Published online Nov 24, 2025. doi: 10.5306/wjco.v16.i11.110792
Revised: June 27, 2025
Accepted: October 13, 2025
Published online: November 24, 2025
Processing time: 159 Days and 11.7 Hours
Dissection of the right paraesophageal lymph node (RPELN) in managing papillary thyroid carcinoma remains a contentious issue. This meta-analysis assesses previously established and novel risk factors associated with RPELN metastasis.
To evaluate previously established and novel risk factors associated with RPELN metastasis in patients with papillary thyroid carcinoma papillary thyroid carci
We searched MEDLINE (via PubMed), ScienceDirect, Scopus and EMBASE up to December 2024. Studies were assessed using the Newcastle-Ottawa Scale. Sta
Of 2444 articles retrieved, 26 were included in our meta-analysis with 16427 patients. The RPELN metastasis rate was 12.98% [95% confidence interval (CI): 12.46%-13.50%]. The pooled results suggested that age < 55 years [odds ratio (OR) = 1.71, 95%CI: 1.35-2.16, P < 0.00001], sex (OR = 0.60, 95%CI: 0.54-0.67, P < 0.00001), tumor size 1 cm (OR = 3.37, 95%CI: 2.69-4.21, P < 0.00001), multifocality (OR = 1.81, 95%CI: 1.49-2.20, P < 0.00001), capsular invasion (OR = 2.94, 95%CI: 2.05-4.20, P < 0.00001), vascular invasion (OR = 2.16, 95%CI: 1.56-2.99, P < 0.00001), extra-thyroid extension (OR = 3.30, 95%CI: 1.82-5.98, P < 0.0001), central lymph node metastasis (OR = 7.77, 95%CI: 4.73-12.76, P < 0.00001), lateral lymph node metastasis (OR = 6.94, 95%CI: 6.11-7.89, P < 0.00001), Hashimoto thyroiditis (OR = 0.79, 95%CI: 0.69-0.92, P = 0.002), micro-calcifications (OR = 2.29, 95%CI: 1.20-4.37, P = 0.01), and echogenicity (OR = 0.62, 95%CI: 0.40-0.98, P = 0.04) should be considered with RPELN metastasis.
The male < 55, tumor size > 1 cm, multifocality, capsular and vascular invasion, extrathyroidal extension, lymph node metastasis, and Hashimoto thyroiditis were significantly associated with RPELN metastasis and should be carefully assessed during dissection.
Core Tip: Several factors significantly associated with right paraesophageal lymph node metastasis include age under 55 years, sex, tumor size of 1 cm, multifocality, capsular invasion, vascular invasion, extra-thyroid extension, central and lateral lymph node metastasis, Hashimoto thyroiditis, microcalcifications, and echogenicity. In contrast, tumor location and margins were not significantly associated. These findings highlight the need for thorough evaluation and proactive surgical intervention in patients with papillary thyroid carcinoma papillary thyroid carcinoma who exhibit these risk factors to manage potential right paraesophageal lymph node involvement.
- Citation: Khawar MMH, Abid MH, Cheema MBA, Khawar M, Shaukat M, Khan MHA, Saifullah M, Noureen R, Khail HA, Qureshi AA, Khokhar MA. Clinicopathological predictors of right para esophageal lymph node metastasis in papillary thyroid carcinoma: A systematic review and meta-analysis. World J Clin Oncol 2025; 16(11): 110792
- URL: https://www.wjgnet.com/2218-4333/full/v16/i11/110792.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i11.110792
The global prevalence of thyroid cancer has witnessed a significant rise over the past few decades, a trend attributed not only to enhanced detection methods[1], but also to an actual increase in cases[2]. The increased availability and use of diagnostic imaging, such as ultrasound and fine-needle aspiration, have contributed to detecting smaller, asymptomatic tumors that might have previously gone unnoticed[3]. Currently, thyroid cancer ranks as the 13th most common cancer worldwide and is the 6th most prevalent among women[4]. Women are three times more commonly affected than men[5]. This surge in prevalence is multifactorial, with key risk factors including radiation exposure, genetic mutations, and hereditary conditions[2]. Among the various types of thyroid cancer, papillary thyroid carcinoma (PTC) is the most prevalent, accounting for up to 90% of all cases[6]. PTC is a well-differentiated thyroid cancer with a generally favorable prognosis. Still, it can invade nearby structures and metastasize to lymph nodes, significantly affecting the patient’s prognosis and quality of life[5]. Recent studies have shown that the molecular profile of PTC, including mutations in the BRAF and RAS genes, can influence its behavior and potential for metastasis[7,8].
While PTC typically demonstrates an excellent long-term survival rate, with over 91% of patients surviving beyond 10 years and more than 87% beyond 15 years[9], its propensity for lymphatic spread remains a critical concern. Cervical lymph node metastasis commonly affects 40%-90% of PTC patients[10]. The metastatic pattern follows an orderly progression, beginning with central lymph nodes before involving other regional nodes[11]. However, although less common, metastasis to more distant lymph nodes, such as the right paraesophageal lymph nodes (RPELN), holds significant clinical importance due to its potential impact on disease prognosis and treatment strategies[12]. The RPELNs, located lateral to the right recurrent laryngeal nerve in the paratracheal area, present a unique challenge in the surgical management of PTC[13]. Dissection of these nodes is often controversial due to the increased risk of postoperative complications and the technical difficulties involved[14]. Understanding the factors that predict RPELN metastasis is crucial for optimizing surgical planning and follow-up strategies.
The studies have explored the clinicopathological factors associated with lymph node metastasis in PTC, identifying tumor size, extrathyroidal extension, multifocality, and aggressive histological variants as potential predictors of nodal involvement[15]. However, specific factors influencing metastasis to the RPELN remain inadequately studied. The unique anatomical location of the RPELN and the associated surgical risks necessitate a comprehensive understanding of the predictive factors for metastasis to these nodes. This study aims to systematically evaluate and synthesize data from multiple studies to identify key clinicopathological predictors of RPELN involvement in PTC. By improving our understanding of these predictive factors, the findings of this study aim to guide more informed surgical decision-making and enhance postoperative monitoring strategies for patients with PTC. The results are expected to contribute to more personalized and effective management approaches, ultimately improving patient outcomes.
This systematic review and meta-analysis adhered to the guidelines outlined by the Preferred Reporting Items for Systematic reviews and Meta-analyses statement and the Cochrane Collaboration[16,17]. The protocol is registered with the international prospective register of systematic reviews (No. CRD42024568357). Ethical approval was unnecessary for this study since it exclusively utilized publicly available data.
The databases searched from inception until December 2024 included MEDLINE (via PubMed), ScienceDirect, Scopus and EMBASE. The following keywords, together with MeSH terms and their combinations, were used to maximize the search accuracy: “thyroid carcinoma”, “papillary thyroid carcinoma”, “PTC”, “thyroid neoplasm”, “posterior to the right recurrent laryngeal nerve”, “right para-esophageal lymph node”, “lymph node metastasis”, “lymphatic spread”, “risk factors”, and “predictors”.
The inclusion criteria: (1) Diagnosis of PTC was validated either during the surgical procedure or through postoperative pathology; (2) Each patient underwent thyroidectomy along with either unilateral or bilateral prophylactic central lymph node dissection; (3) Studies were either prospective or retrospective in design; and (4) Only studies published in English with comprehensive medical records available for data extraction were included.
The exclusion criteria: (1) Articles such as comments, case series, reviews, overviews, and conference abstracts; (2) Duplicate publications; (3) Studies where patients underwent completion thyroidectomy after initial surgery; and (4) Articles lacking complete clinical data or follow-up information.
