Published online Nov 26, 2025. doi: 10.12998/wjcc.v13.i33.112045
Revised: July 23, 2025
Accepted: October 28, 2025
Published online: November 26, 2025
Processing time: 128 Days and 8.9 Hours
Psoriasis is a chronic inflammatory condition related to an increased atherosclerotic cardiovascular disease (ASCVD) risk.
To investigate whether lipoprotein (a) [Lp(a)] levels are increased in patients with psoriasis.
A comprehensive literature search up to January 30, 2025 was conducted utilizing PubMed and Cochrane Library databases. A qualitative synthesis and a meta-analysis on Lp(a) mean differences (MD) between psoriasis cases and healthy controls (HC) was performed. The protocol of this meta-analysis has been re
Eighteen studies with 1650 psoriasis patients and 1621 HC were eligible for qua
Our findings suggest that Lp(a) levels are significantly elevated in psoriasis patients, further adding to their ASCVD risk.
Core Tip: Elevated lipoprotein (a) [Lp(a)] levels represent an atherosclerotic cardiovascular disease risk (ASCVD) factor. The findings of the present systematic review and meta-analysis show a significant increase of Lp(a) levels in psoriasis patients compared with healthy controls. As such, Lp(a) measurement in patients with psoriasis may be of use for a more precise determination of ASCVD risk and treatment goals.
- Citation: Fragkouli MR, Makris A, Mastori-Kourmpani C, Karpettas N, Hadjigeorgiou GF, Tsioutis C, Filippatos TD, Agouridis AP. Lipoprotein (a) levels are elevated in psoriasis: An updated systematic review and meta-analysis. World J Clin Cases 2025; 13(33): 112045
- URL: https://www.wjgnet.com/2307-8960/full/v13/i33/112045.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i33.112045
Psoriasis is a chronic systemic inflammatory skin disease, affecting more than 60 million people worldwide[1]. Central to its etiopathogenesis is an interplay between the innate and adaptive immune systems, while heritability is the major contributor in disease development[2]. Psoriasis-related persistent inflammation is associated with oxidative stress, vascular dysfunction, and lipid abnormalities, all of which are linked to atherosclerotic cardiovascular disease (ASCVD)[3]. Simultaneously, people with psoriasis commonly exhibit ASCVD risk factors including metabolic syndrome (i.e., central obesity, hypertension, glucose resistance, hypertriglyceridemia, and hypoalphalipoproteinemia), diabetes, me
Lipoprotein (a) [Lp(a)] is a low-density lipoprotein (LDL)-like particle synthesized in the liver, whose pathognomic constituent is apolipoprotein (apo) (a). Lp(a) is mostly genetically determined, following an autosomal dominant pattern, and is hardly influenced by lifestyle factors[8]. It possesses pro-inflammatory, pro-atherogenic and pro-thrombotic properties, and is associated with elevated ASCVD risk even in people with an otherwise normal lipid profile[9-11]. Lp(a) levels can be affected by chronic inflammatory conditions, including psoriasis, largely via the effect of the interleukin (IL)-6 axis on the expression of the LPA gene and other inflammation pathways. Consequently, biologic treatments have shown promise in lowering Lp(a) levels[8].
The aim of the present systematic review and meta-analysis is the updated synthesis of published data regarding Lp(a) levels in patients with psoriasis, incorporating all recent evidence.
We performed a qualitative and quantitative synthesis of eligible studies to evaluate whether Lp(a) levels are increased in patients with psoriasis.
An extensive search on PubMed and Cochrane Library databases was conducted until January 30, 2025 using the following search terms: [Lipoprotein (a) OR lp(a)] AND [(psoriasis) OR (psoriatic arthritis)]. The protocol of this systematic review was registered in PROSPERO (No. CRD420250652465) and adheres to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 statement (PRISMA Statement, Ottawa, ON, Canada)[12].
Eligibility criteria for this systematic review included prospective and retrospective observational studies (case control or cohort) that compared adult patients with psoriasis with a control group. The eligibility criteria were defined using the population, intervention, comparators/controls, outcomes, and study design framework, as follows: (1) Population: Patients with psoriasis (including psoriatic arthritis); (2) Intervention: Measurement of Lp(a) levels in psoriasis patients vs controls; (3) Comparator: Healthy controls (HC); (4) Outcome: Lp(a) mean difference between psoriasis patients and HC; and (5) Study design: Observational studies, prospective and retrospective.
Upon deduplication, two independent authors (Fragkouli MR and Agouridis AP) screened and identified records by abstract, title, or both. Relevant full-texts were retrieved and assessed for eligibility based on the aforementioned criteria. Consensus was reached after discussion between reviewers. The following data were collected from eligible studies: (1) Authors; (2) Year of publication; (3) Country; (4) Study design; (5) Population characteristics; and (6) Lp(a) values of psoriasis patients and HC.
The Newcastle-Ottawa Scale (NOS) was applied for each study in order to perform a quality assessment of the included studies[13]. Three primary factors are evaluated by the NOS: (1) Selection; (2) Comparability; and (3) Exposure or outcome. For a more thorough evaluation, these categories are further divided, depending on study design. The highest possible score for each study is 9 points. Good quality studies are indicated by scores of 7-9, while studies scoring 4-6 and 0-3 are at moderate and high risk for bias, respectively.
A meta-analysis was also carried out to enable a quantitative assessment of the included studies. Continuous variables were used to represent outcome estimates. For continuous data, mean differences (MD) and 95%CI of Lp(a) levels among patients with psoriasis and HC were computed. In addition, a sensitivity analysis according to place of origin was performed among the European and Asian populations. Review Manager version 5.0 software (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark, 2008) was used for statistical analysis; a P value of less than 0.05 is deemed statistically significant.
Regarding heterogeneity analysis, the I2 test was used to determine whether statistical heterogeneity exists among the included studies. The heterogeneity was categorized as low, moderate, or high, if the I2 values were 25%, 50%, or more than 75%, respectively. A random effects model was employed when the P value was less than 0.10. The Q test and the I2 statistic were used to assess inter-trial heterogeneity.
