Published online Nov 24, 2025. doi: 10.5306/wjco.v16.i11.110202
Revised: July 2, 2025
Accepted: October 10, 2025
Published online: November 24, 2025
Processing time: 174 Days and 13.5 Hours
Proteases are essential for homeostasis, and their primary function is proteolytic in extracellular and intracellular compartments. The deregulation of expression, abundance, and activity of proteases has been related to several pathologies, including cancer. This deregulation contributes to their pro-tumorigenic activity since they participate in the degradation of extracellular matrix components and adhesion molecules, and the activation of growth factors. However, some proteases, such as ADAM metallopeptidase with thrombospondin type 1 motif 8 and kallikrein-related peptidases 5 and 10, have emerged as tumor suppressors due to their antitumoral actions in specific cancer contexts. In this article, we discuss the antitumoral effects of ADAM metallopeptidase with thrombospondin type 1 motif 8, kallikrein-related peptidases 5 and 10 that have been described to date, suggesting their potential use as novel biomarkers and therapeutic targets in cancer.
Core Tip: In this article, we explore the published information on two proteases of the kallikrein family, kallikrein-related peptidases 5 and 10, and one metallopeptidase with a thrombospondin motif, ADAM metallopeptidase with thrombospondin type 1 motif 8, which are suggested to be both tumor suppressors and protumoral factors, depending on cancer context and stage.
- Citation: Palacios Serrato EG, Medina-Abreu KH, Oropeza-Martínez E, Jacinto-Alemán LF, Macías-Silva M, Tecalco-Cruz AC. ADAMTS-8 and kallikrein-related peptidases 10 and 5 proteases also have a tumor suppression role. World J Clin Oncol 2025; 16(11): 110202
- URL: https://www.wjgnet.com/2218-4333/full/v16/i11/110202.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i11.110202
Proteases belong to a large group of proteins with complex enzymatic functions, mainly performing the hydrolysis of peptides between amino acids in a protein, a process known as proteolysis. Living organisms must break down proteins to obtain amino acid units and synthesize the necessary biomolecules. Some proteins are catalytically inactive and are activated through proteolytic cleavage, revealing an important mechanism of biological regulation by proteases. Cell growth and remodeling processes also require protease participation[1]. In general, proteases are parts of molecular mechanisms that prevent protein accumulation; activate or inhibit the functions of their target proteins; change the localization of some proteins; regulate the release and/or activation of cytokines, growth factors, and peptide hormones; and facilitate cell adhesion and migration[2]. At the physiological level, the participation of proteases is relevant in embryonic development, morphogenesis, skin homeostasis, blood coagulation cascade, and intestinal function[3]. When the protein homeostasis regulated by proteases is inadequate, the organism can develop diseases, such as malignant tumors[1].
Proteases can be classified based on the functional groups located in their active sites, the type of reaction they catalyze, the nature and chemical properties of their catalytic sites, the structure of the protease, and their activation mechanisms. Human proteases include cysteine proteases, serine proteases, metalloproteases, aspartic proteases, and threonine proteases[1]. In humans, 24 matrix metalloproteinase proteins, 21 disintegrin and metalloproteinase (ADAM) proteins, and 19 disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS) proteins have been identified[4-6].
Histidine, glutamic acid, aspartic acid, and lysine are the most common residues found in the active site of metalloproteases[5]. Metalloproteases are characterized by having a metallic ion (Zn2+, Co2+, Mn2+, or Ni2+) coordinated with the amino acid side-chains and a single water molecule at their catalytic site[5]. The zinc-dependent metalloproteinases comprise a superfamily of proteinases, including matrix metalloproteinase proteins, ADAM, and ADAMTS. The ADAM protease structure contains a signal peptide, a pro-domain, a catalytic domain, a disintegrin domain, a cysteine-rich domain, an epidermal growth factor–like domain, a transmembrane domain, and a cytoplasmic tail. ADAMTS differs in the inclusion of a spacer domain and a thrombospondin type-like repeat domain, and the absence of a transmembrane domain[6,7]. In contrast, the active site of serine proteases involves a catalytic triad of serine, histidine, and aspartate[3].
The participation of proteases in cancer has been described as pro-tumorigenic for several years because the target proteins of many proteases are components of the extracellular matrix (ECM), promoting its remodeling and, subsequently, cell migration, invasion, and metastasis, one crucial hallmark of cancer[8-10]. However, some studies suggest that, depending on the cellular context and stage of cancer progression, some proteases could act as tumor suppressors[11]. This work discusses the roles of one metalloprotease [ADAM metallopeptidase with thrombospondin type 1 motif 8 (ADAMTS-8)] and two serine proteases [kallikrein-related peptidases (KLK) 5 and KLK10] in the context of cancer.
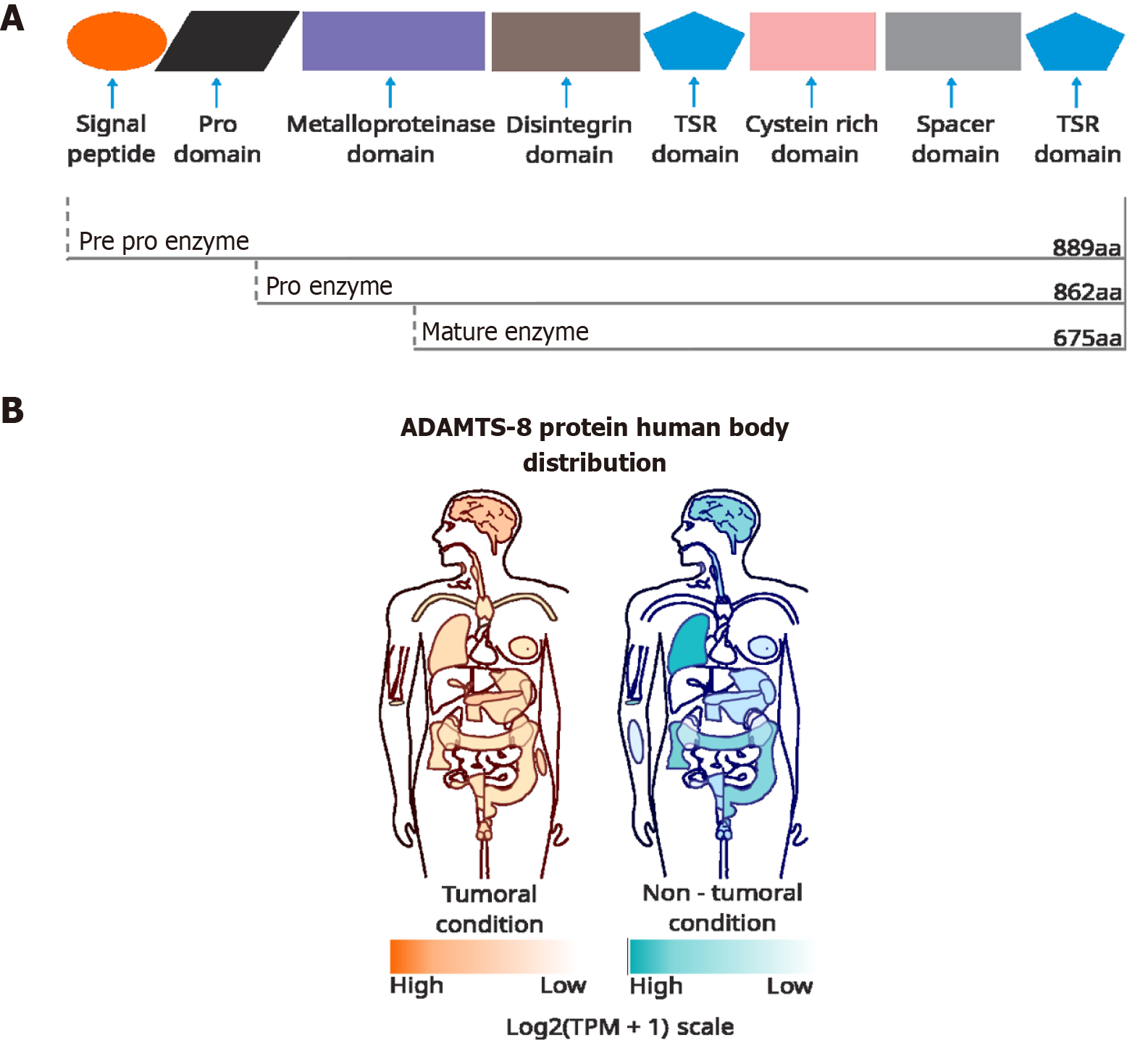
The protease ADAMTS-8 is mainly found in the extracellular space, with predominant expression in the lung and heart[7]. Similar to other members of the ADAMTS family, ADAMTS-8 is produced as a zymogen that consists of a pre-domain (a signal peptide) and a pro-domain that is cleaved by proprotein convertases such as furin, resulting in a mature protein[6]. The ADAMTS protein family includes 19 members and is characterized by ECM metalloproteases with at least one thrombospondin 1-like motif (TSR)[12]. ADAMTS-8 is composed of its respective pre- and pro-domains, a metalloproteinase domain, a disintegrin domain, and an auxiliary domain that comprises a central TSR, a cysteine-rich domain, a cysteine-free spacer domain, and a terminal TSR (Figure 1)[6].
