Published online Oct 16, 2021. doi: 10.12998/wjcc.v9.i29.8864
Peer-review started: May 31, 2021
First decision: June 24, 2021
Revised: July 5, 2021
Accepted: August 23, 2021
Article in press: August 23, 2021
Published online: October 16, 2021
Processing time: 137 Days and 3.3 Hours
Adenomyoepithelioma (AME) of the breast is a rare type of benign breast tumor. Many AMEs show benign behavior, but reports of the malignant type are rare. We present the case of a patient with AME with repeated local recurrences and further malignant transformation.
A 53-year-old woman visited our hospital with a 16-mm palpable mass in the right breast. A core needle biopsy was performed. The pathological diagnosis was AME. Lumpectomy with a safety margin was performed without axillary lymph node dissection (ALND). Two years later, local recurrence developed, and the patient again underwent lumpectomy with a safety margin. The pathology showed malignant AME, and the margin was negative. Eight months later, local recurrence developed again in the same location, and a total mastectomy was performed without ALND. The pathological diagnosis was malignant AME. The patient was disease-free for three years posttreatment.
The treatment of AME requires caution, as it may exhibit repeated recurrences after local excision as well as malignant transformation.
Core Tip: Adenomyoepithelioma (AME) of the breast is a very rare type of benign tumor of the breast. Many AMEs demonstrate benign behavior and are often cured with excision with negative margins, but some AMEs exhibit malignant transformation of the myoepithelium, glandular epithelium, or both. We report the case of a patient with AME with repeated local recurrences and malignant transformation.
