Published online Nov 24, 2025. doi: 10.5306/wjco.v16.i11.108667
Revised: June 22, 2025
Accepted: October 10, 2025
Published online: November 24, 2025
Processing time: 215 Days and 17.8 Hours
Chimeric antigen receptor T (CAR-T) cell therapy represents a major advance in cancer immunotherapy, offering targeted treatment options, particularly for hematologic malignancies. This review comprehensively explores the structural evolution, production processes, and cytotoxic mechanisms underlying CAR-T function. Therapy involves engineering autologous T cells with synthetic rece
Core Tip: This review presents a comprehensive and forward-looking analysis of chimeric antigen receptor T cell (CAR-T) therapy, detailing its structural evolution, manufacturing strategies, and cytotoxic mechanisms. It uniquely integrates emerging advances, such as dual chimeric antigen receptors, SynNotch systems, and universal CAR-T designs. The manuscript also addresses clinical challenges, like antigen escape and cytokine release syndrome, and highlights innovative gene delivery technologies, including viral vectors, CRISPR/Cas9, and RNA-based methods. This work offers a consolidated and insightful resource for researchers and clinicians aiming to advance CAR-T therapy toward safer and more effective cancer treatments.
- Citation: Arjumand S, Raj A, Prattay KMR, Omer HBM, Azam F. Chimeric antigen receptor T cell therapy: Revolutionizing cancer treatment. World J Clin Oncol 2025; 16(11): 108667
- URL: https://www.wjgnet.com/2218-4333/full/v16/i11/108667.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i11.108667
Cancer is a group of diseases characterized by abnormal, uncontrolled cell proliferation along with the ability to invade nearby and distant tissues, through a process known as metastasis, which, if left untreated, can result in a slow and painful death[1]. Cancer is the second leading cause of death worldwide, following heart disease[2]. In the year 2020 alone, approximately 19.3 million cancer cases and 10.0 million cancer-related deaths were recorded[3]. By 2022, these numbers rose to 20.0 million and 9.7 million for new case diagnosis and yearly death toll, respectively. The diagnosis rate is speculated to increase to 35 million by the year 2050[4]. Nevertheless, developing an effective care plan, if not a complete therapeutic cure, has become an essential priority in addressing this global health challenge. To date, cancer treatment methods can be broadly classified into five main types: Surgery, radiotherapy, conventional chemotherapy, molecularly targeted therapy, and immunotherapy[5]. Among these, immunotherapy is the most recent and advanced approach, where the patient's own innate and consequential adaptive immune system is leveraged to amplify or dampen the immune response, thereby achieving a customizable therapeutic outcome for immune-regulated conditions[6]. In the context of cancer immunotherapy, which aims to modify the immune system to better recognize and mount enhanced anti-tumor responses, numerous techniques are under investigation, including immune checkpoint inhibitors, monoclonal antibody (mAb) therapy, cytokine therapy, cancer vaccines, and adoptive cell therapy (ACT)[7]. ACT can be categorized into two types: Non-genetically modified and genetically modified ACT therapies. Non-genetically modified therapies comprise tumor-Infiltrating lymphocyte therapy and natural killer (NK) cell therapy. In contrast, genetically modified approaches include engineered T cell receptor (TCR) therapy and chimeric antigen receptor-T (CAR-T) cell therapy[8].
CAR-T cell therapy is a personalized, gene modification-based cancer immunotherapy primarily used for relapsed or refractory malignancies. It enhances and redirects the natural cytotoxic function of autologous T cells through the expression of a recombinant antigen receptor known as chimeric antigen receptor (CAR), or chimeric immune receptor. CAR is a type of engineered receptor designed to be ectopically expressed on T-lymphocytes to actively target and selectively kill tumor cells that specifically express the corresponding antigens (e.g., CD19, HER-2)[9,10].
A major advantage of CAR-T therapy over other immunotherapies, including artificially expressed endogenous TCR-based T cell therapy, is that they do not require antigen presenting cells (APCs) or major histocompatibility complexes (MHCs) on their surface to present antigen to the CAR receptor. CAR-T cells recognize and directly bind to target tumor antigens in an MHC-independent manner, placing them at a significant advantage in countering the immune evasion mechanisms often employed by neoplastic tumor cells. Thus, the independence from MHC-mediated recognition enables CAR-T cells to target tumor cells regardless of their human leukocyte antigen (HLA) type. Unlike TCR-T cells, which recognize only peptide antigens, CAR-T cells can target a broader range of molecules, including carbohydrates, glycolipids, and peptides. Moreover, the incorporation of different types and numbers of co-stimulatory domains within CAR constructs enables the modulation of oncolytic activity, allowing optimization for specific needs such as safety and persistence. Together, these advantages have made CAR-T therapy one of the most well-accepted therapeutic technologies among scientists, clinicians and patients, with successful clinical trials leading to seven approved therapeutic agents by the European Medicines Agency (EMA) and the United States Food-and-Drug-Administration (FDA) for various hematological malignancies, such as leukemia, lymphoma and multiple myeloma, especially in their relapsed/refractory (R/R) stage[11].
CAR embodies four crucial domains: The extracellular antigen recognition domain (ARD), the spacer or, hinge, the transmembrane domain (TMD), and the intracellular domain, also known as endo domain (Figure 1).
The ARD is the outermost part of the CAR structure, and it specifically recognizes the pre-targeted extracellular-surface-antigens on cancer cells, otherwise known as tumor-associated antigens (TAAs), and ensures a strong bond with the TAAs. Therefore, the ARD is also known as the antigen binding domain.
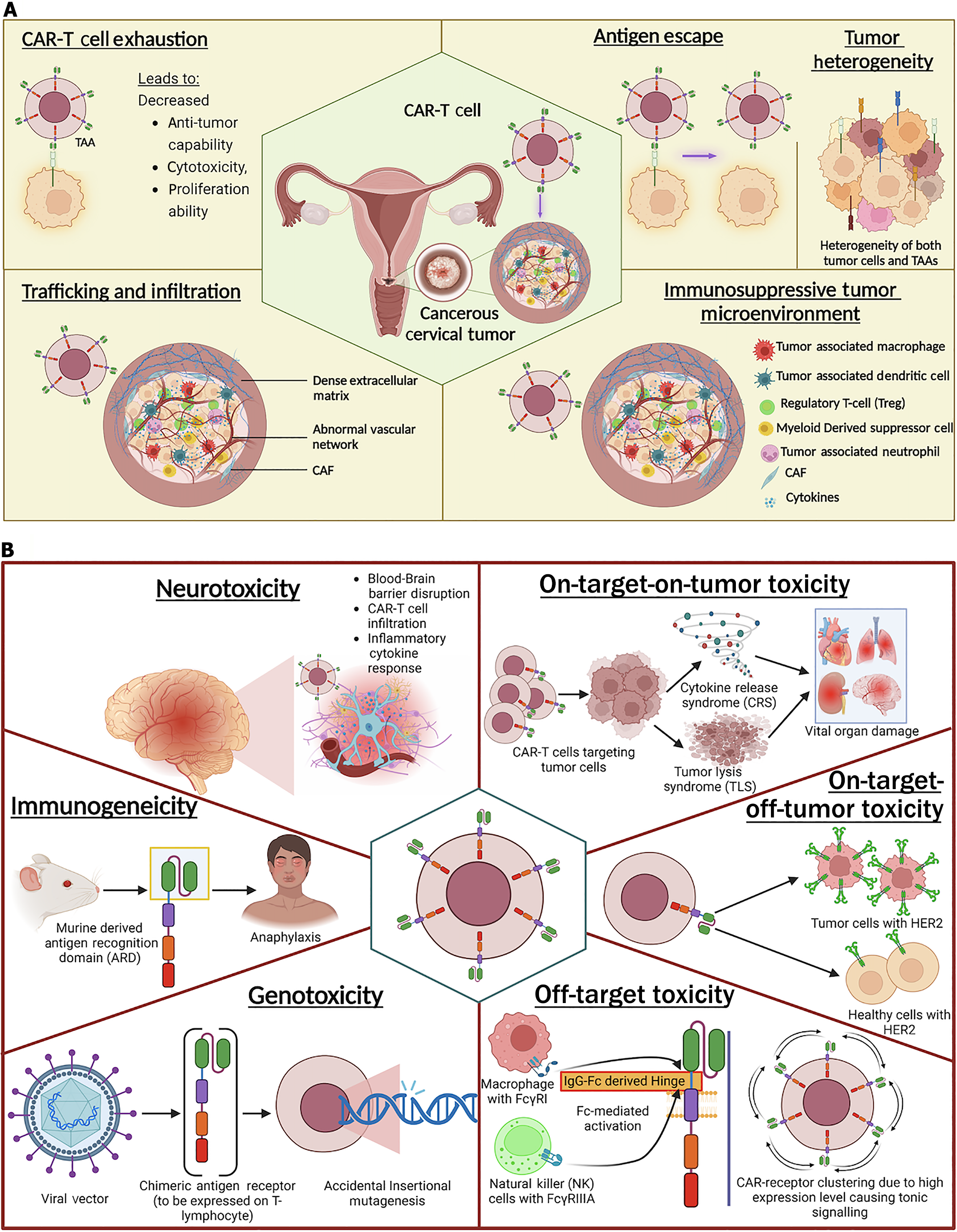
A key methodological consideration in ARD selection is the balance between affinity and specificity for the TAA. While enhanced specificity is generally desirable, affinity must be optimized. Increased specificity reduces the likelihood of off-target toxicity, which can otherwise induce antigen-independent cytotoxicity. However, excessive specificity may also lead CARs to target non-malignant cells that express physiological levels of TAAs, particularly when affinity is suboptimal, making careful calibration of CAR affinity a secondary but essential factor. Excessively high affinity can result in on-target-off-tumor toxicity and premature T-cell exhaustion, whereas insufficient affinity may impair antigen recognition, signal transduction, and full effector CAR-T activation[12].
Among TAAs, CD19 has been extensively studied and successfully targeted in CAR-based therapies. Notably, five of the seven FDA-approved CAR-T therapies, Kymriah, Yescarta, Breyanzi, Tecartus and Aucatzyl, are designed to target CD19 in hematologic malignancies. Ongoing research continues to explore an expanding array of TAAs, with emerging clinical trials generating promising novel targets and valuable insights into CAR-T therapy advances[13].
Different types of ARDs are available to construct desired CARs, which are described below:
Single chain variable fragment: Among the various ARDs, single chain variable fragment (ScFv) is the most common option, consisting of a variable heavy (VH) and variable light (VL) domain connected by a short, flexible polypeptide chain linker. These variable domains are typically derived from mAbs, with ScFvs representing the minimal functional unit responsible for antigen binding, thereby facilitating the synthesis of bindable CAR-T structures[14].
Despite its small size, the linker plays a crucial role in ScFv flexibility, stability, and structural integrity. It determines the orientation of the VH and VL domains, influencing CAR expression, target recognition, and signaling. Linker length and amino acid composition impact ScFv stability, while increased proteolytic stability enhances binding affinity[13].
Affinity and specificity are key determinants of ScFv function, which are influenced by the interaction of variable domain polypeptides and the complementarity-determining regions (CDRs). Notably, ScFvs with identical affinities can exhibit variable effectiveness, and mutations within CDRs can alter epitope recognition, even for the same antigen.
Optimized affinity is critical, as excessive binding strength can lead to toxicity, while lower affinity may be advantageous in certain contexts. For instance, Trastuzumab-derived CAR-T cells engineered with reduced affinity selectively degranulate upon encountering HER2-overexpressing cancer cells, while sparing normal cells that express physiological levels of HER2. This highlights the importance of epitope positioning, antigen density, and avoidance of antigen-independent tonic signaling in ScFv design.
ScFvs are derived from murine, humanized, or fully human mAbs, with each origin influencing specificity and immunogenicity. While murine-derived ScFvs are highly specific, they often cause immunogenicity, leading to CAR-T clearance in clinical settings. Humanized and fully human ScFvs mitigate this problem, as demonstrated in a study where 40% of patients resistant to murine ScFvs responded to humanized variants. However, these alternatives often exhibit reduced specificity due to affinity evolution and in vitro antibody development that lacks in vivo immune surveillance. Emerging approaches, such as human phage display libraries, aim to enhance ScFv precision[14].
Functionally, ScFvs initiate and amplify T-cell cytotoxicity by enabling precise antigen recognition, overcoming tumor immune evasion mechanisms. Unlike native T-lymphocytes, which rely on endogenous antigen presentation, CAR-Ts utilizing ScFvs directly target tumor-specific epitopes, making them a pivotal component in engineered immunotherapies[15].
Other ARD domains: Though ScFv is the most-widely used ARD domain in CAR engineering, there are other types of ARDs available that are used to develop various types of CAR constructs.
(1) Ligand-receptor ARD: These CARs exploit natural ligand-receptor interactions by fusing either a ligand or receptor protein to the CD3-ζ intracellular signaling domain (ISD). This principle is the basis of an advanced next generation CAR, the physiological CAR design, discussed in more detail below. Upon binding to the corresponding receptor or ligand on tumor cells, direct ligand-receptor interactions mediate CAR-T activation, resulting in robust effector function. Such ARDs have been experimentally employed to target stress-induced ligands in various cancers, thereby broadening the potential antigenic scope of CAR-T therapy. For example, CAR-T cells engineered to express the NKG2D receptor have been shown to recognize and eliminate NKG2D ligand-expressing lymphoma cells, while IL13 mutein-based CAR-T cells selectively recognize IL13Rα2+ glioblastoma cells[14]; (2) TCR-mimicking (TCRm) ARD: Though CARs’ MHC-unrestricted antigen binding ability is considered an advantage, it can sometimes restrict T-cells from targeting endogenous target molecules. Thus, TCRm-ARDs enable the recognition of intracellular antigens presented by MHC molecules. For instance, TCRm-ScFv CARs targeting wilms tumor-1 (WT-1) via WT-1/HLA-A*02:01 complexes have demonstrated anti-tumor efficacy in leukemic and ovarian cancer models[16]; and (3) Variable lymphocyte receptors (VLR): Derived from jawless vertebrates, VLR-based ARDs offer broad antigen recognition. Despite potential immunogenicity, VLR-CAR-Ts have shown promising in vitro cytotoxicity[14].
Lastly, various other types of ligands, nanobody, cytokine and peptide-based ARDs are also being explored for enhanced CAR functionality[16].
The hinge, or spacer, is a crucial extracellular component of the CAR structure, functioning as a linkage between the ARD and the intracellular domain via the TMD. It is an amino acid chain derived from immunoglobulin (e.g., IgG1 and IgG4), various clusters of differentiation (e.g., CD8α and CD28), and less commonly, from IgD or CD7. The hinge region plays a pivotal role in enhancing ARD flexibility, thereby overcoming steric hindrance. Its length can be tailored to optimize interactions with TAA, enabling the formation of structured immunological synapses. Furthermore, the hinge contributes to CAR stability, influencing expression levels, signaling efficiency, and overall CAR-T activation[12].
The optimal hinge length is determined by the spatial location of the TAA epitope and the extent of steric hindrance imposed by malignant cells. Longer hinges, such as the mucin-1 (MUC1) hinge, facilitate the recognition of near-membrane antigens, particularly those with complex glycosylation patterns. Conversely, shorter hinges, such as CD8α and CD28, are more effective for targeting far-membrane epitopes. Given these considerations, empirical testing is essential to ensure the appropriate hinge length for novel antigen targets, as a well-adjusted hinge significantly enhances therapeutic outcomes[12].
Again, immunoglobulin-derived short hinges, particularly IgG1 and IgG4, are frequently employed due to their therapeutic efficacy against membrane proximal antigens and modular design, which allows for varying lengths. However, their incorporation also introduces Fc regions capable of interacting with Fcγ receptors (FcγRs), leading to antigen-independent signaling, off-target activation, and reduced persistence in vivo. These issues can be mitigated by removing CH2 domains or mutating them to prevent FcγR binding. Substituting IgG2 residues with IgG1 or IgG4 sequences has been shown to decrease FcγR binding by a factor of 1 × 104.
CD8α- and CD28-derived hinges are also employed in CAR design, particularly in FDA-approved CAR-T therapies such as Kymriah and Yescarta. CD8α hinges naturally pair with their TMDs, whereas CD28 hinges are often incorporated alongside their transmembrane and co-stimulatory domains, promoting stable CAR orientation. One major advantage of these domains is their inherent lack of FcγR interactions, reducing the risk of off-target activation. Additionally, modifications such as substituting cysteine residues with alanine or serine in CD8α hinges can improve CAR dimerization and expression, particularly in NK92 immune cells, showing their potential for future therapeutic applications[15].
The TMD is the gateway to the intracellular segment of the CAR, primarily comprising a hydrophobic α-helical region that spans the plasma membrane, functioning as a liaison between extracellular and intracellular fragments. Beyond its primary role as a structural stabilizer, the TMD influences CAR expression and signaling efficiency. Upon antigen engagement, conformational changes in the TMD facilitate ligand recognition, triggering downstream signaling cascades that ultimately lead to cytokine release, cytotoxic molecule secretion, and activation-induced cell death (AICD).
Although TMDs are often interchanged to complement other CAR components such as ARDs and hinges, their sources remain somewhat limited. For example, CD3ζ was commonly used in first-generation CARs, while CD28, CD8α and CD4 were introduced in later generations. Other TMDs, including CD4, CD7, FcεRIγ, OX40 and H-2Kb, have been used only infrequently.
