Published online Feb 26, 2026. doi: 10.12998/wjcc.v14.i6.118138
Revised: January 11, 2026
Accepted: February 4, 2026
Published online: February 26, 2026
Processing time: 51 Days and 1.1 Hours
Genitourinary sarcomas include testicular sarcomas and are the most common subtype of sarcoma within the genitourinary system. Undifferentiated pleomor
Here we present a 56-year-old male who comes to the urology clinic for left testi
Testicular sarcomas are a rare type of soft tissue sarcoma. The standard treatment of the testicular mass usually begins with radical inguinal orchiectomy. Patients with scrotal sarcomas are at high risk of local and distant recurrence, emphasizing the importance of surgical excision and wide margins. There is little studied reg
Core Tip: Testicular sarcomas are a rare type of soft tissue sarcoma. The standard treatment of the testicular mass usually begins with radical inguinal orchiectomy. Patients with scrotal sarcomas are at high risk of local and distant recurrence, emp
- Citation: Sarver J, Baydoun A, Gudziak M. Undifferentiated testicular pleomorphic sarcoma: A case report. World J Clin Cases 2026; 14(6): 118138
- URL: https://www.wjgnet.com/2307-8960/full/v14/i6/118138.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v14.i6.118138
Testicular sarcomas are the most common subtype of sarcoma within the genitourinary system[1]. Testicular sarcomas are rare, accounting for approximately 1% of soft tissue sarcomas[1]. Undifferentiated pleomorphic sarcoma is a subtype of soft tissue sarcomas that may affect the extremities and retroperitoneum[2]. However, the presence of this subtype within the testicle is rare[3]. Patients usually present with a scrotal or testicular mass and painless scrotal swelling. These sarcomas may behave aggressively, prompting the need for early detection and multidisciplinary treatment[4]. The treatment will usually include surgical resection of the tumor and obtaining negative margins when able[5]. Here we present a case of an undifferentiated pleomorphic testicular sarcoma. This case will explore the presentation and tre
This case is of a 56-year-old male who presents to the urology clinic for left testicular swelling.
The patient initially presented to the clinic for left testicular swelling. He denied any pain in the testicular region. The patient reported the mass increased in size over the last month.
The patient denies any significant past medical history.
The patient denies any significant family history or personal history of prior medical or surgical disease.
On examination, there was a mass appreciated in the left hemiscrotum that was not painful to palpation, and there were no overlying skin changes to the scrotum.