Interestingly, ADAMTS-8 has been implicated in placental development and function, exhibiting strong placental expression in the early stages of gestation[13], and plays a crucial role in the remodeling of the ECM of stromal cells to decidua during pregnancy[14] and in the inhibition of angiogenesis through the inhibition of vascular endothelial growth factor[15]. The tissue inhibitor of metalloproteinase 3 can inhibit the proteolytic activity of ADAMTS-8[16].
In the context of cancer, ADAMTS-8 exhibits a negative correlation with various types of cancer. For instance, the downregulation of ADAMTS-8 has been reported in brain tumors compared to normal tissue[17]. The overexpression of ADAMTS-8 reduces the migration, viability, invasion, and epithelial-mesenchymal transition of glioma cells and reduces brain tumor growth in vivo. Furthermore, the high expression of ADAMTS-8 in primary glioma patients is associated with improved survival compared to patients with low expression and high-grade gliomas[18].
In the case of breast cancer (BC), several studies have found that ADAMTS-8 can have an antitumoral function. Hence, a low expression of ADAMTS-8 in early-stage BC patients correlates with an increased propensity to develop metastasis[19]. Likewise, ADAMTS-8 expression has been found to be lower in tissue derived from BC patients than in adjacent non-tumoral tissue. Additionally, an increase of ADAMTS-8 expression can inhibit proliferation and invasion of BC cells (MDA-MB-231) and induce apoptosis. These results are consistent with those found in in vivo experiments, where the overexpression of ADAMTS-8 reduced tumor growth, suggesting its possible activity as a tumor suppressor[20]. Moreover, ADAMTS-8 overexpression induced cell-cycle arrest, inhibiting BC cell (MDA-MB-231 and BT549) proliferation, migration, and invasion in vitro. ADAMTS-8 expression was higher in normal tissues than in breast tumor tissues by a mechanism associated with ADAMTS-8 gene promoter methylation[21]. In particular, in samples of patients with invasive ductal carcinoma (IDC) human epidermal growth factor receptor 2-positive (HER2+), the level of ADAMTS-8 was higher than in samples with IDC HER2- and in samples from breast fibroadenoma (non-cancerous tissue), suggesting that a positive relationship between ADAMTS-8 and HER2 expression could have a protumoral role in IDC[22].
Other studies have also indicated that the ADAMTS-8 promoter is methylated in non-small-cell lung tumors (NSCLC), glioblastoma, esophageal squamous cell cancer (ESCC), gastric cancer (GC), and colorectal carcinomas (CRC). Thereby, ADAMTS-8 is frequently downregulated in several cancer types via the methylation of the ADAMTS-8 promoter[17,23-25]. At the functional level, ADAMTS-8 typically acts as a tumor suppressor by inhibiting proliferation, migration, and invasion, inducing apoptosis and growth arrest in several tumors, including hepatocellular carcinoma[24,26], CRC[24,27], ESCC[24,28], and NSCLC[24,26]. Despite the downregulation of ADAMTS-8 and its effect as a negative modulator of cancer hallmarks in different cancer types, its molecular target and action mechanisms are still unclear.
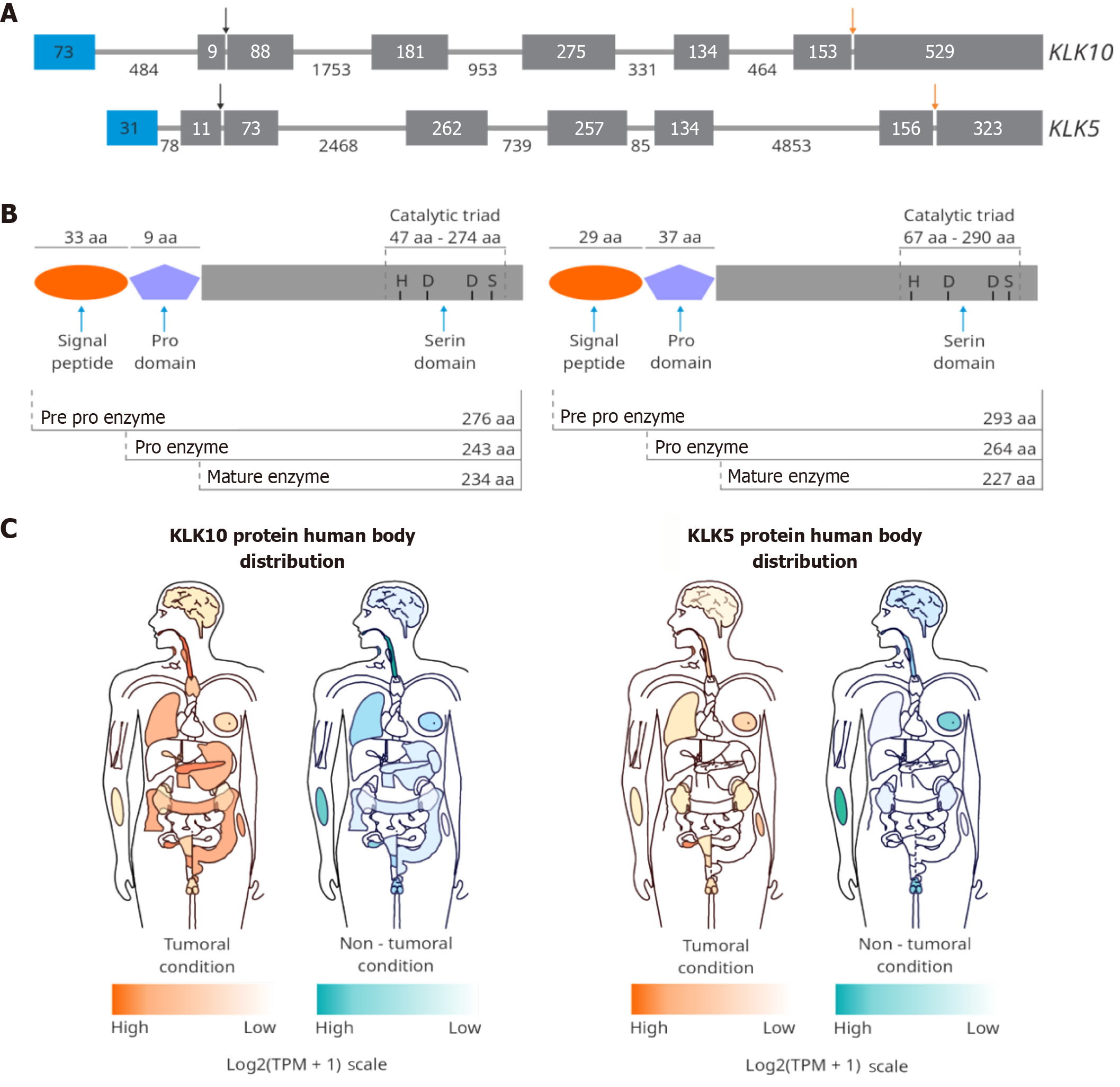
KLKs are members of another protease family. The KLK gene family comprises a cluster of 15 genes on chromosome 19q13.4, which have five conserved exons but differ in non-coding regions and the length of their intron sequences. Hence, the KLK family comprises 15 serine endopeptidases involved in various physiological processes[29]. The expression of KLK can be modulated by steroid hormones, such as androgens, progestins, and estrogens, as well as vitamin D, thyroid hormone, retinoic acid, epigenetic mechanisms, and microenvironment components, among others[30]. KLK genes encode a single-chain pre-pro-proteinase that contains a chymotrypsin or trypsin-like catalytic domain of 224-237 residues, having histidine, aspartic acid, and serine (the catalytic triad) in their catalytic domain[30,31]. All KLKs are synthesized as inactive pre-pro-enzymes and are translocated as inactive zymogens into the endoplasmic reticulum upon removal of the signal peptide. After secretion into the extracellular space, the pro-KLKs are activated by proteolytic release of a pro-peptide from the N-terminus, typically 4-9 amino acids in length. It has been proposed that KLKs can activate themselves[29].
