Published online Nov 24, 2025. doi: 10.5306/wjco.v16.i11.110257
Revised: June 20, 2025
Accepted: October 25, 2025
Published online: November 24, 2025
Processing time: 171 Days and 20.2 Hours
Claudin-6 (CLDN6), a tight junction protein typically restricted to embryonic tissues, is re-expressed in various cancers. However, its prognostic significance in high-grade endometrial carcinoma (HGEC) remains unclear.
To investigate the expression pattern of CLDN6 in HGEC and assess its corre
Immunohistochemical analysis of CLDN6 expression was performed on formalin-fixed, paraffin-embedded tissues from 80 patients diagnosed with HGEC. Associations between CLDN6 expression and histological subtype, the International Federation of Gynecology and Obstetrics (FIGO) stage, depth of myometrial inva
High CLDN6 expression was detected in a subset of HGEC patients and was significantly associated with nonendometrioid histology (P = 0.026), advanced FIGO stage (P = 0.015), deep myometrial invasion (P = 0.038), and recurrence (P = 0.002). While Kaplan-Meier analysis did not reveal a statistically significant difference in disease-free survival or overall survival between the high CLDN6 expression group and the low CLDN6 expression group, multivariate Cox regression revealed that CLDN6 overexpression was an independent predictor of shorter disease-free survival [hazard ratio (HR) = 68.98, P = 0.022] and overall survival (HR = 24.023, P = 0.038).
CLDN6 overexpression is associated with aggressive tumor features and poor clinical outcomes in HGEC, sug
Core Tip: This investigation elucidates the tumor-promoting function of claudin-6 (CLDN6) in high-grade endometrial carcinoma. Immunohistochemical analysis of 80 clinical samples revealed a strong correlation between elevated CLDN6 expression and adverse pathological features, such as the nonendometrioid histologic subtype, extensive myometrial infiltration, lymphovascular space involvement, and regional lymph node dissemination. Multivariate statistical evaluation revealed that CLDN6 expression was an independent prognostic indicator for both disease-free survival and overall survival. Collectively, these results suggest that CLDN6 may serve as a valuable prognostic marker and a viable therapeutic target in the management of aggressive forms of endometrial cancer.
- Citation: Ebrahim NAA, Eissa TS, Hussein MA, Korany OM, Amin NH. Tight junction disruption via claudin-6 overexpression promotes invasion and recurrence in high-grade endometrial tumors. World J Clin Oncol 2025; 16(11): 110257
- URL: https://www.wjgnet.com/2218-4333/full/v16/i11/110257.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i11.110257
Endometrial cancer (EC) is the most frequently diagnosed malignancy of the female reproductive system, with incidence and mortality rates rising steadily worldwide, particularly among postmenopausal women[1]. Despite significant progress in diagnostic techniques and therapeutic strategies, the clinical outcomes for individuals with advanced or recurrent EC remain dismal. The five-year survival rate decreases markedly with disease progression, highlighting the critical need for improved therapeutic interventions[2].
Among the various mechanisms that underlie EC progression, epithelial-mesenchymal transition (EMT) has garnered considerable attention. EMT involves the loss of epithelial characteristics and the acquisition of mesenchymal traits, thereby enhancing the invasive and migratory capabilities of tumor cells and fostering metastatic dissemination[3]. In this context, molecular determinants that regulate EMT and cell adhesion are of particular interest because of their potential role in disease progression and as therapeutic targets.
Tight junction proteins, which are essential for maintaining epithelial cell polarity and tissue integrity, have been increasingly implicated in cancer pathobiology. These complexes consist of integral membrane proteins such as occludins, zonula occludens, and claudins. Members of the claudin family in particular have emerged as pivotal regulators of tumor biology[4].
Claudins, whose expression can be either upregulated or downregulated, play pivotal roles in regulating a wide range of cellular functions. These include intracellular signalling, cell proliferation, paracellular barrier integrity, the transport of growth factors, intercellular adhesion, the maintenance of cell polarity, tissue invasion, and the secretion of soluble prometastatic molecules that can modulate the tumor microenvironment[5]. The expression of these genes is tightly regu
A decrease in claudin expression is frequently indicative of EMT, a physiological process in development and tissue repair that, in the context of cancer, facilitates tumor progression by promoting a more migratory and invasive phe
This disruption not only contributes to tissue disorganization but also increases the exposure of cell surfaces to ex
Although comprehensive pancancer analyses have identified the endometrium as one of the tumor types with the highest prevalence of claudin gene alterations, none of the claudin sequence variants observed in endometrial carcinoma have yet been confirmed as oncogenic driver mutations[8].
Claudin-6 (CLDN6), a less characterized claudin subtype, has recently attracted attention because of its atypical expression profile and association with various cancers. It is expressed primarily during embryogenesis and is largely absent in most adult tissues under physiological conditions[9]. However, its re-expression in malignant tissues suggests a potential oncogenic role. In EC, aberrant overexpression of CLDN6 has been associated with aggressive clinicopathological features, including higher histological grade, advanced stage, and poor patient prognosis[10]. This dysregulated expression may contribute to the disruption of epithelial tight junctions and facilitate EMT, thus enhancing tumor inva
Mechanistic studies have begun to elucidate the signalling pathways through which CLDN6 exerts its oncogenic influence. Notably, CLDN6 has been implicated in the activation of the PI3K/AKT pathway, an axis critical for promoting cell proliferation, survival, and resistance to apoptosis in EC[12]. Furthermore, evidence suggests that CLDN6 may interact with the Wnt/β-catenin signalling cascade, a pathway known for its roles in maintaining stemness, promoting EMT, and driving tumor progression[13-15]. These interactions position CLDN6 as a central player in the modulation of oncogenic signalling.