CD3ζ-based TMDs facilitate heterodimer formation with intracellular CD3ζ signaling domains, lowering the antigen binding threshold and enhancing immunological synapse formation. This results in optimal cell signaling, activation, and cytotoxicity, thereby improving anti-tumor efficacy. Similarly, FcεRIγ TMDs, in conjunction with CD3ζ, have demonstrated comparable stability and signaling properties. However, both TMDs exhibit reduced structural stability compared to CD28-derived TMDs.
On the other hand, CD8α and CD28-derived TMDs are commonly used in both clinical and preclinical CAR designs due to their superior stability and expression levels. CD28-based CARs are associated with robust pro-inflammatory responses, whereas CD8α-based CARs demonstrate enhanced persistence in vivo.
Nevertheless, recent discoveries have highlighted the importance of effective inter-domain pairing. For example, inducible T cell co-stimulator (ICOS)-based TMDs has been proven to work better than CD8α TMDs by enhancing membrane localization, receptor clustering, and signaling synergy, leading to better T-cell persistence and function, especially in CD4+ T cells, resulting in excellent CAR-T tenacity and implied anti-cancer function. Similarly, CD4 TMDs have been found to pair effectively with IgG-based hinge domains, producing cytokine profiles comparable to CD8α-based CARs[15].
Intracellular signaling domain (ISD): The ISD is the most critical component of the CAR structure. To effectively direct T-lymphocyte cytotoxicity toward target cells expressing the intended antigen, it is essential to initiate the requisite signaling cascade via an appropriate ISD following ARD engagement. The ISD is responsible for initiating the downstream signaling cascades that drive T-cell cytotoxic activity and cytokine secretion. CD3ζ and FcRγ serve as the primary ISDs in TCRs and NK cells, respectively, each containing immunoreceptor tyrosine-based activation motifs (ITAMs) that mediate signal transduction. CD3ζ is the predominant ISD used in CARs due to its low activation threshold and high cytokine production. Comparatively, while capable of inducing similar IL-2 secretion and tumor cell death, FcRγ demonstrates inferior cytokine release efficiency. Additionally, alternative signaling domains such as the CD3ζ isoform CD3η, CD3ε and other signaling molecules including the tyrosine kinase Syk, as well as the adaptor proteins DAP10 and DAP12, have been evaluated as potential ISDs to identify the most effective configurations[15].
Co-stimulatory domain (CSD): To design an optimal endodomain, one or more secondary signaling modules, known as costimulatory domains (CSDs), are incorporated alongside the primary ISD to amplify the initial activation signal. These CSDs ensure a robust and sustained cellular response, enhancing T-cell activation, proliferation cytokine secretion, and ultimately, apoptotic cytotoxicity. CSDs are typically derived from CD28 superfamily members, such as CD28 and ICOS, as well as the tumor necrosis factor receptor (TNFR) superfamily, such as 4-1BB (CD137), CD27, and OX40[13]. Among these, CD28 and 4-1BB are the only ones to have received FDA approval, owing to their superior clinical response rates, and are therefore the most widely employed[12].
CD28 and 4-1BB are the only FDA-approved CSDs, and they each havee distinct functional attributes. CD28 induces strong initial activation, enhances cytokine production, including IL-2, IL-4, IL-10, and promotes effector T cell differentiation. Conversely, 4-1BB sustains long-term CAR-T persistence and mitigates exhaustion, making it particularly effective for chronic malignancies. CD28-driven CARs rely on aerobic glycolysis, whereas 4-1BB-driven CARs favor oxidative phosphorylation, impacting T cell metabolism and longevity[15]. Multiple clinical studies have demonstrated that 4-1BB-based memory T cells persist for several years after treatment, while CD28-based T cells diminish within months. This underscores the role of 4-1BB in counteracting T cell exhaustion and prolonging therapeutic efficacy in chronic malignancy[17].
Several other CSDs are under consideration due to promising preclinical findings. CD27 and OX40 enhance persistence and secondary co-stimulation, improving tenacity and potency. However, ICOS provides stronger PI3K stimulation, enhancing signal transduction via the PI3K-Akt pathway. It also promotes CD4+ T cell differentiation into TH1 and TH17 subtypes, improving helper T cell function, T cell grafting, and anti-tumor activity, outperforming CD28 and 4-1BB in resilience. CAR-T therapy benefits from pairing specific T cells with optimal CSDs, such as ICOS for CD4+ and 4-1BB for CD8+ T cells. Emerging candidates like KIR2DS2, NKG2D, DNAM-1 (CD226), DAP10, MYD88 (CD40), CD40 L (CD154), CD244, and GITR (glucocorticoid induced TNFR) etc., are also showing some positive results to be assessed for future prospect[16].
All in all, the selection of ISD and CSD components significantly influences CAR-T efficacy, metabolism, and longevity. Thoughtful pairing of these domains with ARDs and TMDs is essential to optimizing therapeutic outcomes, minimizing adverse effects, and tailoring CAR design to specific malignancies.
Table 1 presents a comparison of the key features across major variants of the CAR components.
| Component | Variant | Key characteristics | Design considerations | Functional impact |
| ARD | Murine ScFv | High specificity; high immunogenicity; low persistence | Suitable for short-term efficacy | Strong initial response; risk of immune rejection |
| Humanized ScFv | Moderate specificity and immunogenicity; improved persistence | Balances efficacy and safety | Enhanced persistence with reduced immunogenicity | |
| Fully human ScFv | Variable specificity; lowest immunogenicity; high persistence | Ideal for long-term or clinical use | Best suited for chronic and relapsed settings | |
| Ligand-receptor ARD | Variable specificity; low immunogenicity; high persistence | High safety and efficacy | Targets stress-induced ligands in solid tumors | |
| TCRm ARD | High specificity; moderate immunogenicity; low persistence | Low safety for chances of off target toxicity; High efficacy | Effective for tumors lacking surface antigens | |
| VLR | High specificity; high immunogenicity; moderate persistence | Low safety; Promising efficacy | Promising in vitro cytotoxicity | |
| Hinge (spacer) domain | MUC1 | Long, no FcγR binding | Effective for membrane-proximal antigens, particularly those with complex glycosylation patterns | modulate immune signaling and enhance tumor-specific responses |
| IgG1/4 | Varying length; FcγR binding | Effective for membrane-proximal antigens | Requires Fc modification to avoid off-target activation | |
| CD8α | Short; no FcγR binding | Useful for far membrane epitopes | Enhances synapse formation with minimal off-target risk | |
| CD28 | Short; no FcγR binding | Useful for far membrane epitopes | Promotes stable orientation of CAR | |
| TMD | CD3ζ | TCR-integrated; part of native TCR complex | May synergize with CD3ζ ISD | Low stability; can support natural TCR-like signaling |
| CD8α | Stable; commonly paired with CD8α hinge | Improves CAR expression | superior stability and expression | |
| CD28 | Robust membrane anchoring | Common in 2nd-generation CARs | superior stability and expression | |
| ICOS | High T-cell persistence and function, especially in CD4+ T cells | excellent CAR-T cell tenacity | Efficient anti-cancer application | |
| CSD | CD28 | Strong initial T cell activation; IL-2, IL-4, IL-10 secretion; promotes effector T cell differentiation; moderate persistence | Drives effector phenotype | Rapid tumor cytotoxicity with limited duration |
| 4-1BB (CD137) | Oxidative phosphorylation; prolonged persistence | Promotes memory T cell formation | Sustained activity and long-term tumor control in chronic malignancies |
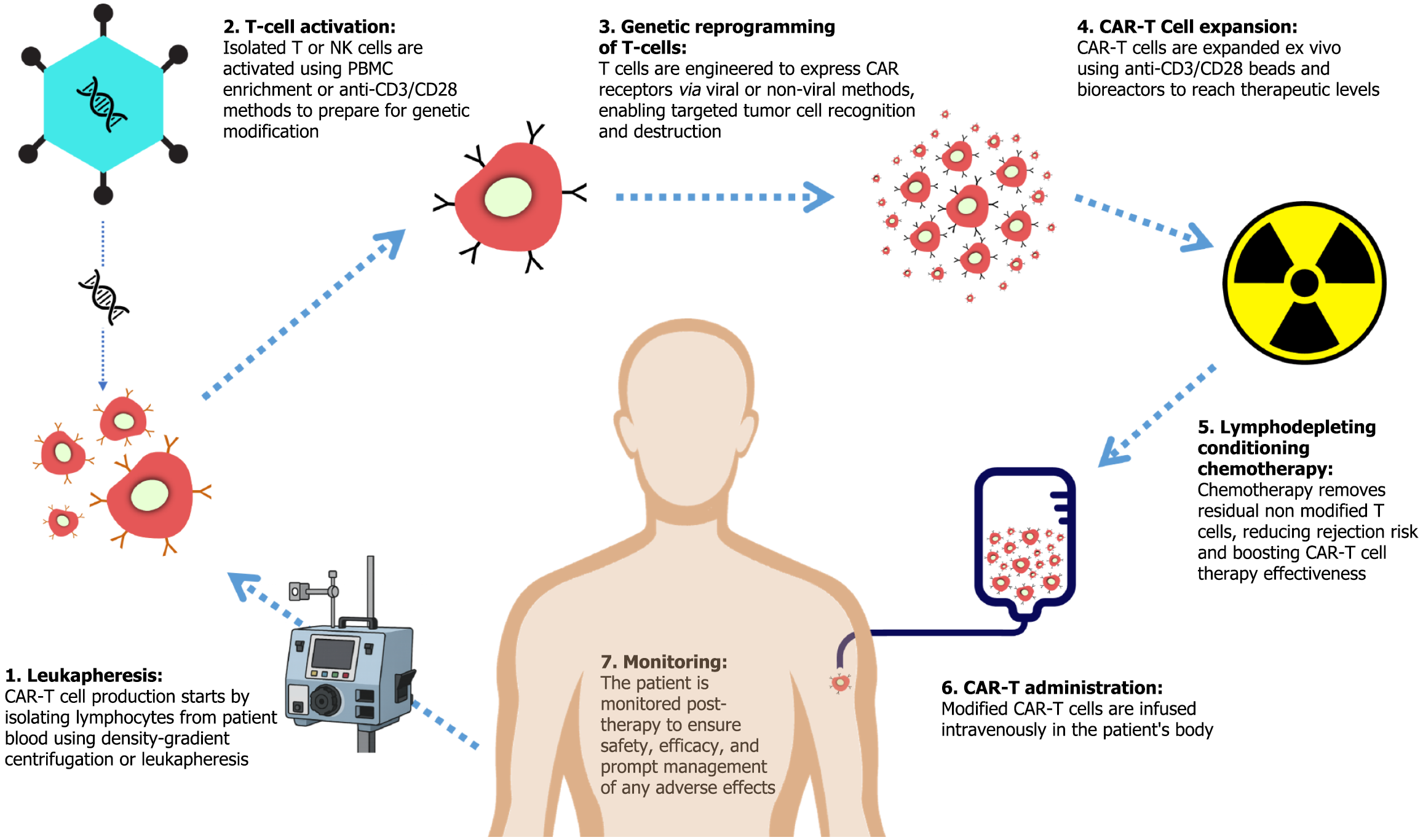
CAR-T therapy is an autologous approach that requires the extraction, genetic modification, expansion, and reinfusion of a patient’s own T cells. The process involves several important steps to ensure the successful production of tumor antigen-targeting CAR-Ts, which are subsequently administered via intravenous infusion (Figure 2).
The first step in CAR-T production involves collecting peripheral blood mononuclear cells (PBMCs), including monocytes, lymphocytes, NK cells, and dendritic cells, from the patient via phlebotomy, followed by separation through density-gradient centrifugation-based apheresis using Ficoll-Paque density gradient media. Alternatively, or as a subsequent step, leukapheresis - a specialized form of apheresis - is employed to directly isolate lymphocytes, including B cells, T cells, and NK cells, from whole blood or PBMCs[18].
Following isolation, CD4+ and CD8+ T cells, or NK cells, when applicable, undergo activation. This can occur naturally within PBMCs or be induced through stimulation with anti-CD3 and anti-CD28 mAb-coated beads, plates, or nanomatrices. This process ensures robust T-cell activation, priming the cells for genetic modification[18].
In this step, T cells are genetically engineered using either viral transduction or non-viral transfection techniques. The CAR-transgene is incorporated into the T-cell genome, resulting in expression of a complete CAR receptor. Lentiviral or γ-retroviral vectors are the most commonly employed gene delivery methods due to their high efficiency and precision. However, non-viral transposon-based systems offer a cost-effective alternative. Upon successful integration of the CAR transgene into the T-cell genome, the cells begin expressing the CAR receptor, enabling antigen recognition and subsequent cytotoxic activity against tumor cells[18].
Once genetically modified, CAR-Ts undergo ex vivo expansion to achieve the necessary quantity for therapeutic efficacy. This is typically achieved through co-culture with anti-CD3 and anti-CD28 mAb-coated beads, followed by bioreactor-based expansion[19].
Prior to CAR-T cell infusion, patients typically undergo lymphodepleting chemotherapy with fludarabine and cyclophosphamide. This regimen depletes residual endogenous T cells, reducing the risk of immune-mediated rejection of the infused CAR-T cells. Additionally, it diminishes immunosuppressive populations such as regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs), thereby improving CAR-T cell engraftment[18].
Lastly, the modified and expanded CAR-Ts (approximately 108 CAR-Ts per standard dosage) are intravenously infused to the patient’s circulatory system to ensure optimal therapeutic response[18].
Following administration, the patient is monitored to track therapy effectiveness and safety, and quick action is taken in case of any adverse effect[19].
The success of CAR therapy depends on the efficient transfer of CAR constructs into patient T cells to enable them to recognize and attack the TAAs, thereby augmenting their therapeutic potential. Several methods of CAR gene delivery have been developed, each with its own advantages and limitations, including major techniques like viral transduction, transposons, and CRISPR/Cas9, as well as a few other non-viral methods.
Viral vector-mediated gene transfer remains one of the most widely used approaches for CAR gene delivery due to its high transduction efficiency and stable gene expression. The predominant viral vectors used for CAR transduction are γ-retroviral and lentiviral vectors. Other viruses, including adenovirus and adeno-associated virus (AAV), have also been explored for gene therapy applications[20]. The viral vectors utilize packaging cell lines to produce stable virus particles for gene delivery. Upon transduction, the viral genome is integrated into the host cell genome, leading to stable expression of therapeutic genes[13].
γ-retroviral vectors: γ-Retroviral vectors, derived from the Retroviridae family, were among the first used in gene therapy. Clinical applications of γ-retroviral vectors have demonstrated their potential, as evidenced by their successful correction of severe combined immunodeficiency-X1 in humans. However, concerns regarding insertional mutagenesis and subsequent leukemia development have been raised, which led to the development of self-inactivating vectors devoid of promoter/enhancer regions, thereby reducing genotoxicity. Despite challenges, γ-retroviral vectors remain a prevalent choice in CAR-T therapy clinical trials[14].
Lentiviral vectors: Lentiviral vectors derived from human immunodeficiency virus type 1 offer distinct advantages over γ-retroviral vectors, particularly in their ability to transduce both dividing and non-dividing cells[15]. Studies have reported lower genotoxicity associated with lentiviral vectors, although concerns regarding deregulated gene expression and clonal dominance persist. Despite these limitations, lentiviral vectors have gained traction in CAR therapy due to their safety profile and efficient transduction capabilities[14].
Adenovirus and AAV: In addition to γ-retrovirus and lentivirus, adenovirus and AAV have been explored for gene therapy applications. Adenovirus exhibits broad tropism and transient gene expression, making it suitable for certain therapeutic interventions. AAV offers a promising vector platform due to its broad serotype diversity and non-pathogenic nature. However, challenges such as episomal transgene persistence limit its utility in long-term gene expression[20].
Safety considerations are paramount in viral transduction-based gene therapy, necessitating stringent adherence to clinical standards. Despite challenges, viral transduction remains a promising avenue for CAR-T therapy and continued advancements in vector design, safety optimization, and manufacturing scalability hold the potential to overcome current limitations and expand the therapeutic utility of viral transduction methods[20].
Transposons, or jumping genes, have emerged as promising non-viral tools for gene therapy, offering an alternative to viral vectors. The two most extensively studied transposon systems in CAR therapy are Sleeping Beauty (SB) and piggyBac (PB), both of which utilize a cut-and-paste mechanism to integrate transgenes into host genomes. The process involves the recognition and binding of the transposase enzyme to the transposon, formation of a synaptic complex, excision of the genetic element, and subsequent reintegration into the target location. Both SB and PB transposases comprise DNA-binding, transposable catalytic, and nuclear localization domains, facilitating efficient gene transfer[15].
Transposon-based gene transfer systems have gained prominence in CAR-T therapy[20]. Compared to viral vectors, transposons offer several advantages, including reduced immunogenicity, lower risk of insertional mutagenesis, and increased cost-effectiveness. SB transposon-based CAR-T therapy is particularly attractive due to its low enhancer activity, non-pathogenic source, and minimal epigenetic alterations at integration sites. Conversely, PB exhibits a larger cargo capacity and higher transposition efficiency, making it a preferred choice for certain applications.
Despite these advantages, transposon-based systems suffer from lower integration efficiency compared to viral vectors, necessitating optimization of transposase expression and delivery methods. Further refinements in transposon design, including the use of synthetic transposons with site-specific targeting, may enhance their safety and efficacy in clinical applications[13].
