Published online Sep 24, 2025. doi: 10.5306/wjco.v16.i9.110068
Revised: June 9, 2025
Accepted: August 25, 2025
Published online: September 24, 2025
Processing time: 117 Days and 22.6 Hours
Postoperative malnutrition, systemic inflammation, and immune dysfunction significantly impair recovery and survival in gastric cancer patients undergoing radical gastrectomy. The Prognostic Immune Nutritional Index (PINI) enables immune-nutritional risk stratification; however, its utility in guiding perioperative nutritional support remains underexplored.
To evaluate whether risk-stratified perioperative nutritional support based on PINI scores improves postoperative recovery, quality of life, and long-term out
In this prospective, randomized controlled trial, 195 patients undergoing radical gastrectomy were stratified into low- (PINI ≤ 1.5), moderate- (1.5 < PINI ≤ 3), and high-risk (PINI > 3) groups. Patients received standard, intensive, or immune-enhancing nutritional support, respectively. Outcomes were assessed at 1 week, 1 month, and 1 year postoperatively and included body mass index (BMI), serum albumin, PINI scores, Pittsburgh Sleep Quality Index (PSQI), Self-Rating Anxiety Scale (SAS), Self-Rating Depression Scale (SDS), Visual Analog Scale (VAS) for pain, EORTC QLQ-C30 for quality of life, complication rates, hospital stay, and survival.
At 1 year, the high-risk group receiving immune-enhancing nutrition demon
PINI-based graded nutritional support significantly enhances postoperative recovery, reduces complications, and improves long-term outcomes following radical gastrectomy. These findings support its integration into precision perioperative care strategies for gastric cancer.
Core Tip: This randomized controlled trial is the first to apply Prognostic Immune Nutritional Index (PINI)-based risk stratification to guide perioperative nutritional support in gastric cancer patients. The tailored interventions significantly improved sleep quality, psychological well-being, nutritional status, and 1-year survival, particularly in high-risk individuals. Our findings highlight the value of individualized immune-enhancing nutrition in optimizing recovery and suggest that PINI may serve as a practical tool for precision perioperative care.
- Citation: Wang G, Pan SJ. Prognostic immune nutritional index-based nutritional stratification enhances recovery and survival in gastric cancer: A randomized controlled trial. World J Clin Oncol 2025; 16(9): 110068
- URL: https://www.wjgnet.com/2218-4333/full/v16/i9/110068.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i9.110068
Gastric cancer remains a leading cause of cancer-related mortality worldwide, with radical gastrectomy serving as the primary curative treatment[1,2]. However, postoperative malnutrition, systemic inflammation, and immune dysfunction continue to pose major challenges, often leading to delayed recovery, increased complications, and poorer long-term outcomes[3]. Traditional nutritional assessments, such as body mass index (BMI) and serum albumin, offer limited insight into the complex interplay between nutrition and immune status, particularly in oncology patients[4].
The Prognostic Immune Nutritional Index (PINI), which combines albumin, transferrin, C-reactive protein (CRP), and α1-acid glycoprotein, provides a more comprehensive assessment of systemic inflammation and metabolic derangement[5]. Elevated PINI scores reflect increased inflammatory activity and catabolic stress, conditions that impair wound healing, compromise immune surveillance, and predispose patients to surgical complications and disease recurrence[6,7]. Mechanistically, PINI integrates both negative (albumin, transferrin) and positive (CRP, α1-AG) acute-phase reactants, serving not only as a prognostic indicator but also as a surrogate marker of modifiable immune-nutritional dysfunction. This underscores its potential utility in guiding personalized perioperative interventions.
Despite its established prognostic value, the use of PINI to stratify and guide perioperative nutritional support in gastric cancer patients has not been thoroughly investigated. Whether such a graded approach could improve short- and long-term outcomes remains an open clinical question.
This study aimed to evaluate the long-term impact of PINI-based graded nutritional support on postoperative recovery in gastric cancer patients undergoing radical gastrectomy. Outcomes of interest included nutritional reconstitution, sleep quality, psychological well-being, pain control, quality of life, complication rates, and survival over a 1-year follow-up. By integrating immune-nutritional risk stratification into perioperative care, this study seeks to provide a precision-based framework for optimizing postoperative recovery and enhancing patient-centered outcomes.
This prospective, randomized controlled trial was conducted at The First Affiliated Hospital of Soochow University between October 2022 and October 2023. The study protocol was approved by the institutional ethics committee (Approval No. SUDA20220906H01) and conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all participants prior to enrollment.
A non-intervention (i.e., no nutritional support) control group was not included due to ethical and clinical considerations. Withholding perioperative nutrition in gastric cancer patients is associated with increased complication rates, delayed recovery, and poorer oncologic outcomes[4]. International clinical guidelines recommend routine nutritional screening and perioperative support in surgical oncology[8]. Thus, all participants received at least standard nutritional support, with interventions stratified according to PINI scores. The rationale is further discussed in the Discussion section.
A total of 195 patients scheduled for elective radical gastrectomy were consecutively enrolled. Sample size was calculated using G*Power 3.1, based on an estimated effect size of 0.4, α = 0.05, and statistical power (1-β) = 0.80. This ensured adequate power to detect clinically relevant intergroup differences in primary outcomes.
Recruitment and screening: All patients were screened according to predefined inclusion and exclusion criteria. Eligible individuals provided written informed consent prior to randomization.