The repertoire of substrates of the human KLK family is vast and still expanding, including hormones, growth factors, ECM proteins, cell receptors, adhesion molecules, and even other types of proteolytic enzymes[32]. The KLK family is involved in many pathological processes, such as inflammation, hypertension, and cancer[32]. In cancer, the KLK family has been related to malignancy promotion but also to the tumor suppression function[11,33-35]. Two KLK protease family members emerging as potential tumor suppressors are KLK10 and KLK5, which are discussed below.
The protein KLK10 is encoded by the KLK10 gene, also known as the normal epithelial cell-specific-1 gene (Figure 2). This protease has 276 amino acids, is N-glycosylated at Asn39 for its secretion as a pro-enzyme, and is activated through cleavage at the position Arg42. KLK10 contains a trypsin-like serine domain as a central domain and is considered a member of the chymotrypsin family of serine S1A peptidases. KLK10 is mainly found in the extracellular space[30,31]. A high expression of KLK10 is detected in the breast, ovary, testis, and prostate[29]. Hormonal stimuli, such as estrogen, androgen, and progestin, can modulate KLK10 expression[36].
KLK10’s role as a tumor suppressor has been evidenced in some cancer types, where the expression of this protease is downregulated (Table 1), and the mechanism responsible seems to be associated with DNA methylation. For instance, the downregulation of KLK10 has been related to hypermethylation in BC and prostate cancer (PCa)[37]. In PCa, overexpression of KLK10 reduces tumor proliferation and glucose metabolism while increasing apoptosis[38] and enhancing sensitivity to radiotherapy[39]. In addition, the reduction or loss of KLK10 mRNA expression correlates with the methylation of KLK10 in CpG islands observed in acute lymphoblastic leukemia[40,41], GC[42], and NSCLC[43].
| Cancer | Expression of KLK10 in the tumor compared with non-tumor conditions | Ref. |
| Breast cancer | Expression depends on stage, grade, and metastasis | [37,44,46-48] |
| Gastric cancer | Expression depends on stage, grade, and metastasis | [42,49,94] |
| Prostate cancer | Decreased | [37,38] |
| Esophageal cancer | Decreased | [52] |
| Acute lymphoblastic leukemia | Decreased | [40,41] |
| Testicular cancer | Decreased | [95] |
| Ovarian cancer | Decreased | [37] |
| Cutaneous melanoma | Increased | [55] |
| Colorectal cancer | Increased | [50,51] |
| Colon cancer | Increased | [96] |
| Pancreatic cancer | Increased | [53,54] |
Although KLK10 was identified as the first serine protease with an antitumoral function in BC, KLK10 seems to play a dual role in the tumorigenesis of mammary tissue. First, decreased KLK10 expression in tumor cell lines and primary breast tumors has been associated with its gene hypermethylation[33]. Furthermore, KLK10 expression is higher in normal breast tissue and benign lesions than in BC tissue, suggesting that KLK10 expression may be a molecular marker of BC[44]. In addition, a decrease in KLK10 expression has been reported in ductal carcinoma in situ and is associated with a high risk of invasive carcinoma[45]. Nevertheless, in BC trastuzumab-resistant cells, KLK10 was found to be elevated, displaying a worse prognosis than in patients with a lower expression of KLK10[46]. A similar scenario occurs in triple-negative BC, since high levels of KLK10 are related to lower survival[47,48]. These studies suggest that KLK10 has a dual role in BC tumorigenesis depending on the subtype of BC.
Protumoral activity of KLK10 has also been reported in different cancer types (Table 1). In GC, KLK10 expression promotes trastuzumab resistance by activating the phosphatidylinositol 3-kinase/protein kinase B pathway[49]. Another study showed that silencing KLK10 reduces cell viability and glucose metabolism and promotes apoptosis in CRC[50]. In this context, high expression of KLK10 reduces survival, mainly in patients with grade I and II cancers[51]. A higher KLK10 expression correlates with chemotherapy resistance using cisplatin in ESCC[52]. Altered KLK10 expression has also been associated with the development of pancreatic adenocarcinomas[53,54] and melanomas[55]. In all these cancers, high KLK10 expression is correlated with poorer survival outcomes. Thus, KLK10 acts as a tumor suppressor but also has a protumoral function depending on the cancer type, subtype, and progression stage.
KLK5 is a protein of 293 amino acids that requires glycosylation on Asn69 for its secretion (Figure 2)[31] and is located in the cytosol, the epidermal lamellar body[56], extracellular space, and secretory granules[57]. The expression of KLK5 is upregulated by estrogens and progestins[58] and inhibited by Zn2+[59]. KLK5 is detected in the skin and in all stratified epithelia[34,60] and is involved in skin desquamation during epidermal differentiation[61,62]. The breast, brain, and testis also express high levels of this protease[29].
KLK5 can self-activate, promote the activation of other KLKs (e.g., KLK7), and cleave ECM components (collagen, fibronectin, and laminin)[32,62]. The expression of KLK5 is lost in advanced-stage human skin cancer[63], but KLK5 downregulation has also been reported in hormone-dependent tumors[35,64-66] and tumors of the reproductive system[34,64,67,68].
In particular, KLK5 plays a role as a tumor suppressor in PCa, since KLK5 expression is decreased in these tumors by androgens and a higher KLK5 expression represents a better prognosis[35,58]. In vaginal tumors, KLK5 deficiency increases resistance to apoptosis[34]. In testicular cancer tissue, KLK5 expression is lower than in early-stage tumors[67], while in ovarian cancer (OC) cell lines, the co-expression of KLK5, 6, and 10 decreased colony formation in soft agar and tumorigenicity in nude mice[69].
In BC, KLK5 expression decreases in all subtypes compared to normal tissue[70-72]. It has been suggested that the oncogene GNA13 negatively regulates KLK5 gene transcription, promoting BC progression[73]. In triple-negative BC cells, overexpression of KLK5 suppresses key epithelial-mesenchymal transition genes, decreasing malignancy[66]. However, it has been suggested that the co-expression of KLK5 with the DSG1 and DSG3 genes is associated with the progression of triple-negative BC[65]. In this BC subtype, the silencing of the KLK5/7 and MFGE8 genes restored sensitivity to selective cyclooxygenase-2 inhibitors, such as celecoxib, significantly reducing primary tumor growth[74]. Additionally, high concentrations of the KLK5 protein have been found in the serum of patients with OC and BC compared with levels in the serum of healthy individuals[75]. These data indicate a duality of KLK5 function in mammary malignant tumorigenesis.