The introduction of CRISPR/Cas9 technology marked a significant advancement in CAR gene delivery. This approach harnesses the potential of Cas9, a type II CRISPR-associated protein, which can be precisely guided to any genomic locus by a single guide RNA (gRNA), functioning as a targeted endonuclease. Cas9 can be delivered into cells using various techniques, such as liposome-mediated transfection, electroporation, chemical transduction, or integration into a viral genome, either in the form of Cas9 protein/gRNA ribonucleoprotein complexes or as a plasmid. Upon delivery, a donor template, typically in plasmid form, aids in integrating the desired transgene into the genome through homology-directed repair. In the context of CAR therapy, CRISPR/Cas9 has been explored for both targeted gene insertion and knockout of inhibitory genes to enhance T-cell functionality[21].
Gene editing for CAR integration: CRISPR/Cas9-mediated insertion of CAR constructs at specific genomic loci, such as the T-cell receptor alpha constant locus, has been shown to enhance CAR-T uniformity and effectiveness in experimental settings. However, the effectiveness of gene editing to integrate CAR remains relatively modest, with current success rates peaking at around 20%, and concerns linger about unintended genetic alterations. Despite these hurdles, ongoing investigations strive to refine CRISPR-based gene editing methods to amplify both the accuracy and efficacy of CAR integration within T cells[21].
Knockout strategies for enhanced CAR function: CRISPR/Cas9 has been utilized to knock out genes encoding immune checkpoint molecules, such as programmed cell death 1 (PD-1), which suppresses T-cell function in the tumor microenvironment (TME). Early-phase clinical trials have demonstrated the feasibility of PD-1 knockout CAR-T therapy. However, precise consequences of removing inhibitory signals from T cells remain unclear, raising concerns about the potential for uncontrolled cell proliferation or severe autoimmunity. Nonetheless, these trials represent crucial steps toward advancing the clinical application of CRISPR/Cas9-mediated CAR therapy and addressing challenges associated with viral vector delivery systems[21].
While viral, transposon and CRISPR/Cas9-based approaches have dominated CAR gene delivery, several other non-viral alternatives have gained interest due to their potential to mitigate genotoxicity and streamline manufacturing processes. Notable among these are electroporation, RNA-based CAR expression, and nanotechnology-driven delivery methods.
Electroporation and RNA-based electroporation: Electroporation is a physical gene transfer method that transiently permeabilizes cell membranes using an electric field, allowing nucleic acid entry into the cytoplasm. Studies found that electroporation of human T cells leads to approximately 40%-60% gene expression and maintains 80% cell viability, which indicates suboptimal transfection efficacy alongside possible cellular stress and apoptosis, necessitating optimization of electroporation parameters to balance transfection efficiency and cell viability[15].
Nevertheless, RNA-based electroporation presents an alternative non-integrative approach, wherein in vitro-tran
RNA-based electroporation technology is being investigated in early clinical trials for CAR therapy, demonstrating promising results in preclinical models of B cell malignancies. Multiple administrations of RNA-based CAR T cells may be required to achieve long-lasting responses, highlighting the need for further optimization and clinical evaluation[22]. Despite challenges such as electroporation-related apoptosis, RNA-based electroporation holds significant potential for enhancing the safety and efficacy of CAR therapy administration[20].
Nanoparticle-based gene delivery: Nanotechnology offers a promising platform for CAR gene delivery, leveraging polymeric nanoparticles, lipid-based carriers, and magnetic nanoparticles for targeted gene transfer. Polymeric nanoparticles, in particular, have demonstrated efficacy in delivering leukemia-specific CAR genes, enabling in situ T-cell modification[23]. Magnetic nanoparticles, such as Fe3O4-based carriers, have been investigated for gene delivery, exhibiting stable cell transfection and plasmid transfection, along with gene silencing and splice correction capabilities. Utilizing nanoparticles for gene delivery presents advantages over viral vectors, such as minimizing nonspecific inflammatory or proliferative effects, thus holding significant potential for enhancing CAR-T therapy efficacy[24].
In this section, we dissect the intricate cellular and molecular processes underpinning the mechanism of action of CAR therapy.
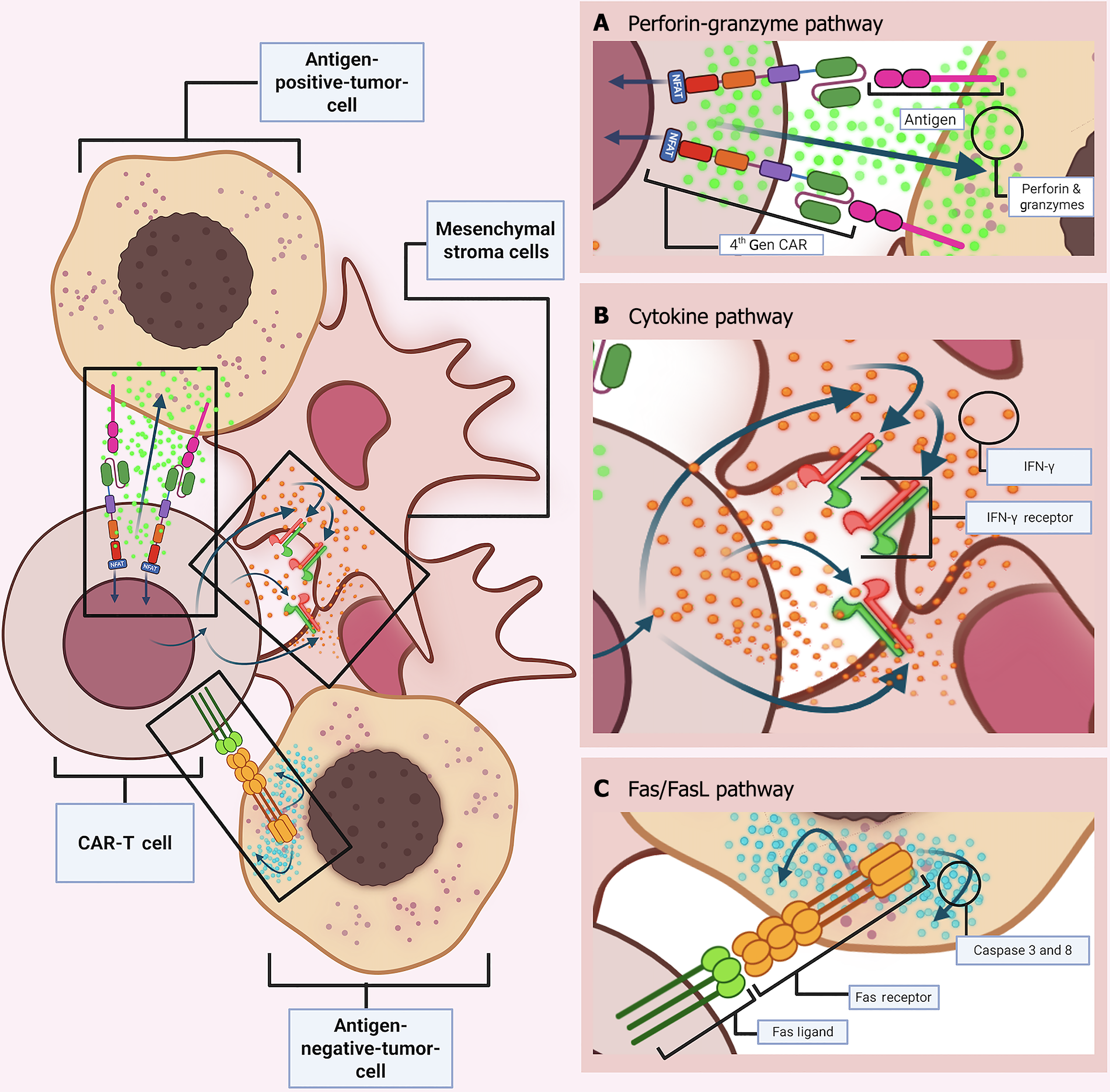
Much like endogenous cytolytic T lymphocyte (CTL), CAR-Ts execute their cytotoxic function through the formation of an immune synapse (IS). This specialized junction forms at the interface between an effector T cell, such as a CTL, and a target cell, which may include APCs such as macrophages, dendritic cells, and B cells, or alternatively, pathogen-infected/malignant cells. The interaction is mediated through TCR-MHC or CAR-TAA binding, respectively. In conventional TCR interaction, the binding occurs between the TCR and the peptide-MHC (pMHC) complex on the antigen-presenting cell. In contrast, CAR-mediated interactions involve CAR binding to the TAA, resulting in the formation of a “non-classical” IS.
The classical IS is defined by a highly organized structure comprising three concentric regions, collectively known as supramolecular activation clusters (SMACs): The central (cSMAC), peripheral (pSMAC), the distal (dSMAC) zones. Within the cSMAC, TCRs and Lck (Lymphocyte Specific Protein Tyrosine Kinase, an Src family kinase) are spatially organized to integrate and amplify activation signals following engagement with the pMHC complex. This eventually results in complete CTL activation through proper downstream signal transduction, following steps such as CD3ζ ITAMS phosphorylation, ZAP70 protein recruitment and mitogen-activated protein kinase cascade activation, as well as the nuclear factor of activated T cells (NFAT) (Figure 3A).
In contrast, the IS formed by CAR-Ts is less spatially organized. The molecular constituents - CAR molecules and Lck - are distributed in submicroscopic clusters rather than being confined to a well-demarcated cSMAC. Furthermore, adhesion molecules such as LFA-1, which in TCR synapses are typically organized in the pSMAC to reinforce synapse stability, are less coherent in CAR synapses. In addition, the actin cytoskeletal structures, typically forming a ring in the dSMAC of classical synapses, are minimized in CAR synapses. Notably, the synaptic interface of CAR-Ts exhibits a reduced radius, which facilitates rapid disengagement following the initiation of cytotoxic signaling (Figure 3A).
Advanced analytical techniques, such as Reverse-Phase-Protein-Array, have revealed that CAR IS takes significantly less time (less than 2 minutes) in eliciting sufficient proximal signals due to protein-kinase-C-delta (PKCδ) proximal signaling protein repression and the absence of a requirement for the bull’s-eye SMAC configuration. Consequently, CAR-Ts achieve accelerated lytic granule mobilization, expediting target cell apoptosis[25].
After IS formation, T cells execute their cytotoxic function through two principal mechanisms. First, they release secretory granules containing cytotoxic molecules such as perforin, granzymes, and cytokines, via directed exocytosis through the IS. Second, they express ligands of the tumor necrosis factor (TNF) superfamily, including Fas ligand (FasL/CD95 L) and TNF-related-apoptosis-inducing-ligand (TRAIL), which engage corresponding receptors on target cells. These mechanisms induce apoptosis either through rapid granule-mediated degranulation or via slower, receptor-dependent TNF signaling pathways. Although both degranulation and TNF-mediated pathways converge on caspase cascade activation, the degranulation pathway is preferred because of its rapid kinetics and spatial precision[26].
Apoptosis, or programmed cell death, is a highly regulated, ATP-dependent process characterized by a cascade of biochemical events. In the context of CAR-T-mediated cytotoxicity, apoptosis can be initiated through multiple routes. The two canonical pathways, the extrinsic (death receptor-mediated) and intrinsic (mitochondrial) pathways, are complemented by the perforin-granzyme degranulation pathway, which is further subdivided into Granzyme A and Granzyme B pathways. While the extrinsic FasL/Fas receptor pathway is generally predominant in conventional CTL-mediated apoptosis, the perforin-granzyme degranulation pathway constitutes the primary mode of cell death induction in CAR-T therapies[26].
Perforin-granzyme degranulation pathway: Effector cells, such as CTLs and NK cells, harbor cytolytic granules that are pre-associated with microtubules. These granules, which are essentially secretory lysosomes, contain biomolecules like perforin and granzymes. Upon IS formation, granules are mobilized toward the cSMAC, where they accumulate near the plasma membrane. Calcium-dependent exocytosis then releases the granules into the synaptic cleft, where degranulation occurs, meaning that the vesicle-bound cytotoxic biomolecules are released and initiate target cell death. Subsequently, perforin induces cell membrane perforation of the target tumor cell, allowing entry of granzymes, serine proteases among which Granzyme A and B are the most crucial. Granzymes then exert their proteolytic functions and trigger apoptotic pathways. Again, calcium influx into the target cell is important for activating intracellular granzyme function[24] (Figure 3B).
(1) Granzyme B-mediated apoptosis: Activated Granzyme B (AGB) proteolytically targets over 300 protein substrates, cleaving specific peptide bonds after aspartic acid residues at defined recognition sites. Among these substrates, procaspases are particularly important for inducing apoptosis. Notably, though AGB can function in a caspase-in
Firstly, AGB can directly activate the executioner caspase-3 (sometimes, along with caspase-6 and -7) from its pro
Alternatively, AGB can indirectly initiate apoptosis by cleaving initiator caspases such as caspase-10/-8/-9/-2, which initiates the entire cascade from beginning to end, converging from multiple stages. Procaspase cleavage by AGB frequently necessitates further homodimerization within multi-protein complexes, such as the death-inducing signaling complex (DISC), the PIDDsome (p53-induced death domain complex), or the apoptosome, in order to achieve complete caspase activation and eventual execution of apoptosis[25] (Figure 3B).
Furthermore, AGB can intersect with the intrinsic mitochondrial pathway. For example, AGB cleaves ‘BH3 interacting domain death agonist (BID)’, a pro-apoptotic member of the BCL-2 family, into its active form, truncated BID (tBID). Upon activation, tBID relocates proximally toward mitochondria and causes mitochondrial outer membrane permeabilization (MOMP) in the presence of the pro-apoptotic proteins BAX and BAK. This occurs through disruption of mi
MOMP induces the release of two distinct groups of pro-apoptotic proteins from the intermembrane space. The first group contains- Cyt-C (cytochrome-c), SMAC/DIABLO (second mitochondria derived activator of caspases/direct IAP binding protein with low pl) and HTR-A2/Omi (high temperature requirement A2/Omi serine peptidase). Likewise, the second group consists of apoptosis inducing factor (AIF), Endonuclease-G (Endo-G) and Caspase Activated DNase (CAD/DFF 40).
Group-1 triggers ‘caspase-dependent intrinsic apoptosis’. Cyt-C catalyzes formation of the apoptosome, a mac
Again, AGB can induce target cell apoptosis through several pathways. For instance, it can disrupt the anti-apoptotic MCL-1/BIM (myeloid cell leukemia-1/BCL-2 interacting mediator of cell death) complex present on the outer mi
Additionally, AGB can promote MOMP by cleaving the anti-apoptotic protein HAX-1, thereby inducing mitochondrial depolarization and triggering the same downstream apoptotic pathway.
Alternatively, AGB can induce apoptosis independently of the caspase cascade by directly cleaving multiple effector-caspase substrates, including BID, and other essential structural and regulatory proteins such as ICAD, DNA-PKCS, NuMa, Lamin B, PARP-1, and tubulin[26].
Finally, all of the caspase-dependent routes converge onto the common execution pathway following the executioner caspase-3 activation, which is of utmost significance for coordinating the final stages of apoptosis. Caspase-3 proteolytically cleaves a wide range of cytoskeletal and nuclear substrates such as actin, tubulin, DNA repair enzymes and lamins. This sequence of events culminates in the hallmark biochemical and morphologic features of apoptosis, e.g., DNA fragmentation, cytoskeletal restructuring, cytoplasmic and chromatic condensation, blebbing and eventual plasma membrane disruption. The resulting apoptotic bodies are subsequently phagocytosed, ensuring efficient clearance of the dying cells[27].
(2) Granzyme A-mediated apoptosis: Unlike AGB, AGA-based cell death strictly functions in a caspase-independent manner by inducing direct mitochondrial damage, initiated from the organelle itself. AGA severely impairs the mitochondrial and resultant nucleolar stability that results in the same aforementioned morphological attributes of apoptosis. However, it induces a distinct type of DNA damage through nick formation, which is initiated by targeting the SET complex and its components[28].
Upon entry into the target cell cytosol through perforation and subsequent calcium-dependent activation, AGA translocates into mitochondria via the TIM/TOM/PAM pathway. Here, the TOM complex (Translocase of the Outer Membrane) gives access to the mitochondria-destined proteins (AGA here) through the outer mitochondrial membrane following prior identification by their distinct N-terminal pre-sequence. Once inside, the TIM complex (Translocase of the Inner Membrane) mediates further passage of the proteins across the inner membrane into the mitochondrial matrix, and throughout the entire inward-directed translocation process, the PAM complex (Pre-sequence Translocase-Associated Motor) provides the necessary mechanical force by converting biochemical energy from ATP into mechanical energy[29] (Figure 3B).
Upon access, AGA directly cleaves NDUFS3 (NADH: Ubiquinone oxidoreductase core subunit S3)[30], a crucial component of the electron transport chain (ETC) Complex-I, otherwise known as NADH: Ubiquinone oxidoreductase. This cleavage disrupts the stability and function of ETC complex-1, resulting in impaired oxidative phosphorylation and the overproduction of reactive oxygen species (ROS). Interestingly, this significant accumulation of ROS within the mitochondrial matrix is generally regarded as a primary indicator of impending apoptosis, even in the absence of preceding MOMP. This phenomenon imposes severe oxidative stress on the cell, particularly on mitochondria, by disrupting the electron transport chain through a self-amplifying positive feedback loop, ultimately leading to markedly reduced ATP production[27].