In addition to these pathways, CLDN6 appears to modulate TGF-β signalling, which governs cell differentiation, immune modulation, and EMT. Dysregulation of this pathway in EC has been linked to tumor progression and immune evasion, and CLDN6 may amplify these effects through its influence on the tumor microenvironment[15,16].
CLDN6 contributes to EC pathogenesis through multiple pathways. As a tight junction protein, it directly impacts the structural integrity of epithelial barriers. Disruption of these junctions not only compromises tissue architecture but also enables cancer cell dissemination by increasing intercellular permeability[16,17]. Additionally, CLDN6 interacts with intracellular scaffolding proteins such as ZO-1, and disturbances in these interactions may trigger oncogenic signalling cascades[11,18]. Functional studies have demonstrated that CLDN6 overexpression can enhance the migration and inva
The aberrant expression of CLDN6 holds significant promise as a biomarker for disease stratification and prognosis. Its upregulation makes it a potential tool for identifying patients at increased risk of recurrence and progression[18,20]. Clinically, measuring CLDN6 levels in tumor samples could help refine treatment strategies and guide personalized management.
Targeting CLDN6 also opens new avenues for therapy. Efforts in other malignancies have demonstrated the feasibility of developing CLDN-targeted agents, including monoclonal antibodies and small-molecule inhibitors. Given its in
In parallel, the evolving molecular classification of EC, as established by The Cancer Genome Atlas, has significantly enhanced our understanding of disease heterogeneity. This system categorizes EC into four prognostic subtypes: POLE ultramutated, microsatellite instability hypermutated, copynumber low (endometrioid) and copynumber high (serous-like), each with distinct genetic profiles and clinical trajectories[19]. This framework not only refines risk assessment but also supports a more individualized approach to therapy, incorporating molecular targets and immunotherapeutic res
Our study aimed to explore the expression pattern and clinical significance of CLDN6 in high-grade endometrial carcinoma, with a particular focus on its association with aggressive histological features and poor prognosis. By characterizing CLDN6 expression in this context, we seek to contribute to the growing body of evidence supporting its role as both a prognostic biomarker and a potential target for future therapeutic development in endometrial cancer.
This retrospective cohort study included 80 patients with histologically confirmed high-grade endometrial carcinoma who underwent surgical management at the National Cancer Institute, Cairo University. Patients whose paraffin blocks or incomplete clinical files were unavailable were excluded. Ethical approval was obtained from the Institutional Review Board (Approval No. PA2502-501-094-197). Comprehensive clinical and pathological data were collected and analysed. Demographic information and treatment outcomes were retrieved from the Department of Epidemiology and Biostatistics, whereas tumor-specific data were accessed from the electronic records of the Department of Oncologic Pathology. Histopathological classification was confirmed through reexamination of hematoxylin and eosin-stained slides to ensure diagnostic consistency. The pathological features assessed included cervical stromal invasion, depth of myometrial invasion (categorized as < 50% or ≥ 50%), lymph node metastasis, lymphovascular space invasion, distant metastasis, surgical margin status, and tumor size, which was dichotomized as < 5 cm or ≥ 5 cm on the basis of the largest dimension and median tumor size. Additional variables included tumor recurrence and a family history of malignancy. Staging was performed according to the International Federation of Gynecology and Obstetrics (FIGO) system: Stage I (tumor confined to the uterus), stage II (involvement of the cervical stroma), stage III (local or regional spread), and stage IV (distant metastasis)[17].
CLDN6 expression was evaluated via immunohistochemistry (IHC) in 4-μm-thick formalin-fixed, paraffin-embedded tissue sections via the Ventana Benchmark Ultra IHC system. Following deparaffinization in xylene and rehydration through graded alcohols, antigen retrieval was performed via citrate buffer (pH 6.0) in a microwave oven for 20 minutes. To block endogenous peroxidase activity, the tissue sections were incubated with 3% hydrogen peroxide for 10 minutes. The sections were subsequently incubated with a primary monoclonal anti-CLDN6 antibody (CLDN6 mouse anti-human monoclonal, OTI5D2) at an optimized dilution. A biotinylated secondary antibody and streptavidin-horseradish peroxidase were applied to amplify the signal. Immunoreactivity was visualized via the use of 3,3'-diaminobenzidine (DAB) as the chromogen, and hematoxylin was used for counterstaining. The slides were then dehydrated, cleared, and mounted for microscopic evaluation. CLDN6 staining was interpreted by three experienced pathologists who were blinded to the clinical and pathological data. The immunoreactivity was scored semiquantitatively on the basis of the staining intensity (0 = negative, 1 = weak, 2 = moderate, 3 = strong) and the percentage of positively stained tumor cells (0 ≤ 1%, 1 = 1%-10%, 2 = 11%-30%, 3 = 31%-50%, 4 ≥ 50%). The final immunoreactivity score (IRS) was obtained by multiplying the intensity score by the percentage score, yielding a total score ranging from 0-12. On the basis of these IRS values, tumors were categorized into two groups: Low CLDN6 expression (IRS < 8) and high CLDN6 expression (IRS ≥ 8) for further statistical analysis. This scoring system is the most common and validated approach used in CLDN6 immunohistochemistry assessments in many previous studies, which have explored its expression in various tumors[3,11,22,23]. Interobserver agreement was quantified via the intraclass correlation coefficient (ICC), which indicated excellent consistency between evaluators (ICC = 0.91). Any differences in interpretation were addressed through collaborative review and consensus.
