Published online Oct 24, 2025. doi: 10.5306/wjco.v16.i10.110126
Revised: June 25, 2025
Accepted: September 17, 2025
Published online: October 24, 2025
Processing time: 148 Days and 0.9 Hours
Catalase (CAT) is a kind of tetrameric protein in the human body, play as a key regulator for controlling oxidative stress. The main function of CAT is to regulate the concentration of hydrogen peroxide (H2O2) by catalyzing the decomposition of H2O2. At present, it is reported that CAT is also involved in regulating the oxidative stress in tumor cells, and its expression level is significantly related to the development of breast cancer (BC). In addition, CAT with different expression patterns, was related in the proliferation, invasion, treatment and prognosis of BC cells. Meanwhile, BC is a common and well-known cancer among women world
Core Tip: Catalase (CAT) plays a critical role in breast cancer (BC) by regulating oxi
- Citation: Liu JW, Chen WJ, Lan YZ, Liu J. Catalase: The golden key to regulate oxidative stress in breast cancer. World J Clin Oncol 2025; 16(10): 110126
- URL: https://www.wjgnet.com/2218-4333/full/v16/i10/110126.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i10.110126
The most common type of cancer in females, breast cancer (BC) has a high mortality rate all over the world, with increased incidence and mortality, brought huge health burden and economic pressure to the people[1]. However, the survival rate of patients with BC at early stage, can be greatly improved after active treatment[2]. Therefore, research on early diagnosis biomarkers for BC is of great significance for patients.
On the other hand, the production of reactive oxygen species (ROS) was indicated as a risk for the incidence of invasive BC with bidirectional effect[3]. It is indicated that ROS has multiple functions that can promote the development of malignant tumors[4], while overproduction of ROS can disrupt the antioxidant system of tumor cells, leading to cell death[5]. Thus, ROS is also thought to be potential targets of invasive BC, while antioxidants prevent such damage caused by ROS. As part of the antioxidant system in the body, many antioxidant enzymes (AE) also enhance immune defenses and reduce the risk of disease and cancer.
Catalase (CAT) is one of the important AE in the human body, which protect the body from damage caused by oxidative stress. It is found that AE represented by CAT are highly expressed to protect cells from excessive formation of ROS[6]. It is well-known that the main functions of CAT are the catalytic decomposition of hydrogen peroxide (H2O2) into water and molecular oxygen (O2)[7], and the concentration of H2O2 in human body adjustment plays an important role. Due to its close association with oxidative stress in the human body, CAT exhibits distinct expression patterns in tumor cells compared to normal cells. Excessive or insufficient expression of CAT can exert varying impacts on tumor growth. It is revealed that there is a significant relationship between the expression level of CAT and BC cells[8], closely related to the proliferation and invasion ability of BC cells[9], which provides new clues for further research on the pathogenesis of BC. This article summarized the effect of CAT on oxidative stress in tumor cells, and discussed its application in the diagnosis and prognosis of BC.
The balance between oxidative damage and antioxidant protection is important to keep homeostasis in normal aerobic cells. Insufficient antioxidant clearance or excessive formation of O2 free radical (FR) results in a condition called oxidative stress. The original definition of oxidative stress was the disruption of the balance between oxidants and antioxidants, which is conducive to the production of oxidants[10]. It is proved that a small amount of oxidant produced endogenously or externally is essential for maintaining balance in cells, tissues, and organisms[11,12]. Hence, this physiological oxidative stress can be regarded as a good factor under certain circumstances[13]. However, when the level of oxidants in the body exceeds normal physiological levels, it can lead to pathological change in the organism. Therefore, the current definition of oxidative stress is added that it can also cause interruption of cellular redox signaling and control, leading to damage[14,15].
ROS are highly reactive molecules produced by organisms in response to normal cellular metabolism and various environmental factors, which can disrupt the structure of nucleic acids, lipids, and proteins, thereby altering their function. Most intracellular ROS originate from the FR superoxide formed by the acquisition of a single electron from O2via the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase or electron leakage in the mitochondrial electron transport chain. In addition, two superoxide molecules can be converted to one non-radical ROS molecule H2O2 and one water molecule by superoxide dismutase (SOD). H2O2 can also accept an electron from free Fe2+ through the Fenton reaction to become a hydroxyl radical[16]. In vivo, ROS are mainly derived from inflammatory cells, mitochondria and peroxisomes. The organelles producing the most H2O2 and superoxide anions, peroxisomes also contain large amounts of antioxidants to balance the ROS produced. In addition, NADPH oxidase is also a major source of intracellular ROS, which catalyzes O2 and NADPH to produce superoxide[17]. Oncogenes have been found to stimulate the production of NADPH oxidase-dependent ROS, necessary for cellular proliferation[18].
Despite the presence of a multifaceted antioxidant enzyme system in human body, there is broad consensus in many studies that redox balance is altered in cancer[19,20]. In the process of cellular metabolism, many short-term and long-term ROS are produced, the ROS produced in this process are relatively stationary, playing an important role in the normal physiological activities of the human body. But in cancer cells, the increase in metabolic rate and relative hypoxia can increase the production of ROS. In addition, oncogenes can also provide the necessary conditions for cell proliferation by stimulating NADPH oxidase-dependent ROS production[18]. Meanwhile, the excessive ROS often induces gene mutations and alternations during transcription processes, ultimately leading to the oncogenesis[21].
ROS is proved to activate a variety of signal transduction pathways that promote tumorigenesis, enhance cell survival, proliferation, migration, and chemotherapy resistance, and lead to DNA damage and genetic instability[22,23]. For example, in inflamed cells, persistent inflammation can cause cells to secrete a large amount of ROS to recruit more activated immune cells, and lead to the imbalance of intracellular immune balance, which eventually leads to precancerous lesions. On this basis, if the amount of ROS produced by cells is higher than the endogenous antioxidant response, it will cause irreversible damage on nucleic acids, proteins and lipids, driving the initiation of carcinogenesis[24]. Compared with normal tissues, the lipid peroxidation in BC tissues was significantly increased, along with increased enzymatic and non-enzymatic antioxidants[25].
Irreversible DNA changes, like point mutations or chromosomal aberrations, drive tumor initiation[26-28]. The 8-hydroxy-deoxyguanosine (8-OHdG), with a mutagenic effect was found to be increased in many different types of cancers[29-31]. The 8-OHdG level in Michigan Cancer Foundation-7 (MCF-7) cells with positive estrogen receptor (ER) was also dramatically higher than that MDA-MB-231 cells with negative ER (9.3-fold)[32], suggesting the important role of ROS in the early stage of carcinogenesis[33].
