Published online Oct 26, 2025. doi: 10.4252/wjsc.v17.i10.110507
Revised: July 17, 2025
Accepted: September 16, 2025
Published online: October 26, 2025
Processing time: 139 Days and 11.4 Hours
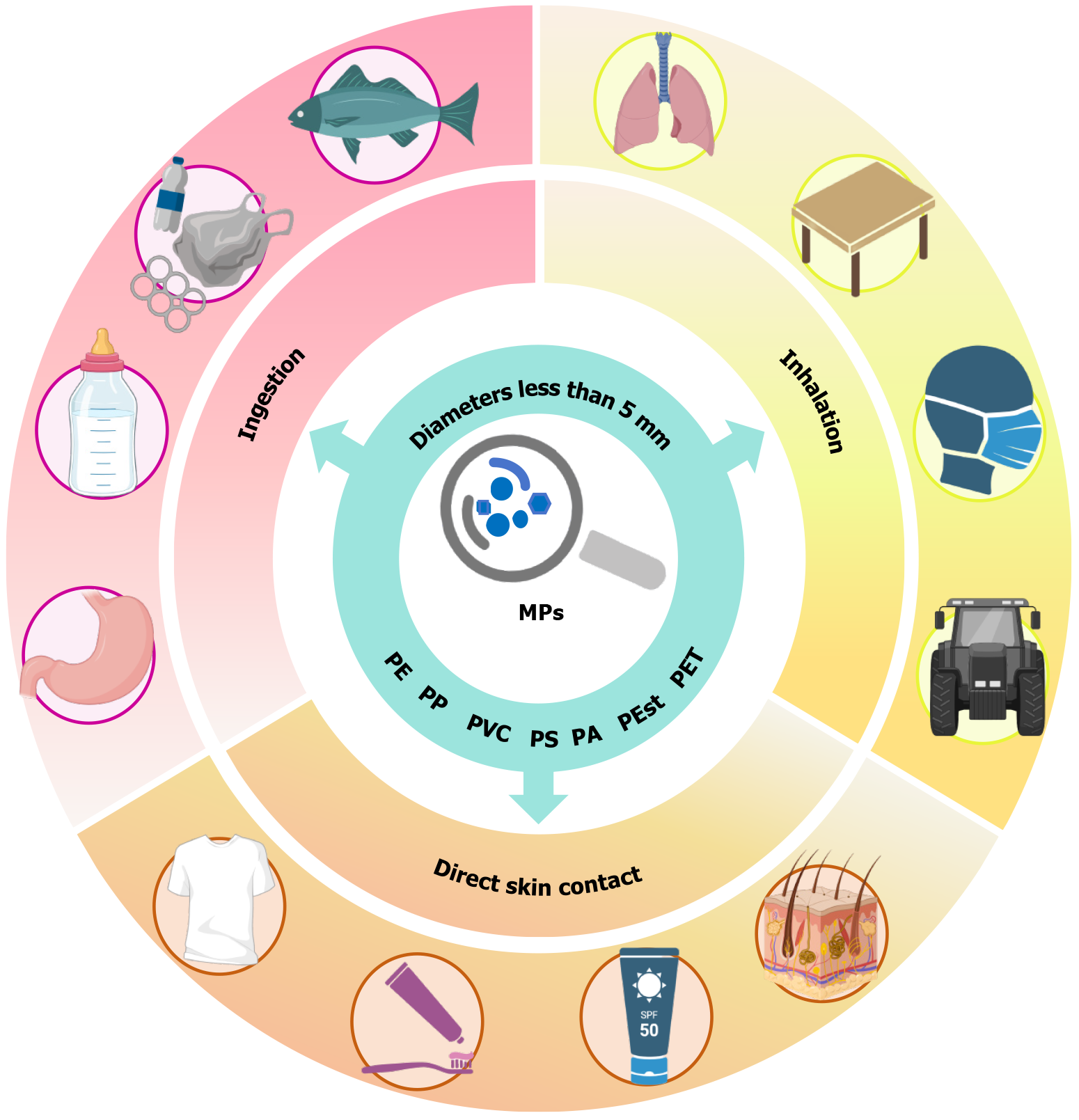
Microplastics (MPs), defined as plastic particles with diameters less than 5 mm, have become significant global environmental contaminants. MPs accumulate in human tissues and organs, raising significant concerns about their potential biological toxicity. Evidence indicates that MPs and associated toxins disrupt stem cell self-renewal, proliferation, and differentiation processes essential for tissue regeneration and systemic homeostasis, yet research on MP-induced stem cell damage remains limited. To identify relevant and recent studies, we searched the PubMed database using title and abstract fields. This review synthesizes current evidence across organ systems, including nervous, hematopoietic, skeletal, and urinary systems, to systematically categorize phenotypic disruptions and un
Core Tip: Microplastics (MPs) have become significant global environmental contaminants. However, the impact of MPs on human health, particularly their effects on stem cells and tissue regeneration, remains poorly understood. This review aims to synthesize existing research on the impact of MPs on stem cell function and tissue regeneration, with a focus on the underlying mechanisms driving these effects. We also discuss the applications and prospects of organoid technology derived from stem cells in the study of MP toxicity.
- Citation: Zheng JH, Li YT, Yang ST, Jia SY, Zheng LW, Wan M. Silent saboteurs: How microplastics disrupt stem cells and tissue regeneration. World J Stem Cells 2025; 17(10): 110507
- URL: https://www.wjgnet.com/1948-0210/full/v17/i10/110507.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v17.i10.110507
The widespread use of plastic products has significantly transformed modern society, providing numerous conveniences. However, the environmental consequences of plastic waste, particularly in the form of microplastics (MPs), have raised serious concerns. MPs, defined as plastic particles less than 5 mm in diameter, have become pervasive pollutants in ecosystems worldwide[1]. These particles are capable of accumulating in various environments, including water[2], soil[3], and air[4], and are increasingly found in organs, human tissues, and bodily fluids, such as liver[5], blood[6], and even placenta[7], highlighting concerns about their potential biological toxicity due to environmental persistence and capacity to carry harmful chemicals[8,9]. While the environmental risks of MPs are well-documented, their specific effects on human health, particularly concerning stem cell function and tissue regeneration, remain poorly understood, despite the critical role of stem cells in repair and homeostasis[10]. Recent evidence suggests MPs may interfere with these vital processes[11], but the precise mechanisms are unclear. This gap in knowledge presents a significant challenge for understanding the extent of the health risks concerning MPs, particularly their potential to disrupt cellular functions critical to tissue regeneration.
Key questions concern the impact of MPs on stem cells. How do MPs interact with stem cells at the molecular and cellular levels, and how do they disrupt stem cell function? What specific biological mechanisms are involved in the damage MPs cause to stem cells, and how do these mechanisms affect cellular processes such as self-renewal, proliferation, and differentiation? What are the broader implications of MP exposure on tissue regeneration, and how might these effects influence long-term health and the development of regenerative medicine? This review aims to synthesize existing research on the impact of MPs on stem cell function and tissue regeneration, with a focus on the underlying mechanisms driving these effects. By exploring how MPs disrupt stem cell behavior through processes such as oxidative stress, mitochondrial dysfunction, and physical damage, the review seeks to clarify the biological risks posed by MPs. In addition, it aims to provide insights into the potential consequences for regenerative medicine and public health, offering a foundation for subsequent research and policy initiatives. The findings presented here are crucial for advancing our understanding of how MPs may compromise cellular functions and hinder tissue repair, ultimately contributing to the formulation of strategies to mitigate the health risks linked to these widespread pollutants.
Our search strategy and information sources are as follows. To identify relevant and recent studies, we searched the PubMed database using title and abstract fields. The following keywords: Microplastics and nanoplastics AND [source OR characteristic OR uptake, OR stem cell* OR progenitor cell* OR pluripotent stem cell* OR mesenchymal stem cell* OR neural stem cell* OR adult stem cell* OR cell differentiat*, OR cell proliferat*]. Only articles published in English were included.
MPs are ubiquitous, and numerous studies have focused on their environmental accumulation. Zhu et al[12] summarized the main environmental sources of MPs into five key categories: Water, soil, atmospheric environment, solid waste, and MP pollution resulting from daily human activities. A study examining the abundance of MPs in marine fish identified nylon nets, fishing gear, and plastic filaments as common sources of MPs in the ocean[13]. Eriksen et al[14] were among the first to report MP pollution in lakes in 2013, and Nava et al[15] assessed the abundance and types of plastic debris in freshwater ecosystems through a standardized cross-national survey. Large-scale sources of MPs in soil include the use of sludge, weathering, decomposition of plastic sheeting in agriculture, and the fragmentation of plastic waste and plastic items in landfills[16]. Sludge and food waste are prominent contributors to solid-waste-related MP pollution[17]. MPs accumulate in the food chain, exposing humans to these particles through dietary intake[18-20], as well as through MPs from everyday products such as feeding bottles[21], plastic food containers[22], and teabags[23]. MPs are now polluting various environmental compartments globally. While the fragmentation of larger plastic items appears to be the dominant source, human activity remains the primary driver of this pollution[1].
MPs are mainly composed of polymers. Therefore, MPs are classified by polymer type as polyethylene (PE), polypropylene, polyvinyl chloride, polystyrene (PS), polyamides, polyesters, and PE terephthalate (PET)[24]. Among these, PE and polypropylene predominate in surveyed polymers, with PET, PS, and polyamides following in abundance[25]. These polymers have different chemical and physical characteristics that determine the differences in the behavior of MPs in the environmental and biological systems.
