Published online Sep 24, 2025. doi: 10.5306/wjco.v16.i9.110087
Revised: June 24, 2025
Accepted: August 5, 2025
Published online: September 24, 2025
Processing time: 117 Days and 17.6 Hours
Bone is the most common site of metastasis in breast cancer, yet limited data exist regarding the precise anatomical distribution of bone metastases by tumor su
To examine the anatomical distribution of the first bone metastases in stage IV breast cancer, stratified by histological subtype. Secondary objectives include analyzing the anatomical distribution of subsequent bone metastases, Metastasis-Free Survival (MFI), Progression-Free Interval (PFI), and overall survival (OS).
A retrospective cohort study was conducted on 107 adult females with stage IV breast cancer and bone metastases between 2013 and 2023. Patients were classified by histological subtype (Luminal A/B, HER2-enriched, and Triple-Negative). First and subsequent bone metastasis locations were identified via computed tomo
Rib metastases were significantly more common in HER2-enriched tumors (80%, P = 0.041), while scapula/clavicle metastases were more prevalent in Triple-Negative cases (37.5%, P = 0.003). Subsequent bone metastases mirrored initial patterns, with pelvic involvement notably higher in HER2-enriched (60%) and luminal B (58%) patients (P = 0.046). No significant differences were found in MFI, PFI, or OS among subtypes. Receptor-based analysis showed no significant variation in bone metastasis locations.
Breast cancer subtypes are associated with suggestive bone metastasis patterns—specifically, rib involvement in HER2-enriched and scapula/clavicle in Triple-Negative cases. While anatomical variations exist, they did not translate into differential survival or fracture risk in this cohort.
Core Tip: This study investigates the anatomical distribution of bone metastases in stage IV breast cancer, stratified by histological subtype. Unlike previous reports focusing on general metastatic trends, our analysis highlights specific skeletal predilections-such as rib involvement in HER2-enriched tumors and scapula/clavicle in triple-negative cases. Despite these patterns, survival outcomes and fracture rates did not significantly differ. These findings support a unified clinical approach while offering new insights that may refine imaging surveillance strategies.
- Citation: Zari DS, Novak R, Haviv O, Ron I, Kaplan B, Awad B, Norman D, Nikomarov D. Anatomical distribution of bone metastases in stage IV breast cancer: According to histological subtype. World J Clin Oncol 2025; 16(9): 110087
- URL: https://www.wjgnet.com/2218-4333/full/v16/i9/110087.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i9.110087
Breast cancer remains the most prevalent cancer among women globally, with approximately 2 million new cases diagnosed annually. Among breast cancer patients, bone is the predominant site of metastasis, followed by lungs, liver, and brain. The metastatic spread significantly impacts patient survival and recurrence rates, underscoring the critical need to understand the relationship between tumor subtypes and metastatic patterns. Breast cancer is categorized into four main subtypes based on immunohistochemistry (IHC) of hormone receptors (HR)-estrogen receptor (ER), progesterone receptor (PR), and HER2. These subtypes-luminal A, luminal B, HER2-enriched, and Triple-Negative-exhibit suggestive metastatic behaviors. Notably, luminal subtypes show a higher propensity for bone metastasis, with up to 71.4% of luminal B tumors primarily metastasizing to bone. While the general metastatic patterns of these subtypes are known, there is a significant gap in our understanding of the specific anatomical distribution of bone metastases. This knowledge is crucial for developing targeted screening and treatment strategies. The primary aim of this study is to examine the anatomical distribution of the first bone metastases in stage IV breast cancer, stratified by histological subtype. Secondary objectives include analyzing the anatomical distribution of subsequent bone metastases, Metastasis-Free Survival (MFI), Progression-Free Interval (PFI), and overall survival (OS).
This retrospective cohort study analyzed medical records of 107 adult females with stage IV breast cancer who presented with bone metastasis at initial diagnosis or during follow-up between January 1, 2013, and December 31, 2023.