Importantly, ROS can also interact with surface and intracellular receptors to modulate and interfere with native signaling pathways[34]. Phosphoinositide 3-kinase (PI3K) pathway, a central signaling pathway, was found to be overactivated in many cancers[35]. It is well-known that activation of protein kinase B (Akt) serves as a critical component in the PI3K signaling pathway, promoting cell proliferation and suppressing apoptosis. Among them, phosphatidylinositol (3,4,5)-trisphosphate (PIP3) enables its translocation to the plasma membrane, by binding Akt. Concurrently, phosphatase and tensin homolog deleted on chromosome ten (PTEN), a negative regulator of this pathway, exhibits constitutive phosphatase activities toward PIP3, converting it into the inactive form, phosphatidylinositol (4,5)-bisphosphate[16]. It is found that ROS can oxidize Cys124, the active site of PTEN, leading to the formation of another protein (Cys71) by disulfide bonds. This conformational change leads to the suppression of PTEN and unblocks the inhibition of PI3K, leaving the PI3K pathway permanently activated, which is a key driver for the development of cancers[36,37].
The expression of selenium-dependent glutathione peroxidase was negatively correlated with ER content in BC cells[38]. It is proved that estrogen is associated with the progression of BC[39,40], which can increase ROS amount in cells[41,42]. Uncoupling proteins (UCPs) can prevent mitochondrial ROS production, serving as a protective factor for normal cells[43,44]. It is explored that estrogen can induce BC by reducing the levels of UCPs and CAT in BC MCF-7 cells, increasing ROS production in mitochondria, indicating that estrogen participates in cancer development by inducing reactive O2 species[45].
On the other hand, it has been found that elevated levels of ROS can also promote anti-tumor signaling under certain conditions, leading to increased oxidative stress and inducing cancer cell death[46,47]. Although ROS typically maintains at a stable high concentration in cancer cells, further increasing ROS can greatly impair their antioxidant capacity and ultimately lead to death driven by oxidative damage. In this case, as normal cells typically have lower levels of oxidants, additional concentrations of ROS are likely to preferentially kill cancer cells rather than non-tumor cells. So-called pro-oxidant therapies take advantage of this important feature[48]. The cytotoxic effect of myricetin on triple-negative BC (TNBC) cells is due to oxidative stress caused by extracellular H2O2 generated by myricetin auto oxidation, leading to the production of ROS through Fenton reaction inside the cells[49]. Interestingly, the induced growth inhibition and ROS generation by fertilized soy milk product can be inhibited by CAT and deferoxamine[50]. In addition, ROS production was also observed in cancer cells treated with paclitaxel[51]. Generally, cancer cells also have the ability to adapt to the elevated ROS levels and clear excess ROS with activating antioxidants for self-protection[52,53]. Overexpression of antioxidants has been found in some tumors to make cancer cells more resistant to subsequent oxidative damage[54,55].
Intrinsic AE are key to protecting cells from FR damage in the human body. Inducible levels of AE in tumor blood vessels can protect the host from high FRs and ROS caused by the tumor[56]. Adenovirus-mediated SOD overexpression can inhibit the growth of BC cells through combination with 1,3-bis(2-chloroethyl)-1-nitrosourea, and achieve complete tumor remission in vivo, providing a new combined treatment regimen for BC treatment[57]. As mentioned previously, ROS can serve as intracellular signaling cascade in the cell of the second messenger, can also play various roles during the oncogenesis[34,48]. In a word, oxidative stress, an imbalance between the production and elimination of ROS, plays an important role in the pathogenesis of various diseases and pathophysiological processes such as BC[58,59]. Therefore, effective utilization and the development of pro-oxidant therapy is a promising strategy for the treatment of malignances.
Undesirable oxidative stress can damage cellular components and lead to the development of cancer[60]. The most important components are the enzymatic antioxidants, which act as the endogenous antioxidant defense system[61]. Among them, CAT and SOD are the important antioxidants in the body, acting as the first line of defense against ROS-mediated damage. When cells are exposed to oxidative stress, SOD can be rapidly induced to catalyze superoxide into O2 and H2O2[62], while CAT can then neutralize H2O2 by splitting it into molecular O2 and water[63].
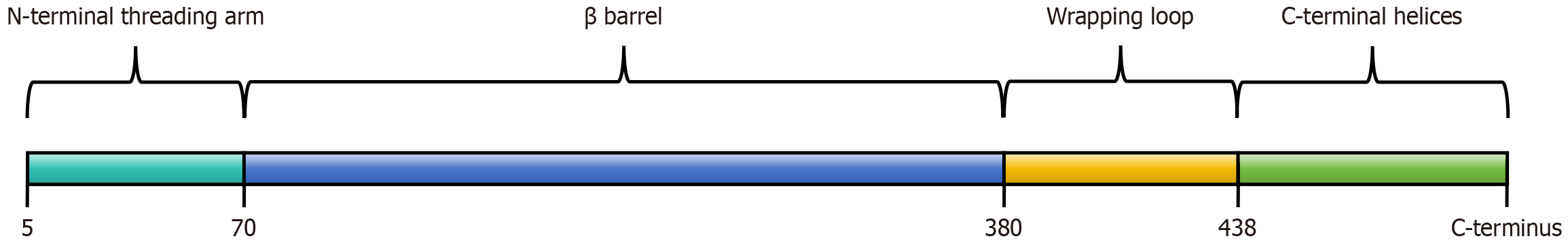
The human CAT gene consists of 12 introns and 13 exons, located on chromosome 11[64]. In human bodies, CAT belongs to a typical heme-containing monofunctional enzyme with an iron protoporphyrin IX prosthetic group that reacts with