- Citation: Oda G, Nakagawa T, Mori M, Fujioka T, Onishi I. Adenomyoepithelioma of the breast with malignant transformation and repeated local recurrence: A case report. World J Clin Cases 2021; 9(29): 8864-8870
- URL: https://www.wjgnet.com/2307-8960/full/v9/i29/8864.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i29.8864
Adenomyoepithelioma (AME) is a very rare type of benign tumor of the breast. Many AMEs demonstrate benign behavior and are often cured with excision with negative margins, but some AMEs exhibit malignant transformation of the myoepithelium, glandular epithelium, or both[1-4]. However, cases of repeated recurrences despite negative excision margins are very rare. We report the case of a patient with malignant transformation after repeated wide local excisions of AME.
A 53-year-old Japanese woman presented with a right breast mass at the site of a previous wide local excision of AME.
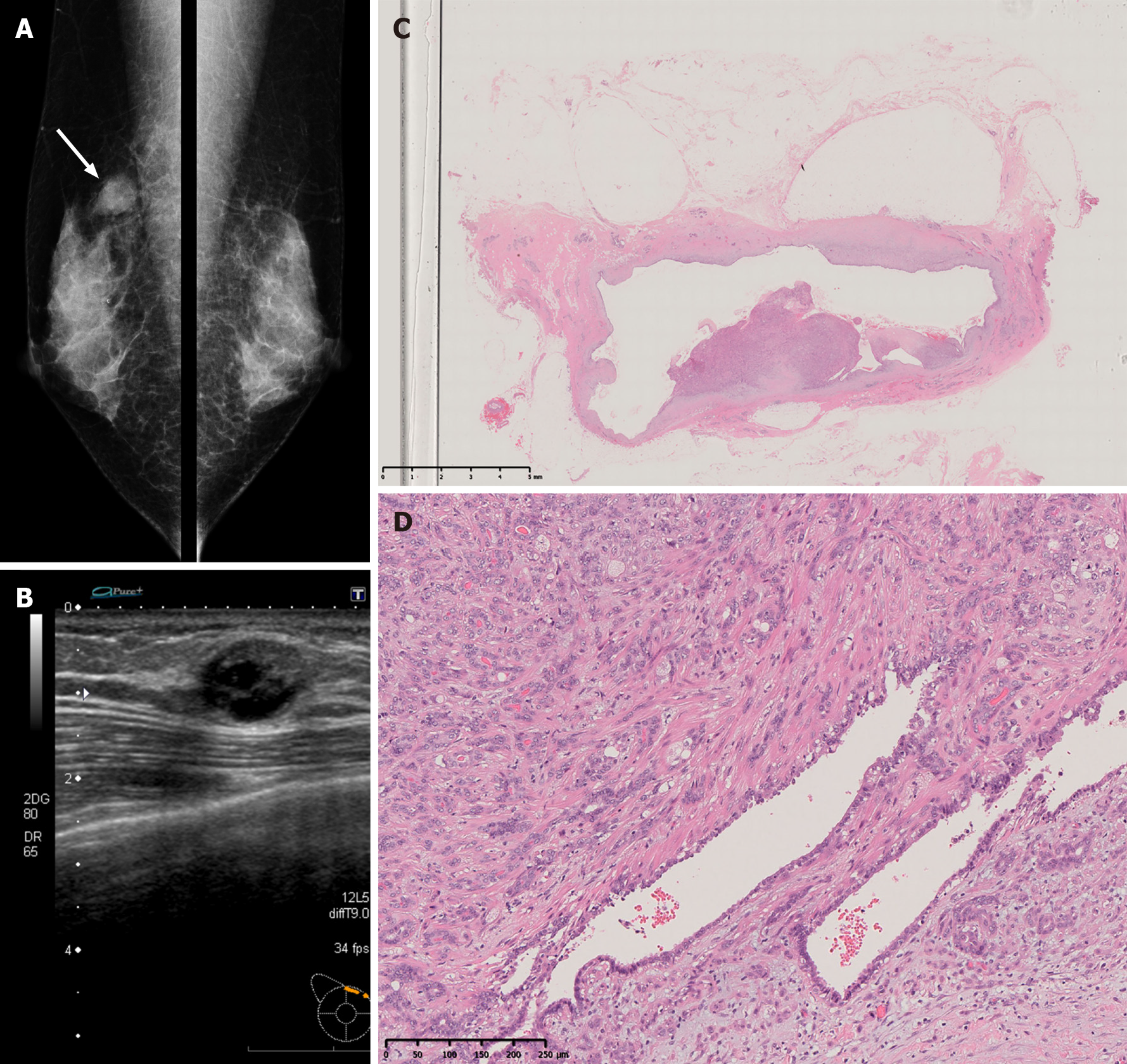
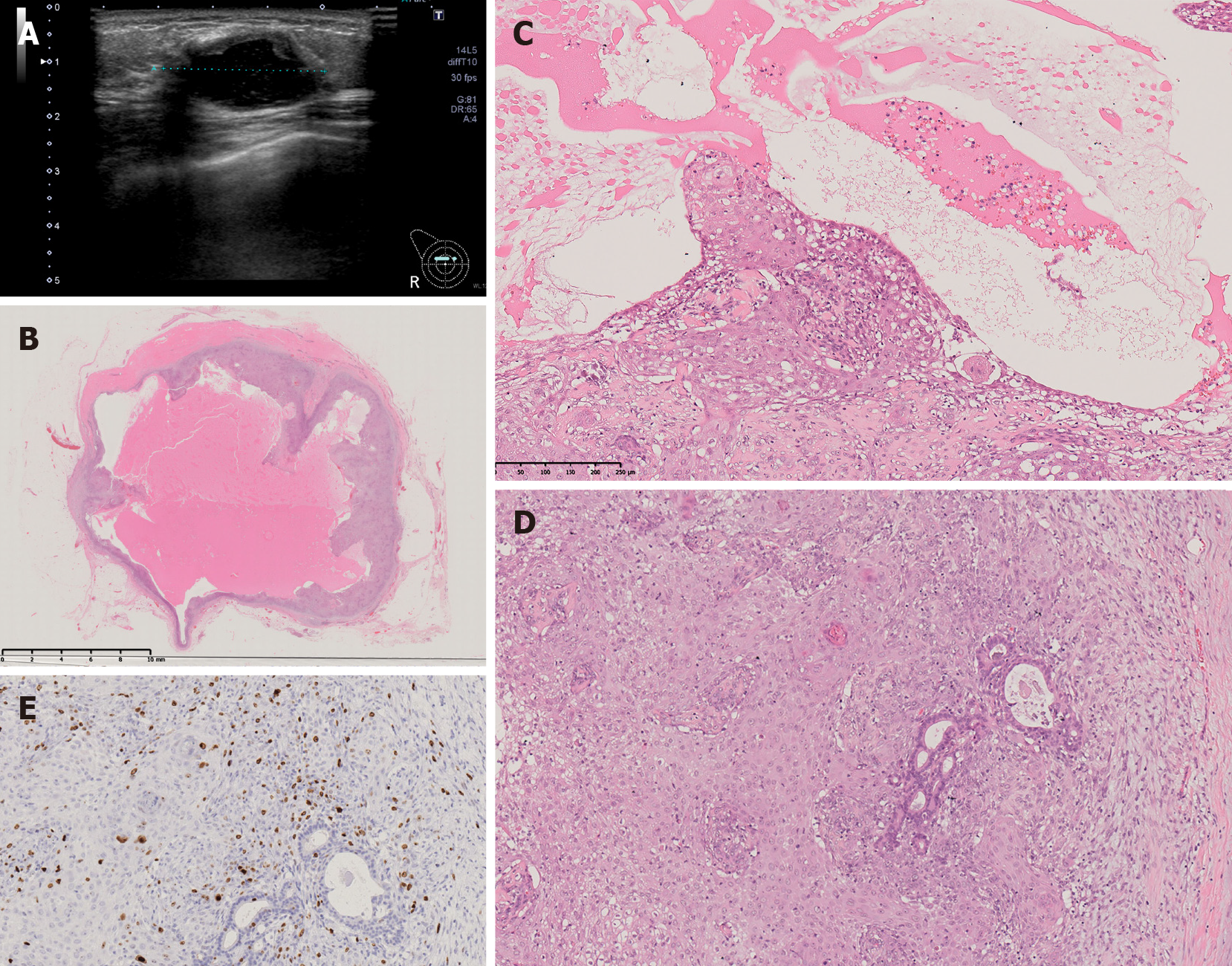
A 53-year-old Japanese woman visited our hospital with a palpable mass approximately 2 cm in size in the upper-inner right breast. The mammogram showed an oval, smooth, and well-defined isodense mass in the upper right breast (Figure 1A). Diagnostic ultrasonography showed a well-defined mass with cystic change, measuring up to 16 mm, at the 2 o’clock position in the right breast (Figure 1B). A core needle biopsy (CNB) was performed, and the lump