The study included adult patients diagnosed with breast cancer who either presented with bone metastases at initial diagnosis or subsequently developed bone metastases during follow-up. Patients under 18 years of age or with a history of other cancers were excluded. HR status for ER and PR was assessed according to CAP/ASCO guidelines, with ≥ 1% nuclear staining classified as positive. HER2 status was determined by IHC, scored as negative (0, +1) or positive (+3). Breast cancer subtypes were defined as Luminal A (ER/PR-positive, HER2-negative, low proliferation-Ki-67 index), Luminal B (ER/PR-positive, HER2-positive, higher proliferation-Ki-67 index), HER2-enriched (ER/PR-negative, HER2-positive, aggressive), and Triple-Negative (ER/PR/HER2-negative, aggressive).
Bone metastases were confirmed using standard imaging modalities: Computed tomography (CT) scan (lytic/sclerotic lesions on axial imaging), 18F-FDG positron emission tomography (PET)/CT (hypermetabolic skeletal lesions with SUVmax ≥ 2.5), or magnetic resonance imaging (T1 hypointense/T2 hyper-intense lesions with contrast enhancement). A board-certified radiologist verified all imaging findings.
Then, the anatomic location of the bone metastasis was classified as skull, pectoral girdle (clavicle/scapula), humerus, sternum, cervical/thoracic/Lumbar/sacral vertebrae, pelvis, femoral head/neck, or femoral shaft.
The following parameters were also examined: The anatomical distribution of subsequent bone metastases. MFI: Time interval from initial breast cancer diagnosis to first bone metastasis; PFI: Time interval from first to second bone metastasis, confirmed by imaging; OS: Time interval from diagnosis to death from any cause or last follow-up.