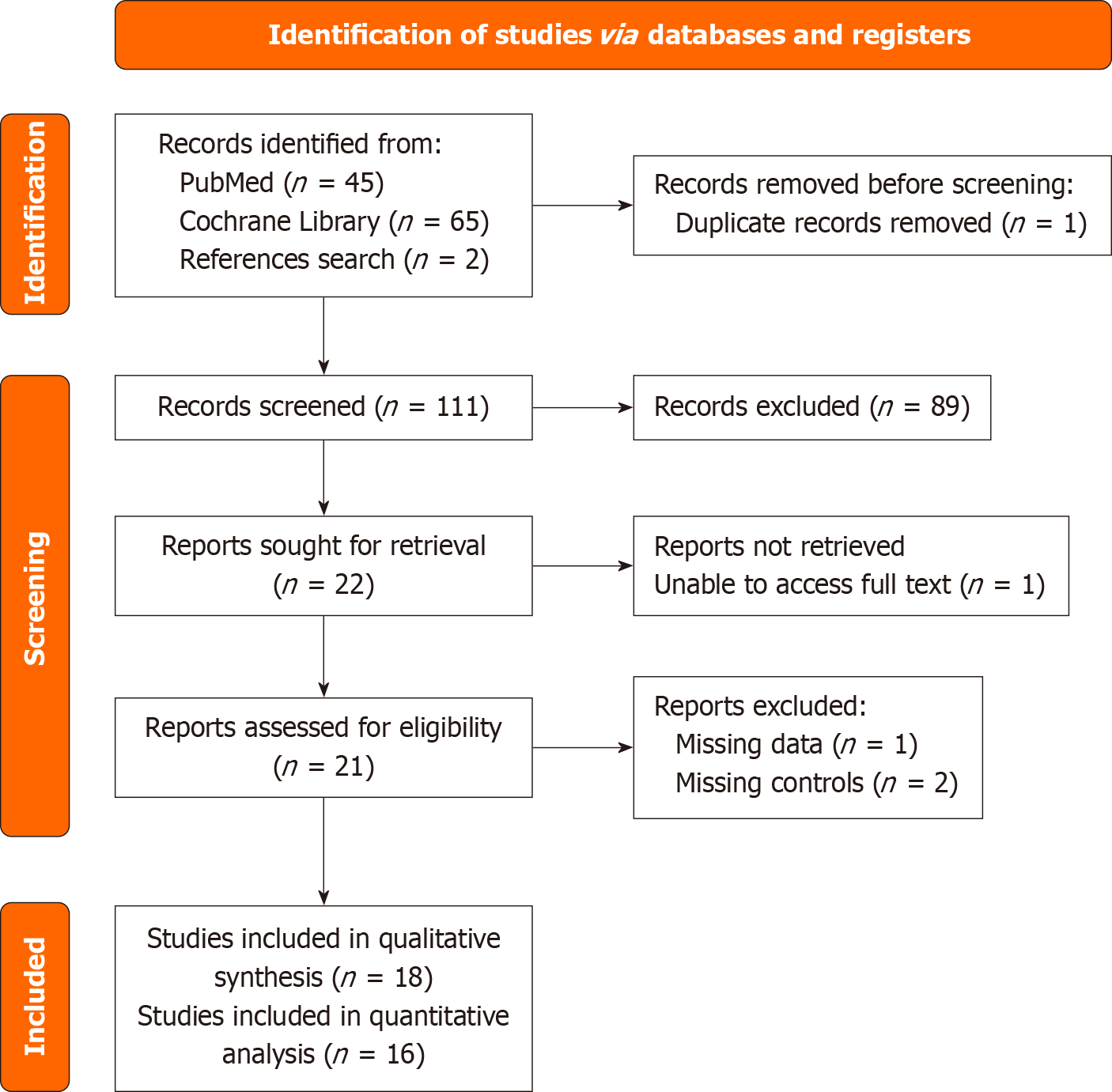
A PRISMA chart was created to summarize the literature search (Figure 1). Upon database search, 112 records were retrieved. Of these, 89 records were eliminated after title and abstract assessment. Following full-text review, 4 more studies were found ineligible for inclusion. Finally, 18 records[14-31] were included in the qualitative synthesis, while 16 were used for quantitative synthesis purposes.
Study characteristics are presented in Table 1[14-31]. All of the 18 included studies were observational (cohort and case control) and performed between 1994 and 2022. A total of 3271 participants were included, of whom 1650 were identified as cases and 1621 were identified as HC. The studies were conducted in the following countries: (1) United States (1 study); (2) India (2 studies); (3) United Kingdom (1 study); (4) Turkey (2 studies); (5) China (2 studies); (6) Portugal (2 studies); (7) Italy (2 studies); (8) Iran (3 studies); (9) Japan (1 study); (10) Poland (1 study); and (11) Greece (1 study). Included studies may be further classified into two distinct groups: European population (5 studies) vs Asian population (10 studies). Three studies[16,21,24] focused on patients with psoriatic arthritis. Reported comorbidities included smoking, alcohol consumption, dyslipidemia and obesity. The mean age of all participants was 42 years, and the male to female ratio was similar between groups, except for two studies[14,18] which enrolled only male participants. Lp(a) levels of both psoriasis cases and HC were available in all 18 studies (Table 2)[14-31].
