating OP[30]. Similarly, BMSC-derived exosomal miR-21-5p improves OP by regulating Kruppel-like factor 3 (KLF3), thus providing a potential therapeutic strategy for OP[31]. Moreover, in a cystathionine β-synthase+/- mouse model, BMSC-derived exosomal lncRNA H19 sponges miR-106, modulating angiopoietin 1-Tie2/nitric oxide signaling to promote osteogenesis and angiogenesis, ultimately mitigating metabolic OP[32]. Another study using an ovariectomized rat model demonstrated that ovariectomized rat BMSC-derived exosomal miR-27a-3p and miR-196b-5p modulate bone remodeling by enhancing osteogenic BMSC differentiation upon overexpression, whereas their suppression impedes osteogenic differentiation and facilitates osteoclast differentiation[33].
Furthermore, in osteolytic diseases, exosomal miR-6924-5p derived from scleraxis-overexpressing platelet-derived growth factor receptor alpha positive BMSCs targets the osteoclastic regulators C-X-C motif chemokine ligand 12 and osteoclast stimulatory transmembrane protein, thereby inhibiting osteoclastogenesis (osteoclast formation) in human monocytes, reducing osteoclast formation, and enhancing tendon-bone healing strength[34]. Conversely, some miRNAs exhibit anti-osteogenic effects. For example, exosomal miR-128-3p derived from aged rat MSCs targets Smad5 and has an anti-osteogenic effect by downregulating osteogenesis-related proteins, including Runt-related transcription factor 2, collagen I, and alkaline phosphatase, suggesting that the miR-128-3p antagomir may facilitate osteogenesis[35]. Altogether, the evidence suggests that MSC-derived exosomal ncRNAs restore the osteoblast-osteoclast balance by stimulating osteoblast-driven bone formation or suppressing osteoclast-mediated bone resorption, thereby maintaining bone homeostasis. These insights highlight the role of MSC-derived exosomal ncRNAs as key regulators of osteogenesis, bone regeneration, and modulation of bone disease[36-39] (Table 1).
| Exosome origin | Exosomal ncRNAs | Targets/pathways | Effects | In vivo models | Ref. |
| BMSCs | miR-150-5p | SOX2 and PI3K/Akt pathway | Induced type H blood vessel angiogenesis and osteogenesis | Female mice | [36] |
| Fucoidan-induced MSCs | miR-146b-5p | TRAF6 and PI3K/Akt/mTOR pathways | Suppressed inflammatory responses, extracellular matrix degradation, and promoted chondrocyte autophagy | Male Sprague-Dawley rats | [37] |
| BMSCs | miR-422a | KLK4 | Inhibited angiogenesis and osteogenesis | Female mice | [38] |
| Synovial MSCs | miR-485-3p | NRP1 and PI3K/Akt pathway | Relieved cartilage damage | N/A | [39] |
Major bone-related disorders include OA, OS, and OP, each with a distinct pathophysiology. OA is a prevalent degenerative disorder that affects the entire synovial joint. It is characterized by hyaline cartilage degeneration, subchondral bone abnormalities, synovial enlargement accompanied by increased vascularity, and tendon and ligament instability[40]. Similarly, OP is characterized by low bone quality and strength and a higher fracture risk. OP is classified into two different types, primary and secondary, based on factors that influence bone metabolism, such as hormonal changes and aging. Primary OP is further classified into type I/postmenopausal and type II/senile. Secondary OP is caused by medications, pathological conditions, and aging and menopause[41]. In contrast, OS is a malignant tumor typically found in the metaphysis of long bones and has the highest incidence in children and adolescents[42,43]. Recent studies highlight the regulatory roles of MSC-derived exosomal ncRNAs in important processes in disease pathophysiology[43-52] (Table 2).
| Diseases | Sources | Exosomal ncRNAs | Targets | Signaling pathways involved | Effects | Ref. |
| OA | BMSCs | lncRNA TUC339 | - | - | Mitigated OA by promoting the transition from M1-type to M2-type macrophage polarization, inhibiting inflammation, and enhancing chondrocyte function | [44] |
| MSCs | lncRNA KLF3-AS1 | - | - | Alleviated OA by promoting chondrocyte proliferation and inhibiting apoptosis, thereby facilitating cartilage repair | [45] | |
| BMSCs | miR-9-5p | 3’ UTR of syndecan-1 | - | Reduced inflammatory markers, decreased oxidative stress, and suppressed expression of OA-associated factors | [46] | |
| BMSCs | miR-135b | - | Downregulation of MAPK6 expression | Promoted M2 polarization of synovial macrophages thereby aiding in cartilage healing | [47] | |
| Adipose MSCs | miR-376c-3p | 3’ UTR of Wnt family member 3 or Wnt family member 9a | Hindering of Wnt/β-catenin pathway | Induced chondrocyte degradation and synovial fibrosis | [48] | |
| Synovial MSCs | miR-320c | - | Modulation of ADAM metalloproteinase domain 19-dependent Wnt signaling | Inhibited extracellular matrix degradation and chondrocyte apoptosis | [49] | |
| BMSCs | circRNA_0001236 | miR-3677-3p | - | Increased the collagen type II alpha 1 chain and sex-determining region Y-box 9 expression and reduced the matrix metalloproteinase-13 expression | [50] | |
| Umbilical cord blood MSCs | lncRNA H19 | miR-29a-3p | - | Decreased the phosphorylation levels of biochemical markers of neuropathic pain (NR1, NR2B, protein kinase C gamma and ERK) in astrocytes | [51] | |
| OP | Human BMSCs | miR-186 | MOB kinase activator 1A | Hippo pathway | Facilitated osteogenesis in postmenopausal OP | [52] |
| OS | BMSCs | lncRNA PVT1 | miR-183-5p | - | Enhanced OS proliferation and migration | [43] |
In addition, MSC-derived exosomal ncRNAs serve as potential diagnostic biomarkers. For instance, microarray analysis of circRNA sequencing profiles in BMSC-derived exosomes from individuals with postmenopausal OP identified five different circRNAs, namely hsa_circ_0009127, hsa_circ_0090759, hsa_circ_0058392, hsa_circ_0090247, and hsa_circ_0049484, that may be potential biomarkers or therapeutic targets[53]. Further studies are required to explore the potential of MSC-derived exosomal ncRNAs as biomarkers. In summary, MSC-derived exosomal ncRNAs play a pivotal regulatory function in bone-related disorder management; however, further detailed studies are essential for their translation into clinical use.
