Published online Oct 16, 2021. doi: 10.12998/wjcc.v9.i29.8858
Peer-review started: May 30, 2021
First decision: June 27, 2021
Revised: June 29, 2021
Accepted: August 16, 2021
Article in press: August 16, 2021
Published online: October 16, 2021
Processing time: 137 Days and 20.4 Hours
Gastrointestinal perforation complicated by subphrenic abscess is a surgical emergency. Its diagnosis relies mainly on X-ray or computed tomography (CT), while the value of ultrasound, especially contrast-enhanced ultrasound (CEUS), has been underestimated.
A 37-year-old man presented with fever and edema of the lower extremities for 10 d. He had a history of laparoscopic repair of gastroduodenal perforation 6 mo prior. His first-time intravenous CEUS indicated a diagnosis of subphrenic abscess. He received antibiotic therapy and ultrasound-guided percutaneous drainage of the abscess. However, second-time intravenous CEUS revealed an unsatisfactory therapeutic effect. Intracavitary CEUS was proposed, and this examination detected communication between the abscess and the stomach. Upper gastrointestinal perforation complicated by fistula formation and subphrenic abscess was diagnosed with the help of CEUS. Abdominal CT and esophagogastroduodenoscopy confirmed the diagnosis. The patient recovered after the perforation was repaired by surgery.
Intravenous and intracavitary CEUS provides helpful information for the diagnosis of upper gastrointestinal perforation complicated by fistula formation and subphrenic abscess.
Core Tip: Gastrointestinal perforation complicated by subphrenic abscess is a surgical emergency that is diagnosed mainly by X-ray or computed tomography. We present a case diagnosed with upper gastrointestinal perforation complicated by fistula formation and subphrenic abscess, with the rare application of intravenous and intracavitary contrast-enhanced ultrasound (CEUS). This case highlights that CEUS provides helpful information for diagnosis, with the advantages of bedside availability, real-time application, convenience, economical aspect, and lack of radiation.
- Citation: Qiu TT, Fu R, Luo Y, Ling WW. Diagnosis of upper gastrointestinal perforation complicated with fistula formation and subphrenic abscess by contrast-enhanced ultrasound: A case report. World J Clin Cases 2021; 9(29): 8858-8863
- URL: https://www.wjgnet.com/2307-8960/full/v9/i29/8858.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i29.8858
Gastrointestinal perforation is a common surgical emergency. Due to leakage of alimentary contents into the peritoneal cavity, most cases are associated with high mortality and morbidity. Classic subdiaphragmatic air on chest X-ray may be absent, and computed tomography (CT) is a more sensitive investigation tool for stable patients[1]. Most of the reported cases present X-ray or CT findings of gastrointestinal perforation[2-4], while few of them have demonstrated the crucial role of ultrasound and contrast-enhanced ultrasound (CEUS) for gastrointestinal perforation diagnosis[5,6]. Here, we report a case of upper gastrointestinal perforation complicated by fistula formation and subphrenic abscess, in which both intravenous CEUS (IVCEUS) and intracavitary CEUS (ICCEUS) were applied and provided the key clue for diagnosis. We present the following case in accordance with the CARE reporting checklist.
A 37-year-old man presented with fever and edema of the lower extremities for 10 d.
The patient had a history of laparoscopic repair of gastroduodenal perforation 6 mo prior.
There was no history of past illness.
There was no personal and family history.
No physical examination was performed.
Laboratory testing showed elevations in leukocyte count, neutrophil ratio, and C-reactive protein level (9.61 × 109/L, 77.6%, and 23.1 mg/L; reference value, 3.5-9.5 × 109/L, 40%-75%, and < 5 mg/L, respectively).