| Ref. | Year | Country | Study design | Population | Comorbidities | Psoriatic patients mean age (mean ± SD) | Controls mean age (mean ± SD) | Age and sex-matched controls |
| Seçkin et al[14] | 1994 | Turkey | Cohort | Psoriasis | Obesity, hypertension, smoking | 36 ± 13 | 36 ± 15 | Male only |
| Jones et al[16] | 2000 | United Kingdom | Case-control | Psoriatic arthritis | Gout | 46 ± 13 | 46 ± 13 | Yes |
| Rocha-Pereira et al[15] | 2001 | Portugal | Case-control | Psoriasis | - | 47 ± 12 | 47 ± 13 | Yes |
| Uyanik et al[17] | 2002 | Turkey | Case-control | Psoriasis | - | 38 ± 5 | - | Yes |
| Pietrzak et al[18] | 2009 | Poland | Cross-sectional | Psoriasis | - | 37 ± 13 | 34 ± 12 | Male only |
| Coimbra et al[19] | 2010 | Portugal | Cohort | Psoriasis | Smoking | 43 ± 15 | 47 ± 15 | Yes |
| Asefi et al[23] | 2012 | Iran | Case-control | Psoriasis | - | 35 ± 11 | 36 ± 13 | Yes |
| Ferretti et al[20] | 2012 | Italy | Case-control | Psoriasis | - | 47 ± 13 | 41 ± 8 | Yes |
| Oliviero et al[21] | 2012 | Italy | Case-control | Psoriatic arthritis | - | 45 ± 15 | - | Yes |
| Nemati et al[22] | 2013 | Iran | Cross-sectional | Psoriasis | - | 35 ± 10 | 33 ± 11 | Yes |
| Asefi et al[25] | 2014 | Iran | Case-control | Psoriasis | - | 35 ± 11 | 36 ± 13 | Yes |
| Papagoras et al[24] | 2014 | Greece | Cross-sectional | Psoriasis | Hypertension, smoking | 48 | 49 | Yes |
| Sunitha et al[26] | 2015 | India | Case-control | Psoriasis | - | 45 ± 14 | 43 ± 11 | Yes |
| Sorokin et al[27] | 2018 | Japan | Cohort | Psoriasis | Dyslipidemia | 50 ± 13 | 40 ± 14 | Yes |
| Wadhwa et al[28] | 2019 | India | Case-control | Psoriasis and psoriatic arthritis | Smoking and alcohol consumption, hypertension, diabetes, metabolic syndrome | 41 ± 14 | 43 ± 5 | Yes |
| Miao et al[29] | 2019 | China | Case-control | Psoriasis, psoriatic arthritis | Obesity, alcohol consumption, smoking, hypertension, hyperlipidemia, diabetes | 52 ± 14 | 50 ± 14 | Yes |
| Garshick et al[31] | 2021 | United States | Case-control | Psoriasis | Obesity | 44 ± 14 | - | Yes |
| Wang et al[30] | 2022 | China | Case-control | Psoriasis and psoriatic arthritis | Obesity, smoking and alcohol consumption, hypertension, diabetes, metabolic syndrome | NA | NA | No |
| Ref. | Year | Psoriasis patients (n) | Psoriasis Lp(a) (mg/dL) | Controls (n) | Controls Lp(a) (mg/dL) | P value |
| Seçkin et al[14] | 1994 | 32 | 13.8 ± 11.8 | 13 | 7.5 ± 8.9 | NS |
| Jones et al[16] | 2000 | 50 | 23.0 ± 20.1 | 50 | 21.8 ± 20.3 | NS |
| Rocha-Pereira et al[15] | 2001 | 48 | 63.8 ± 40.1 | 40 | 31.8 ± 18.1 | < 0.001 |
| Uyanik et al[17] | 2002 | 72 | 27.4 ± 3.6 | 30 | 19.4 ± 4.5 | < 0.01 |
| Pietrzak et al[18] | 2009 | 34 | 34.2 ± 23.6 | 26 | 21.9 ± 14.4 | 0.029 |
| Coimbra et al[19] | 2010 | 34 | 57.0 ± 49.7 | 37 | 34.9 ± 26.4 | ≤ 0.01 |
| Asefi et al[23] | 2012 | 100 | 19.2 ± 16.5 | 100 | 17.1 ± 17.0 | NS |
| Ferretti et al[20] | 2012 | 23 | 29.6 ± 18.3 | 25 | 12.8 ± 2.6 | < 0.001 |
| Oliviero et al[21] | 2012 | 14 | 9.7 | 33 | 5.6 | NS |
| Nemati et al[22] | 2013 | 90 | 19.2 ± 16.5 | 90 | 17.1 ± 15.1 | NS |
| Asefi et al[25] | 2014 | 100 | 19.4 ± 16.9 | 100 | 17.1 ± 17.1 | NS |
| Papagoras et al[24] | 2014 | 56 | 12.5 | 71 | 13.5 | NS |
| Sunitha et al[26] | 2015 | 45 | 28.0 ± 9.1 | 45 | 20.8 ± 7.0 | < 0.001 |
| Sorokin et al[27] | 2018 | 232 | 23.1 ± 19.4 | 20 | 14.7 ± 12.7 | 0.004 |
| Wadhwa et al[28] | 2019 | 132 | 12.6 ± 35.9 | 132 | 4.5 ± 4.0 | < 0.001 |
| Miao et al[29] | 2019 | 222 | 19.0 ± 23.0 | 445 | 15.0 ± 19.0 | 0.028 |
| Garshick et al[31] | 2021 | 35 | 17.0 ± 14.0 | 15 | 9.0 ± 20.0 | 0.02 |
| Wang et al[30] | 2022 | 152 | 14.0 ± 17.5 | 152 | 12.5 ± 15.1 | NS |
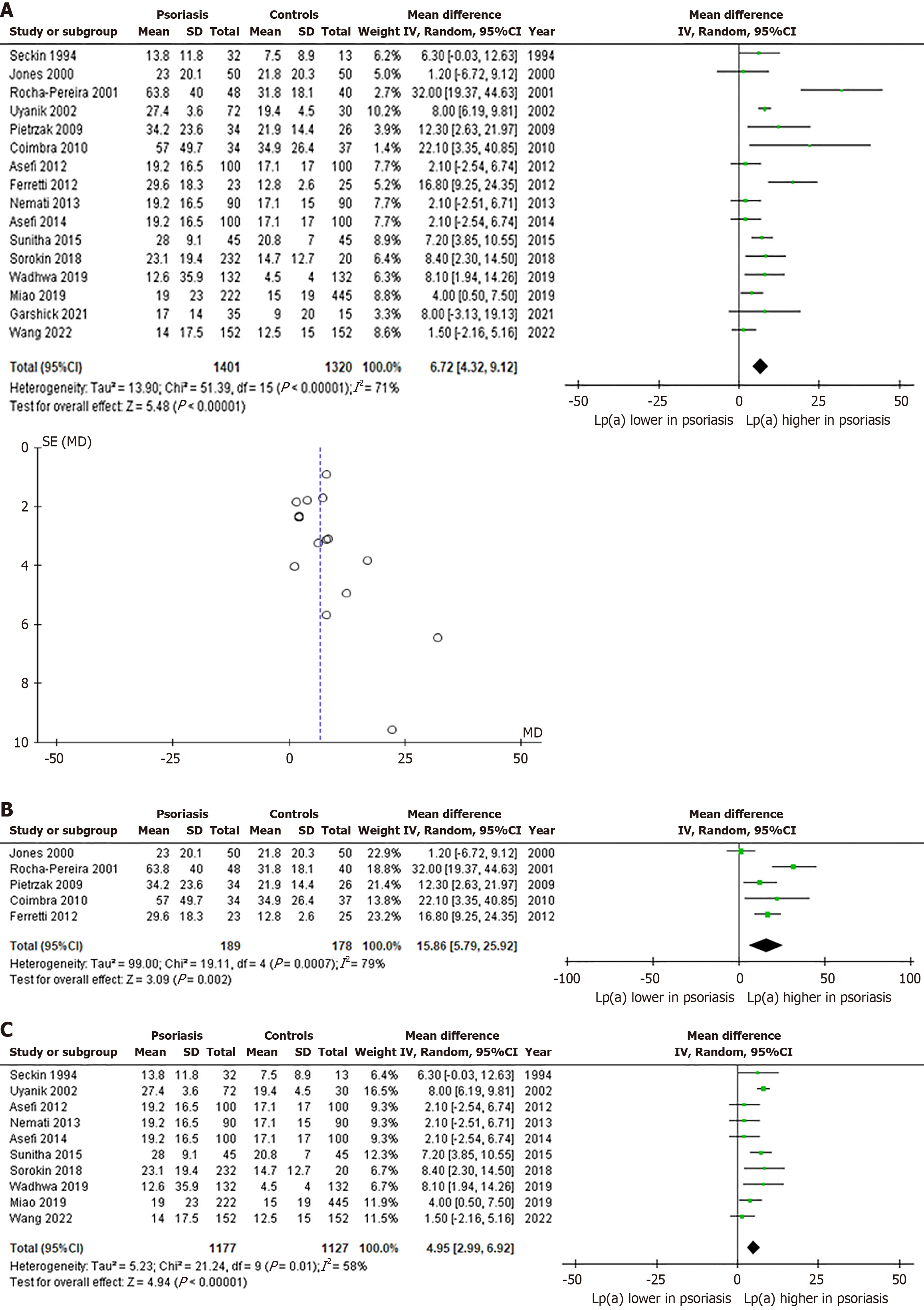