Two authors independently evaluated all the retrieved articles and extracted the relevant data. They first reviewed the titles and abstracts to identify potentially eligible studies. The full texts were subsequently analyzed to verify their suitability for inclusion in the meta-analysis. The extracted data included the first author, publication year, country, study type, and sample size. Additionally, risk factors were categorized into several groups: Demographic details, which in
Statistical analyses were conducted using RevMan version 5.4 (The Cochrane Collaboration, Copenhagen, Denmark). We used the odds ratio (OR) with a 95% confidence interval (CI) as the effect measure. Statistical significance was defined as a P-value of less than 0.05. Heterogeneity was assessed using the Q-test and I2 statistic, with the interpretation of I2 based on the Cochrane Handbook for Systematic Reviews of Interventions[18]. A random-effects model was employed if I2 exceeded 50%; otherwise, a fixed-effects model was used. Sensitivity analysis was conducted to evaluate the influence of individual studies on the pooled effect size[19]. For outcomes with data from 10 or more studies, we assessed publication bias by constructing funnel plots and conducting Egger’s test for funnel plot asymmetry using R version 4.4.
A Newcastle-Ottawa Scale (NOS) was used to evaluate the quality of papers. Two independent authors conducted quality evaluations. NOS consisted of three factors: Patient selection (4 points), the comparability of the groups (2 points), and the ascertainment of the exposure (3 points). The total score ranged from 0 (the worst) to 9 (the best). The quality was interpreted as good (score ≥ 7), moderate (score ≥ 5 to < 7) and poor (score < 5).
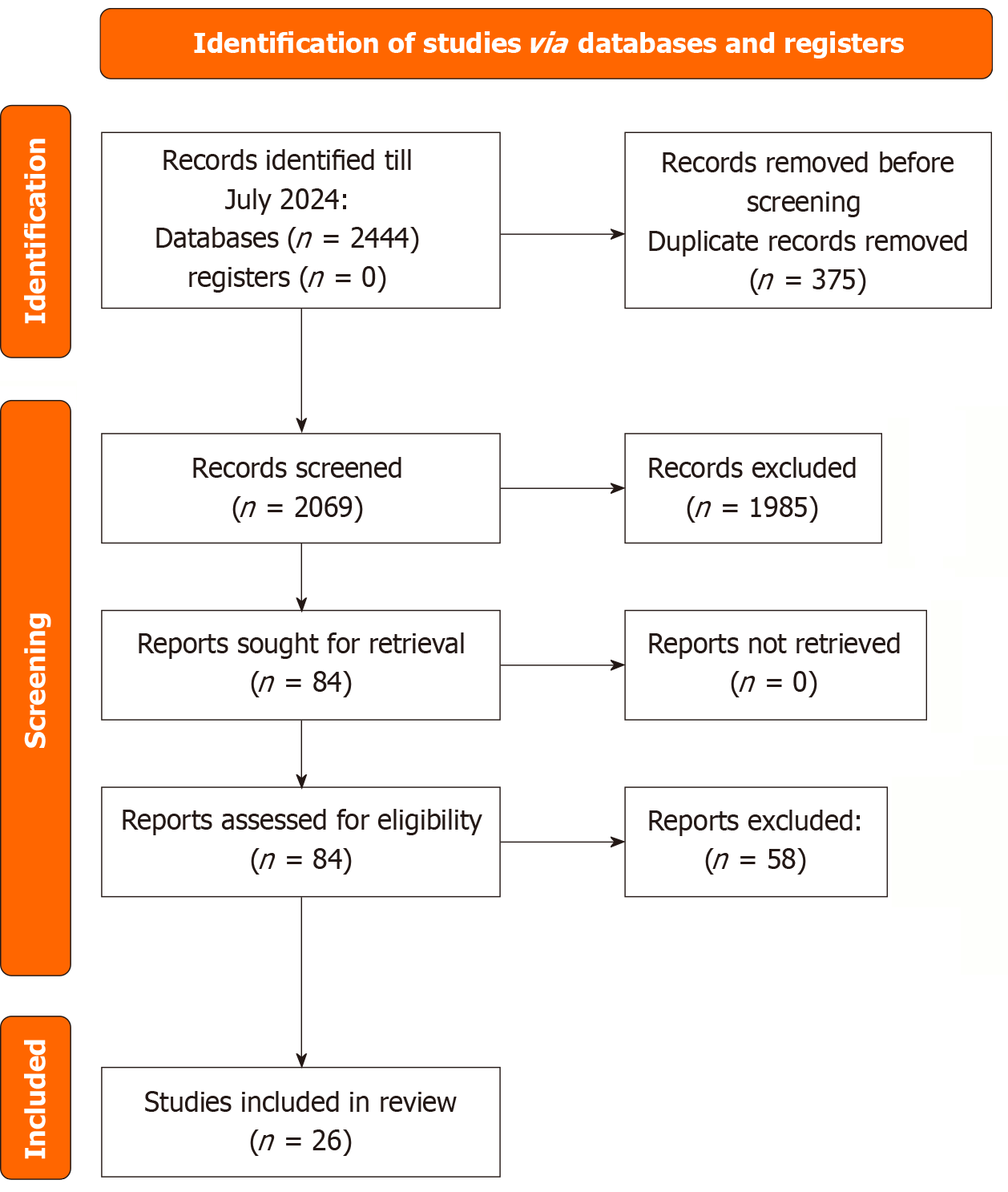
An overview of the study selection process is depicted in Figure 1, following the Preferred Reporting Items for Systematic reviews and Meta-analyses guidelines. The comprehensive literature search identified a total of 2444 articles. After removing duplicates, 2069 titles and abstracts were screened, which led to retrieving 84 full-text articles for further eligibility assessment. Upon detailed evaluation, 26 studies were deemed suitable and were subsequently included in the study[7,8,14,20-42].
The analysis included 26 studies published between 2009 and 2024. These studies comprised a mix of nonrandomized cohorts, encompassing 16427 patients. The prevalence of RPELN metastasis was found to be 12.98% (95%CI: 12.46%-13.50%). Table 1 provides detailed characteristics of each study.