In the natural environment, MPs are constantly changing in form and size[26,27]. They occur as fragments, foam, films, fibers, and beads[28]. Studies indicate that MP fibers elicit greater toxicity than fragments or beads[29]. The shape of MPs can also affect their impact. MP fibers can stress the respiratory system of aquatic animals, while MP beads are more easily ingested and absorbed[30]. While MPs are typically defined as plastic particles with diameters less than 5 mm, environmental MPs span nanometers to millimeters - a size continuum governing their distribution and toxicity[31].
Plastic production incorporates diverse additives - including heavy metals, bisphenol A, brominated flame retardants, per- and polyfluoroalkyl substances, phthalates, and others in precise formulations[9]. These chemicals can leach or migrate into the surrounding environment, often migrating to the plastic surface. Importantly, most MPs have high chemical stability and adsorption capacity, enabling them to accumulate in the environment and act as carriers for contaminants that are passed along the food chain[24].
MPs occur ubiquitously in human tissues, organs, and biofluids[32], primarily entering via ingestion (digestive system), inhalation (respiratory system), and possible dermal exposure[33] (Figure 1). The human placenta has been identified as a pathway for MP exposure to the fetus[7]. The gastrointestinal tract represents the primary route of entry for MPs, with recent studies identifying leaching from plastic food packaging (notably at elevated temperatures), water bottles, and infant bottles. Infants are believed to be at higher exposure levels than adults[34,35]. Atmospheric MPs pose inhalation risks, with evidence indicating indoor emissions from textiles, carpeting, building materials, and furniture[36,37]. Small sizes of MPs may facilitate skin penetration. Plastic particles smaller than 100 nm have been shown to breach the skin barrier, although further research is needed to confirm this for human skin[38,39].
MPs pervade aquatic ecosystems, bioaccumulating in fish tissues and entering human food chains with associated health implications[40]. Evidence indicates ingested MPs resist excretion, bioaccumulating in organs and tissues upon saturation of excretory capacity[41]. Mohamed Nor et al[18] constructed a probabilistic lifetime exposure assessment model for MPs, finding gut and tissue concentrations in adults were 2-6 times higher than in children, with average intestinal particle counts ranging from 300 to 500 per person. However, there are no definitive studies on the long-term health effects of this accumulation, indicating the need for further research into the toxicity, distribution, and health risks of MPs in the human body. Table 1 summarizes existing research evidence on the distribution of MPs in the human body, such as the brain[42], bone[43], bone marrow[44], blood[6], kidney[42], lung[45], placenta[7,46], breast milk[47], liver[42] and other locations[48-50].
| Location | Size of microplastics | Concentration/abundance of microplastics | Testing method | Ref. |
| Brain | 100-200 nm | 4917 μg/g | Py-GC/MS | [42] |
| Bone | 138.86 ± 105.67 μm | 22.9 ± 15.7 particles/g | Raman spectra | [43] |
| Bone marrow | 20-500 μm | 51.29 μg/g | Py-GC/MS | [44] |
| Blood | ≥ 700 nm | 1.6 μg/mL | Py-GC/MS | [6] |
| Kidney | 1-5 μm | 404 μg/g | Py-GC/MS | [42] |
| Lung | 3 μm | 1.42 ± 1.50 MP/g | μFTIR spectroscopy | [45] |
| Placenta | > 1 μm | 126.8 ± 147.5 μg/g | Py-GC/MS | [7,46] |
| Breast milk | 20-50 μm | 20.2 particles/g | Laser infrared imaging spectrometer | [47] |
| Liver | 1-5 μm | 433 μg/g | Py-GC/MS | [42] |
| Testis | 21.76-286.71 μm | 11.60 ± 15.52 particles/g | Py-GC/MS | [48,49] |
| Colon | 1.1 ± 0.3 mm | 28.1 ± 15.4 particles/g | μFTIR spectroscopy | [50] |
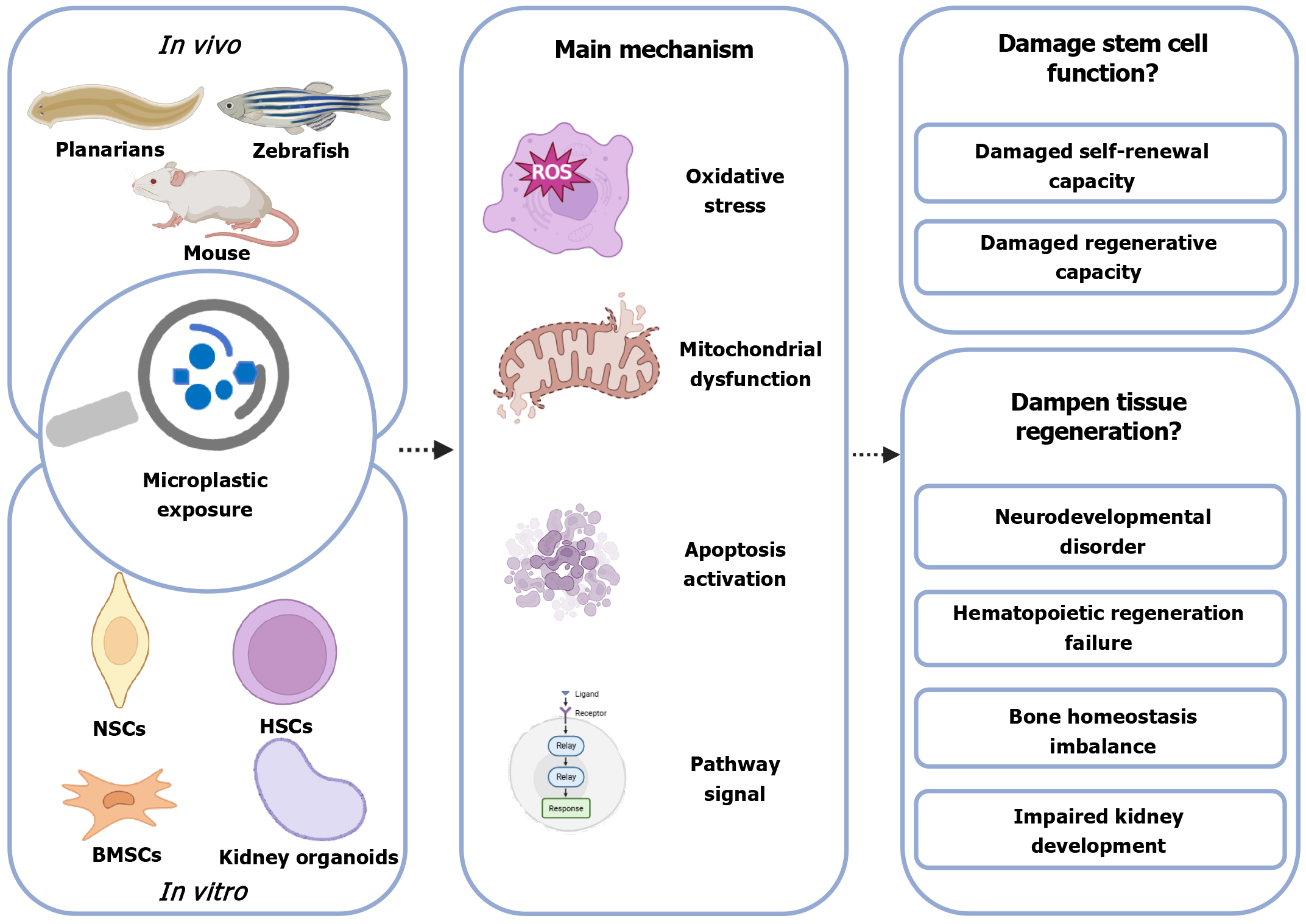
In recent years, MP exposure has been found to adversely affect stem cell function and tissue regeneration (Figure 2). Evidence that MP particles are harmful to stem cells was presented in a study by Gao et al[51] in which they exposed planarians (Dugesia japonica) to an experimental diet in which 100 μg of PS particles were mixed with 1 mg of liver homogenate. After 21 days of exposure, they observed a significant reduction in the growth and germ layer regeneration rates of the planarians compared to the control group, as well as downregulation of the expression of genes related to the regulation of stem cell proliferation and differentiation, including Dj-piwiA and Dj-PCNA. In addition, the enhancement of antioxidant enzyme activities and malondialdehyde confirmed the occurrence of an oxidative stress response. Human cell lines were also used to confirm that MP exposure induced oxidative stress[52]. They selected four types of human cell lines derived from colon (Caco-2), lung (A549 and BEAS-2B) and liver (HepG2) exposed to a single dose of 2 μm PS, which induced oxidative stress and mitochondrial dysfunction after 12 days of exposure, but not for a short period of exposure (2 days). Although animal and cellular studies suggest that MPs pose risks to multiple human organ systems, direct evidence of their health effects in humans remains limited[40,53]. This section will summarize the phenotypes and mechanisms of previous research on MPs on stem cells, categorized by organ system (Figure 3).