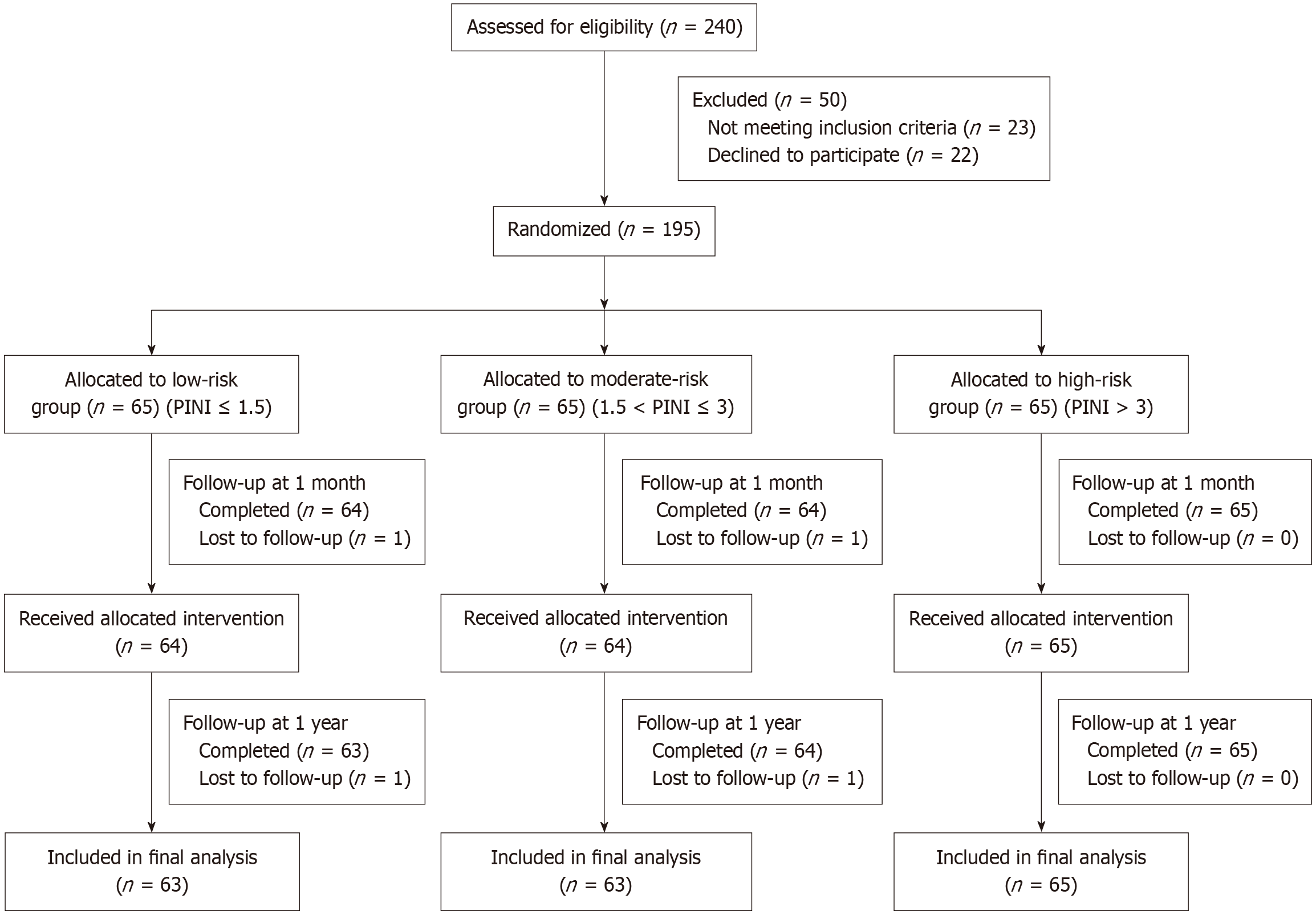
Randomization and allocation: Patients were stratified into low-, moderate-, and high-risk groups based on preoperative PINI scores. Randomization was then conducted within each risk group using a computer-generated block randomization scheme (block size = 6) to ensure allocation balance (Figure 1). To ensure allocation concealment, sequentially numbered, opaque, sealed envelopes were used, prepared and handled by an independent biostatistician not involved in patient recruitment or outcome assessment.
Group allocation was as follows: (1) Low-risk (PINI ≤ 1.5, n = 65): Standard nutritional support; (2) Moderate-risk (1.5 < PINI ≤ 3, n = 65): Intensive nutritional support; and (3) High-risk (PINI > 3, n = 65): Immune-enhancing nutritional support.
Follow-up and analysis: Participants were assessed at baseline, 1 week, 1 month, and 1 year postoperatively. A total of 7 patients were lost to follow-up (low-risk: 2, moderate-risk: 2, high-risk: 0), due to consent withdrawal (n = 2), inter-hospital transfer (n = 1), and loss of contact (n = 1). All analyses were conducted on an intention-to-treat basis. Missing data were addressed using linear mixed models (LMM).
Given the nature of the intervention, blinding of patients and care providers was not feasible. However, to mitigate performance and detection bias, all subjective outcomes—such as sleep quality and psychological measures—were assessed by trained outcome assessors who were blinded to group allocation. Validated and standardized instruments were employed, including the Pittsburgh Sleep Quality Index (PSQI) for sleep assessment and the Hospital Anxiety and Depression Scale for psychological evaluation, each with established scoring systems and proven reliability.
Inclusion criteria: (1) Age 18-75 years; (2) Histologically confirmed gastric adenocarcinoma; (3) Scheduled for R0 radical gastrectomy; (4) ASA physical status classification I-III; (5) Availability of preoperative laboratory data for PINI calculation; and (6) Ability to provide informed consent and comply with follow-up.
Exclusion criteria: (1) Receipt of chemotherapy, radiotherapy, or immunotherapy within 6 months before surgery; (2) Severe organ dysfunction (hepatic, renal, or cardiac failure); (3) Active infection, systemic inflammation, or BMI <
The PINI was calculated as: PINI = (α1-acid glycoprotein × CRP)/(albumin × transferrin), where α1-acid glycoprotein and CRP are acute-phase reactants (mg/L), and albumin (g/L) and transferrin (mg/L) are negative-phase proteins. Based on previously published literature, particularly the seminal work presented in[9], we defined PINI-based risk stratification as follows: (1) Low-risk: PINI ≤ 1.5; (2) Moderate-risk: 1.5 < PINI ≤ 3.0; and (3) High-risk: PINI > 3.0. These thresholds have been shown to predict nutritional and inflammatory risk in oncology patients and have been cited in multiple clinical studies as a valid tool for patient stratification.
All nutritional interventions were administered over a 6-week perioperative period, beginning 14 days preoperatively and continuing for 1 month after surgery. Nutritional strategies were tailored according to PINI-based risk stratification.
Standard nutrition (low-risk group): (1) Energy intake: 25-30 kcal/kg/day; (2) Protein: 1.2-1.5 g/kg/day; and (3) Oral or enteral nutritional supplements as needed.
Intensive nutrition (moderate-risk group): (1) Energy: 30-35 kcal/kg/day; (2) Protein: 1.5-2.0 g/kg/day; and (3) Supplemented with micronutrients and antioxidant vitamins.
Immune-enhancing nutrition (high-risk group): In addition to the above: (1) Glutamine: 0.3-0.5 g/kg/day; (2) Arginine: 9 g/day; and (3) Omega-3 polyunsaturated fatty acids: 2-3 g/day.
Administered orally, enterally, or parenterally, depending on patient tolerance.
Standardized commercial nutritional formulas were used throughout the intervention period. In the high-risk group, patients received Peptisorb Immuno® (Nutricia, Netherlands), an immune-enhancing enteral formula enriched with glutamine, arginine, and omega-3 polyunsaturated fatty acids. For patients in the standard support group, Ensure® (Abbott, United States), a widely used, balanced oral nutritional supplement, was administered as the primary formulation. All products met international clinical standards for enteral nutrition and were adjusted according to individual tolerance and metabolic demands[10].
All patients followed enhanced recovery after surgery (ERAS) protocols, including early mobilization, multimodal analgesia, and infection prevention.
ERAS protocol and compliance: All participants received perioperative care based on a standardized ERAS protocol, which included preoperative education, carbohydrate loading, early ambulation, multimodal analgesia, and early enteral nutrition. ERAS compliance was prospectively monitored using a 16-item checklist covering key elements of the protocol. Adherence was defined as achieving ≥ 80% of the ERAS components. Compliance rates were comparable among the three study groups, minimizing the likelihood of ERAS-related confounding effects.
All outcomes were assessed at baseline, 1 week, 1 month, and 1 year postoperatively.
Sleep quality: PSQI: Higher scores indicate poorer sleep quality[11].
Nutritional status: BMI, serum albumin, and PINI scores[12].
Psychological well-being: Self-Rating Anxiety Scale (SAS) and Self-Rating Depression Scale (SDS)[13,14].
Pain: Visual Analogue Scale (VAS): 0 = no pain; 10 = worst pain[15].
Quality of life: EORTC QLQ-C30 questionnaire[16].
Clinical outcomes: (1) Postoperative complication rate; (2) Length of hospital stay; and (3) 6-month and 1-year overall survival.
Descriptive and inferential statistics: (1) Continuous variables: mean ± SD; analyzed by one-way ANOVA with Bonferroni post hoc tests; (2) Categorical variables: Analyzed using χ2 tests; (3) Repeated-measures ANOVA was used for within-group longitudinal comparisons; (4) Between-group changes (Δ values) were assessed via ANCOVA, adjusting for baseline scores; and (5) Missing data were handled using LMM.