| Ref. | Year | Country | Study design | Metastasis (+) | Metastasis (-) | Total | RPELNM prevalence (%) | Quality |
| Yang et al[7] | 2023 | China | Retrospective cohort study | 24 | 378 | 402 | 0.0597 | 8 |
| Shao et al[8] | 2024 | China | Retrospective cohort study | 45 | 206 | 251 | 0.1792 | 6 |
| Qu et al[14] | 2017 | China | Retrospective cohort study | 20 | 143 | 163 | 0.1226 | 7 |
| Liu et al[20] | 2017 | China | Prospective cohort study | 16 | 129 | 145 | 0.1103 | 8 |
| Lu et al[21] | 2021 | China | Retrospective cohort study | 79 | 403 | 482 | 0.1639 | 7 |
| Zhu et al[22] | 2019 | China | Retrospective cohort study | 111 | 481 | 592 | 0.1875 | 8 |
| Lee et al[23] | 2009 | Korea | Retrospective cohort study | 14 | 109 | 123 | 0.1138 | 7 |
| Bae et al[24] | 2012 | Korea | Retrospective cohort study | 45 | 324 | 369 | 0.1219 | 8 |
| Kim et al[25] | 2012 | Korea | Prospective cohort study | 14 | 229 | 243 | 0.0576 | 8 |
| Ito et al[26] | 2013 | Japan | Retrospective cohort study | 127 | 795 | 922 | 0.1377 | 7 |
| Pinyi et al[27] | 2014 | China | Retrospective cohort study | 108 | 297 | 405 | 0.2666 | 7 |
| Chang et al[28] | 2015 | Korea | Retrospective cohort study | 148 | 5408 | 5556 | 0.0266 | 6 |
| Zhang et al[29] | 2016 | China | Retrospective cohort study | 33 | 213 | 246 | 0.1341 | 7 |
| Luo et al[30] | 2017 | China | Prospective cohort study | 51 | 255 | 306 | 0.1666 | 9 |
| Park et al[31] | 2017 | Korea | Retrospective cohort study | 171 | 936 | 1107 | 0.1544 | 8 |
| Yuan et al[32] | 2017 | China | Retrospective cohort study | 31 | 50 | 81 | 0.3827 | 7 |
| Luo et al[33] | 2018 | China | Prospective cohort study | 102 | 493 | 595 | 0.1714 | 9 |
| Yu et al[34] | 2018 | China | Retrospective cohort study | 158 | 671 | 829 | 0.1905 | 7 |
| Hou et al[35] | 2020 | China | Retrospective cohort study | 96 | 331 | 427 | 0.2248 | 6 |
| Zou et al[36] | 2020 | China | Retrospective cohort study | 127 | 352 | 479 | 0.2651 | 7 |
| Ling et al[37] | 2021 | China | Retrospective cohort study | 30 | 159 | 189 | 0.1587 | 8 |
| Xiao et al[38] | 2022 | China | Retrospective cohort study | 65 | 225 | 290 | 0.2241 | 8 |
| Zhang et al[39] | 2022 | China | Retrospective cohort study | 49 | 641 | 690 | 0.071 | 7 |
| Zhou et al[40] | 2022 | China | Retrospective cohort study | 291 | 271 | 562 | 0.5177 | 7 |
| Gong et al[41] | 2023 | China | Retrospective cohort study | 96 | 307 | 403 | 0.2382 | 6 |
| Shen et al[42] | 2023 | China | Retrospective cohort study | 82 | 488 | 570 | 0.1438 | 7 |
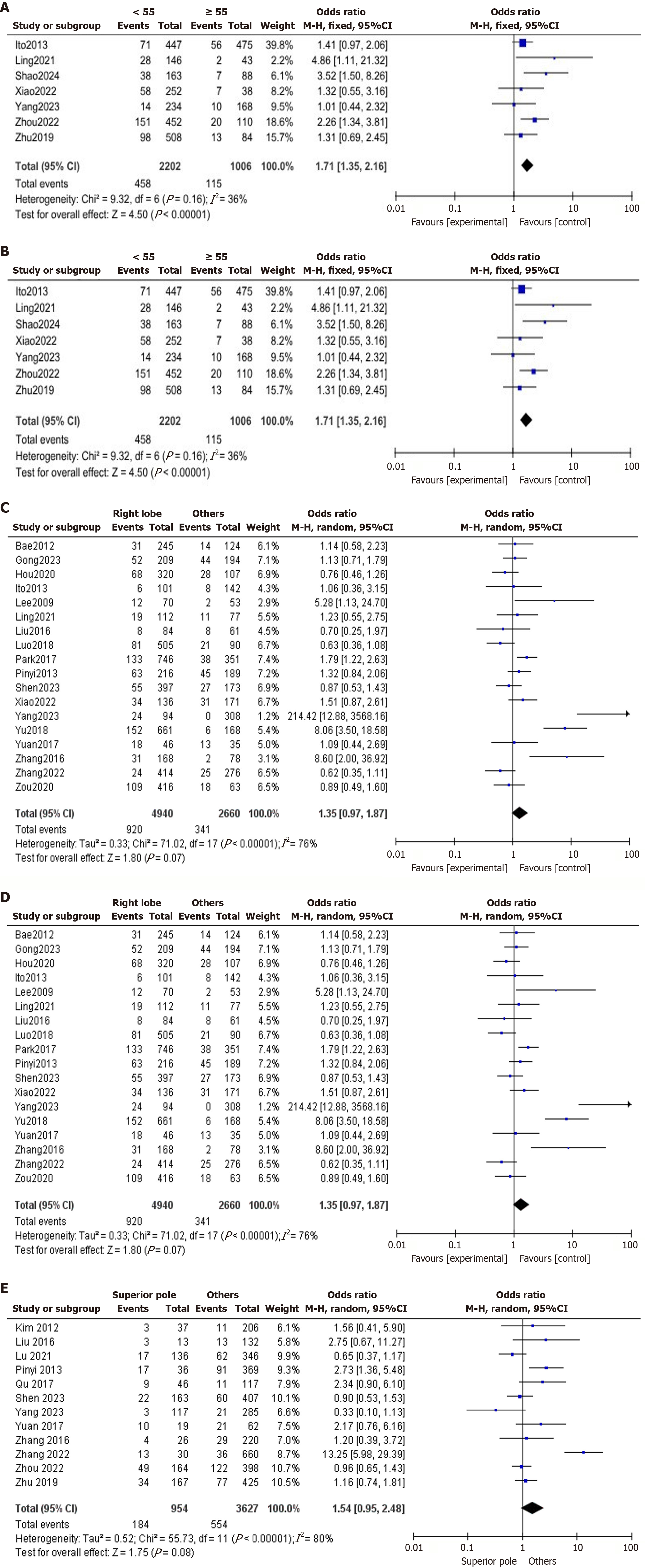
Age: The impact of using 55 years as the age cutoff was examined, analyzing seven studies with a fixed-effects model (I2 = 36%). The analysis revealed that RPELN metastasis was significantly more common in patients under 55 years of age (OR = 1.71, 95%CI: 1.35-2.16, P < 0.00001; Figure 2A). The American Joint Committee on Cancer - tumor-nodes-metastasis staging system underwent a significant revision in its 8th edition, raising the age threshold for high-risk disease-specific mortality under 55 years, which reclassifies many patients as lower risk based solely on their age[43].
Sex: 26 articles involving 16427 subjects were used to analyze the relationship between sex and RPELN metastasis. A fixed-effects model analysis revealed a low heterogeneity (I2 = 13%). The rate of RPELN metastasis was observed to be 11% in women compared to 16.65% in men, highlighting a higher prevalence of RPELN metastasis among male patients (OR = 0.60, 95%CI: 0.54-0.67, P < 0.00001; Figure 2B).
Tumor size: The relationship between tumor size and RPELN metastasis was analyzed across 19 studies comprising 12629 patients. Using a random-effects model, our findings indicate substantial heterogeneity (I2 = 66%). Among patients with tumors larger than 1 cm, 19.95% had RPELN metastasis, compared to 6.82% of patients with tumors measuring 1 cm or smaller. This suggests that tumors greater than 1 cm are significantly linked with RPELN metastasis (OR = 3.37, 95%CI: 2.69-4.21, P < 0.00001; Figure 2C). When the study of Shao et al[8] was excluded, I2 dropped to 41%, indicating that the study was primary source of heterogeneity.
Tumor location: The relationship between tumor location and RPELN metastasis was assessed using a random-effects model across 18 studies involving 7600 patients. Our analysis revealed a higher prevalence of RPELN metastasis in patients with right lobe foci, at 18.62%, compared to 12.81% in patients with tumors located in other regions with substantial heterogeneity among the studies (I2 = 76%). The findings suggest that tumor location in the right lobe may not influence metastasis (OR = 1.35, 95%CI: 0.97-1.87, P = 0.07; Figure 2D). Even after excluding Yu et al’s study[34], the I2 only dropped slightly to 66%, indicating that study was not a major contributor to the heterogeneity.