A quantitative analysis was performed using 16 of the 18 studies, due to insufficient reporting regarding standard deviations in the studies by Oliviero et al[21] and Papagoras et al[24]. The pooled analysis showed significantly higher Lp(a) levels in 1401 patients with psoriasis compared to 1320 HC, with the overall MD being 6.72 mg/dL (95%CI: 4.32-9.12, P < 0.00001, I2 = 71%) (Figure 2A)[14-20,22-23,25-31]. Furthermore, asymmetry is evident in the funnel plot, although it is mainly limited to studies with a larger standard error (Figure 2A)[14-20,22-23,25-31].
Additional sensitivity analyses were performed to ascertain whether region of origin affected our results. Regarding the European sub-population, the pooled analysis showed a pronounced increase in Lp(a) levels in 189 patients with psoriasis vs 178 HC, as reflected by a MD of 15.86 mg/dL (95%CI: 5.79-25.92, P < 0.002, I2 = 79%). (Figure 2B)[15,16,18-20]. On the other hand, the analysis on the Asian sub-population demonstrated a smaller difference in Lp(a) levels between 1177 patients with psoriasis and 1127 HC (MD: 4.95 mg/dL, 95%CI: 2.99-6.92, P < 0.00001, I2 = 58%) (Figure 2C)[14,17,22,23,25-30].
Study quality was evaluated using the NOS for case-control or cohort studies as previously described. The overall mean score of the evaluated studies was 7.7 points, which indicates high quality studies with low risk for bias (Tables 3 and 4)[14-31].
| Ref. | Selection | Comparability | Exposure | Total score | |||||
| Case definition | Case representativeness | Controls selection | Controls definition | Ascertainment | Same method of ascertainment | Non- response rate | |||
| Jones et al[16] | Yes | Yes | Yes | Yes | Yes, yes | Yes | Yes | - | 8/9 |
| Rocha-Pereira et al[15] | Yes | Yes | Yes | Yes | Yes, yes | Yes | Yes | - | 8/9 |
| Uyanik et al[17] | Yes | Yes | Yes | Yes | Yes, yes | Yes | Yes | - | 8/9 |
| Pietrzak et al[18] | Yes | Yes | - | Yes | Yes, yes | Yes | Yes | - | 7/9 |
| Asefi et al[23] | Yes | Yes | Yes | Yes | Yes, yes | Yes | Yes | - | 8/9 |
| Ferretti et al[20] | Yes | Yes | Yes | Yes | Yes, yes | Yes | Yes | - | 8/9 |
| Oliviero et al[21] | Yes | Yes | Yes | Yes | Yes, yes | Yes | Yes | - | 8/9 |
| Nemati et al[22] | Yes | Yes | Yes | Yes | Yes, yes | Yes | Yes | - | 8/9 |
| Asefi et al[25] | Yes | Yes | Yes | Yes | Yes, yes | Yes | Yes | - | 8/9 |
| Papagoras et al[24] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | - | 7/9 |
| Sunitha et al[26] | Yes | Yes | Yes | Yes | Yes, yes | Yes | Yes | - | 8/9 |
| Wadhwa et al[28] | Yes | Yes | Yes | Yes | Yes, yes | Yes | Yes | - | 8/9 |
| Miao et al[29] | Yes | Yes | - | Yes | Yes, yes | Yes | Yes | - | 7/9 |
| Garshick et al[31] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | - | 7/9 |
| Wang et al[30] | Yes | Yes | Yes | Yes | Yes, yes | Yes | Yes | - | 8/9 |
| Ref. | Selection | Comparability | Outcome | Total score | |||||
| Exposed cohort representativeness | Non exposed cohort selection | Exposure ascertainment | Outcome demonstration of interest not present at start of study | Assessment | Follow up long enough | Cohorts follow up adequacy | |||
| Seçkin et al[14] | Yes | Yes | Yes | Yes | Yes, yes | Yes | - | - | 7/9 |
| Coimbra et al[19] | Yes | Yes | Yes | Yes | Yes, yes | Yes | - | Yes | 8/9 |
| Sorokin et al[27] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | - | 7/9 |
Our results support that patients with psoriasis have significantly higher Lp(a) levels compared with HC, suggesting an additional risk factor for ASCVD in these individuals. Our sensitivity analysis indicates that psoriasis patients of European origin have considerably elevated Lp(a) levels compared to their Asian counterparts. Such a racial/ethnic difference regarding Lp(a) concentrations has been previously demonstrated in several studies[32,33].