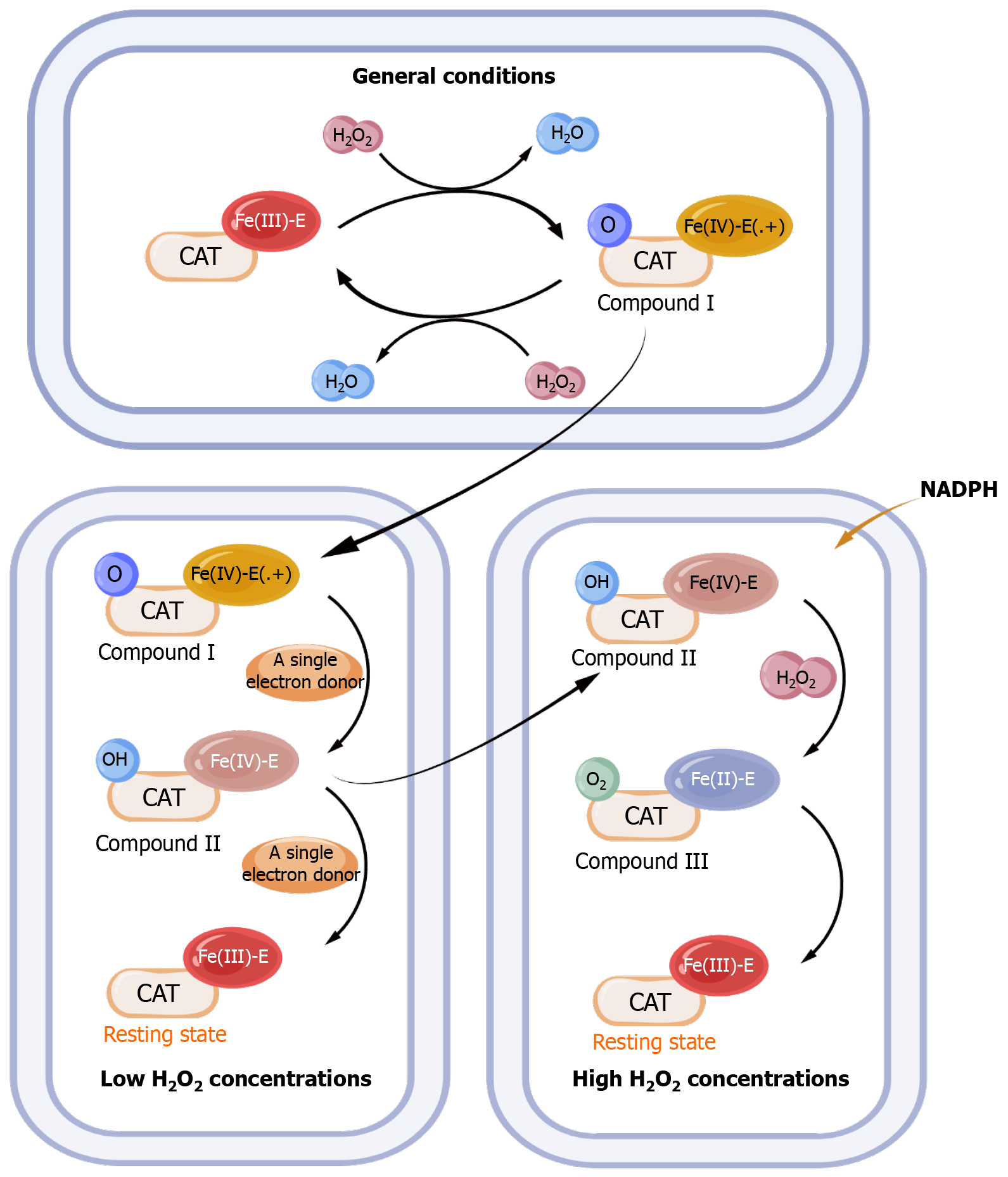
CAT is often first oxidized to a high valent iron intermediate, as compound I, and then reduced to a stable state by the second H2O2 molecule[69]. Compound I is a kind of iron oxide porphyrin cationic radical with a cationic group in its porphyrin[69]. It has been found that with single-electron donors and low H2O2 concentrations, the high valent iron intermediate (compound I) undergoes a one-electron reduction and become an inactive compound intermediate, as compound II. And transition returns to the resting state by another one-electron reduction reaction[70]. In compound I, the porphyrin has a cationic group, while in compound II it lacks a porphyrin cationic group, so compound II does not belong to the conventional iron oxide group[68]. However, at higher H2O2 concentrations, NADPH was found to prevent the formation of this inactive compound II by two-electron reduction process[71]. In the presence of higher concentrations of H2O2 molecules, this inactive compound will also be converted to another inactive intermediate, as compound III, which is intermediate state with an oxyferrous state of iron[72]. And the intermediate, compound III will then return to a quiescent state (Figure 2)[73].
CAT is expressed in almost all tissues of the human body with variable degrees, especially in the liver, kidney and red blood cells, to protect cells against oxidative stress[74]. The expression of human CAT is mainly mediated by peroxisome targeting signals, mainly located in peroxisomes[75]. Functional tetrameric CAT has also been detected in the cytoplasm of human skin fibroblasts[76]. In addition, the functional CAT is also found in cancer and chronic lymphocytic leukemia cell membrane surface, suggesting that CAT production does not necessarily occur exclusively within peroxisomes[77,78]. And this local high CAT level on tumor cell membrane has been suggested to be highly likely related to the dependence of tumor in vivo on increased resistance to exogenous H2O2[79]. Therefore, it is believed that the presence of extracellular CAT is related to the transformation state of malignant cells[80]. An increasing number of studies have also proved that membrane-associated CAT and extracellular CAT are common features of cancer cells, which prevent apoptosis induced by excessive intercellular ROS[81-83]. These observations also open the way for novel anticancer therapies using specific antibodies to target CAT. In addition to membrane-associated CAT, tumor cells have also been found to release soluble CAT, which is suggested with protective effect for tumor cells[84].
CAT is very important for the physiological health of human body. CAT deficiency or dysfunction is associated with different types of diseases. For example, acatalasemia is a rare disease characterized by a deficiency of CAT in red blood cell, which was first identified in 1948 with two mutations in the CAT gene[85-87]. Acatalasemia is usually a benign disease, but sometimes it may lead to the occurrence of other diseases, such as oral gangrene ulcers or essential hypertension[86,88], which may be promoted by phagocytes/bacteria-produced H2O2. Soon, it is pointed out that the mutation of CAT gene results in the change of structure of CAT protein structure, leading to Hungarian acatalasemia[89], while the family members of the Hungarian acatalasemic patient would have a high risk of developing diabetes, which also need further biochemical and genetic analysis[90].
Polymorphisms in the CAT promoter gene are also involved in pathogenesis[91]. The most common ones are -262C/T and -844G/A or -844C/T, affecting the transcription activities of CAT gene, as well as CAT activities[92]. Among them, the variant at -262C/T has significant functional significance, which can affect the binding of AP-2 and Sp-1, and the expression of CAT in red blood cell[93]. In addition, -262C/T polymorphism is also reported to be associated with BC. Compared with individuals with TT genotype, individuals with CC genotype tend to have lower levels of blood CAT, which will lead to oxidative stress, thereby promoting the occurrence of type 1 diabetes (T1D) and making them more susceptible to T1D[93]. Compared with TT and TC genotypes, the activities of CAT in red blood cell with CC genotype is increased, which may reduce the risk of BC[94]. Another one, -844C/T or -844G/A, may reduce the level of CAT by affecting the transcription frequency, and it is closely related to the occurrence of hypertension, but underlying mecha
In summary, CAT plays a key role in combating oxidative stress in normal and malignant cells, as well as in regulating H2O2 levels. The alterations in CAT expression may have widespread effects on the mechanisms of cancer development and pathological processes, which are of high importance to human life[96]. However, the molecular mechanism involved is still unclear, which needs further research.