Multivariate regression: (1) Linear regression models assessed the association between PINI improvements and BMI, PSQI, SAS, SDS, VAS, and QLQ-C30, adjusted for age, tumor stage, and surgical approach; (2) Logistic regression was used to identify predictors of complications and survival; (3) Variables with P < 0.10 in univariate analysis were entered into multivariable models; and (4) Multicollinearity was evaluated using variance inflation factors (VIF < 10).
Survival analysis: (1) Kaplan-Meier curves were constructed for 6- and 12-month survival; (2) Cox proportional hazards models identified survival predictors; (3) Proportional hazards assumption was tested using Schoenfeld residuals; and (4) Hazard ratios were reported, although not statistically significant within the 1-year timeframe.
Statistical threshold: Two-tailed P values < 0.05 were considered statistically significant. Analyses were performed using SPSS version 19.0 (IBM Corp., Armonk, NY, United States).
A total of 195 patients undergoing radical gastrectomy were stratified into low-risk (n = 65), moderate-risk (n = 65), and high-risk (n = 65) groups according to preoperative PINI scores. As shown in Table 1, no statistically significant differences were observed among groups in age, gender, BMI, education level, surgical approach, type of gastrectomy, tumor stage, or comorbidities (all P > 0.05), confirming successful randomization and baseline comparability.
| Variables | Low-risk group (n = 65) | Moderate-risk group (n = 65) | High-risk group (n = 65) | Statistic | P value |
| Age (years) | 58.5 ± 7.9 | 59.1 ± 8.4 | 60.2 ± 8.7 | F = 0.832 | 0.412 |
| Gender | χ² = 0.256 | 0.879 | |||
| Male | 38 (58.5) | 39 (60.0) | 40 (61.5) | ||
| Female | 27 (41.5) | 26 (40.0) | 25 (38.5) | ||
| BMI (kg/m2) | 23.7 ± 2.0 | 23.8 ± 2.1 | 23.5 ± 2.2 | F = 0.356 | 0.701 |
| Education level | χ² = 0.287 | 0.772 | |||
| Junior high or below | 15 (23.1) | 14 (21.5) | 16 (24.6) | ||
| High school | 25 (38.5) | 26 (40.0) | 24 (36.9) | ||
| College or above | 25 (38.5) | 25 (38.5) | 25 (38.5) | ||
| Surgical method | χ² = 0.521 | 0.681 | |||
| Minimally invasive surgery | 50 (76.9) | 48 (73.8) | 51 (78.5) | ||
| Open surgery | 15 (23.1) | 17 (26.2) | 14 (21.5) | ||
| Type of surgery | χ² = 0.132 | 0.936 | |||
| Total gastrectomy | 33 (50.8) | 34 (52.3) | 32 (49.2) | ||
| Partial gastrectomy | 32 (49.2) | 31 (47.7) | 33 (50.8) | ||
| Clinical staging | χ² = 0.217 | 0.897 | |||
| Phase I | 10 (15.4) | 12 (18.5) | 11 (16.9) | ||
| Phase II | 20 (30.8) | 19 (29.2) | 21 (32.3) | ||
| Phase III | 25 (38.5) | 24 (36.9) | 23 (35.4) | ||
| Phase IV | 10 (15.4) | 10 (15.4) | 10 (15.4) | ||
| Comorbidities | χ² = 0.156 | 0.991 | |||
| Hypertension | 15 (23.1) | 16 (24.6) | 17 (26.2) | ||
| Diabetes | 10 (15.4) | 11 (16.9) | 12 (18.5) | ||
| Other | 5 (7.7) | 6 (9.2) | 7 (10.8) |
Postoperative sleep quality significantly improved in all groups over time (P < 0.05, repeated-measures ANOVA). However, patients in the high-risk group receiving immune-enhancing nutrition exhibited the most substantial and sustained improvement. At 1 year postoperatively, PSQI scores were: (1) Low-risk: 6.3 ± 2.4; (2) Moderate-risk: 5.4 ± 2.1; and (3) High-risk: 4.1 ± 1.8 (P < 0.01 vs other groups, Bonferroni-adjusted). Table 2 presents detailed trends across timepoints. These findings underscore the potential role of immune-targeted nutritional strategies in promoting long-term sleep recovery.
| Group | Pre-op PSQI | 1 week post-op | 1 month post-op | 1 year post-op | Improvement | Statistic | P value |
| Low-risk | 10.1 ± 3.2 | 8.5 ± 3.1 | 7.0 ± 2.6 | 6.3 ± 2.4 | -3.8 ± 2.1 | F = 13.56 | 0.032 |
| Moderate-risk | 11.3 ± 4.0 | 8.9 ± 3.5 | 6.7 ± 2.8 | 5.4 ± 2.1 | -5.9 ± 2.5 | F = 15.21 | 0.014 |
| High-risk | 12.6 ± 4.3 | 9.3 ± 3.8 | 5.6 ± 2.3 | 4.1 ± 1.8 | -8.5 ± 2.71 | F = 17.98 | < 0.001 |
BMI: All groups experienced postoperative BMI decline initially, followed by gradual recovery. Notably, the high-risk group achieved weight stability by 1 year, suggesting a protective metabolic effect. BMI changes at 1 year: (1) Low-risk:
| Group | Pre-op BMI | 1W Post-op | 1M Post-op | 1Y Post-op | BMI Δ | Pre-op Albumin | 1W Post-op | 1M Post-op | 1Y Post-op | Albumin Δ | Pre-op PINI | 1W Post-op | 1M Post-op | 1Y Post-op | PINI Δ |
| Low-risk | 23.8 ± 2.0 | 22.9 ± 1.8 | 23.2 ± 1.7 | 23.6 ± 1.5 | -0.2 ± 0.2 | 42.3 ± 4.5 | 43.0 ± 4.3 | 43.8 ± 4.0 | 44.2 ± 3.9 | +1.9 ± 1.2 | 1.2 ± 0.2 | 1.1 ± 0.2 | 0.9 ± 0.2 | 0.8 ± 0.1 | -0.4 ± 0.2 |
| Moderate-risk | 23.6 ± 2.1 | 23.0 ± 2.0 | 23.5 ± 1.8 | 23.9 ± 1.6 | -0.1 ± 0.3 | 41.5 ± 5.2 | 43.5 ± 4.9 | 44.7 ± 4.5 | 45.3 ± 4.3 | +3.8 ± 1.6 | 2.5 ± 0.3 | 2.1 ± 0.3 | 1.8 ± 0.3 | 1.5 ± 0.2 | -1.0 ± 0.3 |
| High-risk | 23.4 ± 2.3 | 23.1 ± 2.1 | 23.7 ± 2.0 | 24.2 ± 1.8 | +0.1 ± 0.21 | 40.2 ± 5.0 | 45.0 ± 4.4 | 46.8 ± 4.2 | 47.5 ± 3.8 | +6.5 ± 2.31 | 4.6 ± 0.4 | 3.8 ± 0.3 | 3.2 ± 0.3 | 2.7 ± 0.2 | -1.9 ± 0.41 |
Serum albumin: Albumin levels increased significantly postoperatively in all groups, with the high-risk group achieving the highest values at 1 year.
