Published online Nov 24, 2025. doi: 10.5306/wjco.v16.i11.106498
Revised: June 17, 2025
Accepted: October 11, 2025
Published online: November 24, 2025
Processing time: 267 Days and 0.8 Hours
Rectal cancer management is currently evolving with the advent of different neoadjuvant treatment strategies and organ preservation strategies. A significant proportion of patients could achieve complete clinical response after neoadjuvant treatment, which often translates to pathologic complete response (pCR) as assessed on surgical specimens after curative intent surgery. Endoscopy plays a significant role in assessing treatment response to neoadjuvant therapies.
To explore the role of endoscopy in predicting subsequent pCR after neoadjuvant treatment in rectal cancer patients.
An extensive literature review was undertaken to identify the criteria used for assessment of endoscopic response and their ability to predict pCR.
Fifteen studies were identified through literature review. The most commonly used endoscopic criteria for evaluation included the presence of a flat white scar and the absence of nodularity or telangiectasia. Information on the timing of en
Endoscopy can be a key prognostic factor in predicting pCR to neoadjuvant treatment in rectal cancer despite significant limitations in currently available data.
Core Tip: Endoscopic assessment of rectal cancer response after neoadjuvant treatment can accurately predict pathologic complete response when specific criteria are applied. The combination of endoscopic features, including the absence of nodularity and ulceration, and the presence of a flat white scar with telangiectasia, achieves a diagnostic accuracy exceeding 80% for predicting pathologic complete response before curative intent surgery. These findings support the use of en
- Citation: Seretis F, Panagaki A, Gkolfakis P, Tziatzios G, Paraskeva K. Endoscopic assessment of rectal cancer response after neoadjuvant chemoradiotherapy: A narrative literature review. World J Clin Oncol 2025; 16(11): 106498
- URL: https://www.wjgnet.com/2218-4333/full/v16/i11/106498.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i11.106498
Rectal cancer presents a common and increasingly complex problem. A new treatment paradigm, total neoadjuvant treatment (TNT), has emerged. It consists of administering all planned chemotherapy and radiotherapy before surgery, either as induction chemotherapy followed by chemoradiotherapy, or as chemoradiotherapy followed by consolidation chemotherapy. This has allowed a significant number of patients to achieve clinical response, as assessed by endoscopy and magnetic resonance imaging (MRI), thus enabling an organ preservation (OP) strategy while avoiding surgery. At the same time, the complexity of rectal cancer treatment algorithms continues to increase. Unfortunately, a simplistic one-size-fits-all approach, still applied in many centers worldwide[1], has been associated with inferior outcomes.
Changes to the rectal cancer treatment paradigm have largely been shaped by the OPRA trial, which randomized patients with stage II/III rectal cancer into either chemoradiotherapy with consolidation chemotherapy or induction chemotherapy with chemoradiotherapy in the neoadjuvant setting. Patients with near-complete or complete responses were offered a watch-and-wait policy, while those who had an incomplete response at restaging or those who had local regrowth while on watchful wait, had surgery with curative intent. Both groups had similar 5-year disease-free-survival, highlighting the safety of the watch-and-wait approach for those patients with complete or near-complete clinical responses (CCRs)[2]. Despite the increased pathologic complete response (pCR) rates achieved with TNT vs traditional chemoradiotherapy regimens, the differences in recurrence or long-term survival gain have not been established[3], suggesting that more accurate tools for tumor response prediction are needed. Notably, pCR achieved through TNT has been associated with inferior survival compared to pCR achieved with conventional regimens of neoadjuvant chemoradiotherapy followed by adjuvant chemotherapy, according to a recent trial[4].
Endoscopy represents a cornerstone of tumor assessment after TNT, classifying patients into those with a CCR, near-complete response (NCR) and incomplete clinical response. This appears to have important prognostic implications for the probability of long-term OP according to results from a subgroup analysis of the OPRA trial[5]. More specifically, CCR was associated with 77% probability at 3 years to achieve OP, vs 40% for NCR. Certain endoscopic features have been described to have predictive value for rectal cancer management after neoadjuvant treatment[6], with variable rates of positive and negative predictive value. A recent consensus[7] tried to further elucidate the definition of NCR, stating that this term should only be used for the first 6 months from the end of neoadjuvant chemoradiotherapy, after which the tumor response should be classified as complete or incomplete. Among the modalities used to assess tumor response, the authors discuss all three [digital rectal examination, endoscopy and MRI, including T2 weighted (commonly known as T2W)-MRI and diffusion-weight imaging (commonly known as DWI) MRI]. However, modalities that evaluate the endoluminal component of the tumor, namely endoscopy and digital rectal examination, appear to be the most in
The purpose of this review is to critically evaluate the existing literature in the context of the aforementioned consensus paper, with the aim of developing a unifying framework for current data and identifying key knowledge gaps. We considered it essential to extract from the literature detailed information on complete treatment plans, including multimodal regimens, and to integrate the timing and role of endoscopic assessments within these strategies to inform clinical decision-making. Our goal was to examine endoscopic response assessment after neoadjuvant therapy holistically, as part of an overall treatment roadmap, rather than as a stand-alone diagnostic tool.
