Published online Sep 24, 2025. doi: 10.5306/wjco.v16.i9.110130
Revised: June 24, 2025
Accepted: August 25, 2025
Published online: September 24, 2025
Processing time: 117 Days and 0.1 Hours
Upper gastrointestinal cancer (UGIC), including esophageal and gastric cancers, poses a major global health challenge due to its high morbidity and mortality. During the preoperative period, patients often face functional decline, malnu
To evaluate the impact of prehabilitation on patients undergoing UGIC surgery and provide a basis for implementation of the prehabilitation compound plan.
A computerized search of databases including Web of Science, PubMed, EMBASE, The Cochrane Library, Cumulative Index to Nursing and Allied Health Literature, China National Knowledge Infrastructure, Wanfang, and Chinese Science and Technology Journal Database was used to collect clinical trials on the impact of prehabilitation on patients undergoing UGIC surgery. After screening, a meta-analysis was conducted using Review Manager 5.0 software, and linear regression analysis was performed on the prehabilitation duration and outcome indicators.
A total of 13 clinical trials were ultimately included, with 8 literature quality evaluations at A level and 5 literature quality evaluations at B level. The meta-analysis results showed that compared with conventional nursing, the prehabilitation group had higher six-minute walk distance, lower postoperative complications and mortality rates, and shorter hospital stays, with statistically significant differences; there were no statistically significant differences in intensive care unit monitoring time and albumin levels between the two groups; regression analysis between prehabilitation duration and outcome indicators showed no significant relationship.
Prehabilitation can improve the perioperative functional ability of patients with UGIC and promote postoperative recovery, but its impact on nutrition, psychology, and quality of life needs to be further explored through more high-quality trials; in addition, further research is needed on the prehabilitation time, location, and specific plan.
Core Tip: This meta-analysis evaluates the impact of prehabilitation-a multidisciplinary intervention including exercise, nutrition, and psychological support-on patients undergoing upper gastrointestinal cancer surgery. Prehabilitation sig
- Citation: Shao X, Zhu YY, Shang B, Cai FJ, Wang XY, Zhou K, Luo CF. Meta-analysis of the impact of prehabilitation on patients undergoing upper gastrointestinal tract tumor surgery. World J Clin Oncol 2025; 16(9): 110130
- URL: https://www.wjgnet.com/2218-4333/full/v16/i9/110130.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i9.110130
Upper gastrointestinal cancer (UGIC) primarily includes esophageal cancer, gastric cancer, and tumors at the gastroesophageal junction. In 2020, the incidence and mortality rates of esophageal and gastric cancer were among the top ten globally and in China, imposing significant medical and economic burdens on society and families[1-3]. Currently, surgery remains the primary treatment modality. However, there is a “window period” between tumor diagnosis and surgery, during which patients often experience a decline in physical activity, increased nutritional risk, and pronounced psychological issues - problems associated with living with cancer[4-6]. This period also presents individual challenges such as insufficient preoperative preparation, increased surgery-related complications, and higher perioperative mortality rate[7,8]. Therefore, targeted preoperative interventions that address functional decline, nutritional depletion, and psychological distress are urgently needed to improve perioperative outcomes in UGIC patients.
In recent years, prehabilitation - defined as a series of interventions implemented before surgery, including exercise, nutritional support, and psychological counseling - has emerged as a promising approach for enhancing surgical readiness and promoting recovery[9]. These interventions are often based on enhanced recovery after surgery (ERAS) protocols and delivered by multidisciplinary teams. While some studies show no difference between prehabilitation and standard care[10], others report significant improvements in physical function, postoperative outcomes, and even survival among patients receiving prehabilitation programs[10,11]. Despite growing international attention, prehabilitation for UGIC remains underexplored, and most existing studies vary widely in intervention type, timing, and evaluation standards. Moreover, no comprehensive meta-analysis has yet focused exclusively on UGIC surgical patients. The Chinese guidelines for ERAS (2021 edition) also include prehabilitation as an important part of preoperative preparation[12], although the evidence supporting it remains relatively weak. Currently, there is a lack of standardized protocols for the timing and methods of prehabilitation, both domestically and internationally. Furthermore, there has been no meta-analysis specifically focused on prehabilitation for UGIC.
This study aims to conduct a meta-analysis of published clinical trials examining the effects of prehabilitation on UGIC surgical patients. By evaluating outcome indicators such as six-minute walk distance (6MWD), complication rate, hospital stay duration, intensive care unit (ICU) monitoring time, mortality, and nutritional status, we hope to provide robust evidence to guide clinical implementation of prehabilitation protocols in this high-risk patient group.
A comprehensive computerized search was performed across multiple databases, including Web of Science, PubMed, EMBASE, The Cochrane Library, Cumulative Index to Nursing and Allied Health Literature, China National Knowledge Infrastructure, Wanfang, and Chinese Science and Technology Journal Database, to identify clinical trials investigating the effects of prehabilitation in patients with UGIC. The search covered all records available from the inception of each database up to December 31, 2022. In English-language databases, the search strategy included the following keyword combinations: (1) Upper gastrointestinal, esophageal, esophagus, esophagogastric, gastric, stomach, duodenal, duo
Inclusion criteria: (1) Population: UGIC patients with a willingness for prehabilitation and stable medical conditions; (2) Intervention and comparison: The experimental group received prehabilitation management, using at least one prehabilitation method such as exercise, nutrition, or psychology; the control group received conventional preoperative care as recommended by the ERAS guidelines; (3) Outcomes: 6MWD, incidence of complications, patient hospitalization duration, ICU monitoring time, postoperative mortality rate, patient nutritional indicators: Albumin (ALB) (g/L); and (4) Studies: Published clinical trials.
Exclusion criteria: (1) Duplicate publications; (2) Animal experiments, mechanistic studies, pharmacology or drug synthesis research; (3) Intervention measures not classified as prehabilitation; (4) Incomplete outcome measures; and (5) Low-quality score in the Joanna Briggs Institute (JBI) literature quality assessment.
This study involved two researchers independently screening the literature, extracting data, and cross-checking. Discrepancies in literature evaluation were resolved through discussion, third-party consultation, or by emailing the original authors. For literature screening, non-clinical trial literature was excluded based on Medical Subject Headings terms and search strategy. Initially, titles were read for preliminary screening to exclude irrelevant literature. Abstracts and full texts were further reviewed for final inclusion. If necessary, study authors were contacted via email or phone to obtain important information for this research. Data extraction included: (1) Basic information of included studies: Study title, first author, publishing journal, impact factor, whether it was a clinical trial, etc; (2) Baseline characteristics of the study subjects and intervention measures; (3) Key elements for bias risk assessment; and (4) Outcome measures of interest, measurement methods, and result data.
Two researchers independently assessed the risk of bias in the included studies and cross-checked the results. The risk of bias was evaluated using the Australian JBI tools for intervention studies randomized controlled trials (RCTs)[13], quasi-experimental studies[14]), categorizing each study’s quality into three levels: A (low bias, fully meeting standards), B (moderate bias, partially meeting standards), C (high bias, fully meeting standards; such studies were excluded). The detailed results of the risk of bias assessments for the included randomized controlled trials and quasi-experimental studies are presented in Supplementary Tables 1 and 2, respectively.
Statistical analysis was performed using RevMan 5.3. For continuous outcomes, mean difference (MD) was used as the effect measure; for dichotomous outcomes, odds ratio (OR) with 95% confidence intervals (CI) was calculated using the Mantel-Haenszel method. Heterogeneity was assessed using the χ2 test (significance level α = 0.1) and quantified with the I2 statistic, with values of 25%, 50%, and 75% indicating low, moderate, and high heterogeneity, respectively. A fixed-effect model was applied when I2 < 50%, and a random-effects model was used if I2 ≥ 50% after excluding substantial clinical heterogeneity. Subgroup analysis, sensitivity analysis, or descriptive analysis was conducted to explore sources of heterogeneity where appropriate. Publication bias was assessed using funnel plots. To evaluate whether the duration of prehabilitation influenced outcomes, linear regression analysis was performed using SPSS 26.0.