Superior pole sublocations: The relationship between tumor location in the superior pole of the right lobe and RPELN metastasis was examined across 12 studies involving 4581 patients. Utilizing a random-effects model, substantial heterogeneity was detected (I2 = 80%). Among patients with tumors situated in the superior pole, 19.28% exhibited RPELN metastasis, compared to 15.27% among those without RPELN metastasis. These findings do not indicate any relation between tumors in the superior pole and RPELN metastasis (OR = 1.54, 95%CI: 0.95-2.48, P = 0.08; Figure 2E). When Zhang et al[39] was excluded, I2 = 51%, indicating a significant drop in heterogeneity. This shows that the study was the main source of heterogeneity.
Middle pole sublocations: The relation between tumors located in the middle pole of the right lobe and RPELN metastasis was investigated across 12 studies involving a total of 4581 patients using a random-effects model. The analysis indicated substantial heterogeneity (I2 = 68%). Among patients with tumors in the middle pole, 16.51% were found to have RPELN metastasis, compared to 15.91% among those without RPELN metastasis. These findings suggest that tumors in the middle pole may not be linked to RPELN metastasis (OR = 1.00, 95%CI: 0.70-1.42, P = 1.00). Excluding Zhang et al’s study[39] resulted in a drop-in heterogeneity to I2 = 58%, suggesting that study may have contributed to the heterogeneity.
Inferior pole sublocations: The relationship between tumors located in the inferior pole of the right lobe and RPELN metastasis was evaluated across 12 studies involving a total of 4581 patients using a fixed-effects model. The analysis revealed moderate heterogeneity (I2 = 45%). Among patients with tumors in the inferior pole, 16.13% were found to have RPELN metastasis, compared to 16.09% among those without RPELN metastasis. These findings suggest that tumors located in the inferior pole show no relation with RPELN metastasis (OR = 0.91, 95%CI: 0.77-1.08, P = 0.28).
Tumor margin: The relationship between tumor margin and RPELN metastasis was analyzed using data from 5 studies involving 2396 patients. A random-effects model revealed moderate heterogeneity (I2 = 57%). Among patients with ill-defined tumor margins, 14.71% had RPELN metastasis, compared to 14.18% with well-defined margins. This indicates that ill-defined tumor margins are unrelated to RPELN metastasis (OR = 0.97, 95%CI: 0.52-1.82, P = 0.93). When the study of Gong et al[41] was excluded, I2 dropped to 0%. This indicating that Gong et al’s study[41] of 2023 was the main source of heterogeneity.
Microcalcification: The relation between microcalcification and RPELN metastasis was assessed across 6 studies involving 2875 patients. Using a random-effects model, the analysis showed considerable heterogeneity (I2 = 86%). Among patients with tumors exhibiting microcalcification, 22.21% had RPELN metastasis, compared to 10% of patients without microcalcification. These findings indicate that microcalcification is linked with RPELN metastasis (OR = 2.29, 95%CI: 1.20-4.37, P = 0.01).
Multifocality: The relationship between tumor multifocality and RPELN metastasis was assessed using data from 22 studies encompassing a total of 14577 patients. A random-effects model revealed substantial heterogeneity (I2 = 61%). Among patients with multifocal tumors, 14.68% had RPELN metastasis, compared to 10.73% of those with unifocal tumors. This analysis indicates that multifocality is significantly linked with RPELN metastasis (OR = 1.81, 95%CI: 1.49-2.20, P < 0.00001). When the study of Pinyi et al’s study[27] was excluded, I2 = 46%, indicating that study was a significant source of heterogeneity.
Capsular invasion: A random-effects model was employed in an analysis involving 5015 patients from 12 studies to assess the link between capsular invasion and RPELN metastasis, revealing substantial heterogeneity (I2 = 75%). The data indicated that 23.986% of patients with capsular invasion had RPELN metastasis, compared to 12.56% of those without RPELN metastasis. The findings suggest a robust relation between capsular invasion and RPELN metastasis (OR = 2.94, 95%CI: 2.05-4.20, P < 0.00001). When the studies of Zhang et al[29], Luo et al[30], and Park et al[31] were excluded, I2 dropped to 48%; however, the study of Park et al[31] was the main source of heterogeneity. On its exclusion, I2 dropped to 68%.
Extrathyroid extension: In an analysis involving 9925 patients from 12 studies to assess the link between extrathyroid extension and the risk of recurrent PTC RPELN metastasis, a random-effects model was employed, revealing considerable heterogeneity (I2 = 89%). The data indicated that 11.03% of patients with extrathyroid extension had RPELN metastasis, compared to 9.25% of those without extrathyroid extension. The findings strongly suggest a robust relation between extrathyroid extension and RPELN metastasis (OR = 3.30, 95%CI: 1.82-5.98, P < 0.0001. When Zhou et al’s study[41] was excluded, I2 dropped to 74%, which is not significantly lower, but this was only identified through sensitivity analysis.
Tumor echogenicity: A fixed-effects model was utilized in a study involving 2871 patients, revealing no heterogeneity (I2 = 0%), to assess the relation between tumor echogenicity and RPELN metastasis across 6 studies. Among patients with tumors displaying echogenicity, 16.85% had RPELN metastasis, compared to 15.72% without tumor echogenicity. These results suggest a significant relation between tumor echogenicity and RPELN metastasis (OR = 0.62, 95%CI: 0.40-0.98, P = 0.04).
Central lymph node metastasis: In an analysis involving 12282 patients from 17 studies to assess the link between central lymph node metastasis and recurrent PTC RPELN metastasis, a random-effects model was employed, revealing considerable heterogeneity (I2 = 88%). The data indicated that 20.06% of patients with central lymph node metastasis had RPELN metastasis, compared to 5.28% of those without central lymph node metastasis. The findings strongly suggest a robust relation between central lymph node metastasis and RPELN metastasis (OR = 7.77, 95%CI: 4.73-12.76, P < 0.00001).
Lateral lymph node metastasis: To evaluate the relation between lateral lymph node metastasis and RPELN metastasis, we analyzed data from 18 studies using a fixed-effects model. The analysis showed moderate heterogeneity (I2 = 42%). The results indicate that lateral lymph node metastasis is a significant risk factor for RPELN metastasis (OR = 6.94, 95%CI: 6.11-7.89, P < 0.00001).
Vascular invasion: The link between vascular invasion and RPELN metastasis was examined across 4 studies comprising 1606 patients. A fixed-effects model analysis revealed low-level heterogeneity (I2 = 32%). The data showed that 31.13% of patients with vascular invasion had RPELN metastasis, compared to 15.35% of those without vascular invasion. This suggests a link between vascular invasion and RPELN metastasis (OR = 2.16, 95%CI: 1.56-2.99, P < 0.00001).
Hashimoto thyroiditis: The relation between Hashimoto thyroiditis and RPELN metastasis was examined using data from 17 studies involving a total of 11229 patients. A fixed-effects model indicated low-level heterogeneity (I2 = 2%). Among patients with Hashimoto thyroiditis, 8.16% had RPELN metastasis, compared to 12.55% of those without the condition. These results suggest that Hashimoto thyroiditis is linked to a lower prevalence of RPELN metastasis (OR = 0.79, 95%CI: 0.69-0.92, P = 0.002).
Based on the NOS quality assessment, the majority of the 26 studies evaluated were classified as good quality, with 22 studies achieving a score of 7 or higher. This includes two studies that attained the highest score of 9, reflecting exceptional quality. Conversely, 4 studies were rated as moderate quality, with scores of 6. These findings indicate a generally robust quality across the included studies, with most meeting or exceeding the threshold for good quality on the NOS (Table 1).