A literature review was conducted in PubMed-indexed journals with search terms “endoscopy AND rectal cancer AND neoadjuvant”, “endoscopic assessment AND rectal cancer AND response”, up to May 15, 2024. Two individual re
An initial search with the aforementioned search terms yielded 1189 articles for review. After exclusion of duplicates (25 articles), 1164 articles were reviewed. A total of 1120 articles were excluded as non-relevant after review of abstracts. Forty-four articles were reviewed as full texts for eligibility. Two studies were excluded because they were single case reports, and eight studies were excluded because they reported on combined outcomes with other modalities. Another 19 of the 44 studies were excluded from final analysis as non-relevant. A manual search of references did not retrieve any additional relevant studies for review. Finally, 15 studies were included for final analysis in the systematic review. Search strategy and results are presented according to Preferred Reporting Items for Systematic Reviews and Meta-analyses (commonly known as PRISMA) 2020 guidelines for systematic reviews[8] in a flowchart, as supplementary data.
Studies were conducted from 2010 onwards to reflect current practice. The majority of studies included locally advanced rectal cancers, namely T3 or T4 tumors or node-positive disease receiving neoadjuvant chemotherapy. The majority of endoscopic assessments were performed at 6-8 weeks after the completion of neoadjuvant treatment. Five studies used deep learning algorithms for assessing endoscopic images. One study used magnifying endoscopy and one study used chromoendoscopy with narrow-band imaging, while the remaining studies used white-light endoscopy. The characteristics of the included studies are presented in Table 1[9-23]. All studies reported the type of neoadjuvant chemoradiotherapy administered to patients.
| Ref. | Year | Number of patients | Stage | Type of neoadjuvant treatment | Time of endoscopic assessment | Endoscopic modality used | Time of surgery | Definition of ECR | pCR | ECR | Accuracy of pCR prediction |
| Lim et al[15] | 2010-2015 | 87 | T3/T4 and/or N+ | 5 × 5 1.8 Gy + 5.4 Gy boost + ChT (5FU, capecitabine or FOLFOX) | 1-2 weeks prior to surgery | N/A | 6-8 weeks after CRT | A flat whitish or reddish scar ulcer, or a flat active/healing stage ulcer with regular edges surrounded by normal mucosa; disappearance of the neoplastic pit pattern without magnification; and disappearance of the neoplastic nodule or stenosis | 21.7% | 17.9% | 88.7% |
| Chino et al[12] | 2013-2015 | 79 | T3/T4 and/or N+ | Neoadjuvant chemoradiotherapy (oral fluoracil + 50.4 Gy), long course 59%, induction chemo (mFOLFOX + bevacizumab) + nCRT for cT4, cN2 or MRF (+) or positive lateral lymph nodes (41%) | 48 days after CRT | WL-C, white light conventional endoscopy; ME, magnifying endoscopy under crystal violet staining | 1-3 days after endoscopy | WL-C: Linear scar/flat scar, completely closed ulcer, no white moss, no protruded nodule. ME: Regenerated pits uniformly arranged, hyper-cellular pits. Wall extension by insufflation: Normal extension | 21.5% | 12.6% | 85% for ME |
| Bengulescu et al[16] | 2015-2017 | 43 | Stage II/III rectal adenocarcinoma | Long course chemoradiotherapy (5FU + RT 504 Gy in 28 fractions) | N/A | N/A | 6-8 weeks after nCRT | Endoscopy good grade (complete response or good response): Erythema; telangectasia; clean ulcer at the base; no elevation | N/A | 67% | N/A |
| Wang et al[17] | 2014-2021 | 214 | LARC | Neoadjuvant chemoradiotherapy not otherwise specified | 6–16 weeks after the completion of NT | Classic endoscopy (Olympus, Tokyo, Japan). ResNet-18 and DenseNet-121 deep learning algorithms | Good response; scarring (flat and white fibrosis with vasculopathy); erythema (erosion with peripheral erythematous mucosal changes) | 23.8% | N/A | Manual method 75%, DenseNet-121: 72.6%, ResNet-18: 71.6% | |
| Ishioka et al[14] | 2012-2017 | 61 | Stage II/II rectal cancer with poor features: MRF < 1 mm, cT4, positive lateral lymph nodes, mesorectal N2 disease and/or tumor requiring abdominoperineal resection | Neoadjuvant chemotherapy (folinic acid, fluorouracil, oxaliplatin + bevacizumab) followed by 50.4 Gy radiotherapy with concurrent S-1 | Median 43 days after end of radiotherapy | White-light endoscopy and chromoendoscopy with indigo carmine dye, followed by magnifying NBI | Median 47 days after end of radiotherapy | Complete response (all 5): White light endoscopy: Ulcer completely closed and linear or flat shape of scar; no residual protruded nodules; preservation of rectal wall distention by insufflation. NBI: Regular or regenerated surface pattern; invisible or isolated vessel pattern (orderly network of micro vessels comprised of thin-caliber vessels) | 31.1% | 11.4% | 70.5% with conventional white-light endoscopy, 75.4% white-light endoscopy + NBI |