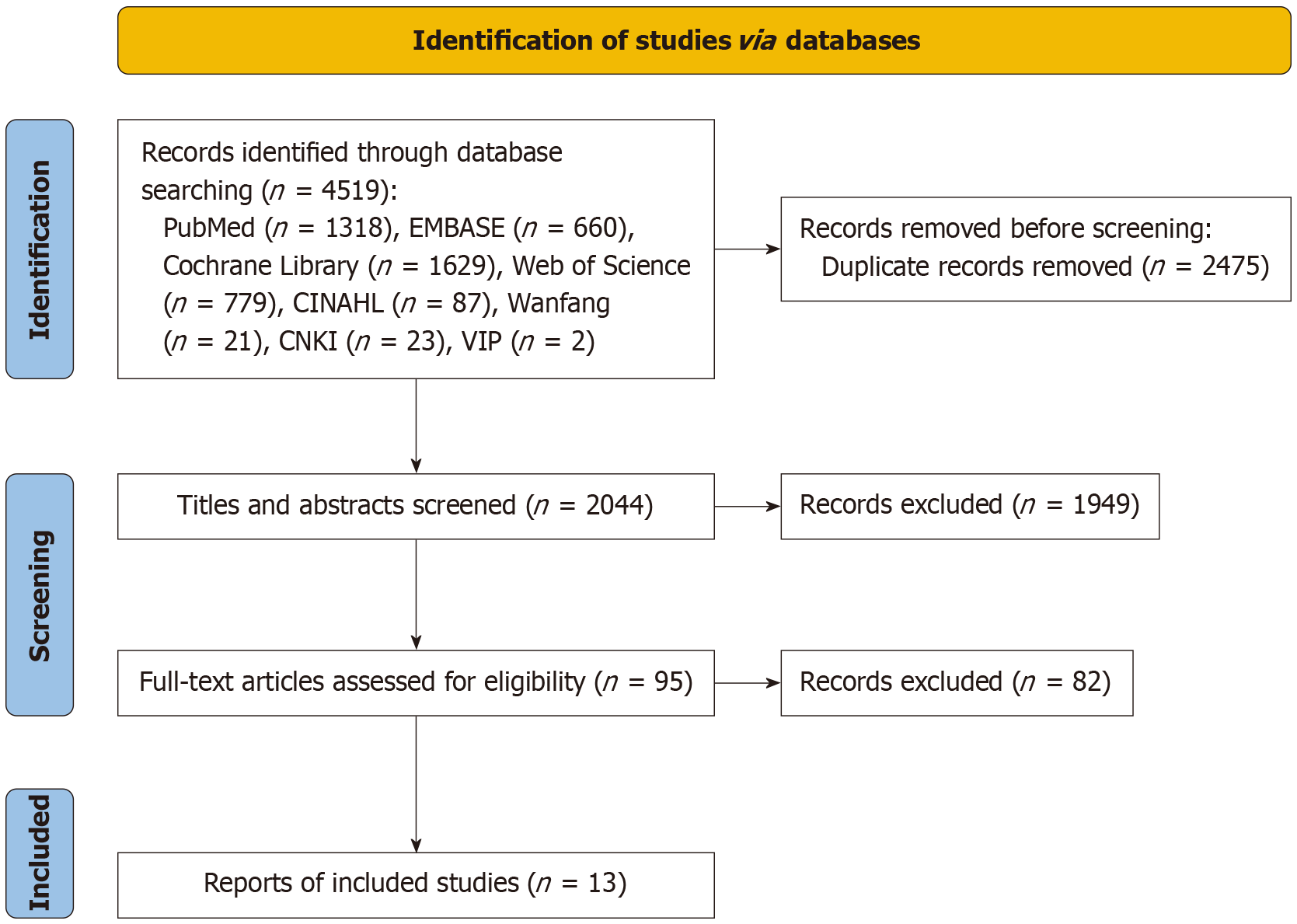
A total of 4519 potentially relevant articles were initially identified through database searches. After a rigorous, multi-step screening process, 13 studies encompassing 1322 patients met the inclusion criteria and were ultimately included in the meta-analysis. Among them, 8 were RCTs[10,11,15-21], while the remaining 5 were quasi-experimental studies[22-26]. The detailed screening process is illustrated in Figure 1, and the basic characteristics of the included studies are summarized in Table 1. Based on the JBI quality assessment, all five quasi-experimental studies were rated as A-level (low risk of bias), whereas the eight RCTs were rated as B-level (moderate risk of bias). A detailed summary of the quality assessment results is provided in Table 1.
| Ref. | Quality | Study design | Number (T/C) | Age (T/C, years), mean ± SD | Participants | Intervention | Time | Location | Outcome1 | |
| T | C | |||||||||
| Minnella et al[16], 2018 | B | RCT | 26/25 | 67.3 ± 7.4/68 ± 11.6 | Esophagogastric resections | Aerobic: 30 minutes moderate - Borg: 12-13, continuous training: 3 day/week. Resistance exercise: Moderate intensity; 3 sets of 8-12 reps with resistance band 1 day/week for 4 weeks) | Standard care as per ERAS | 36 days (17-73) | Home | 1, 2, 3, 4, 5, 10 |
| Nutrition: Provide dietary advice and whey protein supplementation | ||||||||||
| Swaminathan et al[11], 2020 | B | RCT | 29/29 | 56.03 ± 14.95/56.82 ± 11.27 | Gastrectomy | Start inspiratory muscle training based on motivational lung capacity measurement 7 days before surgery, twice a day | Standard care as per ERAS | 7 days | Hospital | 3, 5 |
| Mazzola et al[22], 2017 | A | UCT | 41/35 | 75 (44-90)/75 (59-91) | Frail patients undergoing surgery of the upper gastrointestinal tract | Exercise: Inspirational lung capacity measurement 3 times per day, 30 minutes moderate intensity walking 3 times per week, lasting for 2 weeks | Receiving no special preoperative care | 5-7 days | Hospital | 3, 4, 5, 10 |
| Nutrition: 5-7 days prior to surgery, oral immune nutrition supplementation ± nasal jejunum feeding ± total parenteral nutrition | ||||||||||
| Valkenet et al[17], 2018 | B | RCT | 121/120 | 63.7 ± 7.5 /62.7 ± 8.9 | Esophagectomy | Inspiratory muscle training ≥ 2 weeks, twice a day | Standard care as per ERAS | ≥ 2 weeks | Home | 3, 4, 5, 8, 11 |
| Allen et al[10], 2022 | B | RCT | 26/28 | 65 ± 6/62 ± 9 | Esophagogastric resections | Exercise: 2-3 times a week, with 1 hour of aerobic, resistance, and flexibility training each time | Standard care as per ERAS | 15 weeks | Home + hospital | 4, 5, 8, 9, 11 |
| Nutrition: Calorie and protein intake under the guidance of a nutritionist | ||||||||||
| Psychological: Targeted medical guidance 6 times | ||||||||||
| Yamana et al[18], 2015 | B | RCT | 30/30 | 68.33 ± 7.64/65.9 ± 9.5 | Esophagectomy | Inspiratory, abdominal and lower limb muscle training ≥ 1 week | Receiving no special preoperative care | ≥ 7 days | Hospital | 4, 5 |
| Christensen et al[23], 2019 | A | UCT | 21/29 | 63.9 ± 8.2/65.5 ± 7.3 | Surgery for adenocarcinoma of the gastro-esophageal junction | Approximately 75 minutes of high-intensity aerobic exercise and resistance training twice a week | Standard care as per ERAS | 9 weeks | Home + Hospital | 4, 5, 8 |