was diagnosed as AME. Lumpectomy with safety margins and without axillary lymph node dissection (ALND) was performed. Postoperative pathology confirmed AME. The tumor was a 20-mm cystic lesion, and the cystic wall had nodules or irregular thickening (Figure 1C). The microscopic findings were as follows. Round or spindle-shaped myoepithelium that had proliferated in and around the gland ducts. High mitotic counts were prominent in the myoepithelial component (8/10 high-power fields.) The border of the tumor was relatively clear (Figure 1D). The tumor resection margins were relatively clear with at least 5 mm clearance at the nearest margin. No adjuvant therapy was given. Two years later, the woman returned to our hospital with a palpable mass in the same location. Ultrasound showed a well-defined, oval, low-isoechoic mass on the slightly caudal side of the area of postoperative change (Figure 2A). Vacuum-assisted biopsy was performed, and the diagnosis was recurrent AME. Lumpectomy with safety margins and without ALND was again performed. The tumor was diagnosed as recurrent AME with a proliferation pattern similar to that at the initial surgery. In addition, a diagnosis of malignant transformation of AME was made due to the observation of nuclear atypia, a high mitotic count (approximately 10/10 high-power fields), and invasive growth (Figure 2B-E). Excisional margins were narrow on the side of the pectoralis major muscle, but the tumor was not exposed. No adjuvant therapy was given. Eight months later, the patient presented with a recurrent palpable mass in the same area.
