Published online Nov 24, 2025. doi: 10.5306/wjco.v16.i11.110911
Revised: July 18, 2025
Accepted: October 17, 2025
Published online: November 24, 2025
Processing time: 155 Days and 23.1 Hours
Cardamonin is a natural chalcone that has been extensively investigated for its anticancer activity. However, its clinical relevance is still not explicit, limiting its progression into clinical trials and highlighting a persistent gap between pre
Core Tip: This review summarizes preclinical studies on cardamonin, highlighting that pharmacokinetic studies show poor oral bioavailability, whereas pharmacodynamic findings demonstrate its antiproliferative, anti-metastatic, and chemo
- Citation: Badroon NA, Alsalahi A, Aljaberi MA, Abdul Majid N, Alshawsh MA. Cardamonin as a potential anticancer agent: Preclinical insights and clinical implications. World J Clin Oncol 2025; 16(11): 110911
- URL: https://www.wjgnet.com/2218-4333/full/v16/i11/110911.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i11.110911
Cancer is one of the life-threatening diseases worldwide, ranked as the second most common cause of death[1]. For thousands of years, humans have exploited plants as a source of medicines for treating illnesses[2,3]. Interestingly, 25% of currently prescribed medicines are still derived from plants[3]. Thus, plants still remain as pharmaceutical resources for the discovery of therapeutic agents[3,4]. Chemotherapeutic drugs such as vincristine, vinblastine and other agents, originally isolated from plants, continue to be widely used in clinical oncology[5]. However, their use encounters sig
Flavonoids are natural compounds found in plants, including Silybum marianum, Vaccinium myrtillus, Hypericum perforatum, hawthorn, Ginkgo biloba, Citrus species, and tea[7,8], which have therapeutic values to humans[3]. Some of these flavonoids have been clinically tested for their efficacy in treating several diseases[8]. However, both flavonoids and their synthetic analogues have demonstrated promising chemotherapeutic potential in the preclinical management of various types of cancer[9]. Cardamonin (2’,4’-dihydroxy-6’-methoxychalcone) is a chalcone belonging to the flavonoids class[7]. Despite cardamonin has demonstrated various biological activities with clinical potential against various disorders[10], its anticancer properties have received particular emphasis. Numerous preclinical studies highlight its promising efficacy against several malignancies, including aggressive cancers such as glioblastoma, multiple myeloma, melanoma, nasopharyngeal carcinoma, and ovarian tumors[7,11]. In applied pharmacology, however, the clinical significance of cardamonin remains uncertain and has yet to be incorporated into clinical trials, indicating a gap between preclinical evidence and its translational implications[12].
Building on its emerging therapeutic potential, cardamonin has recently been recognized as a promising candidate for medical applications and subsequently proposed as a potential anticancer chalcone[7,13]. In the light of the growing body of in vitro and in vivo preclinical evidence supporting its anticancer activity, particularly through modulation of multiple cancer-related signaling pathways[13,14], the need to update the current literature is warranted. Unlike previous reviews, this paper focuses exclusively on the anticancer effects of cardamonin across all cancer types studied in preclinical animal and cell models. It presents a cross-comparative analysis of efficacy data, alongside an evaluation of pharmacokinetics and structure-activity relationships that may underlie its therapeutic potential. These insights could enable researchers to address existing gaps and assist future studies aimed at advancing cardamonin towards translational development. Accordingly, this review is an attempt to evaluate whether cardamonin, a promising anticancer agent, is ready to advance from lab-bench to clinical application.
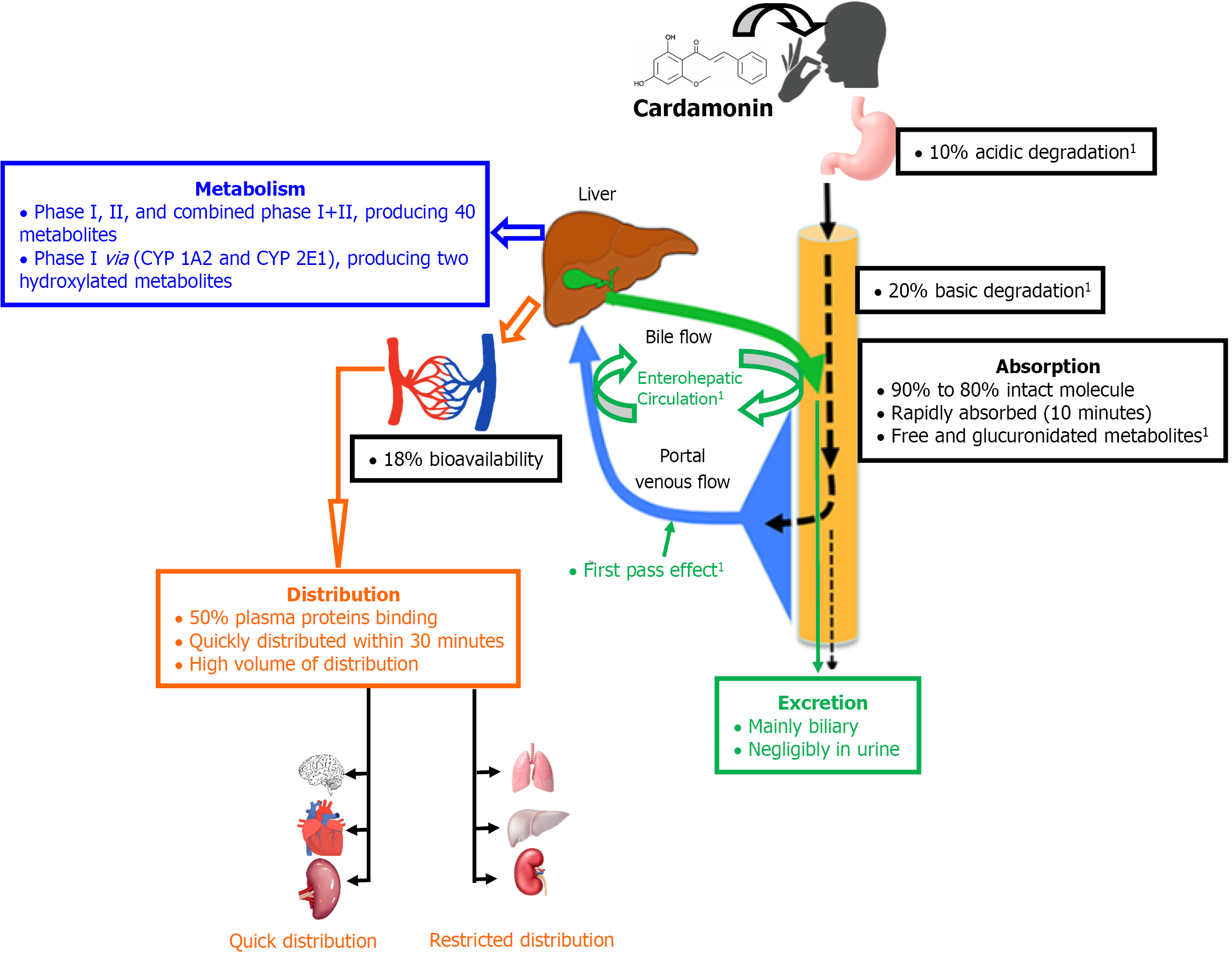
The pharmacokinetic profile of cardamonin (Figure 1) seems to be influenced by its solubility characteristics[15], which is pH-dependent. Despite being partially soluble in aqueous media and minimally soluble in acidic ones, it still retains high ileum permeability[16]. Following oral dosing, the parent molecule resists enzymatic degradation by pepsin and pancreatin. However, 10% and 20% of the intact molecule undergo acidic and basic degradation, respectively. Thus, 80% to 90% of the oral dose of the intact molecule remains available for intestinal absorption[16]. Once absorbed, cardamonin undergoes first-pass metabolism in the liver and enterohepatic circulation[16,17]. Upon absorption, the former barriers significantly reduce the plasma bioavailability of cardamonin to 18%, indicating a poor oral bioavailability[11,12,15]. Additionally, the onset of action of cardamonin is delayed by such barriers, reaching a serum peak concentration of 2 hours[15]. Although cardamonin is rapidly absorbed from the intestine after 10 minutes of oral dosing, detection of free and glucuronidated molecules of cardamonin in plasma indicates that the parent molecule undergoes intestinal metabolism[17]. In the plasma compartment, cardamonin exhibits moderate binding to plasma proteins (< 50%), allowing more than half of the circulating plasma cardamonin to remain unbound and available for distribution into the body fluids and tissues to exert its action within 30 minutes of the oral dosing[16]. Cardamonin, notably, has the advantage of a high volume of distribution, and it is quickly distributed into the liver, kidney and lung, while its distribution into the brain, heart and spleen remains limited[16].
In regard to biotransformation, in vitro evidence suggests that a fraction of cardamonin could be intracellularly metabolized to alpinetin[18]. The in vivo evidence, on the contrary suggests that cardamonin undergoes hepatic clearance through phase I, phase II, and combined phase I and II. This results in producing three key metabolites (two cardamonin-glucuronides and one hydroxy-glucuronide of cardamonin)[19,20]. Additionally, two microsomes (cytochrome P450 1A2 and cytochrome P450 2E1) are responsible for converting the parent molecule into two hydroxylated metabolites during phase I[21], and this conversion involves nicotinamide adenine dinucleotide phosphate hydrogen-dependent enzymes[16]. However, recent investigations indicate that the parent molecule undergoes different major hepatic pathways, including methylation, demethylation, hydrogenation, hydroxylation, dehydroxylation, glucuronidation and sulfation, leading to the identification of up to 40 metabolites in plasma and urine[19]. Finally, cardamonin is mainly excreted through biliary, both as conjugated and unchanged forms via feces, but minimal excretion in urine[16].
Cardamonin is a chalcone structurally characterized by an α,β-unsaturated ketone linking two aromatic rings[12]. Due to the solvent interactions, cardamonin exists as a dimer in aqueous media, which contributes to its poor water solubility, but remains as a monomer in organic solvents (e.g., ethanol, methanol, and dimethyl sulfoxide), making it organic solvent-soluble (lipophilic)[22]. Additionally, the solubility of cardamonin in different media is pH-dependent, where its solubility increases in alkaline media[22]. It can be isolated as a pure trans-isomer, which is more thermodynamically stable than the cis-one[23].