In contrast, KLK5 Levels are increased in uterine cervical cancer[76] and OC, increasing invasion and chemoresistance[77-80]. In gastric adenocarcinoma, patients with higher KLK5 expression were found to have shorter overall survival, enhanced tumor invasion, and nodal metastasis[81]. KLK5 is overexpressed and acts as a pro-tumorigenic factor in lung adenocarcinoma[82,83]. In primary bladder carcinoma, the expression of KLK5 promotes cell migration and invasion[84]. These investigations indicate a pro-tumorigenic role of KLK5 for some cancer types.
Furthermore, as in BC, in oral squamous cell carcinoma, KLK5 has both pro- and antitumor functions. Whereas the loss of KLK5 and KLK7 Leads to a poor clinical prognosis[85,86], the overexpression of KLK5 correlates with metastasis and the formation of more aggressive tumors[87-89] and, consequently, the silencing of KLK5 reduces tumor inflammatory infiltrate[90]. These data suggest that other molecular pathways may modulate the pro- and antitumor activity of KLK5 depending on cancer type (Table 2).
| Cancer | Expression of KLK5 in the tumor compared with non-tumor conditions | Ref. |
| Breast cancer | Expression depends on stage, grade, and metastasis | [65,66,70,74] |
| Oral squamous cell carcinoma | Expression depends on stage, grade, and metastasis | [88,89,97] |
| Vaginal carcinogenesis | Decreased | [34] |
| Prostate cancer | Decreased | [35] |
| Testicular cancer | Decreased | [67] |
| Ovarian cancer | Increased | [77,68] |
| Skin tumorigenesis | Increased | [61] |
| Urinary bladder carcinoma cells | Increased | [84] |
| Uterine cervical cancer | Increased | [76] |
| Lung cancer | Increased | [83] |
| Gastric adenocarcinoma | Increased | [81] |
Tissue remodeling is the reorganization or renewal of tissue structures that can occur during organ development and functional maturity, during wound healing responses, or as a pathogenic process in diseases such as arthritis, asthma, and cancer[1,2]. For this modification, changes in cell function and ECM structure as well as the presence and activation of differential proteases are necessary[2]. Proteases have been implicated in oncogenesis by regulating the proliferation of cancer cells, as extensive proliferation is often accompanied by tissue remodeling. In this context, proteases play critical roles by modulating cell–cell and cell-ECM communication[8,9].
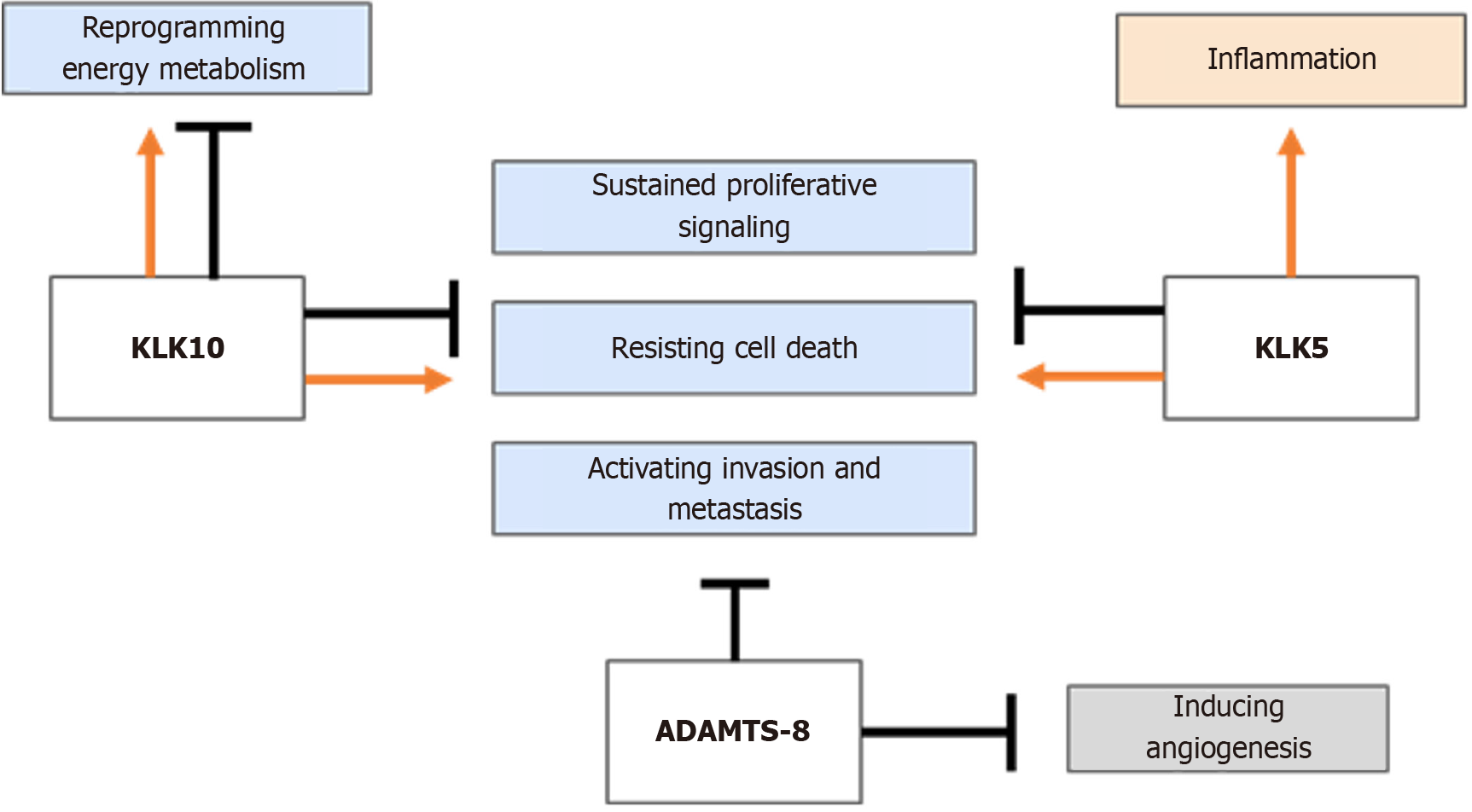
In general terms, proteases have been associated with protumor activity within the tumor microenvironment, thereby enhancing cancer progression. However, evidence indicating that some proteases can have antitumor activities is increasing. ADAMTS-8, KLK10, and KLK5 are three examples of proteases with antitumor functions in some malignant neoplasms (Figure 3). ADAMTS proteins have been reported to exhibit both antitumor and protumor effects, partly attributed to their dual role in angiogenesis[15,17,91]. However, ADAMTS-8 is primarily recognized as an antitumor protease. For instance, low expression of ADAMTS-8 has been associated with a poor prognosis in hepatocellular carcinoma patients, as there is evidence that basal levels of ADAMTS-8 inhibit proliferation and favor apoptosis[26]. It has been suggested that the ADAMTS-8 protease may carry out its antitumor functions by regulating the EGFR/ERK signaling pathway axis in certain cancers[21,24,26]. It has also been reported that ADAMTS-8 exhibits aggrecanase activity, which is involved in the degradation of the ECM in human articular cartilage, but its behavior in a cancer context is still unknown[7,12]. Depending on the cellular context and tumor malignancy, the enzymatic activities of ADAMTS-8 may be modulated differentially, causing it to have a dual role in BC.