No special past medical treatment history.
No family history of breast cancer or other cancers.
An approximately 2 cm palpable mass was observed in the upper-inner area of the right breast.
The patient’s hematology and biochemistry results were all unremarkable.
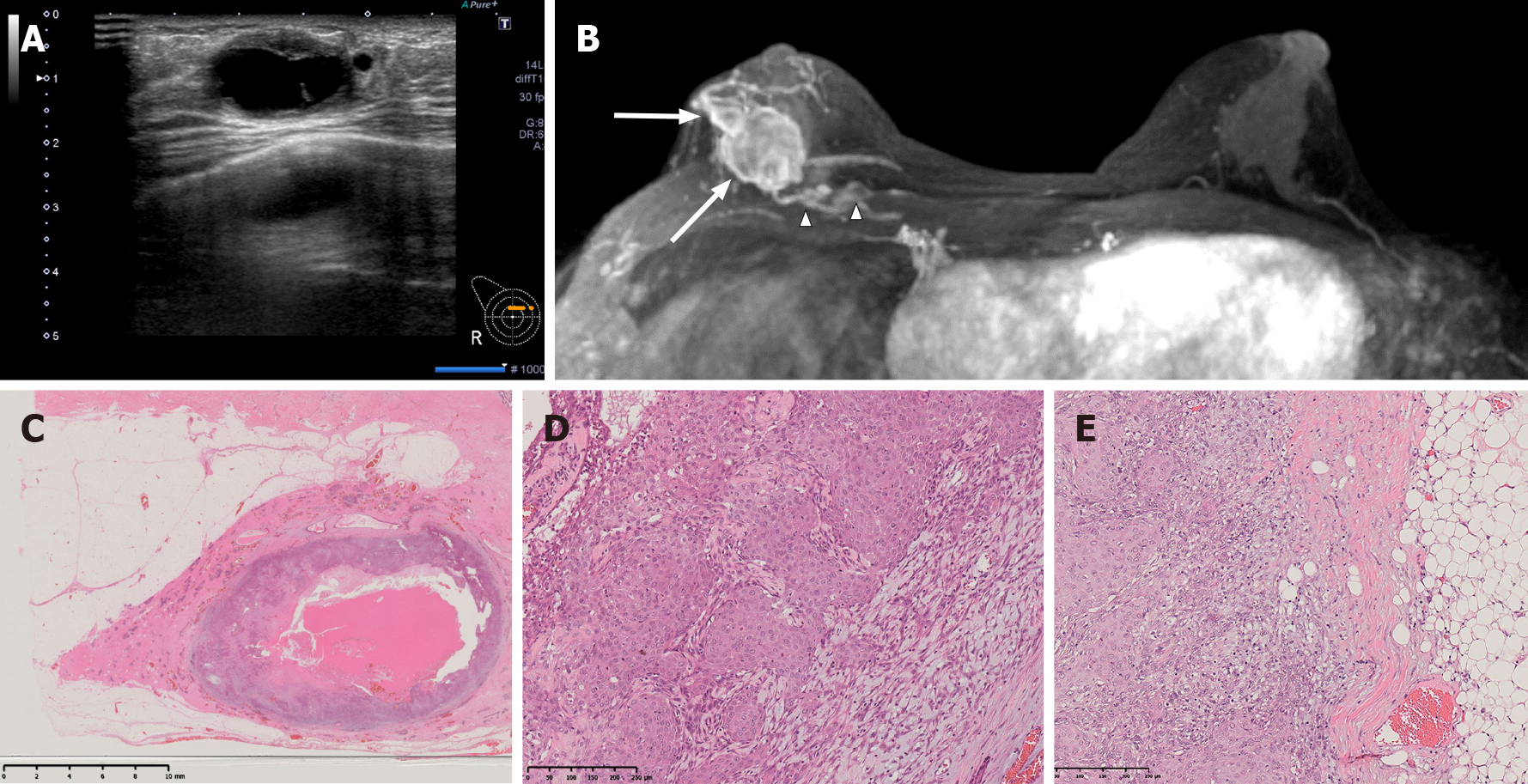
Ultrasound showed a series of masses up to 27 mm in size that had formed on the right side of the previous surgical wound (Figure 3A). The largest of the masses showed no echogenicity, thick walls and internal septal/cystic degeneration. The findings were similar to those of the patient’s previously diagnosed AME. Enhanced breast magnetic resonance imaging (MRI) showed multiple masses up to 25 mm in size in the right inner-upper area (Figure 3B). The masses were cystic with thick walls, similar to the previous tumor, and the patient was diagnosed with recurrence. Two 7-mm nodules were also found within the pectoralis major muscle.