| Kawai et al[18] | 2007-2015 | 198 (186 radical surgery) | Low rectal cancer or T3/4 or N (+) | 50.4 Gy with concurrent fluorouracil | 3-8 weeks after end of CRT | Colonoscopy | 6-8 weeks after end of CRT | Changes in the marginal tumor swelling, classified as almost no change, less than half according to pre-CRT measures, and almost flattened; changes in central ulceration, assessed according to whether reepithelialization of the ulcer was present; a cancer-negative biopsy result | 12.8% in surgery group, 16.7% estimation in the watch-and-wait group | 22.3% in radical surgery group, 50% in watchful waiting | Flattened marginal swelling 69.7%. Ulcer reepithelization 81.2%. Cancer negative biopsy 66.4% |
| Van Der Sande et al[19] | 2012-2015 | 161 (87 surgery, 74 watch and wait) | cT1-2: 21.7%, cT3: 73.3%, cT4: 5%, cNo: 25.5%, cN1: 28.6%, cN2: 46% | Long course CRT or short course RT + interval | 9 weeks after end of radiotherapy median (8-12 weeks) | White light imaging flexible sigmoidoscopy, 3 readers (2 surgeons, 1 gastroenterologist) | 18 weeks interval between endoscopy and surgery | A flat scar; a small flat ulcer (< 1 cm); a large flat ulcer; ulcer with an irregular border; an adenomatous mass; tumorous mass | 16% | 42.8% (20.3% in surgery, 79.7% in watch and wait) | AUC 0.84, 0.80, 0.84 for complete response prediction for each of the 3 readers |
| Haak et al[13] | 2012-2015 | 226 | cT1-2: 22%, cT3: 71%, cT4: 7%, cNo: 24%, cN1: 28%, cN2: 48% | Long course CRT or short course RT + interval | 10 weeks, median: 8-15 weeks | White light imaging flexible endoscopy (EPK-I video processor, Pentax Medical Netherlands, Uithoorn, the Netherlands) + deep learning | 5 weeks, median: 2-10 weeks, between endoscopy and surgery | N/A | 10.6% | 48% | 67%-75% for different convolutional neural network models including endoscopic images and clinical variables |
| Felder et al[20] | N/A survey | 41+17 endoscopic pictures | N/A | Neoadjuvant treatment not otherwise specified | N/A | Two cross-sectional surveys, each containing endoscopic photos of rectal cancers treated with NT. The first survey assessed reproducibility of eight endoscopic criteria using 41 unique endoscopic photos. The second survey included endoscopic pairs of pre-neoadjuvant and post-neoadjuvant treatment photos of 17 patients | N/A | Flat and white scar; telangiectasias; absence of ulceration; absence of nodularity; small mucosal nodules or minor mucosal abnormality; superficial ulceration; mild persisting erythema of the scar; and visible tumor | N/A | N/A | 89% |
| Williams et al[9] | 2014-2020 | 263 | cT1-2: 12.6%, cT3: 75.6%, cT4: 11.8%, cN (+): 63.7% | TNT (INCT-CRT or CRT-CNCT) | 8 ± 4 weeks after TNT completion | Flexible sigmoidoscopy | N/A | Flat scar; telangiectasia; ulcer; nodularity; mucosal irregularity; mild erythema of the scar; visible tumor | N/A | 42.5% | N/A |
| Williams et al[10] | 2012-2020 | 288 | Stage II/III | TNT (INCT-CRT or CRT-CNCT) | 47 days median time from end of TNT | White-light flexible endoscopy with an Olympus scope (model CF-Q160S). Convolutional Neural Network using ResNet-50 | N/A | Flat white scar; telangiectasias; no ulceration; no nodularity | N/A | N/A | AUC 0.99 for training set, AUC 0.98 for main test, AUC 0.92 for local regrowth |
| Chen et al[21] | 2013-2021 | 1000 | T3-4 and / or N (+) | Long course radiotherapy 50 Gy or short course radiotherapy 25 Gy. Concurrent chemotherapy oral/intravenous 5FU or combined with oxaliplatin/irinotecan | 6-8 weeks after NT | White-light endoscopy with endoscopy-based Deep Convolutional Neural Network with a ResNeSt-50 variant | 6-12 weeks after NT | N/A | 21.9% | N/A | 94.21% for training set, 92.13% for validation set, 87.14% for independent set |
| Sohn et al[22] | 2004-2013 | 425 | cT3-4 and/or N (+) | nCRT (45 Gy + 5.4 Gy boost + 5FU/Leucovorin or capecitabine or tegafur/uracil) | Immediately before surgery | White-light endoscopy | 6-8 weeks after nCRT | E-GR: Scarring (the flat and white mucosa with fibrotic changes); telangiectasia (scarring surrounded by small blood vessels); erythema (scarring or erosion with peripheral erythematous mucosal changes) | 10.8% | 11.1% | N/A |
| Han et al[23] | 2004-2013 | 481 | cT3-4 | nCRT (45 Gy + 5.4 Gy boost + 5FU/Leucovorin or capecitabine or capecitabine + irinotecan or tegafur/uracil) | 6-8 weeks after nCRT | White-light endoscopy | 6-8 weeks after nCRT | E-GR: Scarring (the flat and white mucosa with fibrotic changes); telangiectasia (scarring surrounded by small blood vessels); erythema (scarring or erosion with peripheral erythematous mucosal changes) | 11% | N/A | Pathologic good response: ≤ ypT1, positive predictive value of 0.65, negative predictive value of 0.885 |
| Thompson et al[11] | 2012-2017 | 109 | LARC | TNT [ChT (FOLFOX or CAPEOX) + long-course chemoradiotherapy with concurrent 5FU or capecitabine] | 6 weeks after TNT and subsequent follow up visits (3 total selected including first visit) | White-light endoscopy with convolutional neural network VGG-19 | Watch and wait | N/A | N/A | N/A | Training set AUROC 0.83, testing set AUROC 0.83 |
When assessing response to neoadjuvant treatment, pCR as assessed on pathologic examination of specimens after curative intent surgery was set as the gold standard for complete response to neoadjuvant treatment. The goal of the current study was to determine the diagnostic accuracy of endoscopic complete response (ECR) to predict the subsequent “gold standard” of pCR.