Regarding KLK5/10, although they are two members of the KLK family, they differ in their tissue distribution and have different mechanisms of action and participation in physiological processes. Studies to establish connections between KLK10 and other proteases, including KLK5 and ADAMTS-8, especially in pathological contexts such as cancer, are still limited. Structurally, KLK5 and KLK10 are similar, but human KLK10 has a unique longer N-terminus, which may confer a differential role in the same cellular context, such as BC[92]. The oncogenic function of these proteases has been associated with the regulation of the phosphatidylinositol 3-kinase/protein kinase B signaling pathway in specific contexts[46,49,50,52]. Nevertheless, these proteases seem to have an antitumor role in several cancer types. For example, KLK5 can act as a tumor suppressor by repressing the mevalonate pathway in BC[66]. The targets and molecular mechanisms of these proteases, which function as either oncoproteins or tumor suppressors, have not been elucidated to date.
Interestingly, the downregulation of these three proteases in cancer is associated with DNA hypermethylation, a mechanism commonly employed by cancerous cells to silence several tumor suppressors. The tumor suppressor activity of these proteases probably occurs mainly in the early stages of cancer rather than in later stages. The molecular profile of each cancerous tissue type may also define the pro- or antitumor activity of these proteases. Because KLK5 and KLK10 can act as oncoproteins or tumor suppressors, depending on cancer type, these proteases display a dual role in BC. Since mammary malignant tumors are considered a neoplasia collection due to their high tissue and molecular heterogeneity, it is possible to understand that the activity of these KLKs is related to the stage and subtype of BC cells.
Therefore, the function of these proteases as tumor suppressors depends on the cancer stage or metastasis conditions, and they can be turned on or off depending on the tumor microenvironment (Table 3). Together, these data highlight that ADAMTS-8, KLK10, and KLK5 proteases can be considered bona fide tumor suppressors in some cancers.
| Cancer | Expression of ADAMTS-8 | Expression of KLK10 | Expression of KLK5 |
| Breast cancer | 1 | 1 | 1 |
| Prostate cancer | - | - | 2 |
| Testicular cancer | - | 2 | 2 |
| Vaginal cancer | - | - | 2 |
| Ovarian cancer | - | 2 | 3 |
| Gastric cancer | 2 | 1 | 3 |
| Brain cancer | 2 | - | - |
| Lung cancer | 2 | 2 | - |
| Colorectal cancer | 2 | 3 | - |
| Esophageal squamous cell carcinoma | 2 | - | - |
| Hepatocellular carcinoma | 2 | - | - |
| Esophageal cancer | - | 2 | - |
| Acute lymphoblastic leukemia | - | 2 | - |
| Skin cancer | - | 3 | 3 |
| Colon cancer | - | 3 | - |
| Pancreatic cancer | - | 3 | - |
| Oral squamous cell carcinoma | - | - | 1 |
| Urinary bladder carcinoma cells | - | - | 3 |
Despite these findings, the proteolytic targets of these proteases are not entirely known, and consequently, no common substrates have been identified for these proteases. However, the co-expression of KLK5 and KLK10 has been found in tumor cells of squamous cell carcinoma[89], suggesting that they may function cooperatively, participating in a common pathway and possibly sharing some molecular targets. Some tumor-suppressing actions of these proteases may likely occur in a proteolytic activity-independent manner. Therefore, the molecular mechanisms implicated in the tumor suppressor actions of these proteases remain to be investigated.
The mechanisms implicated in the dual role, both pro- and antitumor, of these proteases in a cell context–dependent manner are not clear. Nevertheless, it is known that, in cancer, there is a loss of fine control in the expression of these proteases. Additionally, the deregulation of these proteases may occur in terms of their abundance and activation. Hence, the levels and activity of their inhibitors, activators, and specific substrates, as well as the potential crosstalk between them, may contribute to their ultimate function, promoting or inhibiting tumor progression in particular contexts of cancer.
In conclusion, some proteases exhibit tumor suppressor activity. ADAMTS-8, KLK5, and KLK10 exhibit antitumor activity in malignant tumors and may serve as biomarkers and therapeutic targets in cancer. However, further research is required to detect these proteases in liquid biopsies and to develop assays for their detection. Further studies are also needed to elucidate the mechanisms of action, expression profiles, and activities of these proteases during the progression of specific cancers in order to design novel therapeutic strategies based on these proteases.
Eva G Palacios Serrato, Karen H Medina Abreu and Enrique Oropeza Martínez are postgraduate fellowship recipients from Secretaría de Ciencia, Humanidades, Tecnología e Innovación. We thank Norma Angélica Lira-Rodríguez, and Siankaan Harlem Ziu Lopez Mignon for their help with the figures.
| 1. | Gurumallesh P, Alagu K, Ramakrishnan B, Muthusamy S. A systematic reconsideration on proteases. Int J Biol Macromol. 2019;128:254-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 108] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 2. | Barrett AJ. Proteases. Curr Protoc Protein Sci. 2001;21:Unit 21.1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Hooper NM. Proteases: a primer. Essays Biochem. 2002;38:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Rivera S, Khrestchatisky M, Kaczmarek L, Rosenberg GA, Jaworski DM. Metzincin proteases and their inhibitors: foes or friends in nervous system physiology? J Neurosci. 2010;30:15337-15357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 196] [Cited by in RCA: 192] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 5. | Nagase H. Metalloproteases. Curr Protoc Protein Sci. 2001;21:21.4.1-21.4.13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Apte SS. A disintegrin-like and metalloprotease (reprolysin type) with thrombospondin type 1 motifs: the ADAMTS family. Int J Biochem Cell Biol. 2004;36:981-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 177] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 7. | Burkhard T, Minns AF, Santamaria S. Expression and Purification of Recombinant ADAMTS8. Methods Mol Biol. 2024;2747:55-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 8. | Góra J, Latajka R. Involvement of cysteine proteases in cancer. Curr Med Chem. 2015;22:944-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Mentlein R, Hattermann K, Held-Feindt J. Lost in disruption: role of proteases in glioma invasion and progression. Biochim Biophys Acta. 2012;1825:178-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 10. | Rao JS. Molecular mechanisms of glioma invasiveness: the role of proteases. Nat Rev Cancer. 2003;3:489-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 630] [Cited by in RCA: 648] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 11. | López-Otín C, Matrisian LM. Emerging roles of proteases in tumour suppression. Nat Rev Cancer. 2007;7:800-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 604] [Cited by in RCA: 617] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 12. | Collins-Racie LA, Flannery CR, Zeng W, Corcoran C, Annis-Freeman B, Agostino MJ, Arai M, DiBlasio-Smith E, Dorner AJ, Georgiadis KE, Jin M, Tan XY, Morris EA, LaVallie ER. ADAMTS-8 exhibits aggrecanase activity and is expressed in human articular cartilage. Matrix Biol. 2004;23:219-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 123] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 13. | Kentistou KA, Lim BEM, Kaisinger LR, Steinthorsdottir V, Sharp LN, Patel KA, Tragante V, Hawkes G, Gardner EJ, Olafsdottir T, Wood AR, Zhao Y, Thorleifsson G, Day FR, Ozanne SE, Hattersley AT, O'Rahilly S, Stefansson K, Ong KK, Beaumont RN, Perry JRB, Freathy RM. Rare variant associations with birth weight identify genes involved in adipose tissue regulation, placental function and insulin-like growth factor signalling. Nat Commun. 