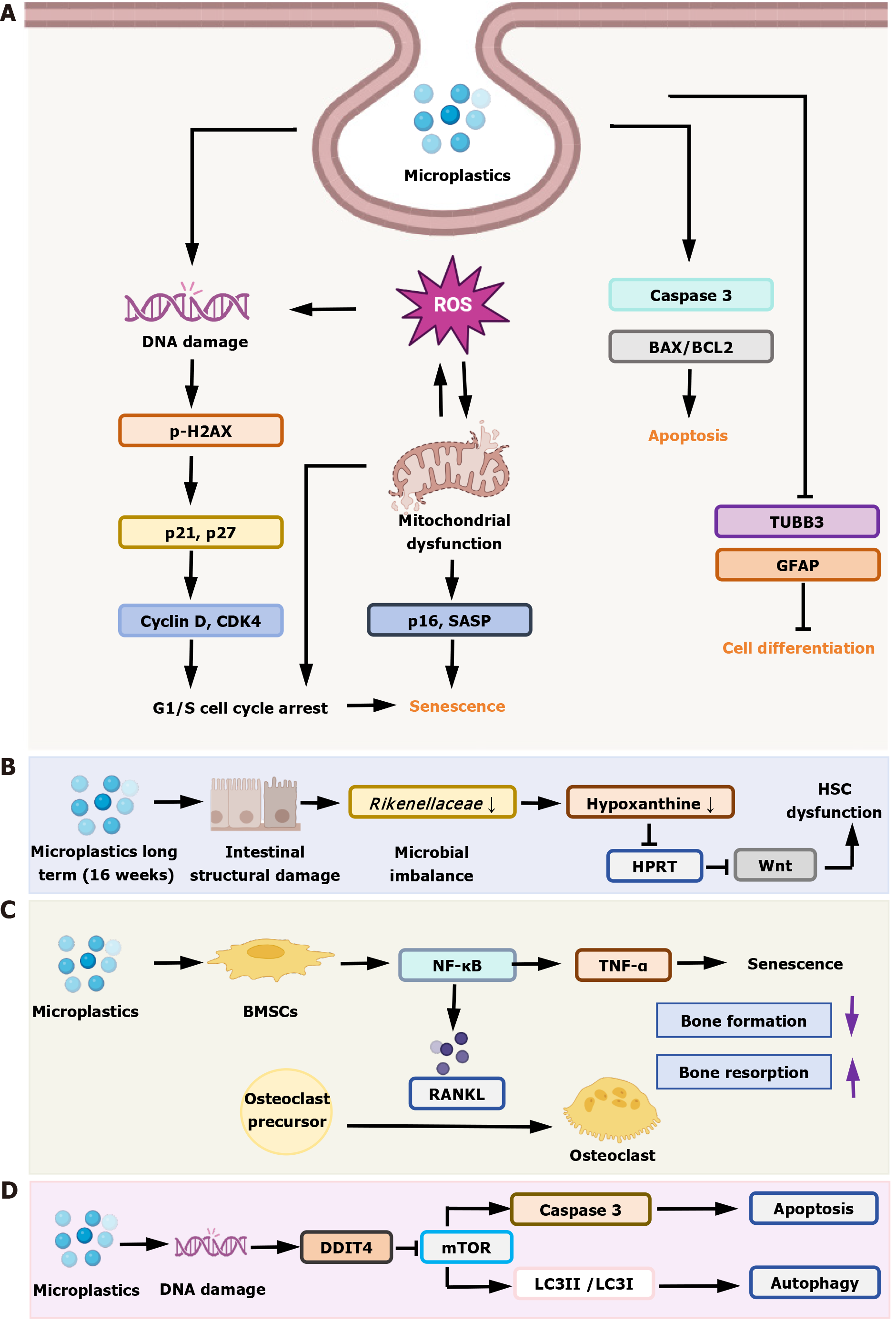
MPs can enter the brain through either the blood-brain barrier or the olfactory route and accumulate in neural cells[54]. Zebrafish embryos exposed to MPs (2 mg/L) and copper (60 and 125 μg/L) for 14 days showed impaired neural stem cell (NSC) function and impaired neural differentiation[55]. This was demonstrated by the downregulation of genes governing neural precursor cell proliferation: SRY-Box transcription factor 2 and proliferating cell nuclear antigen, which inhibited proliferation. Similarly, the downregulation of neuronal differentiation 1 and oligodendrocyte lineage transcription factor 2, genes responsible for motor neuron specialization, impeded terminal differentiation. In addition, the expression of DNA methyltransferases (DNMTs) genes (dnmt1, dnmt3, dnmt5, dnmt6 and dnmt7) was downregulated, suggesting that the expression of neurodevelopment-related genes may be affected by altering DNA methylation patterns. These researchers continued to expose zebrafish adults to MPs (2 mg/L) and copper (25 μg/L) for 30 days[56], and found that expression of the proliferating cell nuclear antigen gene (cell proliferation) was downregulated in the mixed-treatment group, which affected the proliferation of NSCs by inhibiting the cell cycle. At the same time, MPs promoted apoptosis by inhibiting glutathione peroxidase (an antioxidant enzyme with antiapoptotic effects) and activating endogenous and exogenous apoptotic pathways (upregulation of caspase 8, caspase 9, and caspase 3).
MP exposure can adversely affect neurodevelopment in offspring. In an in vivo study in mice[57], exposure of females to PS MPs (500 μg/day) during pregnancy and lactation revealed that MPs could be passed to the offspring via the placenta and breast milk (fluorescent YG signaling), accumulating in developing brain regions. Subsequently, the offspring showed reduced proliferation of hippocampal and cerebellar NSCs (fewer Ki67+ and nestin+ cells) and increased astrocytes (GFAP+ cells), as well as mitochondrial dysfunction, where the MP particles accumulated in mitochondria, leading to disruption of the mitochondrial structure and reduction of ATP production, and affecting energy supply. Another in vivo study exposed rats to PS (2.5 mg/kg/day) during pregnancy and lactation[58], and found that fetal rats had reduced cortical thickness and increased proliferation of cortical cells, but that neural differentiation (glial cells PAX6+, intermediate precursor cells TBR2+) was not affected, and the abnormal increase in superficial neurons may have been associated with deep neuronal apoptosis (upregulation of caspase 3). This regulated apoptosis gene was also mentioned in the zebrafish model above. In another study, neural progenitor cells (NPCs) were exposed to cationic PS nanoplastics (PS-NH3+, 200 μg/mL), and cell cycle arrest, mitochondrial dysfunction, and cellular senescence occurred[59]. The specific changes were that PS-NH3+ caused NPCs to arrest in G1 phase by activating p21 and p27 protein levels (cell cycle inhibitors) and downregulating cyclin D and cyclin dependent kinase 4 (key regulatory proteins in the G1 phase). PS-NH3+ accumulation in mitochondria led to an increase in mitochondrial superoxide, a decrease in membrane potential, inhibition of oxidative phosphorylation, a shift in energy metabolism to glycolysis, and inhibition of cell proliferation. Both together induced senescence of NPCs (upregulation of p16 protein level), reduced NSCs, and inhibited hippocampal neurogenesis and memory function.
In human NSCs (hNS1)[60], 30-nm PS nanoplastics can enter via endocytosis and accumulate in endosomes and vesicles without entering the nuclei. Subsequently, activation of the endogenous apoptotic pathway as well as reduction of cell proliferation were observed via elevated B-cell lymphoma-2 associated X (BAX)/B-cell lymphoma-2 (BCL2) ratio, caspase-3 activation, and downregulation of the proliferation marker Ki-67. Neuronal differentiation was reduced (decreased expression of tubulin beta 3 class III gene) at a high concentration (10 μg/mL), and that the effect of the nanocompound on astrocyte differentiation was not significantly affected. Another study in NSCs of human brain-like organs highlighted mitochondrial dysfunction as a central hub linking MP exposure to abnormal NSC differentiation[61]. MPs can directly target mitochondria, disrupting the structural and functional integrity of mitochondria (morphological abnormalities observed by electron microscopy and dysfunction confirmed by seahorse analysis), leading to an imbalance in reactive oxygen species (ROS) scavenging and disturbances in energy metabolism, and ultimately interfering with the fate of stem cells and inhibiting neuronal maturation. Phenotypic mechanisms and effects of PS MPs on human pluripotent stem-cell-derived cortical spheroids vary by exposure time[62]. Promotion of cell proliferation (increased percentage of Ki67-positive cells), upregulation of neural precursor genes (nestin, paired box 6, and activating transcription factor 4 gene), and upregulation of superoxide dismutase 2 gene expression were seen after short-term exposure (10 days), and no significant toxicity was observed. This suggests that cells respond to short-term MP stimulation by activating antioxidant pathways, counteracting potential toxicity. In contrast, long-term exposure (30 days) resulted in decreased cell viability, downregulation of expression of mature neuronal markers (β-tubulin III and T-box brain transcription factor 1) and cortical layer markers (T-box brain transcription factor 2), and suppression of neural differentiation. In addition to this, leachate released from MPs may exacerbate cell proliferation inhibition and oxidative stress injury by downregulating superoxide dismutase 2 (antioxidant, associated with ROS production) and Ki67 (cell proliferation). MPs (especially at the nanoscale) disrupt neurogenesis by inhibiting neural stem/progenitor cell proliferation, interfering with differentiation, and inducing apoptosis or senescence. Mitochondrial dysfunction and epigenetic regulation are common mechanisms.