The Egger regression test revealed that certain risk factors showed publication bias. Specifically, the middle pole (P = 0.0211), right side tumor location (P < 0.0001), and central lymph node metastasis (P = 0.0488) had significant P-values, indicating potential publication bias. Other factors such as capsular invasion (P = 0.7272), superior pole (P = 0.6624), inferior pole (P = 0.6746), Hashimoto thyroiditis (P = 0.8322), lateral lymph node metastasis (P = 0.9720), multifocality (P = 0.6658), tumor size (P = 0.4404), sex (P = 0.8593), extra-thyroid extension (P = 0.7548), age > 45 (P = 0.7969), and age > 55 (P = 0.2425) did not show significant asymmetry, suggesting a lower likelihood of publication bias for these variables.
In this research endeavor, we extensively analyzed the relationship between 14 distinct risk factors and metastasis to the RPELNs in PTC. Our results emphasize the significance of specific clinicopathological characteristics of PTC in increasing the likelihood of RPELN metastasis, thus underscoring the importance of thorough evaluation and targeted management in PTC patients. We investigated how RPELN metastasis links to demographic variables like age and sex. Previous studies used 45 years as a standard for classification, but the 8th edition of American Joint Committee on Cancer guidelines raised the age stratification to 55 years[43]. Our analysis, using 55 years as the age cut-off, found that patients aged < 55 years have a higher prevalence of RPELN metastasis. This association may be attributed to the more aggressive biological behavior of PTC in younger patients, who often present with larger tumors, extrathyroidal extension, and multifocality, all of which are linked to increased lymph node metastasis. The underlying mechanisms, potentially involving differences in tumor biology or immune response, warrant further investigation. A noteworthy relation was noted between a lower age (< 45 years) and an increased prevalence of RPELN metastasis, as observed in previous studies[44,45]. Though PTC predominantly affects females, men have been more prone to mortality, as discussed by Jonklaas et al[46] and Liu et al[47] report that men should be provided with more aggressive treatments because of poor prognostic outcomes. Compared to women, men with PTC have greater rates of radiation exposure linked to a more aggressive disease. When advising men with PTC, total thyroidectomy with potential central neck dissection should be further explored[48].
Tumor features such as size and location were also evaluated in our study. The cut-off for tumor size was 1 cm. Analysis of our study states that tumor size > 1cm significantly increased RPELN metastasis, which was consistent with the findings of most studies[49,50]. Larger tumors need a more thorough check-up due to a higher likelihood of metastasis[51]. Our analysis identified a higher prevalence of RPELN metastasis in patients with tumors located in the right lobe compared to other sites. The reported prevalence of RPELN metastasis varied widely across studies, ranging from 2% to 51%. This variability is likely attributable to differences in surgical dissection protocols, patient selection, and the thoroughness of lymph node examination. Some studies may have performed routine RPELN dissection, while others only dissected based on preoperative suspicion or intraoperative findings, leading to underreporting in the latter. Additionally, variations in pathological examination techniques could affect detection rates. We further examined the relationship between tumor sublocations and RPELN metastasis. While a trend towards a greater prevalence of RPELN metastasis was observed in tumors located in the superior pole compared to other sublocations, this was not statistically significant in our study. No significant variation in RPELN metastasis rates was detected among tumors in the middle or inferior poles compared to other locations. These findings suggest that although a trend indicates a higher risk of RPELN metastasis with tumors in the superior pole, the data does not provide strong evidence for a clear impact of tumor sublocation on the prevalence of RPELN metastasis. Surgery is recommended for individuals with upper 1/3 tumors exhibiting invasive characteristics and lymph node metastasis. In comparison, monitoring may be appropriate for patients with lower 2/3 tumors that do not exhibit these consequences[52]. Recent studies in our analysis have identified tumor margin as a significant risk factor, a finding not reported by earlier meta-analyses. Ill-defined tumor margins were significantly linked to higher RPELN metastasis. The tumor appears more likely to invade surrounding tissues if it has an infiltrative margin and is positioned peripherally. This aggressiveness is comparable to tumors that have extrathyroid extension[53].
Microcalcification was also taken into consideration as a potential risk factor. Previously, unreported by earlier meta-analyses, recent studies in our analysis have identified microcalcification as a significant risk factor. Associated with larger tumors having aggressive features, microcalcification increases the risk of malignancy[26]. In their study, Lu et al[54] also reported lymph node microcalcification as a significant risk factor for predicting lymph node metastasis. Similar to microcalcification, which was a significant tumor feature in our investigation, multifocality was also related to an increased probability of recurrent RPELN metastasis. This finding aligns with previous research highlighting multifocality as a critical factor in the metastatic process[55]. Specifically, multifocal tumors larger than 1 cm have been shown to hold significant predictive value[56]. Moreover, multifocality has been associated with an increased risk of metastasis and recurrence due to its impact on multiple lymphatic drainage pathways and extrathyroid extension[57]. This enhanced risk reflects the more aggressive nature of multifocal tumors, which necessitates thorough management and vigilant monitoring.
There are, nevertheless, additional significant tumor-related risk factors. Our analysis yielded two other noteworthy risk factors: Capsular invasion and extrathyroid extension. A meta-analysis by Shao et al[15] in 2020 stated the significance of both factors while evaluating the risk of metastasis to RPELN, as seen in our study. For these predictors, high heterogeneity was observed (I2 = 89% for extrathyroid extension and I2 = 75% for capsular invasion), suggesting significant variability across studies, possibly due to differences in diagnostic criteria, surgical techniques, or patient populations. Sensitivity analyses were performed to explore the sources of heterogeneity, but they did not fully resolve the issue, indicating that the strength of association may vary depending on study-specific factors. Thyroid capsular invasion and extrathyroid extension are critical indicators of the extent of tumor infiltration into surrounding tissues. When PTC breaches the thyroid capsule or extends to extrathyroidal structures, the likelihood of metastasis to the RPELN significantly increases. Our analysis revealed a higher prevalence of RPELN metastasis in patients with capsular invasion and extra-thyroid extension. Consequently, thorough lymph node dissection, including RPELN dissection, is imperative to minimize the risk of incomplete surgical resection in cases where capsular invasion or extrathyroid extension is evident. However, RPELN dissection is technically demanding due to its anatomical location adjacent to critical structures such as the recurrent laryngeal nerve and esophagus. This proximity increases the risk of complications, including nerve palsy and esophageal injury. Therefore, surgeons must carefully weigh the benefits of RPELN dissection against the potential risks, particularly in patients without high-risk features. Routine resection should be avoided in the absence of clear indications, and decisions should be individualized based on tumor characteristics, intraoperative findings, and surgical expertise.
This approach ensures more comprehensive disease management and potentially improves patient outcomes. In addition to these invasive characteristics, the echogenicity of the tumor also plays a pivotal role in determining the metastatic potential and overall prognosis of PTC. On ultrasonography, tumors with substantial hypoechogenicity appear noticeably darker and frequently have more aggressive characteristics. This trait is linked to more advanced tumor growth patterns, increased tumor fibrosis, and an increased risk of metastasis[58]. Our study evaluates that increased echogenicity increases RPELN metastasis risk. In 2022, Wang et al[59] reported that heightened tumor echogenicity helps clinicians assess invasive risk and guide decisions on surgical intervention and monitoring.