Descriptive statistics were employed to summarize the demographic and clinicopathological characteristics of the study cohort. Univariable analysis was performed to examine the associations between CLDN6 expression and various clinicopathological variables. χ2 tests or Fisher’s exact tests were used for categorical variables, depending on the expected frequency of observations, with a P value of < 0.05 considered statistically significant. Kaplan-Meier survival curves were generated to estimate overall survival (OS) and disease-free survival (DFS) in relation to CLDN6 expression levels, and log-rank tests were applied to compare survival distributions between the low and high CLDN6 expression groups. To assess the independent prognostic significance of CLDN6 expression, multivariate Cox proportional hazards regression was applied, adjusting for other clinicopathological variables, such as tumor size, lymphovascular invasion, lymph node status, and FIGO stage. Hazard ratios (HRs) with 95% confidence intervals (CIs) were calculated for each variable to determine their independent contributions to DFS and OS. All the statistical analyses were conducted via SPSS version 27 to ensure accurate evaluation and interpretation of the data.
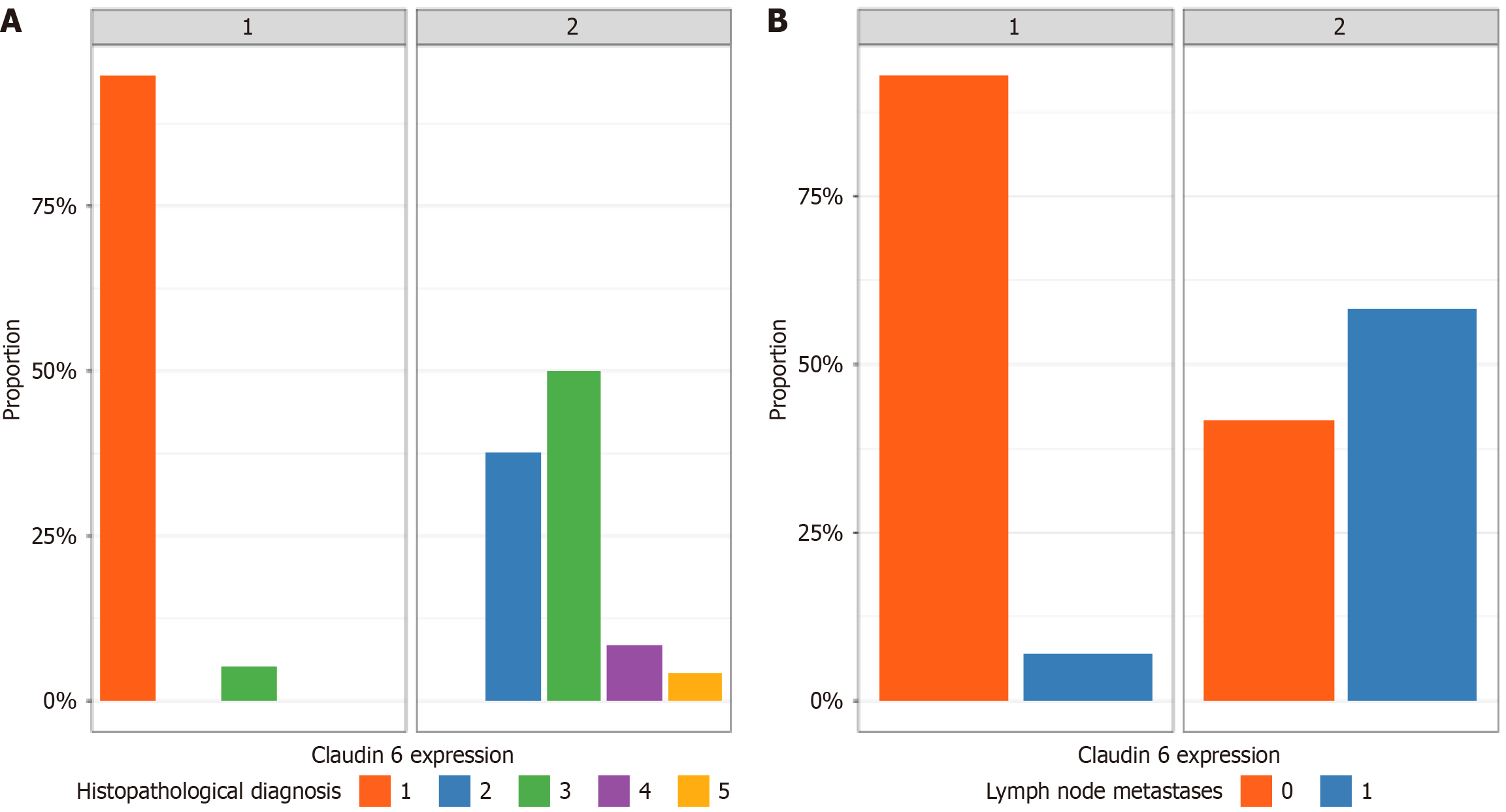
A total of 80 patients who underwent panhysterectomy with or without pelvic lymphadenectomy at the National Cancer Institute, Cairo University, were evaluated in this study. The median age of the patients was 60 years. Table 1 shows the distribution of the clinicopathological characteristics of the study cohort. The distribution of CLDN6 expression revealed that 56 patients (70%) were categorized as CLDN6-low, whereas 24 patients (30%) were considered CLDN6-high (Table 1). Univariate analysis was performed to investigate the relationships between CLDN6 expression and various clinicopathological parameters (Table 1). The results revealed that certain features were significantly associated with CLDN6 status, whereas others were not. Notably, myometrial invasion was significantly correlated, with high CLDN6expression detected in 71% of tumors exhibiting ≥ 50% invasion, whereas