The tumor was 75 mm × 24 mm in size and located in the inner-upper area. The center of the lesion was hollow and cystic, and the lesion contained a jelly-like substance (Figure 3C). The pathological findings were the same as those in the previous recurrence (Figure 3C-E). Epithelium with squamous metaplasia and spindle-shaped myoepithelium arranged in a complex or bundled pattern were present, and there was continuity between the two types of epithelium; the epithelial cells showed prominent nuclear atypia and high mitotic counts that were especially prominent in the squamous epithelial component. There were also findings of invasion of the partially resected pectoralis major muscle and extramammary adipose tissue. The diagnosis of recurrent AME with malignant transformation and squamous differentiation was made.
Total mastectomy and partial resection of the pectoralis muscle were performed without ALND. No adjuvant therapy was given.
Mammogram, breast US, and chest X-ray were performed regularly. The patient was disease-free for three years posttreatment.
AME of the breast is a rare disorder characterized by the simultaneous proliferation of glandular epithelium and myoepithelium. Characteristically, AMEs tend to exhibit benign clinical behavior, although malignant transformation has been reported in a small number of cases. Patients diagnosed with malignant AME over the last 5 years are summarized in Table 1[5-14]. The age distribution ranged from 36 to 78 years (mean 53.0 years). The modalities used for diagnosis and the procedures performed are described below as appropriate.
| No | Ref. | Age | Malignant components | Surgery | Local recurrence | Distant recurrence | Outcome | MMG | US | MRI | Biopsy |
| 1 | Jones et al[5] | 78 | Epithelial and myoepithelial | Lumpectomy→mastectomy+SNB | No | No | 1-yr survival | NA | NA | NA | CNB(suspicious for AME) |
| 2 | Yuan et al[6] | 51 | NA | Lumpectomy→lumpectomy→mastectomy+SNB | Unknowno | Unknown | Unknown | Mass | Cyst-solid space-occupying lesions | Enhanced mass with suspected invasion of surrounding tissue | CNB (suspicious for AME) |
| 3 | Yuan et al[6] | 58 | NA | Lumpectomy→mastectomy | Yes | Yes (bone, carcinomatous pleurisy) | Died after 22 months | NA | NA | NA | CNB (suspicious for AME) |
| 4 | Hempenstall et al[7] | 45 | Epithelial and myoepithelial | Lumpectomy | No | No | Survival (period unknown) | Mass | Heterogeneously hypoechoic mass | NA | CNB (diagnosis of phyllodes tumor) |
| 5 | Watanabe et al[8] | 41 | Epithelial and myoepithelial | Lumpectomy→mastectomy | Yes | Yes (lung) | Unknown | Mass | NA | NA | CNB (diagnosis of papilloma) |
| 6 | Kakkar et al[9] | 36 | Myoepithelial predominant | Lumpectomy+SNB | No | No | 1-yr survival | Mass | NA | Lobulated mass with infiltrative margins | CNB (diagnosis of invasive carcinoma) |
| 7 | Febres-Aldana et al[10] | 47 | Epithelial and myoepithelial | Lumpectomy | No | No | 1-yr survival | Mass | NA | NA | CNB (diagnosis of malignant AME) |
| 8 | Lari et al[11] | 39 | Epithelial and myoepithelial | Lumpectomy→mastectomy+ALND | No | No | Unknown | Ill-defined irregular mass | Low echoic mass | NA | CNB (suspicious for AME) |
| 9 | Moro et al[12] | 64 | Epithelial and myoepithelial | Lumpectomy→mastectomy+ALND | Yes | Yes (lung) | Died 17 months later | NA | Hypoechoic mass with cystic lesion | Multiple masses with invasion of the skin and pectoralis muscle | CNB (diagnosis of AME) |