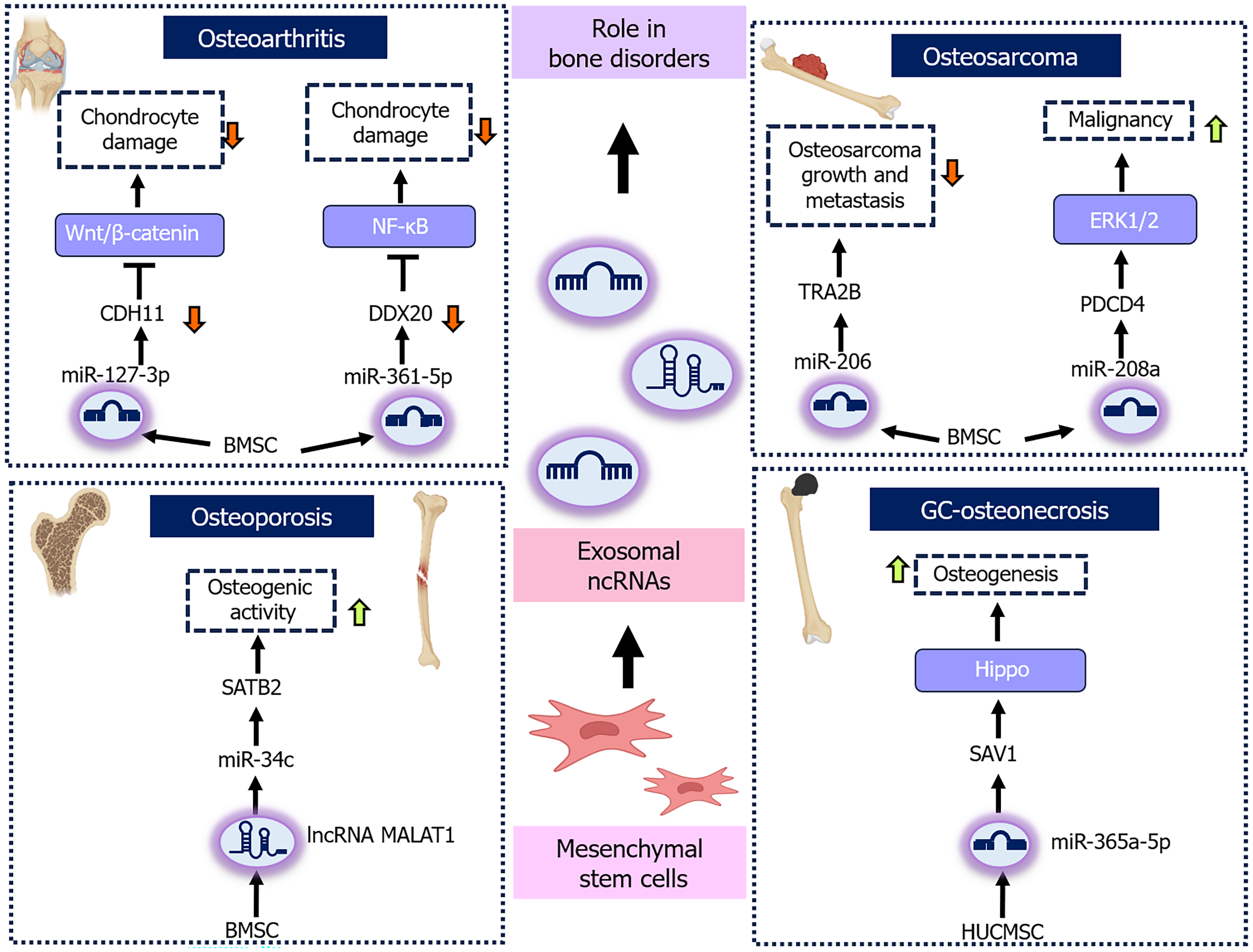
The role of MSC-derived exosomal ncRNAs in modulating bone disorder pathways and molecular targets presents a promising avenue for mitigating disease progression and facilitating the development of more effective treatment strategies. In OA, BMSC-derived exosomal miR-127-3p has been shown to target and downregulate cadherin-11, thereby inhibiting Wnt/β-catenin signaling pathway activation, consequently preventing chondrocyte damage[54]. Similarly, BMSC-derived exosomal miR-361-5p targets Asp-Glu-Ala-Asp-box polypeptide 20, downregulates its expression, and suppresses the nuclear factor kappa B (NF-κB) pathway, thereby mitigating chondrocyte damage and eventually OA[55]. Moreover, human umbilical cord MSC-derived exosomal miR-199a-3p reduces interleukin 1 beta-induced inflammation and apoptosis in chondrocytes by inhibiting the MAPK4/NF-κB signaling pathway, thus slowing OA progression[56]. Additionally, human MSC-derived exosomal lncRNA KLF3-antisense RNA 1 sponges miR-206 to upregulate G-protein-coupled receptor kinase interacting protein-1 (GIT1), thereby promoting chondrocyte proliferation and inhibiting apoptosis, whereas miR-206 overexpression or GIT1 knockdown reverses these effects, highlighting the significance of the lncRNA KLF3- antisense RNA 1/miR-206/GIT1 axis as a key regulator in OA[57]. Similarly, in traumatic OA, BMSC-derived exosomal miR-125a-5p targets E2F transcription factor 2, consequently promoting chondrocyte migration and inhibiting cartilage degeneration[58]. In temporomandibular joint OA, strontium pretreatment of synovial MSCs upregulates apoptosis-linked gene 2-interacting protein X, thereby selectively enhancing the incorporation of therapeutic miRNA into exosomes, including the critical exosomal miR-143-3p, which directly targets major facilitator superfamily domain containing 8 to alleviate chondrocyte ferroptosis and diminish osteoclast-mediated joint pain[59]. In OS, BMSC-derived exosomal miR-206 targets transformer 2β, thereby regulating OS cell proliferation, apoptosis, migration, and invasion, and subsequently suppressing both OS growth and metastasis in vivo[60]. In contrast, BMSC-derived exosomal miR-208a can enhance OS cell malignancy by targeting programmed cell death protein 4 via the extracellular signal-related kinases 1/2 signaling pathway[61]. Additionally, BMSC-derived exosomal lncRNA XIST promotes OS growth and metastasis by binding to miR-655 and downregulating its expression, leading to ATP citrate lyase upregulation, increased lipid accumulation, and β-catenin signaling activation[62]. In OP, BMSC-derived exosomal lncRNA metastasis-associated lung adenocarcinoma transcript-1 sponges miR-34c and upregulates special AT-rich sequence-binding protein 2 expression, thereby enhancing osteogenic activity and mitigating OP symptoms in vivo[63]. Similarly, in osteogenesis-induced human umbilical cord, MSC-derived exosomal miR-328-3p and miR-2110 regulate the MAPK signaling pathway, targeting chordin and tumor necrosis factor-alpha, respectively, regulating osteoclast differentiation, enhancing osteogenesis, and mitigating OP[64]. In glucocorticoid-induced osteonecrosis of the femoral head, exosomal miR-365a-5p derived from human umbilical cord MSCs inhibits salvador homolog 1, which further activates the Hippo signaling pathway, promoting osteogenesis and preventing disease progression[65]. Similarly, in alcohol-induced osteonecrosis of the femoral head, human umbilical cord MSC-derived exosomal miR-25-3p could mitigate this disease by facilitating osteoblast differentiation and inhibiting apoptosis in both BMSCs and in vivo models by suppressing DNA methylation of the miR-25-3p promoter and reduction of gremlin 1 expression[66]. Collectively, these findings underscore the potential of MSC-derived exosomal ncRNAs as an innovative therapeutic strategy for modulating key molecular pathways and targets, offering promising approaches for treating various bone-related disorders (Figure 2).