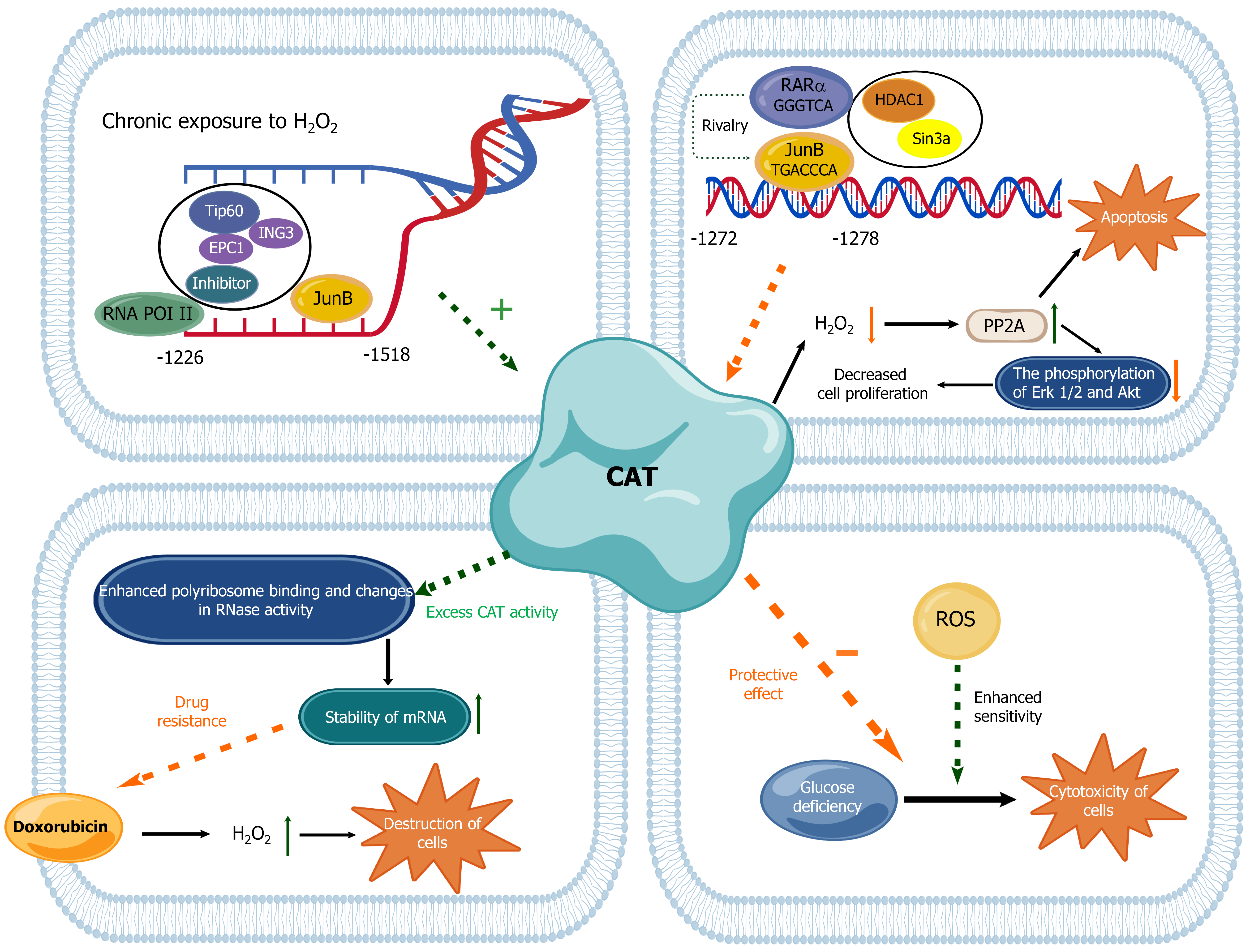
Due to the altered redox balance in cancer, the expression of CAT and other AE is often altered in cancer cell. Transcriptional regulation mainly plays a role through affecting the transcriptional activities of the CAT promoter[68]. In addition, the epigenetic changes represented by DNA methylation and histone modification can also regulate the activity of CAT in tumor cells[97]. In transcriptional regulation, transcription factors generally act through the regulation of chromatin remodeling, which can regulate CAT expression[98]. Studies have found that chromatin remodeling is also a major regulator of CAT expression in BC cells[98,99]. It is confirmed that retinoic acid receptor α (RARα) and Jun B proto-oncogene (JunB) transcription factors function in the control of chromatin remodeling and CAT expression in BC cells[98,100].
The BC MCF-7 resistant to oxidative stress, the so-called Resox cells, was first established by exposing MCF-7 cells to H2O2-generating systems for a long period[101]. These cells exhibited reduced basal levels of ROS and increased CAT expression. On this basis, it was found that there was a new promoter region, at -1518/-1226 site, responsible for regulating CAT expression in Resox cells. And activating protein-1 family members JunB and RARα can mediate the activation and repression of CAT transcription by recruiting coactivator complexes and histone deacetylase-dependent mechanisms to control chromatin remodeling[98]. Therefore, regulation of intracellular oxidative stress by transcription factors through chromatin remodeling in BC may become a novel mechanism for targeting cancer cells.
Due to the bidirectional effect of ROS on tumor cells, CAT also have a similar effect in tumor cells due to its ability to regulate oxidative stress in tumor cells. Firstly, CAT plays a role in preventing the excessive accumulation of oxidants in human bodies, so it can protect cells from the occurrence and development of tumors. It is also found that BC cells with human CAT gene have lower ROS concentration and stronger resistance to H2O2, which confirms that CAT gene also has the ability to eliminate intracellular ROS and maintain intracellular oxidation level[9]. CAT activity is closely related to the growth of human BC cells. Silencing CAT expression resulted in a further increase in the steady-state level of H2O2, which was also accompanied with the increasd growth rate of human BC cells. It is mainly due to the inhibition of protein phosphatase 2A (PP2A) activities by excessive H2O2, leading to the phosphorylation of extracellular signal-regulated kinase 1/2 (ERK 1/2) and Akt, and subsequent proliferation of BC cells. Therefore, overexpression of CAT can induce PP2A-dependent apoptosis of BC cells, thereby inhibiting the occurrence of BC[102].