In three studies, a total neoadjuvant approach was used for rectal cancer management. In studies reported by Williams et al[9,10], patients underwent either chemotherapy with consolidation chemoradiotherapy or long-course chemoradiotherapy followed by chemotherapy as neoadjuvant treatment. In the first report[9], endoscopic criteria used for assessment were reported and the time of endoscopic assessment after TNT was 8 ± 4 weeks. However, the authors did not report any diagnostic accuracy data. In the second report[10], the authors applied convolutional neural network-based algorithms for assessing response at a median time of 47 days after TNT completion. In a study by Thompson et al[11], patients underwent chemotherapy followed by long-course chemoradiotherapy as neoadjuvant treatment. The endoscopic response to treatment was assessed at 6 weeks after completion of TNT and then at regular hospital visits as part of a watch-and-wait protocol. The authors in this study used artificial intelligence to create a deep learning-based model to analyze endoscopic images and reported an area under the receiver operator curve (commonly referred to as AUROC) values of 0.83.
The other 12 studies included here reported on patients receiving traditional neoadjuvant chemoradiotherapy regimens. We noticed a significant variation between studies in the time frames for endoscopic assessment. Two studies[12,13] reported on the performance of endoscopic assessment in relation to the timing of surgery, while two studies[14,15] did not report on the specific timing of endoscopic assessment. The remaining eight studies reported on the timing of endoscopic assessment in relation to the completion of neoadjuvant treatment. Significant variations between time frames and regimens were also noted.
Endoscopic assessment of rectal cancer response after neoadjuvant treatment appears to have a high diagnostic accuracy in predicting pCR. Eleven studies[9,10,12,14,16-21,23] reported on the criteria used to classify endoscopic response of patients. Telangiectasia, flat/white scar, and ulcer with clean base were features consistently used for assessment of complete endoscopic response. The pCR rates ranged from 10.8% to 31.1%. The value of endoscopic assessment in prediction of pCR was expressed as either diagnostic accuracy or area under the curve with different values reported. When extracting the endoscopic criteria used to describe endoscopic CCR from relevant studies, the following criteria were most frequently used: (1) Flat, white scar; (2) Absence of nodularity; (3) Absence of ulceration; and (4) Telangectasia.
We also reviewed endoscopic modalities used to investigate treatment response. Bengulescu et al[16] compared chromoendoscopy to classic white-light imaging and reported better outcomes with chromoendoscopy (75.4% accuracy for pCR prediction for chromoendoscopy vs 70.5% for classic white-light endoscopy). Three studies reported diagnostic accuracy in pCR prediction above the 80% threshold, which is widely considered to be the cutoff value for clinically significant diagnostic accuracy. The diagnostic discriminatory power of some studies was reported as AUROC, with the value consistently above 0.8.
Due to the heterogeneity across studies and data, including the different protocols for endoscopic assessment, it was not feasible to perform meta-analysis. Moreover, due to the variability of endoscopic criteria used to assess CCR among the different studies, it was inappropriate to cumulatively estimate the number of subjects from different studies that meet the definition of complete response. The rates of pCR (if reported) as well as the “ECR” (if reported) are included in the descriptive table. Some studies reported their outcomes using different metrics for the accuracy of endoscopic criteria to predict pCR on subsequent surgical resection, such as area under the curve. Because direct comparisons could not be made, a statistical analysis was not feasible.
Regarding risk of bias, we assessed the methodological quality studies using Quality Assessment of Diagnostic Accuracy Studies (QUADAS)-2 guidelines[24]. Most studies were deemed as high risk of bias for patient selection. Studies that applied deep learning algorithms or chromoendoscopy were deemed as unclear risk for bias due to applicability concerns for the index test. Failure to report accurate endoscopic criteria for ECR assessment or failure to report pCR rates (considered as reference standard), also significantly downgraded the quality of studies for the purposes of our research question, namely whether ECR after neoadjuvant treatment can predict pCR in rectal cancer patients. Results are presented in Table 2[9-23]. All studies identified in our search were retrospective in nature, and thus suffer from potential selection bias. Another form of bias identified in some studies was the lack of comparison to a reference standard, namely pCR for our study. Five studies[10,11,15,18,22] used deep learning algorithms based on convolutional neural networks applied on white-light endoscopy images to enhance diagnostic accuracy. Although all five reported excellent outcomes in terms of diagnostic accuracy, they suffered from applicability concerns and lack of reference standard bias.