Hematopoietic stem cells (HSCs), found in bone marrow and peripheral blood, are multipotent progenitor cells capable of self-renewal that generate all cell types constituting the hematopoietic system[63]. Evidence for the deleterious effects of MP on bone marrow was presented by Sun et al[64] in a study in which mice were given 5-μm fluorescent MPs (0.1 and 0.5 mg/day, ingested orally). The authors observed fluorescent signals in the abdominal and limb bones of the mice, hematotoxicity, downregulation of nicotinamide nucleotide transhydrogenase gene (maintenance of mitochondrial homeostasis) and upregulation of ion channel-related genes (Kcnt2 and Kcns3) in bone marrow cells. Recent studies have found that MPs do not act directly on HSCs, but rather dampen the self-renewal of HSCs by disrupting the gut microbiota-hypoxanthine-Wnt axis[65]. The authors found no difference between the experimental and control groups by co-culturing bone marrow cells with high concentrations of MPs (500 nm, 100 and 250 μg/mL) and by tail vein injection (0.1 μg/100 μL/week, 4 weeks), ruling out a direct effect. The authors found that MPs are enriched in the gastrointestinal tract, disrupting intestinal structure and causing intestinal flora imbalance (significant reduction in the abundance of Rikenellaceae). However, transplanting mouse feces treated with MPs into normal mice reproduced HSC damage, confirming that gut microbiota dysbiosis mediates HSC functional defects. Additionally, RNA sequencing (RNA-seq) of HSCs in the experimental group showed that expression of hypoxanthine-guanine phosphoribosyltransferase, a key enzyme in hypoxanthine metabolism, and Wnt pathway gene (Wnt10a) was downregulated. Addition of WNT10A to the in vitro culture system restored the number, apoptosis rate, and colony-forming ability of HSCs in treated mice, confirming that the Wnt pathway is a key downstream target. Another study on the effects of MP exposure on human CD34+ hematopoietic stem/progenitor cell function showed that MPs inhibit HSC function by disrupting metabolic pathways, particularly the tricarboxylic acid cycle[66]. In this experiment, hematopoietic stem/progenitor cells exposed to MPs (80 nm, 0.1 mg/L, 24 hours) showed abnormal metabolites, such as significantly elevated levels of propionic acid and glutamate oxidation; disruption of the tricarboxylic acid cycle; accumulation of glutamate oxidation, which may affect the differentiation of hematopoietic stem/progenitor cells through the modulation of TET methylcytidine dioxygenase 2 (TET2), and elevated glucose 6-phosphate, which may exacerbate oxidative stress through the pentose phosphate pathway. Via the pentose phosphate pathway, elevated glucose 6-phosphate potentially exacerbates oxidative stress.
Additionally, in an in vivo developmental model of zebrafish, oxidative stress was demonstrated to be a key mediator of damage to HSC function[67]. Zebrafish embryos aged 2 hours were exposed to MPs (20 nm, 2, 5 and 8 mg/L, 46 hours), and RNA-seq showed that MPs altered oxidative enzymes, hydrolase activity, and extracellular matrix composition, and single-cell RNA-seq identified differentially expressed genes with the following changes. Downregulation of angiogenic factor with G patch and FHA domains 1 gene, and abnormal expression of IFI30 (a lysosomal thiol reductase, also a lysosomal oxidoreductase) and peroxiredoxin 1 gene disrupted the cellular redox balance, resulting in impaired HSC function. The reversal of MP-induced tail vein injury by the antioxidant N-acetylcysteine demonstrated that oxidative stress is a key mediator. In summary, the Wnt pathway, oxidative stress, and metabolic disorders may be key mediators of MP damage to hematopoiesis.
Pan et al[68] directly observed the uptake of PS MPs by MC3T3-E1 cells (a pre-osteoblast cell line) using confocal microscopy, confirming that bone cells can take up MPs. Additionally, the authors established a mouse drinking water exposure model (10 mg/L, 6 months) and treated bone marrow mesenchymal stem cells (BMSCs) with different concentrations of PS MPs (15-240 μg/mL, 7 days) in vitro, combined with intervention using the nuclear factor kappa B pathway inhibitor BAY 11-7082[69]. The experiments revealed reduced osteogenesis and enhanced osteoclast activation in mice. MPs activated the nuclear factor kappa B pathway in BMSCs, upregulating downstream inflammatory factors (tumor necrosis factor-α) and accelerating BMSC senescence. Senescent BMSCs overexpressed receptor activator of nuclear factor kappa B ligand, promoting osteoclast formation and ultimately disrupting the balance between bone formation and resorption.
PS nanoplastic exposure (0.3-2.4 mg/mL) induces senescence, promotes adipogenesis, and disrupts cell cycle regulation in human bone marrow-derived mesenchymal stem cell lines[70]. At low concentrations (≤ 2.4 mg/mL), there is no significant cytotoxicity, but it promotes adipogenic differentiation of human bone marrow-derived mesenchymal stem cell lines (upregulation of peroxisome proliferator activated receptor gamma gene). However, MP particles with low crosslinking density degradation exhibit higher toxicity than those with high crosslinking density. Long-term treatment (14 days) in adipogenic culture medium results in increased cell death. Specific effects include upregulation of glutathione peroxidase 3 gene (ROS clearance-related gene) to clear ROS, and downregulation of heat shock protein 70 and X-box binding protein 1 gene (endoplasmic reticulum stress gene). Regarding cell cycle regulation, nanoplastic exposure promotes mitochondrial fusion, inhibits fission, increases the proportion of cells in the S phase, and ultimately promotes adipogenic differentiation via upregulation of mitofusin 2 gene and cyclin D1 gene, and downregulation of fission, mitochondrial 1 gene. At the same time, the upregulation of caspase 3 gene, a gene related to apoptosis, suggests enhanced cell apoptosis, further demonstrating toxicity. The higher toxicity of MP particles with low crosslinking density degradation may be related to their higher surface activity.
Other researchers have studied the cellular differentiation of human BMSCs and adipose mesenchymal stromal cells exposed to two sizes of PET particles (1 and 2.6 μm, 10 μg/mL, 3 days), indicating that their differentiation potential has changed[71]. In this study, MPs induced ROS accumulation and DNA damage (increased histone γH2AX and ATM foci), activated cell cycle inhibitors (p21, p16, and p27), leading to cellular senescence or apoptosis. Additionally, the loss of osteoblast differentiation capacity in BMSCs and the persistence of adipose mesenchymal stromal cells in the early stages of adipocyte differentiation were indicated by increased mRNA levels of peroxisome proliferator-activated receptor γ and decreased lipoprotein lipase levels.
Using human-induced pluripotent stem-cell-derived kidney organoids as a model, the researchers exposed these to 1 mm PS MPs (0-20 μg/mL) during the NPC stage (days 12-22)[72]. They observed a decrease in NPC numbers, reduced activity, increased intracellular ROS, and increased apoptosis (upregulation of caspase 3). Transcriptome analysis revealed significant downregulation of notch receptor 1 gene (NOTCH1), NOTCH2, delta like canonical Notch ligand 1 gene, and jagged canonical Notch ligand 1 gene expression in NPCs, ultimately disrupting nephron pattern formation. Additionally, reduced YAP1 gene expression, associated with impaired cell proliferation, exacerbated organoid growth limitations. Other authors used the same model and concentrations and, in addition to the above findings, supplemented their results with descriptions of the activation of the apoptosis pathway[73]. Activation of the BCL2/BAX/caspase apoptosis pathway reduced expression of the antiapoptotic protein BCL2, increased expression of cytochrome c, proapoptotic proteins BAX and apoptotic peptidase activating factor 1 gene, and activation of caspase 9 and caspase 3, ultimately inducing apoptosis in NPCs. Additionally, differentiation-related genes such as NOTCH1 and jagged canonical Notch ligand 1 were downregulated, leading to abnormal differentiation of renal tubular epithelial cells.
Wang et al[74] explored a new potential mechanism, suggesting that DNA damage inducible transcript 4 (DDIT4) as a key mediator, linking MP exposure to the inhibition of mechanistic target of rapamycin signaling. They also used kidney organoids, with PS MP concentrations set at 1.25-10 μg/mL for 24 hours of treatment, and found that MPs induced upregulation of DDIT4, inhibited mechanistic target of rapamycin activity, and activated autophagy (increased light chain 3 II/Light chain 3 I protein ratio and decreased p62 protein levels). DDIT4 not only promotes autophagy but also induces apoptosis in NPCs by upregulating proapoptotic BAX, caspase 3, and caspase 9, and downregulating antiapoptotic BCL2. Silencing DDIT4 reverses MP-induced autophagy and apoptosis, confirming its role as a key regulatory factor.
Organoids, as a three-dimensional microtissue cultured and self-organized in vitro, have shown potential in the study of environmental toxicology, especially MPs[75,76], because they can highly simulate the structure, cell diversity, and specific functions of organs in vivo[77]. Table 2 summarizes studies about the application of organoids in MP research, detailing the source of organoid, MP type, size, and concentration, and the main biological effects. The prevalence of MPs and their potential biological health risks have attracted widespread attention, but traditional cell lines (two-dimensional) or animal models have significant limitations in studying their complex mechanisms of action. Two-dimensional cells lack physiologically relevant tissue structure and microenvironment, and are difficult to reflect organ level responses[78]. However, animal experiments are costly, long-term, and have species differences[79]. Organoid technology effectively makes up for these shortcomings. Using organoid models derived from stem cells, such as intestine, liver, lung, and kidney, researchers can more realistically evaluate the cellular uptake, tissue-specific accumulation, cytotoxicity, and potential long-term effects, such as metabolic disorders and developmental toxicity, of MPs with different sizes, types, and surface chemical properties and their adsorbed pollutants[80]. In addition, combined with advanced co-culture systems or organ chip technology[81], organoid models are expected to simulate multiorgan interactions and reveal the systemic distribution and metabolic dynamics of MPs in the body[75].