Metastasis to central and lateral compartment nodes can potentially increase the metastatic risk of RPELN. The most common site of lymph node metastasis from the PTC is the central compartment of the neck due to proximity to the thyroid gland and engouement of the gland’s lymphatic drainage through the central lymph node[60]. In their meta-analysis, Shao et al[15] came up with similar findings, potentiating the connection between RPELN metastasis and central lymph node metastasis. Lateral lymph node metastasis in PTC was associated with a worse prognosis in a previous study[61]. That summarizes our point that a worse prognosis in lateral lymph node metastasis could increase the risk of RPELN metastasis. Our study reports that vascular invasion increases the risk of RPELN metastasis. Cancer cells can also metastasize the surrounding vasculature. Increased vascular invasion led to increased RPELN metastasis as per our analysis. Previously not reported by earlier meta-analyses, the recent studies included in our analysis have specifically identified vascular invasion as a crucial risk factor. A study in 2022 reports that vascular invasion in PTC is linked to aggressive pathological characteristics in tumors[62]. Hence, it can be deduced that vascular invasion through blood and lymphatics provides a direct route for cancer cells to spread in surrounding nodes, increasing RPELN metastasis.
The relationship between Hashimoto thyroiditis and PTC was also studied in our analysis. Research suggests that patients with Hashimoto thyroiditis have a greater prevalence of PTC than the general population[63], but it also appears to have a protective role in metastasis. The chronic inflammation caused by Hashimoto’s thyroiditis can modify the tumor microenvironment, making it less conducive for cancer cells to invade and metastasize[64]. In our study, those with Hashimoto thyroiditis had a lower prevalence of RPELN metastasis than those without. A previous meta-analysis by Shao et al[15] also reports no link between increased RPELN metastasis and Hashimoto thyroiditis. In comparison to individuals without Hashimoto thyroiditis, those with coexisting Hashimoto thyroiditis exhibited less aggressive presenting characteristics and had better PTC outcomes[65]. Hence, it is concluded that Hashimoto thyroiditis is not a potential risk factor for RPELN metastasis.
Although a large amount of data was analyzed to draw conclusions, there are still some limitations. The majority of included studies were conducted in Asia, particularly China, which may limit the generalizability of our findings to other populations. There is evidence suggesting that the incidence and behavior of PTC can vary across different ethnic and geographical groups, possibly due to genetic, environmental, or lifestyle factors[65]. Therefore, our results should be interpreted with caution when applied to non-Asian populations, and further studies including diverse cohorts are necessary to confirm these associations. Randomized controlled trials were not included in our studies. Lifestyle and genetic predisposition were not considered. Lastly, differences in diagnostic techniques and sample sizes of each study can lead to variability in prevalence rates.
This meta-analysis highlights key predictors of RPELN metastasis in PTC, including age, tumor size, multifocality, and extrathyroid extension. The observed heterogeneity in some analyses underscores the need for further research to clarify these associations and reduce variability. Future studies should address the sources of heterogeneity identified, such as the impact of individual studies on the overall findings. Additionally, more refined research methodologies, including larger, more homogeneous cohorts, could enhance the precision of risk factor identification. Clinicians should factor in younger age, larger tumor size, multifocality, and extrathyroid extension when assessing RPELN metastasis risk in PTC patients. These characteristics indicate a higher risk and may warrant more intensive surveillance and management. Incorporating these risk factors into preoperative assessments could enhance surgical planning and postoperative follow-up, leading to more personalized treatment and better patient outcomes.
Several factors significantly associated with RPELN metastasis include age under 55 years, sex, tumor size of 1 cm, multifocality, capsular invasion, vascular invasion, extra-thyroid extension, central and lateral lymph node metastasis, Hashimoto thyroiditis, microcalcifications, and echogenicity. In contrast, tumor location and margins were not significantly associated. These findings highlight the need for thorough evaluation and proactive surgical intervention in patients with PTC who exhibit these risk factors to manage potential RPELN involvement.
| 1. | Sajisevi M, Caulley L, Eskander A, Du YJ, Auh E, Karabachev A, Callas P, Conradie W, Martin L, Pasternak J, Golbon B, Rolighed L, Abdelhamid Ahmed AH, Badhey A, Cheung AY, Corsten M, Forner D, Liu JC, Mavedatnia D, Meltzer C, Noel JE, Patel V, Sharma A, Tang AL, Tsao G, Venkatramani M, Williams M, Wrenn SM, Zafereo M, Stack BC Jr, Randolph GW, Davies L. Evaluating the Rising Incidence of Thyroid Cancer and Thyroid Nodule Detection Modes: A Multinational, Multi-institutional Analysis. JAMA Otolaryngol Head Neck Surg. 2022;148:811-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 2. | Bogović Crnčić T, Ilić Tomaš M, Girotto N, Grbac Ivanković S. Risk Factors for Thyroid Cancer: What Do We Know So Far? Acta Clin Croat. 2020;59:66-72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 3. | Costache MI, Iordache S, Karstensen JG, Săftoiu A, Vilmann P. Endoscopic ultrasound-guided fine needle aspiration: from the past to the future. Endosc Ultrasound. 2013;2:77-85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 4. | Kitahara CM, Schneider AB. Epidemiology of Thyroid Cancer. Cancer Epidemiol Biomarkers Prev. 2022;31:1284-1297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 157] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 5. | Toraih EA, Hussein MH, Zerfaoui M, Attia AS, Marzouk Ellythy A, Mostafa A, Ruiz EML, Shama MA, Russell JO, Randolph GW, Kandil E. Site-Specific Metastasis and Survival in Papillary Thyroid Cancer: The Importance of Brain and Multi-Organ Disease. Cancers (Basel). 2021;13:1625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 71] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 6. | Surveillance, Epidemiology, and End Results Program. SEER Cancer Statistics Review (CSR) 1975-2018. 15 Apr 2021. Available from: https://seer.cancer.gov/csr/1975_2018/index.html. |
| 7. | Yang H, Tao L. Lymph Node Posterior to the Right Recurrent Laryngeal Nerve Metastasis in Right Lobe T1a Papillary Thyroid Carcinoma: A Retrospective Cohort Study. Cancer Control. 2023;30:10732748221149819. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 8. | Shao J, Wang X, Yu H, Ding W, Xu B, Ma D, Huang X, Yin H. Preoperative Prediction of Metastatic Lymph Nodes Posterior to the Right Recurrent Laryngeal Nerve in cN0 Papillary Thyroid Carcinoma. Cancer Manag Res. 2024;16:421-429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 9. | Sciuto R, Romano L, Rea S, Marandino F, Sperduti I, Maini CL. Natural history and clinical outcome of differentiated thyroid carcinoma: a retrospective analysis of 1503 patients treated at a single institution. Ann Oncol. 2009;20:1728-1735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 131] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 10. | Tong Y, Li J, Huang Y, Zhou J, Liu T, Guo Y, Yu J, Zhou S, Wang Y, Chang C. Ultrasound-Based Radiomic Nomogram for Predicting Lateral Cervical Lymph Node Metastasis in Papillary Thyroid Carcinoma. Acad Radiol. 2021;28:1675-1684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 11. | Agarwal S, Chand G, Jaiswal S, Mishra A, Agarwal G, Agarwal A, Verma AK, Mishra SK. Pattern and risk factors of central compartment lymph node metastasis in papillary thyroid cancer: a prospective study from an endocrine surgery centre. J Thyroid Res. 2012;2012:436243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Zhao J, Zheng W, Zhong M, Daming L, Guo L. Risk Factors and Prognosis of Retropharyngeal Lymph Node Metastasis from Papillary Thyroid Carcinoma. Altern Ther Health Med. 2024;30:474-479. [PubMed] |