| Ref. | Risk of bias | Applicability concerns | |||||
| Patient selection | Index test | Reference standard | Flow and timing | Patient selection | Index test | Reference standard | |
| Lim et al[15] | High risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Chino et al[12] | High risk | Unclear risk | Unclear risk | Low risk | High risk | High risk | Unclear risk |
| Bengulescu et al[16] | High risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Wang et al[17] | High risk | Low risk | Unclear risk | Low risk | Low risk | Low risk | Unclear risk |
| Ishioka et al[14] | High risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Kawai et al[18] | High risk | Unclear risk | Low risk | Low risk | Low risk | High risk | Low risk |
| Van Der Sande et al[19] | High risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Haak et al[13] | High risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Felder et al[20] | High risk | Unclear risk | Low risk | High risk | Low risk | Unclear risk | Low risk |
| Williams et al[9] | High risk | Low risk | Unclear risk | High risk | Low risk | Unclear risk | Unclear risk |
| Williams et al[10] | High risk | Unclear risk | Unclear risk | High risk | Low risk | Unclear risk | Unclear risk |
| Chen et al[21] | High risk | Unclear risk | Unclear risk | Low risk | Low risk | High risk | Unclear risk |
| Sohn et al[22] | High risk | Unclear risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Han et al[23] | High risk | Low risk | Unclear risk | Low risk | Low risk | Low risk | Unclear risk |
| Thompson et al[11] | High risk | High risk | High risk | High risk | Low risk | High risk | High risk |
The evolving landscape of treatment for locally advanced rectal cancer presents unique challenges in patient mana
A recently published secondary analysis of the OPRA trial[26] suggested that CCR is associated with improved rates of OP, disease-free survival, distant-metastasis free survival and overall survival. The landmark report from Habr-Gama et al[27] from 2010 defined clinical and endoscopic findings of CCR. Findings from this study are widely used as the roadmap when reassessing patients after neoadjuvant treatment, with residual superficial ulceration, irregularity or nodule regarded as equivalent to residual disease. Importantly, the authors concluded that diagnostic biopsies to stage tumor response after treatment were not valuable. In contrast, achieving pCR after neoadjuvant treatment is associated with improved long-term oncologic outcomes, whereas the presence of any residual cancer, even limited to the mucosal (in situ) level, is linked to poorer outcomes[28]. A recent pathological analysis of non-pCR tumors sought to elucidate why accurate staging of tumor response requires diagnostic modalities with submillimeter-level resolution[29]. As
Endoscopy demonstrates a greater ability to accurately identify complete response compared to MRI[32]. Ko et al[33] reported that combining the two modalities increases diagnostic accuracy, despite the fact that they utilized endoscopy with biopsies for their endoscopic assessment of clinical response. In another report from Cho et al[34], neither MRI nor endoscopy had adequate diagnostic accuracy for accurate pCR prediction; thus, the authors suggested a combined approach. We conducted this systematic literature review to estimate the diagnostic accuracy of endoscopic assessment, with an emphasis on specific diagnostic criteria used to define a CCR, as well as the exact time frame in which to perform the endoscopic assessment after neoadjuvant treatment. An increasing body of literature on the combined use of diagnostic modalities has introduced confusion regarding the true diagnostic yield of each modality when applied independently. Combining diagnostic modalities without standardization of technique and criteria can confound results, thus preventing the dissemination of good practices across the medical community. Artificial intelligence and agnostic interpretation of imaging modalities are appealing in terms of diagnostic accuracy, but this effect may be overestimated because the results were not compared with results from studies in which strict criteria were used.
The existing studies suffer from different biases, as demonstrated in our analysis of study quality using the QUADAS-2 guidelines. A recent systematic review[35] on diagnostic performance of MRI and endoscopy for assessing complete response in rectal cancer after neoadjuvant chemoradiotherapy also concluded that the heterogeneity of studies pre
This review revealed heterogeneity in the endoscopic criteria used for prediction and in the specific time frame of assessment. Considering the new definitions[7] and 6 months as the limit to characterize tumor response as either complete or incomplete, such variations in time of assessment become critically important. Another limitation of endoscopic assessment of tumor response after neoadjuvant therapy is its dependence on operator experience, as evaluation, even when specific endoscopic features are systematically assessed, is subject to interobserver variability. No studies have reported on this matter. Furthermore, none have specified whether endoscopic assessments were performed by gastroenterologists or surgeons. Because colorectal surgeons are often responsible for follow-up after neoadjuvant therapy, particularly in the TNT era of watch-and-wait strategies, yet gastroenterologists perform the majority of endoscopic procedures in most centers worldwide, clarifying this distinction is of paramount importance. Therefore, future research should focus on standardization of endoscopic processes, enhancement of training and compliance with time frames dictated by multidisciplinary rectal cancer management guidelines. Artificial intelligence represents a powerful tool when integrated within an appropriate clinical context and applied without deviation from standardized assessment protocols. In our separate review of studies utilizing artificial intelligence, we found that the application of deep learning algorithms to endoscopic images failed to consistently predict pCR.