2025;16:648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 14. | Namli Kalem M, Kalem Z, Bakirarar B, Demircan K. Adamts 1, 4, 5, 8, and 9 in Early Pregnancies. Fetal Pediatr Pathol. 2017;36:387-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Vázquez F, Hastings G, Ortega MA, Lane TF, Oikemus S, Lombardo M, Iruela-Arispe ML. METH-1, a human ortholog of ADAMTS-1, and METH-2 are members of a new family of proteins with angio-inhibitory activity. J Biol Chem. 1999;274:23349-23357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 316] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 16. | Santamaria S, Martin DR, Dong X, Yamamoto K, Apte SS, Ahnström J. Post-translational regulation and proteolytic activity of the metalloproteinase ADAMTS8. J Biol Chem. 2021;297:101323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 17. | Dunn JR, Reed JE, du Plessis DG, Shaw EJ, Reeves P, Gee AL, Warnke P, Walker C. Expression of ADAMTS-8, a secreted protease with antiangiogenic properties, is downregulated in brain tumours. Br J Cancer. 2006;94:1186-1193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 77] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 18. | Zhou B, Liu Y, Ma G, Wang X, Chang B, Lu H, Feng X. ADAMTS8 inhibits glioma development in vitro and in vivo. Folia Neuropathol. 2023;61:144-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 19. | Li Y, Yang X, Sun J, Zhao Y, Zhou Q, Hua B. ADAMTS8 Expression is a Potential Prognostic Biomarker for Postoperative Metastasis in Lymph Node-Negative Early-Stage Invasive Breast Carcinoma Patients. Pharmgenomics Pers Med. 2021;14:1701-1713. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 20. | Zhang K, Tian R, Wang G, Zhang J, Ma H, Hu X, Xi J, Wang G. ADAMTS8 Inhibits Cell Proliferation and Invasion, and Induces Apoptosis in Breast Cancer. Onco Targets Ther. 2020;13:8373-8382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Zhang Q, Kanyomse Q, Luo C, Mo Q, Zhao X, Wang L, Peng W, Ren G. The Prognostic Value of ADAMTS8 and Its Role as a Tumor Suppressor in Breast Cancer. Cancer Invest. 2023;41:119-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 22. | Guo X, Li J, Zhang H, Liu H, Liu Z, Wei X. Relationship Between ADAMTS8, ADAMTS18, and ADAMTS20 (A Disintegrin and Metalloproteinase with Thrombospondin Motifs) Expressions and Tumor Molecular Classification, Clinical Pathological Parameters, and Prognosis in Breast Invasive Ductal Carcinoma. Med Sci Monit. 2018;24:3726-3735. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Dunn JR, Panutsopulos D, Shaw MW, Heighway J, Dormer R, Salmo EN, Watson SG, Field JK, Liloglou T. METH-2 silencing and promoter hypermethylation in NSCLC. Br J Cancer. 2004;91:1149-1154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 54] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | Choi GC, Li J, Wang Y, Li L, Zhong L, Ma B, Su X, Ying J, Xiang T, Rha SY, Yu J, Sung JJ, Tsao SW, Chan AT, Tao Q. The metalloprotease ADAMTS8 displays antitumor properties through antagonizing EGFR-MEK-ERK signaling and is silenced in carcinomas by CpG methylation. Mol Cancer Res. 2014;12:228-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 25. | Chen J, Zhang J, Li X, Zhang C, Zhang H, Jin J, Dai D. Downregulation of ADAMTS8 by DNA Hypermethylation in Gastric Cancer and Its Clinical Significance. Biomed Res Int. 2016;2016:5083841. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Zhao X, Yang C, Wu J, Nan Y. ADAMTS8 targets ERK to suppress cell proliferation, invasion, and metastasis of hepatocellular carcinoma. Onco Targets Ther. 2018;11:7569-7578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 27. | Li L, Yuan S, Zhao X, Luo T. ADAMTS8 is frequently down-regulated in colorectal cancer and functions as a tumor suppressor. Biochem Biophys Res Commun. 2020;524:663-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Wu Z, Shi Y, Ren S, Ju Y, Hu Y, Wu J. ADAMTS8 Inhibits Progression of Esophageal Squamous Cell Carcinoma. DNA Cell Biol. 2020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 29. | Yousef GM, Diamandis EP. The new human tissue kallikrein gene family: structure, function, and association to disease. Endocr Rev. 2001;22:184-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 156] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 30. | Kryza T, Silva ML, Loessner D, Heuzé-Vourc'h N, Clements JA. The kallikrein-related peptidase family: Dysregulation and functions during cancer progression. Biochimie. 2016;122:283-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 31. | Kalinska M, Meyer-Hoffert U, Kantyka T, Potempa J. Kallikreins - The melting pot of activity and function. Biochimie. 2016;122:270-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 86] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 32. | Nauroy P, Nyström A. Kallikreins: Essential epidermal messengers for regulation of the skin microenvironment during homeostasis, repair and disease. Matrix Biol Plus. 2020;6-7:100019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 33. | Goyal J, Smith KM, Cowan JM, Wazer DE, Lee SW, Band V. The role for NES1 serine protease as a novel tumor suppressor. Cancer Res. 1998;58:4782-4786. [PubMed] |
| 34. | Pampalakis G, Zingkou E, Sotiropoulou G. KLK5, a novel potential suppressor of vaginal carcinogenesis. Biol Chem. 2018;399:1107-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 35. | Yousef GM, Scorilas A, Chang A, Rendl L, Diamandis M, Jung K, Diamandis EP. Down-regulation of the human kallikrein gene 5 (KLK5) in prostate cancer tissues. Prostate. 2002;51:126-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 36. | Luo LY, Grass L, Diamandis EP. The normal epithelial cell-specific 1 (NES1) gene is up-regulated by steroid hormones in the breast carcinoma cell line BT-474. Anticancer Res. 2000;20:981-986. [PubMed] |
| 37. | Sidiropoulos M, Pampalakis G, Sotiropoulou G, Katsaros D, Diamandis EP. Downregulation of human kallikrein 10 (KLK10/NES1) by CpG island hypermethylation in breast, ovarian and prostate cancers. Tumour Biol. 2005;26:324-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 38. | Hu J, Lei H, Fei X, Liang S, Xu H, Qin D, Wang Y, Wu Y, Li B. NES1/KLK10 gene represses proliferation, enhances apoptosis and down-regulates glucose metabolism of PC3 prostate cancer cells. Sci Rep. 2015;5:17426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 39. | Hu J, Shen W, Qu Q, Fei X, Miao Y, Huang X, Liu J, Wu Y, Li B. NES1/KLK10 and hNIS gene therapy enhanced iodine-131 internal radiation in PC3 proliferation inhibition. Front Med. 2019;13:646-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 40. | Ahmad SM, Ahmed BS, Khidhir KG, Rahman HS. Prospective quantitative gene expression analysis of kallikrein-related peptidase KLK10 as a diagnostic biomarker for childhood acute lymphoblastic leukemia. PeerJ. 2022;10:e13489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 41. | Roman-Gomez J, Jimenez-Velasco A, Agirre X, Castillejo JA, Barrios M, Andreu EJ, Prosper F, Heiniger A, Torres A. The normal epithelial cell-specific 1 (NES1) gene, a candidate tumor suppressor gene on chromosome 19q13.3-4, is downregulated by hypermethylation in acute lymphoblastic leukemia. Leukemia. 2004;18:362-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 42. | Huang W, Zhong J, Wu LY, Yu LF, Tian XL, Zhang YF, Li B. Downregulation and CpG island hypermethylation of NES1/hK10 gene in the pathogenesis of human gastric cancer. Cancer Lett. 2007;251:78-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 43. | Zhang Y, Song H, Miao Y, Wang R, Chen L. Frequent transcriptional inactivation of Kallikrein 10 gene by CpG island hypermethylation in non-small cell lung cancer. Cancer Sci. 2010;101:934-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 44. | Dhar S, Bhargava R, Yunes M, Li B, Goyal J, Naber SP, Wazer DE, Band V. Analysis of normal epithelial cell specific-1 (NES1)/kallikrein 10 mRNA expression by in situ hybridization, a novel marker for breast cancer. Clin Cancer Res. 2001;7:3393-3398. [PubMed] |