Moreover, one of the crucial ROS, the superoxide anion, upon release into the cytoplasm, catalyzes the translocation of the SET complex from the endoplasmic reticulum to the nucleus[31]. The SET complex normally functions as an important DNA repair and chromatin stability maintaining factor, and it is comprised of several noteworthy components, such as - three DNase nucleases: (1) NM23-H1 (an endonuclease); (2) APE-1 [a base excision repair (BER) endonuclease]; and (3) TREX-1 (3’-5’ exonuclease), as well as other regulatory proteins like- the SET protein (NM23-H1 inhibitor and gene expression modulator), pp32 (transcription and cell cycle regulator) and HMGB2 (DNA-binding, damage recognition and conditional degradation facilitating cofactor)[32].
Counterintuitively, upon translocation into the nucleus, the SET complex converts into a genomic destruction tool. This occurs when AGA sequentially enters the nuclear space following the complex and starts cleaving its components, including the SET protein, and thus, freeing the DNases. Activated NM23-H1 makes single-strand incisions in the target cell DNA, which are again expanded by TREX-1 from the incised 3’ end by continuously removing nucleotides in the 3’ to 5’ direction. Thus, TREX-1 nullifies any attempt of the cell to repair its own DNA, blocking the BER mechanism. Additionally, AGA concurrently cleaves and inactivates APE-1, a catalyst of the BER process. Additionally, it targets HMGB-2, preventing it from stabilizing the distorted DNA structure that further compromises its repair, thereby accelerating apoptotic execution[33].
AGA also aids in eventual chromatin relaxation by catabolizing H1 linker histones and cleaving core histone tails, resulting in a more accessible chromatin structure for DNases activity. Finally, these cumulative AGA-driven events facilitate the formation of single-strand, mega-base DNA segments; in contrast to the shorter and double stranded oligonucleosome fragments generated by AGB-mediated apoptosis[33]. Thus, by cleaving the most crucial elements of the SET complex, AGA disrupts the structural and subsequent functional damage to the target cell DNA, the genetic blueprint of life.
Overall, by inflicting irreversible damage to the composition and functionality of both mitochondria and nucleus, CAR-T-modified cells can selectively target and induce apoptosis through both caspase-dependent granzyme B and caspase-independent granzyme A-based perforin-granzyme pathway (Figure 4A).
Cytokine-mediated pathway: Beyond the direct induction of apoptosis via primary degranulation mechanisms (granzyme A and B-mediated pathway) and secondary death receptor-ligand interaction (Fas/FasL pathway), CAR-Ts can induce cytotoxicity through upregulated cytokine secretion. Cytokine release not only amplifies the direct cytotoxic response but also physically modifies the TME to favor immune-mediated tumor clearance. In the context of solid tumors, where antigen heterogeneity and antigen escape are prevalent, the cytokine-driven mechanism can help overcome these challenges by reducing the reliance on direct antigen dependent cell-cell killing, thereby improving therapeutic efficacy[27].
For instance, Textor and his team showed that anti-HER-2 CAR-Ts engineered to secrete cytokines can sensitize the tumor stroma. The resultant upregulation of interferon-gamma (IFN-γ) receptors on stromal cells facilitates a feedback loop in which pre-secreted IFN-γ further activates cytotoxic mechanisms. In addition, cytokine release can modulate the phenotype of tumor-associated macrophages (TAMs), redirecting them towards an M1-like, pro-inflammatory state that is conducive to tumor cell death[25].
Fourth-generation CAR-Ts, often referred to as T cells redirected for universal cytokine-mediated killing (TRUCKs), are designed to combine antigen-specific recognition with constitutive cytokine secretion (e.g., IL-2 or IL-12). This dual mechanism enhances antitumor activity by simultaneously engaging direct cytotoxic mechanisms and altering the TME to recruit additional immune effector cells. Such an approach is particularly advantageous in settings where the loss of tumor antigens or the downregulation of death receptors poses a challenge to conventional CAR-T function[23] (Figure 4B).
Fas/FasL pathway: The death-receptor/death-ligand, Fas/FasL pathway is an alternative, but less dominant, extrinsic route for CAR-T-driven apoptosis. Although primarily involved in immunoregulation under normal physiological conditions, this calcium-independent pathway can be repurposed by CAR-T cells to induce programmed cell death in tumor cells.
Like the granzyme B pathway, Fas/FasL signaling is dependent on and ultimately converges with the caspase cascade to induce tumor apoptosis. Here, following CAR-TAA binding and subsequent activation, homotrimeric FasL expression gets upregulated on the T-cell surface. Thereafter, FasLs on CAR-T can secondarily bind to the Fas receptors (CD95) on the tumor cell and form a complex. This interaction recruits adaptor proteins, such as Fas-associated death domain, which in turn facilitate DISC assembly. Accordingly, DISC formation leads to pro-caspase-8 recruitment and activation, directly activating caspase-3 from its pro-caspase form. Finally, like AGB, executioner caspase-3 triggers the same downstream cascade of the ‘common execution pathway’, ultimately leading to tumor cell apoptosis.
Even though the Fas/FasL pathway is generally considered a secondary killing mechanism, it can also facilitate primary apoptosis through trans-killing in antigen-negative tumor cells. However, it is possible only when the antigen-positive tumor cell population is nearby to activate the CAR-Ts and enable bystander killing through non-antigen-dependent cell-cell interaction. Hong et al[23] demonstrated that when co-cultured with antigen-positive tumor populations, anti-CD19 and anti-CD30 CAR-Ts can induce apoptosis in antigen-negative cells via Fas/FasL interactions. The Fas/FasL pathway-induced cytotoxity was augmented in antigen-negative cells once the ligand-receptor complex formed. The importance of this pathway was again established when the CD95 gene encoding the Fas-receptor protein was experimentally knocked out from CAR-Ts, significantly decreasing caspase-3 activation and subsequent target cell death (Figure 4C).
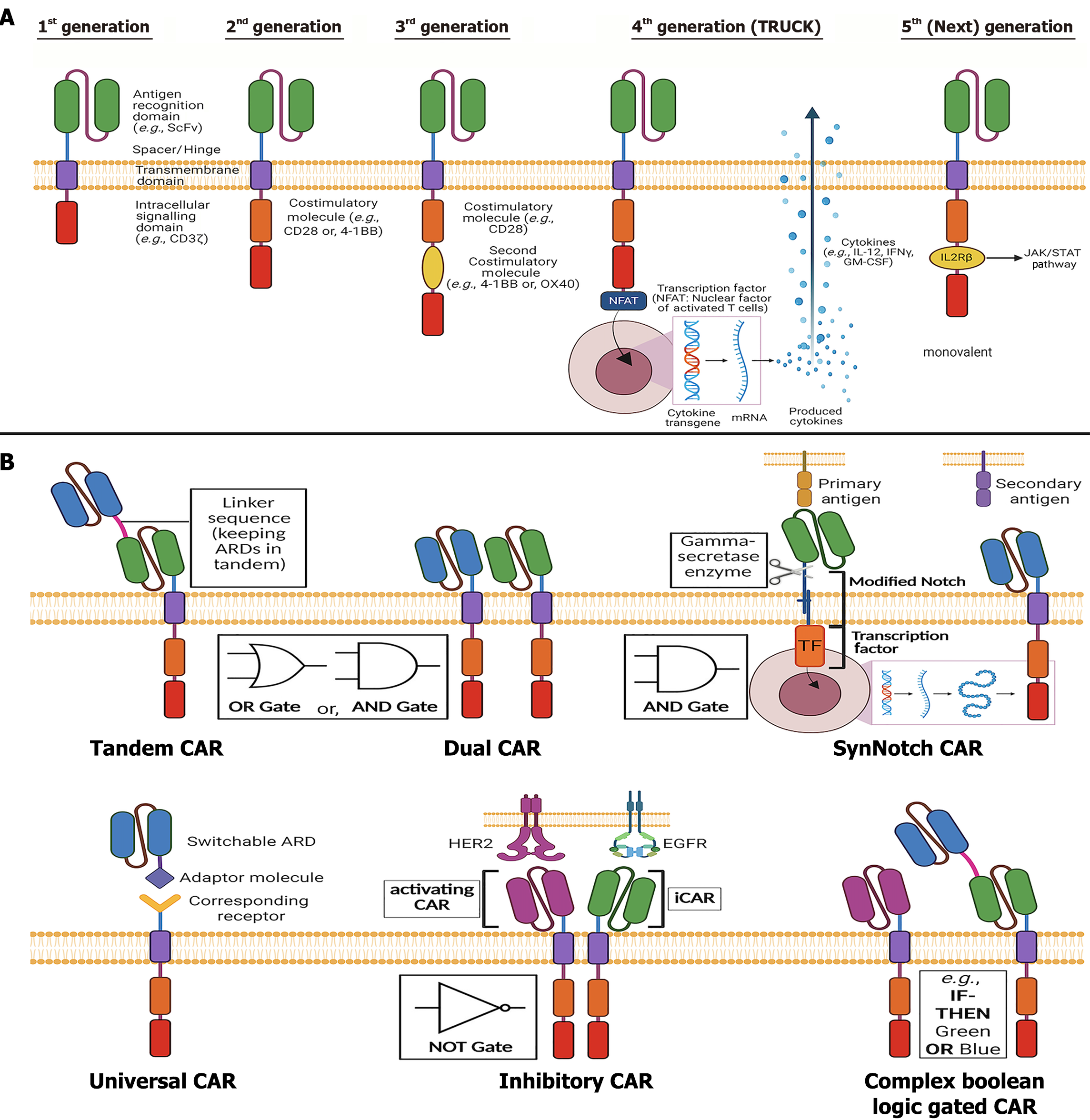
Since their initial development in 1989, CARs have undergone significant advancements, leading to five distinct generations based on the number of CSDs conjoined to the ISD. These modifications have been made to engineer receptor structures with optimal endodomain configurations[20] (Figure 5A).
The first-generation CARs, developed in the late 1990s, lack additional CSDs, making the ISD the sole mediator of T-cell activation following initial recognition through ARD. Although both CD3ζ and FcRγ were considered potential ISDs, CD3ζ was the preferred option, which catalyzed T-cell activation in an MHC-independent pathway through ITAMs[12]. The first fully functional lab-made CAR, initially referred to as the "T-body approach", consisted of an ScFv fused to CD3ζ, marking a pivotal milestone in CAR development[15].
Despite demonstrating promising outcomes in preclinical studies and early-phase clinical trials for hematologic malignancies such as leukemia and lymphoma, as well as solid tumors like neuroblastoma and ovarian cancer, first-generation CARs exhibited limitations. The absence of CSDs resulted in suboptimal activation, limited persistence, and insufficient therapeutic efficacy in later clinical trials[12]. Nevertheless, modest yet beneficial anti-cancer responses were observed with α-CD20-CD3ζ and ScFv-CD3ζ CAR-T therapies of this generation, targeting B-cell lymphoma and neuroblastoma, respectively. Their repeated administration within the neoplastic microenvironment laid the groundwork for the development of subsequent CAR generations[15].
Second generation CAR receptors were developed by adding one single CSD to the CAR molecule, before the ISD, at its endodomain end. This modification substantially enhanced T-cell activation, proliferation, and cytokine production compared to first-generation CARs, making them a superior therapeutic option. In clinical trials targeting TAA, CD19, second-generation CAR-Ts incorporating CD3ζ along with CSD such as CD28 or 4-1BB (CD137) demonstrated significant efficacy in B-cell neoplasia. Notably, a phase I clinical trial reported complete remission (CR) of relapsed or refractory B-cell acute lymphoblastic leukemia (B-ALL) in approximately 90% of patients, highlighting the importance of the CSD in CAR function[15].
Second generation CARs have also been studied in the context of other blood cancers, including B-cell chronic lymphocytic leukemia (B-CLL), diffuse large B-cell lymphoma (also known as the non-Hodgkin lymphoma) and multiple myeloma, along with previously studied B-ALL, all of which showed considerable therapeutic benefits[12]. To mention, all seven approved CAR-T therapies are based on the second generation CAR structure. Moreover, their application is being explored in solid tumors, including glioblastoma, mesothelioma, metastatic sarcoma, and ovarian and pancreatic cancers. Additionally, several alternative CSDs, including CD27, CD40, CD244, ICOS, OX40, DAP10, MYD88, are currently under investigation following promising results in preclinical studies. These efforts highlight the potential to expand the repertoire of effective CSD candidates for future CAR-T cell designs[12].
Building upon the second-generation design, third-generation CARs incorporate an additional CSD within the cytoplasmic region to maximize T-cell activation[12]. Receptors like CD3ζ-CD28-4-1BB and CD3ζ-CD28-OX40 were developed to examine the capacity of T-cell activation, rapid and sustained proliferation, and heightened cytokine release for overall augmented anticancer response[15]. Preclinical studies demonstrated variable outcomes, with some trials reporting superior tumor infiltration, cytotoxic activity, and prolonged T-cell memory. For example, α-CD19-CD3ζ-CD28-4-1BB CAR-Ts exhibited robust antitumor activity in B-CLL, leading to CR in a significant proportion of patients and reducing relapse rates through enhanced memory T-cell formation.
However, in some malignancies such as pancreatic cancer, third-generation CARs did not demonstrate significant advantages over their second-generation counterparts. Furthermore, certain constructs resulted in excessive cytokine release, increasing the risk of cytokine release syndrome (CRS), which can lead to multi-organ failure and mortality[15].
The development of fourth-generation CARs was motivated by the need to mitigate excessive costimulatory activation observed in third-generation CARs. These receptors, commonly referred to as TRUCKs or armored CARs, are also built on the second-generation endo-domain backbone with an integrated NFAT-responsive expression cassette. The NFAT cassettes house a secondary cytokine-expressing transgene such as IL-2, IL-5, IL-12 or IFN-γ etc., that get activated upon TAA-induced CAR activation, resulting in cytokine expression and release. This design enables cytokine secretion in response to antigen stimulation, enhancing the TME’s immunogenicity and facilitating complementary trans-killing of antigen-negative tumor cells[17].
Additionally, these CARs can be engineered to express other immune-modulating molecules, including chemokines, peptides, ligands, receptors, and nanobodies, to augment therapeutic efficacy. The strategic fusion of these immune modulators with the CAR-ISD enables a multifaceted approach to tumor eradication[34]. Dual cytokine expression strategies have emerged. For example, glypican-3 (GPC3)-targeted CAR-Ts co-expressing IL-15 and IL-21 showed superior tumor control in hepatocellular carcinoma xenografts and are being evaluated in clinical trials (NCT04715191, NCT02932956). Beyond cytokines, fourth-generation CARs are now being engineered to express non-cytokine immune-modulating proteins such as PD-1 blocking antibodies, enzymes, transcription factors (TF), and chemokines like IL-7 and CCL19, which improve CAR-T homing and TME penetration[28].
Fifth-generation CARs, also referred to as "next-generation CARs", represent the latest advances in CAR structural engineering, aimed at overcoming the limitations of the previous generations with improved therapeutic efficiency. They can be broadly categorized into two classes - monovalent and multivalent fifth generation constructs. Consistent with the aforementioned classifications, this section will mostly focus on monovalent fifth generation CARs.
Monovalent fifth generation CARs are structurally based on the second generation CAR endo-domain. However, instead of additionally incorporating a second CSD or secondary cytokine expressing transgene, a truncated cytokine receptor domain e.g., the IL2Rβ of the IL-2 receptor, is integrated in the structure. Subsequently, following an antigen-dependent activation, the IL2Rβ domain promotes the JAK/STAT signaling pathway, with a preferential activation of STAT3 and STAT5. This downstream signaling cascade enhances T cell proliferation, persistence and overall survival through this additional support of targeted pro-inflammatory cytokine expression in the TME.
Conversely, advanced CAR-T strategies such as "on/off switch CARs" and "logic-gated CARs”, discussed in depth in the next chapter, fall under the very same fifth generation category but can be classified as multivalent constructs. These CARs are designed to target tumors through multi-antigen recognition, addressing challenges such as antigen loss and tumor heterogeneity. Overall, these next-generation developmental approaches reflect the broader evolution of CAR-T therapy into a more precise, controllable, adaptable and safer treatment strategy[34].
Table 2 summarize a comparative analysis of all five generations of CAR structure, focusing on their structural differences, key characteristics, prominent limitations, approved products and pivotal clinical trials[29-38].
| CAR generation | Intracellular domain structure | Key features and limitations | Pivotal trials1 | Approved/trial phase |
| 1st | ISD (e.g., CD3ζ); No CSD | Weak activation, limited proliferation and persistence; low clinical efficacy | None | None |
| 2nd | ISD + 1 CSD (e.g., CD28 or 4-1BB) | Enhanced activation, cytokine release, persistence, and clinical efficacy. Limited long-term persistence | ELIANA: NCT01029366, NCT02435849[31]; (Kymirah), Zuma-1: NCT02348216 | All 7 FDA-approved CAR-T therapies |
| 3rd | ISD + 2 CSD (e.g., CD28 and 4-1BB) | Mixed results; no clear benefit over 2nd-gen; increased risk of severe CRS | HD-CAR-1 trial: (NCT03676504), ENABLE-1: (NCT04049513) | Phase I/II, Phase I |
| 4th | (2nd generation CAR: ISD + 1 CSD) + secondary transgene (e.g., NFAT) for cytokine secretion | Inducible cytokine expression; potential to overcome antigen loss; risk of on-target/off-tumor effects | NCT03778346; NCT03542799 | Phase I, Phase I |
| 5th | 2nd Generation CAR + IL-2 membrane receptor ISD (IL2Rβ here) | Integrates ISD, CSD, and cytokine signaling; monovalent design limits antigen coverage | NCT05665062; NCT05666635 | Early Phase I, early Phase I, |
As mentioned above, beyond the conventional CAR structure with varied generations, including the fifth generation monovalent CARs, may other sophisticated approaches have been adopted over the years, which fall under the umbrella of fifth generation multivalent CAR design. These next generation constructs are founded on the same core principle of antigen targeting but integrate components of diverse biomolecules, such as the Notch receptor, Tag protein, naturally occurring ligand-receptor pairs or, even synthetic elements. Thus, this evolving category is demonstrating a shift toward customizable and modular designs to ensure enhanced precision, precaution and performance. Often, these modifications help the CAR-Ts through either minimizing on-target/off-tumor (OT/OT) effect, one of the most prominent adverse effects of CAR-T therapy, or increasing range of targetable antigens as per requirement. Some of these advanced CAR-Ts are discussed below (Figure 5B).