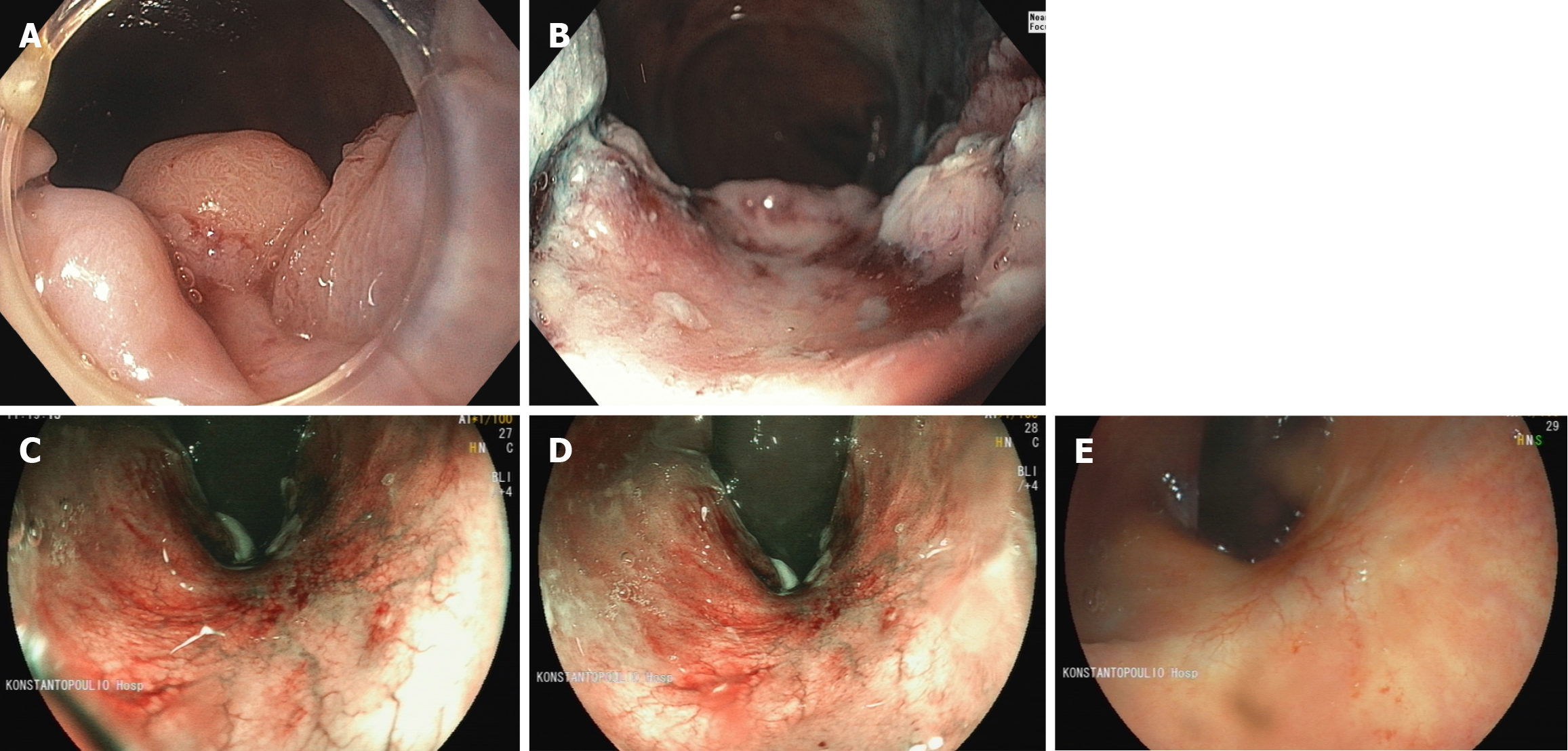
We have provided exemplary endoscopic images from patients undergoing watchful waiting after neoadjuvant chemoradiation for rectal cancer (Figure 1). We have also systematically used the absence of nodularity, telangiectasia, and a completely flat white scar with no irregularities as endoscopic predictors of complete response.
Endoscopic assessment of rectal cancer response after neoadjuvant (chemo)radiotherapy, when performed using standardized criteria for defining CCR, can predict pCR with good diagnostic accuracy. The endoscopic features most consistently associated with tumor response include a flat scar, absence of nodularity or ulceration, and the presence of telangiectasias. The use of deep learning algorithms applied to endoscopic images may further enhance diagnostic performance. However, existing studies are limited by various forms of bias and exhibit significant heterogeneity in study protocols, criteria, and response definitions.
| 1. | Ballal DS, Vispute TP, Saklani AP. The conundrum of total neoadjuvant therapy in rectal cancer. Colorectal Dis. 2024;26:1068-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 2. | Verheij FS, Omer DM, Williams H, Lin ST, Qin LX, Buckley JT, Thompson HM, Yuval JB, Kim JK, Dunne RF, Marcet J, Cataldo P, Polite B, Herzig DO, Liska D, Oommen S, Friel CM, Ternent C, Coveler AL, Hunt S, Gregory A, Varma MG, Bello BL, Carmichael JC, Krauss J, Gleisner A, Guillem JG, Temple L, Goodman KA, Segal NH, Cercek A, Yaeger R, Nash GM, Widmar M, Wei IH, Pappou EP, Weiser MR, Paty PB, Smith JJ, Wu AJ, Gollub MJ, Saltz LB, Garcia-Aguilar J. Long-Term Results of Organ Preservation in Patients With Rectal Adenocarcinoma Treated With Total Neoadjuvant Therapy: The Randomized Phase II OPRA Trial. J Clin Oncol. 2024;42:500-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 192] [Article Influence: 96.0] [Reference Citation Analysis (0)] |
| 3. | Zwart WH, Temmink SJD, Hospers GAP, Marijnen CAM, Putter H, Nagtegaal ID, Blomqvist L, Kranenbarg EM, Roodvoets AGH, Martling A, van de Velde CJH, Glimelius B, Peeters KCMJ, van Etten B, Nilsson PJ; Collaborative investigators. Oncological outcomes after a pathological complete response following total neoadjuvant therapy or chemoradiotherapy for high-risk locally advanced rectal cancer in the RAPIDO trial. Eur J Cancer. 2024;204:114044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 26] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 4. | Goffredo P, Suraju MO, Mott SL, Troester AM, Weaver L, Mishra A, Sokas C, Hassan I. ASO Visual Abstract: Pathologic Complete Response, Total Neoadjuvant Therapy, and the Survival Paradox in Locally Advanced Rectal Cancer. Ann Surg Oncol. 2024;31:5971-5972. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Socha J, Glynne-Jones R, Bujko K. Oncological risks associated with the planned watch-and-wait strategy using total neoadjuvant treatment for rectal cancer: A narrative review. Cancer Treat Rev. 2024;129:102796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 6. | Erozkan K, Liska D, Oktem A, Alipouriani A, Schabl L, Valente MA, Miller JA, Purysko AS, Steele SR, Gorgun E. The role of combining interim and final analysis by using endoscopic and radiologic methods in total neoadjuvant treatment. Am J Surg. 2025;241:116104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Custers PA, Beets GL, Bach SP, Blomqvist LK, Figueiredo N, Gollub MJ, Martling A, Melenhorst J, Ortega CD, Perez RO, Smith JJ, Lambregts DMJ, Beets-Tan RGH, Maas M. An International Expert-Based Consensus on the Definition of a Clinical Near-Complete Response After Neoadjuvant (Chemo)radiotherapy for Rectal Cancer. Dis Colon Rectum. 2024;67:782-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 8. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44932] [Cited by in RCA: 51000] [Article Influence: 10200.0] [Reference Citation Analysis (2)] |