| Liu et al[19], 2020 | B | RCT | 26/24 | 62.04 ± 5.12/64.58 ± 5.87 | Esophagectomy | Preoperative nutritional support one week in advance and home enteral nutrition one month in advance | Standard care as per ERAS | 5w | Home+ Hospital | (3, 4, 5, 6, 7, 8, 10, 11) |
| Dai et al[20], 2022 | B | RCT | 48/46 | 55.21±9.87/ 54.73±9.26 | Gastrectomy | Exercise: Aerobic exercise for about 20 minutes three times a week, a group of resistance exercise per day, 2-3 respiratory muscle training sessions per day | Standard care as per ERAS | 6-8 days | Hospital | 1, 2, 3, 4, 5, 6, 7, 8 |
| Nutrition: Calorie and whey protein intake under the guidance of a nutritionist | ||||||||||
| Psychology: Personalized Psychological Counseling | ||||||||||
| Li et al[24], 2021 | A | UCT | 84/84 | 59.23 ± 6.13/58.1 ± 4.76 | Surgery for esophageal gastric junction adenocarcinoma | Exercise: Approximately 30 minutes of moderate intensity aerobic exercise three times a week, 30 minutes of resistance training once a week, and 2-3 respiratory muscle training sessions per day | Standard care as per ERAS | 9-11 weeks | Home + Hospital | 1, 2, 4, 5, 6, 7 |
| Nutrition: Medication treats symptoms, adjusts body mass, and balances diet | ||||||||||
| Guo et al[21], 2018 | B | RCT | 50/50 | 62.94 ± 6.35/64.88 ± 5.85 | Esophagectomy | Exercise: Climbing stairs, walking, using an inhaler to assist in inspiratory muscle training, and abdominal breathing training | Standard care as per ERAS | 7-9 days | Hospital | 4, 5, 11 |
| Nutrition: Enteral nutrition after admission, carbohydrate intake 12 hours before surgery | ||||||||||
| Psychology: Pre health compound case education | ||||||||||
| Xie et al[25], 2019 | A | UCT | 128/128 | 61.54 ± 8.12/62.82 ± 7.93 | Esophagectomy | Exercise: Coughing, blowing balloons, walking, and training with respiratory function exercise equipment | Standard care as per ERAS | Hospitalization until surgery | Hospital | 5, 6, 7, 11 |
| Nutrition: Enteral nutrition 3 days before surgery, carbohydrate intake 12 hours before surgery | ||||||||||
| Psychology: Pre health compound case education | ||||||||||
| Li et al[26], 2019 | A | UCT | 34/30 | 60.43 ± 10.21/61.30 ± 11.12 | Esophagectomy | Exercise: Climbing stairs, 6-minute walk test, and respiratory function training | Standard care as per ERAS | 7 days | Hospital | 4, 5, 6, 7 |
| Nutrition: High protein diet should be given 7 days before surgery, semi liquid and enteral nutrition powder should be given 3 days before surgery, and carbohydrates should be supplemented 1 day and on the same day before surgery | ||||||||||
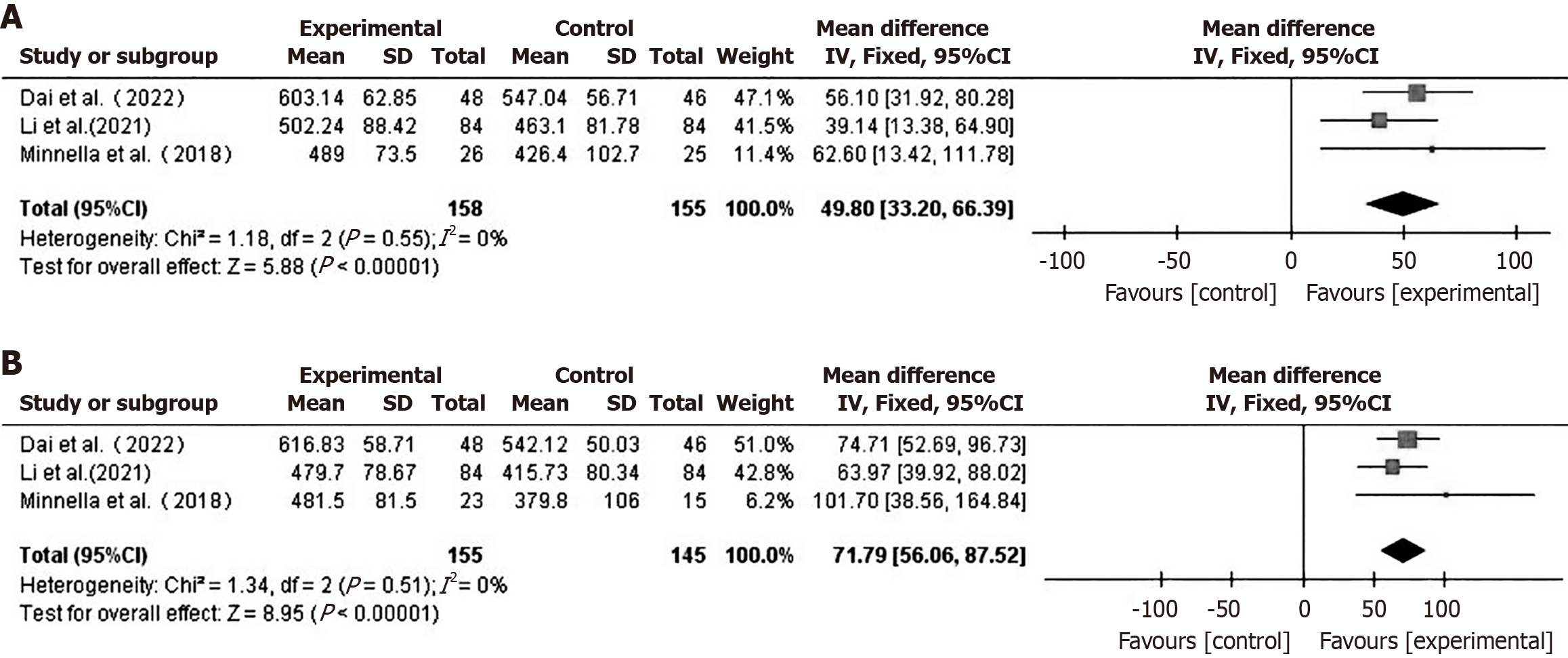
Preoperative and postoperative functional ability - 6MWD: A total of 3 studies[16,20,24] compared preoperative and postoperative 6MWD between the two groups. Using a fixed-effect model (I2 = 0%), the preoperative 6MWD in the prehabilitation group was higher than that in the control group (MD = 49.80, 95%CI: 33.20-66.39, P < 0.001) (Figure 2A). The postoperative 6MWD in the prehabilitation group was also higher than that in the control group (MD = 71.79, 95%CI: 56.06-87.52, P < 0.001) (Figure 2B).