| 45. | Yunes MJ, Neuschatz AC, Bornstein LE, Naber SP, Band V, Wazer DE. Loss of expression of the putative tumor suppressor NES1 gene in biopsy-proven ductal carcinoma in situ predicts for invasive carcinoma at definitive surgery. Int J Radiat Oncol Biol Phys. 2003;56:653-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 46. | Wang Z, Ruan B, Jin Y, Zhang Y, Li J, Zhu L, Xu W, Feng L, Jin H, Wang X. Identification of KLK10 as a therapeutic target to reverse trastuzumab resistance in breast cancer. Oncotarget. 2016;7:79494-79502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 47. | Liu Y, Gong W, Preis S, Dorn J, Kiechle M, Reuning U, Magdolen V, Dreyer TF. A Pair of Prognostic Biomarkers in Triple-Negative Breast Cancer: KLK10 and KLK11 mRNA Expression. Life (Basel). 2022;12:1517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 48. | Sahoo B, Pinnix Z, Sims S, Zelikovsky A. Identifying Biomarkers Using Support Vector Machine to Understand the Racial Disparity in Triple-Negative Breast Cancer. J Comput Biol. 2023;30:502-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 49. | Tang L, Long Z, Zhao N, Feng G, Guo X, Yu M. NES1/KLK10 promotes trastuzumab resistance via activation of PI3K/AKT signaling pathway in gastric cancer. J Cell Biochem. 2018;119:6398-6407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 50. | Wei H, Dong C, Shen Z. Kallikrein-related peptidase (KLK10) cessation blunts colorectal cancer cell growth and glucose metabolism by regulating the PI3K/Akt/mTOR pathway. Neoplasma. 2020;67:889-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 51. | Luo YC, Lv YL, He RX, Shi XX, Jiang T. Kallikrein-related peptidase 10 predicts prognosis and mediates tumor immunomodulation in colorectal cancer. Biochem Biophys Res Commun. 2023;689:149217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 52. | Li L, Xu N, Fan N, Meng Q, Luo W, Lv L, Ma W, Liu X, Liu L, Xu F, Wang H, Mao W, Li Y. Upregulated KLK10 inhibits esophageal cancer proliferation and enhances cisplatin sensitivity in vitro. Oncol Rep. 2015;34:2325-2332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 53. | Candido JB, Maiques O, Boxberg M, Kast V, Peerani E, Tomás-Bort E, Weichert W, Sananes A, Papo N, Magdolen V, Sanz-Moreno V, Loessner D. Kallikrein-Related Peptidase 6 Is Associated with the Tumour Microenvironment of Pancreatic Ductal Adenocarcinoma. Cancers (Basel). 2021;13:3969. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 54. | Zhang M, Jiang L, Liu XY, Liu FX, Zhang H, Zhang YJ, Tang XM, Ma YS, Wu HY, Diao X, Yang C, Liu JB, Fu D, Zhang J, Yu H. KLK10/LIPH/PARD6B/SLC52A3 are promising molecular biomarkers for the prognosis of pancreatic cancer through a ceRNA network. Heliyon. 2024;10:e24287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 55. | Chikhaoui A, Jones M, Režen T, Ben Ahmed M, Naouali C, Komel R, Zghal M, Boubaker S, Abdelhak S, Yacoub-Youssef H. Inflammatory landscape in Xeroderma pigmentosum patients with cutaneous melanoma. Sci Rep. 2022;12:13854. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 56. | Ishida-Yamamoto A, Deraison C, Bonnart C, Bitoun E, Robinson R, O'Brien TJ, Wakamatsu K, Ohtsubo S, Takahashi H, Hashimoto Y, Dopping-Hepenstal PJ, McGrath JA, Iizuka H, Richard G, Hovnanian A. LEKTI is localized in lamellar granules, separated from KLK5 and KLK7, and is secreted in the extracellular spaces of the superficial stratum granulosum. J Invest Dermatol. 2005;124:360-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 121] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 57. | Yamasaki K, Schauber J, Coda A, Lin H, Dorschner RA, Schechter NM, Bonnart C, Descargues P, Hovnanian A, Gallo RL. Kallikrein-mediated proteolysis regulates the antimicrobial effects of cathelicidins in skin. FASEB J. 2006;20:2068-2080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 353] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 58. | Yousef GM, Diamandis EP. The new kallikrein-like gene, KLK-L2. Molecular characterization, mapping, tissue expression, and hormonal regulation. J Biol Chem. 1999;274:37511-37516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 74] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 59. | Debela M, Goettig P, Magdolen V, Huber R, Schechter NM, Bode W. Structural basis of the zinc inhibition of human tissue kallikrein 5. J Mol Biol. 2007;373:1017-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 60. | Meyer-Hoffert U, Wu Z, Schröder JM. Identification of lympho-epithelial Kazal-type inhibitor 2 in human skin as a kallikrein-related peptidase 5-specific protease inhibitor. PLoS One. 2009;4:e4372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 61. | Brattsand M, Stefansson K, Lundh C, Haasum Y, Egelrud T. A proteolytic cascade of kallikreins in the stratum corneum. J Invest Dermatol. 2005;124:198-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 230] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 62. | Ekholm IE, Brattsand M, Egelrud T. Stratum corneum tryptic enzyme in normal epidermis: a missing link in the desquamation process? J Invest Dermatol. 2000;114:56-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 153] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 63. | Pampalakis G, Zingkou E, Kaklamanis L, Spella M, Stathopoulos GT, Sotiropoulou G. Elimination of KLK5 inhibits early skin tumorigenesis by reducing epidermal proteolysis and reinforcing epidermal microstructure. Biochim Biophys Acta Mol Basis Dis. 2019;1865:165520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 64. | Yousef GM, Scorilas A, Katsaros D, Fracchioli S, Iskander L, Borgono C, Rigault de la Longrais IA, Puopolo M, Massobrio M, Diamandis EP. Prognostic value of the human kallikrein gene 15 expression in ovarian cancer. J Clin Oncol. 2003;21:3119-3126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 65. | Song Y, Bai G, Li X, Zhou L, Si Y, Liu X, Deng Y, Shi Y. Bioinformatics analysis of human kallikrein 5 (KLK5) expression in metaplastic triple-negative breast cancer. Cancer Innov. 2023;2:376-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 66. | Pampalakis G, Obasuyi O, Papadodima O, Chatziioannou A, Zoumpourlis V, Sotiropoulou G. The KLK5 protease suppresses breast cancer by repressing the mevalonate pathway. Oncotarget. 2014;5:2390-2403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 67. | Yousef GM, Obiezu CV, Jung K, Stephan C, Scorilas A, Diamandis EP. Differential expression of Kallikrein gene 5 in cancerous and normal testicular tissues. Urology. 2002;60:714-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 68. | Dorn J, Magdolen V, Gkazepis A, Gerte T, Harlozinska A, Sedlaczek P, Diamandis EP, Schuster T, Harbeck N, Kiechle M, Schmitt M. Circulating biomarker tissue kallikrein-related peptidase KLK5 impacts ovarian cancer patients' survival. Ann Oncol. 2011;22:1783-1790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 69. | Pépin D, Shao ZQ, Huppé G, Wakefield A, Chu CW, Sharif Z, Vanderhyden BC. Kallikreins 5, 6 and 10 differentially alter pathophysiology and overall survival in an ovarian cancer xenograft model. PLoS One. 2011;6:e26075. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 70. | Feng Y, Li X, Sun B, Wang Y, Zhang L, Pan X, Chen X, Wang X, Wang J, Hao X. Evidence for a transcriptional signature of breast cancer. Breast Cancer Res Treat. 