Descriptive statistics on terms of mean, standard deviation, median, percentages, and ranges were calculated to the whole parameters in the study. Normal distribution of continuous parameters was tested by the Kolmogorov-Smirnov test. As a result of these tests, we used ANOVA or Kruskal-Wallis tests for differences between groups with adjustment for multiple comparisons. For categorical parameters, we used the Pearson χ2. The Kaplan-Meier curve was used to analyze time-to-event (time from first metastasis to second metastasis). The Kaplan-Meier curve graphically represents the survival rate. Time is plotted on the x-axis, and the survival rate is plotted on the y-axis. P < 0.05 was considered sig
The final cohort comprised 107 adult female patients who were diagnosed with stage IV breast cancer and bone metastasis. Among them, 82 (76.6%) were luminal A, 12 (11.2%) were luminal B, 8 (7.4%) were Triple-Negative, and 5 (4.6%) were HER2-enriched. The patient's demographics are reported in Table 1.
| Luminal A (n = 82) | Luminal B (n = 12) | Triple neg (n = 8) | HER 2 enrich (n = 5) | P value | |
| Age at diagnosis | 59.8 ± 12.4 | 58.7 ± 11.65 | 62.2 ± 15.8 | 56.21 ± 11.0 | 0.85 |
| Metastasis-free survival (median, years) | 2.55 (0.05-7.39) | 2.75 (0.65-7.12) | 1.14 (0.09-5.98) | 0.37 (0.05-8.89) | 0.98 |
| BMI | 26.8 ± 5.9 | 24.4 ± 5.7 | 26.3 ± 4.8 | 25.4 ± 8.3 | 0.58 |
| Death | 59 (72.8%) | 9 (75%) | 8 (100%) | 3 (60%) | 0.33 |
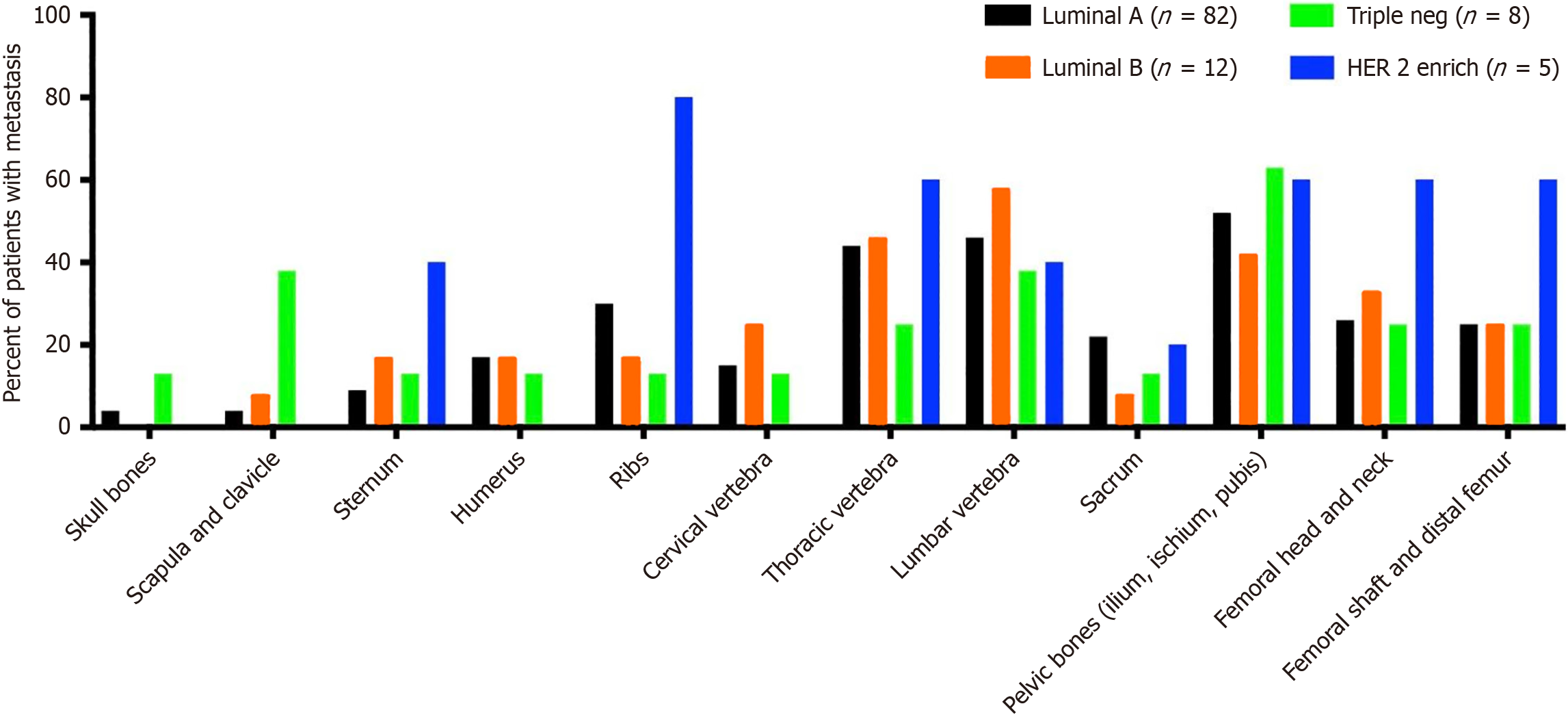
The distribution of the first bone metastasis varied among breast cancer subtypes. Patients with HER2-enriched tumors exhibited a higher prevalence of rib metastases (80%) compared to other subtypes (P = 0.041). The scapula and clavicle were significantly more affected in Triple-Negative patients (37.5%) compared to HER2-enriched (0%), luminal A (3.7%), and luminal B (8.3%) subtypes (P = 0.003). Other skeletal sites that were examined showed no significant differences between subtypes as shown (Figure 1; Table 2).