CLDN6 expression was detected in 38% of tumors in the low-expression group (P = 0.010). Additionally, lymph node metastasis was strongly associated with CLDN6 positivity, present in 58% of CLDN6-high tumors vs only 7.1% of those with low expression (P < 0.001). Lymphovascular invasion was also significantly related, occurring in 83% of high CLDN6 cases compared with 57% of low CLDN6 cases (P = 0.024). In contrast, other factors, including cervical invasion, tumor size, patient age, margin status, distant metastasis, tumor recurrence, FIGO stage, death events, and family history, were not significantly associated with CLDN6 expression (P > 0.05 for all). These findings highlight the potential of CLDN6 as a marker linked to aggressive pathological features in high-grade endometrial carcinoma.
| Parameter | Total (n = 80) | CLDN6 low (n = 56) | CLDN6 high (n = 24) | P value |
| Age group | 0.19 | |||
| ≤ 50 | 39 (49) | 30 (54) | 9 (38) | |
| > 50 | 41 (51) | 26 (46) | 15 (62) | |
| Family history of cancer | 1 | |||
| Absent | 68 (85) | 47 (84) | 21 (88) | |
| Present | 12 (15) | 9 (16) | 3 (12) | |
| Diagnosis type | < 0.001 | |||
| High grade Endometrioid | 53 (66) | 53 (95) | 0 (0) | |
| Serous | 9 (11) | 0 (0) | 9 (38) | |
| Carcinosarcoma | 15 (19) | 3 (5.4) | 12 (50) | |
| Clear | 2 (2.5) | 0 (0) | 2 (8.3) | |
| Poorly differentiated | 1 (1.2) | 0 (0) | 1 (4.2) | |
| Tumor size | 0.2 | |||
| < 5 cm | 42 (52) | 32 (57) | 10 (42) | |
| ≥ 5 cm | 38 (48) | 24 (43) | 14 (58) | |
| Cervical invasion | 0.35 | |||
| Absent | 62 (78) | 45 (80) | 17 (71) | |
| Present | 18 (22) | 11 (20) | 7 (29) | |
| Myometrial invasion | < 0.010 | |||
| < 50% | 42 (52) | 35 (62) | 7 (29) | |
| ≥ 50% | 38 (48) | 21 (38) | 17 (71) | |
| Lymphovascular invasion | 0.024 | |||
| Absent | 28 (35) | 24 (43) | 4 (17) | |
| Present | 52 (65) | 32 (57) | 20 (83) | |
| Lymph node metastasis | < 0.001 | |||
| Absent | 62 (78) | 52 (93) | 10 (42) | |
| Present | 18 (22) | 4 (7.1) | 14 (58) | |
| Distant metastasis | 1 | |||
| Absent | 76 (95) | 53 (95) | 23 (96) | |
| Present | 4 (5) | 3 (5.4) | 1 (4.2) | |
| Surgical margin status | 0.51 | |||