On the contrary, CAT can also perform a protective or even promoting effect on tumor cells in some cases. In general, high concentrations of H2O2 are toxic to most eukaryotic and prokaryotic cells. Taking advantage of this property, anti-tumor antibiotics containing quinones, such as doxorubicin, can destroy tumor cells by stimulating the production of
The decreased biological activities of CAT in BC are often accompanied with the increased steady-state level of H2O2 in cells[102]. So increased oxidative stress markers and diminished antioxidant defense systems are also considered factors associated with the occurrence and progression of BC[106]. The expression of CAT in BC is affected by many factors and is closely related to the development and prognosis of BC (Figure 3)[73].
However, it remains unclear whether there are significant differences in the activity of CAT among various molecular subtypes of BC. It can be speculated that CAT activity may be closely associated with the levels of oxidative stress, metabolic properties, and clinical prognosis of each subtype. Maycotte et al[107] found that TNBC cells exhibited lower levels of CAT compared with MCF7 cells. The low CAT activity may be related to the high level of oxidative stress in TNBC cells[107]. Since TNBC cells generally have a high capacity for ROS generation, low CAT activity may limit their ability to scavenge H2O2, resulting in elevated levels of intracellular oxidative stress. However, the specific reasons and results need further research.
Normally, when ROS produced within cells increases, the cells are exposed to a pro-oxidative environment for a long time, which is closely related to tumor invasiveness[108,109]. It is proved that ROS at a certain concentration can act as a second messenger to participate in and stimulate tumor growth and metastasis pathways [p38/mitogen-activated protein kinase (MAPK)][4]. The concentration of AE such as CAT in the mitochondria of cells can reduce the oxidative stress produced by mitochondria, thereby controlling the concentration of ROS[110]. And this may also affect the aggressive phenotype of BC to a certain extent.
However, at present, the correlation between CAT and BC aggressiveness is not clear. By comparing the tumor tissues of mitochondrially targeted CAT (mCAT)-positive and mCAT-negative PyMT mice with different parameters, it was found that only 13% of the tumors from the McAt-positive PyMT mice exhibited a grade 3 histological aggressive phenotype, whereas 63% of the tumors from the McAt-negative PyMT mice had a grade 3 histological aggressive phenotype[9]. This indicated that mitochondrial CAT has the ability to reduce matrix infiltration and cell motility and migration to a certain extent. It is speculated that mCAT may activate different factors by altering ROS-dependent signaling pathways, thereby promoting epithelial-mesenchymal transition and tumor metastasis[111]. However, its specific mechanism may need further research.
Distant metastasis of advanced BC is often the main cause of death in patients with BC[112]. The main affected organs of metastatic BC are lung, brain, liver and bone[113,114]. According to previous studies, different metastatic cancers show different characteristics when the metastatic sites are different[115,116]. In metastatic BC, the expression of CAT and ROS-related proteins can also be affected by the metastatic site[117]. Interestingly, it is found that the expression of CAT is low in bone metastases, and the expression of stromal glutathione S-transferase π is high in the metastatic BC in bone and liver, along with high proportion of ER expression and inactive ROS states[116]. According to previous studies, the expression patterns of BC biomarkers (ER, PR, and human epidermal growth factor receptor 2) also vary according to the site of metastasis[118]. The majority of bone metastases predominantly exhibit hormone receptor positive[119]. Importantly, the inhibition of ER expression can lead to increased ROS production in BC cells[120]. Consequently, the lower CAT expression observed in bone metastases and the variations in ROS status across different metastatic sites are likely attributable to differences in hormone receptor status.
As an important modulator in ROS regulation, it is found that the negative expression of CAT has a great correlation with the short overall survival (OS) of the patients[121]. The gene mutation caused by oxidative stress will also change the prognosis of BC after treatment. It has been demonstrated that BC patients with genotypes leading to high levels of ROS exhibit superior OS compared to those with genotypes associated with lower ROS levels[122]. This suggests that genetic variants contributing to increased oxidative stress may potentiate the efficacy of chemotherapy or radiotherapy, thereby enhance treatment outcomes and improve patient survival.
Progestins are equally important for BC progression. It is reported that progesterone and various progestin-potent anabolic steroids (MPA and tibolone) can effectively induce CAT activity, which can counteract the H2O2 induced cell growth in BC cells and normal human breast epithelial cells. Among them, progesterone inhibits cell proliferation mainly by mediating progesterone receptor (PR) B subtype to effectively induce CAT activity[123]. Therefore, in order to provide better treatment and prognosis of BC, the evaluation of PR is essential. In addition, ER-α also can mediate estradiol to reduce the CAT activity through antioxidant effect of BC cells, indicating the potential role of steroid hormones in ROS metabolism and oncogenesis[124,125].
Regarding the common polymorphism, a C262T substitution, in the upstream of CAT transcription start site, the T allele leads to higher levels of CAT expression in red blood cell by enhancing promoter activity, which may influence the host's response to oxidative stress[126]. However, the specific key transcription factors need to be further identified. Interestingly, genetic polymorphisms, the CAT TT and MnSOD CC genotypes were found to decrease mortality risk in BC patients[122], potentially due to heightened oxidative stress and cytotoxicity associated with these polymorphisms. And the specific mechanism needs to be further studied. Collectively, these findings indicate that patients undergoing radiotherapy and chemotherapy for BC may achieve improved survival outcomes. Importantly, it is indicated that patients with TNBC, the aggressive molecular type of BC, who exhibit high CAT expression tend to have a lower N stage, reduced tumor recurrence rates, and prolonged OS[127]. It is plausible that CAT overexpression in TNBC protects normal cells from elevated ROS-induced toxicity and enhances therapeutic sensitivity, thereby improving patient survival[127].
As mentioned above, oxidative stress is caused by the imbalance of ROS, participating in the pathogenesis of various diseases, including BC[128]. The high production of O2 FRs and low CAT activities in BC indicate the high oxidative stress in BC patients, predicting AE (especially CAT) as the promising therapeutic strategy for BC[129]. Therefore, as a ROS-associated protein, the expression of CAT has been regarded as a potential strategy for treating patients with BC.
Most chemotherapeutic agents utilized in BC therapy exert their effects, by modulating ROS homeostasis. Taxanes, including paclitaxel and docetaxel, are frequently employed for the therapy of patients with BC[130]. Their primarily function by stabilizing the GDP-tubulin complex, thereby inhibiting microtubule dynamics and inducing mitotic arrest and apoptosis. Additionally, taxanes can disrupt mitochondrial respiratory chain function and impair normal electron transport, leading to the generation of superoxide radicals[131]. Consequently, excessive superoxide production may enhance the cytotoxic effects of these drugs on tumor cells, contributing to their therapeutic efficacy.