| First bone metastasis | Luminal A (n = 82) | Luminal B (n = 12) | Triple neg (n = 8) | HER 2 enrich (n = 5) | P value |
| Skull bones | 3/82 (3.7) | 0 | 1/8 (12.5) | 0 | 0.5 |
| Scapula and clavicle | 3/82 (3.7) | 1/12 (8.3) | 3/8 (37.5) | 0 | 0.003 |
| Sternum | 7/82 (8.5) | 2/12 (17) | 1/8 (12.5) | 2/5 (40) | 0.16 |
| Humerus | 14/82 (17) | 2/12 (17) | 1/8 (12.5) | 0 | 0.77 |
| Ribs | 24/82 (30) | 2/12 (17) | 1/8 (12.5) | 4/5 (80) | 0.041 |
| Cervical vertebra | 12/82 (15) | 3/12 (25) | 1/8 (12.5) | 0 | 0.60 |
| Thoracic vertebra | 36/82 (44) | 5/12 (45.5) | 2/8 (25) | 3/5 (60) | 0.64 |
| Lumbar vertebra | 38/82 (46) | 7/12 (58) | 3/8 (37.5) | 2/5 (40) | 0.79 |
| Sacrum | 18/82 (22) | 1/12 (8) | 1/8 (12.5) | 1/5 (20) | 0.68 |
| Pelvic bones (ilium, ischium, pubis) | 43/82 (52) | 5/12 (42) | 5/8 (62.5) | 3/5 (60) | 0.80 |
| Femoral head and neck | 21/82 (26) | 4/12 (33) | 2/8 (25) | 3/5 (60) | 0.39 |
| Femoral shaft and distal femur | 20/82 (25) | 3/12 (25) | 2/8 (25) | 3/5 (60) | 0.38 |
The pattern of subsequent bone metastases followed a similar distribution, with pelvic bone involvement being more common in HER2-enriched (60%) and luminal B (58%) groups compared to luminal A (38%) (Table 3).
| Second bone metastasis | Luminal A (n = 82) | Luminal B (n = 12) | Triple neg (n = 8) | HER 2 enrich (n = 5) | P value |
| Without bone progression | 16/82 (19.5) | 3/12 (25) | 3/8 (37.5) | 1/5 (20) | 0.68 |
| Skull bones | 7/82 (9) | 0 | 0 | 0 | 0.50 |
| Scapula and clavicle | 6/82 (7) | 1/12 (8) | 1/8 (12.5) | 0 | 0.36 |
| Sternum | 7/82 (9) | 0 | 0 | 0 | 0.50 |
| Humerus | 10/82 (12.5) | 2/12 (17) | 2/8 (25) | 0 | 0.60 |
| Ribs | 28/82 (35) | 2/12 (17) | 0 | 1/5 (20) | 0.13 |
| Cervical vertebra | 19/82 (24) | 3/12 (25) | 1/8 (12.5) | 1/5 (20) | 0.90 |
| Thoracic vertebra | 35/82 (44) | 3/12 (25) | 2/8 (25) | 2/5 (40) | 0.50 |
| Lumbar vertebra | 28/82 (35) | 6/12 (50) | 2/8 (25) | 0 | 0.24 |
| Sacrum | 17/82 (21) | 3/12 (25) | 0 | 1/5 (20) | 0.53 |
| Pelvic bones (ilium, ischium, pubis) | 31/82 (38) | 7/12 (58) | 0 | 3/5 (60) | 0.046 |
| Femoral head and neck | 25/82 (31) | 2/12 (17) | 3/8 (37.5) | 1/5 (20) | 0.68 |
| Femoral shaft and distal femur | 24/82 (30) | 2/12 (17) | 2/8 (25) | 1/5 (20) | 0.77 |
The median MFI time was lowest in the HER2-enriched group (4.0 months), while luminal B patients exhibited the longest period (median 34 months), though differences were not statistically significant (P = 0.89). Mortality was highest in Triple-Negative patients (100%), followed by luminal B (75%) and luminal A (72.8%), but without significant variation (P = 0.33), Table 1.