| 10 | Zhang et al[13] | 64 | Malignant degeneration of the myoepithelium | Lumpectomy+SNB | No | No | 1-yr survival | Mass | Hypoechoic mass | NA | Excisional biopsy(diagnosis of ductal carcinoma) |
| 11 | Parikh et al[14] | 61 | Epithelial and myoepithelial | Lumpectomy | No | No | Unknown | FAD | Heterogeneously hypoechoic | NA | CNB(suspicious for AME) |
| 12 | Our case | 53 | Myoepithelial predominant | Lumpectomy→lumpectomy→mastectomy | Yes | No | 2-yr survival | Mass | Mass with cystic change | Multiple masses with cystic change with invasion of the pectoralis muscle | CNB(diagnosis of AME) |
The malignant transformation of AME is indicated by features such as prominent cytological atypia, an elevated mitotic index, necrosis, and metastasis. Because of the biphasic nature of the tumor, carcinomas may arise from the glandular epithelium, myoepithelium, or both. In the present case, the proliferation of the myoepithelium was initially more prominent than that of the glandular epithelium in the initial surgical specimen. At the time of recurrence, the glandular epithelium was eradicated, and the tumor was mainly myoepithelium and epithelium with squamous metaplasia. The proportion of myoepithelium and glandular epithelium proliferation differs from case to case. The case of a patient with different proportions in the metastatic site and the primary tumor has also been reported[12]. Malignant transformation at the time of local recurrence has been reported, but the number of cases is small[8,12,15]. Our patient experienced repeated local recurrences despite negative margins and eventually required a total mastectomy. Most AMEs can be treated by local excision, but local recurrences have been found to occur 8 mo to 5 years after the initial excision[8,12]. Recurrence despite negative surgical margins is very rare[12]
There were no specific mammography findings indicative of a diagnosis of AME described in previous studies. Most reports describe a hypoechoic mass on US, but there have been cases with cystic degeneration, as in the present case[6,12]. Although MRI has only been described for a few AME patients, MRI is useful in some cases, such as for our patient, because it can reveal invasion of the surrounding tissue[6,9,12]. Although most patients undergo preoperative CNB, care should be taken to avoid misdiagnoses, as the pathological findings may indicate other tumors, including phyllodes tumors[7], papillomas[8] or noninvasive carcinomas[13].
There are no clear treatment guidelines for AME. Lumpectomy with a safety margin or quadrantectomy is often performed, and total mastectomy is sometimes performed for large or suspected malignant tumors[5,6,8,11-13,16]. Incomplete resection or malignant transformation is a risk factor for local recurrence, but it is important to note that local recurrence can occur even with clearly negative margins in a benign lesion, as observed in our patient. It will become clearer as more cases are reported which patients should undergo total mastectomy and whether reconstruction is possible after resection. Axillary lymph node metastases are rare. Cases of axillary dissection have been reported[11,12], but the data on the indication for this approach and its efficacy are inconclusive. There are no data about adjuvant radiotherapy and/or chemotherapy. Therefore, adjuvant therapy was not administered in the present case.