Tumor markers were drawn and showed alpha-fetoprotein (AFP) 2.1 and human chorionic gonadotropin (hCG) < 5.
He initially underwent a scrotal ultrasound that showed an 8.8 cm × 6.1 cm × 7.8 cm hypervascular mass. The right tes
The patient was diagnosed with a left testicular mass, and the plan was to proceed to the operating room for left radical orchiectomy.
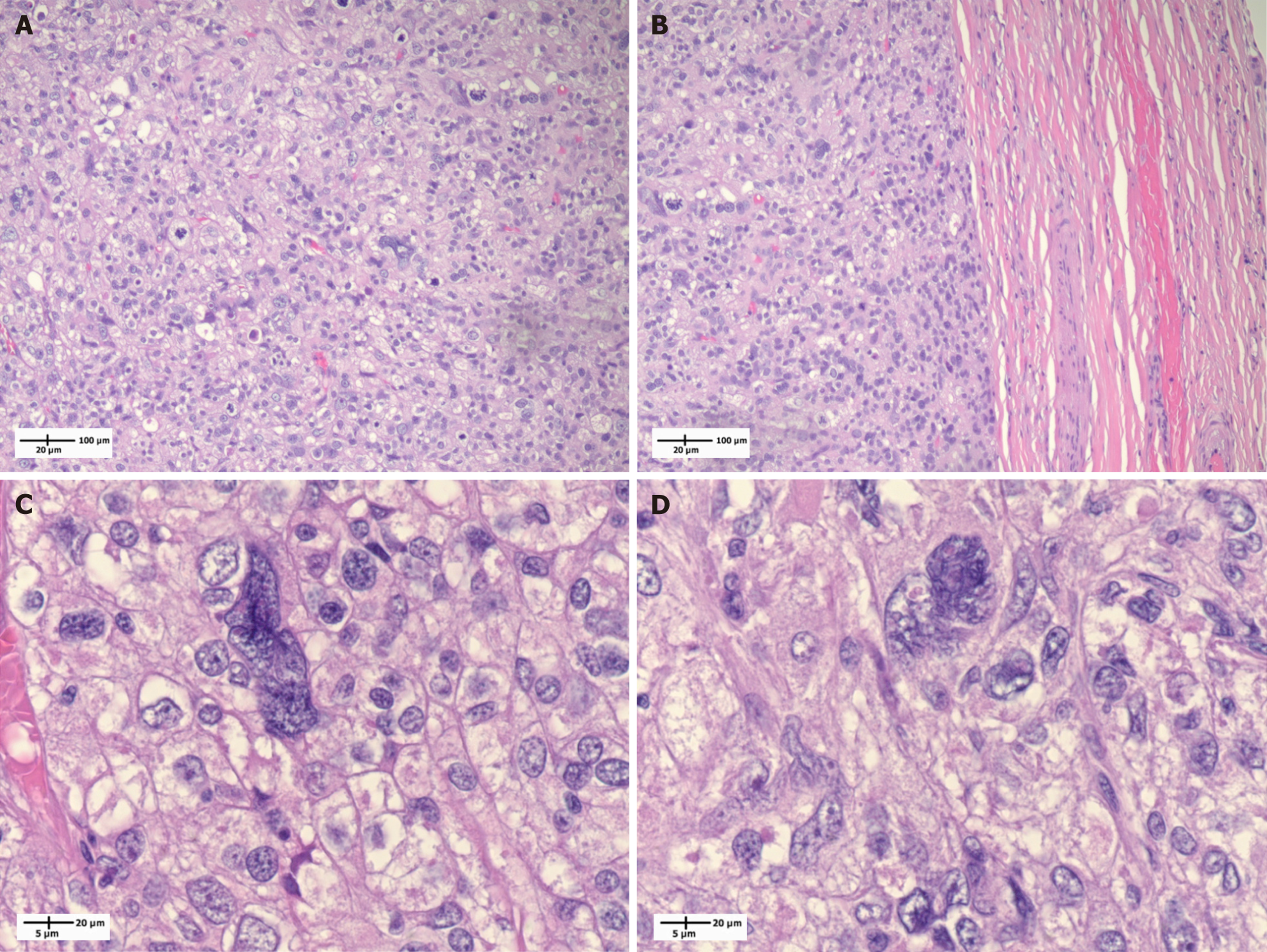
The patient then underwent left radical orchiectomy via an inguinal approach. Pathology revealed undifferentiated pleomorphic sarcoma (grade 3), 9.5 cm in size, and it was limited to the testicle (Figure 1A and B). The surgical margins were negative. His final pathological stage was pT1bNx. On pathologic staining, the pathology slides show a haphazardly growing sheet-like proliferation of spindle cells exhibiting marked nuclear pleomorphism. Additionally, large, bizarre, atypical forms and tumor giant cells were noted (Figure 1C and D). Tumor cells were stained negative for pankeratin, CK5/6, GATA binding protein 3, S100, CD30, CD117, octamer-binding transcription factor 3/4, inhibin, AFP, sal-like 4, desmin, murine double minute 2, and beta-hCG. Tumor cells show patchy positivity for vimentin on staining. Overall, these findings are compatible with an undifferentiated pleomorphic sarcoma.
A follow-up positron emission tomography CT (PET-CT) scan was obtained based on the recommendations of medical oncology to better characterize the lung nodules seen on the initial CT scan. The PET-CT showed no evidence of hyper
Testicular sarcomas are a rare type of soft tissue sarcoma[1]. These sarcomas may arise from the scrotum, epididymis, spermatic cord, and tunica vaginalis[6]. The standard treatment of the testicular mass usually begins with radical inguinal orchiectomy. Patients with scrotal and testicular sarcomas are at high risk of local and distant recurrence, emphasizing the importance of surgical excision and wide margins[7]. As noted in the National Comprehensive Cancer Network guidelines, obtaining appropriate oncological margins for these patients is important, and consideration for adjuvant therapies may be warranted[8]. Dotan et al[9] noted that unfavorable prognostic variables for genitourinary sarcomas include metastasis at presentation, high tumor grade, lack of leiomyosarcoma and liposarcoma elements, large tumor size, incomplete surgical resection, and positive surgical margins. The patient presented in this case underwent prompt radical inguinal orchiectomy after his tumor was diagnosed and had negative margins in the pathological specimen.