| Organoid | Source | Type of MPs | Size of MPs | Concentration of MPs | Biological effects | Ref. |
| Cardiac organoids | hESC | PS | 28-29 nm | 5 and 20 μg/mL | MPs impeded differentiation of hESCs; autophagy and p38/ERK MAPK signaling pathways | [86] |
| hPSCs | PS | 956 nm | 0.025-2.5 μg/mL | Cardiotoxic; destruction of mitochondrial function | [87] | |
| Cerebral organoids | hiPSCs | PS | 25-60 nm | 50-200 ng/mL | MPs enter mitochondria and damage morphology and function; impair neural development and cause apoptosis | [61] |
| Endometrial organoids | Endometrial epithelial gland cells | PS | 2 μm | 5 and 50 μg/mL | Apoptotic responses and disrupted growth pattern | [88] |
| Hepatobiliary organoids | Hepatic progenitor cells | PS | 1 μm | 2.5 μg/mL | Hepatotoxicity; hepatic metabolism dysregulation MPs were localized in the bile duct area | [89] |
| Intestinal organoids | HIMEC | PS | 25 nm | 0.67 μg/mL | No significant toxicity at 60 μg/mL; downregulated IFI6 (immunosuppressive and antiviral functions) expression | [90] |
| HIMEC | PS | 196 and 211 nm | 100 μg/mL | Validate MPs exposure impaired intestinal stem cells via Notch and Wnt/β-catenin signaling pathway under OS conditions | [91] | |
| HIMEC | PS, PTFE, PMMA | 100 nm | 50 μg/mL | Metabolic toxicity of three MPs can be predicted using nontargeted metabolomics | [92] | |
| HIMEC | PS | 135-141 nm | 60 μg/mL | Benzo[a]pyrene co-exposure inhibit intestinal stem cells differentiation by OS pathway; Notch signaling pathway | [93] | |
| hiPSCs | PS | 50 nm | 10 and 100 μg/mL | MPs accumulation was positively correlated with exposure time and concentration; ROS | [94] | |
| Kidney organoids | hiPSCs | PS | 1 μm | 2.5 μg/mL | Nephrotoxicity; MPs induce nephrotoxicity through DDIT4-mediated autophagy and apoptosis | [74] |
| hiPSCs | PS | 97.77 nm | 200-800 μg/mL | Inhibitory effects of MPs on kidney organoids growth were concentration- and time-dependent | [95] | |
| hiPSCs | PS | 1 μm | 2.5 μg/mL | Lead to increased ROS and mitochondrial damage in nephron progenitors; induce glycolysis inhibition contributing to nephrogenesis disruption | [96] | |
| hiPSCs | PS | 1 μm | 10 μg/mL | Nephrotoxicity; impair cell membrane integrity, disturbed mitochondrial homeostasis; proliferation and apoptosis; Notch signaling | [72] | |
| Liver organoids | hESC | PS | 1-5 μm | 50 particles/mL | Low-dose of aged MPs induced reductive stress with disrupted mitochondrial respiratory chain function | [97] |
| hESC | PS | 1 μm | 20-200 ng/mL | Induce hepatic injury via ferroptosis; small size has high cytotoxicity | [98] | |
| hESC | PS | 1 μm | 50 ng/mL | Due to physical contact and damage to cells, PS-induced hepatotoxic effects may be more significant than BPA | [99] | |
| Lung organoids | Lung tissues | PS | 40 nm | 100 μg/mL | PS-NPs facilitate sustained transitional cell presence and accumulation | [100] |
| Lung tissues | Nylon | 1-10 μm | 1-100 μg/mL | MPs made by nylon inhibit developing airway organoids; inhibit Hoxa5 can restore organoid | [101] | |
| Retinal organoids | hESC | PS | < 0.2 mm | 0.01-1 mg/mL | Neurotoxicity of MPs; triphenyl phosphate co-exposure exerted higher neurotoxicity | [102] |
Organoid technology is still in its infancy and has many limitations. For instance, the reproducibility of the morphology and function of the obtained three-dimensional organoid system remains a major bottleneck. Organoids show heterogeneity and variability among organoid-forming cells, organoids in the same dish, and individual patients[82]. If not controlled, the repeatability and robustness of the system may be endangered. MPs accumulated in the body may cause chronic toxicity[69,83], and the experiment requires long-term exposure. However, the lifespan and function of organoids are limited[84], which may limit the research of organoids in the chronic toxicity of MPs. Although organoids still need to be improved in simulating immune response and vascularization, they are becoming an indispensable and promising research tool in elucidating the biological effect mechanism of MPs, assessing health risks, and developing targeted protection strategies, promoting the development of MPs toxicology research to be more physiologically relevant, efficient, and predictable.
Firstly, different research teams use different experimental models, types of MPs, and exposure conditions to obtain different research results. For instance, several studies demonstrated that MPs interact with planarian tissues, adversely affecting regeneration processes[8,51]. However, Gambino et al[85] found that chronic alimentary exposure to MPs did not affect regeneration in freshwater planarians. This may be because there are many kinds of MPs with different physicochemical properties and different mechanisms of action on organisms. At the same time, the difference in experimental conditions also affects the consistency of research results, making our comprehensive understanding of MPs limited. Secondly, many studies use MP doses and exposure durations that poorly reflect actual environmental pollution levels. This makes it difficult for us to accurately evaluate the true impact of MPs on stem cell and tissue regeneration, as well as determine their specific effect threshold at different doses and exposure times. Finally, there are limitations to organoid technology. Current organoids exist as single organs, and more research is needed to investigate how they can simulate the actual process of MP exposure.
Health and environmental risks: As emerging contaminants, MPs threaten stem cell integrity and tissue regeneration, representing a multifactorial risk to human health and ecosystem stability. Therefore, in-depth investigation into the mechanisms and extent of MPs impacts is crucial for developing effective prevention strategies and safeguarding human health and ecosystems.
Future research direction: Future research should pay more attention to the interaction mechanism between MPs and stem cells, and explore in depth how MPs affect the function of stem cells through processes such as cell membranes and interactions with intracellular biomolecules. At the same time, research on stem cells of different tissue types should be strengthened to understand the specific effects of MPs on tissue regeneration. In addition, establishing more accurate experimental models and analysis methods, strengthening the exploration of new technologies such as organoids, and improving the reliability and comparability of research results.
Interdisciplinary collaboration and suggestions: Given the complexity of the MP issue, it is necessary for experts from multiple disciplines such as environmental science, medicine, and materials science to collaborate across disciplines and jointly tackle this challenge. At the same time, it is necessary to strengthen the supervision of MP pollution, enhance environmental education for the public, and raise public awareness of the hazards of MP pollution.
This article critically reviews MP characterization, stem cell responses and regenerative impairment, molecular mechanisms, and organoid platforms for MP risk assessment. Current evidence, though limited, reveals that MP exposure disrupts stem cell self-renewal, proliferation, and differentiation processes essential for tissue regeneration. Key evidence reveals mitochondrial dysfunction (e.g., ATP depletion and superoxide accumulation), epigenetic dysregulation (altered DNA methylation of neurodevelopmental genes), and cell cycle disruption (G1/S arrest via p21 and p27 activation) as primary drivers of toxicity. Organ-specific phenotypes include neural differentiation failure, hematopoietic suppression via inhibition of metabolic and Wnt pathways, mesenchymal stem cell senescence-driven bone remodeling imbalance, and renal progenitor apoptosis triggered by NOTCH/DDIT4 dysregulation. Stem-cell-derived organoids validate these effects, demonstrating their utility in modeling organotypic toxicity of MPs. However, research remains scarce, particularly regarding chronic exposure and molecular drivers. Future work must prioritize mechanistic depth to elucidate MP - stem cell interactions and their regenerative impacts.