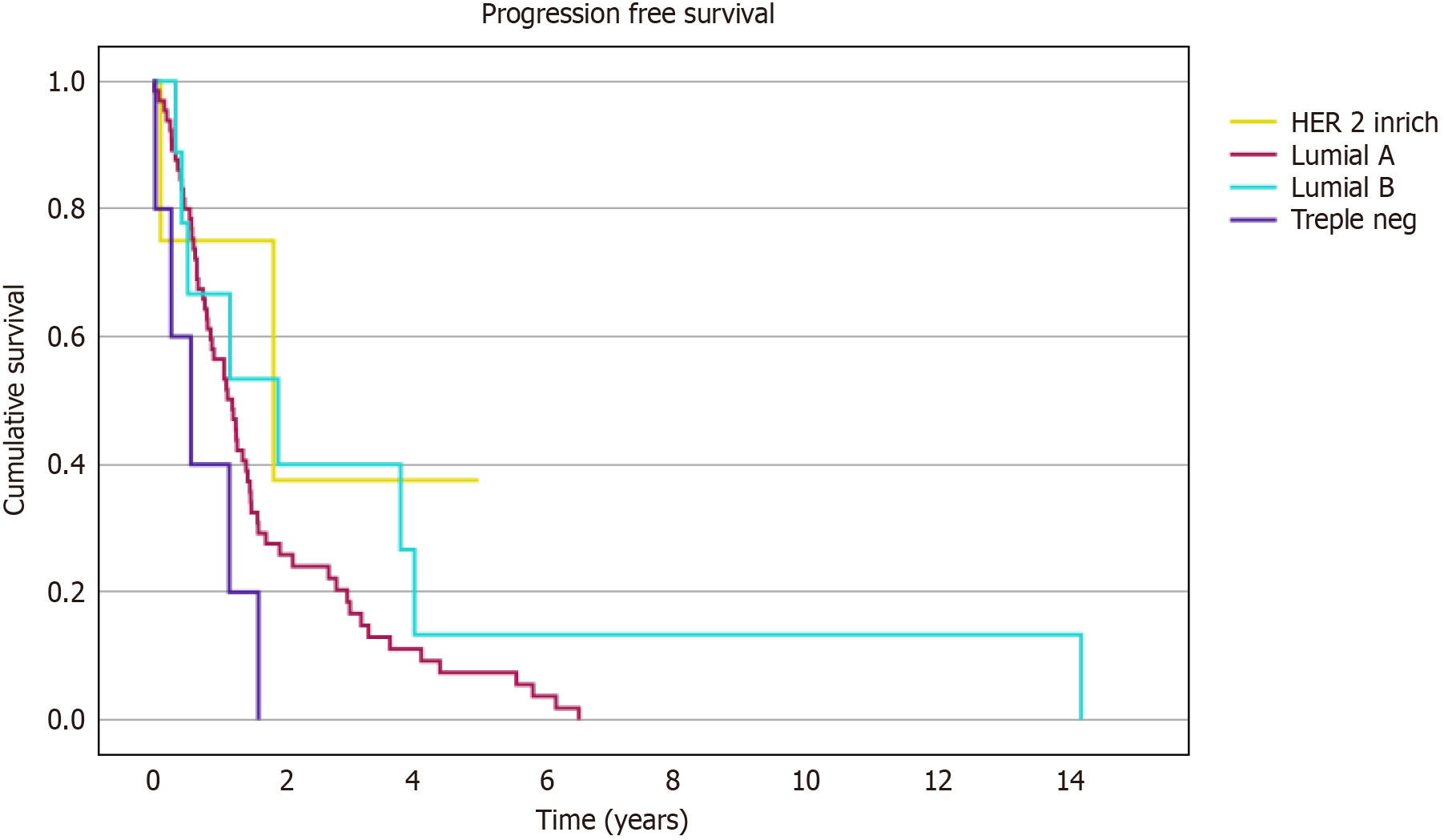
The PFI, defined as the time between the first and second bone metastases, is illustrated by the Kaplan-Meier curve in Figure 2. Pairwise Log-Rank tests revealed no statistically significant differences in survival intervals between breast cancer subtypes. However, trends approaching significance were observed in comparisons involving Triple-Negative vs HER2-enriched (P = 0.062) and Luminal B subtypes (P = 0.057).
Regarding pathologic fracture/impending fracture in the common anatomical sites, there was no significant difference among the different groups (Table 4).
| Pathological fracture | Luminal A (n = 82) | Luminal B (n = 12) | Triple neg (n = 8) | HER 2 enrich (n = 5) | P value |
| Femoral head and neck | 16 (20) | 4 (33) | 2 (25) | 1 (20) | 0.75 |
| Spinal vertebrae | 12 (15) | 1 (8) | 0 | 1 (20) | 0.77 |
| Humerus | 5 (6) | 1 (8) | 1 (12.5) | 0 | 0.78 |
| Other1 | 8 (10) | 0 | 1 (12.5) | 0 | 0.63 |
| Impending hip fracture | 29 (35) | 6 (50) | 3 (37.5) | 0 | 0.28 |
A receptor-based analysis revealed no significant differences in bone metastasis locations between HER2-positive, PR-positive, and ER-positive groups (Table 5). MFI was shortest in HER2-positive patients (1.61 years) and longest in PR-positive patients (3.98 years), but without significant differences.