| 13. | Shaha AR. Right paraesophageal lymph node metastasis. Eur J Surg Oncol. 2016;42:79-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Qu Y, Zhang H, Zhang P, Dong W, He L, Sun W, Liu J. Risk factors and the preoperative assessment of right para-oesophageal lymph node metastasis in right lobe papillary thyroid carcinoma: A case series. Int J Surg. 2017;42:123-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Shao L, Sun W, Zhang H, Zhang P, Wang Z, Dong W, He L, Zhang T, Qin Y. Risk factors for right paraesophageal lymph node metastasis in papillary thyroid carcinoma: A meta-analysis. Surg Oncol. 2020;32:90-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9207] [Cited by in RCA: 8343] [Article Influence: 521.4] [Reference Citation Analysis (2)] |
| 17. | Chaimani A, Caldwell DM, Li T, Higgins JPT, Salanti G. Chapter 11: Undertaking network meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Chichester (UK): John Wiley & Sons, 2019: 285–320. [DOI] [Full Text] |
| 18. | Higgins JPT, Li T, Deeks JJ. Chapter 6: Choosing effect measuresand computing estimates of effect. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Chichester (UK): John Wiley & Sons, 2019: 143–176. [DOI] [Full Text] |
| 19. | PennState Eberly College of Science. 16.8-Random Effects/Sensitivity Analysis. [cited 16 August 2024]. Available from: https://online.stat.psu.edu/stat509/lesson/16/16.8. |
| 20. | Liu C, Chen T, Zeng W, Wang S, Xiong Y, Liu Z, Huang T. Reevaluating the prognostic significance of male gender for papillary thyroid carcinoma and microcarcinoma: a SEER database analysis. Sci Rep. 2017;7:11412. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 21. | Lu KN, Zhang Y, Da JY, Zhou TH, Zhao LQ, Peng Y, Pan G, Shi JJ, Zhou L, Ni YQ, Luo DC. A Novel Scoring System for Predicting the Metastases of Posterior Right Recurrent Laryngeal Nerve Lymph Node Involvement in Patients With Papillary Thyroid Carcinoma by Preoperative Ultrasound. Front Endocrinol (Lausanne). 2021;12:738138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 22. | Zhu J, Huang R, Hu D, Dou Y, Ren H, Yang Z, Deng C, Xiong W, Wang D, Mao Y, Li X, Su X. Individualized Prediction Of Metastatic Involvement Of Lymph Nodes Posterior To The Right Recurrent Laryngeal Nerve In Papillary Thyroid Carcinoma. Onco Targets Ther. 2019;12:9077-9084. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Lee BJ, Lee JC, Wang SG, Kim YK, Kim IJ, Son SM. Metastasis of right upper para-esophageal lymph nodes in central compartment lymph node dissection of papillary thyroid cancer. World J Surg. 2009;33:2094-2098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Bae SY, Yang JH, Choi MY, Choe JH, Kim JH, Kim JS. Right paraesophageal lymph node dissection in papillary thyroid carcinoma. Ann Surg Oncol. 2012;19:996-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Kim YS, Park WC. Clinical predictors of right upper paraesophageal lymph node metastasis from papillary thyroid carcinoma. World J Surg Oncol. 2012;10:164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Ito Y, Fukushima M, Higashiyama T, Kihara M, Takamura Y, Kobayashi K, Miya A, Miyauchi A. Incidence and predictors of right paraesophageal lymph node metastasis of N0 papillary thyroid carcinoma located in the right lobe. Endocr J. 2013;60:389-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 27. | Pinyi Z, Bin Z, Jianlong B, Yao L, Weifeng Z. Risk factors and clinical indication of metastasis to lymph nodes posterior to right recurrent laryngeal nerve in papillary thyroid carcinoma: a single-center study in China. Head Neck. 2014;36:1335-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Chang H, Yoo RN, Kim SM, Kim BW, Lee YS, Lee SC, Chang HS, Park CS. The Clinical Significance of the Right Para-Oesophageal Lymph Nodes in Papillary Thyroid Cancer. Yonsei Med J. 2015;56:1632-1637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 29. | Zhang L, Liu H, Xie Y, Xia Y, Zhang B, Shan G, Li X. Risk factors and indication for dissection of right paraesophageal lymph node metastasis in papillary thyroid carcinoma. Eur J Surg Oncol. 2016;42:81-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Luo DC, Xu XC, Ding JW, Zhang Y, Peng Y, Pan G, Zhang W. Clinical value and indication for the dissection of lymph nodes posterior to the right recurrent laryngeal nerve in papillary thyroid carcinoma. Oncotarget. 2017;8:79897-79905. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 31. | Park YM, Lee SM, Kim DW, Shin SC, Lee BJ. Predictive factors of right paraesophageal lymph node metastasis in papillary thyroid carcinoma: Single center experience and meta-analysis. PLoS One. 2017;12:e0177956. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 32. | Yuan J, Li J, Chen X, Zhong Z, Chen Z, Yin Y, Du J, Cong S, Wu Z. Predictors of lymph nodes posterior to the right recurrent laryngeal nerve metastasis in patients with papillary thyroid carcinoma: A retrospective study. Medicine (Baltimore). 2017;96:e7908. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 33. | Luo Y, Xu XC, Shen J, Shi JJ, Lu S, He W, Lei JY, Luo DC. Model of lymph node metastasis posterior to the right recurrent laryngeal nerve in papillary thyroid carcinoma. Cancer Manag Res. 2018;10:2449-2455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 34. | Yu QA, Ma DK, Liu KP, Wang P, Xie CM, Wu YH, Dai WJ, Jiang HC. Clinicopathologic risk factors for right paraesophageal lymph node metastasis in patients with papillary thyroid carcinoma. J Endocrinol Invest. 2018;41:1333-1338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 35. | Hou J, Shan H, Zhang Y, Fan Y, Wu B. Risk factors of metastasis to the lymph nodes posterior to the right recurrent laryngeal nerve in papillary thyroid carcinoma. Eur Arch Otorhinolaryngol. 2020;277:881-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 36. | Zou M, Wang YH, Dong YF, Lai XJ, Li JC. Clinical and sonographic features for the preoperative prediction of lymph nodes posterior to the right recurrent laryngeal nerve metastasis in patients with papillary thyroid carcinoma. J Endocrinol Invest. 2020;43:1511-1517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 37. | Ling Y, Zhang L, Li K, Zhao Y, Zhao J, Jia L, Wang Y, Kang H. Carbon nanoparticle-guided intraoperative lymph node biopsy predicts the status of lymph nodes posterior to right recurrent laryngeal nerve in cN0 papillary thyroid carcinoma. Gland Surg. 2021;10:1554-1563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 38. | Xiao X, Wu Y, Zou L, Chen Y, Zhang C. Value of dissection of lymph nodes posterior to the right recurrent laryngeal nerve in patients with cN(0) papillary thyroid carcinoma. Gland Surg. 2022;11:1204-1211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 39. | Zhang S, Xu C, Yang B, Yan D. Nomogram combining preoperative ultrasonography with clinical features for predicting lymph nodes posterior to the right recurrent laryngeal nerve metastasis in patients with papillary thyroid cancer. Acta Endocrinol (Buchar). 