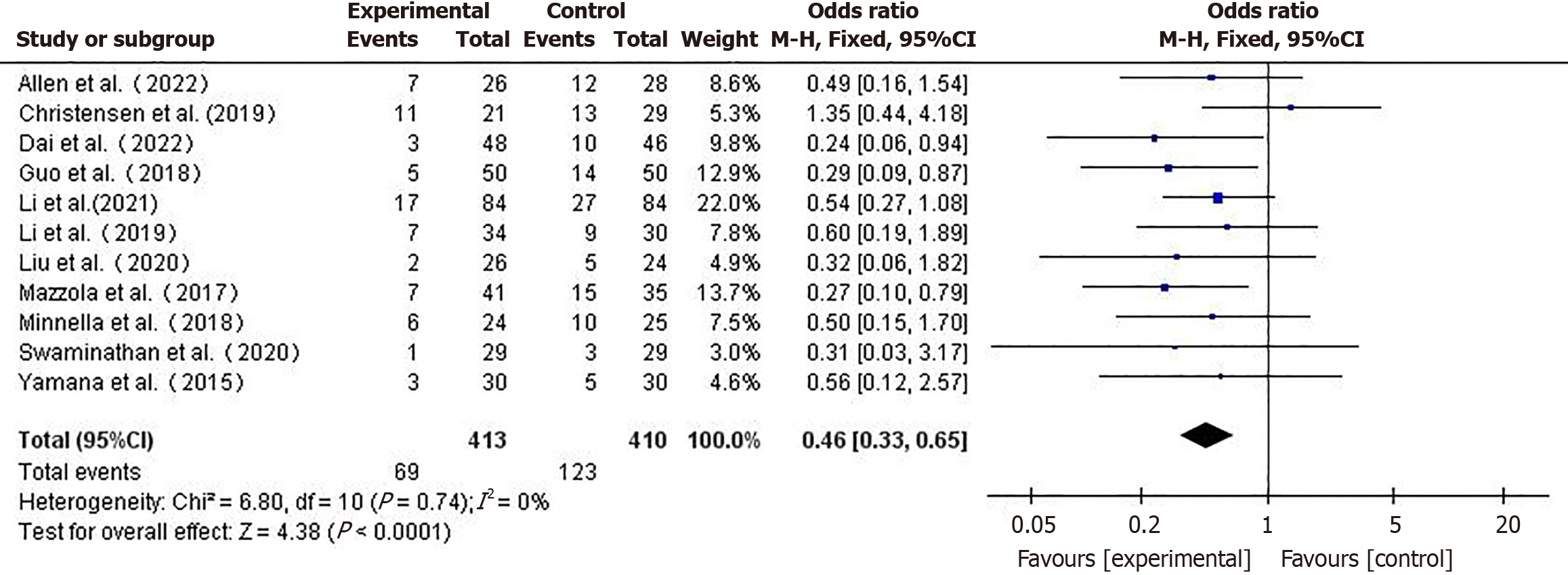
Incidence of complications: A total of 11 studies[10,11,16,18-24,26] reported the incidence of complications, A fixed-effect model (I2 = 0%) was used for the meta-analysis. The incidence of complications in the prehabilitation group was lower than in the control group (OR = 0.46, 95%CI: 0.33-0.65, P < 0.001) (Figure 3).
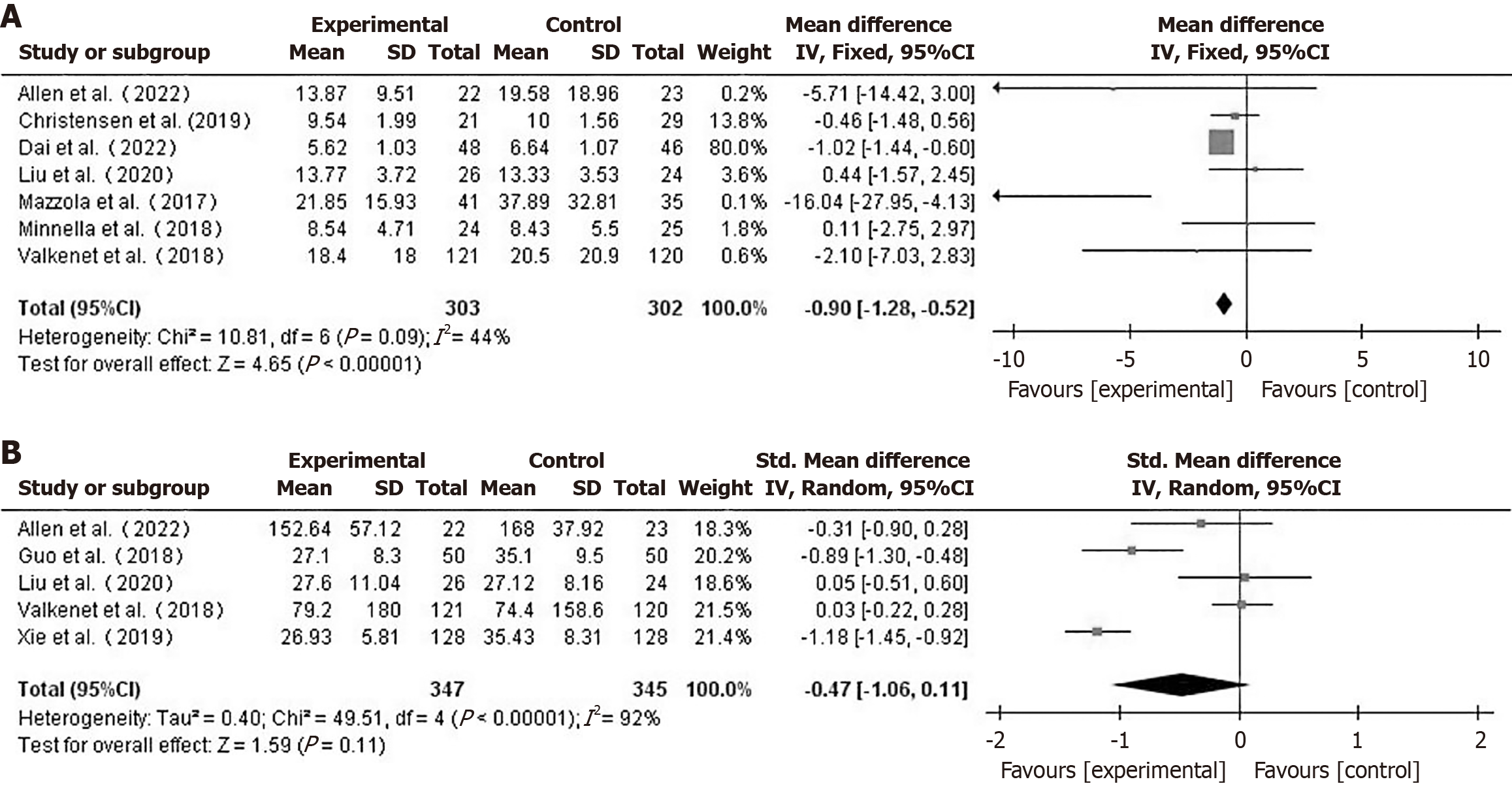
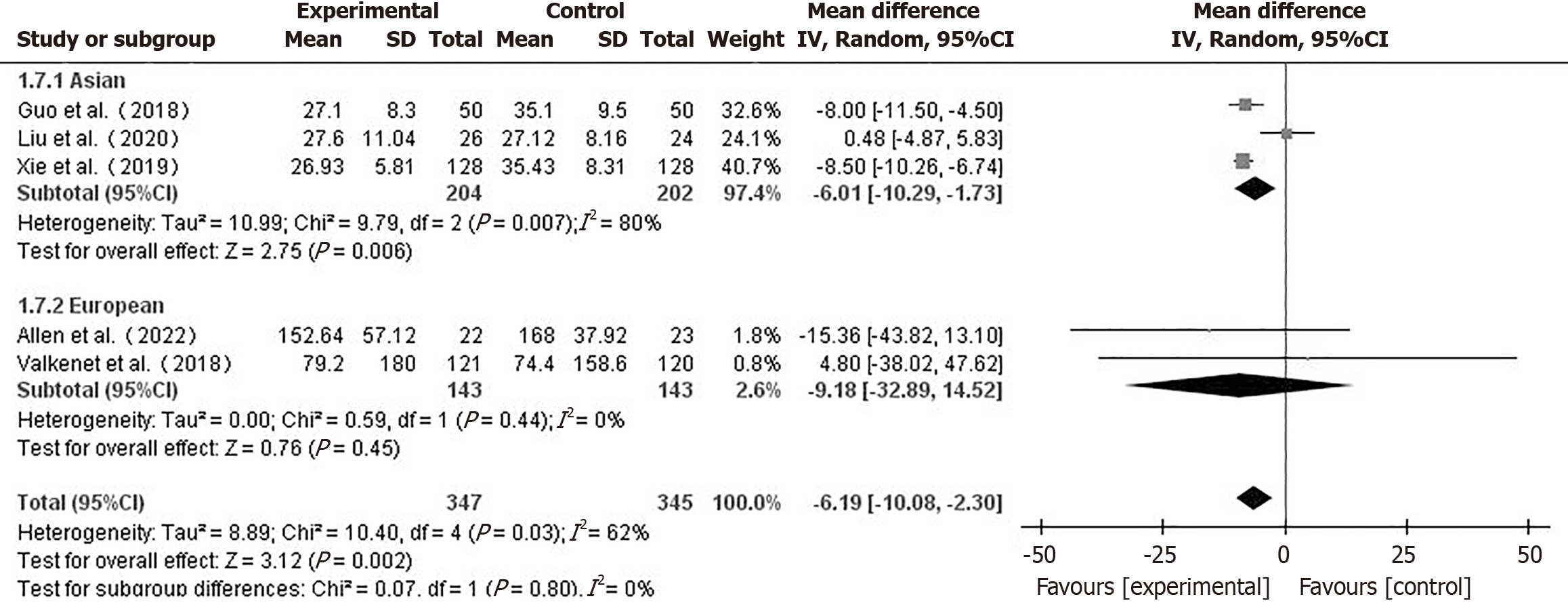
Hospital stay and ICU care duration: Seven papers[10,16,17,19,20,22,23] reported on the length of postoperative hospitalization of patients. Using a fixed-effects model (I2 = 44%), hospital stay for the prehabilitation group was shorter than that for the control group (MD = -0.90, 95%CI: -1.28 to -0.52, P < 0.001) (Figure 4A). In a comparison between the two groups, a total of 5 studies[10,17,19,21,25] which reported the ICU care duration were included. Using a random-effects model (I2 = 92%), the difference in ICU care duration between the two groups was not statistically significant (MD = -0.47, 95%CI:
Subgroup analysis by geographic region (Figure 5) revealed that the Asian subgroup showed a significant reduction in ICU stay (MD = -6.01, 95%CI: -10.27 to -1.73, P = 0.006), while the European subgroup showed no significant difference (MD = -9.18, 95%CI: -35.89 to 14.52, P = 0.45). This suggests prehabilitation may be more effective in reducing ICU stay for Asian patients.
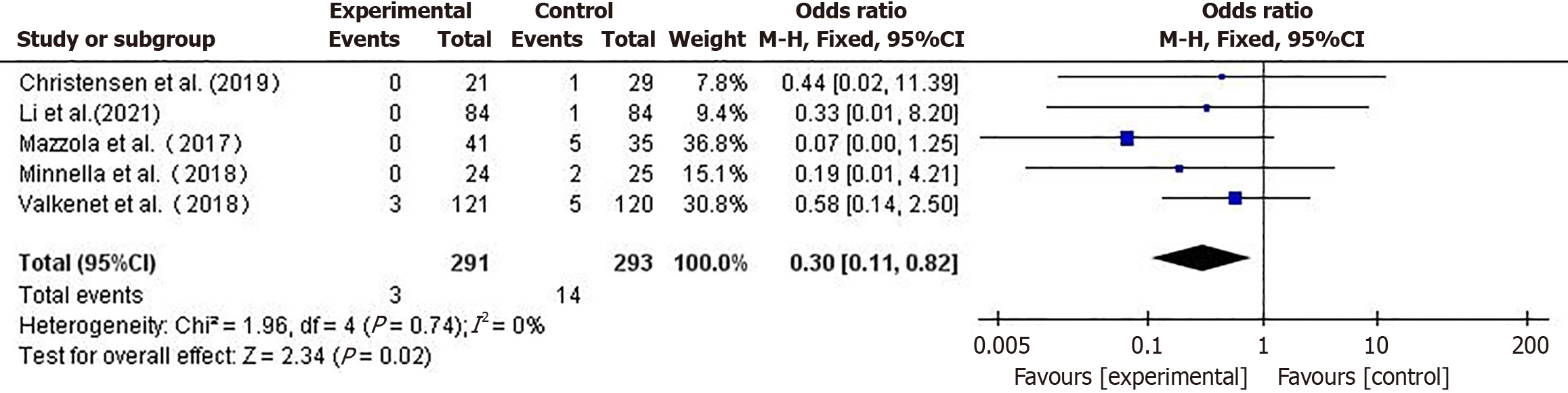
30-day postoperative mortality rate: Five studies[16,17,22-24] compared postoperative mortality between the two groups of patients. Using a fixed-effects model (I2 = 0%), the mortality rate in the prehabilitation group was lower than that in the control group (OR = 0.30, 95%CI: 0.11-0.81, P = 0.02) (Figure 6).
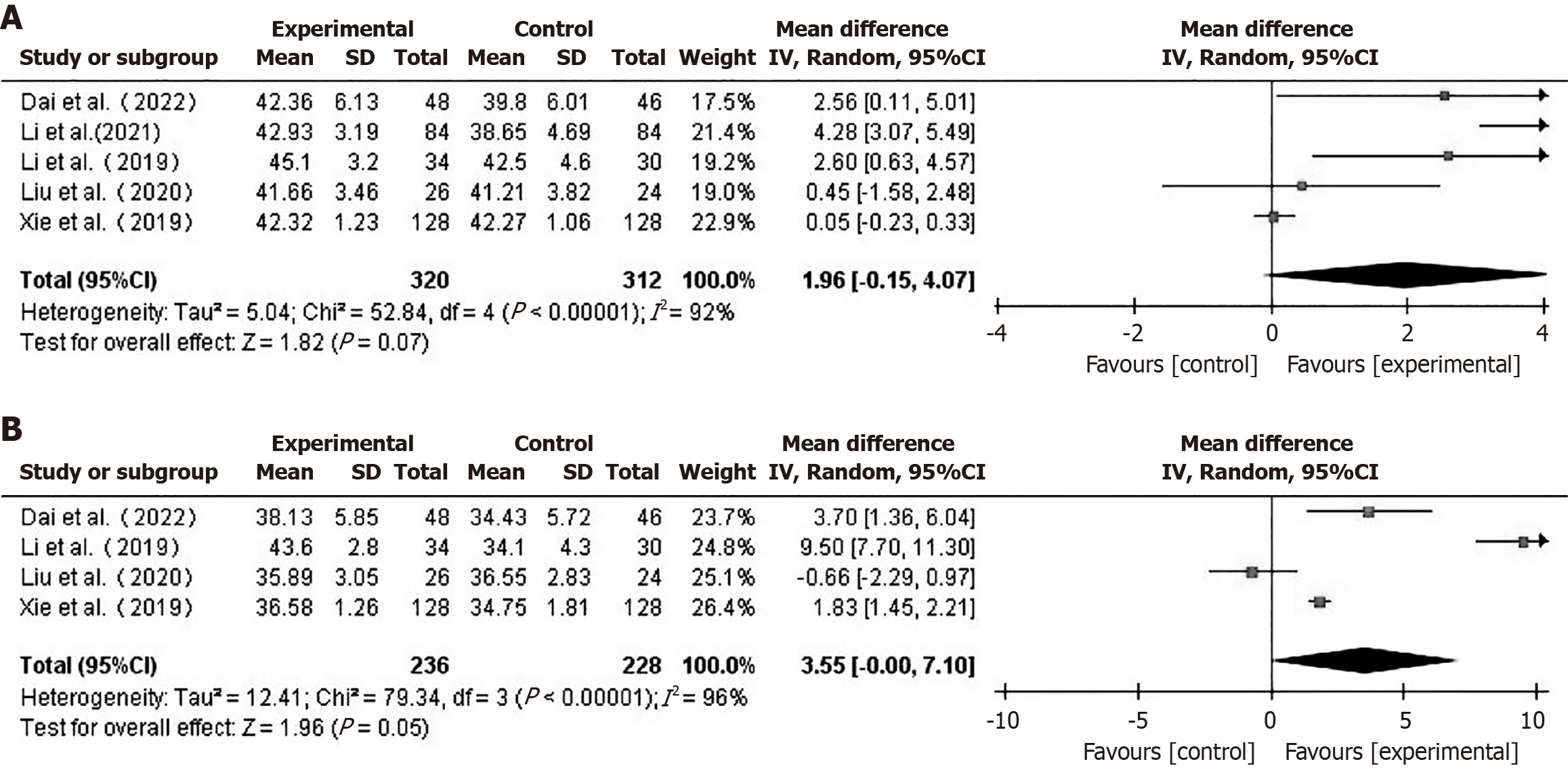
Nutritional indicator: ALB: Five studies[19,20,24-26] reported preoperative ALB levels in patients. Using a random-effects model (I2 = 92%), the difference in preoperative ALB between the two groups was not statistically significant (MD = 1.96, 95%CI: -0.15 to 4.07, P = 0.07) (Figure 7A). Four studies[19,20,25,26] compared postoperative ALB levels between the two groups of patients. Using a random-effects model (I2 = 96%), there was no statistically significant difference in ALB levels one week post-operatively between the two groups (MD = 3.55, 95%CI: -0.00 to 7.10, P = 0.05) (Figure 7B).