Preclinical studies have substantiated the therapeutic efficacy of MSC-derived exosomes in various bone disorders, such as OA and OP. In OA models, exosomes derived from the infrapatellar fat pad, synovial membrane, or chondrogenically induced MSCs have been shown to have anti-inflammatory effects, inhibit cartilage degradation, and stimulate matrix synthesis[67-69]. These effects are largely mediated by exosomal ncRNAs, including miR-100-5p, miR-140-5p, and regulatory lncRNAs that target critical pathways like mammalian target of rapamycin, Wnt/β-catenin, and G-protein coupled receptor kinase interacting protein[70]. Although preclinical animal models have demonstrated the therapeutic potential of MSC-derived exosomes, variability in exosome source, dosage, and administration routes contribute to inconsistent and non-reproducible outcomes. Moreover, the limited ability of these models to recapitulate the complexity of human pathophysiology presents significant challenges for clinical translation[71].
Clinical trials investigating exosomal ncRNAs are limited, with an even smaller number focused specifically on MSC-derived exosomes. This constraint underscores the need for additional investigations into their potential in diverse therapeutic contexts, particularly in bone regeneration. Based on data obtained from ClinicalTrials.gov, no registered study to date has directly evaluated the therapeutic role of ncRNAs within MSC-derived exosomes. A related study assessed MSC-derived exosomes in patients with OA and discussed the presence of bioactive molecules, including ncRNAs, within the exosomal cargo[72]. However, it does not clarify which bioactive molecules drive the therapeutic effect or which specific ncRNAs are involved. While some trials have focused on exosomal ncRNAs, one completed trial specifically investigated dysregulated miRNAs present in the circulating exosomes of patients with bone metastases and identified biomarkers to predict bone metastasis risk[73]. Another trial focused on profiling exosomal RNA as a biomarker of lung metastasis in patients with primary high-grade OS[74]. Moreover, a phase 1 trial evaluated the safety of allogeneic MSC-derived exosomes administered via intra-articular injection in the knees of individuals with mild to moderate symptomatic OA[75]. Nevertheless, data regarding patient-related factors, costs, and legal regulations remain unclear, which is a significant limitation.
The clinical implementation of MSC-derived exosomal ncRNAs requires robust preclinical and clinical trials to evaluate their safety, efficacy, dosing strategies, and delivery methods. However, the clinical translation of MSC-derived exosomal ncRNAs faces several challenges. Scalability remains a major concern, as producing exosomes in appropriate quantities while maintaining consistent quality poses significant technical challenges. Additionally, the heterogeneity of MSC-derived exosomes from different tissue sources remains a critical gap in advancing this therapeutic approach, underscoring the need for standardized production protocols[76,77]. Additional barriers include inefficient ncRNA loading, content variability, and the presence of immunogenic components such as major histocompatibility complex molecules. Furthermore, the limited understanding of receptor specificity, biodistribution, pharmacokinetics, and off-target effects complicates delivery strategies, emphasizing the need for optimized dosing and repeated administration protocols to achieve sustained therapeutic effects[78]. Several signaling pathways, including Wnt/β-catenin, Notch, MAPK, Janus kinase/signal transducer and activator of transcription, and NF-κB, are critically involved in bone-related disorders[79]. However, regulation by MSC-derived exosomal ncRNAs remains poorly understood and warrants further investigation. Numerous studies have suggested that exosomal ncRNAs could serve as potential biomarkers for various diseases. A study by Zhi et al[80] suggests that exosomal hsa_circ_0006859 is a circulating biomarker of postmenopausal OP. Similarly, the synovial fluid-derived exosomal lncRNA prostate cancer gene expression marker 1 could be a biomarker for differentiating early from later stages of OA[81]. Nevertheless, further research is necessary to identify specific ncRNAs with reliable diagnostic potential that can be utilized as noninvasive biomarkers for bone-related disorders.