Dual CAR-Ts incorporate two distinct ARDs expressed on two separate CAR constructs on a single T cell, enabling them to simultaneously target two antigens (TAA) on a malignant cell. Like traditional CARs, these dual CARs are equipped with ISDs (e.g., CD3ζ), co-stimulatory domains (CSDs), a hinge, and a TMD. However, their two ARDs (e.g., ScFv or VHH) provide enhanced targeting flexibility and safety by utilizing “AND” or “OR” logic gates[19]. The AND gate requires simultaneous binding to both antigens, amplifying activation signals and leading to precise killing of target cells. In contrast, the OR gate compensates for antigen loss or mutation, allowing activation upon binding to either antigen[15]. Notable examples include bispecific CD19-CD20 and CD19-CD22 CAR-T therapies, currently under clinical trials for leukemias (e.g., R/R B-ALL, CLL) and lymphomas (e.g., SLL, NHL). CD19-CD22 CAR-T therapy has demonstrated strong efficacy against R/R B-ALL, efficiently targeting malignant cells expressing either antigen. A Phase 1 clinical study reported a 28.57% CR rate in seven B-ALL/DLBCL patients[36].
In solid tumors, AND-gated dual CAR-Ts have shown promising results in targeting CEA+ and MSLN+ pancreatic adenocarcinoma (AsPC-1) cell lines in xenogeneic models. The robust tumor-killing effect did not affect monospecific TAA+ healthy cells, highlighting improved precision and reduced off-target effects. Preclinical studies targeting solid tumors such as breast, pancreatic, prostate, ovarian, and glioblastoma multiforme have employed dual CAR-Ts (e.g., ERB2-MUC1, MUC1-PSCA, PSMA-CD19/PSCA, Meso-Fra, and EGFR-EGFRvIII). These studies consistently demonstrated enhanced tumor cytotoxicity, cytokine production (e.g., IL-2, IFN-γ), T cell proliferation, persistence, and reduced off-target effects, solidifying dual CAR-T therapy as a promising approach for improved therapeutic outcomes[19].
Tandem CAR-Ts represent another bispecific CAR-T strategy, enabling simultaneous and synergistic signal transduction upon antigen binding. Unlike Dual CAR-Ts, Tandem CAR-Ts incorporate two ARDs (e.g., ScFv) within a single CAR structure, linked in tandem by flexible amino acid sequences (e.g., serine, glycine). This design simplifies manufacturing, making it a more cost-effective and streamlined alternative[15]. Tandem CAR-Ts can function as either an AND gate, requiring simultaneous recognition of both antigens for precise activation and reduced off-target toxicity, or as an OR gate, where binding to either antigen is sufficient to activate T cells. This adaptability addresses challenges like tumor immune evasion caused by antigen heterogeneity or loss[19].
Several Tandem CAR-T therapies have demonstrated therapeutic success against hematological malignancies, including leukemias and lymphomas, as well as solid tumors such as ovarian and breast cancers, glioblastoma, and multiple myeloma. One pioneering example is the CD19-HER2 Tandem CAR-T, which exhibited effective antigen detection and cytotoxicity against both CD19+ Raji lymphoma cells and HER2+ Daoy medulloblastoma cells separately in an OR gate manner. Furthermore, this CAR-T construct could be selectively activated in an AND gate manner when co-cultured with target cells, resulting in cytokine release (e.g., IFN-γ, IL-2). Notably, it exhibited no cytotoxic activity against antigen-negative MDA-MB-468 cells, underscoring its precision[39]. Similarly, CD19-CD20 Tandem CAR-Ts showed enhanced AND gate-based cytotoxicity in leukemic cells, with increased expression of IFN-γ, TNF-α, IL-2, and GM-CSF[40]. Other examples include CD19-CD22, CD19-CD133, CD19-CD79a, CD70-B7H3, Her2-IL13Rα2, and EGFRvIII-IL13Rα2 Tandem CAR-Ts. These therapies, evaluated in preclinical and clinical studies, demonstrated reduced antigen escape, selective targeting of dual-antigen-positive cancer cells, robust cytokine production, and improved safety and efficacy. These findings establish Tandem CAR-Ts as promising anti-tumor agents with superior persistence and therapeutic outcomes compared to single-antigen CAR-Ts in vitro and xenogeneic in vivo models[41,42].
SynNotch is an innovative AND-gated CAR-T therapy designed to collaboratively recognize multiple antigens. It can also function as an IF-THEN gated approach when required. Inspired by wild-type Notch receptor proteins, which regulate cellular processes such as embryonic development and tissue regeneration, SynNotch mimics the modular structure of natural Notch proteins[43]. The wild-type Notch receptor consists of three components: An extracellular domain that binds ligands (e.g., Delta, Jagged), a TMD, and an intracellular domain that functions as a TF upon activation[44,45]. SynNotch receptors are engineered on T cells to target specific antigens. Ligand-receptor engagement cleaves synthetic TFs (e.g., Gal4-VP64, tetR-VP64), inducing CAR expression against a secondary antigen. Full T cell activation occurs only when both the SynNotch receptor and CAR engage their respective antigens, ensuring precise cis-killing of dual-antigen-expressing tumor cells while reducing off-target toxicity through AND gating[46].
SynNotch CAR-Ts have shown efficacy in solid tumors with antigen heterogeneity (e.g., lung adenocarcinoma, mesothelioma, ovarian, breast, colon, pancreatic cancers) and hematological malignancies (e.g., ALL, CLL). Various designs include CD19-SynNotch receptors triggering anti-mesothelin CAR-Ts, GFP-SynNotch receptors activating anti-CD19 CAR-Ts, and EpCAM- or B7H3-SynNotch receptors stimulating anti-ROR1 CAR-Ts for breast cancer models[19,47]. Beyond CAR expression, SynNotch receptors can also induce cytokine release or mAb production. For instance, a study demonstrated that IL-10 secretion alongside CAR expression using Axl-SynNotch interaction in lung, breast, pancreatic, and colon cancers[48]. Additionally, SynNotch CAR-Ts can perform trans-killing of antigen-negative cells near antigen-positive tumor cells within the TME, making it an effective strategy against immune escape mechanisms[49]. In glioblastoma, where antigens like EGFRvIII are highly specialized but heterogeneous, SynNotch CAR-Ts have been used in a "prime and kill" approach. SynNotch receptors targeting EGFRvIII or MOG prime T cells, which then express tandem OR-gated CARs targeting EphA2 or IL13Rα2. This enables cis-killing of dual-antigen-expressing cells and trans-killing of nearby cells, leading to comprehensive tumor eradication and long-term persistence[48].
Universal CAR-T (Uni-CAR) therapy addresses challenges of structural rigidity and antigen inflexibility in conventional CAR-Ts. Uni-CARs are based on a split structure that enables adaptability[19]. In this design, a soluble tumor-antigen-targeting ligand (TL) directly binds to the TAA, while a universal immune receptor (UIR) on the T cell indirectly engages the antigen through the TL. The UIR comprises an extracellular adapter domain (EAD) and an ISD. The EAD recognizes a specific "tag" present on the TL, forming a bridge between the TL and ISD. This connection triggers full T cell activation, leading to programmed malignant cell death[18]. Four types of Uni-CAR systems have been developed, each utilizing a unique UIR structure: (1) Tag-Specific UIR: Directly binds to a tag on the TL; (2) Anti-Tag Specific UIR: Uses an anti-tag antibody for tag recognition; (3) Bi-Specific Immune Receptor: Simultaneously engages two distinct tags, enhancing flexibility; and (4) Antibody-dependent cellular cytotoxicity-based immune receptor: Relies on antibody interactions to mediate cytotoxicity.
This modular approach allows Uni-CAR-Ts to adapt to different TAAs without the requirement for new CAR synthesis, offering a flexible and cost-effective therapeutic strategy[50].
Inhibitory CAR-T (i-CAR-T) therapy has been developed to protect healthy cells from CAR-T-induced cytotoxicity, while improving the ability of CAR-Ts to distinguish between malignant and non-malignant cells. This approach employs a bicistronic Dual-CAR system, where two independent CARs are expressed on a single T cell[51].
The first CAR is a conventional structure targeting TAAs and mediating standard cytotoxicity. In contrast, the second CAR is inhibitory, designed using immunoregulatory molecules such as PD-1, PDL-1, and CTLA-4, which act as natural immune checkpoints. This inhibitory CAR incorporates the endogenous signaling domains of these molecules, paired with an extracellular recognition domain specifically targeting an antigen exclusively expressed on healthy cells. This design prevents T cell activation when the inhibitory CAR engages its antigen, even if the conventional CAR binds to a TAA[15].
Prominent examples of i-CAR-T therapies include: (1) iHLA-A2-HER2-CAR-T therapy: This anti-HER2 CAR-T therapy includes an HLA-A2-targeting i-CAR. In vitro and in vivo studies in immunodeficient mouse models demonstrated potent cytotoxicity against HLA-A2-negative tumor cells while sparing HLA-A2-positive healthy cells[52]; and (2) iKP-19-CAR-T therapy: This therapy combines an anti-CD19 CAR with an HLA-C1-targeting inhibitory CAR (iKP CAR). The inhibitory CAR uses KIR (killer-cell immunoglobulin-like receptor) as the ARD and PD-1 as the ISD. In vitro and xenogeneic in vivo studies showed selective cytotoxicity against CD19(+)HLA-C1(-) Burkitt’s lymphoma cells while sparing CD19(+)HLA-C1(+) normal B cells, thereby reducing off-target effects such as B-cell aplasia[53].
This dual-CAR strategy demonstrates significant potential in enhancing precision and safety in CAR-T therapies, ensuring selective targeting of malignant cells while preserving healthy tissues.
Physiological CAR-T therapy was developed to address the issue of immunogenicity associated with murine-derived ARDs (e.g., ScFv) in CARs expressed on human T cells. Immunogenicity, the immune system's response to foreign entities, can reduce CAR-T persistence and effectiveness, potentially leading to anaphylaxis. Unlike standard CARs, physiological CARs incorporate naturally occurring receptor-ligand systems as ARDs instead of conventional ScFv. These natural receptor-ligand interactions provide a more straightforward signaling cascade, leading to faster T cell activation and cytotoxicity while mitigating immunogenicity[19,15].
Several physiological CAR-T therapies have been studied: (1) CD70-CD27 CAR-T therapy: CAR with a CD27 receptor ARD has been designed to target CD70-expressing tumor cells. This system demonstrated rapid cytotoxicity of CD70(+) tumor cells while preventing AICD in CAR-Ts[54]; (2) NKG2D CAR-T therapy: Human NKG2D receptor ARD to target NKG2D-ligand-positive lymphoma cells, achieving robust tumor eradication with enhanced cytokine production[55]; (3) HER3 and HER4 CAR-T therapy: CARs using HER3 and HER4 receptors have been developed to target heregulin-presenting breast tumor cells[56]; (4) IL13-Zetakine CAR-T therapy: CAR-Ts expressing IL13-zetakine receptors have been engineered to effectively target IL13Rα2-positive glioblastoma cells[57]; (5) VEGF ligand CAR-T therapy: VEGF ligand-expressing CAR-Ts were utilized to target VEGF-receptor-positive tumor cells[58]; and (6) NKp30 CAR-T therapy: CAR-Ts were created using the NKp30 receptor to target B7-H6 ligand-positive tumor cells, demonstrating strong cytotoxicity and cytokine release[59].
These physiological CAR designs provide improved tumor eradication through efficient cytotoxicity, elevated cytokine production, and enhanced persistence, overcoming the limitations of conventional CAR-T therapies[19].
In addition to the CAR-T types described earlier, numerous innovative CAR designs are under preclinical and clinical investigation, showing promise in enhancing efficiency, safety, and efficacy for cancers that remain challenging to manage. Some of these advanced CARs include: (1) Trivalent CAR-Ts: These CAR-Ts express three independent CARs on a single T cell or include three ARDs linked in tandem, enabling simultaneous multi-antigen targeting[60]; (2) Boolean Logic-Gated CAR-Ts: These CAR-Ts employ programmable logic gates such as AND, OR, NOT, IF-NOT, and IF-BETTER gates. By combining multiple CARs, they enable precise and efficient multi-targeting based on predefined antigen recognition logic[61]; (3) Chimeric Switch Receptor (CSR) CAR: CSR CARs reverse the function of inhibitory CARs by designing the extracellular domain of immune checkpoints (e.g., PD-1, CTLA-4) to pair with conventional ISDs (e.g., CD28-TM/CD28-ISD). This design converts immunosuppressive checkpoint signals into T cell activation signals[62]; (4) Synthetic TCR and Antigen Receptor (STAR) CAR: STAR CARs integrate the ISD of a TCR with the extracellular structure of a CAR, combining the TCR's strong signal transduction with the CAR's antigen recognition capabilities[63]; and (5) On/Off switch CAR-Ts: These CARs are split structures where the ISD and co-stimulatory domain assemble only in the presence of a specific drug, functioning as an "On-switch" to activate T cells in the presence of TAAs. Conversely, "Off-switch" CARs use a molecule to trigger CAR protein degradation, halting T cell activity[64].
Additional examples of next-generation CAR-T therapies include: (1) Photon- or ultrasound-switchable CARs: Activated via external light or ultrasound signals for precise control[65]; (2) Suicide gene-based CARs: Incorporate genes like HSV-tk, iCasp9, or EGFRt to induce CAR-T apoptosis, if needed[66]; (3) NK CARs: CARs designed for NK cells to enhance innate immune responses[67]; and (4) CRISPR CARs: Utilize CRISPR technology for precise genome editing to improve CAR function or overcome resistance mechanisms[68].
These advanced CAR-T therapies demonstrate significant potential in addressing existing challenges, improving antigen targeting, enhancing antitumor activity, and broadening the application of CAR-T therapy for various cancers.
While CAR-T therapy has achieved remarkable success in hematologic malignancies, alternative cell-based immunotherapies such as CAR-engineered NK (CAR-NK) and CAR-macrophage (CAR-M) therapies are emerging as promising strategies to address current limitations, particularly in solid tumors. CAR-NK cells offer several advantages over CAR-Ts. They exhibit intrinsic cytotoxicity, secrete immunoregulatory cytokines like IFN-γ, and are less likely to induce graft-vs-host disease (GvHD), making them suitable for off-the-shelf allogeneic therapies. Additionally, CAR-NK cells exhibit a lower risk of cytokine release syndrome and neurotoxicity, common adverse events associated with CAR-T therapies[69]. Recent clinical trials using cord blood-derived CD19-directed CAR-NK cells have shown favorable outcomes in B-cell malignancies, with high response rates and minimal toxicity[70]. Engineered CAR-NK platforms such as FT596 and NKX101 are undergoing clinical development to target CD19 and NKG2D ligands, respectively, further demonstrating the translational potential of CAR-NK therapies[69].
CAR-M represent another novel therapeutic avenue. Unlike T or NK cells, macrophages have innate phagocytic abilities and naturally infiltrate solid tumor tissues. Engineered CAR-Ms not only induce antigen-specific phagocytosis but also remodel the TME by secreting pro-inflammatory cytokines and converting M2-like immunosuppressive macrophages into tumoricidal M1 phenotypes. The first-in-human phase I trial of CT-0508, a HER2-specific CAR-M product, reported promising safety and early signs of efficacy in solid tumors[71,72]. Together, CAR-NK and CAR-M therapies hold significant promise in overcoming limitations posed by CAR-Ts, particularly in solid tumors and in patients where autologous T cell collection is not feasible[73].
The regulatory approval of CAR-T therapies marks a transformative step in personalized cancer treatment, particularly in hematologic malignancies. In 2017, the United Staes FDA approved the first CAR-T product, tisagenlecleucel (Kymriah), for pediatric and young adult patients with relapsed or refractory B-ALL, followed by approvals of axicabtagene ciloleucel (Yescarta), brexucabtagene autoleucel (Tecartus), and lisocabtagene maraleucel (Breyanzi) for various forms of lymphoma and multiple myeloma. These approvals were granted under accelerated pathways due to compelling clinical efficacy, with the EMA similarly endorsing these products under conditional marketing authorization[74]. Regulatory bodies require stringent manufacturing controls, patient monitoring for CRS and neurotoxicity, and long-term follow-up for potential genotoxicity. The FDA has also released guidance documents to facilitate the development of cell and gene therapies, emphasizing the need for robust clinical trial design, potency assays, and comparability protocols for autologous products[75].