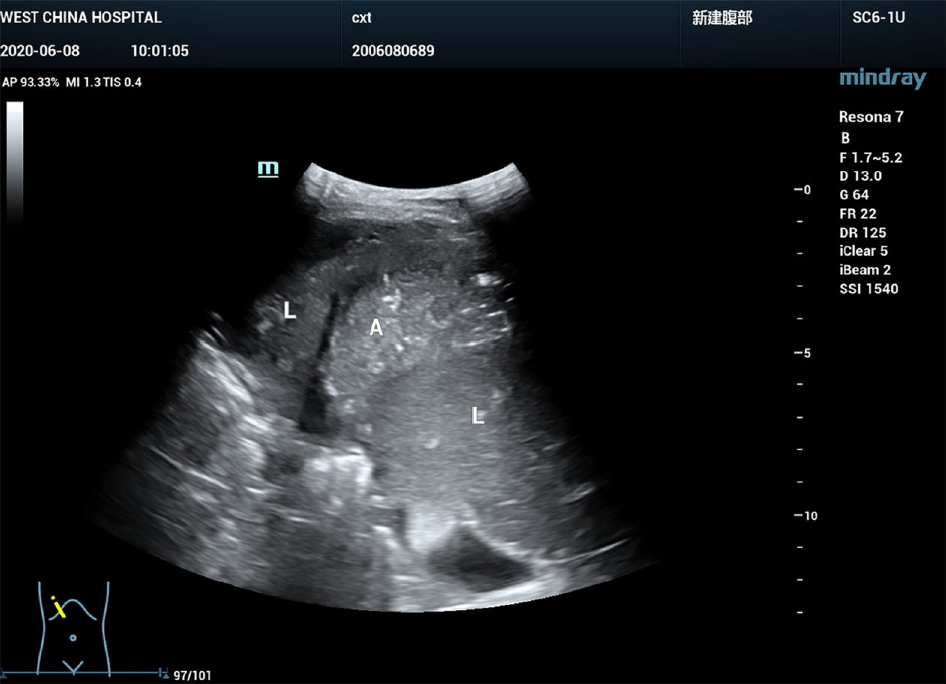
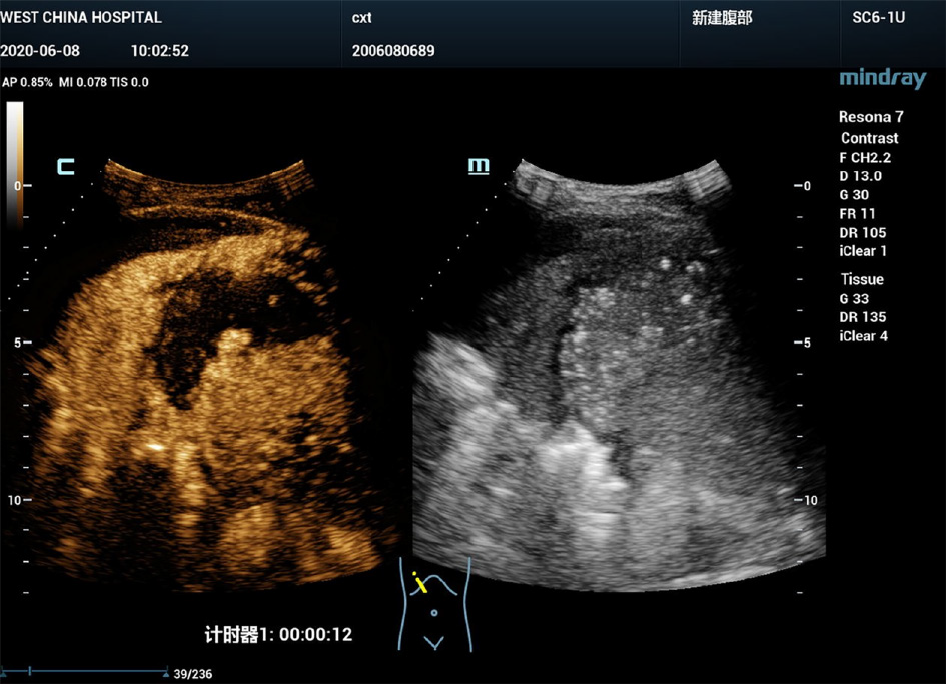
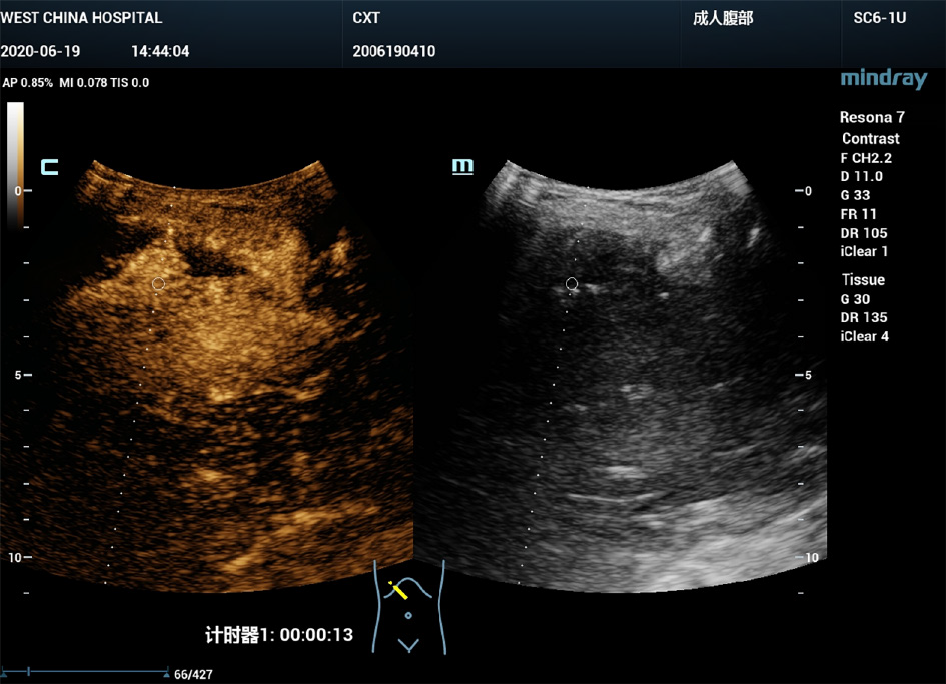
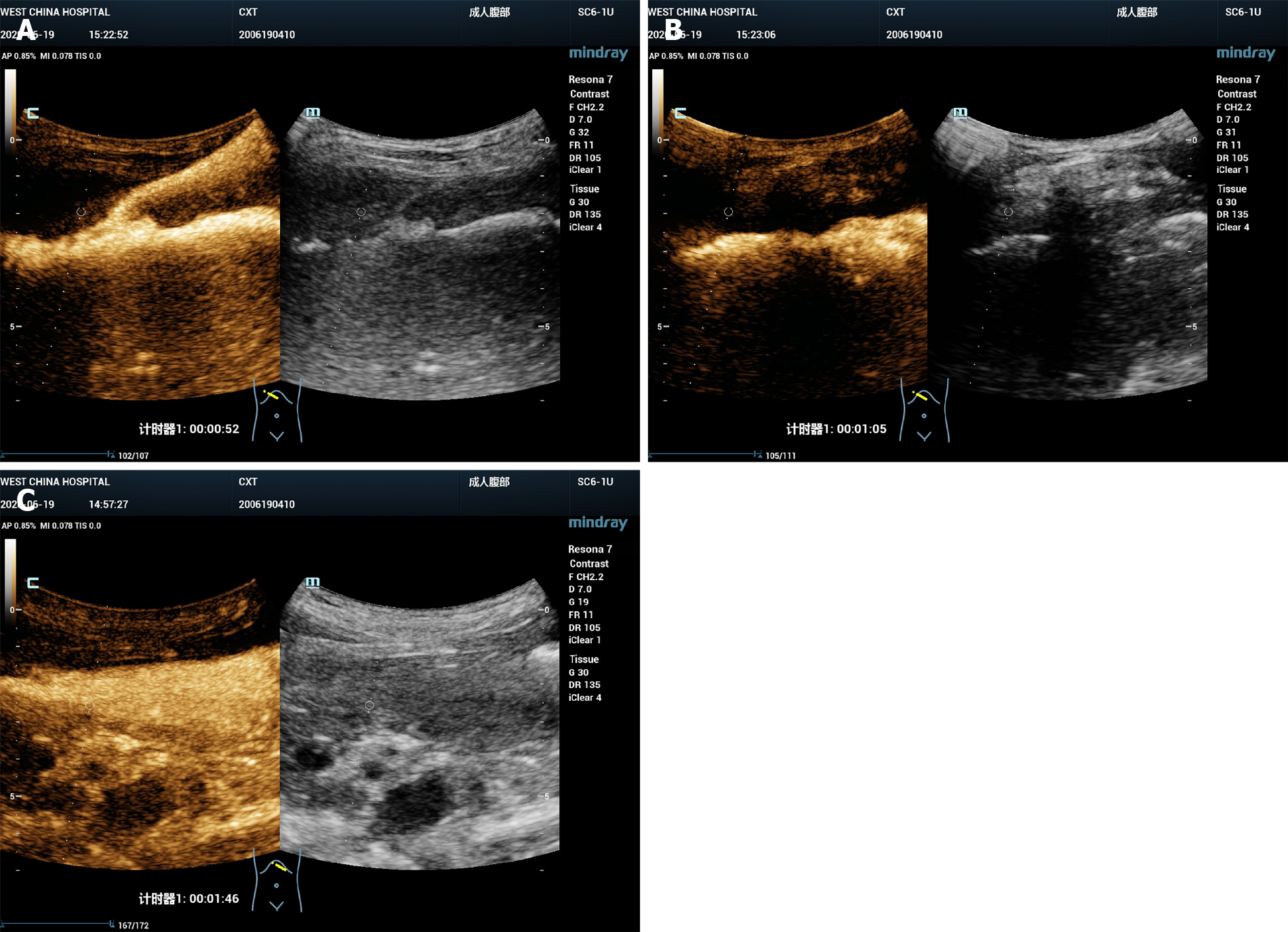
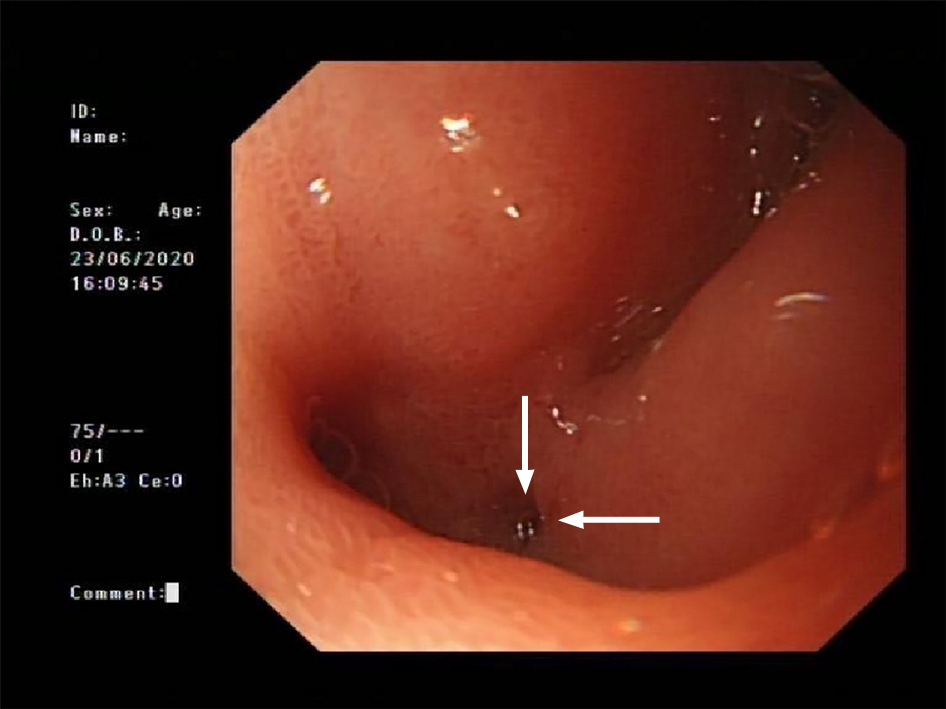
B-mode ultrasound revealed a 6.0 cm × 3.0 cm right subphrenic mass with a heterogeneous echo (Figure 1). The mass displayed a large-scale nonenhancement area with slightly enhanced septa inside for the first IVCEUS (Figure 2). Right subphrenic abscess was diagnosed. The patient received antibiotic therapy and ultrasound-guided percutaneous drainage of the abscess. Nine days after treatment, second-time IVCEUS was performed to evaluate the therapeutic effects. No significant change in abscess size occurred after treatment (before treatment, 6.0 cm × 3.0 cm; after treatment, 6.0 cm × 1.7 cm) (Figure 3). However, the drainage volume from the catheter was 100-200 mL each day. In this situation, ICCEUS was proposed. SonoVue (0.2 mL) and 20 mL of saline were injected via the drainage tube. Enhancement was observed from the drainage tube to the stomach through the early to late phase (Figure 4 and Video), which indicated communication between the abscess and stomach. Esophagogastroduodenoscopy (Figure 5) was performed to confirm the diagnosis of duodenal bulbar perforation.
Both ICCEUS and esophagogastroduodenoscopy indicated a diagnosis of duodenal bulbar perforation.
The patient received another 2 wk of antibiotic therapy, drainage of the subphrenic abscess, and jejunum enteral nutrition. The perforation was repaired by surgery. Intervention adherence of the patient was good according to daily medical records.
No adverse or unanticipated events were recorded during the in-hospital days.