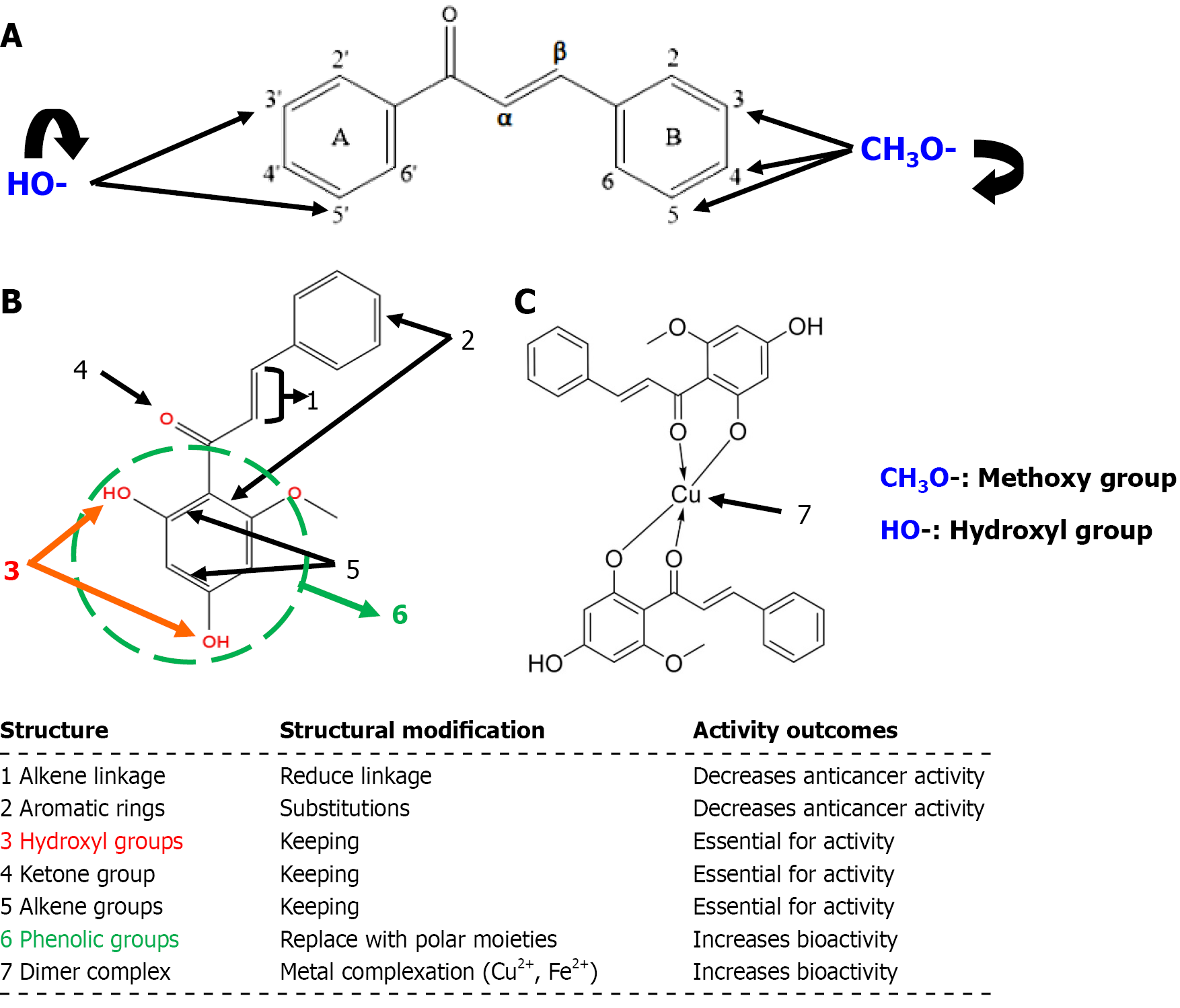
The structure-activity relationship of chalcones varies depending on the therapeutic target[24]. In the context of anticancer activity, methoxy substitutions at the 3, 4 and 5 positions of the B-ring enhance cytotoxicity[24]. Similarly, the presence of hydroxyl groups at the 3’ and 5’ positions of the A-ring increase cytotoxicity. However, the α,β-unsaturated carbonyl bond has been proven to be essential for bioactivity (Figure 2A)[24].
Inserting different substitutions on the aromatic rings of the parent molecule of cardamonin and reducing the alkene linkage decrease the anticancer activity (Figure 2B)[25]. Conversely, the structure-activity relationship of 20 cardamonin analogues indicates that the modification of the hydroxyl group and the presence of ketone and alkene groups are essential for bioactivity (Figure 2B)[24,26]. Additionally, substituting the phenolic groups with more polar moieties or forming complexes with bivalent metal ions (e.g., copper or iron) enhances its bioactivity (Figure 2C)[26].
Preclinical studies suggest that cardamonin is generally well tolerated in animal models at doses ranging from 5 mg/kg to 2000 mg/kg when administered over 28 days. Moreover, cardamonin has demonstrated substantial protective effects against the cytotoxicity of various anticancer agents and has improved survival rates in certain cancer-induced animal models. For example, every-other-day intraperitoneal administration of 30 mg/kg of cardamonin for 20 days in SUM190-xenograft male nude mice was not associated with notable adverse effects[27]. Similarly, a daily intraperitoneal dose of
Furthermore, while in vitro studies have demonstrated cardamonin’s antiproliferative effects against different types of cancer cell lines, most studies have not assessed its cytotoxicity on normal cells, representing a major key limitation of the preclinical studies that are included in this review. Nonetheless, few studies have reported that cardamonin exhibits relatively low cytotoxicity against normal cells[30,31].
The in vitro studies have shown that cardamonin exhibits antiproliferative effects on several breast cancer cells line through attenuating cellular viability and arresting cell cycle growth at G2/M phase (Table 1)[27,32-37]. Similarly, in vivo evidence from breast cancer-induced animals indicated that cardamonin could inhibit the growth of breast-induced cancer through inhibiting overexpression of testes-specific protease 50 (TSP50)-xenograft cells, downregulating mRNA expression of cancer stem cells (CSCs)-associated genes, blocking Toll-like receptor 3 (TLR3) on the induced-tumorigenic CSC, suppressing hypoxia-inducible factor (HIF)-1α mediated cell metabolism or inducing apoptosis (Table 2)[27,32,33,35,37,38].
| Cell lines | Effects | Molecular mechanism | Ref. |
| Human hepatocellular carcinoma (HepG2) | (1) Inhibited cellular viability; (2) Arrested cell cycle at G1 phase; (3) Induced apoptosis by accumulating mitochondrial ROS, upregulating caspases expression (caspase 3/7, caspase-8 and caspase-9), upregulating expression of proapoptotic proteins (FADD, Fas, TRIAL, HIF-1, cleaved caspase-3) and downregulating expression of antiapoptotic proteins (HSP60, HSP27 and HSP70, XIAP, catalase, clusterin, and surviving); and (4) Inhibited cellular colonization | Deactivated NF-κB signalling pathway by blocking nuclear translocation of NF-κB and increasing phosphorylation of p53 | [61] |
| (1) Inhibited cellular viability; and (2) Induced apoptosis by cell shrinkage and chromatin condensation | Blocked TSP50-mediated NF-κB signaling pathway by downregulating protein and mRNA expressions of TSP50 | [35] | |
| Human mesenchymal triple-negative breast cancer cells (SUM190; TLR3 activation-enhanced CSC phenotypes) | Inhibited mammosphere formation by blocking TLR3 on the induced CSCs by downregulating protein expression of associated stem-cell genes (OCT4 and c-Myc) | Deactivated concurrently Wnt/β-catenin and NF-κB signalling pathways by concurrent inhibition of nuclear translocations of both β-catenin and NF-κB | [27] |
| Human mesenchymal triple-negative breast cancer (SUM190 and MDA-MB231), SUM190 (inflammatory breast cancer cell line, invasive ductal carcinoma, ER-PR-HER2-/+) and CAMA-1 cells (adenocarcinoma, ER+/PR-, oncogenic mutations in PTEN and p53, in-frame mutation in E-cadherin gene) and MCF-7 cells (tolerant to 5-FU, doxorubicin, or paclitaxel) | (1) Augmented antimetastatic effects of 5-FU, doxorubicin or paclitaxel; (2) Inhibited mammosphere formation through inhibiting cellular colonization and abolishing self-renewal capacity of CSCs; and (3) Downregulated mRNA expression of CSCs-associated genes (ALDH1, SOX2, c-Myc, OCT4, NANOG) and CSCs-associated histone modifier genes (EZH2, SETDB1, SMYD3) | Deactivated NF-κB and STAT3 signalling pathways by downregulating protein expressions of NF-κB/IKβα and STAT3, respectively | [38] |
| MDA-MB-231 cells (transfected with pGL3-TSP50 promoter plasmid) | (1) Inhibited cellular viability; (2) Arrested cell cycle at G2/M-phase by upregulating expression of CDK inhibitor (p21) in TSP50-overexpressed cells; and (3) Induced mitochondrial-dependent apoptosis as appeared by cells shrinkage and chromatin condensation with DNA fragmentations, upregulating expression of pro-apoptotic proteins (BAX, activated caspase-9, cleaved caspase-3) and downregulating expression of the anti-apoptotic protein BCL-2 | Blocked TSP50-mediated NF-κB signaling pathway through downregulating expression of p65 nuclear with downregulating protein and mRNA expression of TSP50 | [35] |
| Triple negative breast cancer cells (BT-549, ER-negative) | (1) Inhibited cellular viability; (2) Arrested cell cycle at G2/M phase; (3) Induced mitochondrial apoptosis by upregulating expression of cytochrome c, proapoptotic proteins (BAX, cleaved caspase 3 and cleaved PARP) and downregulating expression of the antiapoptotic protein BCL-2; (4) Inhibited cellular colonization; and (5) Retarded invasion by blocking EMT through upregulating protein expression of the epithelia marker E-cadherin and downregulating protein expression of the mesenchymal markers N-cadherin and vimentin | Deactivated Wnt/β-catenin signaling pathway by downregulating protein expression of β-catenin and β-catenin downstream targets (cyclin D1, c-Myc, VEGF, and CDK-4) | [37] |
| Human TNBC cell line (MDA-MB-231) | (1) Inhibited cellular viability; (2) Arrested cell cycle at G2/M phase; (3) Induced mitochondrial apoptosis through accumulating ROS, downregulating expressions of the anti-apoptotic proteins BCL-2 and upregulating expressions of pro-apoptotic proteins (BAX, cleaved caspase-3 and PARP); and (4) Metabolic reprograming through inhibiting HIF-1α by downregulating protein and mRNA expressions of HIF-1α and HIF-1α target genes (PDHK1 and LDHA) | Deactivated mTOR signalling pathway by downregulating protein expressions of mTOR and S6K | [32] |
| (1) Inhibited cellular viability; (2) Arresting cell cycle at G2/M phase; (3) Induced mitochondrial apoptosis through accumulating ROS, upregulating expression of pro-apoptotic proteins (cleaved caspase-3, PARP, and BAX) and downregulating expression of the antiapoptotic protein BCL-2; and (4) Activated FOXO3a by upregulating protein expression of FOXO3a and its target genes (p21, p27, Bim), and upstreaming JNK | Activated JNK/mTOR signalling pathway | [33] | |
| Human ER positive breast cancer cells (MCF-7); resistant to Rapamycin and its analogues, mTOR-inhibitors resistant cells) | Inhibited cellular viability of mTOR resistant cells | Deactivated mTOR signaling pathway by downregulating protein expressions of mTOR, S6K1, and raptor | [36] |
| Human TNBC cells (MDA-231 and MDA-468) | (1) Inhibited cellular viability; and (2) Desensitized cells by blocking tumor resistant through downregulating expression of PD-L1, and CCL2 | Deactivated JAK/STAT axis signaling pathway by downregulating mRNA expression of MUC1, JAK1, and STAT3; deactivated NF-κB signaling pathway by downregulating protein expressions of NF-κB1 (p50), NF-κB2 (p52) and Nrf2 | [34] |
| Human colorectal cancer cells (HCT116, lack p53) | (1) Inhibited cellular viability; and (2) Induced apoptosis (early and late stages) by increasing ROS formation, and upregulating expression of antiapoptotic proteins (BID, BAX, cleaved caspase-8, cleaved caspase 9, and cleaved caspase 3 and cleaved PARP), and downregulating expression of antiapoptotic proteins (cIAP-1, cFLIP, XIAP, BCL-2 and surviving) | Activated CHOP and SP1-dependent TRAIL by upregulating expressions of proteins and mRNA of TRAIL-DR4 and TRAIL-DR5 (death receptors) and upregulating protein expressions of CHOP and SP1 (specificity protein 1) | [58] |
| Human colon adenocarcinoma cells (SW480) | (1) Inhibited cellular viability; and (2) Arrested cell cycle at G2/M phase | Deactivated Wnt/β-catenin signaling pathway by down-regulating protein expressions of β-catenin and expressions of β-catenin dependent genes (c-Myc, and cyclin D1) | [57] |
| Human colorectal cancer cells (HCT116) | (1) Inhibited cellular viability; (2) Arrested cell growth at G2/M phase; and (3) Induced autophagic cellular death by increasing level of LC3 proteins (LC3-II) and increasing autophagosomes | Activated JNK signaling pathway by increasing phosphorylation of JNK isomers (JNK1 and JNK2) and upregulating protein and mRNA expressions of p53 | [54] |
| Human colorectal cancer cells (SW620) | (1) Inhibited cellular viability; (2) Arrested cell cycle at S phase; and (3) Induced mitochondrial apoptosis by increasing number of apoptotic cells, and increasing ROS generation | Activated JNK signalling pathway by increasing phosphorylation of JNK and p38 | [53] |
| Human colorectal cancer cells (HCT116) (TSP50 expressing, 5-flourouracil- resistant) | Augmented the antiproliferative effect of 5-FU to inhibit cellular viability, induce apoptosis by increasing activity of caspase-3 and -9, upregulating expression of the proapoptotic protein BAX and downregulating expressions of stem-cell associated proteins (c-Myc and OCT4) | Blocked the activated TSP50-mediated by NF-κB signaling pathway through downregulation of protein expressions of cyclin E, TSP50 and NF-κB | [55] |