| Negative | 78 (98) | 55 (98) | 23 (96) | |
| Positive | 2 (2.5) | 1 (1.8) | 1 (4.2) | |
| FIGO stage | 0.32 | |||
| Stage I | 58 (72) | 43 (77) | 15 (62) | |
| Stage II | 9 (11) | 6 (11) | 3 (12) | |
| Stage III | 9 (11) | 4 (7.1) | 5 (21) | |
| Stage IV | 4 (5) | 3 (5.4) | 1 (4.2) | |
| Tumor recurrence | 0.26 | |||
| Absent | 63 (79) | 46 (82) | 17 (71) | |
| Present | 17 (21) | 10 (18) | 7 (29) | |
| Death events | 0.41 | |||
| Alive | 52 (65) | 38 (68) | 14 (58) | |
| Deceased | 28 (35) | 18 (32) | 10 (42) |
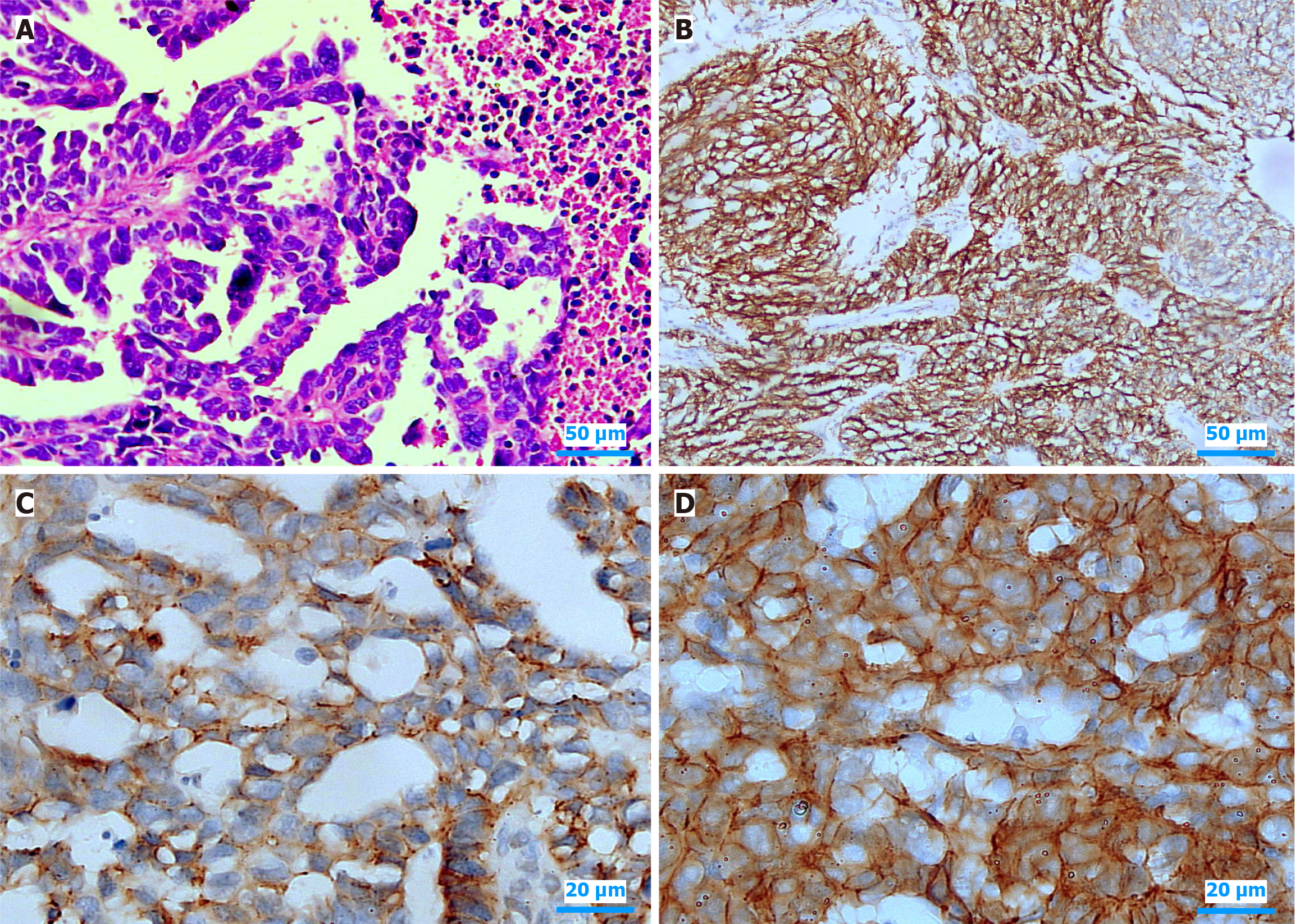
As illustrated in Figure 1, serous carcinoma of the endometrium displays distinct histopathological features and variable CLDN6 expression patterns, with immunohistochemistry revealing both diffuse and localized membranous staining. Furthermore, Figure 2 summarizes the distribution of CLDN6 across various high-grade endometrial carcinoma subtypes and its association with lymph node metastasis, underscoring the potential relevance of CLDN6 as a marker of aggressive tumor behavior and metastatic propensity.
Logistic regression analysis of CLDN6 expression in high-grade endometrial cancer identified several critical predictors from histopathological variables, with particular emphasis on histopathological diagnosis and lymphovascular invasion. The analysis revealed that the histopathological diagnosis had a substantial effect on predicting CLDN6 overexpression. Notably, several carcinomas presented the highest odds ratio for CLDN6 overexpression. Its odds ratio was exceptionally high, at approximately 188 million (P < 0.001), underscoring its dominant role in predicting CLDN6 positivity within this subgroup. Additionally, lymphovascular invasion emerged as a key predictor that significantly influences CLDN6 expression. Patients with lymphovascular invasion were markedly more likely to overexpress CLDN6 (P = 0.002), further emphasizing the critical role of vascular involvement in shaping the molecular profile of the tumor. The odds ratio for lymphovascular invasion approached zero, suggesting a profound impact on CLDN6 positivity, which aligns with the aggressive nature of tumors exhibiting vascular spread.