To conclude, we report a rare case of a patient with AME with repeated local recurrences and further malignant transformation despite negative excisional margins. AME may recur repeatedly even after lumpectomy with safety margins, as in the present case, and the patients should be carefully monitored.
| 1. | Korolczuk A, Amarowicz M, Bąk K, Korobowicz E, Koncewicz T. Adenomyoepithelioma of the breast with late pulmonary metastases - case report and review of the literature. J Cardiothorac Surg. 2016;11:121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 2. | Awamleh AA, Gudi M, Shousha S. Malignant adenomyoepithelioma of the breast with lymph node metastasis: a detailed immunohistochemical study. Case Rep Pathol. 2012;2012:305858. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Lee S, Oh SY, Kim SH, Lee JH, Kim DC, Cho SH, Lee M, Kim HJ. Malignant Adenomyoepithelioma of the Breast and Responsiveness to Eribulin. J Breast Cancer. 2015;18:400-403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Ahmadi N, Negahban S, Aledavood A, Daneshbod K, Daneshbod Y. Malignant adenomyoepithelioma of the breast: a review. Breast J. 2015;21:291-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Jones M, Fletcher J. Malignant adenomyoepithelioma of the breast. Pathology. 2017;49:322-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Yuan Z, Qu X, Zhang ZT, Jiang WG. Lessons From Managing the Breast Malignant Adenomyoepithelioma and the Discussion on Treatment Strategy. World J Oncol. 2017;8:126-131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (2)] |
| 7. | Hempenstall LE, Saxena M, Donaldson E. Malignant adenomyoepithelioma with multifocal adenosquamous carcinoma of the breast: A case report. Breast J. 2019;25:731-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Watanabe S, Otani T, Iwasa T, Takahama T, Takeda M, Sakai K, Nishio K, Ito A, Nakagawa K. A Case of Metastatic Malignant Breast Adenomyoepithelioma With a Codon-61 Mutation of HRAS. Clin Breast Cancer. 2019;19:e589-e592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Kakkar A, Jangra K, Kumar N, Sharma MC, Mathur SR, Deo SS. Epithelial-myoepithelial carcinoma of the breast: A rare type of malignant adenomyoepithelioma. Breast J. 2019;25:1273-1275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Febres-Aldana CA, Mejia-Mejia O, Krishnamurthy K, Mesko T, Poppiti R. Malignant transformation in a Breast Adenomyoepithelioma Caused by Amplification of c-MYC: A Common pathway to Cancer in a Rare Entity. J Breast Cancer. 2020;23:93-99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Lari EA, Lari AA, Alsaeed T. Malignant adenomyoepithelioma of the breast: A case report. Int J Surg Case Rep. 2020;72:56-58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Moro K, Sakata E, Nakahara A, Hashidate H, Gabriel E, Makino H. Malignant adenomyoepithelioma of the breast. Surg Case Rep. 2020;6:118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Zhang Z, Wang Y, Xie X, Peng J, Hong J, Bi L, Yang M. Malignant adenomyoepithelioma of the breast: A case report. Medicine (Baltimore). 2021;100:e24461. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Parikh P, Jameel Z, Falcon S, Rosa M, Kiluk J, Hoover S, Soliman H, Ataya D. Adenomyoepithelioma of the breast: Case series and literature review. Clin Imaging. 2021;75:157-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 15. | Zhang L, Qin G, He Z, Chen W, Yang L. The mammography and MRI manifestations of adenomyoepithelioma of the breast. Clin Radiol. 2016;71:235-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Ito R, Ota D, Ando S, Mori M, Fukuuchi A. A case of adenomyoepithelioma with myoepithelial carcinoma of the breast. Clin Case Rep. 2019;7:930-934. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Meshikhes AW S-Editor: Ma YJ L-Editor: A P-Editor: Guo X