Platinum complexes, such as cisplatin, are widely used in clinical settings for the management of TNBC[130]. Their primary mechanism of action involves the formation of DNA adducts, which impede replication and trigger apoptosis. Moreover, platinum complexes can induce substantial ROS production via mitochondrial or NADPH oxidase pathways, resulting in significant side effects in patients[132].
Anthracyclines, including doxorubicin and epirubicin, represent standard therapeutic options for BC patients[130]. These agents inhibit topoisomerase II, thereby disrupting the synthesis of DNA and RNA, subsequently inducing cell death[48]. Furthermore, doxorubicin penetrates the inner mitochondrial membrane, competing with coenzyme Q10 in the electron transport chain, promoting the formation of superoxide radicals. The elevated ROS levels may augment drug-induced cytotoxicity against tumor cells[133].
Although the current understanding of CAT function and mechanism in tumorigenesis is still limited, the reported features of CAT in BC formation and progression make it a central factor in many therapeutic modalities.
The accumulation of ROS caused by enzyme inhibition is beneficial to the death of cancer cells and can be used as an anti-cancer drug. However, only a few CAT inhibitor drugs have been reported, which primarily interact with key amino acid residues in the catalytic site or the cofactor NADPH. Ferroptosis is a kind of cell death caused by excessive lipid peroxidation induced by ROS[134]. Recently, a novel CAT inhibitor, benzaldehyde thiourea derivatives, a derivative of benzaldehyde thioaminolone, has been designed to inhibit CAT activity by binding to NADPH-binding sites. This interaction induces endoplasmic reticulum stress and subsequent autophagy in DU145 castrate-resistant prostate cancer cells, leading to the degradation of ferritin heavy chain 1 and Fe2+ accumulation. The results promote the Fenton reaction and reactive hydroxyl radicals (•OH) production, ultimately inducing ferroptosis in tumor cells. And when the same experiment was performed in BC cells (MCF-7), similar results were obtained. Therefore, the inhibition of CAT may become a new strategy to induce siderosis of cancer cells through the dual regulation of ROS levels and ferroptosis[135].
Flavonoids are polyphenolic substances widely present in fruits and vegetables, with antioxidant, anti-inflammatory and anti-cancer activities[136,137]. The enzyme inhibitory properties of flavonoids have been studied recently. The investigation found that when the CAT interact with flavonoids, its alpha helix structure will be lost, which inhibits CAT activity[138]. In addition, in flavonols such as myricetin and quercetin, the higher number of hydroxyl groups in the B ring may contribute to their stronger inhibitory ability to CAT. This observed inhibition of CAT can lead to a rise in intracellular ROS levels and ultimately trigger a higher rate of apoptosis[138]. This can also be an important anti-cancer mechanism and provide a basis for the development of new anticancer drugs. Besides, it is suggested that Halymenia durvillei (HD), which is consumed in the diet of Korean women, has a BC risk reduction efficacy[139]. Recently, HD ethanolic extract was found to reduce the expression of CAT mRNA levels and induce ROS production in tumor cells, leading to cell cycle arrest in BC[140]. To some extent, it adds a new understanding of CAT in the targeted therapy of BC cells.
As a classic CAT inhibitor, 3-aminotriazole (3-AT) has been shown to exert anti-tumor effects through multiple pathways in cancer. Early studies have shown that 3-AT can inhibit γ-radiation-induced lymphoma and neutron radiation-induced ovarian tumors in animal models[141]. It may be due to the inhibition of CAT expression by 3-AT, which promotes the increase of oxidative stress level and enhances the sensitivity of tumor to radiation. Moreover, 3-AT could delay the appearance of murine mammary tumor virus (MUMTV)-driven breast tumors and even achieve long-term tumor-free status in animal models[141]. This suggests that the effect of 3-AT is background-dependent. By itself, it causes cancer by inhibiting CAT leading to H2O2 accumulation, but when combined with other carcinogenic factors (such as MuMTV and radiation), it can exert anti-tumor effect. In addition, with the development of nanoparticle technology, the combination of nanoparticles and 3-AT has further promoted the treatment of tumors in recent years. For example, nano-metal organic frameworks loaded 3-AT targeted delivery system can release 3-AT and chemotherapeutic drugs through pH response. Among them, 3-AT can promote the efficacy of chemodynamic therapy by inhibiting the activity of CAT to achieve the dual synergistic effect of chemodynamic therapy and chemotherapy[142]. It has shown efficient tumor suppression in models such as BC. Although 3-AT has shown potential in preclinical studies, its long-term toxicity and optimal dosing regimen are still unclear and need to be further explored.
There are also compounds with the ability to enhance CAT activities. On this basis, they can also play a role in the treatment of tumors. For example, Autocrine human growth hormone (hGH) can specifically regulate the gene expression of CAT by activating the p44/42 MAPK (MAPK, ERK 1/2) signaling pathway. It has been shown that binding of hGH to its transmembrane receptor induces sustained activation of ERK 1/2 through a Janus kinase 2-dependent phosphorylation cascade[143]. Activated ERK 1/2 further translocalizes to the nucleus and binds to transcription factors in the promoter region of the CAT gene, thereby enhancing its transcriptional activities[144]. Notably, the selective mitogen-activated protein kinase inhibitor PD098059 completely abolished hGH-induced transcriptional stimulation of CAT genes by inhibiting the phosphorylation of ERK 1/2 (IC50 = 10 μM), confirming the necessity of ERK signaling in this regulation.
As one of the polyphenols, resveratrol was initially identified as a potential CAT activator due to its ability to activate sirtuins and fork head transcription factors of the O class transcription factors. At specific concentrations, resveratrol has been shown to enhance CAT activity and inhibit cell proliferation in human cancer cell lines[145]. Furthermore, metformin has been demonstrated to form hydrogen bonds with CAT and interacts with it. This interaction results in elevated CAT activity in mouse liver and provides protection against CCl4-induced liver injury[146]. Notably, recent studies have revealed that the combination of metformin and resveratrol exhibits a synergistic effect, which can mitigate the progression of TNBC by enhancing CAT activity[147].
In addition, non-enzymatic antioxidants such as curcumin, vitamin C, and plant polyphenols can also play a role in treating cancer by blocking FR chain reactions[148]. For example, curcumin has antioxidant activity and antiproliferation effect, which can significantly reduce the level of oxidative stress by regulating CAT activities, thus inhibiting the progress of BC[149]. Further research has found that the anticancer effect of curcumin is to some extent related to the copper mediated ROS induced selective cell death mechanism in cancer cells[150].