Serum albumin at 1 year: (1) Low-risk: 44.2 ± 3.9 g/L; (2) Moderate-risk: 45.3 ± 4.3 g/L; and (3) High-risk: 47.5 ± 3.8 g/L (P < 0.01). As shown in Table 3, serum albumin levels followed a positive trend over time, particularly in the high-risk group.
PINI score improvement: All groups showed reductions in PINI scores, indicating systemic immune-nutritional improvement. The high-risk group had the largest decrease. PINI change at 1 year: (1) Low-risk: -0.4 ± 0.2; (2) Moderate-risk: -1.0 ± 0.3; and (3) High-risk: -1.9 ± 0.4 (P < 0.01, linear regression). PINI score improvements across follow-up intervals are also reported in Table 3.
Anxiety and depression scores (SAS and SDS) decreased significantly over time in all groups (P < 0.01), with the most pronounced improvement in the high-risk group. SAS scores at 1 year: (1) Low-risk: 40.9 ± 6.8; (2) Moderate-risk: 41.1 ± 7.9; and (3) High-risk: 37.2 ± 6.4 (P < 0.01). SDS scores at 1 year: (1) Low-risk: 39.8 ± 7.3; (2) Moderate-risk: 38.9 ± 7.7; and (3) High-risk: 35.6 ± 6.1 (P < 0.01). Table 4 presents the evolution of psychological status, as assessed by SAS and SDS scores, at each follow-up point.
| Group | Pre-op SAS | 1W | 1M | 1Y | SAS Δ | Pre-op SDS | 1W | 1M | 1Y | SDS Δ |
| Low-risk | 46.2 ± 8.3 | 44.5 ± 7.8 | 42.7 ± 7.1 | 40.9 ± 6.8 | -5.3 ± 2.2 | 45.8 ± 7.9 | 43.9 ± 7.5 | 41.6 ± 7.0 | 39.8 ± 7.3 | -6.0 ± 2.4 |
| Moderate-risk | 48.4 ± 9.0 | 45.9 ± 8.7 | 43.8 ± 8.4 | 41.1 ± 7.9 | -7.3 ± 2.6 | 47.5 ± 8.6 | 44.5 ± 8.3 | 42.1 ± 7.9 | 38.9 ± 7.7 | -8.6 ± 2.9 |
| High-risk | 50.1 ± 9.5 | 44.3 ± 8.2 | 39.6 ± 7.2 | 37.2 ± 6.4 | -12.9 ± 3.11 | 49.9 ± 9.3 | 44.1 ± 8.1 | 39.3 ± 7.5 | 35.6 ± 6.1 | -12.6 ± 4.21 |
Postoperative pain intensity decreased significantly in all groups. The high-risk group reported the greatest reduction in VAS scores at 1 year, suggesting an anti-inflammatory effect. VAS scores at 1 year: (1) Low-risk: 3.2 ± 1.5; (2) Moderate-risk: 2.9 ± 1.4; and (3) High-risk: 2.1 ± 1.3 (P < 0.01). See Table 5. These findings support the analgesic benefit of tailored immune-nutritional support.
| Group | Pre-op VAS | 1 week post-op | 1 month post-op | 1 year post-op | Improvement | Statistic | P value |
| Low-risk | 6.9 ± 2.3 | 5.1 ± 2.0 | 3.9 ± 1.8 | 3.2 ± 1.5 | -3.7 ± 1.3 | F = 10.87 | 0.028 |
| Moderate-risk | 7.2 ± 2.5 | 5.3 ± 2.1 | 3.7 ± 1.9 | 2.9 ± 1.4 | -4.3 ± 1.5 | F = 11.45 | 0.014 |
| High-risk | 7.5 ± 2.6 | 4.8 ± 1.9 | 3.2 ± 1.6 | 2.1 ± 1.3 | -5.4 ± 1.61 | F = 13.78 | < 0.001 |
All groups reported improvement in quality of life over time. The high-risk group showed the most significant gains.
QLQ-C30 global score at 1 year: (1) Low-risk: 75.0 ± 7.6; (2) Moderate-risk: 73.8 ± 6.9; and (3) High-risk: 78.2 ± 8.0 (P < 0.01). Table 6 illustrates domain-specific subscale trends. These improvements underscore the holistic recovery benefit of immune-enhancing nutrition.
| Group | Pre-op QOL | 1 week post-op | 1 month post-op | 1 year post-op | Improvement | Statistic | P value |
| Low-risk | 58.7 ± 11.1 | 64.3 ± 10.4 | 70.1 ± 8.9 | 75.0 ± 7.6 | 16.3 ± 4.8 | F = 7.86 | 0.031 |
| Moderate-risk | 53.5 ± 9.2 | 62.4 ± 8.3 | 67.2 ± 7.4 | 73.8 ± 6.9 | 20.3 ± 5.1 | F = 10.13 | 0.012 |
| High-risk | 49.8 ± 13.0 | 63.5 ± 10.5 | 71.1 ± 8.9 | 78.2 ± 8.0 | 28.4 ± 6.31 | F = 15.49 | < 0.001 |
Complication rates: Despite exhibiting the poorest baseline immune-nutritional status, patients in the high-risk group receiving immune-enhancing nutrition experienced the lowest postoperative complication rate: (1) Low-risk group: 10.0% (7/65); (2) Moderate-risk group: 16.7% (11/65); and (3) High-risk group: 3.3% (2/65) (P < 0.01, logistic regression). As shown in Table 7, this unexpected but clinically meaningful finding underscores the protective immunomodulatory effects of targeted nutritional support. Components such as glutamine, arginine, and omega-3 fatty acids may mitigate postoperative stress, enhance wound healing, and reduce infectious complications.
| Group | Complication rate | Mean hospital stay (days) | 6-month survival rate | 1-year survival rate | Statistic | P value |
| Low-risk | 10.0% (7/65) | 12.3 ± 3.1 | 96.7% (63/65) | 93.8% (61/65) | χ² = 6.42 | 0.004 |
| Moderate-risk | 16.7% (11/65) | 11.2 ± 2.9 | 93.3% (61/65) | 90.2% (59/65) | F = 6.89 | 0.003 |
| High-risk | 3.3% (2/65) | 9.8 ± 2.4 | 100% (65/65) | 98.5% (64/65) | χ² = 1.89 | 0.192 |
Length of hospital stay: Hospitalization duration was also significantly shorter in the high-risk group, suggesting accelerated postoperative recovery and improved readiness for discharge: (1) Low-risk group: 12.3 ± 3.1 days; (2) Moderate-risk group: 11.2 ± 2.9 days; and (3) High-risk group: 9.8 ± 2.4 days (P < 0.01, linear regression).
As shown in Table 7, these findings reflect the capacity of immune-enhancing nutritional interventions to attenuate the physiological stress response, promote early mobilization, and facilitate gastrointestinal functional recovery. Nutrients such as glutamine and omega-3 fatty acids may modulate systemic inflammation and enhance tissue repair, ultimately shortening the trajectory to discharge readiness.
Survival outcomes: Although 6-month survival rates did not differ significantly among groups, the high-risk group demonstrated a significantly higher 1-year survival rate, despite their initially poorer nutritional and inflammatory status: 6-month survival: (1) Low-risk: 96.7%; (2) Moderate-risk: 93.3%; and (3) High-risk: 100% (P > 0.05). 1-year survival: (1) Low-risk: 93.8%; (2) Moderate-risk: 90.2%; and (3) High-risk: 98.5% (P < 0.05). As shown in Table 7, this favorable trend suggests that early, PINI-guided correction of immune-nutritional deficits may contribute to long-term oncologic resilience. Mechanistically, improved nutritional status, inflammatory control, and postoperative recovery may synergize to enhance tumor surveillance, reduce complications, and delay recurrence. These results reaffirm the critical role of personalized, risk-adapted nutrition as a modifiable and clinically actionable factor in the long-term prognosis of gastric cancer patients.