As of 2024, only seven CAR-T therapies have received marketing authorization from the FDA and EMA (including the European Economic Area), starting with Tisagenlecleucel's approval in 2017. These therapies primarily target CD19, a widely expressed pan-B-cell marker, and are approved for use against seven B-cell hematologic malignancies with remarkable clinical outcomes. CD19 is expressed across all stages of B-cell development, from progenitors to plasma cells, allowing precise targeting and significantly reducing on-target, off-tumor toxicity in B-cell leukemias and lymphomas. However, this specificity leads to the elimination of the entire B-cell population, resulting in B lymphocyte aplasia and subsequent immunodeficiency due to hypogammaglobulinemia. Fortunately, this can be effectively managed through intravenous immunoglobulin infusion therapy to restore antibody levels[8,18]. Additionally, B-cell maturation antigen (BCMA) forms the basis of two approved therapies, with other antigens like CD20, CD22, HER2, EGFRvIII, and IL-13Rα2 under investigation for both hematological and solid tumors. Notably, all approved CAR-T therapies are currently indicated for blood-related cancers. Hematologic malignancies offer superior target accessibility due to the absence of a solid extracellular matrix, a key challenge in treating solid tumors. This highlights the continued focus on optimizing CAR-T therapies for broader applications while maintaining their efficacy and safety[18]. A summary of FDA- and EMA-approved CAR-T therapies, including their manufacturers, targets, indications, and approval details, is presented in Table 3[76,77].
| Product name (generic name) | Manufacturer | Target antigen | ISD | CSD | Vector | Indications | Pivotal clinical trials | Regulatory approval |
| Kymriah (Tisagenlecleucel) | Novartis Pharmaceuticals Corporation | CD-19 | CD3ζ | 4-1BB | Lentiviral | B-ALL, LBCL, HGBCL, FL | NCT01626495, ENSIGN (NCT02228096), ELIANA (NCT02435849) | FDA: August 30, 2017; EMA: August 23, 2018 |
| Yescarta (Axicabtagene ciloleucel) | Kite Pharma, Inc. | CD-19 | CD3ζ | CD28 | γ-retroviral | PMBCL, LBCL, HGBCL, FL | ZUMA-1 (NCT02348216), ZUMA-5 (NCT03105336), ZUMA-7 (NCT03391466), ZUMA-23 (NCT05371093) | FDA: October 18, 2017; EMA: August 23, 2018 |
| Tecartus (Brexucabtagene autoleucel) | Kite Pharma, Inc. | CD-19 | CD3ζ | CD28 | γ-retroviral | MCL, B-ALL (FDA only) | ZUMA-2 (NCT02601313), ZUMA-3 (NCT02614066) | FDA: Jul 24, 2020; EMA: December 14, 2020 |
| Breyanzi (Lisocabtagene maraleucel) | Juno Therapeutics, Inc. | CD-19 | CD3ζ | 4-1BB | Lentiviral | FL3B, LBCL, HGBCL, PMBCL | TRANSCEND NHL 001 (NCT02631044), TRANSCEND CLL 004 (NCT03331198), TRANSCEND FL (NCT04245839) | FDA: February 5, 2021; EMA: April 4, 2022 |
| Abecma (Idecabtagene vicleucel) | Celgene Corporation | BCMA | CD3ζ | 4-1BB | Lentiviral | MM | KarMMA (NCT03361748), KarMMA-2 (NCT03601078), KarMMA-3 (NCT03651128) | FDA: March 26, 2021; EMA: August 18, 2021 |
| Carvykti (Ciltacabtagene autoleucel) | Johnson & Johnson Innovative Medicine | BCMA | CD3ζ | 4-1BB | Lentiviral | MM | CARTITUDE-1 (NCT03548207), CARTITUDE-2 (NCT04133636), CARTITUDE-4 (NCT04181827) | FDA: February 28, 2022; EMA: May 25, 2022 |
| Aucatzyl (Obecabtagene autoleucel) | Autolus Inc. | CD19 | CD3ζ | 4-1BB | Lentiviral | B-ALL | FELIX (NCT04404660) | FDA: November 8, 2024 |
Despite growing success, challenges remain in harmonizing global regulatory standards and addressing complexities in scaling up manufacturing, cost-effectiveness, and post-marketing surveillance. As next-generation and non-T cell platforms enter clinical testing, adaptive regulatory frameworks will be critical to ensure both innovation and patient safety.
CAR-T therapy in cancer treatment presents significant challenges and potential toxicities (Figure 6). Issues such as CAR-T exhaustion, antigen escape due to tumor heterogeneity, and difficulties in trafficking and infiltration into tumor sites are prominent. Moreover, toxicities like CRS, tumor lysis syndrome (TLS), neurotoxicity, and immunogenicity require careful management. This section aims to examine these complexities, offering insights into strategies for optimizing CAR-T therapy's efficacy while minimizing associated risks.
While CAR-T therapy has shown remarkable success in treating various hematological malignancies, it continues to face significant challenges, especially in the case of targeting solid tumors. These limitations arise from both the intrinsic dysfunctions of CAR-Ts, as well as the extrinsic barriers imposed by the TME, which together affect CAR-T therapy efficacy and persistence (Figure 6A).
Understanding these limitations is essential for the development of next-generation CAR designs with safer and improved clinical outcome.
CAR-T exhaustion: CAR-T exhaustion is a major challenge, particularly in solid tumors like lung cancer. It occurs due to persistent antigen exposure, increased inhibitory receptors, and suppressive immune cells and cytokines in the TME. The NR4A TF family plays a key role in this process, limiting CAR-T function in solid tumors[78]. Recent studies have demonstrated that knocking out all three NR4A genes (NR4A1/2/3) in CAR-Ts enhances their mitochondrial oxidative phosphorylation, leading to improved persistence and antitumor activity in solid tumor models, including lung cancer. These NR4A triple knockout CAR-Ts exhibit reduced expression of exhaustion markers such as PD-1, TIM-3, and LAG-3, and maintain a memory-like phenotype under continuous antigen exposure. Mechanistically, NR4A ablation improves mitochondrial fitness, thereby sustaining energy production and enhancing the durability of CAR-Ts within the TME. These findings suggest that targeting NR4A TF could be a promising strategy to overcome CAR-T exhaustion in solid tumors[79].
Antigen escape: Antigen escape is another major challenge in CAR-T therapy, largely due to antigen heterogeneity. In solid tumors like lung cancer, antigen loss and resistance mechanisms contribute to relapse[78]. Cancer cells evade therapy by partially or completely losing target antigen expression, reducing CAR-T effectiveness[12]. For example, CD19-targeted CAR-T therapy in ALL and BCMA-targeted CAR-T therapy in multiple myeloma led to disease recurrence due to antigen escape. Similarly, IL13Ra2-targeted CAR-T therapy in glioblastoma resulted in relapse due to reduced IL13Ra2 expression[78]. To counteract antigen escape, approaches like dual CAR constructs and tandem CARs targeting multiple antigens are being explored[12]. Advanced tandem and bispecific CAR designs, along with next-generation sequencing-based surveillance, have been proposed to improve durability of response and detect escape variants early in therapy[80].
Trafficking and infiltration: CAR-T therapy faces significant challenges in effectively trafficking and infiltrating solid tumors like lung cancer. Chemokines, chemokine receptors, adhesion molecules, and the TME influence T-cell infiltration[12]. Additionally, lung cancer's fibrotic stroma, abnormal collagen deposition, and cancer-associated fibroblasts create physical barriers that hinder CAR-T penetration and efficacy[78]. To overcome these barriers, alternative delivery methods, such as local administration, have been proposed. Preclinical studies demonstrated that intraventricular injection improved CAR-T therapy outcomes in breast cancer brain metastases and glioblastoma. Other promising strategies include engineering CAR-Ts to express chemokine receptors that match tumor-derived chemokines and modifying CAR-Ts to degrade extracellular matrix components, improving infiltration and antitumor efficacy[12]. Recent studies also highlight the use of matrix-remodeling enzymes, such as heparanase and collagenase, co-expressed in CAR-Ts to facilitate deeper tumor penetration. Moreover, CAR-Ts can be engineered to express dominant-negative receptors that resist TME-imposed signaling barriers, enhancing their migratory capabilities. Novel nanoparticle-guided or microdevice-based local delivery approaches are also under investigation for precise CAR-T placement within tumor tissues. These innovations emphasize the need to combine physical, genetic, and delivery-based strategies to optimize CAR-T localization and efficacy in solid tumors[81].
Immunosuppressive TME: The TME in solid tumors is highly immunosuppressive, limiting CAR-T function. MDSCs, TAMs, and Tregs contribute to this suppression by secreting factors like TGF-β, IL-10, ARG-1, iNOS, COX2, PGE2, FAP, and PD-L1, which regulate metabolism, cytokine networks, and immune checkpoints[72]. Additionally, the TME is oxidative, hypoxic, acidic, and nutrient-starved, further impairing immune responses[46]. Checkpoint pathways like PD-1 and CTLA-4 weaken antitumor immunity, fostering an environment that supports tumor progression. Strategies to counteract TME suppression include combination immunotherapy with CAR-Ts and checkpoint inhibitors, enhancing T-cell persistence and function. Engineering CAR-Ts to resist immunosuppressive signals or deliver immunostimulatory signals holds promise for improving therapeutic efficacy in the hostile TME[8]. Recent studies found that arming CAR-Ts with dominant-negative TGF-β receptors or PD-1 switch receptors can enhance their activity within suppressive TMEs. Novel CAR designs incorporating cytokine secretion modules, such as IL-12 or IL-18, have shown improved local immune activation and tumor regression. Additionally, targeted depletion of immunosuppressive stromal elements using bispecific CAR-T constructs is gaining attention for improving tumor infiltration and persistence. These strategies collectively aim to transform the TME from an immunosuppressive to an immunostimulatory landscape, offering new therapeutic opportunities for treating solid tumors[8,76].
Genetic modification of T cells to enhance their cancer-fighting ability also increases the risk of toxicities, making their classification crucial in clinical trials. These adverse events are categorized as:
On-target-on-tumor toxicity: This includes CRS and TLS. CRS is a common toxicity marked by fever, nausea, fatigue, and cardiac dysfunction. Elevated IL-6 levels correlate with severity, and severe cases can lead to shock and multi-organ failure. Management includes high-dose corticosteroids and IL-6 receptor antagonists like tocilizumab[46,74].
TLS results from rapid tumor destruction, causing metabolic imbalances and renal failure. Elevated phosphate, potassium, and uric acid levels are key indicators[12]. Management includes hydration, uric acid-lowering agents, and electrolyte monitoring[72].
On-target-off-tumor toxicity: It occurs when CAR-Ts attack normal tissues expressing the targeted antigen. CAR-Ts targeting carbonic anhydrase IX in renal cell carcinoma caused cholestasis[77]. HER2/neu-specific CAR-Ts led to fatal respiratory failure due to antigen recognition in lung tissue[72]. CD19-targeted CAR-T therapy induces B-cell aplasia, which is manageable with gamma globulin infusion[46].
Off-target-off-tumor toxicity: This occurs when CAR-Ts attack unintended antigens or self-activate due to Fc receptor interactions. Though cross-reactive antigen recognition has not been observed, an enhanced-affinity TCR therapy led to fatal cardiac toxicity, emphasizing the need for caution[12].
Neurotoxicity: Common in CD19-specific CAR-T therapy, neurotoxicity presents as confusion, seizures, and encephalopathy[12]. Cytokine-mediated inflammation and blood-brain barrier dysfunction may contribute[72]. Management involves corticosteroids, IL-1 receptor antagonists, and GM-CSF neutralization, though tocilizumab remains uncertain in efficacy[46].
Immunogenicity: CAR-T therapy can trigger immune responses due to mouse-derived mAbs in CAR constructs, leading to anaphylaxis[12]. Humanizing ScFvs or using humanized antibodies reduces immunogenicity and improves CAR-T persistence[48].
Genotoxicity- insertional mutagenesis: Viral vectors used in CAR-T therapy may integrate into oncogenic regions, posing a risk of insertional mutagenesis. Although no cases have been reported, the long-term persistence of CAR-Ts makes this a future concern[12,80].
GvHD: GvHD occurs in transplant recipients, where donor-derived CAR-Ts attack host tissues. Though rare in early CAR-T trials, a chronic GvHD case was managed with corticosteroids. Genome editing strategies, such as disabling αβ T-cell receptors, help mitigate GvHD risks[46].
The possible toxicities of CAR-T therapies are illustrated in Figure 6B.
As CAR-T therapy continues to evolve, identifying novel targetable antigens is crucial for expanding its applicability, particularly in solid tumors and refractory hematologic malignancies. Several promising antigens are currently under investigation to overcome antigen escape, enhance tumor specificity, and improve clinical efficacy. GPC3, highly expressed in hepatocellular carcinoma, has shown encouraging preclinical and clinical outcomes[78]. Other emerging antigens include IL13Rα2 in glioblastoma, CD70 in hematologic and solid malignancies, and mesothelin in pancreatic and ovarian cancers, all of which are undergoing clinical trials to assess their therapeutic value. B7-H3 (CD276), a pan-cancer antigen overexpressed in various solid tumors (e.g., neuroblastoma, osteosarcoma), has demonstrated robust antitumor activity in preclinical models, with early-phase trials showing manageable safety profiles[79,12]. Another emerging target, CLDN18.2, has gained attention for its high expression in gastric and pancreatic cancers, with Phase I/II trials (NCT04404595) reporting partial responses in heavily pretreated patients[80]. CDH17, a gut-specific marker, is being explored for colorectal and gastric cancers, with preclinical studies showing potent tumor regression in xenograft models[81]. In hematologic malignancies, CD38 is under investigation for relapsed/refractory multiple myeloma, with dual-targeting CAR-Ts (CD38/BCMA) showing enhanced efficacy in overcoming antigen escape[82]. GPRC5D, another myeloma-associated antigen, has shown remarkable responses in early trials (NCT04555551), including patients refractory to BCMA-directed therapies[83]. Efforts are also underway to target TROP2, a glycoprotein overexpressed in triple-negative breast and lung cancers, with armored CAR-Ts incorporating cytokine secretion to improve persistence[84].
These efforts aim to refine CAR-T therapy by targeting tumor-specific markers while minimizing off-tumor effects, paving the way for more effective and durable treatment options[11,85-87]. The following table (Table 4) summarizes the most promising target antigens currently being explored in clinical trials, along with their associated cancers, study phases, and outcomes.
| Target specific CAR-T | Targeted cancer | Prominent clinical trials | Trial phase | Study outcome | Ref. |
| Hematologic cancers | |||||
| CD20-CAR | Leukemias and lymphomas (e.g., R/R B-NHL) | NCT03277729, NCT04007029, NCT04697940, NCT01735604 | Phase 1/2 | NCT03277729: High CR+, ORR+ and safety profile; NCT01735604: 54.55% CR+, 18.18% SD+, 180 days median PFS | [88] |
| CD22-CAR | Leukemias and lymphomas (e.g., R/R B-ALL) | NCT03262298, NCT05470777, NCT055078227, NCT04088890 | Phase 1/2 | NCT03262298: Dose-reliant anti-tumor effect; NCT04088890: 100% CR+ effect in CD19-treated recurrence | [89] |
| CD30-CAR | R/R Hodgkin’s lymphoma | NCT02690545, NCT02917083 | Phase 1/2 | 59% CR+; 72% ORR; high safety profile | [90] |
| NKG2D-Ligand based CAR | R/R- (TLL, AML, MDS) | NCT02203825 | Phase 1 | High antitumor efficacy | [91] |
| CD33-CAR | R/R AML | NCT03971799 | Phase 1/2 | Not completed | [92] |
| CD123-CAR | R/R AML | NCT04014881 | Phase 1 | Not completed | [92] |
| CLL-1.CD33 and/or, CD123-CAR | R/R AML | NCT04010877 | Phase 1/2 | Not completed | [92] |
| FLT3-CAR | R/R AML | NCT05023707 | Phase 1/2 | Strong anti-tumor effect and low OT/OT toxicity | [93] |
| IL-1RAP-CAR | CML | NCT02842320 | Phase 1 | Anti-leukemic cytotoxicity against antigen expressing cells | [94] |
| CD7-CAR | R/R TLL | NCT04689659 | Phase 1/2 | Effective in vivo CAR-T cell expansion, High CR+, feasible safety profile | [95] |
| CD4-CAR | R/R T-cell lymphoma | NCT04712864 | Phase 1 | Powerful anti-tumor cytotoxicity | [96] |
| CD38-CAR | R/R AML | NCT04351022 | Phase 1/2 | 66.7% CR+, 240 days OS | [97] |
| CD5-CAR | R/R T-cell lymphoma | NCT03081910 | Phase 1 | Moderate response and safety profile | [98] |
| Solid cancers | |||||
| HER2-CAR | CNS tumor | NCT03500991 | Phase 1 | Specially localized immune response | [99] |
| HER2-CAR | HER2+ breast, gastric, pancreatic cancer | NCT04650451, NCT04430595 | Phase 1/2 | NCT01935843: 4.5 month of PR+, 45.45% SD+, 146 days median PFS+ | [100] |
| Allogenic NKG2D-CAR (CYAD-101) | mCRC | NCT03692429, NCT04991948 | Phase 1 | NCT03692429: 13.33% CR+, 60% SD, 46.67% with quarterly SD and 119 days median PFS | [101] |
| IL13Rα2-CAR | Glioblastoma | NCT04003649, NCT04661384, NCT02208362, NCT01935843 | Phase 1 | NCT01935843: Tumor shrinkage maintenance for 7.49 months; but antigen downgrading based tumor re-occurrence | [29] |
| GPC3-CAR | HCC | NCT03198546, NCT02395250, NCT03146234 | Phase 1/2 | NCT02395250 and NCT03146234: 18.18% PR+, highest 3.68 years OS+, 50.3% and 10.5% OS+ rate in 6 and 36 months | [85] |
| EGFR806-CAR | Advanced solid tumors | NCT03618381 | Phase 1 | Not completed | [92] |
| MUC1/PD-1-CAR | NSCLC | NCT03525782 | Phase 1/2 | Not completed | [92] |
| GD2-CAR | Brain cancer | NCT04099797 | Phase 1 | Enrolling participants | [92] |
| Mesothelin-CAR | Lung, pancreatic, ovarian, and cervical cancer | NCT01583686 | Phase 1/2 | Enrolling participants | [92] |
| CEA-CAR | Lung, colorectal, breast, stomach, and pancreatic cancer | NCT02349724 | Phase 1 | Enrolling participants | [92] |
| CD19.B7H3-CAR | Advanced solid tumors | NCT04483778 | Phase 1 | Enrolling participants | [92] |
| TnMUC1-CAR | R/R solid tumors | NCT04025216 | Phase 1 | Not completed | [92] |
CAR-T therapy represents a significant advancement in cancer immunotherapy, involving the genetic modification of a patient’s T cells to target specific TAAs. This personalized approach has demonstrated substantial clinical efficacy, particularly in hematologic malignancies such as relapsed or refractory B-cell cancers, leading to multiple FDA approvals. CAR-Ts are engineered to combine antigen recognition and T-cell activation within a single synthetic receptor, enabling precise and potent tumor cell eradication. Over time, CAR constructs have evolved from first- to fifth-generation designs, incorporating enhanced ISDs to improve persistence, specificity, and antitumor activity. Emerging strategies, including dual and tandem CARs, logic-gated and armored CARs, and advancements in gene delivery technologies such as CRISPR/Cas9 and transposon-based systems, have addressed several limitations, including antigen escape and off-tumor toxicity. Nonetheless, significant challenges remain, particularly in the treatment of solid tumors, where immunosuppressive microenvironments, heterogeneous antigen expression, and limited T-cell infiltration hinder therapeutic efficacy. Additionally, adverse events such as cytokine release syndrome, neurotoxicity, and long-term cytopenias, along with high costs and manufacturing complexity, present ongoing barriers. Current research is focused on refining CAR-T design, improving safety and scalability, and expanding therapeutic applications through allogeneic or universal CAR-T platforms. Despite these limitations, CAR-T therapy continues to hold promise for durable responses and long-term disease control, marking a pivotal step toward more effective and individualized cancer treatment.