| Human colon cancer cells (HT-29 and SW-460) | Inhibited cellular viability through downregulating mRNA expression of the proliferative factor Ki67 | Deactivated STAT3 signaling pathway by downregulating protein expression of STAT3 and its upstream protein JAK2 | [60] |
| Human colorectal cancer cells (HCT116) | Inhibiting cellular viability | Reversed the inflammatory conditions by downregulating mRNA expression of IL-6 and TNF-α | [59] |
| Human colorectal adenocarcinoma cells (HT-29) and human colorectal carcinoma cells (HCT116) | (1) Inhibited cellular viability; and (2) Hindered cells migration and invasion | Blocked ADRβ2/EMT by downregulating mRNA expression of ADRβ2 and protein expression of matrix metalloproteases (MMP-2 and MMP-9 N-cadherin) | [56] |
| Lewis lung carcinoma cells (LLC) | (1) Inhibited cellular viability; and (2) Hindered cells invasion and migration by downregulating protein expression of snail and upregulating protein expression of E-cadherin | Deactivated mTOR signaling pathway by inhibiting phosphorylation of mTOR and S6K1 | [71] |
| Non-small-cell lung cancer (H460) | (1) Inhibited cellular viability; (2) Arrested cell cycle at G2/M phase by downregulating protein expression of cyclin D1 and CDK4; (3) Induced apoptosis by upregulating proapoptotic proteins (BAX and cleaved caspase-3) and downregulating expression of the antiapoptotic proteins BCL-2; (4) Inhibited cellular colonization; and (5) Hindered cells migration and invasion | Deactivated PI3K/Akt/mTOR signaling pathway by downregulating protein expression of Akt and mTOR, modulating EMT by upregulating E-cadherin with N-cadherin) and downregulating EMT promotion transcription factor (ZEB1) | [64] |
| Human gastric cancer 5-FU resistant cells (BGC-823/5-FU) | (1) Restored inhibitory effect of 5-FU on cellular viability, overcoming P-glycoprotein-mediated resistance by downregulating protein expression of P-glycoprotein as well as blocking P-glycoprotein efflux pump through increasing levels of accumulated intracellular Rh-123; (2) Augmented antiproliferative effect of 5-FU through inducing apoptosis (more increase in number of apoptotic cells); and (3) Arrested cell cycle growth at G1 phase | Deactivated Wnt/β-catenin signaling pathway by downregulating protein expression of β-catenin, mRNA expression of stem cell-associated genes (MDR1, CD44, ALDH1, OCT4, c-Myc), Wnt target genes (β-catenin, TCF4 and cyclinD1) and blocking complex formation of β-catenin/TCF4 | [73] |
| Human multiple myeloma cells (U266 and ARH-77) | (1) Inhibited cellular viability; and (2) Induced apoptosis by upregulating expression of proapoptotic proteins (cleaved caspase-3 and PARP) and downregulating expression of antiapoptotic proteins (BCL-2, BCL-xL, survivin, XIAP, cIAP-1 and cIAP-2) | Deactivated NF-κB signaling pathway by blocking phosphorylation of NF-κB p65, downregulating protein expression of Iκβα and IKKβ and products of NF-κB regulating-genes (ICAM-1, COX-2, VEGF) | [67] |
| Mouse leukemia cells (WEHI-3) | (1) Inhibited cellular viability; (2) Arrested cell cycle at G0/G1 phase; and (3) Induced mitochondria-dependent apoptosis (early and late stage) by increasing accumulation of mitochondrial ROS, upregulating expression of proapoptotic proteins (BAX, cytochrome c, AIF, Endo G, Apaf-1 and cleaved PARP), downregulating expression of antiapoptotic proteins (BCL-2), upregulating mRNA expressions of DAP, TMBIM4 transmembrane, ATG5, and downregulating mRNA expression of DDIT3, DDIT4, BAG6, BCL-2 L13 and BRAT1 | Deactivated mitochondria-dependent and ER stress signaling pathways | [66] |
| Lymphoma cells (SUDHL-4 and OCI-Ly7) | Inhibited cellular viability | Deactivated mTOR signaling pathway by inhibiting phosphorylation of mTORC1 and its downstream substrate S6K1 and downregulating expression of raptor | [65] |
| Human prostate tumor cell line (PC-3) | (1) Inhibited cellular viability; and (2) Induced apoptosis by increasing DNA fragmentation | Deactivated NF-κB signaling pathway by downregulating mRNA expression of NF-κB1 | [50] |
| Human androgen independent prostate cancer cells (DU145) | (1) Inhibited cellular viability by down regulating expression of complex cell cycle progression proteins (cyclin D1/CDK4, cyclin E/CDK2), and down regulating mRNA expression of cyclin D1; (2) Induced apoptosis by upregulating expression of proapoptotic proteins (cleaved caspase 3 and cleaved PARP), downregulating expression of the antiapoptotic protein BCL-2 and upregulating protein expression of caspase-8 and 9; and (3) Hindered cells invasion and migration | Deactivated STAT3 signaling pathway by decreasing phosphorylation of STAT3, suppressing activity of STAT3 to bind DNA, nuclear pool depletion of STAT3, while downregulating expression of upstream STAT3 kinase (JAK2) and downregulating protein expression of oncogenes products (BCL-xL, BCL-2, XIAP, VEGF, COX-2, and MMP-9) | [49] |
| Human melanoma cell (A375) | (1) Inhibited cellular viability; (2) Induced apoptosis by upregulating expression of proapoptotic proteins (cleaved caspase-3 and cleaved PARP); and (3) Hindered cells invasion | No signaling pathway was reported | [18] |
| (1) Inhibited cellular viability; (2) Induced apoptosis by upregulating pro-apoptotic proteins expression (BAX, cleaved PARP, cleaved caspase 9, cleaved caspase 8) and downregulating expression of the antiapoptotic proteins BCL-2; and (3) Inhibited cells migration and invasion | Deactivated NF-κB signaling pathway by downregulating protein expression of p65 NF-κB | [70] | |
| Human epithelial ovarian cancer cells (SKOV3 and CoCl2-mimicked hypoxic cells) | (1) Inhibited cellular viability; and (2) Anti-angiogenesis and inhibited HIF 1 by downregulating mRNA and protein expressions of VEGF of HIF-1α and HIF-2α, respectively | Deactivated mTOR by downregulating protein expression of mTOR and S6K1 | [47] |
| Human epithelial ovarian cancer cells (SKOV3, cisplatin sensitive) | (1) Augmented the antiproliferative effect of cisplatin through inhibiting cellular viability, arresting cells growth at G2/M phase, and inducing apoptosis by downregulating protein expression of anti-apoptotic proteins (BCL-2, XIAP and survivin); and (2) Augmented antimetastatic effect of cisplatin through inhibiting cellular colonization | Deactivated mTOR signaling pathway by inhibiting phosphorylation of mTOR and its downstream target S6K | [72] |
| (1) Inhibited cellular viability; (2) Induced autophagy by increasing autophagosomes and upregulating protein expression of an autophagy marker LC3-II; and (3) Induced apoptosis by upregulating expression of proapoptotic proteins (cleaved PARP and cleaved caspase-3) | Deactivated mTOR signaling pathway by downregulating protein expression of raptor and decreasing phosphorylation level of S6K1 | [44] | |
| (1) Induced autophagy by evidenced by upregulating protein expression of autophagy markers (LC3-II and LAMP1); and (2) Metabolic programming by decreasing lactate secretion and inhibiting activity of hexokinase and lactate dehydrogenase | Deactivated mTOR signaling pathway by deactivating phosphorylation of mTOR and S6K1 | [45] | |
| Inhibited cellular viability | Deactivated mTOR signaling pathway by inhibiting phosphorylation of mTOR and S6K1, while downregulating protein expression of raptor | [47] | |
| Human epithelial ovarian cancer cells (SKOV3) | (1) Inhibited cellular viability; and (2) Induced autophagy by increasing number of autophagosomes and upregulation protein expression of LC3II (an autophagy marker) | Deactivation mTOR signaling pathway by inhibiting phosphorylation of mTOR and S6K1 through upregulating mRNA expression of DAP1 that negatively regulated autophagy | [41] |
| (1) Inhibited cellular viability; (2) Arrested cell cycle at G2/M phase by upregulating expressions of cyclin D1, cyclin B1 and p-histone H3 with downregulating expressions of cyclin A, p-cdc2 and Myt1; (3) Induced apoptosis (early and late) by downregulating expression of antiapoptotic proteins (MCL-1, BCL-2) and upregulating expressions of proapoptotic proteins (BAX, and cleaved caspase 3); and (4) Inhibited cellular colonization | Deactivated mTOR signaling pathway by downregulating protein expressions of mTOR, PRAS40, raptor and RagC, in association with deactivating NF-κB signaling pathway by downregulating protein expression of NF-κB, IKKα and IKKβ | [43] | |
| Paclitaxel-resistant ovarian cancer cells (SKOV3-Taxol) | Enhanced the antiproliferative effect of paclitaxel through enhancing inhibiting cellular viability, arresting cell cycle at G2/M phase, and inducing apoptosis by downregulating mRNA expression of MDR1 and protein expression of P-gp | Deactivated NF-κB signaling pathway by downregulating protein expression of p65 NF-κB | [40] |
| Ovarian cancer cells (SKOV3) with induced TAMs | (1) Inhibited cellular viability by inhibiting M-2 polarization of TAMs through downregulating protein expressions of pro-tumorigenic factors (MMP2 and MMP9), and mRNA expressions of IL-6 and VEGF-α; and (2) Hindered cells migration and invasion | Deactivation mTOR signaling pathway by inhibiting phosphorylation of mTOR and S6K1 and downregulating expression of raptor; deactivated STAT3 signaling pathway by inhibiting phosphorylation of STAT3 | [39] |
| Human ovarian cancer SKOV3 and A2780 cells | (1) Inhibited cellular viability of both cell lines; (2) Inducing oxidative stress through increasing liberation of cellular ROS (little stronger in SKOV3 than that in A2780); and (3) Hindered SKOV3 cells migration through inducing ROS liberation by partial abolishing the reactive oxygen scavenger NAC | Deactivated mTOR signalling pathways pathway by inhibiting phosphorylation of S6K1 at Thr389 and mTORC1 at Ser2448 with downregulating expression of raptor and p-ERK1/2 | [48] |
| Human ovarian SKOV3 and A2780 cells | (1) Inhibited cellular viability; (2) Induced apoptosis by reducing MMP (inducing mitochondrial damage) and downregulating mRNA expression and proteins of SREBP1, FASN, ACC and ACLY; (3) Inhibited activity of CPT-1 (i.e., inhibited lipogenesis); and (4) Inhibited cellular colonization | Deactivated mTOR signalling pathway via raptor by inhibiting phosphorylation of mTOR, S6K1, and 4E-BP1 and expression of raptor | [42] |