A sensitivity analysis of the meta-analysis comparing ALB between the two groups was performed. After excluding the study by Xie et al[25], the results indicated higher preoperative ALB in the prehabilitation group, using a random-effects model (MD = 2.58, 95%CI: 0.84-4.33, P = 0.004). The results for ALB one week postoperatively remained stable after sequentially excluding individual studies.
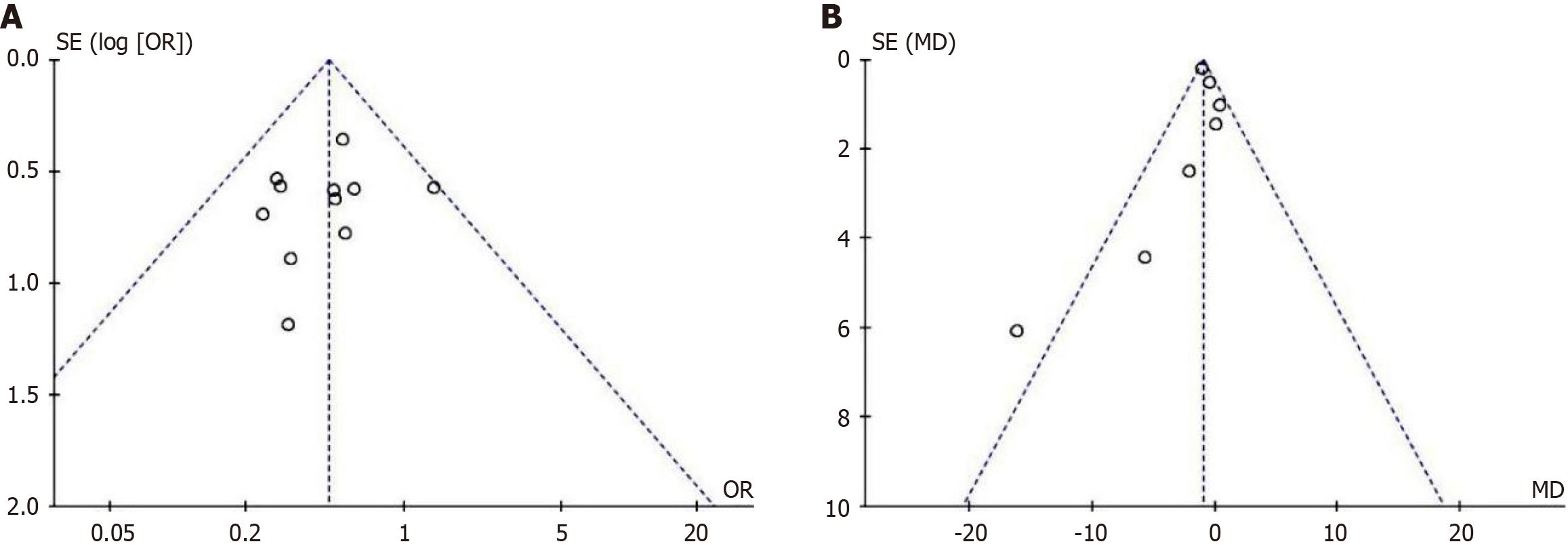
Publication bias was tested for the main outcome indicators. Funnel plots for the incidence of complications (Figure 8A) and hospital stay duration (Figure 8B) showed a largely symmetrical distribution on both sides, suggesting a lower likelihood of significant publication bias. For other outcome indicators, the number of studies included was too small, making the construction of funnel plots less meaningful.
Finally, we performed a linear regression analysis between the implementation time of prehabilitation and outcome indicators. The results in Table 2 show that no significant correlation was found between any of the indicators and the implementation time of prehabilitation (P > 0.05).
| Outcome | R2 | P value |
| Preoperative 6MWD | 0.61 | 0.43 |
| 6MWD at 4 weeks after surgery | 0.72 | 0.36 |
| Post-operative complications | 0.20 | 0.23 |
| Length of stay | 0.03 | 0.72 |
| ICU stay | 0.71 | 0.16 |
| 30-day mortality after surgery | 0.22 | 0.43 |
| ALB at 1 day before surgery | 0.49 | 0.30 |
| ALB at 7 days post-surgery | 0.52 | 0.48 |
This study conducted a comprehensive systematic review and meta-analysis to investigate the impact of prehabilitation on perioperative outcomes in patients who underwent UGIC surgery. The findings suggest that prehabilitation significantly improves pre- and postoperative functional status, reduces postoperative complications and 30-day mortality, and shortens hospital stay, thereby has substantial clinical value. However, its effects on ICU stay, nutritional status, psychological outcomes, and quality of life (QoL) remain inconclusive and require further analysis considering study heterogeneity and methodological variation.
Prehabilitation enhances physical reserves and postoperative resilience by integrating planned exercise training, nutritional support, and psychological interventions. Our findings indicate that patients in the prehabilitation group had significantly greater 6MWD both before and after surgery compared to controls, demonstrating a clear functional benefit. These results align with previous studies by Piraux et al[27] and Michael et al[28], which reported that structured prehabilitation improves physical capability and accelerates recovery. Additionally, prehabilitation significantly reduced postoperative complication rates and 30-day mortality and shortened hospital stays, underscoring its key role within the ERAS framework.
Although the overall effect on ICU length of stay was not significant, subgroup analysis revealed a statistically significant reduction in ICU stay among Asian populations, but not in European cohorts. These regional differences may be related to disparities in adherence, intervention intensity, or ICU management practices. Given the limited number of studies and the high heterogeneity (I2 = 92%), further validation in large-scale studies is warranted.