Although testicular sarcomas are rare, there are certain pathological characteristics that are specific to this tumor sub
Adjuvant techniques such as retroperitoneal lymph node dissections for these sarcoma cases remain controversial[11]. Some studies report high regional recurrence in subtypes of genitourinary sarcomas and may require retroperitoneal lymph node dissections, including those with rhabdomyosarcoma, fibrous histiocytoma, or fibrosarcoma elements[12]. However, regional nodal recurrence for paratesticular soft tissue sarcoma is rare[7]. Likewise, there is little studied regarding the integration of radiotherapy and chemotherapy for these cases as a neoadjuvant or adjuvant therapy[4]. However, some studies show improvement with radiation and chemotherapy in this patient cohort. Le Doussal et al[13] found that combined radiotherapy and chemotherapy reduced the risk of local recurrence and prolonged patient survival in those with pleomorphic sarcoma[13]. Additionally, Ülker et al[10] report no recurrence at 2-year follow-up for patients who received three courses of chemotherapy for pleomorphic sarcomas[10]. There still remains a gap in the literature and practice for these therapies in these patients; radical orchiectomy remains the important first step in the treatment pathway. There may eventually be a role for immunotherapy for the treatment of sarcomas[14]. In this study, our patient was followed postoperatively without nodal or distant disease as evidenced by his initial PET-CT scan after surgery. This follow-up PET-CT scan was obtained by medical oncology to better characterize the lung nodules seen on the initial CT scan. On PET-CT, there was no evidence of hypermetabolic lymph nodes or masses in the abdomen or pelvis, and the scan noted multiple sub-centimeter lung nodules with no tracer uptake. At this time, he has not received any adjuvant th
Genitourinary sarcomas are a rare entity. The mainstay of treatment remains initial inguinal orchiectomy for those who present with a testicle. As previously reported, with adequate surgical treatment including complete surgical resection, patients may achieve prolonged disease-specific survival[9]. The patient in this study underwent a radical inguinal orchiectomy with negative margins. Pathology revealed testicular pleomorphic sarcoma. His initial tumor markers pre
This case highlights the presentation and treatment of a patient with a rare phenotype of testicular pleomorphic sarcoma treated by radical inguinal orchiectomy. His surgical margins were negative and follow up PET-CT did not reveal any occult metastatic disease. He is healing appropriately after surgery and has not received adjuvant or neoadjuvant the
| 1. | Pollock R, Randall L, O’Sullivan B. Sarcoma Oncology: A Multidisciplinary Approach. 1st ed. People’s Medical Publishing House. 2019. |
| 2. | Weiss SW, Enzinger FM. Malignant fibrous histiocytoma: an analysis of 200 cases. Cancer. 1978;41:2250-2266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 3. | Han JP, Luo WX, Zeng JW, Ma WX, Zhao Q, Xie YQ, Zhang ZC. Recurrent testicular undifferentiated pleomorphic sarcoma. Asian J Androl. 