| Pancreas cancer cells (PANC-1 and SW1990) | (1) Augmented the antiproliferative effect of gemcitabine through inhibiting cellular viability, inducing apoptosis by upregulating expression of pro-apoptotic proteins (BAX and cleaved caspase 3), while downregulating expression of the anti-apoptotic protein BCL-2; and (2) Augmented inhibitory effect of gemcitabine on cellular colonization | Deactivated mTOR signaling pathway by inhibiting the phosphorylation of PI3K, AKT, and mTOR; modulated FOXO3a-FOXM1 axis by upregulating expression of FOXO3a and downregulating expression of FOXM1 | [63] |
| Human pancreatic cancer cell line (BxPC3). | (1) Inhibited cellular viability; (2) Induced DNA damaged by enhancing chromatin condensation, nuclear shrinkage and fragmentation though increasing formation of γ-H2AX (one of the histone family member X); (3) Inducing apoptosis by upregulating expression of the pro-apoptotic protein Bax and downregulating anti-apoptotic protein BCL-2, involving iintrinsic or extrinsic pathways as evidenced by increasing expression of cleaved caspase-3 and cytochrome C; and (4) Inhibited cellular colonization | No signaling pathway was reported | [62] |
| Human esophageal cancer cells (EC-9706 and TE10) | (1) Inhibited cellular viability by downregulating protein expression of the proliferative marker PCNA; (2) Induced apoptosis by increasing dense chromatin-containing cells, proportion of apoptotic cells (at early and late stages), upregulating pro-apoptotic proteins expression (BAD, BAX, cleaved PARP, and cleaved caspase-3) and downregulating expression of anti-the apoptotic protein BCL-2; (3) Inhibited cellular colonization; and (4) Hindered cells invasion and migration through inversing EMT by upregulating protein expression of E-cadherin and downregulating protein expression of N-cadherin, vimentin, snail, MMP2, MMP7, and MMP9 | Deactivated PI3K/AKT/mTOR signaling pathway by inhibiting phosphorylation and downregulation of the expression of PI3K and its downstream effector AKT | [30] |
| Human osteosarcoma cells (143B and MG63) | (1) Inhibited cellular viability; (2) Arrested cell cycle at G2/M phase by downregulating expression of cell cycle-related proteins (cyclin D1, c-Myc, and PCNA); (3) Induced apoptosis by upregulating expression of proapoptotic proteins (BAX, BAD, cleaved caspase 3, and cleaved PARP) and downregulating expression of the antiapoptotic protein BCL-2; and (4) Hindered cells invasion and migration by reversing EMT process through downregulating protein expressions of MMP-2, N-cadherin, snail and vimentin | Activated JNK signaling pathway by increasing phosphorylation of p38 MPAK and JNK | [31] |
| Human glioblastoma stem cells (CD133+ GSCs) | (1) Inhibited cellular viability by downregulating cell cycle regulator protein (cyclin D1), and VEGF; and (2) Induced apoptosis by upregulating protein expression of intrinsic apoptosis pathway (caspases 3, caspases 9, and PARP), and downregulating expression of antiapoptotic proteins (BCL-2, BCL-xL, MCL-1, surviving) | Deactivated STAT3 signaling pathway by decreasing phosphorylation level of STAT3, and downregulating expressions of STAT3 downstream proteins (BCL-xL, BCL-2, MCL-1, survivin, and VEGF) | [69] |
| Human bladder cancer cells (T24 and UM-UC-3) | (1) Iinhibited cellular viability; (2) Inducing apoptosis (no evidence); (3) Blocked glycolysis by decreasing glucose uptake, ATP generation and lactate production; and (4) Induced oxidative stress by increasing liberation of cellular ROS through upregulating Nrf2 and NQO1genes | Deactivated PI3K/AKT/mTOR pathway by reducing phosphorylation of PI3K, AKT and mTOR | [51] |
| Human bladder cancer cells (HT1376 and HTB-9) | (1) Inhibited cellular viability; (2) Arrested cell cycle growth by blocking transition of G0/G1 phase to S phase; and (3) Induced apoptosis by increasing cellular apoptotic rate and upregulating protein expression of ESR1 | Deactivated PI3K/AKT/mTOR signaling pathway by downregulation expression of ESR1 | [52] |
| Cancer type | Model | Cancer induction | Dosage | Mechanism | Ref. |
| Liver | Athymic nude mice | HepG2-xenograft | Single oral daily of 25 mg/kg or 50 mg/kg for 24 days | Deactivated NF-κB signalling pathway by downregulating protein expression of PCNA, Ki-67 NF-κB-p65 and Ikkβ | [29] |
| Breast | BALB/c female mic | 4T1-pcDNA3-TSP50-luc -xenograft | Single IP dose of 100 μg/50 μL every-three-days for 15 days | Inhibited overexpression of endogenous TSP50 | [35] |
| Athymic nude mice | SUM190-xenograft | Single IP dose of 25 mg/kg every-other-day for 17 days with doxorubicin | Abolished doxorubicin-enriched CSCs through downregulating mRNA expression of CSCs-associated genes (ALDH1, SOX2, OCT4, NANOG) | [38] | |
| Athymic nude mice | SUM190 and SUM149-xenograft | Single IP dose of 30 mg/kg every-other-day for 20 days | Blocked TLR3 on the induced-tumorigenic cancer stem cells | [27] | |
| Female nude mice | MDA-MB-231 cells xenograft | Single daily IP dose of 3 mg/kg for 28 days | Suppressed HIF-1α mediated-cell metabolism | [32] | |
| Female nude mice | MDA-MB-231 cells xenograft | Single IP dose of 30 mg/kg every-other-day for 28 days | Inducing apoptosis by upregulating expression of p21, p27, and Bim, and increasing the level of cleaved caspase 3, while cyclin D1 level decreased; activated FOXO3a-JNK signalling pathway by upregulating expressions of JNK and FOXO3a | [33] | |
| BALB/c mic | 4T1-xenograft | Single oral daily dose of 5 mg/kg for 30 days | Retarded tumour growth (no further mechanistic information) | [37] | |
| Colorectal | BALB/c nude mice | HT29-xenograft | Single IP dose of 25 mg/kg every-two-days per week for 28 days | Hindered metastasis to lungs by downregulating expression of ADRB2 and matrix metalloproteases (MMP-2 and MMP-9 N-cadherin) | [56] |
| C57BL/6 mice | Azoxymethane-induced | Single oral daily dose of 40 mg/kg every-other-day for 90 days | Downregulated expression Ki67; deactivated STAT signaling pathway by downregulating protein expression of JAK2, STAT1, STAT3 and STAT5 | [60] | |
| C57Bl/6 mice | Azoxymethane-induced | Single oral dose of 10 mg/kg five days per week for 30 weeks | Stopped colorectal cancer cells proliferation by downregulating Ki-67 index; deactivated Wnt/β-catenin and NF-κB signaling pathways; modulated expression of microRNAs | [53] | |
| C57Bl/6 mice | Azoxymethane-induced | Single oral dose of 10 mg/kg either from day 1 to day 140 or from day 50 to day 140 | Reduced risk of colitis-induced colon cancer by reducing tumors number, increasing colon shrinkage and alleviating inflammatory insult; blocked accumulation of immune cells; deactivated NF-κB and iNOS signalling pathways; protected mice against inflammation-associated colitis by modulating expression of microRNAs | [59] | |
| Lung (LLC cell) | C57BL/6 mice | LLC-xenograft | Single daily IP dose of 3.5 mg/kg, 7 mg/kg, or 10.5 mg/kg for 20 days | Reduced tumour size; hindered metastasis through inhibiting lung colonization of LLC cells | [71] |
| Lung (NSCLC) | BALB/c nude mice | H460-xenograft | Single daily IP dose of 5 mg/kg for 14 days | Downregulated expression of Ki-67 in lungs, and deactivated PI3K/Akt/mTOR signalling pathway by downregulating protein expression of Akt and mTOR | [64] |
| Gastric (5-FU-resistan) | BALB/c nude mice | BGC-823/5-FU xenograft | Single IP dose of 25 mg/kg twice per week for 30 days | Superior retarding tumour growth (no further mechanistic information) | [73] |
| Esophageal cancer | Male nude mice | EC9706 xenograft | Single oral dose of 5 mg/kg, 15 mg/kg, or 25 mg/kg every-three-days for 26 days | Downregulated protein expression of PCNA, BCL-2, vimentin, PI3K, and Akt; deactivated mTOR signalling pathway | [30] |
| Pancreas | Athymic BALB/c nude mice | PANC-1 and SW1990 xenograft | Single daily IP dose of 5 μg/kg for 42 days | Modulating FOXO3a/FOXM1 axis pathway through upregulating expression of FOXO3a; deactivated FOXM1/AKT/mTOR signalling pathway by lowering phosphorylation of PI3K, AKT, and mTOR | [63] |
| Ovarian | Female BALB/c nude mic | SKOV3 or a mix of SKOV3 and M2 macrophages xenograft | Single daily IP dose of 5 mg/kg or 25 mg/kg for 15 days | Downregulated protein expression of Ki67, CD163 and CD206; deactivated mTOR signalling pathway | [39] |
| BALB/c nude mice | SKOV3 and PDC xenograft | Single daily IP dose of 20 mg/kg for 13 days | Induced apoptosis; deactivated mTOR and NF-κB signaling pathways | [43] | |
| Female BALB/c nude mice | SKOV3 xenograft | Single oral daily dose of 15 mg/kg or 30 mg/kg for 20 days | Downregulated expression of Ki-67, raptor and FASN, activated mTOR signaling pathway | [42] | |
| Leukemia | Male BALB/c mice | WEHI-3 xenograft | 5 mg/kg, IP, once every two days for 14 days and 42 days | Improved animal survival after 42 days, decreased population of CD3 (T cells), CD11b (monocytes) and macrophages-3 after 14 days, increased population of CD19 (B cells), and enhanced macrophages phagocytic ability | [68] |
| Lymphoma | Female SCID mice | SUDHL-4 cells expressing mutant; RRAGC xenograft | Single oral daily dose of 15 mg/kg for 30 days | Sensitized RagCT90N-mutant highly to cardamonin | [65] |
| Osteosarcoma | Female BALB/c nude mice | 143B xenograft | Single oral dose of 5 mg/kg, 15 mg/kg, or 25 mg/kg every-two-days for 20 days | Deactivated mTOR signalling pathway, inhibited level of PCNA, BCL-2 and vimentin, and increased phosphorylation level of p38 MAPK and JNK | [31] |
In ovarian cancers, cardamonin has shown significant in vitro antiproliferative effects against ovarian cancer cell lines (SKOV3 and A2780) under various conditions through decreasing cellular viability, suppressing HIF-1 expression, arresting cells growth at G2/M phase, inducing apoptosis, inducing autophagy, modulating metabolic programming, inhibiting M-2 polarization of tumor-associated macrophages or inducing oxidative stress (Table 1)[39-48]. Consistently, cardamonin also inhibits the growth of ovarian-induced cancer in animal models through downregulating the protein expression of Ki-67, cluster of differentiation (CD) 163, and CD206 and inducing apoptosis (Table 2)[39,42,43].