The follow-up duration for the studied cohort ranged from 12 to 95 months, with a median follow-up of 73 months and an interquartile range of 54.8 to 82.2 months. Kaplan-Meier survival analysis was performed to investigate the impact of CLDN6 expression levels on OS. The analysis revealed that patients with low CLDN6 expression presented a longer mean survival time of 79.13 months (95%CI: 72.67-85.59) than did those with high CLDN6 expression, who presented a mean survival of 69.27 months (95%CI: 57.00-81.53). However, this observed difference did not reach statistical sig
The median DFS for the entire study population was 70.0 months, with an interquartile range of 49.5-81.2 months. To further assess the prognostic relevance of CLDN6 expression for DFS, Kaplan-Meier survival estimates were generated for the two subgroups. Among the 80 patients, 56 presented low CLDN6 expression (with 10 events and 82.1% censored observations), whereas 24 presented high expression levels (with 7 events and 70.8% censored observations). The mean estimated DFS was notably longer in the low-expression group [82.9 months (95%CI: 76.1-89.8)] than in the high-expression group [72.4 months (95%CI: 59.8-85.0)]. Despite this apparent difference, the log-rank test did not reveal sta
Multivariate Cox proportional hazards regression analysis was performed to assess the factors influencing DFS and OS. CLDN6 expression demonstrated a statistically significant independent prognostic impact. Specifically, high CLDN6 expression was associated with a markedly increased hazard of recurrence, with a HR of 68.98 (95%CI: 1.82-2617.56, P = 0.022). These findings suggest that tumors with high CLDN6 expression are at substantially greater risk of disease relapse than are those with low CLDN6 expression, even after adjusting for critical clinicopathologic parameters, including tumor size, lymphovascular invasion, lymph node status, stage, and margin status. Moreover, high CLDN6 expression emerged as a statistically significant predictor of overall survival in multivariate analysis (P = 0.038). Specifically, the hazard ratio for patients with high CLDN6 expression was estimated at 24.023 (95%CI: 1.193-483.611), indicating a 24-fold increased risk of death relative to those with low expression after adjusting for other covariates in the model.
This study highlights the crucial role of CLDN6 in determining the clinicopathological characteristics and prognosis of high-grade endometrial carcinoma (HGEC). Elevated CLDN6 expression is significantly correlated with aggressive histological subtypes, increased incidence of lymphovascular invasion (LVI), lymph node metastases, and deep myometrial infiltration—all of which are well-recognized indicators of unfavourable prognosis in endometrial cancer[7,8].
CLDN6 belongs to the claudin family, which is integral to tight junctions that preserve epithelial polarity and regulate paracellular transport[4,15]. Among the claudin family members, CLDN6 stands out for its distinctive role in activating cell adhesion pathways and modulating nuclear receptor signalling. Research has shown that changes in claudin expression can compromise the integrity of tight junctions (TJs), resulting in increased paracellular permeability. This breakdown of barrier function disrupts cell polarity, creating a microenvironment that facilitates greater access to nutrients and growth factors for tumor cells. Additionally, the weakening of intercellular adhesion may increase the capacity of cancer cells to invade surrounding tissues and promote metastasis[15,24-27].
Although normally repressed in adult tissues, CLDN6 is reactivated in various malignancies, where it has been linked to disrupted cell adhesion, enhanced motility, and epithelial-mesenchymal transition (EMT)—a key mechanism in tumor invasion and dissemination[3,9,15]. CLDN6 is overexpressed in endometrial carcinoma. Experimental deletion of the CLDN6 gene has been shown to reduce both the proliferation and migratory capacity of HEC-1-B endometrial cancer cells, likely through suppression of the PI3K/Akt/mTOR signalling pathway. The aberrant expression of CLDN6 in our HGEC samples is consistent with previous studies in other epithelial cancers, supporting its role as an oncofetal protein and a potential mediator of tumor progression[10,12,11,17,24].
Dysregulated expression of CLDN6 contributes to the progression of endometrial cancer by engaging a complex adhesion-dependent signalling cascade. CLDN6 initiates the activation of Src family kinases (SFKs) through interactions involving its second extracellular loop and key tyrosine residues, Y196 and Y200—both of which are essential for downstream signalling. This activation triggers the PI3K pathway, subsequently leading to the phosphorylation and ac
In our cohort, tumors with high CLDN6 expression were predominantly nonendometrioid in type, especially serous carcinoma and carcinosarcoma subtypes, which are well known for their aggressive clinical behavior and poor prognosis. Multivariate logistic regression identified the histological subtype as the strongest predictor of elevated CLDN6 expression, suggesting preferential expression in biologically aggressive tumors[6,7,15]. These findings align with experimental evidence indicating that CLDN6 enhances proliferation, invasion, and EMT in endometrial cancer cells via estrogen receptor α signalling and activation of the PI3K/AKT/mTOR pathway[11,15,25].
We also observed a significant link between CLDN6 overexpression and key metastatic features, including LVI, nodal metastases, and deep myometrial penetration. These findings imply a functional role for CLDN6 in facilitating tumor progression through both vascular and stromal pathways. In addition, in vitro studies have demonstrated that CLDN6 promotes cancer cell migration and invasion, while silencing CLDN6 reduces these traits by downregulating critical signalling pathways such as the PI3K/AKT/mTOR pathway[18,15]. Additionally, CLDN6 expression has been associated with increased motility and increased activity of matrix metalloproteinases, which promote extracellular matrix degra
Although CLDN6 expression was strongly associated with aggressive pathological features, Kaplan-Meier survival analysis did not reveal statistically significant differences in OS or DFS based on CLDN6 Levels alone. However, multivariate Cox regression—adjusting for key clinical variables—identified high CLDN6 expression as an independent prognostic marker for both OS and DFS. Patients with CLDN6 overexpression presented a markedly increased risk of death (24-fold) and disease recurrence (nearly 69-fold), highlighting its potential utility as a prognostic biomarker.