Several strategies are currently being developed to address translational barriers associated with exosome-based therapy. For instance, local exosome administration is generally preferred over systemic delivery to ensure higher exosome concentration at the target injury site and reduce off-target effects[82,83]. Likewise, exosomes can be engineered to carry therapeutic molecules, such as small interfering RNAs or peptides, via electroporation or membrane modification, which is another approach aimed at improving targeting and therapeutic efficacy[84].
Furthermore, exosome isolation involves techniques such as size-exclusion chromatography, ultrafiltration, and immunoaffinity. Although size-exclusion chromatography and ultrafiltration are efficient, they diminish exosome purity and yield. In contrast, immunoaffinity ensures high specificity and depends on certain surface markers. A combined approach can help mitigate these limitations[70]. Although different strategies have been investigated, the precise mechanisms by which exosomal ncRNAs function are still not fully understood.
In bone-related applications, in addition to the delivery strategies mentioned, other approaches include the incorporation of exosomes into biomaterial scaffolds - such as hydrogels, collagen, poly(lactic-co-glycolic acid), and tricalcium phosphate - which can facilitate sustained release and improve scaffold osteoconductivity[85,86]. Additionally, the osteogenic potential of exosomes can be enhanced through genetic modification of parental MSCs (e.g., overexpression of osteogenic miRNAs such as miR-375), environmental priming (e.g., hypoxia or tumor necrosis factor alpha exposure), and mechanical stimuli (e.g., low-density pulsed ultrasound)[87-89]. Through multidisciplinary research and technological innovations, MSC-derived exosomal ncRNAs may become a next-generation, cell-free therapeutic platform for bone repair and regeneration.
In conclusion, MSC-derived exosomal ncRNAs have emerged as a promising cell-free therapeutic approach that plays a key role in maintaining bone homeostasis and slowing the progression of bone-related diseases by targeting critical molecular pathways and signaling networks. In addition to their therapeutic potential, these ncRNAs hold significant promise as biomarkers for early detection of bone diseases. Nevertheless, its clinical value should be validated in patient populations. However, this study has several limitations and areas that require further investigation. First, most studies on MSC-derived exosomal ncRNAs have focused on miRNAs, with limited research on lncRNAs, including linear lncRNAs and circRNAs. Second, the networks based on competing endogenous RNAs remain largely unexplored, which warrants further research. Third, preclinical validation should extend beyond rodent models to complex large animal systems. Fourth, well-designed clinical trials with larger patient cohorts should be conducted. Although several strategies and challenges are discussed in the clinical implications section, it is imperative to attain a more profound understanding of the regulatory mechanisms of MSC-derived exosomal ncRNAs, develop standardized protocols, and conduct thorough preclinical and clinical validation.
| 1. | Florencio-Silva R, Sasso GR, Sasso-Cerri E, Simões MJ, Cerri PS. Biology of Bone Tissue: Structure, Function, and Factors That Influence Bone Cells. Biomed Res Int. 2015;2015:421746. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 769] [Cited by in RCA: 1151] [Article Influence: 104.6] [Reference Citation Analysis (0)] |
| 2. | Camacho NP, Carroll P, Raggio CL. Fourier transform infrared imaging spectroscopy (FT-IRIS) of mineralization in bisphosphonate-treated oim/oim mice. Calcif Tissue Int. 2003;72:604-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Marie PJ. Osteoblast dysfunctions in bone diseases: from cellular and molecular mechanisms to therapeutic strategies. Cell Mol Life Sci. 2015;72:1347-1361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 4. | Ding DC, Shyu WC, Lin SZ. Mesenchymal stem cells. Cell Transplant. 2011;20:5-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 621] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 5. | Pham PV. MSCs, but not mesenchymal stem cells. Biomed Res Ther. 2024;11:6797-6800. [DOI] [Full Text] |
| 6. | Fodor WL. Tissue engineering and cell based therapies, from the bench to the clinic: the potential to replace, repair and regenerate. Reprod Biol Endocrinol. 2003;1:102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 87] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 7. | Huerta CT, Ortiz YY, Liu ZJ, Velazquez OC. Methods and Limitations of Augmenting Mesenchymal Stem Cells for Therapeutic Applications. Adv Wound Care (New Rochelle). 2023;12:467-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 8. | Plaisance C, Chow L, Impastato R, Williams ZJ, Sabino I, Sikes KJ, Santangelo KS, Dow S, Pezzanite LM. Innate immune pathway activated mesenchymal stromal cells improve function and histologic outcomes in a rodent osteoarthritis model. Front Bioeng Biotechnol. 2025;13:1525969. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Lee BW, Lee JJ, Jung JY, Ju JH. Intra-Articular Injection of Human Bone Marrow-Derived Mesenchymal Stem Cells in Knee Osteoarthritis: A Randomized, Double-Blind, Controlled Trial. Cell Transplant. 2025;34:9636897241303275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 10. | Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367:eaau6977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6920] [Cited by in RCA: 7556] [Article Influence: 1259.3] [Reference Citation Analysis (4)] |
| 11. | Ansari FJ, Tafti HA, Amanzadeh A, Rabbani S, Shokrgozar MA, Heidari R, Behroozi J, Eyni H, Uversky VN, Ghanbari H. Comparison of the efficiency of ultrafiltration, precipitation, and ultracentrifugation methods for exosome isolation. Biochem Biophys Rep. 2024;38:101668. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 48] [Reference Citation Analysis (0)] |