| First bone metastasis | ER (n = 94) | PR (n = 59) | HER 2 (n = 16) | P value |
| Skull bones | 3/94 (3.2) | 1/59 (1.7) | 0 | 1.00 |
| Scapula and clavicle | 4/94 (4.2) | 3/59 (5) | 1/16 (6.2) | 0.93 |
| Sternum | 9/94 (9.6) | 5/59 (8.5) | 4/16 (25) | 0.14 |
| Humerus | 16/94 (17.0) | 10/59 (16.9) | 2/16 (12.5) | 0.89 |
| Ribs | 26/94 (28.0) | 14/59 (24.1) | 6/16 (37.5) | 0.56 |
| Cervical vertebra | 15/94 (16.0) | 10/59 (16.9) | 3/16 (18.8) | 0.96 |
| Thoracic vertebra | 41/94 (43.6) | 23/59 (38.9) | 8/16 (50) | 0.62 |
| Lumbar vertebra | 45/94 (47.9) | 25/59 (42.4) | 8/16 (50) | 0.76 |
| Sacrum | 19/94 (20.2) | 10/59 (16.9) | 2/16 (12.5) | 0.72 |
| Pelvic bones (ilium, ischium, pubis) | 48/94 (51.1) | 27/59 (45.8) | 8/16 (50) | 0.81 |
| Femoral head and neck | 25/94 (26.6) | 18/59 (30.5) | 7/16 (43.8) | 0.37 |
| Femoral shaft and distal femur | 23/94 (24.7) | 16/59 (27.6) | 6/16 (37.5) | 0.56 |
This study investigated the anatomical distribution of bone metastases in stage IV breast cancer patients. Rib metastases were significantly more common in HER2-enriched tumors, while scapula/clavicle metastases were more prevalent in Triple-Negative cases. Subsequent bone metastases mirrored initial patterns, with pelvic involvement notably higher in HER2-enriched and luminal B patients. Nevertheless, there was no significant difference regarding pathologic fractures or impending fractures in the common anatomical sites. Furthermore, receptor-based analysis showed no significant variation in bone metastasis locations, and no significant differences were found in MFI, PFI, or OS among subtypes.
According to the American Cancer Society, luminal A accounts for 73% of breast cancer cases in the United States, luminal B for 11%, Triple-Negative for 12%, and HER2-enriched for 4%[1-7]. In this study, luminal A made up 76%, luminal B 11%, Triple-Negative 8%, and HER2-enriched 5%. The main objective of this study was to define the anatomical distribution pattern of different subtypes of metastatic breast cancer. While metastatic patterns of breast cancer subtypes are well documented, data on the specific anatomical distribution of bone metastases remains limited[8-12].
Pareek et al[12] investigated the incidence of bone metastases and their correlation with HR status in a cohort of 262 patients. They reported a bone metastasis rate of 25.25%, with a higher prevalence in ER-positive tumors. The spine and pelvis were the most frequently affected sites. A study by Siregar et al[6], this examined 65 female patients with bone-only metastatic breast cancer to evaluate the distribution of bone metastasis sites across histological subtypes and tumor grades. Their study has found that vertebral and costal metastases were significantly associated with higher histological grades and differed by cancer subtype, suggesting more aggressive tumors tend to involve a broader range of bone sites. Corroborating with Siregar et al[6] and Pareek et al[12] results, this study has shown that HER2-enriched tumors have a strong tendency for rib metastases (P = 0.041). Triple-Negative breast cancer had significantly more scapula and clavicle metastases (37.5%) than other subtypes (P = 0.003). Conversely, receptor-based analysis showed no significant variation in bone metastasis locations. These findings underscore the complexity of metastatic patterns in breast cancer, where certain subtypes exhibit site-specific tendencies despite an overall lack of significant differences based on receptor status alone[11-13].