Our findings align with previous work by Kojima et al[11], who reported a correlation between CLDN6 upregulation and high tumor grade, deep myometrial invasion, and adverse survival outcomes in endometrial cancer (EC) patients[10,11]. Moreover, recent transcriptomic analyses have revealed the broader role of claudins in modulating the tumor microenvironment, immune evasion, and therapeutic resistance[14,15,17]. Notably, CLDN6 has garnered attention as a tumor-specific antigen with applications in antibody-drug conjugates and CAR-T-cell therapies[19]. Its restricted expression in normal tissues and high prevalence in aggressive EC subtypes make it an attractive candidate for targeted intervention[11,20].
Interestingly, a subset of tumors with high CLDN6 expression did not experience recurrence or mortality during follow-up, suggesting biological heterogeneity. This observation may reflect interactions between CLDN6 and other molecular pathways, including hormonal signalling, immune checkpoints, and angiogenesis—all areas currently under investigation[11,15,16].
In a 2023 study, Martina et al[27] reported on CLDN6-23-ADC, an investigational antibody-drug conjugate specifically designed to target CLDN6. Preclinical models of endometrial cancer demonstrated that treatment with CLDN6-23-ADC resulted in marked tumor regression, underscoring its potential as a promising and highly selective therapeutic strategy[27].
Notably, CLDN6 expression, assessed via IHC, has emerged as a potential predictive biomarker for therapy selection, independent of tumor histology. This broadens the therapeutic applicability of CLDN6-23-ADC across various CLDN6-positive malignancies.
Building on these encouraging preclinical results, Konecny et al[28] initiated a first-in-human phase I clinical trial (NCT05103683) to evaluate the safety, tolerability, pharmacokinetics, and preliminary efficacy of CLDN6-23-ADC (TORL-1-23) in patients with advanced solid tumors. The ongoing trial includes individuals with CLDN6-expressing ovarian, endometrial, and other solid tumors, representing a pivotal step in the clinical translation of CLDN6-targeted therapies. If successful, this approach could usher in a new era of precision medicine for treating aggressive gynecologic and other CLDN6-positive cancers[27].
In summary, our data support the role of CLDN6 as a key player in the progression of HGEC. Its expression is closely tied to poor prognostic features and retains independent prognostic significance. Evaluating CLDN6 in routine diagnostic workflows may enhance risk stratification and inform personalized treatment strategies. Furthermore, CLDN6 holds promise as a therapeutic target, particularly for serous and carcinosarcoma subtypes, which remain challenging to treat with conventional approaches.
Despite the strengths of this study, certain limitations should be acknowledged. These include its retrospective nature, the modest sample size, and the lack of functional validation using in vitro or in vivo experimental models. These limitations highlight areas for future research to further clarify the clinical relevance and therapeutic potential of CLDN6.
This study highlights the critical role of CLDN6 as both a potential prognostic biomarker and a therapeutic target in HGEC. Elevated CLDN6 expression was significantly linked to aggressive clinicopathological features, including non
| 1. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12667] [Cited by in RCA: 15523] [Article Influence: 2587.2] [Reference Citation Analysis (6)] |
| 2. | Urick ME, Bell DW. Clinical actionability of molecular targets in endometrial cancer. Nat Rev Cancer. 2019;19:510-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 305] [Article Influence: 43.6] [Reference Citation Analysis (0)] |
| 3. | Lee Y, Kim HS. Clinicopathological Significance of Claudin-6 Immunoreactivity in Low-grade, Early-stage Endometrioid Endometrial Carcinoma. In Vivo. 2025;39:367-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 4. | Capoferri D, Bignotti E, Ravaggi A, Mitola S, Romani C. Finding the junction between claudins and endometrial carcinoma. Biochim Biophys Acta Rev Cancer. 2023;1878:189019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 5. | Wang DW, Zhang WH, Danil G, Yang K, Hu JK. The role and mechanism of claudins in cancer. Front Oncol. 2022;12:1051497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 52] [Reference Citation Analysis (0)] |
| 6. | Corsini M, Ravaggi A, Odicino F, Santin AD, Ravelli C, Presta M, Romani C, Mitola S. Claudin3 is localized outside the tight junctions in human carcinomas. Oncotarget. 2018;9:18446-18453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Goebel EA, Vidal A, Matias-Guiu X, Blake Gilks C. The evolution of endometrial carcinoma classification through application of immunohistochemistry and molecular diagnostics: past, present and future. Virchows Arch. 2018;472:885-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 8. | Zhang P, Li S, Zhao P, Guo Z, Lu S. Comparative Physiological Analysis Reveals the Role of NR-Derived Nitric Oxide in the Cold Tolerance of Forage Legumes. Int J Mol Sci. 2019;20:1368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Hana C, Thaw Dar NN, Galo Venegas M, Vulfovich M. Claudins in Cancer: A Current and Future Therapeutic Target. Int J Mol Sci. 2024;25:4634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 10. | Kojima M, Sugimoto K, Kobayashi M, Ichikawa-Tomikawa N, Kashiwagi K, Watanabe T, Soeda S, Fujimori K, Chiba H. Aberrant Claudin-6-Adhesion Signaling Promotes Endometrial Cancer Progression via Estrogen Receptor α. Mol Cancer Res. 2021;19:1208-1220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 11. | Kojima M, Sugimoto K, Tanaka M, Endo Y, Kato H, Honda T, Furukawa S, Nishiyama H, Watanabe T, Soeda S, Fujimori K, Chiba H. Prognostic Significance of Aberrant Claudin-6 Expression in Endometrial Cancer. Cancers (Basel). 