2022;18:333-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 40. | Zhou M, Duan Y, Ye B, Wang Y, Li H, Wu Y, Chen P, Zhu J, Jing C, Wu Y, Wang X. Pattern and Predictive Factors of Metastasis in Lymph Nodes Posterior to the Right Recurrent Laryngeal Nerve in Papillary Thyroid Carcinoma. Front Endocrinol (Lausanne). 2022;13:914946. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 41. | Gong Y, Zuo Z, Tang K, Xu Y, Zhang R, Peng Q, Niu C. Multimodal predictive factors of metastasis in lymph nodes posterior to the right recurrent laryngeal nerve in papillary thyroid carcinoma. Front Endocrinol (Lausanne). 2023;14:1187825. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 42. | Shen B, Zhou C, Xu C, Yang B, Wu X, Fu X, Liu S, Sun J, Xie Y, Zhu Z. Ultrasound-based Radiomics for Predicting Metastasis in the Lymph Nodes Posterior to the Right Recurrent Laryngeal Nerve in Patients with Papillary Thyroid Cancer. Curr Med Imaging. 2024;20:e15734056257332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 43. | Kaliszewski K, Diakowska D, Nowak Ł, Wojtczak B, Rudnicki J. The age threshold of the 8th edition AJCC classification is useful for indicating patients with aggressive papillary thyroid cancer in clinical practice. BMC Cancer. 2020;20:1166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 44. | Li C, Xiang J, Wang Y. Risk Factors for Predicting Lymph Nodes Posterior to Right Recurrent Laryngeal Nerve (LN-prRLN) Metastasis in Thyroid Papillary Carcinoma: A Meta-Analysis. Int J Endocrinol. 2019;2019:7064328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 45. | Liu LS, Liang J, Li JH, Liu X, Jiang L, Long JX, Jiang YM, Wei ZX. The incidence and risk factors for central lymph node metastasis in cN0 papillary thyroid microcarcinoma: a meta-analysis. Eur Arch Otorhinolaryngol. 2017;274:1327-1338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 46. | Jonklaas J, Nogueras-Gonzalez G, Munsell M, Litofsky D, Ain KB, Bigos ST, Brierley JD, Cooper DS, Haugen BR, Ladenson PW, Magner J, Robbins J, Ross DS, Skarulis MC, Steward DL, Maxon HR, Sherman SI; National Thyroid Cancer Treatment Cooperative Study Group. The impact of age and gender on papillary thyroid cancer survival. J Clin Endocrinol Metab. 2012;97:E878-E887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 157] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 47. | Remer LF, Lee CI, Picado O, Lew JI. Sex Differences in Papillary Thyroid Cancer. J Surg Res. 2022;271:163-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 48. | Chisholm EJ, Kulinskaya E, Tolley NS. Systematic review and meta-analysis of the adverse effects of thyroidectomy combined with central neck dissection as compared with thyroidectomy alone. Laryngoscope. 2009;119:1135-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 129] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 49. | Guo G, Meng Y, Tan W, Xia Y, Cheng C, Chen X, Gu Z. Induction of Apoptosis Coupled to Endoplasmic Reticulum Stress through Regulation of CHOP and JNK in Bone Marrow Mesenchymal Stem Cells from Patients with Systemic Lupus Erythematosus. J Immunol Res. 2015;2015:183738. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 50. | Machens A, Holzhausen HJ, Dralle H. The prognostic value of primary tumor size in papillary and follicular thyroid carcinoma. Cancer. 2005;103:2269-2273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 248] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 51. | Tejpar S, Stintzing S, Ciardiello F, Tabernero J, Van Cutsem E, Beier F, Esser R, Lenz HJ, Heinemann V. Prognostic and Predictive Relevance of Primary Tumor Location in Patients With RAS Wild-Type Metastatic Colorectal Cancer: Retrospective Analyses of the CRYSTAL and FIRE-3 Trials. JAMA Oncol. 2017;3:194-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 441] [Cited by in RCA: 544] [Article Influence: 60.4] [Reference Citation Analysis (0)] |
| 52. | Lee WK, Lee J, Kim H, Lee SG, Choi SH, Jeong S, Kwon HJ, Jung SG, Jo YS. Peripheral location and infiltrative margin predict invasive features of papillary thyroid microcarcinoma. Eur J Endocrinol. 2019;181:139-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 53. | Ye ZQ, Gu DN, Hu HY, Zhou YL, Hu XQ, Zhang XH. Hashimoto's thyroiditis, microcalcification and raised thyrotropin levels within normal range are associated with thyroid cancer. World J Surg Oncol. 2013;11:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 54. | Lu J, Liao J, Chen Y, Li J, Huang X, Zhang H, Zhang B. Risk factor analysis and prediction model for papillary thyroid carcinoma with lymph node metastasis. Front Endocrinol (Lausanne). 2023;14:1287593. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 55. | Del Rio P, Loderer T, Giuffrida M, Cozzani F, Rossini M, Bonfili D, Bonati E. Multifocality in patients treated for papillary Thyroid Carcinoma: a preliminary analysis of related risk factors. Acta Biomed. 2021;92:e2021017. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 56. | Kim KJ, Kim SM, Lee YS, Chung WY, Chang HS, Park CS. Prognostic significance of tumor multifocality in papillary thyroid carcinoma and its relationship with primary tumor size: a retrospective study of 2,309 consecutive patients. Ann Surg Oncol. 2015;22:125-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 57. | Kim HJ, Sohn SY, Jang HW, Kim SW, Chung JH. Multifocality, but not bilaterality, is a predictor of disease recurrence/persistence of papillary thyroid carcinoma. World J Surg. 2013;37:376-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 118] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 58. | Lim YS, Lee YS, Lee JC, Son SM, Shin DH, Kim SS, Kim IJ, Lee BJ. Ultrasound Echogenicity of Papillary Thyroid Cancer Is Affected by Tumor Growth Patterns and Tumor Fibrosis. In Vivo. 2021;35:1633-1640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 59. | Wang G, Nie F, Wang Y, Wang P, Wang L, Fan X, Ma Z. Value of Echogenic Foci in Diagnosing Papillary Thyroid Carcinoma and Predicting Aggressive Biological Behavior. J Ultrasound Med. 2022;41:1237-1245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 60. | Gimm O, Rath FW, Dralle H. Pattern of lymph node metastases in papillary thyroid carcinoma. Br J Surg. 1998;85:252-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 142] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 61. | Gao L, Li X, Xia Y, Liu R, Liu C, Shi X, Wu Y, Ma L, Jiang Y. Large-Volume Lateral Lymph Node Metastasis Predicts Worse Prognosis in Papillary Thyroid Carcinoma Patients With N1b. Front Endocrinol (Lausanne). 2021;12:815207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 62. | Reilly J, Faridmoayer E, Lapkus M, Pastewski J, Sun F, Elassar H, Studzinski DM, Callahan RE, Czako P, Nagar S. Vascular invasion predicts advanced tumor characteristics in papillary thyroid carcinoma. Am J Surg. 2022;223:487-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 63. | Lun Y, Wu X, Xia Q, Han Y, Zhang X, Liu Z, Wang F, Duan Z, Xin S, Zhang J. Hashimoto's thyroiditis as a risk factor of papillary thyroid cancer may improve cancer prognosis. Otolaryngol Head Neck Surg. 2013;148:396-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 101] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 64. | Zeng B, Min Y, Feng Y, Xiang K, Chen H, Lin Z. Hashimoto's Thyroiditis Is Associated With Central Lymph Node Metastasis in Classical Papillary Thyroid Cancer: Analysis from a High-Volume Single-Center Experience. Front Endocrinol (Lausanne). 2022;13:868606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 65. | Xu S, Huang H, Qian J, Liu Y, Huang Y, Wang X, Liu S, Xu Z, Liu J. Prevalence of Hashimoto Thyroiditis in Adults With Papillary Thyroid Cancer and Its Association With Cancer Recurrence and Outcomes. JAMA Netw Open. 2021;4:e2118526. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 94] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/