Samarah Arjumand extends her sincere gratitude to BRAC University and the School of Pharmacy for supporting her undergraduate research project. She also acknowledges Faruque Azam for his guidance and supervision throughout the project. The authors thank Dr. Md Rabiul Islam for his valuable insights and helpful suggestions on the manuscript.
| 1. | National Cancer Institute. Definition of cancer - NCI Dictionary of Cancer Terms. [cited 3 March 2025]. Available from: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/cancer. |
| 2. | National Center for Health Statistics. FastStats - Leading Causes of Death. [cited March 2025]. Available from: https://www.cdc.gov/nchs/fastats/leading-causes-of-death.htm. |
| 3. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 68404] [Article Influence: 13680.8] [Reference Citation Analysis (201)] |
| 4. | Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5690] [Cited by in RCA: 12374] [Article Influence: 6187.0] [Reference Citation Analysis (6)] |
| 5. | Falzone L, Salomone S, Libra M. Evolution of Cancer Pharmacological Treatments at the Turn of the Third Millennium. Front Pharmacol. 2018;9:1300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 377] [Cited by in RCA: 578] [Article Influence: 72.3] [Reference Citation Analysis (0)] |
| 6. | Naran K, Nundalall T, Chetty S, Barth S. Principles of Immunotherapy: Implications for Treatment Strategies in Cancer and Infectious Diseases. Front Microbiol. 2018;9:3158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 7. | Cancer Research UK. Targeted cancer drugs and immunotherapy. [cited 3 March 2025]. Available from: https://www.cancerresearchuk.org/about-cancer/treatment/targeted-cancer-drugs-immunotherapy. |
| 8. | Cancer Research Institute. Adoptive Cell Therapy. [cited 3 March 2025]. Available from: https://www.cancerresearch.org/treatment-types/adoptive-cell-therapy. |
| 9. | Zhang C, Durer S, Thandra KC, Kasi A. Chimeric Antigen Receptor T-Cell Therapy. 2022 Oct 3. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. [PubMed] |
| 10. | Chen Q, Guo X, Ma W. Opportunities and challenges of CD47-targeted therapy in cancer immunotherapy. Oncol Res. 2023;32:49-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 53] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 11. | Muhammad N, Mao Q, Xia H. CAR T-cells for cancer therapy. Biotechnol Genet Eng Rev. 2017;33:190-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 12. | Sterner RC, Sterner RM. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 2021;11:69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 326] [Cited by in RCA: 1869] [Article Influence: 373.8] [Reference Citation Analysis (12)] |
| 13. | Guedan S, Calderon H, Posey AD Jr, Maus MV. Engineering and Design of Chimeric Antigen Receptors. Mol Ther Methods Clin Dev. 2019;12:145-156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 179] [Cited by in RCA: 351] [Article Influence: 43.9] [Reference Citation Analysis (0)] |
| 14. | Oldham RAA, Medin JA. Practical considerations for chimeric antigen receptor design and delivery. Expert Opin Biol Ther. 2017;17:961-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Mohanty R, Chowdhury CR, Arega S, Sen P, Ganguly P, Ganguly N. CAR T cell therapy: A new era for cancer treatment (Review). Oncol Rep. 2019;42:2183-2195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 79] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 16. | Rafiq S, Hackett CS, Brentjens RJ. Engineering strategies to overcome the current roadblocks in CAR T cell therapy. Nat Rev Clin Oncol. 2020;17:147-167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 435] [Cited by in RCA: 1057] [Article Influence: 151.0] [Reference Citation Analysis (0)] |
| 17. | Fraietta JA, Lacey SF, Orlando EJ, Pruteanu-Malinici I, Gohil M, Lundh S, Boesteanu AC, Wang Y, O'Connor RS, Hwang WT, Pequignot E, Ambrose DE, Zhang C, Wilcox N, Bedoya F, Dorfmeier C, Chen F, Tian L, Parakandi H, Gupta M, Young RM, Johnson FB, Kulikovskaya I, Liu L, Xu J, Kassim SH, Davis MM, Levine BL, Frey NV, Siegel DL, Huang AC, Wherry EJ, Bitter H, Brogdon JL, Porter DL, June CH, Melenhorst JJ. Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat Med. 2018;24:563-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 768] [Cited by in RCA: 1369] [Article Influence: 171.1] [Reference Citation Analysis (0)] |
| 18. | De Marco RC, Monzo HJ, Ojala PM. CAR T Cell Therapy: A Versatile Living Drug. Int J Mol Sci. 2023;24:6300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 63] [Reference Citation Analysis (0)] |
| 19. | Tahmasebi S, Elahi R, Khosh E, Esmaeilzadeh A. Programmable and multi-targeted CARs: a new breakthrough in cancer CAR-T cell therapy. Clin Transl Oncol. 2021;23:1003-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 20. | Miliotou AN, Papadopoulou LC. CAR T-cell Therapy: A New Era in Cancer Immunotherapy. Curr Pharm Biotechnol. 2018;19:5-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 307] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 21. | Liu X, Zhang Y, Cheng C, Cheng AW, Zhang X, Li N, Xia C, Wei X, Liu X, Wang H. CRISPR-Cas9-mediated multiplex gene editing in CAR-T cells. Cell Res. 2017;27:154-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 311] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 22. | Huang S, Xu J, Baran N, Ma W. Advancing the next generation of cancer treatment with circular RNAs in CAR-T cell therapy. Biomed Pharmacother. 2024;181:117753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 20] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 23. | Smith TT, Stephan SB, Moffett HF, McKnight LE, Ji W, Reiman D, Bonagofski E, Wohlfahrt ME, Pillai SPS, Stephan MT. In situ programming of leukaemia-specific T cells using synthetic DNA nanocarriers. Nat Nanotechnol. 2017;12:813-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 413] [Cited by in RCA: 580] [Article Influence: 64.4] [Reference Citation Analysis (0)] |
| 24. | Alijani HQ, Pourseyedi S, Torkzadeh-Mahani M, Khatami M. Porous α-Fe(2)O(3) nanocarriers: Biosynthesis and in vitro gene delivery applications. Heliyon. 2024;10:e28676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 25. | Benmebarek MR, Karches CH, Cadilha BL, Lesch S, Endres S, Kobold S. Killing Mechanisms of Chimeric Antigen Receptor (CAR) T Cells. Int J Mol Sci. 2019;20:1283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 303] [Cited by in RCA: 379] [Article Influence: 54.1] [Reference Citation Analysis (0)] |
| 26. | Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35:495-516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10243] [Cited by in RCA: 9951] [Article Influence: 523.7] [Reference Citation Analysis (0)] |
| 27. | Chen Q, Lu L, Ma W. Efficacy, Safety, and Challenges of CAR T-Cells in the Treatment of Solid Tumors. Cancers (Basel). 2022;14:5983. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 28. | Tang L, Pan S, Wei X, Xu X, Wei Q. Arming CAR-T cells with cytokines and more: Innovations in the fourth-generation CAR-T development. Mol Ther. 2023;31:3146-3162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 101] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 29. | Brown CE, Alizadeh D, Starr R, Weng L, Wagner JR, Naranjo A, Ostberg JR, Blanchard MS, Kilpatrick J, Simpson J, Kurien A, Priceman SJ, Wang X, Harshbarger TL, D'Apuzzo M, Ressler JA, Jensen MC, Barish ME, Chen M, Portnow J, Forman SJ, Badie B. Regression of Glioblastoma after Chimeric Antigen Receptor T-Cell Therapy. N Engl J Med. 2016;375:2561-2569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1255] [Cited by in RCA: 1427] [Article Influence: 142.7] [Reference Citation Analysis (0)] |
| 30. | Frey NV, Shaw PA, Hexner EO, Pequignot E, Gill S, Luger SM, Mangan JK, Loren AW, Perl AE, Maude SL, Grupp SA, Shah NN, Gilmore J, Lacey SF, Melenhorst JJ, Levine BL, June CH, Porter DL. Optimizing Chimeric Antigen Receptor T-Cell Therapy for Adults With Acute Lymphoblastic Leukemia. J Clin Oncol. 2020;38:415-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 212] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 31. | Lieberman J. Granzyme A activates another way to die. Immunol Rev. 2010;235:93-104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 177] [Cited by in RCA: 180] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 32. | Starkova T, Polyanichko A, Tomilin AN, Chikhirzhina E. Structure and Functions of HMGB2 Protein. Int J Mol Sci. 2023;24:8334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 50] [Reference Citation Analysis (0)] |
| 33. | Schubert ML, Schmitt A, Hückelhoven-Krauss A, Neuber B, Kunz A, Waldhoff P, Vonficht D, Yousefian S, Jopp-Saile L, Wang L, Korell F, Keib A, Michels B, Haas D, Sauer T, Derigs P, Kulozik A, Kunz J, Pavel P, Laier S, Wuchter P, Schmier J, Bug G, Lang F, Gökbuget N, Casper J, Görner M, Finke J, Neubauer A, Ringhoffer M, Wolleschak D, Brüggemann M, Haas S, Ho AD, Müller-Tidow C, Dreger P, Schmitt M. Treatment of adult ALL patients with third-generation CD19-directed CAR T cells: results of a pivotal trial. J Hematol Oncol. 2023;16:79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 34. | Weinkove R, George P, Fyfe R, Hurley A, Dasyam N, Nouri Y, Ostapowicz T, Mullins S, Giunti G, Lavender B, Mester B, Bollard CM, Perera T, Jina H, D'souza A, Qin L, Ritchie DS, Frampton CM, Perret R, Li P, Hermans I. A Phase 1 Dose Escalation and Expansion Trial of Third-Generation CD19-Directed CAR T-Cells Incorporating CD28 and Toll-like Receptor 2 (TLR2) Co-Stimulation for Relapsed or Refractory B-Cell Non-Hodgkin Lymphomas (ENABLE-1). Blood. 2024;144:2067-2067. [DOI] [Full Text] |
| 35. | Duan D, Wang K, Wei C, Feng D, Liu Y, He Q, Xu X, Wang C, Zhao S, Lv L, Long J, Lin D, Zhao A, Fang B, Jiang J, Tang S, Gao J. The BCMA-Targeted Fourth-Generation CAR-T Cells Secreting IL-7 and CCL19 for Therapy of Refractory/Recurrent Multiple Myeloma. Front Immunol. 2021;12:609421. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 36. | Tiangeng. Phase I & II Study of EGFR-IL12-CART Cells for Patients With Metastatic Colorectal Cancer. [accessed 2025 March 3]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: http://clinicaltrials.gov/show/NCT03542799 ClinicalTrials.gov Identifier: NCT03542799. |
| 37. | Palomba ML, Caimi PF, Mei M, Hernandez-Ilizaliturri F, Shouse G, Winter AM, Yedinak G, Mou D, Huang N, Moua A, Oft M, Aspuria P, Mehta-Damani A, Rizvi N, Unabia S, Taylor P, Mcleroy J, Cortese MJ. A Phase 1 Study to Evaluate the Safety and Tolerability of a Combination Autologous CD19 CAR T Cell Therapy (SYNCAR-001) and Orthogonal IL-2 (STK-009) in Subjects with Relapsed or Refractory CD19 Expressing Hematologic Malignancies (NCT05665062). Blood. 2024;144:3453-3453. [DOI] [Full Text] |
| 38. | Gong J, Shen L, Zang A, Zhou H, Tang J, Liu D, Jia Y, Wang B. A phase I open-label study of a novel IL-2Rβ/γ cytokine agonist, LTC004, in patients with advanced or metastatic solid tumors. J Clin Oncol. 2024;42:2652-2652. [DOI] [Full Text] |
| 39. | Grada Z, Hegde M, Byrd T, Shaffer DR, Ghazi A, Brawley VS, Corder A, Schönfeld K, Koch J, Dotti G, Heslop HE, Gottschalk S, Wels WS, Baker ML, Ahmed N. TanCAR: A Novel Bispecific Chimeric Antigen Receptor for Cancer Immunotherapy. Mol Ther Nucleic Acids. 2013;2:e105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 293] [Cited by in RCA: 373] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 40. | Hamieh M, Mansilla-Soto J, Rivière I, Sadelain M. Programming CAR T Cell Tumor Recognition: Tuned Antigen Sensing and Logic Gating. Cancer Discov. 2023;13:829-843. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 106] [Reference Citation Analysis (0)] |
| 41. | Zhang H, Zhu S, Deng W, Li R, Zhou H, Xiong H. The landscape of chimeric antigen receptor T cell therapy in breast cancer: Perspectives and outlook. Front Immunol. 2022;13:887471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 42. | Schmidts A, Srivastava AA, Ramapriyan R, Bailey SR, Bouffard AA, Cahill DP, Carter BS, Curry WT, Dunn GP, Frigault MJ, Gerstner ER, Ghannam JY, Kann MC, Larson RC, Leick MB, Nahed BV, Richardson LG, Scarfò I, Sun J, Wakimoto H, Maus MV, Choi BD. Tandem chimeric antigen receptor (CAR) T cells targeting EGFRvIII and IL-13Rα2 are effective against heterogeneous glioblastoma. Neurooncol Adv. 2023;5:vdac185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 47] [Reference Citation Analysis (0)] |
| 43. | Mao H, Ito Y. 4.19 Growth Factors and Protein-Modified Surfaces and Interfaces. In: Ducheyne P, editor. Comprehensive Biomaterials II. Elsevier, 2017: 321-359. [DOI] [Full Text] |
| 44. | Khamaisi B, Luca VC, Blacklow SC, Sprinzak D. Functional Comparison between Endogenous and Synthetic Notch Systems. ACS Synth Biol. 2022;11:3343-3353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 45. | Steinbuck MP, Winandy S. A Review of Notch Processing With New Insights Into Ligand-Independent Notch Signaling in T-Cells. Front Immunol. 2018;9:1230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 46. | Morsut L, Roybal KT, Xiong X, Gordley RM, Coyle SM, Thomson M, Lim WA. Engineering Customized Cell Sensing and Response Behaviors Using Synthetic Notch Receptors. Cell. 2016;164:780-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 788] [Cited by in RCA: 759] [Article Influence: 75.9] [Reference Citation Analysis (0)] |
| 47. | Abbott RC, Hughes-Parry HE, Jenkins MR. To go or not to go? Biological logic gating engineered T cells. J Immunother Cancer. 2022;10:e004185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 36] [Reference Citation Analysis (0)] |
| 48. | Choe JH, Watchmaker PB, Simic MS, Gilbert RD, Li AW, Krasnow NA, Downey KM, Yu W, Carrera DA, Celli A, Cho J, Briones JD, Duecker JM, Goretsky YE, Dannenfelser R, Cardarelli L, Troyanskaya O, Sidhu SS, Roybal KT, Okada H, Lim WA. SynNotch-CAR T cells overcome challenges of specificity, heterogeneity, and persistence in treating glioblastoma. Sci Transl Med. 2021;13:eabe7378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 373] [Article Influence: 74.6] [Reference Citation Analysis (0)] |
| 49. | Simon S, Bugos G, Salter AI, Riddell SR. Synthetic receptors for logic gated T cell recognition and function. Curr Opin Immunol. 2022;74:9-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 50. | Minutolo NG, Hollander EE, Powell DJ Jr. The Emergence of Universal Immune Receptor T Cell Therapy for Cancer. Front Oncol. 2019;9:176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 75] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 51. | Hashem Boroojerdi M, Rahbarizadeh F, Safarzadeh Kozani P, Kamali E, Safarzadeh Kozani P. Strategies for having a more effective and less toxic CAR T-cell therapy for acute lymphoblastic leukemia. Med Oncol. 2020;37:100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 52. | Bassan D, Weist M, Weinberger L, Yi J, Adams G, Kim T, Schnair C, Vucci K, Tabak S, Chaim N, Bujanover N, Lopesco Y, Levy-kanfo L, Foord O, Johnston J, Calzone F, Kendell R, Sharbi-Yunger A. 316 Next generation CAR-T incorporating an inhibitory CAR (iCAR) for increased safety in solid tumors. J Immunother Cancer. 2022;10 (Suppl 2):A332-A332. [DOI] [Full Text] |