Since cancer cells generally exhibit more ROS than normal cellular tissues[151], pro-oxidant therapies in cancer treatment are also increasingly being developed. This selectivity arises because normal cells, which typically maintain low oxidative stress levels, are equipped to manage increased ROS without experiencing oxidative damage or cell death[48]. Consistently, high doses of vitamin C exhibit effective cytotoxicity toward cancer cells due to their pro-oxidative effects. Specifically, high-dose vitamin C treatment has been shown to elevate the levels of upstream metabolites in the glycolytic pathway and the tricarboxylic acid cycle, while simultaneously reducing adenosine triphosphate levels and adenylate energy charge in MCF-7 cells[152]. One of the pro-oxidant therapies is a combination of ascorbic acid and menadione (ASC/MEN). Interestingly, overexpression of CAT instead protects cancer cells from ASC/MEN-mediated cell death, thereby reducing patient survival[8]. In addition, other studies have found that arsenic trioxide can also reduce the level of CAT in cancer cells and increase the sensitivity of BC cells to ASC/MEN therapy[153]. Therefore, for therapy strategy, the effect of CAT overexpression on the resistance of pro-oxidant drugs should also be considered.
Recently, the strategy of nanotechnology based on AE has shown breakthrough potential in the treatment of BC, which can specifically destroy the redox defense system through the precise delivery of functionalized nanoparticles and multiple mechanisms of action. For example, anti-SOD2 antibody-modified gold nanoparticles (AuNPs), loaded with siRNA to impair the antioxidant defense of tumor cells by silencing SOD 2 gene expression, can localize BC tissues by dual antibody-mediated active targeting and enhanced permeation retention effect. At the same time, the local surface plasmon resonance effect generated by AuNPs under near-infrared light excitation can directly trigger the excessive production of mitochondrial ROS in BC cells, and realize the spatiotemporal synergy of gene silencing and physical therapy[154].
According to the acidic microenvironment of BC cells, superparamagnetic iron oxide nanoparticles (SPIONs) can be enriched in the tumor site under the guidance of magnetic targeting and release the iron ions in response to the pH value and synergies with β-lapachone lead to accumulation of H2O2, thereby increasing the oxidative stress in cancer cells. In addition, SPIONs can further amplify the toxicity of H2O2 through the Fenton reaction and generate highly •OH, leading to DNA damage and cell death in cancer cells. However, the expression of CAT in cells or the administration of iron chelators can block the therapeutic synergy[61,155]. Therefore, the combination of SPION and CAT inhibitory drugs (such as 3-AT) may significantly enhance the level of oxidative stress in cells and improve the therapeutic effect to BC. This synergistic effect provides a new strategy for the targeted therapy of BC.
In addition to affecting the oxidative toxicity of SPIONs to tumor cells, the combination of cat and nanoparticles can also improve the therapeutic effect of chemotherapy and radiotherapy to a certain extent. The tumor microenvironment (such as hypoxia) is usually the main factor limiting the two cancer treatments, chemotherapy and radiotherapy[156,157]. It is reported that CAT encapsulated in liposomes composed of cisplatin (IV)-prodrug coupled phospholipids to form CAT@Pt (IV)-liposomes, can be used to enhance chemoradiotherapy for cancer. CAT@Pt (IV) is loaded into liposomes, where the enzyme activity is preserved and well protected, and is able to trigger the breakdown of H2O2 produced by tumor cells, thereby generating additional O2 to alleviate hypoxia. In vivo tumor treatment further demonstrated that the use of these CAT@Pt (IV) liposomal nanoparticles could significantly improve the therapeutic efficacy of chemoradiotherapy[158].
Developed multifunctional integrated nanoplatforms (such as Au@Fe3O4 Janus particles) are engineered to not only enhance penetration into dense BC tissues, but also integrate multiple mechanisms such as antioxidant enzyme inhibition, cascade catalysis, and photothermal/photodynamic therapy. Precise disruption of the redox network of BC cells at the single-cell level[159]. These strategies systematically interfere with the antioxidant defense system of BC cells through spatially and temporally resolved drug release and energy conversion, providing innovative therapeutic paradigms for reversing tumor drug resistance and targeting metabolic vulnerability.
Generally, the special role of CAT in BC has been paid more and more attention by scholars. Based on the expression of CAT in tumor cells, more and more therapeutic strategies are being proposed. With the continuous development of related fields, CAT is likely to become a potential therapeutic target in the future.
Due to changes in CAT expression and activity can lead to changes in the level of ROS in cells, so many studies have shown that CAT plays a dual role in cancer, it can be used as tumor suppressor proteins, and also can be used as tumor growth protein in promoting survival. At present, investigation of CAT in BC are deepening and expanding the understanding of its molecular mechanism and principle. As the understanding of CAT function continues to deepen, it is believed that CAT is likely to become a new choice for BC treatment in the future. In conclusion, the study of CAT in the context of BC is an expanding field that will enhance our understanding of BC. A further understanding of the complex roles of CAT in cancer is a major challenge for future studies of CAT, especially its dual regulation in different cancer types and its impact on resistance to pro-oxidant drugs.