| 9. | Williams H, Thompson HM, Lin ST, Verheij FS, Omer DM, Qin LX, Garcia-Aguilar J; OPRA Consortium. Endoscopic Predictors of Residual Tumor After Total Neoadjuvant Therapy: A Post Hoc Analysis From the Organ Preservation in Rectal Adenocarcinoma Trial. Dis Colon Rectum. 2024;67:369-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 10. | Williams H, Thompson HM, Lee C, Rangnekar A, Gomez JT, Widmar M, Wei IH, Pappou EP, Nash GM, Weiser MR, Paty PB, Smith JJ, Veeraraghavan H, Garcia-Aguilar J. Assessing Endoscopic Response in Locally Advanced Rectal Cancer Treated with Total Neoadjuvant Therapy: Development and Validation of a Highly Accurate Convolutional Neural Network. Ann Surg Oncol. 2024;31:6443-6451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 11. | Thompson HM, Kim JK, Jimenez-Rodriguez RM, Garcia-Aguilar J, Veeraraghavan H. Deep Learning-Based Model for Identifying Tumors in Endoscopic Images From Patients With Locally Advanced Rectal Cancer Treated With Total Neoadjuvant Therapy. Dis Colon Rectum. 2023;66:383-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 12. | Chino A, Konishi T, Ogura A, Kawachi H, Osumi H, Yoshio T, Kishihara T, Ide D, Saito S, Igarashi M, Akiyoshi T, Ueno M, Fujisaki J. Endoscopic criteria to evaluate tumor response of rectal cancer to neoadjuvant chemoradiotherapy using magnifying chromoendoscopy. Eur J Surg Oncol. 2018;44:1247-1253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Haak HE, Gao X, Maas M, Waktola S, Benson S, Beets-Tan RGH, Beets GL, van Leerdam M, Melenhorst J. The use of deep learning on endoscopic images to assess the response of rectal cancer after chemoradiation. Surg Endosc. 2022;36:3592-3600. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Ishioka M, Chino A, Ide D, Saito S, Igarashi M, Nagasaki T, Akiyoshi T, Nagayama S, Fukunaga Y, Ueno M, Kawachi H, Yamamoto N, Fujisaki J, Konishi T. Adding Narrow-Band Imaging to Chromoendoscopy for the Evaluation of Tumor Response to Neoadjuvant Therapy in Rectal Cancer. Dis Colon Rectum. 2021;64:53-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Lim SG, Kim YB, Oh SY. Clinical Significance of the Endoscopic Finding in Predicting Complete Tumor Response to Preoperative Chemoradiation Therapy in Rectal Cancer. World J Surg. 2016;40:3029-3034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Bengulescu I, Radu P, Iorga C, Bratucu M, Pasnicu C, Garofil D, Popa F, Strambu V. The Value of Endoscopy as a Predictive Factor when Evaluating the Clinical Response to Neoadjuvant Chemoradiotherapy for Patients with Rectal Cancer. Chirurgia (Bucur). 2020;115:373-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Wang A, Zhou J, Wang G, Zhang B, Xin H, Zhou H. Deep learning of endoscopic features for the assessment of neoadjuvant therapy response in locally advanced rectal cancer. Asian J Surg. 2023;46:3568-3574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 18. | Kawai K, Ishihara S, Nozawa H, Hata K, Kiyomatsu T, Morikawa T, Fukayama M, Watanabe T. Prediction of Pathological Complete Response Using Endoscopic Findings and Outcomes of Patients Who Underwent Watchful Waiting After Chemoradiotherapy for Rectal Cancer. Dis Colon Rectum. 2017;60:368-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 19. | van der Sande ME, Maas M, Melenhorst J, Breukink SO, van Leerdam ME, Beets GL. Predictive Value of Endoscopic Features for a Complete Response After Chemoradiotherapy for Rectal Cancer. Ann Surg. 2021;274:e541-e547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 20. | Felder SI, Patil S, Kennedy E, Garcia-Aguilar J. Endoscopic Feature and Response Reproducibility in Tumor Assessment after Neoadjuvant Therapy for Rectal Adenocarcinoma. Ann Surg Oncol. 2021;28:5205-5223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Chen X, Chen J, He X, Xu L, Liu W, Lin D, Luo Y, Feng Y, Lian L, Hu J, Lan P. Endoscopy-Based Deep Convolutional Neural Network Predicts Response to Neoadjuvant Treatment for Locally Advanced Rectal Cancer. Front Physiol. 2022;13:880981. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 22. | Sohn DK, Han KS, Kim BC, Hong CW, Chang HJ, Baek JY, Kim MJ, Park SC, Oh JH, Kim DY. Endoscopic assessment of tumor regression after preoperative chemoradiotherapy as a prognostic marker in locally advanced rectal cancer. Surg Oncol. 2017;26:453-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Han KS, Sohn DK, Kim DY, Kim BC, Hong CW, Chang HJ, Kim SY, Baek JY, Park SC, Kim MJ, Oh JH. Endoscopic Criteria for Evaluating Tumor Stage after Preoperative Chemoradiation Therapy in Locally Advanced Rectal Cancer. Cancer Res Treat. 2016;48:567-573. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, Leeflang MM, Sterne JA, Bossuyt PM; QUADAS-2 Group. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6953] [Cited by in RCA: 10349] [Article Influence: 689.9] [Reference Citation Analysis (3)] |