Prehabilitation showed a trend toward improved preoperative nutritional status but did not significantly affect postoperative ALB levels, which showed high heterogeneity (I2 = 96%). This may result from inconsistent timing of assessment, varied nutritional interventions, metabolic changes post-surgery, and patient-specific factors such as age, cancer type, and treatment. Notably, ALB is a nonspecific marker influenced by inflammation and fluid status, limiting its accuracy for nutritional evaluation[29]. Future studies should adopt more reliable tools like the Patient-Generated Subjective Global Assessment or prealbumin[30].
Although some studies, such as the study by Allen et al[10], reported improvements in psychological well-being using the Beck Inventory, there was a lack of consistent psychological interventions and assessment tools across studies, limiting meta-analytic capability. Given the prevalence of psychological distress among UGIC patients, future research should incorporate validated scales like the Hospital Anxiety and Depression Scale, Patient Health Questionnaire-9, or Generalized Anxiety Disorder 7-item Scale to quantify psychological benefits. Likewise, although some trials reported improved QoL, heterogeneity in assessment tools (e.g., European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-Core 30, short form 36 health survey, Functional Assessment of Cancer Therapy-General) prevented data pooling[23]. Standardized cancer-specific instruments such as the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-Core 30[31] are recommended for future studies.
The duration of prehabilitation ranged from 1 week to 4 weeks, with interventions conducted at home or in hospitals and utilizing either single- or multi-modal strategies. Linear regression found no significant association between duration and outcomes, suggesting that the implementation of prehabilitation itself may be more important than the exact length of intervention. In China, short preoperative hospitalization and patient reluctance to delay surgery-especially in rural populations-pose practical challenges[28]. Thus, developing simplified, low-cost, and telehealth-supported home-based programs tailored to China’s healthcare context is essential[32,33].
The ERAS guidelines, including the 2021 Chinese edition, recommend multimodal prehabilitation combining exercise, nutrition, and psychological support[12]. International prospective studies such as the PREHAB trial are exploring standardized structures for implementation[34]. In China, an integrated “hospital-community-home” management model could enhance accessibility and scalability. We propose that future protocols emphasize multidisciplinary collaboration, flexible delivery strategies, and patient-centered design to establish robust, standardized, and context-sensitive prehabilitation pathways.
The included studies had diverse surgical backgrounds, tumor stages, and histories of chemotherapy and radiotherapy. Although all followed guidelines for perioperative care, baseline consistency is unclear. The patient populations, tumor characteristics, and intervention modalities varied significantly among studies, contributing to notable clinical heterogeneity and limiting the consistency and generalizability of the findings. Despite our attempt to perform subgroup analyses based on tumor stage, surgical methods, and types of prehabilitation interventions, these efforts were hindered by insufficient and inconsistent reporting in the included studies. Most studies did not provide detailed stratification information, making subgroup comparisons infeasible. Additionally, there were differences in the evaluation indicators among the studies, and inconsistent use of outcome metrics further limited data comparability. The methodologies of the included studies were not always clearly described. Despite attempts to contact original authors via email or phone, some included literature lacked clear descriptions, potentially leading to implementation and mea
These limitations may affect the internal and external validity of the study findings. For instance, unclear intervention protocols or outcome definitions in original studies could introduce selection and measurement bias, while heterogeneity in patient populations may limit the generalizability of the conclusions to broader clinical settings. Moreover, potential publication bias and the absence of long-term outcome data constrain the ability to assess the durability of prehabilitation benefits. Therefore, further high-quality, large-scale randomized controlled trials are needed to confirm our findings.
Given the limitations identified in this meta-analysis, future research should aim to establish standardized, multimodal prehabilitation protocols tailored to UGIC patients. High-quality, multicenter RCTs are needed to determine the most effective timing and components of prehabilitation. In addition, more robust tools and unified outcome measures should be developed to assess the psychological and QoL effects of prehabilitation, which remain insufficiently explored. Studies focusing on home-based vs hospital-based delivery models will also be essential to guide practical implementation strategies, particularly in different healthcare systems.
Prehabilitation management in UGIC patients has high applicability for improving preoperative functional reserves and promoting postoperative recovery. This study indicates that the impact of prehabilitation on nutrition, psychology, and QoL requires further research. Future studies should consider incorporating validated and sensitive tools for nutritional assessment (e.g., Patient-Generated Subjective Global Assessment, prealbumin) and psychological evaluation (e.g., Hospital Anxiety and Depression Scale, Patient Health Questionnaire-9), to ensure robust measurement of these critical dimensions. Additionally, there are no uniform standards for the timing and setting of prehabilitation. More large-sample, high-quality RCTs are needed to verify the effects of different prehabilitation timings and settings on UGIC patients, while also focusing on changes in patients' nutritional status, psychological well-being, and QoL.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 68565] [Article Influence: 13713.0] [Reference Citation Analysis (201)] |
| 2. | Liu ZC, Li ZX, Zhang Y, Zhou T, Zhang JY, You WC, Pan KF, Li WQ. [Interpretation on the report of Global Cancer Statistics 2020]. Zhongliu Zonghe Zhiliao Dianzi Zazhi. 2021;7:1-13. [DOI] [Full Text] |
| 3. | Qiu H, Cao S, Xu R. Cancer incidence, mortality, and burden in China: a time-trend analysis and comparison with the United States and United Kingdom based on the global epidemiological data released in 2020. Cancer Commun (Lond). 2021;41:1037-1048. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 349] [Cited by in RCA: 693] [Article Influence: 138.6] [Reference Citation Analysis (0)] |
| 4. | Cong M, Shi H. Consensus of Chinese experts on exercise therapy for cancer patients. Sci Sin -Vitae. 2022;52:1-16. [DOI] [Full Text] |
| 5. | Zhu L, Gao J, Bo DX, Liang Y, Yang Z, Liu RR, Zhang H. [Prevalence of nutritional risk in patients with esophageal cancer in China: A meta-analysis]. Xiandai Yufang Yixue. 2020;47:4447-4451. |
| 6. | Liu JH, Zhang N, Cui YC. [Research progress on psychosocial factors on the incidence and prognosis of cancer]. Zhonghua Zhongliu Fangzhi Zazhi. 2020;27:750-754. [DOI] [Full Text] |
| 7. | Bausys A, Mazeikaite M, Bickaite K, Bausys B, Bausys R, Strupas K. The Role of Prehabilitation in Modern Esophagogastric Cancer Surgery: A Comprehensive Review. Cancers (Basel). 2022;14:2096. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 8. | Liu ZJ, Zhang L, Liu HS, Cang J, Wang TL, Min S, Chen LX, Chen W, Li SQ, Huang YG; Working Group of "Expert Consensus on Prehabilitation Management of Thoracic Surgery"; Chinese Society of Anesthesiology. [Expert Consensus on Prehabilitation Management for Enhanced Recovery in Patients Undergoing Thoracic Surgery (2022)]. Xiehe Yixue Zazhi. 2022;13:387-401. [DOI] [Full Text] |