In prostate cancer, in vitro studies reported that cardamonin exerted antiproliferative effects on the human prostate tumor cell line (PC-3) and human androgen-independent prostate cancer cells (DU145) through inhibition of cellular viability and induction of apoptosis (Table 1)[49,50]. Similarly, cardamonin also demonstrated antiproliferative effects against human bladder epithelium immortalized cells (SV-HUC-1) and human bladder cancer cells (HT1376 and HTB-9) through inhibition of cellular viability, cell cycle arrest at G0/G1 phase to S phase, induction of apoptosis, inhibition of glycolysis and induction of oxidative stress (Table 2)[51,52].
The in vitro studies have shown that cardamonin demonstrates antiproliferative effects against several colorectal cancer cell lines through inhibiting cellular viability and inducing apoptosis, as well as oxidative stress, arresting the cell cycle at G2/M phase and S phase, and inducing autophagy cellular death (Table 1)[53-60]. Meanwhile, the in vivo evidence indicated that cardamonin inhibited the tumor growth of colorectal-induced cancer in animal models through downregulating expression of the antiproliferative marker (Ki-67) and ameliorating the associated colitis (Table 2)[53,59,60]. Addi
Cardamonin also demonstrated in vitro antiproliferative effects against the human pancreatic cancer cell line (BxPC3) through inhibiting cellular viability and induction of apoptosis (Table 1)[62]. Similarly, the in vivo evidence indicated that cardamonin could potentially inhibit the growth of pancreatic tumors in an animal model with biologically-PANC-1 and SW1990 cells xenograft through modulating forkhead box O3a/forkhead box M1 axis pathway (Table 2)[63]. Addi
An in vitro study demonstrated that cardamonin suppressed the proliferation of Lewis lung carcinoma cells (LLC) through inhibition of cellular viability and suppression of the proliferation of non-small-cell lung cancer (H460) through cell cycle arrest at G2/M phase and induction of apoptosis (Table 1)[64]. Meanwhile, cardamonin suppressed the growth of LLC cell xenografts in an animal model through reduction of lung colonization of LLC and inhibited the growth of H460 cell xenografts through decreasing the proliferation marker Ki-67 (Table 2)[64].
In an in vitro study, cardamonin demonstrated antiproliferative effects against lymphoma cells (SUDHL-4 and OCI-Ly7) through inhibiting cellular viability[65]. Mouse leukemia cells (WEHI-3) also demonstrated inhibition of cellular viability, cell cycle arrest at G0/G1 phase and induction of apoptosis[66], and in human multiple myeloma cells (U266 and ARH-77) through inhibition of cellular viability and induction of apoptosis (Table 1)[67]. In an animal-based study, cardamonin inhibited the growth of murine acute myelomonocytic leukemia cells (WEHI-3)-xenograft and lymphoma cells (SUDHL-4)-xenograft through enhancing the phagocytic ability of macrophages, decreasing the population of CD3 (T cells), CD11b (monocytes), and macrophage surface antigen 3 (macrophages), while increasing the population of CD19 (B cells; Table 2)[65,68].
Some in vitro studies demonstrated that cardamonin possesses antiproliferative effect against human osteosarcoma cells (143B and MG63) through inhibiting cellular viability, arresting the cell cycle at G2/M phase and inducing apoptosis (Table 1)[31]. Meanwhile, cardamonin has also been shown to inhibit the growth of human osteosarcoma cells (143B)-xenograft through inhibiting the level of PCNA, B cell lymphoma 2, and vimentin while increasing the phosphorylation level of c-Jun N-terminal kinase (JNK) and p38 mitogen-activated protein kinase (Table 2)[31].
A single study has investigated the antitumor effects of cardamonin in glioblastoma, showing activity against human glioblastoma stem cells (CD133 + GSCs) by inhibiting cellular viability and inducing apoptosis[69]. In addition, two studies reported that cardamonin exerted antiproliferative effects against human melanoma cells (A375) through similar mechanisms of viability inhibition and apoptosis induction (Table 1)[18,70].
Some in vitro studies showed that cardamonin exerted an anti-metastatic effect through inhibiting the cellular coloniza
Cardamonin was shown to suppress the migration and invasion of different cancer cell lines, including human osteosarcoma cells (143B and MG63) by reversing epithelial-mesenchymal transition (EMT)[31], human esophageal cancer cells (EC-9706 and TE10) by inversing EMT[30], human ovarian cancer (SKOV3 and A2780) by inducing the liberation of reactive oxygen species[48], ovarian cancer cells (SKOV3; with induced tumour-associated macrophage)[39], human melanoma cells (A375)[70], human melanoma cells (A375)18], human androgen-independent prostate cancer cells (DU145)[49], non-small-cell lung cancer cells (H460)[64], LLC by downregulating the protein expression of snail, while upregulating the protein expression of E-cadherin[71], human colorectal adenocarcinoma cells (HT-29 and HCT116)[58], and triple-negative breast cancer cells (BT-549, estrogen receptor-negative) by blocking EMT (Table 1)[37].
In animal-based studies, cardamonin inhibited the metastasis of colorectal cancer cells (HT29) to lungs through down-regulating expression of adrenoceptor beta-2 and matrix metalloproteases[56], and LLC through inhibition of lung colonization of LLC cells (Table 2)[71].
Cardamonin augmented the anti-metastatic effects of cisplatin through inhibition of cellular viability and cellular colonization in human epithelial ovarian cancer cells (SKOV3, cisplatin sensitive; Table 1)[72]. In the context of therapy-resistant breast cancer, working on breast cancer cells with TLR3-activated CSCs suggested that cardamonin could block the tumorigenic-promoting effect of TLR3 to activate CSCs in breast cancer cells (Table 1)[27]. Additionally, this in vitro evidence was corroborated by an in vivo study, where the antitumor effects of cardamonin on induced CSCs in breast cancer xenograft models were abolished when TLR3 was blocked on the tumorigenic CSCs (Table 2)[27]. Similarly, the in vitro evidence from another study also showed that breast cancer cells tolerant to 5-fluorouracil (5-FU), doxorubicin, or paclitaxel supported the CSCs survival[38], which was reversed after a combination of 5-FU, doxorubicin, or paclitaxel with cardamonin, resulting in the inhibition of mammosphere formation and abolishing the self-renewal capacity of CSCs (Table 1)[38]. The aforementioned in vitro evidence was supported by the in vivo evidence from breast cancer-xenografted animals, showing that cardamonin with doxorubicin suppressed tumor growth and eliminated doxorubicin-enriched CSCs[38]. In addition, the in vitro evidence showed that rapamycin-resistant breast cancer cells [mammalian target of rapamycin (mTOR)-resistant breast cancer MCF-7 cells] were susceptible to the antiproliferative effect of cardamonin through exerting a potent inhibition of cellular viability (Table 1)[41].
The in vitro evidence from colorectal cancer cells (HCT116; deficient p53) showed that cardamonin augmented the antiproliferative effect of tumor necrosis factor-related apoptosis-inducing ligand against colorectal cancer by inhibiting cellular viability and inducing apoptosis[58]. In human colorectal cancer cells (HCT116; TSP50 expressing, 5-FU-resistant), the results from in vitro studies showed that cardamonin augmented the antiproliferative effect of 5-FU by enhancing the inhibition of cellular viability and induction of apoptosis[55]. Similarly, cardamonin augmented the antiproliferative effect of cisplatin or paclitaxel by inhibiting cellular viability, arresting cells growth at the G2/M phase and inducing apoptosis in SKOV3 cells (Table 1)[40,72].
Some in vitro studies demonstrated that cardamonin is able to restore the sensitivity of 5-FU-resistant human gastric cancer cells (BGC-823/5-FU) by enhancing the inhibitory effect of 5-FU on cellular viability, overcoming the P-glyco
The majority of in vitro and in vivo preclinical studies suggested that the anticancer effect of cardamonin involves the nuclear factor kappa-light-chain-enhancer of activated B cells NF-κB signaling pathway[29,35,43,50,55,61,67], while others suggested that its anticancer activity involves mTOR signaling pathway[36,39,42-48,71]. Other studies have indicated the anticancer activity of cardamonin through the involvement of signal transducer and activator of transcription 3 (STAT3) signaling pathway[34,49,60,69], Wnt/β-catenin signaling pathways[37,57,73], or mitochondria-dependent and estrogen receptor stress signaling pathways[66]. Additional preclinical studies suggest that cardamonin exerts its action through activation of the JNK signaling pathway[31,33,54]. Moreover, several studies indicated that cardamonin exerts its anticancer activity via a concurrent inhibition of the activated Wnt/β-catenin and NF-κB signaling pathways[53], NF-κB and mTOR signaling pathways[47], NF-κB and STAT3 signaling pathways or mTOR and STAT3 signaling pathways[38,39].
The preclinical studies also suggest other specific and promising strategies to be targeted by cardamonin for treating different types of cancers through inhibition of the overexpressed TSP50[35,55], modulating the metabolism of cancers cells through inhibition of HIF-1α[32], activation of forkhead box O3a/forkhead box M1 axis[33,63], modulating the expressions of microRNAs[53], blocking adrenoreceptor beta 2[56], activating C/EBP homologous protein and specificity protein 1-dependent tumor necrosis factor-related apoptosis-inducing ligand[58], reversing the inflammatory conditions[59], or eliminating the tumorigenic activity of CSCs[38].
Cardamonin is a naturally occurring bioactive chalcone with a broad spectrum of biological activities[74], and it has been suggested as a promising candidate to combat cancer via multiple cancer signaling pathways[13]. This review, hence, focuses on the preclinical anticancer activity of cardamonin, given the critical need to advance cancer research and identify new prospects for the development of future anticancer therapeutics. Additionally, investigating the anticancer activities of botanicals remains highly relevant, as approximately 25% of the prescribed medicines are still derived from plants[75]. Notably, 128 anticancer drugs were released on the market from 1981 to 2010, out of which 12 were natural products, while 32 were derivatives from natural products[76]. Furthermore, over 100 natural product-derived compounds are presently under clinical trials, with a similar number is in preclinical development[77]. Furthermore, the emergence of multidrug resistance to the available chemotherapeutics constitutes an extra motivation for researchers to explore alternative therapeutic options, particularly natural products’ compounds[78].
Cardamonin can be isolated from several plant species; however, the chemical laboratory synthesis of the compound has become more practical than plant isolation in terms of time, effort, and cost. Moreover, cardamonin can be isolated as a trans-isomer, which is more thermodynamically stable than the cis-one[23], and contributes to the predictability of its pharmacokinetic and pharmacodynamic properties[79-81]. The two factors together constitute preliminary pillars to support the feasibility of investigating the anticancer activity of cardamonin against several types of cancer.