| 25. | Giunta EF, Bregni G, Pretta A, Deleporte A, Liberale G, Bali AM, Moretti L, Troiani T, Ciardiello F, Hendlisz A, Sclafani F. Total neoadjuvant therapy for rectal cancer: Making sense of the results from the RAPIDO and PRODIGE 23 trials. Cancer Treat Rev. 2021;96:102177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 26. | Thompson HM, Omer DM, Lin S, Kim JK, Yuval JB, Verheij FS, Qin LX, Gollub MJ, Wu AJ, Lee M, Patil S, Hezel AF, Marcet JE, Cataldo PA, Polite BN, Herzig DO, Liska D, Oommen S, Friel CM, Ternent CA, Coveler AL, Hunt SR, Garcia-Aguilar J; OPRA Consortium. Organ Preservation and Survival by Clinical Response Grade in Patients With Rectal Cancer Treated With Total Neoadjuvant Therapy: A Secondary Analysis of the OPRA Randomized Clinical Trial. JAMA Netw Open. 2024;7:e2350903. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 76] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 27. | Habr-Gama A, Perez RO, Wynn G, Marks J, Kessler H, Gama-Rodrigues J. Complete clinical response after neoadjuvant chemoradiation therapy for distal rectal cancer: characterization of clinical and endoscopic findings for standardization. Dis Colon Rectum. 2010;53:1692-1698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 337] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 28. | Kohrman NM, Wlodarczyk JR, Ding L, McAndrew NP, Algaze SD, Cologne KG, Lee SW, Koller SE. Rectal Cancer Survival for Residual Carcinoma In Situ Versus Pathologic Complete Response After Neoadjuvant Therapy. Dis Colon Rectum. 2024;67:920-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 29. | van der Stel SD, van den Berg JG, Snaebjornsson P, Seignette IM, Witteveen M, Grotenhuis BA, Beets GL, Post AL, Ruers TJM. Size and depth of residual tumor after neoadjuvant chemoradiotherapy in rectal cancer - implications for the development of new imaging modalities for response assessment. Front Oncol. 2023;13:1209732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 30. | Verrijssen AS, Guillem J, Perez R, Bujko K, Guedj N, Habr-Gama A, Houben R, Goudkade D, Melenhorst J, Buijsen J, Vanneste B, Grabsch HI, Bellezzo M, Paiva Fonseca G, Verhaegen F, Berbee M, Van Limbergen EJ. Microscopic intramural extension of rectal cancer after neoadjuvant chemoradiation: A meta-analysis based on individual patient data. Radiother Oncol. 2020;144:37-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 31. | Deidda S, Spolverato G, Capelli G, Bao RQ, Bettoni L, Crimì F, Zorcolo L, Pucciarelli S, Restivo A. Limits of Clinical Restaging in Detecting Responders After Neoadjuvant Therapies for Rectal Cancer. Dis Colon Rectum. 2023;66:957-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 32. | Rojas MA, Cataneo J, Gagnon-Konamna M, Borsuk DJ, Jarzabek AJ, Marecik SJ, Park JJ, Kochar K. Correlation of Tumor Response Between Flexible Sigmoidoscopy and Magnetic Resonance Imaging in Patients Undergoing Neoadjuvant Therapy for Locally Advanced Rectal Cancer: A Retrospective Review. Am Surg. 2023;89:2595-2599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 33. | Ko HM, Choi YH, Lee JE, Lee KH, Kim JY, Kim JS. Combination Assessment of Clinical Complete Response of Patients With Rectal Cancer Following Chemoradiotherapy With Endoscopy and Magnetic Resonance Imaging. Ann Coloproctol. 2019;35:202-208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 34. | Cho MS, Kim H, Han YD, Hur H, Min BS, Baik SH, Cheon JH, Lim JS, Lee KY, Kim NK. Endoscopy and magnetic resonance imaging-based prediction of ypT stage in patients with rectal cancer who received chemoradiotherapy: Results from a prospective study of 110 patients. Medicine (Baltimore). 2019;98:e16614. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 35. | Munk NE, Bondeven P, Pedersen BG. Diagnostic performance of MRI and endoscopy for assessing complete response in rectal cancer after neoadjuvant chemoradiotherapy: a systematic review of the literature. Acta Radiol. 2023;64:20-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 36. | Dijkstra EA, Nilsson PJ, Hospers GAP, Bahadoer RR, Meershoek-Klein Kranenbarg E, Roodvoets AGH, Putter H, Berglund Å, Cervantes A, Crolla RMPH, Hendriks MP, Capdevila J, Edhemovic I, Marijnen CAM, van de Velde CJH, Glimelius B, van Etten B; Collaborative Investigators. Locoregional Failure During and After Short-course Radiotherapy Followed by Chemotherapy and Surgery Compared With Long-course Chemoradiotherapy and Surgery: A 5-Year Follow-up of the RAPIDO Trial. Ann Surg. 2023;278:e766-e772. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 235] [Article Influence: 78.3] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/