Pearson correlation analysis demonstrated strong associations between improvements in PINI and key recovery parameters (Table 8): (1) ΔPINI vs ΔPSQI: r = 0.68, P < 0.01; (2) ΔPINI vs ΔSAS: r = 0.65, P < 0.01; (3) ΔPINI vs ΔSDS: r = 0.61, P < 0.01; (4) ΔPINI vs ΔQLQ-C30: r = 0.72, P < 0.01; and (5) ΔPINI vs complication rate: R = -0.59, P < 0.01.
| Variable | Pearson correlation coefficient (r) | P value | Interpretation |
| PSQI score improvement | 0.68 | < 0.01 | Better sleep quality |
| SAS score reduction | 0.65 | < 0.01 | Lower anxiety levels |
| SDS score reduction | 0.61 | < 0.01 | Reduced depressive symptoms |
| EORTC QLQ-C30 improvement | 0.72 | < 0.01 | Better quality of life |
| Postoperative complication rate | -0.59 | < 0.01 | Lower risk of postoperative complications |
These results further support the central hypothesis that PINI-based graded nutritional support enhances not only physiological recovery, but also psychological and functional outcomes following gastrectomy.
This study systematically evaluated the long-term efficacy of PINI-based graded nutritional support in patients undergoing radical gastrectomy. The results demonstrated that individualized nutritional interventions significantly improved sleep quality, nutritional status, psychological well-being, pain management, and quality of life, particularly in high-risk patients with elevated preoperative PINI scores. These findings highlight the critical role of immune-enhancing nutrition in optimizing postoperative recovery and long-term outcomes.
This study is among the first to integrate PINI-based risk stratification into perioperative nutritional management for gastric cancer patients undergoing radical gastrectomy. Unlike conventional nutritional interventions that apply uniform protocols, this graded approach tailors nutritional support based on individualized immune-nutritional status, op
Our findings demonstrated that high-risk patients (PINI > 3) receiving immune-enhancing nutrition experienced the most significant improvements in multiple domains, including nutritional status (BMI, albumin levels, PINI scores), complication rates, sleep quality, and psychological well-being. This aligns with prior research suggesting that perioperative immune-enhancing nutrition modulates inflammatory responses and enhances metabolic recovery[17,18].
Additionally, the high-risk group exhibited the highest 1-year survival rate (98.5%), suggesting that targeted nu
Postoperative sleep disturbances are common in gastric cancer patients due to surgical stress, systemic inflammation, and psychological distress[21]. This study found that PINI-based graded nutritional support significantly improved sleep quality, particularly in the high-risk group. Several mechanisms may explain this effect:
Modulation of systemic inflammation: (1) Elevated CRP and pro-inflammatory cytokines [e.g., interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α)] are known to impair sleep regulation and circadian rhythms[22]; and (2) Immune-enhancing nutrition has been shown to reduce systemic inflammation, potentially mitigating its disruptive effects on sleep[23].
Energy metabolism and fatigue reduction: (1) Gastric cancer surgery often results in energy depletion and metabolic stress, contributing to postoperative fatigue; and (2) Adequate perioperative nutrition supports mitochondrial function and ATP production, thereby promoting better sleep-wake cycles[24].
Psychological stress reduction: (1) PINI score improvement correlated strongly with reductions in anxiety (r = 0.65) and depression (r = 0.61, P < 0.01); and (2) These findings suggest that better immune-nutritional status alleviates psychological distress, which in turn improves sleep quality[25].
These findings reinforce the importance of targeted nutritional support in addressing postoperative sleep dysfunction, a key factor influencing overall recovery and quality of life.
Postoperative complications such as infections, thrombosis, and impaired wound healing are closely linked to nutritional and immune status. In this study, the high-risk group exhibited the lowest complication rate (3.3%), despite having the poorest preoperative nutritional status (P < 0.01 compared to other groups).
These findings are consistent with prior studies demonstrating that perioperative immune-enhancing nutrition reduces surgical complications[4,26]. Possible contributing mechanisms include: (1) Enhanced protein synthesis and tissue repair: Glutamine and arginine support collagen synthesis and immune cell proliferation, accelerating wound healing[27]; (2) Anti-inflammatory and Immunomodulatory Effects: Omega-3 fatty acids suppress pro-inflammatory cytokines, reducing systemic inflammation and infection risk[28]; and (3) Improved Gut Barrier Function: Malnutrition disrupts gut mi
These findings underscore the importance of proactive nutritional interventions in reducing postoperative morbidity, particularly for high-risk patients with compromised immune function.
This study confirmed that immune-enhancing nutrition contributes to significant improvements in anxiety (SAS), depression (SDS), and pain management (VAS scores). (1) High-risk patients experienced the greatest reductions in SAS
These results align with research indicating that malnutrition exacerbates psychological distress, while adequate nutrition stabilizes neuroendocrine function[31].
Furthermore, nutritional support may enhance endogenous opioid release and modulate pain perception, explaining the greater reduction in VAS scores among high-risk patients[32].
Postoperative quality of life (QOL) is a key determinant of long-term recovery in gastric cancer patients. The findings from this study demonstrate that patients receiving immune-enhancing nutrition (high-risk group) experienced the greatest improvements across multiple dimensions of the EORTC QLQ-C30 scores, particularly in physical, emotional, and role functioning domains[4,25]. These effects can be attributed to the following mechanisms:
Multitargeted benefits of immune nutrition: (1) Nutritional optimization enhances physical function, reducing fatigue, appetite loss, and pain, which are major barriers to postoperative rehabilitation[33,34]; and (2) Modulation of systemic inflammation alleviates inflammatory symptoms that negatively impact QOL[35,36].
Reduction in complication burden: (1) Lower Postoperative Complication Rates: Patients receiving IEN had significantly lower rates of complications, including infections, delayed wound healing, and anastomotic leaks[8,37]; and (2) Correlation with Improved QOL: The reduction in complications correlated with improved QOL scores (r = -0.59, P < 0.01), emphasizing the relationship between clinical outcomes and patient-reported well-being[38].
Positive feedback loop between psychological and physiological recovery: (1) Improvement in psychological well-being (SAS, SDS scores) correlated with better QOL scores (r = 0.72, P < 0.01); and (2) This suggests that reducing postoperative distress not only enhances mental health but also accelerates physical recovery, forming a self-reinforcing cycle of rehabilitation[39,40].
These findings align with previous research indicating that perioperative nutrition plays a fundamental role in long-term patient-centered outcomes[41].
A key limitation of the present study is that we did not longitudinally measure additional inflammatory biomarkers, such as IL-6, TNF-α, or high-sensitivity C-reactive protein, beyond the components of the PINI index. This was due to financial and logistical constraints during the study period. Although PINI provides an integrative estimate of systemic inflammation and nutritional status, the lack of comprehensive inflammatory profiling limits mechanistic interpretation. Future studies should incorporate dynamic measurements of immune and inflammatory markers to better elucidate the underlying biological effects of targeted nutritional interventions.