2020;12:2748. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 12. | Jeon H, Sterpi M, Mo C, Bteich F. Claudins: from gatekeepers of epithelial integrity to potential targets in hepato-pancreato-biliary cancers. Front Oncol. 2024;14:1454882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 13. | Piestrzeniewicz-Ulanska D, Brys M, Semczuk A, Rechberger T, Jakowicki JA, Krajewska WM. TGF-beta signaling is disrupted in endometrioid-type endometrial carcinomas. Gynecol Oncol. 2004;95:173-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Salvador E, Burek M, Förster CY. Tight Junctions and the Tumor Microenvironment. Curr Pathobiol Rep. 2016;4:135-145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 15. | Du H, Yang X, Fan J, Du X. Claudin 6: Therapeutic prospects for tumours, and mechanisms of expression and regulation (Review). Mol Med Rep. 2021;24:677. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 16. | Borowczak J, Łaszczych D, Olejnik K, Michalski J, Gutowska A, Kula M, Bator A, Sekielska-Domanowska M, Makarewicz R, Marszałek A, Szylberg Ł, Bodnar M. Tight Junctions and Cancer: Targeting Claudin-1 and Claudin-4 in Thyroid Pathologies. Pharmaceuticals (Basel). 2024;17:1304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 17. | Cao X, He GZ. Knockdown of CLDN6 inhibits cell proliferation and migration via PI3K/AKT/mTOR signaling pathway in endometrial carcinoma cell line HEC-1-B. Onco Targets Ther. 2018;11:6351-6360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 18. | Vonniessen B, Tabariès S, Siegel PM. Antibody-mediated targeting of Claudins in cancer. Front Oncol. 2024;14:1320766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 19. | Zheng W. Molecular Classification of Endometrial Cancer and the 2023 FIGO Staging: Exploring the Challenges and Opportunities for Pathologists. Cancers (Basel). 2023;15:4101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 34] [Reference Citation Analysis (0)] |
| 20. | Concin N, Matias-Guiu X, Vergote I, Cibula D, Mirza MR, Marnitz S, Ledermann J, Bosse T, Chargari C, Fagotti A, Fotopoulou C, Gonzalez Martin A, Lax S, Lorusso D, Marth C, Morice P, Nout RA, O'Donnell D, Querleu D, Raspollini MR, Sehouli J, Sturdza A, Taylor A, Westermann A, Wimberger P, Colombo N, Planchamp F, Creutzberg CL. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int J Gynecol Cancer. 2021;31:12-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1245] [Cited by in RCA: 1303] [Article Influence: 260.6] [Reference Citation Analysis (0)] |
| 21. | Baker-Rand H, Kitson SJ. Recent Advances in Endometrial Cancer Prevention, Early Diagnosis and Treatment. Cancers (Basel). 2024;16:1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 41] [Reference Citation Analysis (0)] |
| 22. | Lu YZ, Li Y, Zhang T, Han ST. Claudin-6 is down-regulated in gastric cancer and its potential pathway. Cancer Biomark. 2020;28:329-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Tao D, Guan B, Li H, Zhou C. Expression patterns of claudins in cancer. Heliyon. 2023;9:e21338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 24. | Yin C, Li XB. Unlocking early detection: How screening can save lives from cervical cancer. World J Clin Oncol. 2025;16:102456. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 25. | Zhang BL, Gao W, He L, Liu XT, Wang ZM, Tan L. Functional heterogeneity and clinical implications of CD4+ T cell subtypes in high-grade serous ovarian carcinoma. World J Clin Oncol. 2025;16:104138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 26. | Saito A, Yoshida H, Nishikawa T, Yonemori K. Human epidermal growth factor receptor 2 targeted therapy in endometrial cancer: Clinical and pathological perspectives. World J Clin Oncol. 2021;12:868-881. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 27. | McDermott MSJ, O'Brien NA, Hoffstrom B, Gong K, Lu M, Zhang J, Luo T, Liang M, Jia W, Hong JJ, Chau K, Davenport S, Xie B, Press MF, Panayiotou R, Handly-Santana A, Brugge JS, Presta L, Glaspy J, Slamon DJ. Preclinical Efficacy of the Antibody-Drug Conjugate CLDN6-23-ADC for the Treatment of CLDN6-Positive Solid Tumors. Clin Cancer Res. 2023;29:2131-2143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 28. | Konecny GE, Wahner Hendrickson AE, Winterhoff B, Chander C, Bilic S, Davenport S, Chung A, Miller L, Press MF, Letrent SP, Slamon DJ. Initial results of dose finding in a first-in-human phase 1 study of a novel Claudin 6 (CLDN6) targeted antibody drug conjugate (ADC) TORL-1-23 in patients with advanced solid tumors. J Clin Oncol. 2023;41:3082-3082. [DOI] [Full Text] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/