According to international guidelines and expert consensus statements, Lp(a) measurement should be performed at least once during an individual's lifetime, since the finding of an elevated Lp(a) concentration could redefine their cardiovascular risk assessment[34,35]. As mentioned above, Lp(a) concentration is predominantly determined by genetics (90%) in more than any other lipoprotein[9]. Indeed, the Kringle IV repeat polymorphism determines the variability in Lp(a) concentrations. Several frequent and rare functional single-nucleotide polymorphisms (SNPs) profoundly modify the inverse correlation of isoform size and eventually, the Lp(a) concentrations, explaining the variations among in
Our findings are in line with the results of a 2019 systematic review and meta-analysis of 49 studies by Ramezani et al[36]. In their meta-analysis focused on Lp(a) levels, 8 studies were included with 446 psoriasis patients and 369 HC, and showed that Lp(a) levels were higher by 8.51 mg/dL in patients vs HC (95%CI: 4.86-12.17, P < 0.00001)[36]. Comparably, our updated meta-analysis, which included 16 studies with 3271 psoriasis patients, corroborated these results, although the MD was found to be lower. Regarding classic lipid profile parameters, Ramezani et al[36] showed significant ele
An increased incidence of major adverse cardiovascular events (MACE) including myocardial infarction and stroke has been demonstrated in patients with psoriasis[37]. Postulated mechanisms include a synergy between traditional ASCVD risk factors and inflammation-related mechanisms[38]. Importantly, six studies have shown that psoriasis is associated with an increased risk of atherosclerosis and MACE in an independent, dose-response relation to inflammatory activity[37,39-43]. Therefore, traditional cardiovascular disease scores tend to underestimate the effect of disease-related factors on ASCVD risk[44] and, as such, proposals for a 1.5 risk multiplier have been made when using these scores[45,46].
Several pathophysiological mechanisms are common between psoriasis and atherosclerosis. The two diseases are characterized by an activation of effector T cells, specifically T helper (Th) 1 and Th17 cells. On the one hand, psoriasis is related to dendritic cell-derived secretion of IL-12 and IL-23, which lead to differentiation of T cells into Th1 and Th17 types, respectively. Within the psoriatic plaque, Th1 cells produce tumor necrosis factor-alpha (TNF-α) and interferon-gamma (IFN-γ), inducing keratinocyte proliferation, upregulation of adhesion molecules, and angiogenesis. Th17 cells release IL-17 and IL-22, producing similar results. Further, T regulatory cell inhibition coexists with Th1 and Th17 pathway overactivation. On the other hand, atheromatic plaque-related endothelial activation is also associated with the production of IL-12 and IL-23 by macrophages and dendritic cells. In this context, Th1 cells promote plaque buildup, and Th17 cells induce plaque angiogenesis and instability[47,48]. A study by Mehta et al[49] pointed towards a dominant role of the Th1 cascade, particularly IFN-γ, in inflammation-induced atherogenesis. Moreover, systemic inflammation participates in insulin resistance and causes lipoprotein oxidation, foam cell formation and, hence, atherogenesis propagation[50]. Given the shared mechanisms between psoriasis and atherosclerosis, treatments may aim in both directions. Efficacious psoriasis treatments with anti-atherogenic properties include methotrexate, anti-TNF, anti-IL-12/23 and anti-IL-17 agents[50-52]. However, the role of such therapies on ASCVD is yet to be determined[53].
The elevated ASCVD risk in patients with psoriasis is further delineated by the higher prevalence of co-morbid risk factors including metabolic syndrome, type 2 diabetes, dyslipidemia, and smoking compared to the general population[4-6]. In fact, incidence of metabolic syndrome and other risk factors is analogous to psoriasis severity, while disease activity and treatment response are associated with the occurrence of such comorbidities[53-56]. Of note, the link between psoriasis and traditional cardiovascular risk factors is independent of confounders like age, sex or smoking, and is determined by aberrations in common pathophysiological pathways and genetic factors[57,58].
In regard to dyslipidemia, the aforementioned lipoprotein level alterations [i.e., elevated Lp(a), TCHOL, triglycerides, LDL-C, apo B and low HDL-C] are explained by comorbid metabolic diseases (e.g. obesity), systemic inflammation, as well as type of received treatment[29]. Specifically, Lp(a) levels have been shown to correlate with Psoriasis Area Severity Index score, an indicator of disease severity, while Lp(a) decreases have been demonstrated secondary to administration of anti-inflammatory agents such as methotrexate and anti-TNF, pointing toward the involvement of a pathway other than the IL-6 axis[14,18,30,59]. This provides further evidence in favor of the inflammation hypothesis connecting psoriasis and ASCVD[18]. Disease severity is also associated with Lp(a) peroxidation and oxidative stress[20], while high Lp(a) levels affect chemotaxis and expression of adhesion molecules, and, therefore, T-cell migration[22]. Nevertheless, a Mendelian randomization analysis by Ti et al[60] showed no causal genetic association between psoriasis and Lp(a) levels.
The present systematic review has certain limitations. It relies on observational studies, which, albeit of high quality as per the quality assessment, may suffer from confounding and bias. Furthermore, our meta-analysis has a high heterogeneity, which may be attributed to non-standardized Lp(a) measurement methods. Finally, the funnel plot presents relative asymmetry, which may possibly suggest publication bias. However, the asymmetry is limited to studies with a larger standard error, pointing toward random inter-study differences.