The spine is the most common site of bone metastasis in breast cancer patients[14], with a high rate of metastases in the thoracic and lumbar vertebrae across all histological subtypes. Our study shows that 25%-60% of patients with metastatic breast cancer exhibit spinal involvement (Tables 1 and 2), consistent with previous findings that spinal lesions account for approximately two-thirds of all bone metastases[13,14]. Several explanations have been proposed regarding the patterns of cancer cell dissemination from the primary tumor to the spine. One suggested mechanism involves invasion via the venous drainage system. Each vertebral body is drained by a basivertebral vein, which subsequently drains into the epidural venous plexus-a valveless venous network within the spinal canal-previously proposed as a pathway facilitating metastatic spread due to the absence of venous valves[14,15].
The ribs are richly vascularized via anterior and posterior intercostal arteries, supplying both periosteum and marrow. This high vascularity, combined with the heightened angiogenic activity observed in HER2-positive breast cancers-marked by overexpression of vascular endothelial growth factor and aggressive neovascularization[15]-provides a plausible biological explanation for the increased predilection of rib metastases in this subgroup.
Current literature suggests that patients suffering from pathological fractures (PF) are at increased risk of death[16-19]. A systematic review by Lamo-Espinosa et al[17] analyzed data from 15464 metastatic cancer patients and found that PF significantly increase the risk of mortality and reduce the mean survival of patients with metastatic cancer. This study observed the rate of PFs or impending fractures and inspected the disease progression. No significant differences regarding pathologic fractures or impending fractures were found, and no significant differences were found in MFI, PFI, or OS among subtypes. The lack of significant differences in the remaining anatomical sites between the groups, as well as the comparable rates of prophylactic fixation procedures and survival outcomes, provides important insight into the underlying characteristics of the disease. These findings indicate that current preventive strategies and clinical decision-making approaches remain appropriate and do not require substantial modification for this patient population.
Despite the strengths of our study-including its well-defined cohort and precise anatomical classification of bone metastases-several limitations merit discussion. The retrospective design introduces inherent biases, notably potential variability in imaging modalities and interpretation. Furthermore, the sample size-especially in the HER2-enriched and TNBC subgroups, is small, which may limit statistical power and generalizability. We also could not fully account for confounding factors such as systemic treatment history, genetic predisposition, or tumor microenvironment influences.
Although we observed subtype-specific metastatic patterns (e.g., rib in HER2, scapula/clavicle in TNBC), these did not correspond to differential survival. This may be due to subtype-dependent systemic therapies, such as HER2-targeted agents-and bone-modifying drugs potentially mitigating survival differences.
Lastly Routine bone scans may not adequately cover scapular and clavicular regions, potentially reducing detection sensitivity in TNBC patients. Advanced imaging modalities, such as SPECT/CT or PETCT, may improve detection in these anatomical sites.
In conclusion, breast cancer histological subtypes are associated with suggestive patterns of bone metastasis, specifically rib involvement in HER2-enriched and the scapula/clavicle in Triple-Negative cases. Despite these differences, survival outcomes and fracture rates were not significantly impacted by subtype. These findings support a more nuanced approach to surveillance while reinforcing the validity of current preventive strategies. Future research should explore underlying biological mechanisms and larger cohorts to validate these observations.
Thanks to Romy Schindler for editing assistance. Thanks to Ronit Leiba for statistical analysis assistance.