| 1. | Thompson RC, Courtene-Jones W, Boucher J, Pahl S, Raubenheimer K, Koelmans AA. Twenty years of microplastic pollution research-what have we learned? Science. 2024;386:eadl2746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 445] [Article Influence: 222.5] [Reference Citation Analysis (0)] |
| 2. | Wang S, Chen H, Zhou X, Tian Y, Lin C, Wang W, Zhou K, Zhang Y, Lin H. Microplastic abundance, distribution and composition in the mid-west Pacific Ocean. Environ Pollut. 2020;264:114125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 123] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 3. | Yang L, Zhang Y, Kang S, Wang Z, Wu C. Microplastics in soil: A review on methods, occurrence, sources, and potential risk. Sci Total Environ. 2021;780:146546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 365] [Article Influence: 73.0] [Reference Citation Analysis (0)] |
| 4. | Wright SL, Ulke J, Font A, Chan KLA, Kelly FJ. Atmospheric microplastic deposition in an urban environment and an evaluation of transport. Environ Int. 2020;136:105411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 535] [Article Influence: 89.2] [Reference Citation Analysis (0)] |
| 5. | Horvatits T, Tamminga M, Liu B, Sebode M, Carambia A, Fischer L, Püschel K, Huber S, Fischer EK. Microplastics detected in cirrhotic liver tissue. EBioMedicine. 2022;82:104147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 385] [Article Influence: 96.3] [Reference Citation Analysis (0)] |
| 6. | Leslie HA, van Velzen MJM, Brandsma SH, Vethaak AD, Garcia-Vallejo JJ, Lamoree MH. Discovery and quantification of plastic particle pollution in human blood. Environ Int. 2022;163:107199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 503] [Cited by in RCA: 1671] [Article Influence: 417.8] [Reference Citation Analysis (0)] |
| 7. | Ragusa A, Svelato A, Santacroce C, Catalano P, Notarstefano V, Carnevali O, Papa F, Rongioletti MCA, Baiocco F, Draghi S, D'Amore E, Rinaldo D, Matta M, Giorgini E. Plasticenta: First evidence of microplastics in human placenta. Environ Int. 2021;146:106274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 734] [Cited by in RCA: 1540] [Article Influence: 308.0] [Reference Citation Analysis (0)] |
| 8. | Liu Z, You XY. Recent progress of microplastic toxicity on human exposure base on in vitro and in vivo studies. Sci Total Environ. 2023;903:166766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 71] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 9. | Yu Y, Kumar M, Bolan S, Padhye LP, Bolan N, Li S, Wang L, Hou D, Li Y. Various additive release from microplastics and their toxicity in aquatic environments. Environ Pollut. 2024;343:123219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 53] [Reference Citation Analysis (0)] |
| 10. | Kolios G, Moodley Y. Introduction to stem cells and regenerative medicine. Respiration. 2013;85:3-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 312] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 11. | Dhaka V, Singh S, Anil AG, Sunil Kumar Naik TS, Garg S, Samuel J, Kumar M, Ramamurthy PC, Singh J. Occurrence, toxicity and remediation of polyethylene terephthalate plastics. A review. Environ Chem Lett. 2022;20:1777-1800. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 111] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 12. | Zhu Y, Che R, Zong X, Wang J, Li J, Zhang C, Wang F. A comprehensive review on the source, ingestion route, attachment and toxicity of microplastics/nanoplastics in human systems. J Environ Manage. 2024;352:120039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 56] [Reference Citation Analysis (0)] |
| 13. | Karbalaei S, Golieskardi A, Hamzah HB, Abdulwahid S, Hanachi P, Walker TR, Karami A. Abundance and characteristics of microplastics in commercial marine fish from Malaysia. Mar Pollut Bull. 2019;148:5-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 114] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 14. | Eriksen M, Mason S, Wilson S, Box C, Zellers A, Edwards W, Farley H, Amato S. Microplastic pollution in the surface waters of the Laurentian Great Lakes. Mar Pollut Bull. 2013;77:177-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1126] [Cited by in RCA: 961] [Article Influence: 73.9] [Reference Citation Analysis (0)] |
| 15. | Nava V, Chandra S, Aherne J, Alfonso MB, Antão-Geraldes AM, Attermeyer K, Bao R, Bartrons M, Berger SA, Biernaczyk M, Bissen R, Brookes JD, Brown D, Cañedo-Argüelles M, Canle M, Capelli C, Carballeira R, Cereijo JL, Chawchai S, Christensen ST, Christoffersen KS, de Eyto E, Delgado J, Dornan TN, Doubek JP, Dusaucy J, Erina O, Ersoy Z, Feuchtmayr H, Frezzotti ML, Galafassi S, Gateuille D, Gonçalves V, Grossart HP, Hamilton DP, Harris TD, Kangur K, Kankılıç GB, Kessler R, Kiel C, Krynak EM, Leiva-Presa À, Lepori F, Matias MG, Matsuzaki SS, McElarney Y, Messyasz B, Mitchell M, Mlambo MC, Motitsoe SN, Nandini S, Orlandi V, Owens C, Özkundakci D, Pinnow S, Pociecha A, Raposeiro PM, Rõõm EI, Rotta F, Salmaso N, Sarma SSS, Sartirana D, Scordo F, Sibomana C, Siewert D, Stepanowska K, Tavşanoğlu ÜN, Tereshina M, Thompson J, Tolotti M, Valois A, Verburg P, Welsh B, Wesolek B, Weyhenmeyer GA, Wu N, Zawisza E, Zink L, Leoni B. Plastic debris in lakes and reservoirs. Nature. 2023;619:317-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 180] [Article Influence: 60.0] [Reference Citation Analysis (0)] |
| 16. | Rochman CM. Microplastics research-from sink to source. Science. 2018;360:28-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1115] [Cited by in RCA: 730] [Article Influence: 91.3] [Reference Citation Analysis (0)] |
| 17. | Shi Y, Chai J, Xu T, Ding L, Huang M, Gan F, Pi K, Gerson AR, Yang J. Microplastics contamination associated with low-value domestic source organic solid waste: A review. Sci Total Environ. 2023;857:159679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 18. | Mohamed Nor NH, Kooi M, Diepens NJ, Koelmans AA. Lifetime Accumulation of Microplastic in Children and Adults. Environ Sci Technol. 2021;55:5084-5096. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 318] [Article Influence: 63.6] [Reference Citation Analysis (0)] |
| 19. | Yuan Z, Nag R, Cummins E. Human exposure to micro/nano-plastics through vegetables, fruits, and grains - A predictive modelling approach. J Hazard Mater. 2024;480:135900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 20. | Wang Y, Nan X, Sun H, Shi Y, Miao J, Li Y, Han X, Zhang N, Wang H, Ren N, Zhao X, Liu B. From insects to mammals! Tissue accumulation and transgenerational transfer of micro/nano-plastics through the food chain. J Hazard Mater. 2024;480:136424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 21. | Li D, Shi Y, Yang L, Xiao L, Kehoe DK, Gun'ko YK, Boland JJ, Wang JJ. Microplastic release from the degradation of polypropylene feeding bottles during infant formula preparation. Nat Food. 2020;1:746-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 316] [Article Influence: 52.7] [Reference Citation Analysis (0)] |
| 22. | Fadare OO, Wan B, Guo LH, Zhao L. Microplastics from consumer plastic food containers: Are we consuming it? Chemosphere. 2020;253:126787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 174] [Article Influence: 29.0] [Reference Citation Analysis (8)] |
| 23. | Banaei G, García-Rodríguez A, Tavakolpournegari A, Martín-Pérez J, Villacorta A, Marcos R, Hernández A. The release of polylactic acid nanoplastics (PLA-NPLs) from commercial teabags. Obtention, characterization, and hazard effects of true-to-life PLA-NPLs. J Hazard Mater. 2023;458:131899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 32] [Reference Citation Analysis (0)] |
| 24. | Alijagic A, Suljević D, Fočak M, Sulejmanović J, Šehović E, Särndahl E, Engwall M. The triple exposure nexus of microplastic particles, plastic-associated chemicals, and environmental pollutants from a human health perspective. Environ Int. 2024;188:108736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 56] [Reference Citation Analysis (0)] |
| 25. | Zhang Y, Lyu L, Tao Y, Ju H, Chen J. Health risks of phthalates: A review of immunotoxicity. Environ Pollut. 2022;313:120173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 66] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 26. | Fang Z, Sallach JB, Hodson ME. Size- and concentration-dependent effects of microplastics on soil aggregate formation and properties. J Hazard Mater. 2024;465:133395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 42] [Reference Citation Analysis (0)] |
| 27. | Andrady AL, Barnes PW, Bornman JF, Gouin T, Madronich S, White CC, Zepp RG, Jansen MAK. Oxidation and fragmentation of plastics in a changing environment; from UV-radiation to biological degradation. Sci Total Environ. 2022;851:158022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 136] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 28. | Burns EE, Boxall ABA. Microplastics in the aquatic environment: Evidence for or against adverse impacts and major knowledge gaps. Environ Toxicol Chem. 2018;37:2776-2796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 376] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 29. | Qiao R, Deng Y, Zhang S, Wolosker MB, Zhu Q, Ren H, Zhang Y. Accumulation of different shapes of microplastics initiates intestinal injury and gut microbiota dysbiosis in the gut of zebrafish. Chemosphere. 2019;236:124334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 587] [Article Influence: 83.9] [Reference Citation Analysis (11)] |
| 30. | Stienbarger CD, Joseph J, Athey SN, Monteleone B, Andrady AL, Watanabe WO, Seaton P, Taylor AR, Brander SM. Direct ingestion, trophic transfer, and physiological effects of microplastics in the early life stages of Centropristis striata, a commercially and recreationally valuable fishery species. Environ Pollut. 2021;285:117653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 31. | Bucci K, Tulio M, Rochman CM. What is known and unknown about the effects of plastic pollution: A meta-analysis and systematic review. Ecol Appl. 2020;30:e02044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 301] [Article Influence: 50.2] [Reference Citation Analysis (0)] |
| 32. | Sorci G, Loiseau C. Should we worry about the accumulation of microplastics in human organs? EBioMedicine. 2022;82:104191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 33. | Winiarska E, Jutel M, Zemelka-Wiacek M. The potential impact of nano- and microplastics on human health: Understanding human health risks. Environ Res. 2024;251:118535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 146] [Article Influence: 73.0] [Reference Citation Analysis (0)] |