| 12. | Yang Q, Li S, Ou H, Zhang Y, Zhu G, Li S, Lei L. Exosome-based delivery strategies for tumor therapy: an update on modification, loading, and clinical application. J Nanobiotechnology. 2024;22:41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 91] [Article Influence: 45.5] [Reference Citation Analysis (0)] |
| 13. | Zhang Z, Shi C, Wang Z. The physiological functions and therapeutic potential of exosomes during the development and treatment of polycystic ovary syndrome. Front Physiol. 2023;14:1279469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Gurunathan S, Kang MH, Jeyaraj M, Qasim M, Kim JH. Review of the Isolation, Characterization, Biological Function, and Multifarious Therapeutic Approaches of Exosomes. Cells. 2019;8:307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 843] [Cited by in RCA: 848] [Article Influence: 121.1] [Reference Citation Analysis (0)] |
| 15. | An W, Zhang W, Qi J, Xu W, Long Y, Qin H, Yao K. Mesenchymal stem cells and mesenchymal stem cell-derived exosomes: a promising strategy for treating retinal degenerative diseases. Mol Med. 2025;31:75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 16. | Ren YZ, Ding SS, Jiang YP, Wen H, Li T. Application of exosome-derived noncoding RNAs in bone regeneration: Opportunities and challenges. World J Stem Cells. 2022;14:473-489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (2)] |
| 17. | Li H, Zheng Q, Xie X, Wang J, Zhu H, Hu H, He H, Lu Q. Role of Exosomal Non-Coding RNAs in Bone-Related Diseases. Front Cell Dev Biol. 2021;9:811666. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Hassan MQ, Tye CE, Stein GS, Lian JB. Non-coding RNAs: Epigenetic regulators of bone development and homeostasis. Bone. 2015;81:746-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 83] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 19. | Yin B, Ma Q, Song C, Zhao L, Yu F, Wang C, Shi Y, Ye L. Exosome-Derived Noncoding RNAs as a Promising Treatment of Bone Regeneration. Stem Cells Int. 2021;2021:6696894. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 20. | Das CK, Jena BC, Banerjee I, Das S, Parekh A, Bhutia SK, Mandal M. Exosome as a Novel Shuttle for Delivery of Therapeutics across Biological Barriers. Mol Pharm. 2019;16:24-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 189] [Article Influence: 23.6] [Reference Citation Analysis (1)] |
| 21. | Huang X, Wu W, Jing D, Yang L, Guo H, Wang L, Zhang W, Pu F, Shao Z. Engineered exosome as targeted lncRNA MEG3 delivery vehicles for osteosarcoma therapy. J Control Release. 2022;343:107-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 129] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 22. | Kuang H, Ma J, Chi X, Fu Q, Zhu Q, Cao W, Zhang P, Xie X. Integrated Osteoinductive Factors─Exosome@MicroRNA-26a Hydrogel Enhances Bone Regeneration. ACS Appl Mater Interfaces. 2023;15:22805-22816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 37] [Reference Citation Analysis (0)] |
| 23. | Zhang Y, Xie Y, Hao Z, Zhou P, Wang P, Fang S, Li L, Xu S, Xia Y. Umbilical Mesenchymal Stem Cell-Derived Exosome-Encapsulated Hydrogels Accelerate Bone Repair by Enhancing Angiogenesis. ACS Appl Mater Interfaces. 2021;13:18472-18487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 188] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 24. | Mohamed AM. An overview of bone cells and their regulating factors of differentiation. Malays J Med Sci. 2008;15:4-12. [PubMed] |
| 25. | Luo D, Xie W, He X, Zhou X, Ye P, Wang P. Exosomal miR-590-3p derived from bone marrow mesenchymal stem cells promotes osteoblast differentiation and osteogenesis by targeting TGFBR1. In Vitro Cell Dev Biol Anim. 2025;61:46-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 26. | Ma S, Li S, Zhang Y, Nie J, Cao J, Li A, Li Y, Pei D. BMSC-Derived Exosomal CircHIPK3 Promotes Osteogenic Differentiation of MC3T3-E1 Cells via Mitophagy. Int J Mol Sci. 2023;24:2785. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 27. | Huang Y, Zhang X, Zhan J, Yan Z, Chen D, Xue X, Pan X. Bone marrow mesenchymal stem cell-derived exosomal miR-206 promotes osteoblast proliferation and differentiation in osteoarthritis by reducing Elf3. J Cell Mol Med. 2021;25:7734-7745. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 28. | Wu D, Chang X, Tian J, Kang L, Wu Y, Liu J, Wu X, Huang Y, Gao B, Wang H, Qiu G, Wu Z. Bone mesenchymal stem cells stimulation by magnetic nanoparticles and a static magnetic field: release of exosomal miR-1260a improves osteogenesis and angiogenesis. J Nanobiotechnology. 2021;19:209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 169] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 29. | Zhai M, Zhu Y, Yang M, Mao C. Human Mesenchymal Stem Cell Derived Exosomes Enhance Cell-Free Bone Regeneration by Altering Their miRNAs Profiles. Adv Sci (Weinh). 2020;7:2001334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 189] [Cited by in RCA: 186] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 30. | Qiu M, Zhai S, Fu Q, Liu D. Bone Marrow Mesenchymal Stem Cells-Derived Exosomal MicroRNA-150-3p Promotes Osteoblast Proliferation and Differentiation in Osteoporosis. Hum Gene Ther. 2021;32:717-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 79] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 31. | You M, Ai Z, Zeng J, Fu Y, Zhang L, Wu X. Bone mesenchymal stem cells (BMSCs)-derived exosomal microRNA-21-5p regulates Kruppel-like factor 3 (KLF3) to promote osteoblast proliferation in vitro. Bioengineered. 2022;13:11933-11944. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 32. | Behera J, Kumar A, Voor MJ, Tyagi N. Exosomal lncRNA-H19 promotes osteogenesis and angiogenesis through mediating Angpt1/Tie2-NO signaling in CBS-heterozygous mice. Theranostics. 2021;11:7715-7734. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 115] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 33. | Lai G, Zhao R, Zhuang W, Hou Z, Yang Z, He P, Wu J, Sang H. BMSC-derived exosomal miR-27a-3p and miR-196b-5p regulate bone remodeling in ovariectomized rats. PeerJ. 2022;10:e13744. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 34. | Feng W, Jin Q, Ming-Yu Y, Yang H, Xu T, You-Xing S, Xu-Ting B, Wan C, Yun-Jiao W, Huan W, Ai-Ning Y, Yan L, Hong T, Pan H, Mi-Duo M, Gang H, Mei Z, Xia K, Kang-Lai T. MiR-6924-5p-rich exosomes derived from genetically modified Scleraxis-overexpressing PDGFRα(+) BMMSCs as novel nanotherapeutics for treating osteolysis during tendon-bone healing and improving healing strength. Biomaterials. 2021;279:121242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 35. | Xu T, Luo Y, Wang J, Zhang N, Gu C, Li L, Qian D, Cai W, Fan J, Yin G. Exosomal miRNA-128-3p from mesenchymal stem cells of aged rats regulates osteogenesis and bone fracture healing by targeting Smad5. J Nanobiotechnology. 2020;18:47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 103] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 36. | Wu F, Song C, Zhen G, Jin Q, Li W, Liang X, Xu W, Guo W, Yang Y, Dong W, Jiang A, Kong P, Yan J. Exosomes derived from BMSCs in osteogenic differentiation promote type H blood vessel angiogenesis through miR-150-5p mediated metabolic reprogramming of endothelial cells. Cell Mol Life Sci. 2024;81:344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 37. | Lou C, Jiang H, Lin Z, Xia T, Wang W, Lin C, Zhang Z, Fu H, Iqbal S, Liu H, Lin J, Wang J, Pan X, Xue X. MiR-146b-5p enriched bioinspired exosomes derived from fucoidan-directed induction mesenchymal stem cells protect chondrocytes in osteoarthritis by targeting TRAF6. J Nanobiotechnology. 2023;21:486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 38. | He Q, Zhuang Q, Deng Z, Wang H, Yue J, Jiang L, You W, Sun W, Xie W. Inhibiting angiogenesis and osteogenesis by targeted regulation of KLK4 via the exosomal MiR-422a derived from bone mesenchymal stem cells. Biotechnol Bioproc E. 2025;30:262-274. [DOI] [Full Text] |
| 39. | Qiu M, Xie Y, Tan G, Wang X, Huang P, Hong L. Synovial mesenchymal stem cell-derived exosomal miR-485-3p relieves cartilage damage in osteoarthritis by targeting the NRP1-mediated PI3K/Akt pathway: Exosomal miR-485-3p relieves cartilage damage. Heliyon. 2024;10:e24042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 18] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 40. | Yao Q, Wu X, Tao C, Gong W, Chen M, Qu M, Zhong Y, He T, Chen S, Xiao G. Osteoarthritis: pathogenic signaling pathways and therapeutic targets. Signal Transduct Target Ther. 2023;8:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 790] [Cited by in RCA: 736] [Article Influence: 245.3] [Reference Citation Analysis (0)] |
| 41. | Amarnath SS, Kumar V, Das SL. Classification of Osteoporosis. Indian J Orthop. 2023;57:49-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 42. | Lin YH, Jewell BE, Gingold J, Lu L, Zhao R, Wang LL, Lee DF. Osteosarcoma: Molecular Pathogenesis and iPSC Modeling. Trends Mol Med. 2017;23:737-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 123] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 43. | Zhao W, Qin P, Zhang D, Cui X, Gao J, Yu Z, Chai Y, Wang J, Li J. Long non-coding RNA PVT1 encapsulated in bone marrow mesenchymal stem cell-derived exosomes promotes osteosarcoma growth and metastasis by stabilizing ERG and sponging miR-183-5p. Aging (Albany NY). 2019;11:9581-9596. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 100] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 44. | Shen X, Qin J, Wei Z, Liu F. Bone marrow mesenchymal stem cell exosome-derived lncRNA TUC339 influences the progression of osteoarthritis by regulating synovial macrophage polarization and chondrocyte apoptosis. Biomed Pharmacother. 2023;167:115488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 34] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 45. | Liu Y, Zou R, Wang Z, Wen C, Zhang F, Lin F. Exosomal KLF3-AS1 from hMSCs promoted cartilage repair and chondrocyte proliferation in osteoarthritis. Biochem J. 2018;475:3629-3638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 143] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 46. | Jin Z, Ren J, Qi S. Exosomal miR-9-5p secreted by bone marrow-derived mesenchymal stem cells alleviates osteoarthritis by inhibiting syndecan-1. Cell Tissue Res. 2020;381:99-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 95] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 47. | Wang R, Xu B. TGF-β1-modified MSC-derived exosomal miR-135b attenuates cartilage injury via promoting M2 synovial macrophage polarization by targeting MAPK6. Cell Tissue Res. 2021;384:113-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 94] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 48. | Li F, Xu Z, Xie Z, Sun X, Li C, Chen Y, Xu J, Pi G. Adipose mesenchymal stem cells-derived exosomes alleviate osteoarthritis by transporting microRNA -376c-3p and targeting the WNT-beta-catenin signaling axis. Apoptosis. 2023;28:362-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 47] [Reference Citation Analysis (0)] |
| 49. | Kong R, Zhang J, Ji L, Yu Y, Gao J, Zhao D. Synovial mesenchymal stem cell-derived exosomal microRNA-320c facilitates cartilage damage repair by targeting ADAM19-dependent Wnt signalling in osteoarthritis rats. Inflammopharmacology. 2023;31:915-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 50. | Mao G, Xu Y, Long D, Sun H, Li H, Xin R, Zhang Z, Li Z, Yang Z, Kang Y. Exosome-transported circRNA_0001236 enhances chondrogenesis and suppress cartilage degradation via the miR-3677-3p/Sox9 axis. Stem Cell Res Ther. 2021;12:389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 90] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 51. | Yang Q, Yao Y, Zhao D, Zou H, Lai C, Xiang G, Wang G, Luo L, Shi Y, Li Y, Yang M, Huang X. LncRNA H19 secreted by umbilical cord blood mesenchymal stem cells through microRNA-29a-3p/FOS axis for central sensitization of pain in advanced osteoarthritis. Am J Transl Res. 2021;13:1245-1256. [PubMed] |