| 1. | Pang L, Gan C, Xu J, Jia Y, Chai J, Huang R, Li A, Ge H, Yu S, Cheng H. Bone Metastasis of Breast Cancer: Molecular Mechanisms and Therapeutic Strategies. Cancers (Basel). 2022;14:5727. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 42] [Reference Citation Analysis (0)] |
| 2. | Jiang X, Chen G, Sun L, Liu C, Zhang Y, Liu M, Liu C. Characteristics and survival in bone metastatic breast cancer patients with different hormone receptor status: A population-based cohort study. Front Oncol. 2022;12:977226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 3. | Wu Q, Li J, Zhu S, Wu J, Chen C, Liu Q, Wei W, Zhang Y, Sun S. Breast cancer subtypes predict the preferential site of distant metastases: a SEER based study. Oncotarget. 2017;8:27990-27996. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 157] [Cited by in RCA: 264] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 4. | Pulido C, Vendrell I, Ferreira AR, Casimiro S, Mansinho A, Alho I, Costa L. Bone metastasis risk factors in breast cancer. Ecancermedicalscience. 2017;11:715. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 80] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 5. | Marie L, Braik D, Abdel-Razeq N, Abu-Fares H, Al-Thunaibat A, Abdel-Razeq H. Clinical Characteristics, Prognostic Factors and Treatment Outcomes of Patients with Bone-Only Metastatic Breast Cancer. Cancer Manag Res. 2022;14:2519-2531. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 6. | Siregar KB, Al Anas M. Unveiling bone metastasis: Exploring histological subtypes of breast cancer in Indonesia's tertiary referral hospital. Cancer Treat Res Commun. 2023;37:100764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 7. | Howlader N, Cronin KA, Kurian AW, Andridge R. Differences in Breast Cancer Survival by Molecular Subtypes in the United States. Cancer Epidemiol Biomarkers Prev. 2018;27:619-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 412] [Article Influence: 51.5] [Reference Citation Analysis (0)] |
| 8. | Xiao W, Zheng S, Yang A, Zhang X, Zou Y, Tang H, Xie X. Breast cancer subtypes and the risk of distant metastasis at initial diagnosis: a population-based study. Cancer Manag Res. 2018;10:5329-5338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 130] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 9. | Arciero CA, Guo Y, Jiang R, Behera M, O'Regan R, Peng L, Li X. ER(+)/HER2(+) Breast Cancer Has Different Metastatic Patterns and Better Survival Than ER(-)/HER2(+) Breast Cancer. Clin Breast Cancer. 2019;19:236-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 84] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 10. | Ahn SG, Lee HM, Cho SH, Lee SA, Hwang SH, Jeong J, Lee HD. Prognostic factors for patients with bone-only metastasis in breast cancer. Yonsei Med J. 2013;54:1168-1177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 11. | Liede A, Jerzak KJ, Hernandez RK, Wade SW, Sun P, Narod SA. The incidence of bone metastasis after early-stage breast cancer in Canada. Breast Cancer Res Treat. 2016;156:587-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 12. | Pareek A, Singh OP, Yogi V, Ghori HU, Tiwari V, Redhu P. Bone metastases incidence and its correlation with hormonal and human epidermal growth factor receptor 2 neu receptors in breast cancer. J Cancer Res Ther. 2019;15:971-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Briasoulis E, Karavasilis V, Kostadima L, Ignatiadis M, Fountzilas G, Pavlidis N. Metastatic breast carcinoma confined to bone: portrait of a clinical entity. Cancer. 2004;101:1524-1528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Peterson KA, Zehri AH, Lee KE, Kittel CA, Evans JK, Wilson JL, Hsu W. Current trends in incidence, characteristics, and surgical management of metastatic breast cancer to the spine: A National Inpatient Sample analysis from 2005 to 2014. J Clin Neurosci. 2021;91:99-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Kumar R, Yarmand-Bagheri R. The role of HER2 in angiogenesis. Semin Oncol. 2001;28:27-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 148] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 16. | Saad F, Lipton A, Cook R, Chen YM, Smith M, Coleman R. Pathologic fractures correlate with reduced survival in patients with malignant bone disease. Cancer. 2007;110:1860-1867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 448] [Cited by in RCA: 473] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 17. | Lamo-Espinosa JM, Mariscal G, Gómez-Álvarez J, Khalil I, San-Julián M. Survival impact of pathological fractures in metastatic cancer: a comprehensive meta-analysis. Arch Orthop Trauma Surg. 2025;145:253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Mendes D, Alves C, Afonso N, Cardoso F, Passos-Coelho JL, Costa L, Andrade S, Batel-Marques F. The benefit of HER2-targeted therapies on overall survival of patients with metastatic HER2-positive breast cancer--a systematic review. Breast Cancer Res. 2015;17:140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 147] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 19. | Gampenrieder SP, Rinnerthaler G, Greil R. Bone-targeted therapy in metastatic breast cancer - all well-established knowledge? Breast Care (Basel). 2014;9:323-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/