Although no statistically significant differences were observed in 6-month survival rates among the three groups, the high-risk group demonstrated superior short-term recovery outcomes, including shorter hospital stays, lower postoperative complication rates, and improved functional recovery. These early postoperative benefits suggest that graded nutritional support may contribute to long-term survival advantages by optimizing the early recovery phase[42,43].
Acceleration of early postoperative recovery: The high-risk group had the shortest hospital stay (9.8 ± 2.4 days) compared to the moderate-risk (11.2 ± 2.9 days) and low-risk groups (12.3 ± 3.1 days, P < 0.01).This reduction in hospital stay can be attributed to: (1) Faster resolution of surgical stress and systemic inflammation, facilitated by immune-enhancing nutrition[44]; (2) Lower rates of postoperative complications (3.3% vs 16.7% in the moderate-risk group and 10.0% in the low-risk group, P < 0.01), reducing the need for prolonged hospitalization[45]; and (3) Shorter hospital stays are not only beneficial for cost reduction but also reduce the risk of nosocomial infections and hospital-related complications[46].
Reduction in postoperative metabolic and immune dysregulation: Postoperative metabolic stress leads to catabolism, muscle loss, and immune suppression, increasing the risk of delayed recovery. Precision nutritional interventions in the high-risk group led to: (1) Better preservation of lean body mass (lower BMI reduction at 1 year: +0.1 ± 0.2 kg/m2vs -0.3 ± 0.3 kg/m2 in the low-risk group, P < 0.01); (2) Higher albumin levels at 1 year (47.5 ± 3.8 g/L vs 44.2 ± 3.9 g/L in the low-risk group, P < 0.01), indicating superior protein metabolism and immune function; and (3) This suggests that nutritional modulation of inflammatory and metabolic responses contributes to more stable postoperative recovery, reducing the likelihood of late complications[4,47].
Potential long-term survival benefits: (1) At 1-year follow-up, survival rates were significantly higher in the high-risk group (98.5%) compared to the moderate-risk (90.2%) and low-risk (93.8%) groups (P < 0.05); (2) While 6-month survival rates did not differ significantly, the trend toward improved 1-year survival suggests that early nutritional optimization may have a lasting impact on patient outcomes; and (3) These findings align with previous studies demonstrating that perioperative nutritional support can improve long-term oncologic outcomes by mitigating cancer-related cachexia and immune dysfunction[4].
Although the 1-year survival rate was notably improved in the high-risk group receiving immune-enhancing nutrition, this finding should be interpreted with caution. Patients in this group had worse baseline nutritional and inflammatory status, and despite statistical adjustment, residual confounding cannot be fully excluded. Moreover, the follow-up period was relatively short, and survival estimates should be considered preliminary. This result is therefore hypothesis-generating rather than confirmatory, and future studies with larger sample sizes and extended follow-up are needed to validate the long-term survival benefits of risk-stratified nutritional interventions.
Implications for long-term prognosis and future research: While this study provides preliminary evidence suggesting that immune-enhancing nutrition may offer survival benefits, further research with extended follow-up periods (3-5 years) and disease-free survival (DFS) assessments is essential to confirm its long-term impact. Future investigations should focus on: (1) Mechanistic Insights: Exploring the role of immune-enhancing nutrition in modulating the tumor microenvironment and systemic inflammation, which may influence cancer progression and recurrence[48]; (2) Long-Term Dietary Adherence: Evaluating how sustained post-discharge nutritional adherence affects long-term survival, metabolic stability, and quality of life; and (3) Comprehensive Oncologic Outcomes: Assessing 3-year and 5-year DFS and overall survival (OS) to determine whether early postoperative benefits translate into improved long-term prognosis[49].
In summary, although immediate postoperative survival rates were comparable across groups, the superior early recovery, lower complication rates, and improved metabolic stability observed in the high-risk group suggest that PINI-based nutritional interventions may contribute to long-term survival benefits. However, further large-scale, multi-center trials with longer follow-up are needed to validate these findings.
From a practical perspective, the implementation of immune-enhancing nutritional support raises important considerations. These specialized formulas—such as those enriched with glutamine, arginine, and omega-3 fatty acids—are often more costly and less widely available than standard nutritional options. However, their selective use in high-risk patients may represent a cost-effective strategy, as improved nutritional and immunological recovery may help reduce pos
This study did not include a non-intervention control group due to ethical and clinical considerations: (1) Withholding perioperative nutrition in gastric cancer patients would be unethical, given its well-established benefits in reducing complications and improving recovery[4]; (2) Prior studies have shown that malnourished cancer patients without nutritional support experience higher infection rates, prolonged hospital stays, and increased mortality[50]; and (3) Instead of a placebo group, a stratified nutritional support model was implemented, allowing for meaningful com
To account for potential biases, multivariable regression analysis was performed to adjust for confounding variables, ensuring the robustness of findings.
While this study provides compelling evidence supporting PINI-based graded nutritional support, several limitations should be acknowledged, along with directions for future research.
Strengths of this study: (1) First study to integrate PINI-based risk stratification into perioperative nutritional ma
Limitations: Despite the strengths of this study, several limitations should be acknowledged: (1) Single-center design and limited generalizability. This study was conducted at a single academic medical center, which may limit the generalizability of the findings to broader populations. A multi-center trial across diverse healthcare settings would enhance external validity and confirm the reproducibility of the results; (2) Limited follow-up duration. The follow-up period was restricted to one year, which may not fully capture long-term survival benefits or potential late complications. Future research should incorporate longer follow-up periods (e.g., 3-year, 5-year DFS and OS) to better assess the sustained effects of PINI-based graded nutritional support on long-term oncologic and functional outcomes; (3) Lack of Detailed Dietary Intake Assessment. While this study focused on perioperative nutritional interventions, long-term dietary habits were not systematically assessed. Future studies should include comprehensive dietary intake monitoring to evaluate nutrient adherence and its impact on long-term recovery and quality of life; and (4) Absence of Cost-Effectiveness Analysis. The economic feasibility of graded nutritional support was not analyzed in this study. Conducting a cost-effectiveness analysis would provide valuable insights for healthcare policymakers, helping to determine the financial sustainability of implementing personalized nutritional strategies in clinical practice. By addressing these limitations in future research, a more comprehensive understanding of the clinical, economic, and long-term benefits of PINI-based nutritional interventions can be achieved.
Future directions: (1) Multi-center randomized controlled trials should be conducted to validate findings across diverse populations; (2) Exploration of biomarkers (e.g., inflammatory cytokines, gut microbiota composition) could provide a mechanistic understanding of immune-enhancing nutrition; and (3) Investigation of the impact of long-term nutritional adherence on DFS and OS would be valuable for guiding postoperative nutritional recommendations.
This randomized trial provides compelling evidence that PINI-guided graded nutritional support improves postoperative recovery following radical gastrectomy, especially in high-risk patients. By integrating immune-enhancing nutrition, patients achieved better physical, psychological, and functional outcomes, including lower complication rates, reduced hospital stay, and improved 1-year survival.