| 9. | Carli F, Gillis C, Scheede-Bergdahl C. Promoting a culture of prehabilitation for the surgical cancer patient. Acta Oncol. 2017;56:128-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 136] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 10. | Allen S, Sultan J. ASO Author Reflections: A Randomized Controlled Trial to Address the Effect of Prehabilitation During Neoadjuvant Therapy on Cardiopulmonary Fitness, Muscle Mass and Quality of Life in the Oesophagogastric Cancer Patient. Ann Surg Oncol. 2022;29:1851-1852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 11. | Swaminathan N, Kundra P, Ravi R, Kate V. ERAS protocol with respiratory prehabilitation versus conventional perioperative protocol in elective gastrectomy- a randomized controlled trial. Int J Surg. 2020;81:149-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 12. | Chinese Society of Surgery; Chinese Medical Association; Chinese Society of Anesthesiology; Chinese Medical Association; Zhao YP, Huang YG, Cao H, Chen YJ, Gu XP, Min S, Peng SJ, Wang DX, Yao HW. [Clinical practice guidelines for enhanced recovery after surgery in China (2021 edition)]. Zhongguo Shiyong Waike Zazhi. 2021;41:961-992. |
| 13. | Zhou YF, Gu Y, Hu Y, Xing WJ. [Quality assessment tool of different types of research for JBI evidence based health care-quality evaluation of intervention study]. Hushi Jinxiu Zazhi. 2018;33:24-26. [DOI] [Full Text] |
| 14. | Zhou YF, Gu Y, Hu Y, Xing WJ. [JBI evidence-based health center's quality assessment tool for different types of research: Quality evaluation of intervention research]. Hushi Jinxiu Zazhi. 2018;33:112-113. [DOI] [Full Text] |
| 15. | Luo DH, Wan X, Liu JM, Tong TJ. [How to estimate the sample mean and standard deviation from the sample size, median, extremes or quartiles]. Zhongguo Xunzhen Yixue Zazhi. 2017;17: 1350-1356. [DOI] [Full Text] |
| 16. | Minnella EM, Awasthi R, Loiselle SE, Agnihotram RV, Ferri LE, Carli F. Effect of Exercise and Nutrition Prehabilitation on Functional Capacity in Esophagogastric Cancer Surgery: A Randomized Clinical Trial. JAMA Surg. 2018;153:1081-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 380] [Article Influence: 47.5] [Reference Citation Analysis (0)] |
| 17. | Valkenet K, Trappenburg JCA, Ruurda JP, Guinan EM, Reynolds JV, Nafteux P, Fontaine M, Rodrigo HE, van der Peet DL, Hania SW, Sosef MN, Willms J, Rosman C, Pieters H, Scheepers JJG, Faber T, Kouwenhoven EA, Tinselboer M, Räsänen J, Ryynänen H, Gosselink R, van Hillegersberg R, Backx FJG. Multicentre randomized clinical trial of inspiratory muscle training versus usual care before surgery for oesophageal cancer. Br J Surg. 2018;105:502-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 85] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 18. | Yamana I, Takeno S, Hashimoto T, Maki K, Shibata R, Shiwaku H, Shimaoka H, Shiota E, Yamashita Y. Randomized Controlled Study to Evaluate the Efficacy of a Preoperative Respiratory Rehabilitation Program to Prevent Postoperative Pulmonary Complications after Esophagectomy. Dig Surg. 2015;32:331-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 19. | Liu K, Ji S, Xu Y, Diao Q, Shao C, Luo J, Zhu Y, Jiang Z, Diao Y, Cong Z, Hu L, Qiang Y, Shen Y. Safety, feasibility, and effect of an enhanced nutritional support pathway including extended preoperative and home enteral nutrition in patients undergoing enhanced recovery after esophagectomy: a pilot randomized clinical trial. Dis Esophagus. 2020;33:doz030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | Dai T, Bi QQ, Wu DQ, Tang JJ. [Application effect study of three mode pre-rehabilitation nursing strategy in laparoscopic radical gastrectomy for gastric cancer]. Zhongguo Shiyong Huli Zaizhi. 2022;38:924-930. |
| 21. | Guo Z, Ji SG, Xu Y, Cong ZZ, Hu LW, Shen Y. [The effect of preoperative prehabilitation on nutritional status and body composition of patients with esophageal cancer after operation]. Changwai Yu Changnei Yingyang. 2018;25:156-160. |
| 22. | Mazzola M, Bertoglio C, Boniardi M, Magistro C, De Martini P, Carnevali P, Morini L, Ferrari G. Frailty in major oncologic surgery of upper gastrointestinal tract: How to improve postoperative outcomes. Eur J Surg Oncol. 2017;43:1566-1571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 61] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 23. | Christensen JF, Simonsen C, Banck-Petersen A, Thorsen-Streit S, Herrstedt A, Djurhuus SS, Egeland C, Mortensen CE, Kofoed SC, Kristensen TS, Garbyal RS, Pedersen BK, Svendsen LB, Højman P, de Heer P. Safety and feasibility of preoperative exercise training during neoadjuvant treatment before surgery for adenocarcinoma of the gastro-oesophageal junction. BJS Open. 2019;3:74-84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 24. | Li L, Zhang YH, Huang YL, Li SM. [Effect of preoperative prehabilitation in patients receiving surgical treatment for adenocarcinoma of the esophagogastric junction]. Hulixue Zazhi. 2021;36:80-83. [DOI] [Full Text] |
| 25. | Xie Q, Wang Q, Lei M, Liu XP. [Effect of Preoperative Prehabilitation on Nutritional Indicators, Body Composition, Blood Glucose, Inflammatory Response and Immune Function of Patients with Esophageal Cancer after Operation]. Linchuang Wuzhen Wuzhi. 2019;32:87-93. [DOI] [Full Text] |
| 26. | Li HC, Tong F, Ying YH, Pan HH, Zhao W. [Prehabilitation program in perioperative period for patients with esophageal cancer]. Zhejiang Yixue. 2019;41:1301-1304. [DOI] [Full Text] |
| 27. | Piraux E, Caty G, Reychler G. Effects of preoperative combined aerobic and resistance exercise training in cancer patients undergoing tumour resection surgery: A systematic review of randomised trials. Surg Oncol. 2018;27:584-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 83] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 28. | Michael CM, Lehrer EJ, Schmitz KH, Zaorsky NG. Prehabilitation exercise therapy for cancer: A systematic review and meta-analysis. Cancer Med. 2021;10:4195-4205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 100] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 29. | Keller U. Nutritional Laboratory Markers in Malnutrition. J Clin Med. 2019;8:775. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 199] [Cited by in RCA: 477] [Article Influence: 68.1] [Reference Citation Analysis (0)] |
| 30. | Fu Z, Zhang R, Wang KH, Cong MH, Li T, Weng M, Guo ZQ, Li ZN, Li ZP, Wang C, Xu HX, Song CH, Zhuang CL, Zhang Q, Li W, Shi HP; INSCOC Study Group. Development and validation of a Modified Patient-Generated Subjective Global Assessment as a nutritional assessment tool in cancer patients. J Cachexia Sarcopenia Muscle. 2022;13:343-354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 27] [Reference Citation Analysis (0)] |
| 31. | Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman SB, de Haes JC. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9802] [Cited by in RCA: 11919] [Article Influence: 361.2] [Reference Citation Analysis (0)] |
| 32. | Waterland JL, Ismail H, Amin B, Granger CL, Denehy L, Riedel B. Patient acceptance of prehabilitation for major surgery: an exploratory survey. Support Care Cancer. 2021;29:779-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 33. | Burnett C, Bestall JC, Burke S, Morgan E, Murray RL, Greenwood-Wilson S, Williams GF, Franks KN. Prehabilitation and Rehabilitation for Patients with Lung Cancer: A Review of Where we are Today. Clin Oncol (R Coll Radiol). 2022;34:724-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 34. | Meric-Bernstam F, Bahleda R, Hierro C, Sanson M, Bridgewater J, Arkenau HT, Tran B, Kelley RK, Park JO, Javle M, He Y, Benhadji KA, Goyal L. Futibatinib, an Irreversible FGFR1-4 Inhibitor, in Patients with Advanced Solid Tumors Harboring FGF/FGFR Aberrations: A Phase I Dose-Expansion Study. Cancer Discov. 2022;12:402-415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 206] [Article Influence: 51.5] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/