The preclinical pharmacokinetic profile of cardamonin has been identified in several studies[15,18-20,82]. However, more studies are warranted to improve its bioavailability in the future[11], as the evidence suggests that cardamonin exhibits poor oral bioavailability (18%), where it undergoes several barriers before reaching systemic circulation[11,14,17]. Additionally, the evidence indicates that the parent molecule of cardamonin undergoes intestinal metabolism[16], which is considered a common absorption-limiting barrier for flavonoids[83]. This drawback of poor oral bioavailability suggests an alternative route of administration to a parenteral one[83]. This review therefore recommends evaluating the parenteral route for cardamonin in healthy animal models with direct comparison with the oral route. To enhance the bioavailability of cardamonin, nanoparticle-based formulations have been investigated as a promising strategy to overcome its pharmacokinetic limitations and potentially improve its anticancer efficacy. In this context, cardamonin was formulated into nanoparticles using Lycium barbarum polysaccharide as a natural nanocarrier, which facilitated sustained drug release and demonstrated efficacy against breast cancer[84]. Additionally, applying nanocarriers such as liposomes, polymeric nanoparticles and micelles could improve oral bioavailability of poorly soluble chalcones and flavonoids through enhancing their solubility and intestinal permeation as well as protecting them against gastrointestinal de
Evidence indicates that the parent molecule of cardamonin undergoes hepatic metabolism[15,19-21]. However, the metabolites of the parent molecule have yet to be identified, and this is critical where biotransformation renders the parent molecule pharmacologically toxic, active, or even inactive, and in many cases, these prototypes may not be the significant circulating compounds to show the effects after oral dosing[19]. Thus, identifying the circulating metabolites of cardamonin is essential in order to determine whether the prototype molecule or its metabolites are responsible for anticancer activity[19].
A study also indicates that cardamonin undergoes enterohepatic circulation and is mainly biliary excreted via faces[15,16]. Therefore, the former evidence is of high clinical importance, as it increases the concentration of cardamonin in the liver, which may enhance its anticancer activity against hepatic cancer or enhance the opportunity of hepatic adverse effects. These findings warrant further studies on cardamonin in liver cancer, particularly since it has the advantage of a high volume of distribution and is easily distributed into the liver[15].
The evidence indicates that cardamonin is readily distributed into the liver, kidney and lung, while its distribution is limited to the brain, heart and spleen[15], which could navigate the interest focusing into investigating the anticancer activity of cardamonin in cancers of the liver, lung and kidney rather than the spleen, brain and heart. This review also recommends further pharmacokinetic investigations, including the use of guinea pigs as an alternative animal model, as their metabolic profile more closely resembles that of humans. In contrast, mouse and rat hepatic microsomes generate the hydroxylated metabolite of cardamonin at only around 50% of human levels[15].
From the perspective of structure-activity relationship, inserting different substitutions on the aromatic rings and reducing the alkene linkage to inhibit the NF-κB signaling pathway directly decrease the anticancer activity of carda
From the viewpoint of preclinical safety, the evaluation of in vitro and in vivo toxicity is vitally paramount in predicting the possible adverse effects in humans after exposure to chemicals[88,89]. In addition, the evaluation of the toxicity of a compound in animal models is crucial in characterizing the potential result of its toxic effects and ensuring its safety profile prior to clinical applications[89]. Therefore, the limited availability of in vivo toxicity studies for cardamonin is highlighted in the literature to address the significant gap between the medicinally beneficial effects of cardamonin and the possible risks that could arise upon shifting the preclinical setting to human application. Accordingly, conducting further in vivo acute, subacute, and chronic toxicity studies of cardamonin using experimental animal models (male and female) are highly recommended. Numerous preclinical studies have been conducted to investigate the anticancer activities of cardamonin in cancer cells line and cancer-induced animals, and the collected evidence from this review suggests that cardamonin possesses substantial anticancer activity. However, the findings of this review indicate that most of the conducted studies are likely to support the anticancer activity of cardamonin against breast cancers[27,32,33,35-38], colorectal cancers[47,53-55,57-60] and ovarian cancers[39-41,44,47,72]. The former conclusion does not imply that the anticancer activities of cardamonin against other cancers (liver, prostatic, gastric, pancreatic, or leukemia) lack efficacy but rather reflect the current research emphasis and distribution within the available literature or most studies conducted. Nonetheless, it is noteworthy that this review did not evaluate the internal validity of the included studies. Rather, it aimed to synthesize existing evidence to identify theoretical and practical gaps, thereby informing future research directions on the anticancer potential of cardamonin. The major trends of anticancer activity of cardamonin from the conducted studies indicate that cardamonin has antiproliferative, anti-metastatic and chemosensitizing effects. This indicates that cardamonin may possess antiproliferative and anti-metastatic properties in naive tumors. Concurrently, it can be used with a standard chemotherapeutic agent to augment the anticancer activity or overcome the chemoresistance to those agents. This body of evidence diverges the efforts of the investigators to conduct studies depending on the accumulated evidence about the anticancer activity of cardamonin against a specific type of cancer. Hence, cardamonin has demonstrated broad anticancer activity in preclinical settings. However, a cross-comparative analysis can help prioritize which cancer types respond most effectively to cardamonin (Table 3). In liver cancer, the in vitro hepatocellular carcinoma model demonstrated a moderate half-maximal inhibitory concentration (IC50; 17.1 μmol/L)[61], but a robust in vivo inhibition (65%)[29], marking this evidence as particularly promising for further translational work. In breast cancer, the MDAMB231 cells showed a sub30 μmol/L IC50 and up to 50% tumor inhibition in an animal model[32,59], and this merits further in vivo priority investigation, while the 4T1-Luc cells in vitro model showed a high IC50 (86 μmol/L) despite substantial tumor suppression[35], suggesting limited cellular potency and a need for optimization. The nonsmall cell lung cancer (LLC) animal model showed strong in vivo inhibition (84%)[70], but the absence of matching in vitro IC50 data highlights a gap that must be addressed. Esophageal and ovarian carcinoma models consistently combined low micromolar IC50 values with substantial tumor suppression[30], supporting their classification as promising candidates. Finally, the A2780 ovarian cells model lacked in vivo validation, indicating a clear next step of animal testing before advancing[42]. Overall, this comparative framework helps prioritize hepatocellular, breast (MDAMB231), esophageal, and ovarian (SKOV3) indications for focused preclinical development. However, once sufficient studies (either in vitro or in vivo) focused on specific cancer types become available, researchers should aim to conduct a systematic review and meta-analysis dedicated to evaluating the anticancer activity, toxicity, and safety profiles of cardamonin in a more quantitative and rigorous manner. This will help generate precise evidence to support policymakers in making well-informed decisions.
| Cancer type | Cell studies | Animal studies | Future prospective | ||||
| Cells line | IC50 (μM) | Ref. | Induced cancer | Inhibition | Ref. | ||
| Liver | HepG2 cells | 17.1 | [61] | HepG2-xenograft | 65.2% | [29] | Promising |
| 53 | [35] | ||||||
| Breast | 4T1 Luc cells | 85.96 | [35] | 4T1 Luc-xenograft | Substantial (graphic presentation) | [35] | Not promising in vitro |
| MDA-MB-231 cells | 45.6 | [35] | |||||
| MDA-MB-231 cells | 25.5 | [32] | MDA-MB-231 xenograft | Mild (≤ 50%; graphic presentation) | [32] | Promising | |
| Lung | LCC cells | NR | [71] | LLC-xenograft | 84.3% | [71] | Needs in vitro testing |
| Esophageal | EC9706 cells | 8.7 | [30] | EC9706-xenograft | Substantial (graphic presentation) | [30] | Promising |
| Ovary | SKOV3 cells | 8.04 | [43] | SKOV3 and PDC xenograft | Substantial (graphic presentation) | [43] | Promising |
| PDC cells | 14.9 | ||||||
| SKOV3 cells | 23.4 | [42] | SKOV3-xenograft | Substantial (graphic presentation) | [42] | Promising | |
| A2780 cells | 28.8 | ||||||
A notable limitation in the current body of research is inconsistency or lack of proper selection of cancer cells line with limited consideration of the cancer stage and sensitivity of the cancer cells, especially to cardamonin, which suggests that study designs are often influenced by authors’ preferences for novelty, potentially affecting the translational value of the findings. Nevertheless, this review infers that the feasibility of investigating the combination of cardamonin with other standard chemotherapy in several malignancies would be rational to provide a strong basis for its clinical trials to determine whether cardamonin possesses the same antineoplastic effects in humans and the doses at which the compound is effective[11]. This review highlights the extensive recruitment of cancer cell lines for investigating the anticancer activity of cardamonin in contrast to the relatively limited number of animal-based studies. Thus, this review advocates the incorporation of animal models to complement human tumor cell lines to facilitate a more comprehensive understanding of the complex processes underlying cancer metastasis in living systems[90].
Preclinical pharmacokinetics and pharmacodynamics evidence indicate that advancing cardamonin as a viable anticancer agent for clinical application remains premature due to several experimental shortcomings. Notably, pursuing structural analogues prior to establishing the parent compound’s in vivo efficacy or identifying its bio-transformed metabolites is lacks scientific justification. Cardamonin’s poor oral bioavailability, as demonstrated in preclinical studies, suggests that alternative delivery routes (e.g., parenteral administration) or enhanced formulation may be required. The underlying molecular mechanisms of the anticancer activity of cardamonin are not fully elucidated, with current findings suggesting broad inhibition of multiple signaling pathways, including NF-κB, mTOR, STAT3, and Wnt/β-catenin, or a concurrent suppression of the activated Wnt/β-catenin and NF-κB, NF-κB and mTOR, NF-κB and STAT3, or mTOR and STAT3, which needs further mechanistic clarification. Safety data are also limited, with a lack of comprehensive in vivo toxicity studies, highlighting a considerable gap between the medicinal benefits of cardamonin and the possible risks in clinical contexts. Despite these gaps, cardamonin has shown promising antiproliferative, anti-metastatic, and chemo sensitizing effects, particularly against breast, colorectal, and ovarian cancers. However, the feasibility of investigating the combination of cardamonin with other standard chemotherapies may offer a more robust foundation for future clinical translation.