The present systematic review and meta-analysis suggest a significant increase of Lp(a) levels in psoriasis patients compared with HC. Elevated Lp(a) represents an ASCVD risk factor additional to the metabolic derangement frequently encountered in psoriasis. As such, Lp(a) measurement in psoriatic patients may be of use for a more precise determination of cardiovascular risk and treatment goals. Finally, given the pro-inflammatory effects of Lp(a) and its association with the severity of systemic inflammation, our results highlight the possible cardiovascular benefit of disease-modifying anti-inflammatory treatments in such patients, yet further research is required in this direction.
| 1. | Griffiths CEM, Armstrong AW, Gudjonsson JE, Barker JNWN. Psoriasis. Lancet. 2021;397:1301-1315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 1513] [Article Influence: 302.6] [Reference Citation Analysis (0)] |
| 2. | Ghazizadeh R, Shimizu H, Tosa M, Ghazizadeh M. Pathogenic mechanisms shared between psoriasis and cardiovascular disease. Int J Med Sci. 2010;7:284-289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 3. | Osigwe PC, Agomoh CE, Osigwe IS, Akumiah FK. The Association Between Psoriasis and Atherosclerotic Cardiovascular Disease: A Systematic Review and Meta-Analysis of Observational Studies. Cureus. 2024;16:e63379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 4. | Choudhary S, Pradhan D, Pandey A, Khan MK, Lall R, Ramesh V, Puri P, Jain AK, Thomas G. The Association of Metabolic Syndrome and Psoriasis: A Systematic Review and Meta-Analysis of Observational Study. Endocr Metab Immune Disord Drug Targets. 2020;20:703-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 5. | Blegvad C, Nybo Andersen AM, Groot J, Zachariae C, Barker J, Skov L. Clinical characteristics including cardiovascular and metabolic risk factors in adolescents with psoriasis. J Eur Acad Dermatol Venereol. 2020;34:1516-1523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Wu JJ, Kavanaugh A, Lebwohl MG, Gniadecki R, Merola JF. Psoriasis and metabolic syndrome: implications for the management and treatment of psoriasis. J Eur Acad Dermatol Venereol. 2022;36:797-806. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 128] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 7. | Yamazaki F. Psoriasis: Comorbidities. J Dermatol. 2021;48:732-740. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 144] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 8. | Makris A, Barkas F, Sfikakis PP, Liberopoulos E, Filippatos TD, Ray KK, Agouridis AP. Lipoprotein(a), Interleukin-6 inhibitors, and atherosclerotic cardiovascular disease: Is there an association? Atheroscler Plus. 2023;54:1-6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 9. | Kronenberg F. Lipoprotein(a). Handb Exp Pharmacol. 2022;270:201-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 10. | Krueger JG, Bowcock A. Psoriasis pathophysiology: current concepts of pathogenesis. Ann Rheum Dis. 2005;64 Suppl 2:ii30-ii36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 229] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 11. | Späh F. Inflammation in atherosclerosis and psoriasis: common pathogenic mechanisms and the potential for an integrated treatment approach. Br J Dermatol. 2008;159 Suppl 2:10-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 151] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 12. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44932] [Cited by in RCA: 50771] [Article Influence: 10154.2] [Reference Citation Analysis (2)] |
| 13. | Wells GA, Wells G, Shea B, Shea B, O'Connell D, Peterson J, Welch Losos M, Tugwell P, Ga SW, Zello G, Petersen JA. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. United States: Semantic Scholar, 2014. |
| 14. | Seçkin D, Tokgözoğlu L, Akkaya S. Are lipoprotein profile and lipoprotein (a) levels altered in men with psoriasis? J Am Acad Dermatol. 1994;31:445-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Rocha-Pereira P, Santos-Silva A, Rebelo I, Figueiredo A, Quintanilha A, Teixeira F. Dislipidemia and oxidative stress in mild and in severe psoriasis as a risk for cardiovascular disease. Clin Chim Acta. 2001;303:33-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 145] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 16. | Jones SM, Harris CP, Lloyd J, Stirling CA, Reckless JP, McHugh NJ. Lipoproteins and their subfractions in psoriatic arthritis: identification of an atherogenic profile with active joint disease. Ann Rheum Dis. 2000;59:904-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 66] [Reference Citation Analysis (0)] |
| 17. | Uyanik BS, Ari Z, Onur E, Gündüz K, Tanülkü S, Durkan K. Serum lipids and apolipoproteins in patients with psoriasis. Clin Chem Lab Med. 2002;40:65-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 55] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Pietrzak A, Kadzielewski J, Janowski K, Roliński J, Krasowska D, Chodorowska G, Paszkowski T, Kapeć E, Jastrzebska I, Tabarkiewicz J, Lotti T. Lipoprotein (a) in patients with psoriasis: associations with lipid profiles and disease severity. Int J Dermatol. 2009;48:379-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Coimbra S, Oliveira H, Reis F, Belo L, Rocha S, Quintanilha A, Figueiredo A, Teixeira F, Castro E, Rocha-Pereira P, Santos-Silva A. Psoriasis therapy and cardiovascular risk factors: a 12-week follow-up study. Am J Clin Dermatol. 2010;11:423-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Ferretti G, Bacchetti T, Campanati A, Simonetti O, Liberati G, Offidani A. Correlation between lipoprotein(a) and lipid peroxidation in psoriasis: role of the enzyme paraoxonase-1. Br J Dermatol. 2012;166:204-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 21. | Oliviero F, Lo Nigro A, Bernardi D, Giunco S, Baldo G, Scanu A, Sfriso P, Ramonda R, Plebani M, Punzi L. A comparative study of serum and synovial fluid lipoprotein levels in patients with various arthritides. Clin Chim Acta. 2012;413:303-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 22. | Nemati H, Khodarahmi R, Rahmani A, Ebrahimi A, Amani M, Eftekhari K. Serum lipid profile in psoriatic patients: correlation between vascular adhesion protein 1 and lipoprotein (a). Cell Biochem Funct. 