| 52. | Li L, Zhou X, Zhang JT, Liu AF, Zhang C, Han JC, Zhang XQ, Wu S, Zhang XY, Lv FQ. Exosomal miR-186 derived from BMSCs promote osteogenesis through hippo signaling pathway in postmenopausal osteoporosis. J Orthop Surg Res. 2021;16:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 53. | Fu M, Fang L, Xiang X, Fan X, Wu J, Wang J. Microarray analysis of circRNAs sequencing profile in exosomes derived from bone marrow mesenchymal stem cells in postmenopausal osteoporosis patients. J Clin Lab Anal. 2022;36:e23916. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 54. | Dong J, Li L, Fang X, Zang M. Exosome-Encapsulated microRNA-127-3p Released from Bone Marrow-Derived Mesenchymal Stem Cells Alleviates Osteoarthritis Through Regulating CDH11-Mediated Wnt/β-Catenin Pathway. J Pain Res. 2021;14:297-310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 55. | Tao Y, Zhou J, Wang Z, Tao H, Bai J, Ge G, Li W, Zhang W, Hao Y, Yang X, Geng D. Human bone mesenchymal stem cells-derived exosomal miRNA-361-5p alleviates osteoarthritis by downregulating DDX20 and inactivating the NF-κB signaling pathway. Bioorg Chem. 2021;113:104978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 56. | Chen LQ, Ma S, Yu J, Zuo DC, Yin ZJ, Li FY, He X, Peng HT, Shi XQ, Huang WJ, Li Q, Wang J. Human umbilical cord mesenchymal stem cell-derived exosomal miR-199a-3p inhibits the MAPK4/NF-κB signaling pathway to relieve osteoarthritis. World J Stem Cells. 2025;17:103919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 57. | Liu Y, Lin L, Zou R, Wen C, Wang Z, Lin F. MSC-derived exosomes promote proliferation and inhibit apoptosis of chondrocytes via lncRNA-KLF3-AS1/miR-206/GIT1 axis in osteoarthritis. Cell Cycle. 2018;17:2411-2422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 272] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 58. | Xia Q, Wang Q, Lin F, Wang J. miR-125a-5p-abundant exosomes derived from mesenchymal stem cells suppress chondrocyte degeneration via targeting E2F2 in traumatic osteoarthritis. Bioengineered. 2021;12:11225-11238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 59. | Yuan W, Liu J, Zhang Z, Ye C, Zhou X, Yi Y, Wu Y, Li Y, Zhang Q, Xiong X, Xiao H, Liu J, Wang J. Strontium-Alix interaction enhances exosomal miRNA selectively loading in synovial MSCs for temporomandibular joint osteoarthritis treatment. Int J Oral Sci. 2025;17:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 60. | Zhang H, Wang J, Ren T, Huang Y, Liang X, Yu Y, Wang W, Niu J, Guo W. Bone marrow mesenchymal stem cell-derived exosomal miR-206 inhibits osteosarcoma progression by targeting TRA2B. Cancer Lett. 2020;490:54-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 105] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 61. | Qin F, Tang H, Zhang Y, Zhang Z, Huang P, Zhu J. Bone marrow-derived mesenchymal stem cell-derived exosomal microRNA-208a promotes osteosarcoma cell proliferation, migration, and invasion. J Cell Physiol. 2020;235:4734-4745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 90] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 62. | Zhu G, Xia Y, Zhao Z, Li A, Li H, Xiao T. LncRNA XIST from the bone marrow mesenchymal stem cell derived exosome promotes osteosarcoma growth and metastasis through miR-655/ACLY signal. Cancer Cell Int. 2022;22:330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 63. | Yang X, Yang J, Lei P, Wen T. LncRNA MALAT1 shuttled by bone marrow-derived mesenchymal stem cells-secreted exosomes alleviates osteoporosis through mediating microRNA-34c/SATB2 axis. Aging (Albany NY). 2019;11:8777-8791. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 230] [Cited by in RCA: 233] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 64. | Yahao G, Xinjia W. The Role and Mechanism of Exosomes from Umbilical Cord Mesenchymal Stem Cells in Inducing Osteogenesis and Preventing Osteoporosis. Cell Transplant. 2021;30:9636897211057465. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 65. | Kuang MJ, Zhang KH, Qiu J, Wang AB, Che WW, Li XM, Shi DL, Wang DC. Exosomal miR-365a-5p derived from HUC-MSCs regulates osteogenesis in GIONFH through the Hippo signaling pathway. Mol Ther Nucleic Acids. 2021;23:565-576. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 66. | Tang Z, Xu X, Shi W, Ren X, Luo H, Xu Y, Li C. Huc-MSC-derived exosomes alleviates alcohol-induced osteonecrosis of the femoral head through targeting the miR-25-3p/GREM1 axis. Genomics. 2025;117:110996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 67. | Zhu Y, Wang Y, Zhao B, Niu X, Hu B, Li Q, Zhang J, Ding J, Chen Y, Wang Y. Comparison of exosomes secreted by induced pluripotent stem cell-derived mesenchymal stem cells and synovial membrane-derived mesenchymal stem cells for the treatment of osteoarthritis. Stem Cell Res Ther. 2017;8:64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 202] [Cited by in RCA: 337] [Article Influence: 37.4] [Reference Citation Analysis (1)] |
| 68. | Wu J, Kuang L, Chen C, Yang J, Zeng WN, Li T, Chen H, Huang S, Fu Z, Li J, Liu R, Ni Z, Chen L, Yang L. miR-100-5p-abundant exosomes derived from infrapatellar fat pad MSCs protect articular cartilage and ameliorate gait abnormalities via inhibition of mTOR in osteoarthritis. Biomaterials. 2019;206:87-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 426] [Article Influence: 60.9] [Reference Citation Analysis (0)] |
| 69. | Mao G, Zhang Z, Hu S, Zhang Z, Chang Z, Huang Z, Liao W, Kang Y. Exosomes derived from miR-92a-3p-overexpressing human mesenchymal stem cells enhance chondrogenesis and suppress cartilage degradation via targeting WNT5A. Stem Cell Res Ther. 2018;9:247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 184] [Cited by in RCA: 367] [Article Influence: 45.9] [Reference Citation Analysis (0)] |