| 1. | Ng SH, Kaur P, Tan LLC, Koh MYH, Ho AHY, Hum A, Tan WS. Healthcare Expenditure Trajectories in the Last 5 Years of Life: A Retrospective Cohort Study of Decedents with Advanced Cancer and End-Stage Organ Diseases. Pharmacoecon Open. 2025;9:661-670. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 2. | Verma SK, Rai S, Sidhu VK, Chitara D, Kumar P. Overview and History Of Natural Products. In: Kesharwani RK, Kumar P, Keservani RK, editors. The Nature of Nutraceuticals: History, Properties, Sources, and Nanotechnology. 1st ed. New York: Apple Academic Press, 2025: 17. [DOI] [Full Text] |
| 3. | Hasnat H, Shompa SA, Islam MM, Alam S, Richi FT, Emon NU, Ashrafi S, Ahmed NU, Chowdhury MNR, Fatema N, Hossain MS, Ghosh A, Ahmed F. Flavonoids: A treasure house of prospective pharmacological potentials. Heliyon. 2024;10:e27533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 89] [Reference Citation Analysis (0)] |
| 4. | Kapoor B, Gulati M, Gupta R, Singh SK, Gupta M, Nabi A, Chawla PA. A Review on Plant Flavonoids as Potential Anticancer Agents. Curr Org Chem. 2021;25:737-747. [RCA] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 5. | Chunarkar-Patil P, Kaleem M, Mishra R, Ray S, Ahmad A, Verma D, Bhayye S, Dubey R, Singh HN, Kumar S. Anticancer Drug Discovery Based on Natural Products: From Computational Approaches to Clinical Studies. Biomedicines. 2024;12:201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 124] [Article Influence: 62.0] [Reference Citation Analysis (0)] |
| 6. | Makhija P, Handral HK, Mahadevan G, Kathuria H, Sethi G, Grobben B. Black cardamom (Amomum subulatum Roxb.) fruit extracts exhibit apoptotic activity against lung cancer cells. J Ethnopharmacol. 2022;287:114953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 7. | Wang Y, An J, Zhou J, Chang L, Zhang Q, Peng F. Hydroxysafflor yellow A: a natural pigment with potential anticancer therapeutic effect. Front Pharmacol. 2024;15:1495393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 8. | Firenzuoli F, Gori L, Crupi A, Neri D. [Flavonoids: risks or therapeutic opportunities?]. Recenti Prog Med. 2004;95:345-351. [PubMed] |
| 9. | Pyo Y, Kwon KH, Jung YJ. Anticancer Potential of Flavonoids: Their Role in Cancer Prevention and Health Benefits. Foods. 2024;13:2253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 10. | Adelusi TI, Du L, Chowdhury A, Xiaoke G, Lu Q, Yin X. Signaling pathways and proteins targeted by antidiabetic chalcones. Life Sci. 2021;284:118982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Ramchandani S, Naz I, Dhudha N, Garg M. An overview of the potential anticancer properties of cardamonin. Explor Target Antitumor Ther. 2020;1:413-426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Daimary UD, Parama D, Rana V, Banik K, Kumar A, Harsha C, Kunnumakkara AB. Emerging roles of cardamonin, a multitargeted nutraceutical in the prevention and treatment of chronic diseases. Curr Res Pharmacol Drug Discov. 2021;2:100008. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 13. | Bano S, Majumder A, Srivastava A, Nayak KB. Deciphering the Potentials of Cardamom in Cancer Prevention and Therapy: From Kitchen to Clinic. Biomolecules. 2024;14:1166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 14. | Nawaz J, Rasul A, Shah MA, Hussain G, Riaz A, Sarfraz I, Zafar S, Adnan M, Khan AH, Selamoglu Z. Cardamonin: A new player to fight cancer via multiple cancer signaling pathways. Life Sci. 2020;250:117591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 81] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 15. | Jaiswal S, Sharma A, Shukla M, Lal J. Gender-related pharmacokinetics and bioavailability of a novel anticancer chalcone, cardamonin, in rats determined by liquid chromatography tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2015;986-987:23-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Jaiswal S, Shukla M, Sharma A, Rangaraj N, Vaghasiya K, Malik MY, Lal J. Preclinical pharmacokinetics and ADME characterization of a novel anticancer chalcone, cardamonin. Drug Test Anal. 2017;9:1124-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Wu Y, Jing R, Jia G, Wang Y, Yu T, Zhang W. Measurement of Free and Glucuronidated Cardamonin in Rat Plasma and Bile Using UPLC-MS/MS and Its Application to a Pharmacokinetic and Bile Excretion Study. Chem Res Chin Univ. 2019;35:962-966. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Berning L, Scharf L, Aplak E, Stucki D, von Montfort C, Reichert AS, Stahl W, Brenneisen P. In vitro selective cytotoxicity of the dietary chalcone cardamonin (CD) on melanoma compared to healthy cells is mediated by apoptosis. PLoS One. 2019;14:e0222267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 19. | Dong F, Wang S, Yang A, Li Q, Wang Y, Dai L, Tao Y, Wei X, Zhang J. Systematic screening and characterization of cardamonin metabolites using UHPLC-Q-Exactive Orbitrap MS after oral administration to rats. Arab J Chem. 2020;13:8768-8782. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Zenger K, Agnolet S, Schneider B, Kraus B. Biotransformation of Flavokawains A, B, and C, Chalcones from Kava (Piper methysticum), by Human Liver Microsomes. J Agric Food Chem. 2015;63:6376-6385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 21. | He YQ, Yang L, Liu Y, Zhang JW, Tang J, Su J, Li YY, Lu YL, Wang CH, Yang L, Wang ZT. Characterization of cardamonin metabolism by P450 in different species via HPLC-ESI-ion trap and UPLC-ESI-quadrupole mass spectrometry. Acta Pharmacol Sin. 2009;30:1462-1470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Mehmood A, Sun Y, Chen X. Cardamonin: Advances on Resources, Biosynthesis Pathway, Bioavailability, Bioactivity, and Pharmacology. In: Xiao J, editor. Handbook of Dietary Flavonoids. Cham: Springer, 2023: 1-38. [DOI] [Full Text] |
| 23. | Gomes MN, Muratov EN, Pereira M, Peixoto JC, Rosseto LP, Cravo PVL, Andrade CH, Neves BJ. Chalcone Derivatives: Promising Starting Points for Drug Design. Molecules. 2017;22:1210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 231] [Cited by in RCA: 241] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 24. | Alshazly O, Abuo-Rahma GEA, Mohamed MFA, Abdel-Aziz M. Amide linked chalcone derivatives, a promising class of compounds with versatile biological effects. RSC Adv. 2025;15:19043-19068. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 25. | Srinivasan B, Johnson TE, Lad R, Xing C. Structure-activity relationship studies of chalcone leading to 3-hydroxy-4,3',4',5'-tetramethoxychalcone and its analogues as potent nuclear factor kappaB inhibitors and their anticancer activities. J Med Chem. 2009;52:7228-7235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 141] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 26. | Break MKB, Hossan MS, Khoo Y, Qazzaz ME, Al-Hayali MZK, Chow SC, Wiart C, Bradshaw TD, Collins H, Khoo TJ. Discovery of a highly active anticancer analogue of cardamonin that acts as an inducer of caspase-dependent apoptosis and modulator of the mTOR pathway. Fitoterapia. 2018;125:161-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 27. | Jia D, Yang W, Li L, Liu H, Tan Y, Ooi S, Chi L, Filion LG, Figeys D, Wang L. β-Catenin and NF-κB co-activation triggered by TLR3 stimulation facilitates stem cell-like phenotypes in breast cancer. Cell Death Differ. 2015;22:298-310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 28. | Voon FL, Sulaiman MR, Akhtar MN, Idris MF, Akira A, Perimal EK, Israf DA, Ming-Tatt L. Cardamonin (2',4'-dihydroxy-6'-methoxychalcone) isolated from Boesenbergia rotunda (L.) Mansf. inhibits CFA-induced rheumatoid arthritis in rats. Eur J Pharmacol. 2017;794:127-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (3)] |
| 29. | Badroon N, Abdul Majid N, Al-Suede FSR, Nazari V M, Giribabu N, Abdul Majid AMS, Eid EEM, Alshawsh MA. Cardamonin Exerts Antitumor Effect on Human Hepatocellular Carcinoma Xenografts in Athymic Nude Mice through Inhibiting NF-κβ Pathway. Biomedicines. 2020;8:586. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 30. | Wang Z, Liu H, Hu Q, Shi L, Lü M, Deng M, Luo G. Cardamonin inhibits the progression of oesophageal cancer by inhibiting the PI3K/AKT signalling pathway. J Cancer. 2021;12:3597-3610. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 31. | Zhang L, Yang C, Huang Y, Huang H, Yuan X, Zhang P, Ye C, Wei M, Wang Y, Luo X, Luo J. Corrigendum to "Cardamonin inhibits the growth of human osteosarcoma cells through activating P38 and JNK signaling pathway" 134 (February) (2021) 111155. Biomed Pharmacother. 2025;182:117777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 32. | Jin J, Qiu S, Wang P, Liang X, Huang F, Wu H, Zhang B, Zhang W, Tian X, Xu R, Shi H, Wu X. Cardamonin inhibits breast cancer growth by repressing HIF-1α-dependent metabolic reprogramming. J Exp Clin Cancer Res. 2019;38:377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 172] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 33. | Kong W, Li C, Qi Q, Shen J, Chang K. Cardamonin induces G2/M arrest and apoptosis via activation of the JNK-FOXO3a pathway in breast cancer cells. Cell Biol Int. 2020;44:177-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 34. | Mendonca P, Kaur S, Kirpal B, Soliman KF. Cardamonin anticancer effects through the modulation of the tumor immune microenvironment in triple-negative breast cancer cells. Am J Cancer Res. 2024;14:5644-5664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 35. | Mi XG, Song ZB, Sun LG, Bao YL, Yu CL, Wu Y, Li YX. Cardamonin inhibited cell viability and tumorigenesis partially through blockade of testes-specific protease 50-mediated nuclear factor-kappaB signaling pathway activation. Int J Biochem Cell Biol. 2016;73:63-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 36. | Niu P, Li J, Chen H, Zhu Y, Zhou J, Shi D. Antiproliferative effect of cardamonin on mTOR inhibitorresistant cancer cells. Mol Med Rep. 2020;21:1399-1407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 37. | Shrivastava S, Jeengar MK, Thummuri D, Koval A, Katanaev VL, Marepally S, Naidu VGM. Cardamonin, a chalcone, inhibits human triple negative breast cancer cell invasiveness by downregulation of Wnt/β-catenin signaling cascades and reversal of epithelial-mesenchymal transition. Biofactors. 2017;43:152-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 38. | Jia D, Tan Y, Liu H, Ooi S, Li L, Wright K, Bennett S, Addison CL, Wang L. Cardamonin reduces chemotherapy-enriched breast cancer stem-like cells in vitro and in vivo. Oncotarget. 2016;7:771-785. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 39. | Chen H, Huang S, Niu P, Zhu Y, Zhou J, Jiang L, Li D, Shi D. Cardamonin suppresses pro-tumor function of macrophages by decreasing M2 polarization on ovarian cancer cells via mTOR inhibition. Mol Ther Oncolytics. 2022;26:175-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 40. | Ding Q, Niu P, Zhu Y, Chen H, Shi D. Cardamonin inhibits the expression of P-glycoprotein and enhances the anti-proliferation of paclitaxel on SKOV3-Taxol cells. J Nat Med. 2022;76:220-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 41. | Nie X, Chen H, Niu P, Zhu Y, Zhou J, Jiang L, Li D, Lin M, Chen Z, Shi D. DAP1 negatively regulates autophagy induced by cardamonin in SKOV3 cells. Cell Biol Int. 2020;44:2192-2201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 42. | Niu P, Li D, Chen H, Zhu Y, Zhou J, Zhang J, Liu Y. Cardamonin suppresses mTORC1/SREBP1 through reducing Raptor and inhibits de novo lipogenesis in ovarian cancer. PLoS One. 2025;20:e0322733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 43. | Ruibin J, Bo J, Danying W, Jianguo F, Linhui G. Cardamonin induces G2/M phase arrest and apoptosis through inhibition of NF-κB and mTOR pathways in ovarian cancer. Aging (Albany NY). 2020;12:25730-25743. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 44. | Shi D, Niu P, Heng X, Chen L, Zhu Y, Zhou J. Autophagy induced by cardamonin is associated with mTORC1 inhibition in SKOV3 cells. Pharmacol Rep. 2018;70:908-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 45. | Shi D, Zhao D, Niu P, Zhu Y, Zhou J, Chen H. Glycolysis inhibition via mTOR suppression is a key step in cardamonin-induced autophagy in SKOV3 cells. BMC Complement Altern Med. 2018;18:317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 46. | Shi D, Zhu Y, Niu P, Zhou J, Chen H. Raptor mediates the antiproliferation of cardamonin by mTORC1 inhibition in SKOV3 cells. Onco Targets Ther. 2018;11:757-767. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 47. | Xue ZG, Niu PG, Shi DH, Liu Y, Deng J, Chen YY. Cardamonin Inhibits Angiogenesis by mTOR Downregulation in SKOV3 Cells. Planta Med. 2016;82:70-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 48. | Zhu Y, Wang S, Niu P, Chen H, Zhou J, Jiang L, Li D, Shi D. Raptor couples mTORC1 and ERK1/2 inhibition by cardamonin with oxidative stress induction in ovarian cancer cells. PeerJ. 2023;11:e15498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 49. | Zhang J, Sikka S, Siveen KS, Lee JH, Um JY, Kumar AP, Chinnathambi A, Alharbi SA, Basappa, Rangappa KS, Sethi G, Ahn KS. Cardamonin represses proliferation, invasion, and causes apoptosis through the modulation of signal transducer and activator of transcription 3 pathway in prostate cancer. Apoptosis. 2017;22:158-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 70] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 50. | Pascoal AC, Ehrenfried CA, Lopez BG, de Araujo TM, Pascoal VD, Gilioli R, Anhê GF, Ruiz AL, Carvalho JE, Stefanello ME, Salvador MJ. Antiproliferative activity and induction of apoptosis in PC-3 cells by the chalcone cardamonin from Campomanesia adamantium (Myrtaceae) in a bioactivity-guided study. Molecules. 2014;19:1843-1855. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 51. | Li P, Tang C, Fu D, Xu X, Ge J, Jia R. Cardamonin suppresses glycolysis and induces oxidative stress by inhibiting PI3K/AKT/mTOR pathway in bladder cancer cells. Trop J Pharm Res. 2023;22:1541-1546. [DOI] [Full Text] |
| 52. | Zhang P, Song D, Fang Z, Sun D, Wang L, Shi L, Gao L, Jiang X. Cardamomin Inhibits the Proliferation and Tumorigenesis of Bladder Cancer by ESR1 in PI3K/AKT Pathway. Biochem Genet. 2025;63:2946-2966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 53. | James S, Aparna JS, Paul AM, Lankadasari MB, Mohammed S, Binu VS, Santhoshkumar TR, Reshmi G, Harikumar KB. Cardamonin inhibits colonic neoplasia through modulation of MicroRNA expression. Sci Rep. 2017;7:13945. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 54. | Kim YJ, Kang KS, Choi KC, Ko H. Cardamonin induces autophagy and an antiproliferative effect through JNK activation in human colorectal carcinoma HCT116 cells. Bioorg Med Chem Lett. 2015;25:2559-2564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 55. | Lu S, Lin C, Cheng X, Hua H, Xiang T, Huang Y, Huang X. Cardamonin reduces chemotherapy resistance of colon cancer cells via the TSP50/NF-κB pathway in vitro. Oncol Lett. 2018;15:9641-9646. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 56. | Lu T, Zheng C, Fan Z. Cardamonin suppressed the migration, invasion, epithelial mesenchymal transition (EMT) and lung metastasis of colorectal cancer cells by down-regulating ADRB2 expression. Pharm Biol. 2022;60:1011-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 57. | Park S, Gwak J, Han SJ, Oh S. Cardamonin suppresses the proliferation of colon cancer cells by promoting β-catenin degradation. Biol Pharm Bull. 2013;36:1040-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 58. | Yadav VR, Prasad S, Aggarwal BB. Cardamonin sensitizes tumour cells to TRAIL through ROS- and CHOP-mediated up-regulation of death receptors and down-regulation of survival proteins. Br J Pharmacol. 2012;165:741-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 59. | James S, Aparna JS, Babu A, Paul AM, Lankadasari MB, Athira SR, Kumar SS, Vijayan Y, Namitha NN, Mohammed S, Reshmi G, Harikumar KB. Cardamonin Attenuates Experimental Colitis and Associated Colorectal Cancer. Biomolecules. 2021;11:661. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (1)] |
| 60. | Hou S, Yuan Q, Yu N, Liu B, Huang G, Yuan X. Cardamonin attenuates chronic inflammation and tumorigenesis in colon. Cell Cycle. 2019;18:3275-3287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 61. | Badroon NA, Abdul Majid N, Alshawsh MA. Antiproliferative and Apoptotic Effects of Cardamonin against Hepatocellular Carcinoma HepG2 Cells. Nutrients. 2020;12:1757. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 62. | Xu XW, Zheng JJ, Gao DS, Hu QR. Cardamonin Induces Apoptosis and Suppresses the Proliferation of BxPC3 Cells. Indian J Pharm Sci. 2022;84:62-68. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 63. | Sun H, Zhang N, Jin Y, Xu H. Cardamonin Promotes the Apoptosis and Chemotherapy Sensitivity to Gemcitabine of Pancreatic Cancer Through Modulating the FOXO3a-FOXM1 Axis. Dose Response. 2021;19:15593258211042163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 64. | Zhou X, Zhou R, Li Q, Jie X, Hong J, Zong Y, Dong X, Zhang S, Li Z, Wu G. Cardamonin inhibits the proliferation and metastasis of non-small-cell lung cancer cells by suppressing the PI3K/Akt/mTOR pathway. Anticancer Drugs. 2019;30:241-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 65. | Liu Y, Zhu Y, Chen H, Zhou J, Niu P, Shi D. Raptor mediates the selective inhibitory effect of cardamonin on RRAGC-mutant B cell lymphoma. BMC Complement Med Ther. 2023;23:336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 66. | Liao NC, Shih YL, Chou JS, Chen KW, Chen YL, Lee MH, Peng SF, Leu SJ, Chung JG. Cardamonin Induces Cell Cycle Arrest, Apoptosis and Alters Apoptosis Associated Gene Expression in WEHI-3 Mouse Leukemia Cells. Am J Chin Med. 2019;47:635-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 67. | Qin Y, Sun CY, Lu FR, Shu XR, Yang D, Chen L, She XM, Gregg NM, Guo T, Hu Y. Cardamonin exerts potent activity against multiple myeloma through blockade of NF-κB pathway in vitro. Leuk Res. 2012;36:514-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 68. | Liao NC, Shih YL, Ho MT, Lu TJ, Lee CH, Peng SF, Leu SJ, Chung JG. Cardamonin induces immune responses and enhances survival rate in WEHI-3 cell-generated mouse leukemia in vivo. Environ Toxicol. 2020;35:457-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 69. | Wu N, Liu J, Zhao X, Yan Z, Jiang B, Wang L, Cao S, Shi D, Lin X. Cardamonin induces apoptosis by suppressing STAT3 signaling pathway in glioblastoma stem cells. Tumour Biol. 2015;36:9667-9676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 70. | Yue Y, Liu L, Liu P, Li Y, Lu H, Li Y, Zhang G, Duan X. Cardamonin as a potential treatment for melanoma induces human melanoma cell apoptosis. Oncol Lett. 2020;19:1393-1399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 71. | Niu PG, Zhang YX, Shi DH, Liu Y, Chen YY, Deng J. Cardamonin Inhibits Metastasis of Lewis Lung Carcinoma Cells by Decreasing mTOR Activity. PLoS One. 2015;10:e0127778. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 72. | Niu P, Shi D, Zhang S, Zhu Y, Zhou J. Cardamonin enhances the anti-proliferative effect of cisplatin on ovarian cancer. Oncol Lett. 2018;15:3991-3997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 73. | Hou G, Yuan X, Li Y, Hou G, Liu X. Cardamonin, a natural chalcone, reduces 5-fluorouracil resistance of gastric cancer cells through targeting Wnt/β-catenin signal pathway. Invest New Drugs. 2020;38:329-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 74. | Gonçalves LM, Valente IM, Rodrigues JA. An overview on cardamonin. J Med Food. 2014;17:633-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 102] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 75. | Sharma P, Gupta K, Khandai SK, Malik S, Thareja S. Phytometabolites as modulators of breast cancer: a comprehensive review of mechanistic insights. Med Oncol. 2024;41:45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 76. | Newman DJ, Cragg GM. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J Nat Prod. 2012;75:311-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3569] [Cited by in RCA: 3219] [Article Influence: 229.9] [Reference Citation Analysis (0)] |
| 77. | Goel B, Jain SK. Semisynthesis: Bridging natural products and novel anticancer therapies. Eur J Med Chem Rep. 2024;12:100218. [DOI] [Full Text] |
| 78. | El makawy AI, Abdel-Aziem SH, Mohammed SE, Ibrahim FM, Abd El-Kader HA, Sharaf HA, Youssef DA, Mabrouk DM. Exploration of tumor growth regression of quinoa and chia oil nanocapsules via the control of PIK3CA and MYC expression, anti-inflammation and cell proliferation inhibition, and their hepatorenal safety in rat breast cancer model. Bull Natl Res Cent. 2024;48:7. [DOI] [Full Text] |
| 79. | Chhabra N, Aseri ML, Padmanabhan D. A review of drug isomerism and its significance. Int J Appl Basic Med Res. 2013;3:16-18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 94] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 80. | McConathy J, Owens MJ. Stereochemistry in Drug Action. Prim Care Companion J Clin Psychiatry. 2003;5:70-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 159] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 81. | Nguyen LA, He H, Pham-Huy C. Chiral drugs: an overview. Int J Biomed Sci. 2006;2:85-100. [PubMed] [DOI] [Full Text] |
| 82. | He W, Jiang Y, Zhang X, Zhang Y, Ji H, Zhang N. Anticancer cardamonin analogs suppress the activation of NF-kappaB pathway in lung cancer cells. Mol Cell Biochem. 2014;389:25-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 83. | González R, Ballester I, López-Posadas R, Suárez MD, Zarzuelo A, Martínez-Augustin O, Sánchez de Medina F. Effects of flavonoids and other polyphenols on inflammation. Crit Rev Food Sci Nutr. 2011;51:331-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 382] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 84. | Liu X, Guo Y, Wang X, Wang X, Gong T, Wang X, Xia Y, Zheng W, Guo Y, Han M. Preparation of cardamonin and IR780 Co-loaded on Lycium barbarum polysaccharide nanoparticles and anti-tumor efficacy evaluation. J Drug Deliv Sci Technol. 2024;99:106004. [RCA] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 85. | Smeu A, Marcovici I, Dehelean CA, Dumitrel SI, Borza C, Lighezan R. Flavonoids and Flavonoid-Based Nanopharmaceuticals as Promising Therapeutic Strategies for Colorectal Cancer-An Updated Literature Review. Pharmaceuticals (Basel). 2025;18:231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 86. | Ahmad S, Israf DA, Lajis NH, Shaari K, Mohamed H, Wahab AA, Ariffin KT, Hoo WY, Aziz NA, Kadir AA, Sulaiman MR, Somchit MN. Cardamonin, inhibits pro-inflammatory mediators in activated RAW 264.7 cells and whole blood. Eur J Pharmacol. 2006;538:188-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 65] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 87. | Roehrer S, Stork V, Ludwig C, Minceva M, Behr J. Analyzing bioactive effects of the minor hop compound xanthohumol C on human breast cancer cells using quantitative proteomics. PLoS One. 2019;14:e0213469. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 88. | Olson H, Betton G, Robinson D, Thomas K, Monro A, Kolaja G, Lilly P, Sanders J, Sipes G, Bracken W, Dorato M, Van Deun K, Smith P, Berger B, Heller A. Concordance of the toxicity of pharmaceuticals in humans and in animals. Regul Toxicol Pharmacol. 2000;32:56-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1359] [Cited by in RCA: 1270] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 89. | Gupta R, Polaka S, Rajpoot K, Tekade M, Sharma MC, Tekade RK. Importance of toxicity testing in drug discovery and research. In: Tekade RK, editor. Pharmacokinetics and Toxicokinetic Considerations. Cambridge (MA): Elsevier, 2022: 117-144. [DOI] [Full Text] |
| 90. | Katt ME, Placone AL, Wong AD, Xu ZS, Searson PC. In Vitro Tumor Models: Advantages, Disadvantages, Variables, and Selecting the Right Platform. Front Bioeng Biotechnol. 2016;4:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 371] [Cited by in RCA: 522] [Article Influence: 52.2] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/