| 53. | Tao L, Farooq MA, Gao Y, Zhang L, Niu C, Ajmal I, Zhou Y, He C, Zhao G, Yao J, Liu M, Jiang W. CD19-CAR-T Cells Bearing a KIR/PD-1-Based Inhibitory CAR Eradicate CD19(+)HLA-C1(-) Malignant B Cells While Sparing CD19(+)HLA-C1(+) Healthy B Cells. Cancers (Basel). 2020;12:2612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 54. | Shaffer DR, Savoldo B, Yi Z, Chow KK, Kakarla S, Spencer DM, Dotti G, Wu MF, Liu H, Kenney S, Gottschalk S. T cells redirected against CD70 for the immunotherapy of CD70-positive malignancies. Blood. 2011;117:4304-4314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 146] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 55. | Zhang T, Lemoi BA, Sentman CL. Chimeric NK-receptor-bearing T cells mediate antitumor immunotherapy. Blood. 2005;106:1544-1551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 136] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 56. | Muniappan A, Banapour B, Lebkowski J, Talib S. Ligand-mediated cytolysis of tumor cells: use of heregulin-zeta chimeras to redirect cytotoxic T lymphocytes. Cancer Gene Ther. 2000;7:128-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 57. | Kahlon KS, Brown C, Cooper LJ, Raubitschek A, Forman SJ, Jensen MC. Specific recognition and killing of glioblastoma multiforme by interleukin 13-zetakine redirected cytolytic T cells. Cancer Res. 2004;64:9160-9166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 289] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 58. | Niederman TM, Ghogawala Z, Carter BS, Tompkins HS, Russell MM, Mulligan RC. Antitumor activity of cytotoxic T lymphocytes engineered to target vascular endothelial growth factor receptors. Proc Natl Acad Sci U S A. 2002;99:7009-7014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 93] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 59. | Zhang T, Wu MR, Sentman CL. An NKp30-based chimeric antigen receptor promotes T cell effector functions and antitumor efficacy in vivo. J Immunol. 2012;189:2290-2299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 112] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 60. | Zhao J, Zheng M, Ma L, Guan T, Su L. From spear to trident: Upgrading arsenal of CAR-T cells in the treatment of multiple myeloma. Heliyon. 2024;10:e29997. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 61. | Nolan-Stevaux O, Smith R. Logic-gated and contextual control of immunotherapy for solid tumors: contrasting multi-specific T cell engagers and CAR-T cell therapies. Front Immunol. 2024;15:1490911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 62. | Liang Y, Liu H, Lu Z, Lei W, Zhang C, Li P, Liang A, Young KH, Qian W. CD19 CAR-T expressing PD-1/CD28 chimeric switch receptor as a salvage therapy for DLBCL patients treated with different CD19-directed CAR T-cell therapies. J Hematol Oncol. 2021;14:26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 63. | Stenger D, Stief TA, Kaeuferle T, Willier S, Rataj F, Schober K, Vick B, Lotfi R, Wagner B, Grünewald TGP, Kobold S, Busch DH, Jeremias I, Blaeschke F, Feuchtinger T. Endogenous TCR promotes in vivo persistence of CD19-CAR-T cells compared to a CRISPR/Cas9-mediated TCR knockout CAR. Blood. 2020;136:1407-1418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 138] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 64. | Lu L, Xie M, Yang B, Zhao WB, Cao J. Enhancing the safety of CAR-T cell therapy: Synthetic genetic switch for spatiotemporal control. Sci Adv. 2024;10:eadj6251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 55] [Reference Citation Analysis (0)] |
| 65. | Huang Z, Wu Y, Allen ME, Pan Y, Kyriakakis P, Lu S, Chang YJ, Wang X, Chien S, Wang Y. Engineering light-controllable CAR T cells for cancer immunotherapy. Sci Adv. 2020;6:eaay9209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 130] [Article Influence: 21.7] [Reference Citation Analysis (20)] |
| 66. | Guercio M, Manni S, Boffa I, Caruso S, Di Cecca S, Sinibaldi M, Abbaszadeh Z, Camera A, Ciccone R, Polito VA, Ferrandino F, Reddel S, Catanoso ML, Bocceri E, Del Bufalo F, Algeri M, De Angelis B, Quintarelli C, Locatelli F. Inclusion of the Inducible Caspase 9 Suicide Gene in CAR Construct Increases Safety of CAR.CD19 T Cell Therapy in B-Cell Malignancies. Front Immunol. 2021;12:755639. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (11)] |
| 67. | Gong Y, Klein Wolterink RGJ, Wang J, Bos GMJ, Germeraad WTV. Chimeric antigen receptor natural killer (CAR-NK) cell design and engineering for cancer therapy. J Hematol Oncol. 2021;14:73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 281] [Article Influence: 56.2] [Reference Citation Analysis (0)] |
| 68. | Khoshandam M, Soltaninejad H, Hamidieh AA, Hosseinkhani S. CRISPR, CAR-T, and NK: Current applications and future perspectives. Genes Dis. 2024;11:101121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 69. | Chu J, Gao F, Yan M, Zhao S, Yan Z, Shi B, Liu Y. Natural killer cells: a promising immunotherapy for cancer. J Transl Med. 2022;20:240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 118] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 70. | Liu E, Marin D, Banerjee P, Macapinlac HA, Thompson P, Basar R, Nassif Kerbauy L, Overman B, Thall P, Kaplan M, Nandivada V, Kaur I, Nunez Cortes A, Cao K, Daher M, Hosing C, Cohen EN, Kebriaei P, Mehta R, Neelapu S, Nieto Y, Wang M, Wierda W, Keating M, Champlin R, Shpall EJ, Rezvani K. Use of CAR-Transduced Natural Killer Cells in CD19-Positive Lymphoid Tumors. N Engl J Med. 2020;382:545-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 920] [Cited by in RCA: 1622] [Article Influence: 270.3] [Reference Citation Analysis (18)] |
| 71. | Klichinsky M, Ruella M, Shestova O, Lu XM, Best A, Zeeman M, Schmierer M, Gabrusiewicz K, Anderson NR, Petty NE, Cummins KD, Shen F, Shan X, Veliz K, Blouch K, Yashiro-Ohtani Y, Kenderian SS, Kim MY, O'Connor RS, Wallace SR, Kozlowski MS, Marchione DM, Shestov M, Garcia BA, June CH, Gill S. Human chimeric antigen receptor macrophages for cancer immunotherapy. Nat Biotechnol. 2020;38:947-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 1066] [Article Influence: 177.7] [Reference Citation Analysis (0)] |
| 72. | Mercogliano MF, Bruni S, Mauro FL, Schillaci R. Emerging Targeted Therapies for HER2-Positive Breast Cancer. Cancers (Basel). 2023;15:1987. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 97] [Reference Citation Analysis (0)] |
| 73. | Li J, Chen P, Ma W. The next frontier in immunotherapy: potential and challenges of CAR-macrophages. Exp Hematol Oncol. 2024;13:76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 49] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 74. | Giorgioni L, Ambrosone A, Cometa MF, Salvati AL, Magrelli A. CAR-T State of the Art and Future Challenges, A Regulatory Perspective. Int J Mol Sci. 2023;24:11803. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (1)] |
| 75. | S S KD, Joga R, Srivastava S, Nagpal K, Dhamija I, Grover P, Kumar S. Regulatory landscape and challenges in CAR-T cell therapy development in the US, EU, Japan, and India. Eur J Pharm Biopharm. 2024;201:114361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 76. | European Medicines Agency. European Medicines Agency (EMA). [cited 3 March 2025]. Available from: https://www.ema.europa.eu/en/homepage. |
| 77. | United States Food and Drug Administration. FDA. 2025. [cited 5 March 2025]. Available from: https://www.fda.gov/. |
| 78. | Kandra P, Nandigama R, Eul B, Huber M, Kobold S, Seeger W, Grimminger F, Savai R. Utility and Drawbacks of Chimeric Antigen Receptor T Cell (CAR-T) Therapy in Lung Cancer. Front Immunol. 2022;13:903562. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 79. | Yin X, He L, Guo Z. T-cell exhaustion in CAR-T-cell therapy and strategies to overcome it. Immunology. 2023;169:400-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 36] [Reference Citation Analysis (0)] |
| 80. | Lin H, Yang X, Ye S, Huang L, Mu W. Antigen escape in CAR-T cell therapy: Mechanisms and overcoming strategies. Biomed Pharmacother. 2024;178:117252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 45] [Reference Citation Analysis (0)] |
| 81. | Hong M, Talluri S, Chen YY. Advances in promoting chimeric antigen receptor T cell trafficking and infiltration of solid tumors. Curr Opin Biotechnol. 2023;84:103020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 82. | Yang Z, Ha B, Wu Q, Ren F, Yin Z, Zhang H. Expanding the horizon of CAR T cell therapy: from cancer treatment to autoimmune diseases and beyond. Front Immunol. 2025;16:1544532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 83. | Zhang K, Chen H, Li F, Huang S, Chen F, Li Y. Bright future or blind alley? CAR-T cell therapy for solid tumors. Front Immunol. 2023;14:1045024. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 27] [Reference Citation Analysis (0)] |
| 84. | Sun S, Hao H, Yang G, Zhang Y, Fu Y. Immunotherapy with CAR-Modified T Cells: Toxicities and Overcoming Strategies. J Immunol Res. 2018;2018:2386187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 120] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 85. | Shi D, Shi Y, Kaseb AO, Qi X, Zhang Y, Chi J, Lu Q, Gao H, Jiang H, Wang H, Yuan D, Ma H, Wang H, Li Z, Zhai B. Chimeric Antigen Receptor-Glypican-3 T-Cell Therapy for Advanced Hepatocellular Carcinoma: Results of Phase I Trials. Clin Cancer Res. 2020;26:3979-3989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 278] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 86. | Mackensen A, Haanen JBAG, Koenecke C, Alsdorf W, Wagner-Drouet E, Borchmann P, Heudobler D, Ferstl B, Klobuch S, Bokemeyer C, Desuki A, Lüke F, Kutsch N, Müller F, Smit E, Hillemanns P, Karagiannis P, Wiegert E, He Y, Ho T, Kang-Fortner Q, Schlitter AM, Schulz-Eying C, Finlayson A, Flemmig C, Kühlcke K, Preußner L, Rengstl B, Türeci Ö, Şahin U. CLDN6-specific CAR-T cells plus amplifying RNA vaccine in relapsed or refractory solid tumors: the phase 1 BNT211-01 trial. Nat Med. 2023;29:2844-2853.. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 185] [Cited by in RCA: 173] [Article Influence: 57.7] [Reference Citation Analysis (0)] |
| 87. | Yang Y, Vedvyas Y, Alcaina Y, Trumper SJ, Babu DS, Min IM, Tremblay JM, Shoemaker CB, Jin MM. Affinity-tuned mesothelin CAR T cells demonstrate enhanced targeting specificity and reduced off-tumor toxicity. JCI Insight. 2024;9:e186268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 88. | Sallman DA, Brayer J, Sagatys EM, Lonez C, Breman E, Agaugué S, Verma B, Gilham DE, Lehmann FF, Davila ML. NKG2D-based chimeric antigen receptor therapy induced remission in a relapsed/refractory acute myeloid leukemia patient. Haematologica. 2018;103:e424-e426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 89. | Rennert P, Su L, Dufort F, Birt A, Sanford T, Wu L, Ambrose C, Lobb R. A Novel CD19-Anti-CD20 Bridging Protein Prevents and Reverses CD19-Negative Relapse from CAR19 T Cell Treatment In Vivo. Blood. 2019;134:252-252. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 90. | Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, Chew A, Gonzalez VE, Zheng Z, Lacey SF, Mahnke YD, Melenhorst JJ, Rheingold SR, Shen A, Teachey DT, Levine BL, June CH, Porter DL, Grupp SA. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371:1507-1517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4147] [Cited by in RCA: 4328] [Article Influence: 360.7] [Reference Citation Analysis (0)] |
| 91. | Shadman M, Yeung C, Redman MW, Lee SY, Lee DH, Ramachandran A, Ra S, Marzbani EA, Graf SA, Warren EH, Gopal AK, Chapuis AG, Bar M, Green DJ, Gauthier J, Cassaday RD, Kiem H, Turtle CJ, Maloney DG, Till BG. Third Generation CD20 Targeted CAR T-Cell Therapy (MB-106) for Treatment of Patients with Relapsed/Refractory B-Cell Non-Hodgkin Lymphoma. Blood. 2020;136:38-39. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 92. | Asmamaw Dejenie T, Tiruneh G/Medhin M, Dessie Terefe G, Tadele Admasu F, Wale Tesega W, Chekol Abebe E. Current updates on generations, approvals, and clinical trials of CAR T-cell therapy. Hum Vaccin Immunother. 2022;18:2114254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 114] [Reference Citation Analysis (0)] |
| 93. | Driouk L, Gicobi JK, Kamihara Y, Rutherford K, Dranoff G, Ritz J, Baumeister SHC. Chimeric Antigen Receptor T Cells Targeting NKG2D-Ligands Show Robust Efficacy Against Acute Myeloid Leukemia and T-Cell Acute Lymphoblastic Leukemia. Front Immunol. 2020;11:580328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 94. | Maiorova V, Mollaev MD, Vikhreva P, Kulakovskaya E, Pershin D, Chudakov DM, Kibardin A, Maschan MA, Larin S. Natural Flt3Lg-Based Chimeric Antigen Receptor (Flt3-CAR) T Cells Successfully Target Flt3 on AML Cell Lines. Vaccines (Basel). 2021;9:1238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 95. | Fry TJ, Shah NN, Orentas RJ, Stetler-Stevenson M, Yuan CM, Ramakrishna S, Wolters P, Martin S, Delbrook C, Yates B, Shalabi H, Fountaine TJ, Shern JF, Majzner RG, Stroncek DF, Sabatino M, Feng Y, Dimitrov DS, Zhang L, Nguyen S, Qin H, Dropulic B, Lee DW, Mackall CL. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat Med. 2018;24:20-28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 761] [Cited by in RCA: 1090] [Article Influence: 121.1] [Reference Citation Analysis (0)] |
| 96. | Hill L, Rouce RH, Smith TS, Yang L, Srinivasan M, Zhang H, Perconti S, Mehta B, Dakhova O, Randall J, Grilley B, Heslop HE, Brenner MK, Mamonkin M. CD5 CAR T-Cells for Treatment of Patients with Relapsed/Refractory CD5 Expressing T-Cell Lymphoma Demonstrates Safety and Anti-Tumor Activity. Biol Blood Marrow Transplant. 2020;26:S237. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 97. | Warda W, Larosa F, Neto Da Rocha M, Trad R, Deconinck E, Fajloun Z, Faure C, Caillot D, Moldovan M, Valmary-Degano S, Biichle S, Daguindau E, Garnache-Ottou F, Tabruyn S, Adotevi O, Deschamps M, Ferrand C. CML Hematopoietic Stem Cells Expressing IL1RAP Can Be Targeted by Chimeric Antigen Receptor-Engineered T Cells. Cancer Res. 2019;79:663-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 72] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 98. | Cui Q, Qian C, Xu N, Kang L, Dai H, Cui W, Song B, Yin J, Li Z, Zhu X, Qu C, Liu T, Shen W, Zhu M, Yu L, Wu D, Tang X. CD38-directed CAR-T cell therapy: a novel immunotherapy strategy for relapsed acute myeloid leukemia after allogeneic hematopoietic stem cell transplantation. J Hematol Oncol. 2021;14:82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 103] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 99. | Mamonkin M, Rouce RH, Tashiro H, Brenner MK. A T-cell-directed chimeric antigen receptor for the selective treatment of T-cell malignancies. Blood. 2015;126:983-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 266] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 100. | Feng K, Liu Y, Guo Y, Qiu J, Wu Z, Dai H, Yang Q, Wang Y, Han W. Phase I study of chimeric antigen receptor modified T cells in treating HER2-positive advanced biliary tract cancers and pancreatic cancers. Protein Cell. 2018;9:838-847. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 250] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 101. | Prenen H, Dekervel J, Hendlisz A, Anguille S, Awada A, Cerf E, Lonez C, Breman E, Dheur M, Alcantar-Orozco E, Gilham DE, Flament A, Lehmann F, Van Cutsem E. Updated data from alloSHRINK phase I first-in-human study evaluating CYAD-101, an innovative non-gene edited allogeneic CAR-T in mCRC. J Clin Oncol. 2021;39:74-74. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/