CEUS includes intravenous or intracavitary administration of ultrasound contrast agents. IVCEUS helps characterize the enhancement pattern of the focal lesion itself. ICCEUS allows identification of the needle or catheter position, delineation of any cavity or duct, and improved tracking of a fistula. Reported detection and classification of fistulas by ICCEUS include vesico-intestinal fistulas, anal fistulas, and rectovaginal fistulas[6]. In this case, the patient had a history of laparoscopic repair of gastroduodenal perforation 6 mo prior. Recurrent gastroduodenal perforation caused a right subphrenic abscess via a fistula, which has seldom been reported through literature review[7,8]. This type of fistula is characterized by a large volume of digestive fluid leakage, including gastric and intestinal fluids, which are relatively corrosive and may lead to complicated intra-abdominal infection[9]. It was noted by the ultrasound doctor that the relatively large drainage volume (100-200 mL each day) mismatched the relatively small and unchanged size of the abscess based on the findings of two-time IVCEUS. Therefore, ICCEUS was proposed, and a fistula between the subphrenic abscess and upper gastrointestinal tract was diagnosed by ICCEUS, which illuminated the way for further diagnosis and treatment. According to a previous study[10], the sensitivity, specificity, and accuracy of the diagnosis of gastrointestinal fistulas by ICCEUS were 72.7%, 95.0%, and 83.3%, respectively. It can improve the ability to diagnose post-surgical gastrointestinal fistulas as a novel technique and may also play an important role in interventional treatment and follow-up. The patient appreciated the help of CEUS, and the subsequent corresponding treatment was effective from his perspective.
However, ultrasound has its own pitfalls. It is strongly operator-dependent and not suitable for obese patients or those with subcutaneous emphysema. Although the accuracy in detecting the perforation site between CT and CEUS has not been compared, ultrasound may be particularly useful in cases where the radiation burden needs to be limited, notably in children and pregnant women.
In summary, CEUS may be used as a problem-solving tool for the diagnosis of gastrointestinal perforation complicated by fistula and/or abscess formation due to its lack of radiation, capability for real-time imaging of various planes, and availability at the bedside.
| 1. | Weledji EP. An Overview of Gastroduodenal Perforation. Front Surg. 2020;7:573901. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (1)] |
| 2. | Borofsky S, Taffel M, Khati N, Zeman R, Hill M. The emergency room diagnosis of gastrointestinal tract perforation: the role of CT. Emerg Radiol. 2015;22:315-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Toprak H, Yilmaz TF, Yurtsever I, Sharifov R, Gültekin MA, Yiğman S, Yildiz Ş. Multidetector CT findings in gastrointestinal tract perforation that can help prediction of perforation site accurately. Clin Radiol. 2019;74:736.e1-736.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Shin D, Rahimi H, Haroon S, Merritt A, Vemula A, Noronha A, LeBedis CA. Imaging of Gastrointestinal Tract Perforation. Radiol Clin North Am. 2020;58:19-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Coppolino F, Gatta G, Di Grezia G, Reginelli A, Iacobellis F, Vallone G, Giganti M, Genovese E. Gastrointestinal perforation: ultrasonographic diagnosis. Crit Ultrasound J. 2013;5 Suppl 1:S4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 6. | Sidhu PS, Cantisani V, Dietrich CF. The EFSUMB guidelines and recommendations for the clinical practice of contrast-enhanced ultrasound (CEUS) in non-hepatic applications: update 2017 (long version). Ultraschall Med. 2018;39:e2-e44. |
| 7. | Hanaoka Y, Sugimoto D, Nakamura H, Yamashita H, Igarashi H, Ogata I. A case of left subphrenic abscess caused by gastric ulcer penetration that was successfully treated with endoscopic transgastric drainage. Nihon Shokakibyo Gakkai Zasshi. 2016;113:2035-2041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Hamura R, Haruki K, Kumagai Y, Shiba H, Wakiyama S, Yanaga K. Subphrenic abscess due to Clostridium perfringens after hepatic resection for hepatocellular carcinoma following emphysematous cholecystitis: Report of a case. Int J Surg Case Rep. 2020;67:86-90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Li X, Wu X, Bai B, Yu D, Yu P, Zhao Q. [Research progress of the open abdomen in the treatment of gastrointestinal fistula with complicated intra-abdominal infection]. Zhonghua Weichangwaike Zazhi. 2018;21:1446-1450. [PubMed] |
| 10. | Xu EJ, Zhang M, Li K, Su ZZ, Long YL, Zeng QJ, Guo HY, Zheng RQ. Intracavitary Contrast-Enhanced Ultrasound in the Management of Post-Surgical Gastrointestinal Fistulas. Ultrasound Med Biol. 2018;44:502-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Maglangit SACA, Priyadarshi RN S-Editor: Wu YXJ L-Editor: Wang TQ P-Editor: Guo X