| 34. | Senathirajah K, Attwood S, Bhagwat G, Carbery M, Wilson S, Palanisami T. Estimation of the mass of microplastics ingested - A pivotal first step towards human health risk assessment. J Hazard Mater. 2021;404:124004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 555] [Article Influence: 111.0] [Reference Citation Analysis (0)] |
| 35. | Elizalde-Velázquez GA, Gómez-Oliván LM. Microplastics in aquatic environments: A review on occurrence, distribution, toxic effects, and implications for human health. Sci Total Environ. 2021;780:146551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 121] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 36. | Cui J, Chen C, Gan Q, Wang T, Li W, Zeng W, Xu X, Chen G, Wang L, Lu Z, Li J, Jin B. Indoor microplastics and bacteria in the atmospheric fallout in urban homes. Sci Total Environ. 2022;852:158233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 37. | Dris R, Gasperi J, Mirande C, Mandin C, Guerrouache M, Langlois V, Tassin B. A first overview of textile fibers, including microplastics, in indoor and outdoor environments. Environ Pollut. 2017;221:453-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 658] [Cited by in RCA: 731] [Article Influence: 81.2] [Reference Citation Analysis (0)] |
| 38. | Schneider M, Stracke F, Hansen S, Schaefer UF. Nanoparticles and their interactions with the dermal barrier. Dermatoendocrinol. 2009;1:197-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 292] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 39. | Bastyans S, Jackson S, Fejer G. Micro and nano-plastics, a threat to human health? Emerg Top Life Sci. 2022;6:411-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 40. | Ali N, Katsouli J, Marczylo EL, Gant TW, Wright S, Bernardino de la Serna J. The potential impacts of micro-and-nano plastics on various organ systems in humans. EBioMedicine. 2024;99:104901. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 170] [Article Influence: 85.0] [Reference Citation Analysis (1)] |
| 41. | Pitt JA, Kozal JS, Jayasundara N, Massarsky A, Trevisan R, Geitner N, Wiesner M, Levin ED, Di Giulio RT. Uptake, tissue distribution, and toxicity of polystyrene nanoparticles in developing zebrafish (Danio rerio). Aquat Toxicol. 2018;194:185-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 417] [Article Influence: 52.1] [Reference Citation Analysis (0)] |
| 42. | Nihart AJ, Garcia MA, El Hayek E, Liu R, Olewine M, Kingston JD, Castillo EF, Gullapalli RR, Howard T, Bleske B, Scott J, Gonzalez-Estrella J, Gross JM, Spilde M, Adolphi NL, Gallego DF, Jarrell HS, Dvorscak G, Zuluaga-Ruiz ME, West AB, Campen MJ. Bioaccumulation of microplastics in decedent human brains. Nat Med. 2025;31:1114-1119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 219] [Cited by in RCA: 233] [Article Influence: 233.0] [Reference Citation Analysis (1)] |
| 43. | Yang Q, Peng Y, Wu X, Cao X, Zhang P, Liang Z, Zhang J, Zhang Y, Gao P, Fu Y, Liu P, Cao Z, Ding T. Microplastics in human skeletal tissues: Presence, distribution and health implications. Environ Int. 2025;196:109316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 30] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 44. | Guo X, Wang L, Wang X, Li D, Wang H, Xu H, Liu Y, Kang R, Chen Q, Zheng L, Wu S, Guo Z, Zhang S. Discovery and analysis of microplastics in human bone marrow. J Hazard Mater. 2024;477:135266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 55] [Reference Citation Analysis (0)] |
| 45. | Jenner LC, Rotchell JM, Bennett RT, Cowen M, Tentzeris V, Sadofsky LR. Detection of microplastics in human lung tissue using μFTIR spectroscopy. Sci Total Environ. 2022;831:154907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 679] [Article Influence: 169.8] [Reference Citation Analysis (0)] |
| 46. | Garcia MA, Liu R, Nihart A, El Hayek E, Castillo E, Barrozo ER, Suter MA, Bleske B, Scott J, Forsythe K, Gonzalez-Estrella J, Aagaard KM, Campen MJ. Quantitation and identification of microplastics accumulation in human placental specimens using pyrolysis gas chromatography mass spectrometry. Toxicol Sci. 2024;199:81-88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 177] [Cited by in RCA: 124] [Article Influence: 62.0] [Reference Citation Analysis (0)] |
| 47. | Liu S, Guo J, Liu X, Yang R, Wang H, Sun Y, Chen B, Dong R. Detection of various microplastics in placentas, meconium, infant feces, breastmilk and infant formula: A pilot prospective study. Sci Total Environ. 2023;854:158699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 203] [Article Influence: 67.7] [Reference Citation Analysis (0)] |
| 48. | Zhao Q, Zhu L, Weng J, Jin Z, Cao Y, Jiang H, Zhang Z. Detection and characterization of microplastics in the human testis and semen. Sci Total Environ. 2023;877:162713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 225] [Reference Citation Analysis (0)] |
| 49. | Hu CJ, Garcia MA, Nihart A, Liu R, Yin L, Adolphi N, Gallego DF, Kang H, Campen MJ, Yu X. Microplastic presence in dog and human testis and its potential association with sperm count and weights of testis and epididymis. Toxicol Sci. 2024;200:235-240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 155] [Cited by in RCA: 98] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 50. | Ibrahim YS, Tuan Anuar S, Azmi AA, Wan Mohd Khalik WMA, Lehata S, Hamzah SR, Ismail D, Ma ZF, Dzulkarnaen A, Zakaria Z, Mustaffa N, Tuan Sharif SE, Lee YY. Detection of microplastics in human colectomy specimens. JGH Open. 2021;5:116-121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 362] [Cited by in RCA: 292] [Article Influence: 58.4] [Reference Citation Analysis (0)] |
| 51. | Gao T, Sun B, Xu Z, Chen Q, Yang M, Wan Q, Song L, Chen G, Jing C, Zeng EY, Yang G. Exposure to polystyrene microplastics reduces regeneration and growth in planarians. J Hazard Mater. 2022;432:128673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 52. | Peng M, Vercauteren M, Grootaert C, Rajkovic A, Boon N, Janssen C, Asselman J. Cellular and bioenergetic effects of polystyrene microplastic in function of cell type, differentiation status and post-exposure time. Environ Pollut. 2023;337:122550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 53. | Chartres N, Cooper CB, Bland G, Pelch KE, Gandhi SA, BakenRa A, Woodruff TJ. Effects of Microplastic Exposure on Human Digestive, Reproductive, and Respiratory Health: A Rapid Systematic Review. Environ Sci Technol. 2024;58:22843-22864. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 46] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 54. | Eisen A, Pioro EP, Goutman SA, Kiernan MC. Nanoplastics and Neurodegeneration in ALS. Brain Sci. 2024;14:471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 55. | Santos D, Luzio A, Bellas J, Monteiro SM. Microplastics- and copper-induced changes in neurogenesis and DNA methyltransferases in the early life stages of zebrafish. Chem Biol Interact. 2022;363:110021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 29] [Reference Citation Analysis (0)] |
| 56. | Santos D, Luzio A, Félix L, Cabecinha E, Bellas J, Monteiro SM. Microplastics and copper induce apoptosis, alter neurocircuits, and cause behavioral changes in zebrafish (Danio rerio) brain. Ecotoxicol Environ Saf. 2022;242:113926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 57. | Jeong B, Baek JY, Koo J, Park S, Ryu YK, Kim KS, Zhang S, Chung C, Dogan R, Choi HS, Um D, Kim TK, Lee WS, Jeong J, Shin WH, Lee JR, Kim NS, Lee DY. Maternal exposure to polystyrene nanoplastics causes brain abnormalities in progeny. J Hazard Mater. 2022;426:127815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 176] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 58. | Tian L, Zhang Y, Chen J, Liu X, Nie H, Li K, Liu H, Lai W, Shi Y, Xi Z, Lin B. Effects of nanoplastic exposure during pregnancy and lactation on neurodevelopment of rat offspring. J Hazard Mater. 2024;474:134800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 31] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 59. | Yang S, Lee S, Lee Y, Cho JH, Kim SH, Ha ES, Jung YS, Chung HY, Kim MS, Kim HS, Chang SC, Min KJ, Lee J. Cationic nanoplastic causes mitochondrial dysfunction in neural progenitor cells and impairs hippocampal neurogenesis. Free Radic Biol Med. 2023;208:194-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 29] [Reference Citation Analysis (0)] |
| 60. | González-Caballero MC, de Alba González M, Torres-Ruiz M, Iglesias-Hernández P, Zapata V, Terrón MC, Sachse M, Morales M, Martin-Folgar R, Liste I, Cañas-Portilla AI. Internalization and toxicity of polystyrene nanoplastics on inmortalized human neural stem cells. Chemosphere. 2024;355:141815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 29] [Reference Citation Analysis (0)] |
| 61. | Tao M, Wang C, Zheng Z, Gao W, Chen Q, Xu M, Zhu W, Xu L, Han X, Guo X, Liu Y. Nanoplastics exposure-induced mitochondrial dysfunction contributes to disrupted stem cell differentiation in human cerebral organoids. Ecotoxicol Environ Saf. 2024;285:117063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 62. | Hua T, Kiran S, Li Y, Sang QA. Microplastics exposure affects neural development of human pluripotent stem cell-derived cortical spheroids. J Hazard Mater. 2022;435:128884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 77] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 63. | Hawley RG, Ramezani A, Hawley TS. Hematopoietic stem cells. Methods Enzymol. 2006;419:149-179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 64. | Sun R, Xu K, Yu L, Pu Y, Xiong F, He Y, Huang Q, Tang M, Chen M, Yin L, Zhang J, Pu Y. Preliminary study on impacts of polystyrene microplastics on the hematological system and gene expression in bone marrow cells of mice. Ecotoxicol Environ Saf. 2021;218:112296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 65. | Jiang L, Ye Y, Han Y, Wang Q, Lu H, Li J, Qian W, Zeng X, Zhang Z, Zhao Y, Shi J, Luo Y, Qiu Y, Sun J, Sheng J, Huang H, Qian P. Microplastics dampen the self-renewal of hematopoietic stem cells by disrupting the gut microbiota-hypoxanthine-Wnt axis. Cell Discov. 2024;10:35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 42] [Reference Citation Analysis (0)] |