2010;122:65-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 71. | Li X, Liu J, Wang Y, Zhang L, Ning L, Feng Y. Parallel underexpression of kallikrein 5 and kallikrein 7 mRNA in breast malignancies. Cancer Sci. 2009;100:601-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 72. | Yousef GM, Yacoub GM, Polymeris ME, Popalis C, Soosaipillai A, Diamandis EP. Kallikrein gene downregulation in breast cancer. Br J Cancer. 2004;90:167-172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 72] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 73. | Teo CR, Casey PJ, Rasheed SA. The GNA13-RhoA signaling axis suppresses expression of tumor protective Kallikreins. Cell Signal. 2016;28:1479-1488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 74. | Tian J, Wang V, Wang N, Khadang B, Boudreault J, Bakdounes K, Ali S, Lebrun JJ. Identification of MFGE8 and KLK5/7 as mediators of breast tumorigenesis and resistance to COX-2 inhibition. Breast Cancer Res. 2021;23:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 75. | Yousef GM, Polymeris ME, Grass L, Soosaipillai A, Chan PC, Scorilas A, Borgoño C, Harbeck N, Schmalfeldt B, Dorn J, Schmitt M, Diamandis EP. Human kallikrein 5: a potential novel serum biomarker for breast and ovarian cancer. Cancer Res. 2003;63:3958-3965. [PubMed] |
| 76. | Chang JS, Kim N, Kim JY, Do SI, Cho Y, Kim HS, Kim YB. Kallikrein 5 overexpression is associated with poor prognosis in uterine cervical cancer. J Gynecol Oncol. 2020;31:e78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 77. | Loessner D, Quent VM, Kraemer J, Weber EC, Hutmacher DW, Magdolen V, Clements JA. Combined expression of KLK4, KLK5, KLK6, and KLK7 by ovarian cancer cells leads to decreased adhesion and paclitaxel-induced chemoresistance. Gynecol Oncol. 2012;127:569-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 78. | Loessner D, Goettig P, Preis S, Felber J, Bronger H, Clements JA, Dorn J, Magdolen V. Kallikrein-related peptidases represent attractive therapeutic targets for ovarian cancer. Expert Opin Ther Targets. 2018;22:745-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 79. | Gong W, Liu Y, Seidl C, Diamandis EP, Kiechle M, Drecoll E, Kotzsch M, Magdolen V, Dorn J. Quantitative assessment and clinical relevance of kallikrein-related peptidase 5 mRNA expression in advanced high-grade serous ovarian cancer. BMC Cancer. 2019;19:696. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 80. | Prezas P, Arlt MJ, Viktorov P, Soosaipillai A, Holzscheiter L, Schmitt M, Talieri M, Diamandis EP, Krüger A, Magdolen V. Overexpression of the human tissue kallikrein genes KLK4, 5, 6, and 7 increases the malignant phenotype of ovarian cancer cells. Biol Chem. 2006;387:807-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 69] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 81. | Abuduhadeer X, Xu X, Aihesan K, Yilihamu M, Zhao Y, Zhang W. Clinical significance of kallikrein 5 as a novel prognostic biomarker in gastric adenocarcinoma. J Clin Lab Anal. 2021;35:e23958. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 82. | Ma H, Hockla A, Mehner C, Coban M, Papo N, Radisky DC, Radisky ES. PRSS3/Mesotrypsin and kallikrein-related peptidase 5 are associated with poor prognosis and contribute to tumor cell invasion and growth in lung adenocarcinoma. Sci Rep. 2019;9:1844. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 83. | Planque C, de Monte M, Guyetant S, Rollin J, Desmazes C, Panel V, Lemarié E, Courty Y. KLK5 and KLK7, two members of the human tissue kallikrein family, are differentially expressed in lung cancer. Biochem Biophys Res Commun. 2005;329:1260-1266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 84. | Shinoda Y, Kozaki K, Imoto I, Obara W, Tsuda H, Mizutani Y, Shuin T, Fujioka T, Miki T, Inazawa J. Association of KLK5 overexpression with invasiveness of urinary bladder carcinoma cells. Cancer Sci. 2007;98:1078-1086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 85. | Gonzalez HE, Gujrati M, Frederick M, Henderson Y, Arumugam J, Spring PW, Mitsudo K, Kim HW, Clayman GL. Identification of 9 genes differentially expressed in head and neck squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 2003;129:754-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 80] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 86. | Ye H, Yu T, Temam S, Ziober BL, Wang J, Schwartz JL, Mao L, Wong DT, Zhou X. Transcriptomic dissection of tongue squamous cell carcinoma. BMC Genomics. 2008;9:69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 220] [Cited by in RCA: 265] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 87. | Jiang R, Shi Z, Johnson JJ, Liu Y, Stack MS. Kallikrein-5 promotes cleavage of desmoglein-1 and loss of cell-cell cohesion in oral squamous cell carcinoma. J Biol Chem. 2011;286:9127-9135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 88. | Kang Y, Chen J, Li X, Luo M, Chen H, Cui B, Wang L, Lv D, Feng Y, Zhang P. Salivary KLK5 and uPA are potential biomarkers for malignant transformation of OLK and OLP. Cancer Biomark. 2021;31:317-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 89. | Pettus JR, Johnson JJ, Shi Z, Davis JW, Koblinski J, Ghosh S, Liu Y, Ravosa MJ, Frazier S, Stack MS. Multiple kallikrein (KLK 5, 7, 8, and 10) expression in squamous cell carcinoma of the oral cavity. Histol Histopathol. 2009;24:197-207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 90. | Johnson JJ, Miller DL, Jiang R, Liu Y, Shi Z, Tarwater L, Williams R, Balsara R, Sauter ER, Stack MS. Protease-activated Receptor-2 (PAR-2)-mediated Nf-κB Activation Suppresses Inflammation-associated Tumor Suppressor MicroRNAs in Oral Squamous Cell Carcinoma. J Biol Chem. 2016;291:6936-6945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 91. | Zhang Y, Hu K, Qu Z, Xie Z, Tian F. ADAMTS8 inhibited lung cancer progression through suppressing VEGFA. Biochem Biophys Res Commun. 2022;598:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 92. | Debela M, Magdolen V, Bode W, Brandstetter H, Goettig P. Structural basis for the Zn2+ inhibition of the zymogen-like kallikrein-related peptidase 10. Biol Chem. 2016;397:1251-1264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 93. | Tang Z, Li C, Kang B, Gao G, Li C, Zhang Z. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017;45:W98-W102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5550] [Cited by in RCA: 7438] [Article Influence: 826.4] [Reference Citation Analysis (1)] |
| 94. | Li A, Li Y, Li Y, Zhang M, Zhang H, Chen F. Identification and validation of key genes associated with pathogenesis and prognosis of gastric cancer. PeerJ. 2023;11:e16243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 95. | Luo LY, Rajpert-De Meyts ER, Jung K, Diamandis EP. Expression of the normal epithelial cell-specific 1 (NES1; KLK10) candidate tumour suppressor gene in normal and malignant testicular tissue. Br J Cancer. 2001;85:220-224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 58] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 96. | Kato K, Noda T, Kobayashi S, Sasaki K, Iwagami Y, Yamada D, Tomimaru Y, Takahashi H, Uemura M, Asaoka T, Shimizu J, Doki Y, Eguchi H. KLK10 derived from tumor endothelial cells accelerates colon cancer cell proliferation and hematogenous liver metastasis formation. Cancer Sci. 2024;115:1520-1535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 97. | Alves MG, Kodama MH, da Silva EZM, Gomes BBM, da Silva RAA, Vieira GV, Alves VM, da Fonseca CK, Santana AC, Cecílio NT, Costa MSA, Jamur MC, Oliver C, Cunha TM, Bugge TH, Braz-Silva PH, Colli LM, Sales KU. Relative expression of KLK5 to LEKTI is associated with aggressiveness of oral squamous cell carcinoma. Transl Oncol. 2021;14:100970. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/