2013;31:36-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Asefi M, Vaisi-Raygani A, Bahrehmand F, Kiani A, Rahimi Z, Nomani H, Ebrahimi A, Tavilani H, Pourmotabbed T. Paraoxonase 1 (PON1) 55 polymorphism, lipid profiles and psoriasis. Br J Dermatol. 2012;167:1279-1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 24. | Papagoras C, Markatseli TE, Saougou I, Alamanos Y, Zikou AK, Voulgari PV, Kiortsis DN, Drosos AA. Cardiovascular risk profile in patients with spondyloarthritis. Joint Bone Spine. 2014;81:57-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 25. | Asefi M, Vaisi-Raygani A, Khodarahmi R, Nemati H, Rahimi Z, Vaisi-Raygani H, Tavilani H, Pourmotabbed T. Methylentetrahydrofolatereductase (rs1801133) polymorphism and psoriasis: contribution to oxidative stress, lipid peroxidation and correlation with vascular adhesion protein 1, preliminary report. J Eur Acad Dermatol Venereol. 2014;28:1192-1198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 26. | Sunitha S, Rajappa M, Thappa DM, Chandrashekar L, Munisamy M, Revathy G, Priyadarssini M. Comprehensive lipid tetrad index, atherogenic index and lipid peroxidation: Surrogate markers for increased cardiovascular risk in psoriasis. Indian J Dermatol Venereol Leprol. 2015;81:464-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Sorokin AV, Kotani K, Elnabawi YA, Dey AK, Sajja AP, Yamada S, Ueda M, Harrington CL, Baumer Y, Rodante JA, Gelfand JM, Chen MY, Joshi AA, Playford MP, Remaley AT, Mehta NN. Association Between Oxidation-Modified Lipoproteins and Coronary Plaque in Psoriasis. Circ Res. 2018;123:1244-1254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 28. | Wadhwa D, Mahajan VK, Mehta KS, Chauhan PS, Yadav RS, Bhushan S, Sharma V, Sharma A, Sharma A, Chauhan S. Malondialdehyde, lipoprotein-a, lipoprotein ratios, comprehensive lipid tetrad index and atherogenic index as surrogate markers for cardiovascular disease in patients with psoriasis: a case-control study. Arch Dermatol Res. 2019;311:287-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 29. | Miao C, Li J, Li Y, Zhang X. Obesity and dyslipidemia in patients with psoriasis: A case-control study. Medicine (Baltimore). 2019;98:e16323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 30. | Wang B, Deng H, Hu Y, Han L, Huang Q, Fang X, Yang K, Wu S, Zheng Z, Yawalkar N, Zhang Z, Yan K. The difference of lipid profiles between psoriasis with arthritis and psoriasis without arthritis and sex-specific downregulation of methotrexate on the apolipoprotein B/apolipoprotein A-1 ratio. Arthritis Res Ther. 2022;24:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 31. | Garshick MS, Drenkova K, Barrett T, Schwartzbard A, Weintraub H, Scher J, Fisher E, Berger J. Lipoprotein(a) is elevated in psoriasis and associated with cardiovascular risk. J Am Coll Cardiol. 2021;77:1580. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 32. | Paré G, Çaku A, McQueen M, Anand SS, Enas E, Clarke R, Boffa MB, Koschinsky M, Wang X, Yusuf S; INTERHEART Investigators. Lipoprotein(a) Levels and the Risk of Myocardial Infarction Among 7 Ethnic Groups. Circulation. 2019;139:1472-1482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 257] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 33. | Mehta A, Jain V, Saeed A, Saseen JJ, Gulati M, Ballantyne CM, Virani SS. Lipoprotein(a) and ethnicities. Atherosclerosis. 2022;349:42-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 81] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 34. | Kronenberg F, Mora S, Stroes ESG, Ference BA, Arsenault BJ, Berglund L, Dweck MR, Koschinsky M, Lambert G, Mach F, McNeal CJ, Moriarty PM, Natarajan P, Nordestgaard BG, Parhofer KG, Virani SS, von Eckardstein A, Watts GF, Stock JK, Ray KK, Tokgözoğlu LS, Catapano AL. Lipoprotein(a) in atherosclerotic cardiovascular disease and aortic stenosis: a European Atherosclerosis Society consensus statement. Eur Heart J. 2022;43:3925-3946. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 900] [Cited by in RCA: 775] [Article Influence: 193.8] [Reference Citation Analysis (0)] |
| 35. | Kronenberg F, Bedlington N, Ademi Z, Geantă M, Silberzahn T, Rijken M, Kaal A, Harada-Shiba M, Chen Z, Thanassoulis G, Eliasen B, Eiselé JL, Wiegman A, Ballantyne CM, Broome E, Calabrò M, Corral P, Dol A, Donato LJ, Evans E, Funabashi S, Gouni-Berthold I, Ibarluzea IG, Johnson N, Lane J, Mora S, Nordestgaard BG, Pećin I, Kaal-Poppelaars R, Langlois MR, Ray KK, Rodenbach A, Santos RD, Stroes ESG, Tada H, Vrablík M, Winokur M, Yoshida M, Nicholls SJ, Daccord M. The Brussels International Declaration on Lipoprotein(a) Testing and Management. Atherosclerosis. 2025;406:119218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 29] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 36. | Ramezani M, Zavattaro E, Sadeghi M. Evaluation of serum lipid, lipoprotein, and apolipoprotein levels in psoriatic patients: a systematic review and meta-analysis of case-control studies. Postepy Dermatol Alergol. 2019;36:692-702. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 37. | Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296:1735-1741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1246] [Cited by in RCA: 1479] [Article Influence: 74.0] [Reference Citation Analysis (0)] |
| 38. | Masson W, Lobo M, Molinero G. Psoriasis and Cardiovascular Risk: A Comprehensive Review. Adv Ther. 2020;37:2017-2033. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 137] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 39. | Mehta NN, Azfar RS, Shin DB, Neimann AL, Troxel AB, Gelfand JM. Patients with severe psoriasis are at increased risk of cardiovascular mortality: cohort study using the General Practice Research Database. Eur Heart J. 2010;31:1000-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 665] [Article Influence: 39.1] [Reference Citation Analysis (0)] |