These findings support incorporating PINI-guided nutritional protocols into routine perioperative care to enhance oncologic and functional outcomes in gastric cancer. Future work should focus on extending survival analysis, cost-benefit evaluation, and biomarker-driven strategies to advance the field of precision perioperative nutrition.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 68549] [Article Influence: 13709.8] [Reference Citation Analysis (201)] |
| 2. | Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396:635-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1150] [Cited by in RCA: 3295] [Article Influence: 549.2] [Reference Citation Analysis (6)] |
| 3. | Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H. Gastric cancer. Lancet. 2016;388:2654-2664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1282] [Cited by in RCA: 1504] [Article Influence: 150.4] [Reference Citation Analysis (1)] |
| 4. | Ludwig RB, Paludo J, Fernandes D, Scherer F. Lesser time of preoperative fasting and early postoperative feeding are safe? Arq Bras Cir Dig. 2013;26:54-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Cederholm T, Jensen GL, Correia MITD, Gonzalez MC, Fukushima R, Higashiguchi T, Baptista G, Barazzoni R, Blaauw R, Coats A, Crivelli A, Evans DC, Gramlich L, Fuchs-Tarlovsky V, Keller H, Llido L, Malone A, Mogensen KM, Morley JE, Muscaritoli M, Nyulasi I, Pirlich M, Pisprasert V, de van der Schueren MAE, Siltharm S, Singer P, Tappenden K, Velasco N, Waitzberg D, Yamwong P, Yu J, Van Gossum A, Compher C; GLIM Core Leadership Committee; GLIM Working Group. GLIM criteria for the diagnosis of malnutrition - A consensus report from the global clinical nutrition community. Clin Nutr. 2019;38:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 795] [Cited by in RCA: 1879] [Article Influence: 234.9] [Reference Citation Analysis (0)] |
| 6. | Cashin AG, McAuley JH. Clinimetrics: Physiotherapy Evidence Database (PEDro) Scale. J Physiother. 2020;66:59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 603] [Article Influence: 100.5] [Reference Citation Analysis (0)] |
| 7. | Lobo DN, Gianotti L, Adiamah A, Barazzoni R, Deutz NEP, Dhatariya K, Greenhaff PL, Hiesmayr M, Hjort Jakobsen D, Klek S, Krznaric Z, Ljungqvist O, McMillan DC, Rollins KE, Panisic Sekeljic M, Skipworth RJE, Stanga Z, Stockley A, Stockley R, Weimann A. Perioperative nutrition: Recommendations from the ESPEN expert group. Clin Nutr. 2020;39:3211-3227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 181] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 8. | Ma M, Zheng Z, Zeng Z, Li J, Ye X, Kang W. Perioperative Enteral Immunonutrition Support for the Immune Function and Intestinal Mucosal Barrier in Gastric Cancer Patients Undergoing Gastrectomy: A Prospective Randomized Controlled Study. Nutrients. 2023;15:4566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 9. | Aviram M, Rosenblat M, Gaitini D, Nitecki S, Hoffman A, Dornfeld L, Volkova N, Presser D, Attias J, Liker H, Hayek T. Pomegranate juice consumption for 3 years by patients with carotid artery stenosis reduces common carotid intima-media thickness, blood pressure and LDL oxidation. Clin Nutr. 2004;23:423-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 415] [Cited by in RCA: 371] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 10. | Braga M, Ljungqvist O, Soeters P, Fearon K, Weimann A, Bozzetti F; ESPEN. ESPEN Guidelines on Parenteral Nutrition: surgery. Clin Nutr. 2009;28:378-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 407] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 11. | Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17520] [Cited by in RCA: 23554] [Article Influence: 636.6] [Reference Citation Analysis (0)] |
| 12. | Kanda M, Fujii T, Kodera Y, Nagai S, Takeda S, Nakao A. Nutritional predictors of postoperative outcome in pancreatic cancer. Br J Surg. 2011;98:268-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 481] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 13. | Zung WW. A rating instrument for anxiety disorders. Psychosomatics. 1971;12:371-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2251] [Cited by in RCA: 2941] [Article Influence: 53.5] [Reference Citation Analysis (1)] |
| 14. | ZUNG WW. A SELF-RATING DEPRESSION SCALE. Arch Gen Psychiatry. 1965;12:63-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5900] [Cited by in RCA: 6283] [Article Influence: 209.4] [Reference Citation Analysis (0)] |
| 15. | Diop JPD, Diallo RN, Bourdon-Huguenin V, Dem A, Diouf D, Dieng MM, Ba SA, Dia Y, Ka S, Mbengue B, Thiam A, Faye O, Diop PA, Sobol H, Dieye A. Novel BRCA2 pathogenic variant c.5219 T > G; p.(Leu1740Ter) in a consanguineous Senegalese family with hereditary breast cancer. BMC Med Genet. 2019;20:73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman SB, de Haes JC. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9802] [Cited by in RCA: 11919] [Article Influence: 361.2] [Reference Citation Analysis (0)] |
| 17. | Wang HX, Wang CC, Yang W, Gao LL, Yu SQ. Prognostic value of preoperative prognostic nutritional index in stage III gastric cancer after curative resection: a retrospective cohort study. Asia Pac J Clin Nutr. 2018;27:540-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 18. | Hasegawa T, Yatagai N, Furukawa T, Wakui E, Saito I, Takeda D, Kakei Y, Sakakibara A, Nibu KI, Akashi M. The prospective evaluation and risk factors of dysphagia after surgery in patients with oral cancer. J Otolaryngol Head Neck Surg. 2021;50:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 19. | Yang Y, Gao P, Song Y, Sun J, Chen X, Zhao J, Ma B, Wang Z. The prognostic nutritional index is a predictive indicator of prognosis and postoperative complications in gastric cancer: A meta-analysis. Eur J Surg Oncol. 2016;42:1176-1182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 198] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 20. | Sun K, Chen S, Xu J, Li G, He Y. The prognostic significance of the prognostic nutritional index in cancer: a systematic review and meta-analysis. J Cancer Res Clin Oncol. 2014;140:1537-1549. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 311] [Cited by in RCA: 318] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 21. | Antunes FP, Costa Mda C, Paim JS, Vieira-da-Silva LM, Cruz AA, Natividade M, Barreto ML. [Social inequalities in spatial distribution of hospital admissions due to respiratory diseases]. Cad Saude Publica. 2013;29:1346-1356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Imaralu JO, Ani IF, Onuoha CE, Grillo EO, Oguntade FA, Nwankpa CC. A Five-Year Review of Laparoscopic Gynaecological Surgeries in a Private-Owned Teaching Hospital, in Nigeria. West Afr J Med. 2022;39:111-118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 23. | Song GM, Tian X, Liang H, Yi LJ, Zhou JG, Zeng Z, Shuai T, Ou YX, Zhang L, Wang Y. Role of Enteral Immunonutrition in Patients Undergoing Surgery for Gastric Cancer: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Medicine (Baltimore). 2015;94:e1311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 24. | Zhang X, Yang L, Hou L, Liu J, Zhu H, Zhang J. Effect of a psychological nursing intervention on quality of life and cognitive function in patients with gastric carcinoma: A randomised controlled trial. Eur J Cancer Care (Engl). 2020;29:e13292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Zhang X, Chen X, Yang J, Hu Y, Li K. Effects of nutritional support on the clinical outcomes of well-nourished patients with cancer: a meta-analysis. Eur J Clin Nutr. 2020;74:1389-1400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Zhang L, Liu X, Tong F, Zou R, Peng W, Yang H, Liu F, Yang D, Huang X, Yi L, Wen M, Jiang L. Cognitive behavioral therapy for anxiety and depression in cancer survivors: a meta-analysis. Sci Rep. 2022;12:21466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 33] [Reference Citation Analysis (0)] |