2023;25:541-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 4. | Unlü Y, Huq GE, Ozyalvaçli G, Zengin M, Koca SB, Yücetas U, Bozkurt ER, Behzatoğlu K. Paratesticular sarcomas: A report of seven cases. Oncol Lett. 2015;9:308-312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | Gronchi A, Miah AB, Dei Tos AP, Abecassis N, Bajpai J, Bauer S, Biagini R, Bielack S, Blay JY, Bolle S, Bonvalot S, Boukovinas I, Bovee JVMG, Boye K, Brennan B, Brodowicz T, Buonadonna A, De Álava E, Del Muro XG, Dufresne A, Eriksson M, Fagioli F, Fedenko A, Ferraresi V, Ferrari A, Frezza AM, Gasperoni S, Gelderblom H, Gouin F, Grignani G, Haas R, Hassan AB, Hecker-Nolting S, Hindi N, Hohenberger P, Joensuu H, Jones RL, Jungels C, Jutte P, Kager L, Kasper B, Kawai A, Kopeckova K, Krákorová DA, Le Cesne A, Le Grange F, Legius E, Leithner A, Lopez-Pousa A, Martin-Broto J, Merimsky O, Messiou C, Mir O, Montemurro M, Morland B, Morosi C, Palmerini E, Pantaleo MA, Piana R, Piperno-Neumann S, Reichardt P, Rutkowski P, Safwat AA, Sangalli C, Sbaraglia M, Scheipl S, Schöffski P, Sleijfer S, Strauss D, Strauss S, Sundby Hall K, Trama A, Unk M, van de Sande MAJ, van der Graaf WTA, van Houdt WJ, Frebourg T, Casali PG, Stacchiotti S; ESMO Guidelines Committee, EURACAN and GENTURIS. Soft tissue and visceral sarcomas: ESMO-EURACAN-GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up(☆). Ann Oncol. 2021;32:1348-1365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 701] [Article Influence: 140.2] [Reference Citation Analysis (2)] |
| 6. | Lioe TF, Biggart JD. Tumours of the spermatic cord and paratesticular tissue. A clinicopathological study. Br J Urol. 1993;71:600-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 68] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Chowdhry VK, Kane JM 3rd, Wang K, Joyce D, Grand'Maison A, Mann GN. Testicular, Spermatic Cord, and Scrotal Soft Tissue Sarcomas: Treatment Outcomes and Patterns of Failure. Sarcoma. 2021;2021:8824301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | von Mehren M, Kane JM, Agulnik M, Bui MM, Carr-Ascher J, Choy E, Connelly M, Dry S, Ganjoo KN, Gonzalez RJ, Holder A, Homsi J, Keedy V, Kelly CM, Kim E, Liebner D, McCarter M, McGarry SV, Mesko NW, Meyer C, Pappo AS, Parkes AM, Petersen IA, Pollack SM, Poppe M, Riedel RF, Schuetze S, Shabason J, Sicklick JK, Spraker MB, Zimel M, Hang LE, Sundar H, Bergman MA. Soft Tissue Sarcoma, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022;20:815-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 251] [Reference Citation Analysis (0)] |
| 9. | Dotan ZA, Tal R, Golijanin D, Snyder ME, Antonescu C, Brennan MF, Russo P. Adult genitourinary sarcoma: the 25-year Memorial Sloan-Kettering experience. J Urol. 2006;176:2033-8; discussion 2038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 138] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 10. | Ülker V, Atalay HA, Çakır Ç, Sargan A. Giant malignant fibrous histiocytoma of the testis. Turk J Urol. 2018;44:79-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 11. | Bansal D, Varghese J, Babu M, Mehta N, Rathore RS, Mehta S, Pillai BS, Sam MP, Moorthy K. Leiomyosarcoma Presenting as a Scrotal Mass. Urol Case Rep. 2016;7:42-44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Catton CN, Cummings BJ, Fornasier V, O'Sullivan B, Quirt I, Warr D. Adult paratesticular sarcomas: a review of 21 cases. J Urol. 1991;146:342-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 65] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Le Doussal V, Coindre JM, Leroux A, Hacene K, Terrier P, Bui NB, Bonichon F, Collin F, Mandard AM, Contesso G. Prognostic factors for patients with localized primary malignant fibrous histiocytoma: a multicenter study of 216 patients with multivariate analysis. Cancer. 1996;77:1823-1830. [PubMed] [DOI] [Full Text] |
| 14. | Wu JT, Nowak E, Imamura J, Leng J, Shepard D, Campbell SR, Scott J, Nystrom L, Mesko N, Schwartz GK, Burke ZDC. Immunotherapy in the Treatment of Undifferentiated Pleomorphic Sarcoma and Myxofibrosarcoma. Curr Treat Options Oncol. 2025;26:891-909. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/