| 70. | Tan F, Li X, Wang Z, Li J, Shahzad K, Zheng J. Clinical applications of stem cell-derived exosomes. Signal Transduct Target Ther. 2024;9:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 293] [Cited by in RCA: 400] [Article Influence: 200.0] [Reference Citation Analysis (0)] |
| 71. | Lotfy A, AboQuella NM, Wang H. Mesenchymal stromal/stem cell (MSC)-derived exosomes in clinical trials. Stem Cell Res Ther. 2023;14:66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 290] [Article Influence: 96.7] [Reference Citation Analysis (0)] |
| 72. | Dehghani L. Mesenchymal Stem Cells Derived Exosomes in Osteoarthritis Patients. [accessed 2025 Sep 15]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/study/NCT06466850 ClinicalTrials.gov Identifier: NCT06466850. |
| 73. | Giavaresi G. Identification and Characterization of Predictive Factors of Onset of Bone Metastases in Cancer Patients (PreMetOn). [accessed 2025 Apr 25]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/study/NCT03895216 ClinicalTrials.gov Identifier: NCT03895216. |
| 74. | Shen Y. Circulating Exosome RNA in Lung Metastases of Primary High-Grade Osteosarcoma. [accessed 2025 Apr 25]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/study/NCT03108677 ClinicalTrials.gov Identifier: NCT03108677. |
| 75. | Universidad de los Andes, Chile. Intra-articular Injection of UC-MSC Exosome in Knee Osteoarthritis (EXO-OA01). [accessed 2025 Apr 25]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/study/NCT06431152 ClinicalTrials.gov Identifier: NCT06431152. |
| 76. | Rezaie J, Feghhi M, Etemadi T. A review on exosomes application in clinical trials: perspective, questions, and challenges. Cell Commun Signal. 2022;20:145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 427] [Article Influence: 106.8] [Reference Citation Analysis (0)] |
| 77. | Shi H, Yang Y, Xing H, Jia J, Xiong W, Guo S, Yang S. Exosomal non-coding RNAs: Emerging insights into therapeutic potential and mechanisms in bone healing. J Tissue Eng. 2024;15:20417314241286606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 78. | Wang M, Yu F, Ding H, Wang Y, Li P, Wang K. Emerging Function and Clinical Values of Exosomal MicroRNAs in Cancer. Mol Ther Nucleic Acids. 2019;16:791-804. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 151] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 79. | Wu Z, Li W, Jiang K, Lin Z, Qian C, Wu M, Xia Y, Li N, Zhang H, Xiao H, Bai J, Geng D. Regulation of bone homeostasis: signaling pathways and therapeutic targets. MedComm (2020). 2024;5:e657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 42] [Reference Citation Analysis (0)] |
| 80. | Zhi F, Ding Y, Wang R, Yang Y, Luo K, Hua F. Exosomal hsa_circ_0006859 is a potential biomarker for postmenopausal osteoporosis and enhances adipogenic versus osteogenic differentiation in human bone marrow mesenchymal stem cells by sponging miR-431-5p. Stem Cell Res Ther. 2021;12:157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 78] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 81. | Zhao Y, Xu J. Synovial fluid-derived exosomal lncRNA PCGEM1 as biomarker for the different stages of osteoarthritis. Int Orthop. 2018;42:2865-2872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 104] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 82. | Lai CP, Mardini O, Ericsson M, Prabhakar S, Maguire C, Chen JW, Tannous BA, Breakefield XO. Dynamic biodistribution of extracellular vesicles in vivo using a multimodal imaging reporter. ACS Nano. 2014;8:483-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 544] [Cited by in RCA: 723] [Article Influence: 60.3] [Reference Citation Analysis (0)] |
| 83. | Cataldi M, Vigliotti C, Mosca T, Cammarota M, Capone D. Emerging Role of the Spleen in the Pharmacokinetics of Monoclonal Antibodies, Nanoparticles and Exosomes. Int J Mol Sci. 2017;18:1249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 206] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 84. | Cooper JM, Wiklander PB, Nordin JZ, Al-Shawi R, Wood MJ, Vithlani M, Schapira AH, Simons JP, El-Andaloussi S, Alvarez-Erviti L. Systemic exosomal siRNA delivery reduced alpha-synuclein aggregates in brains of transgenic mice. Mov Disord. 2014;29:1476-1485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 286] [Cited by in RCA: 418] [Article Influence: 34.8] [Reference Citation Analysis (9)] |
| 85. | Narayanan R, Huang CC, Ravindran S. Hijacking the Cellular Mail: Exosome Mediated Differentiation of Mesenchymal Stem Cells. Stem Cells Int. 2016;2016:3808674. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 195] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 86. | Li W, Liu Y, Zhang P, Tang Y, Zhou M, Jiang W, Zhang X, Wu G, Zhou Y. Tissue-Engineered Bone Immobilized with Human Adipose Stem Cells-Derived Exosomes Promotes Bone Regeneration. ACS Appl Mater Interfaces. 2018;10:5240-5254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 337] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 87. | Chen S, Tang Y, Liu Y, Zhang P, Lv L, Zhang X, Jia L, Zhou Y. Exosomes derived from miR-375-overexpressing human adipose mesenchymal stem cells promote bone regeneration. Cell Prolif. 2019;52:e12669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 252] [Cited by in RCA: 252] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 88. | Lu Z, Chen Y, Dunstan C, Roohani-Esfahani S, Zreiqat H. Priming Adipose Stem Cells with Tumor Necrosis Factor-Alpha Preconditioning Potentiates Their Exosome Efficacy for Bone Regeneration. Tissue Eng Part A. 2017;23:1212-1220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 154] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 89. | Marrelli M, Codispoti B, Shelton RM, Scheven BA, Cooper PR, Tatullo M, Paduano F. Dental Pulp Stem Cell Mechanoresponsiveness: Effects of Mechanical Stimuli on Dental Pulp Stem Cell Behavior. Front Physiol. 2018;9:1685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 93] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/