| 27. | Odom MJ, Grace KA, Brigido MK, Theyyunni NR, Kessler RA, Greineder CF. Shoulder Pseudodislocation Associated with Calcific Tendinitis/Bursitis and Diagnosed by Point of Care Ultrasound. J Emerg Med. 2020;58:72-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 28. | Zhang X, Wang H, Zhang Y, Liu Y, Wang Z, Du Q, Xie C. Danggui Sini decoction for treating diabetic peripheral neuropathy: A protocol of systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore). 2020;99:e20482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Marano L, Porfidia R, Pezzella M, Grassia M, Petrillo M, Esposito G, Braccio B, Gallo P, Boccardi V, Cosenza A, Izzo G, Di Martino N. Clinical and immunological impact of early postoperative enteral immunonutrition after total gastrectomy in gastric cancer patients: a prospective randomized study. Ann Surg Oncol. 2013;20:3912-3918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 84] [Article Influence: 6.5] [Reference Citation Analysis (1)] |
| 30. | Silver JK, Baima J. Cancer prehabilitation: an opportunity to decrease treatment-related morbidity, increase cancer treatment options, and improve physical and psychological health outcomes. Am J Phys Med Rehabil. 2013;92:715-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 443] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 31. | Jiang N, Deng JY, Ding XW, Ke B, Liu N, Zhang RP, Liang H. Prognostic nutritional index predicts postoperative complications and long-term outcomes of gastric cancer. World J Gastroenterol. 2014;20:10537-10544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 113] [Cited by in RCA: 124] [Article Influence: 10.3] [Reference Citation Analysis (1)] |
| 32. | Burghardt J, Beutel ME, Hasenburg A, Schmutzer G, Brähler E. Declining Sexual Activity and Desire in Women: Findings from Representative German Surveys 2005 and 2016. Arch Sex Behav. 2020;49:919-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 33. | Osland E, Hossain MB, Khan S, Memon MA. Effect of timing of pharmaconutrition (immunonutrition) administration on outcomes of elective surgery for gastrointestinal malignancies: a systematic review and meta-analysis. JPEN J Parenter Enteral Nutr. 2014;38:53-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 95] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 34. | Matsui R, Sagawa M, Inaki N, Fukunaga T, Nunobe S. Impact of Perioperative Immunonutrition on Postoperative Outcomes in Patients with Upper Gastrointestinal Cancer: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients. 2024;16:577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 35. | Wang G, Pan S. The impact of sleep interventions combined with enhanced nutritional support on sleep quality, nutritional status, pain management, psychological well-being, and quality of life in postoperative colon cancer patients. J Cancer Res Clin Oncol. 2025;151:50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 36. | Zhao H, Liu C, Ruan G, Zheng X, Chen Y, Lin S, Liu X, Shi J, Li X, Li S, Shi H. The quality of life impacting factors in malnourished patients with gastric cancer. Front Oncol. 2024;14:1336859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 37. | Li Y, Bi X, Zhao J, Huang Z, Zhou J, Li Z, Zhang Y, Li M, Chen X, Hu X, Chi Y, Zhao D, Zhao H, Cai J. CEA Level, Radical Surgery, CD56 and CgA Expression Are Prognostic Factors for Patients With Locoregional Gastrin-Independent GNET. Medicine (Baltimore). 2016;95:e3567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 38. | Fujitani K, Tsujinaka T, Fujita J, Miyashiro I, Imamura H, Kimura Y, Kobayashi K, Kurokawa Y, Shimokawa T, Furukawa H; Osaka Gastrointestinal Cancer Chemotherapy Study Group. Prospective randomized trial of preoperative enteral immunonutrition followed by elective total gastrectomy for gastric cancer. Br J Surg. 2012;99:621-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 128] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 39. | Sasahara M, Kanda M, Ito S, Mochizuki Y, Teramoto H, Ishigure K, Murai T, Asada T, Ishiyama A, Matsushita H, Tanaka C, Kobayashi D, Fujiwara M, Murotani K, Kodera Y. The Preoperative Prognostic Nutritional Index Predicts Short-Term and Long-Term Outcomes of Patients with Stage II/III Gastric Cancer: Analysis of a Multi-Institution Dataset. Dig Surg. 2020;37:135-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 40. | Oh HM, Son CG. The Risk of Psychological Stress on Cancer Recurrence: A Systematic Review. Cancers (Basel). 2021;13:5816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 41. | August DA, Huhmann MB; American Society for Parenteral and Enteral Nutrition (A. S.P.E.N.) Board of Directors. A.S.P.E.N. clinical guidelines: nutrition support therapy during adult anticancer treatment and in hematopoietic cell transplantation. JPEN J Parenter Enteral Nutr. 2009;33:472-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 337] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 42. | Braga M, Gianotti L, Vignali A, Cestari A, Bisagni P, Di Carlo V. Artificial nutrition after major abdominal surgery: impact of route of administration and composition of the diet. Crit Care Med. 1998;26:24-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 127] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 43. | Rou WS, Lee BS, Moon HS, Lee ES, Kim SH, Lee HY. Risk factors and therapeutic results of early local recurrence after transcatheter arterial chemoembolization. World J Gastroenterol. 2014;20:6995-7004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 44. | Wang G, Zhang Q, Pan S. Investigation of negative emotions and sleep quality in gastric cancer patients and intervention strategies. Front Neurol. 2025;16:1536736. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 45. | Gianotti L, Braga M, Nespoli L, Radaelli G, Beneduce A, Di Carlo V. A randomized controlled trial of preoperative oral supplementation with a specialized diet in patients with gastrointestinal cancer. Gastroenterology. 2002;122:1763-1770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 315] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 46. | Matsui R, Sagawa M, Sano A, Sakai M, Hiraoka SI, Tabei I, Imai T, Matsumoto H, Onogawa S, Sonoi N, Nagata S, Ogawa R, Wakiyama S, Miyazaki Y, Kumagai K, Tsutsumi R, Okabayashi T, Uneno Y, Higashibeppu N, Kotani J. Impact of Perioperative Immunonutrition on Postoperative Outcomes for Patients Undergoing Head and Neck or Gastrointestinal Cancer Surgeries: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Ann Surg. 2024;279:419-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 22] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 47. | de Tapia B, Bonnin M, Valles C, Mozas C, Herrera D, Sanz M, Nart J. Clinical outcomes and associated factors in the treatment of peri-implant mucositis, combining mechanical debridement and prosthesis modification: A 30-month follow-up prospective case series. J Clin Periodontol. 2022;49:1357-1365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 48. | Calder PC. Immunonutrition in surgical and critically ill patients. Br J Nutr. 2007;98 Suppl 1:S133-S139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 92] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 49. | Khin NA, Yang P, Hung HM, Maung-U K, Chen YF, Meeker-O'Connell A, Okwesili P, Yasuda SU, Ball LK, Huang SM, O'Neill RT, Temple R. Regulatory and scientific issues regarding use of foreign data in support of new drug applications in the United States: an FDA perspective. Clin Pharmacol Ther. 2013;94:230-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 50. | Jie B, Jiang ZM, Nolan MT, Efron DT, Zhu SN, Yu K, Kondrup J. Impact of nutritional support on clinical outcome in patients at nutritional risk: a multicenter, prospective cohort study in Baltimore and Beijing teaching hospitals. Nutrition. 2010;26:1088-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 86] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 51. | Bozzetti F, Braga M, Gianotti L, Gavazzi C, Mariani L. Postoperative enteral versus parenteral nutrition in malnourished patients with gastrointestinal cancer: a randomised multicentre trial. Lancet. 2001;358:1487-1492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 306] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/