| 40. | Gelfand JM, Dommasch ED, Shin DB, Azfar RS, Kurd SK, Wang X, Troxel AB. The risk of stroke in patients with psoriasis. J Invest Dermatol. 2009;129:2411-2418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 331] [Cited by in RCA: 412] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 41. | Ahlehoff O, Gislason GH, Jørgensen CH, Lindhardsen J, Charlot M, Olesen JB, Abildstrøm SZ, Skov L, Torp-Pedersen C, Hansen PR. Psoriasis and risk of atrial fibrillation and ischaemic stroke: a Danish Nationwide Cohort Study. Eur Heart J. 2012;33:2054-2064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 178] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 42. | Ludwig RJ, Herzog C, Rostock A, Ochsendorf FR, Zollner TM, Thaci D, Kaufmann R, Vogl TJ, Boehncke WH. Psoriasis: a possible risk factor for development of coronary artery calcification. Br J Dermatol. 2007;156:271-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 265] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 43. | Balci DD, Balci A, Karazincir S, Ucar E, Iyigun U, Yalcin F, Seyfeli E, Inandi T, Egilmez E. Increased carotid artery intima-media thickness and impaired endothelial function in psoriasis. J Eur Acad Dermatol Venereol. 2009;23:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 148] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 44. | Mehta NN, Krishnamoorthy P, Yu Y, Khan O, Raper A, Van Voorhees A, Troxel AB, Gelfand JM. The impact of psoriasis on 10-year Framingham risk. J Am Acad Dermatol. 2012;67:796-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 45. | Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, Cooney MT, Corrà U, Cosyns B, Deaton C, Graham I, Hall MS, Hobbs FDR, Løchen ML, Löllgen H, Marques-Vidal P, Perk J, Prescott E, Redon J, Richter DJ, Sattar N, Smulders Y, Tiberi M, van der Worp HB, van Dis I, Verschuren WMM, Binno S; ESC Scientific Document Group. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J. 2016;37:2315-2381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5080] [Cited by in RCA: 4816] [Article Influence: 481.6] [Reference Citation Analysis (1)] |
| 46. | Agca R, Heslinga SC, Rollefstad S, Heslinga M, McInnes IB, Peters MJ, Kvien TK, Dougados M, Radner H, Atzeni F, Primdahl J, Södergren A, Wallberg Jonsson S, van Rompay J, Zabalan C, Pedersen TR, Jacobsson L, de Vlam K, Gonzalez-Gay MA, Semb AG, Kitas GD, Smulders YM, Szekanecz Z, Sattar N, Symmons DP, Nurmohamed MT. EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann Rheum Dis. 2017;76:17-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 957] [Cited by in RCA: 915] [Article Influence: 101.7] [Reference Citation Analysis (0)] |
| 47. | Armstrong AW, Voyles SV, Armstrong EJ, Fuller EN, Rutledge JC. A tale of two plaques: convergent mechanisms of T-cell-mediated inflammation in psoriasis and atherosclerosis. Exp Dermatol. 2011;20:544-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 105] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 48. | Piaserico S, Orlando G, Messina F. Psoriasis and Cardiometabolic Diseases: Shared Genetic and Molecular Pathways. Int J Mol Sci. 2022;23:9063. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 49. | Mehta NN, Teague HL, Swindell WR, Baumer Y, Ward NL, Xing X, Baugous B, Johnston A, Joshi AA, Silverman J, Barnes DH, Wolterink L, Nair RP, Stuart PE, Playford M, Voorhees JJ, Sarkar MK, Elder JT, Gallagher K, Ganesh SK, Gudjonsson JE. IFN-γ and TNF-α synergism may provide a link between psoriasis and inflammatory atherogenesis. Sci Rep. 2017;7:13831. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 101] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 50. | Merzel Šabović EK, Starbek Zorko M, Janić M. Killing Two Birds with One Stone: Potential Therapies Targeting Psoriasis and Atherosclerosis at the Same Time. Int J Mol Sci. 2022;23:6648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 51. | Yang ZS, Lin NN, Li L, Li Y. The Effect of TNF Inhibitors on Cardiovascular Events in Psoriasis and Psoriatic Arthritis: an Updated Meta-Analysis. Clin Rev Allergy Immunol. 2016;51:240-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 96] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 52. | Piros ÉA, Szilveszter B, Vattay B, Maurovich-Horvat P, Szalai K, Dósa E, Merkely B, Holló P. Novel anti-inflammatory therapies to reduce cardiovascular burden of psoriasis. Dermatol Ther. 2021;34:e14721. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 53. | Langan SM, Seminara NM, Shin DB, Troxel AB, Kimmel SE, Mehta NN, Margolis DJ, Gelfand JM. Prevalence of metabolic syndrome in patients with psoriasis: a population-based study in the United Kingdom. J Invest Dermatol. 2012;132:556-562. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 288] [Cited by in RCA: 352] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 54. | Ogdie A, Schwartzman S, Eder L, Maharaj AB, Zisman D, Raychaudhuri SP, Reddy SM, Husni E. Comprehensive treatment of psoriatic arthritis: managing comorbidities and extraarticular manifestations. J Rheumatol. 2014;41:2315-2322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 55. | Di Minno MN, Peluso R, Iervolino S, Russolillo A, Lupoli R, Scarpa R; CaRRDs Study Group. Weight loss and achievement of minimal disease activity in patients with psoriatic arthritis starting treatment with tumour necrosis factor α blockers. Ann Rheum Dis. 2014;73:1157-1162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 180] [Cited by in RCA: 179] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 56. | Cui P, Li D, Shi L, Yan H, Li T, Liu C, Wang W, Zheng H, Ding N, Li X, Li R, Shi Y, Wang X, Fu H, Qiu Y, Li R, Shi D. Cardiovascular comorbidities among patients with psoriasis: a national register-based study in China. Sci Rep. 2024;14:19683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 57. | Cohen AD, Sherf M, Vidavsky L, Vardy DA, Shapiro J, Meyerovitch J. Association between psoriasis and the metabolic syndrome. A cross-sectional study. Dermatology. 2008;216:152-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 175] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 58. | Patrick MT, Li Q, Wasikowski R, Mehta N, Gudjonsson JE, Elder JT, Zhou X, Tsoi LC. Shared genetic risk factors and causal association between psoriasis and coronary artery disease. Nat Commun. 2022;13:6565. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 59. | Sattar N, Crompton P, Cherry L, Kane D, Lowe G, McInnes IB. Effects of tumor necrosis factor blockade on cardiovascular risk factors in psoriatic arthritis: a double-blind, placebo-controlled study. Arthritis Rheum. 2007;56:831-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 86] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 60. | Ti Y, Xu D, Qin X, Hu Y, Xu Y, Zhao Q, Bu P, Li J. Mendelian randomization analysis does not support a causal influence between lipoprotein(A) and immune-mediated inflammatory diseases. Sci Rep. 2025;15:3834. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/