| 66. | Guo X, Cheng C, Chen L, Cao C, Li D, Fan R, Wei X. Metabolomic characteristics in human CD34(+) hematopoietic stem/progenitor cells exposed to polystyrene nanoplastics. Food Chem Toxicol. 2023;177:113817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 67. | Chen J, Lu C, Xie W, Cao X, Zhang J, Luo J, Li J. Exposure to Nanoplastics Cause Caudal Vein Plexus Damage and Hematopoietic Dysfunction by Oxidative Stress Response in Zebrafish (Danio rerio). Int J Nanomedicine. 2024;19:13789-13803. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 68. | Pan C, Wu Y, Hu S, Li K, Liu X, Shi Y, Lin W, Wang X, Shi Y, Xu Z, Wang H, Chen H. Polystyrene microplastics arrest skeletal growth in puberty through accelerating osteoblast senescence. Environ Pollut. 2023;322:121217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 44] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 69. | Pan C, Hong R, Wang K, Shi Y, Fan Z, Liu T, Chen H. Chronic exposure to polystyrene microplastics triggers osteoporosis by breaking the balance of osteoblast and osteoclast differentiation. Toxicology. 2025;510:154017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 70. | Im GB, Kim YG, Jo IS, Yoo TY, Kim SW, Park HS, Hyeon T, Yi GR, Bhang SH. Effect of polystyrene nanoplastics and their degraded forms on stem cell fate. J Hazard Mater. 2022;430:128411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 71. | Najahi H, Alessio N, Squillaro T, Conti GO, Ferrante M, Di Bernardo G, Galderisi U, Messaoudi I, Minucci S, Banni M. Environmental microplastics (EMPs) exposure alter the differentiation potential of mesenchymal stromal cells. Environ Res. 2022;214:114088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 72. | Zhou B, Wei Y, Chen L, Zhang A, Liang T, Low JH, Liu Z, He S, Guo Z, Xie J. Microplastics exposure disrupts nephrogenesis and induces renal toxicity in human iPSC-derived kidney organoids. Environ Pollut. 2024;360:124645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 73. | Zhang A, Wang Y, Xue Q, Yao J, Chen L, Feng S, Shao J, Guo Z, Zhou B, Xie J. Polystyrene microplastics disrupt kidney organoid development via oxidative stress and Bcl-2/Bax/caspase pathway. Chem Biol Interact. 2025;419:111642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 74. | Wang Y, Zhang A, Liang T, Chen L, Feng S, Zhao Z, Jing Z, Lv J, Xie J, Zhou B. Polystyrene microplastics induce nephrotoxicity through DDIT4-mediated autophagy and apoptosis. Ecotoxicol Environ Saf. 2025;294:118066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 75. | Simian M, Bissell MJ. Organoids: A historical perspective of thinking in three dimensions. J Cell Biol. 2017;216:31-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 323] [Cited by in RCA: 451] [Article Influence: 45.1] [Reference Citation Analysis (0)] |
| 76. | Hu W, Lazar MA. Modelling metabolic diseases and drug response using stem cells and organoids. Nat Rev Endocrinol. 2022;18:744-759. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 60] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 77. | Wang H, Xu T, Yin D. Emerging trends in the methodology of environmental toxicology: 3D cell culture and its applications. Sci Total Environ. 2023;857:159501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 27] [Reference Citation Analysis (0)] |
| 78. | Silvani S, Figliuzzi M, Remuzzi A. Toxicological evaluation of airborne particulate matter. Are cell culture technologies ready to replace animal testing? J Appl Toxicol. 2019;39:1484-1491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 79. | Ehrenfellner B, Zissler A, Steinbacher P, Monticelli FC, Pittner S. Are animal models predictive for human postmortem muscle protein degradation? Int J Legal Med. 2017;131:1615-1621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 80. | Cong J, Wu J, Fang Y, Wang J, Kong X, Wang L, Duan Z. Application of organoid technology in the human health risk assessment of microplastics: A review of progresses and challenges. Environ Int. 2024;188:108744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 81. | Zhang T, Yang S, Ge Y, Yin L, Pu Y, Gu Z, Chen Z, Liang G. Unveiling the Heart's Hidden Enemy: Dynamic Insights into Polystyrene Nanoplastic-Induced Cardiotoxicity Based on Cardiac Organoid-on-a-Chip. ACS Nano. 2024;18:31569-31585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 19] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 82. | Zhao Z, Chen X, Dowbaj AM, Sljukic A, Bratlie K, Lin L, Fong ELS, Balachander GM, Chen Z, Soragni A, Huch M, Zeng YA, Wang Q, Yu H. Organoids. Nat Rev Methods Primers. 2022;2:94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 560] [Article Influence: 140.0] [Reference Citation Analysis (0)] |
| 83. | Jin H, Yan M, Pan C, Liu Z, Sha X, Jiang C, Li L, Pan M, Li D, Han X, Ding J. Chronic exposure to polystyrene microplastics induced male reproductive toxicity and decreased testosterone levels via the LH-mediated LHR/cAMP/PKA/StAR pathway. Part Fibre Toxicol. 2022;19:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 190] [Article Influence: 47.5] [Reference Citation Analysis (0)] |
| 84. | Hofer M, Lutolf MP. Engineering organoids. Nat Rev Mater. 2021;6:402-420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 278] [Cited by in RCA: 737] [Article Influence: 147.4] [Reference Citation Analysis (0)] |
| 85. | Gambino G, Falleni A, Nigro M, Salvetti A, Cecchettini A, Ippolito C, Guidi P, Rossi L. Dynamics of interaction and effects of microplastics on planarian tissue regeneration and cellular homeostasis. Aquat Toxicol. 2020;218:105354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 86. | Li J, Weng H, Liu S, Li F, Xu K, Wen S, Chen X, Li C, Nie Y, Liao B, Wu J, Kantawong F, Xie X, Yu F, Li G. Embryonic exposure of polystyrene nanoplastics affects cardiac development. Sci Total Environ. 2024;906:167406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 87. | Zhou Y, Wu Q, Li Y, Feng Y, Wang Y, Cheng W. Low-dose of polystyrene microplastics induce cardiotoxicity in mice and human-originated cardiac organoids. Environ Int. 2023;179:108171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 70] [Reference Citation Analysis (0)] |
| 88. | Qin X, Cao M, Peng T, Shan H, Lian W, Yu Y, Shui G, Li R. Features, Potential Invasion Pathways, and Reproductive Health Risks of Microplastics Detected in Human Uterus. Environ Sci Technol. 2024;58:10482-10493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 67] [Reference Citation Analysis (0)] |
| 89. | Li P, Miyamoto D, Adachi T, Hara T, Soyama A, Matsushima H, Imamura H, Kanetaka K, Gu W, Eguchi S. Mitigation of polystyrene microplastic-induced hepatotoxicity in human hepatobiliary organoids through bile extraction. Ecotoxicol Environ Saf. 2024;288:117330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 90. | Kharaghani D, DeLoid GM, He P, Swenor B, Bui TH, Zuverza-Mena N, Tamez C, Musante C, Verzi M, White JC, Demokritou P. Toxicity and absorption of polystyrene micro-nanoplastics in healthy and Crohn's disease human duodenum-chip models. J Hazard Mater. 2025;490:137714. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 91. | Shaoyong W, Sun L, Gan Y, Jin H, Wang W, Yin L, Wang Y, Jin M. Sight of Aged Microplastics Adsorbing Heavy Metal Exacerbated Intestinal Injury: A Mechanistic Study of Autophagy-Mediated Toxicity Response. ACS Nano. 2024;18:28849-28865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 19] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 92. | Xuan L, Luo J, Qu C, Guo P, Yi W, Yang J, Yan Y, Guan H, Zhou P, Huang R. Predictive metabolomic signatures for safety assessment of three plastic nanoparticles using intestinal organoids. Sci Total Environ. 2024;913:169606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 93. | Shaoyong W, Jin H, Jiang X, Xu B, Liu Y, Wang Y, Jin M. Benzo [a] pyrene-loaded aged polystyrene microplastics promote colonic barrier injury via oxidative stress-mediated notch signalling. J Hazard Mater. 2023;457:131820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 36] [Reference Citation Analysis (0)] |
| 94. | Hou Z, Meng R, Chen G, Lai T, Qing R, Hao S, Deng J, Wang B. Distinct accumulation of nanoplastics in human intestinal organoids. Sci Total Environ. 2022;838:155811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 68] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 95. | Chen L, Han B, Yang S, Guo L, Zhao L, Liu P, Hong X, Zhao Y, Peng Y, Qi S, Hu L, Chen Y. Toxicological effects and mechanisms of renal injury induced by inhalation exposure to airborne nanoplastics. J Hazard Mater. 2025;488:137393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 96. | Zhou B, Zhang A, Wang Y, Feng S, Xue Q, Liu Z, Zhao H, Jing Z, Xie J. Microplastics induce human kidney development retardation through ATP-mediated glucose metabolism rewiring. J Hazard Mater. 2025;486:137002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 97. | Cheng W, Chen H, Zhou Y, You Y, Lei D, Li Y, Feng Y, Wang Y. Aged fragmented-polypropylene microplastics induced ageing statues-dependent bioenergetic imbalance and reductive stress: In vivo and liver organoids-based in vitro study. Environ Int. 2024;191:108949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 98. | Cheng W, Zhou Y, Chen H, Wu Q, Li Y, Wang H, Feng Y, Wang Y. The iron matters: Aged microplastics disrupted the iron homeostasis in the liver organoids. Sci Total Environ. 2024;906:167529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 99. | Cheng W, Zhou Y, Xie Y, Li Y, Zhou R, Wang H, Feng Y, Wang Y. Combined effect of polystyrene microplastics and bisphenol A on the human embryonic stem cells-derived liver organoids: The hepatotoxicity and lipid accumulation. Sci Total Environ. 2023;854:158585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 42] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 100. | Yang S, Ge Y, Zhang T, Yin L, Pu Y, Chen Z, Liang G. Dynamic non-coding RNA biomarker reveals lung injury and repair induced by polystyrene nanoplastics. Environ Int. 2025;195:109266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 101. | Song S, van Dijk F, Vasse GF, Liu Q, Gosselink IF, Weltjens E, Remels AHV, de Jager MH, Bos S, Li C, Stoeger T, Rehberg M, Kutschke D, van Eck GWA, Wu X, Willems SH, Boom DHA, Kooter IM, Spierings D, Wardenaar R, Cole M, Nawijn MC, Salvati A, Gosens R, Melgert BN. Inhalable Textile Microplastic Fibers Impair Airway Epithelial Differentiation. Am J Respir Crit Care Med. 2024;209:427-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 40] [Reference Citation Analysis (0)] |
| 102. | Li M, Gao X, Lan Y, Pan Y, Yuan Y, Wu Z, Faiola F, Zhu L, Tang J, Gong J, Wang B. Revealing the neurodevelopmental toxicity of face mask-derived microplastics to humans based on neural organoids. J Hazard Mater. 2025;492:138084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/