| 1. | Momenimovahed Z, Salehiniya H. Epidemiological characteristics of and risk factors for breast cancer in the world. Breast Cancer (Dove Med Press). 2019;11:151-164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 394] [Cited by in RCA: 438] [Article Influence: 62.6] [Reference Citation Analysis (0)] |
| 2. | Zeng H, Chen W, Zheng R, Zhang S, Ji JS, Zou X, Xia C, Sun K, Yang Z, Li H, Wang N, Han R, Liu S, Li H, Mu H, He Y, Xu Y, Fu Z, Zhou Y, Jiang J, Yang Y, Chen J, Wei K, Fan D, Wang J, Fu F, Zhao D, Song G, Chen J, Jiang C, Zhou X, Gu X, Jin F, Li Q, Li Y, Wu T, Yan C, Dong J, Hua Z, Baade P, Bray F, Jemal A, Yu XQ, He J. Changing cancer survival in China during 2003-15: a pooled analysis of 17 population-based cancer registries. Lancet Glob Health. 2018;6:e555-e567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 795] [Cited by in RCA: 1087] [Article Influence: 135.9] [Reference Citation Analysis (3)] |
| 3. | Bai RK, Leal SM, Covarrubias D, Liu A, Wong LJ. Mitochondrial genetic background modifies breast cancer risk. Cancer Res. 2007;67:4687-4694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 186] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 4. | Nishikawa M. Reactive oxygen species in tumor metastasis. Cancer Lett. 2008;266:53-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 297] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 5. | Klaunig JE, Wang Z. Oxidative stress in carcinogenesis. Curr Opin Toxicol. 2018;7:116-121. [RCA] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 6. | Hawk MA, McCallister C, Schafer ZT. Antioxidant Activity during Tumor Progression: A Necessity for the Survival of Cancer Cells? Cancers (Basel). 2016;8:92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 7. | Nandi A, Yan LJ, Jana CK, Das N. Role of Catalase in Oxidative Stress- and Age-Associated Degenerative Diseases. Oxid Med Cell Longev. 2019;2019:9613090. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 220] [Cited by in RCA: 656] [Article Influence: 93.7] [Reference Citation Analysis (0)] |
| 8. | Glorieux C, Dejeans N, Sid B, Beck R, Calderon PB, Verrax J. Catalase overexpression in mammary cancer cells leads to a less aggressive phenotype and an altered response to chemotherapy. Biochem Pharmacol. 2011;82:1384-1390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 118] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 9. | Goh J, Enns L, Fatemie S, Hopkins H, Morton J, Pettan-Brewer C, Ladiges W. Mitochondrial targeted catalase suppresses invasive breast cancer in mice. BMC Cancer. 2011;11:191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 125] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 10. | Sies H. Oxidative stress: oxidants and antioxidants. Exp Physiol. 1997;82:291-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2006] [Cited by in RCA: 1970] [Article Influence: 67.9] [Reference Citation Analysis (8)] |
| 11. | Halliwell B. Understanding mechanisms of antioxidant action in health and disease. Nat Rev Mol Cell Biol. 2024;25:13-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 306] [Article Influence: 153.0] [Reference Citation Analysis (0)] |
| 12. | Fujii J, Homma T, Osaki T. Superoxide Radicals in the Execution of Cell Death. Antioxidants (Basel). 2022;11:501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 165] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 13. | Sies H. Oxidative stress: a concept in redox biology and medicine. Redox Biol. 2015;4:180-183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1535] [Cited by in RCA: 2000] [Article Influence: 181.8] [Reference Citation Analysis (7)] |
| 14. | Sies H, Berndt C, Jones DP. Oxidative Stress. Annu Rev Biochem. 2017;86:715-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1456] [Cited by in RCA: 2435] [Article Influence: 270.6] [Reference Citation Analysis (1)] |
| 15. | Pisoschi AM, Pop A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur J Med Chem. 2015;97:55-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1244] [Cited by in RCA: 1650] [Article Influence: 150.0] [Reference Citation Analysis (0)] |
| 16. | Sullivan LB, Chandel NS. Mitochondrial reactive oxygen species and cancer. Cancer Metab. 2014;2:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 431] [Cited by in RCA: 571] [Article Influence: 47.6] [Reference Citation Analysis (0)] |
| 17. | Tafani M, Sansone L, Limana F, Arcangeli T, De Santis E, Polese M, Fini M, Russo MA. The Interplay of Reactive Oxygen Species, Hypoxia, Inflammation, and Sirtuins in Cancer Initiation and Progression. Oxid Med Cell Longev. 2016;2016:3907147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 188] [Cited by in RCA: 266] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 18. | Irani K, Xia Y, Zweier JL, Sollott SJ, Der CJ, Fearon ER, Sundaresan M, Finkel T, Goldschmidt-Clermont PJ. Mitogenic signaling mediated by oxidants in Ras-transformed fibroblasts. Science. 1997;275:1649-1652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1262] [Cited by in RCA: 1217] [Article Influence: 42.0] [Reference Citation Analysis (7)] |
| 19. | Cordani M, Butera G, Pacchiana R, Masetto F, Mullappilly N, Riganti C, Donadelli M. Mutant p53-Associated Molecular Mechanisms of ROS Regulation in Cancer Cells. Biomolecules. 2020;10:361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 100] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 20. | Dando I, Cordani M, Dalla Pozza E, Biondani G, Donadelli M, Palmieri M. Antioxidant Mechanisms and ROS-Related MicroRNAs in Cancer Stem Cells. Oxid Med Cell Longev. 2015;2015:425708. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 21. | Perillo B, Di Donato M, Pezone A, Di Zazzo E, Giovannelli P, Galasso G, Castoria G, Migliaccio A. ROS in cancer therapy: the bright side of the moon. Exp Mol Med. 2020;52:192-203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 639] [Cited by in RCA: 1477] [Article Influence: 246.2] [Reference Citation Analysis (0)] |
| 22. | Moloney JN, Cotter TG. ROS signalling in the biology of cancer. Semin Cell Dev Biol. 2018;80:50-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 754] [Cited by in RCA: 1406] [Article Influence: 156.2] [Reference Citation Analysis (0)] |
| 23. | Wu WS. The signaling mechanism of ROS in tumor progression. Cancer Metastasis Rev. 2006;25:695-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 529] [Cited by in RCA: 571] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 24. | Murata M, Thanan R, Ma N, Kawanishi S. Role of nitrative and oxidative DNA damage in inflammation-related carcinogenesis. J Biomed Biotechnol. 2012;2012:623019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 137] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 25. | Kumaraguruparan R, Subapriya R, Viswanathan P, Nagini S. Tissue lipid peroxidation and antioxidant status in patients with adenocarcinoma of the breast. Clin Chim Acta. 2002;325:165-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 62] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 26. | Bakhoum SF, Compton DA. Chromosomal instability and cancer: a complex relationship with therapeutic potential. J Clin Invest. 2012;122:1138-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 210] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 27. | Pei H, Dai Y, Yu Y, Tang J, Cao Z, Zhang Y, Li B, Nie J, Hei TK, Zhou G. The Tumorigenic Effect of lncRNA AFAP1-AS1 is Mediated by Translated Peptide ATMLP Under the Control of m(6) A Methylation. Adv Sci (Weinh). 2023;10:e2300314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 37] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 28. | Liu M, Hong Y, Duan X, Zhou Q, Chen J, Liu S, Su J, Han L, Zhang J, Niu B. Unveiling the metal mutation nexus: Exploring the genomic impacts of heavy metal exposure in lung adenocarcinoma and colorectal cancer. J Hazard Mater. 2024;461:132590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 29. | Todorović S, Ćeranić MS, Tošković B, Diklić M, Mitrović Ajtić O, Subotički T, Vukotić M, Dragojević T, Živković E, Oprić S, Stojiljkovic M, Gačić J, Čolaković N, Crnokrak B, Čokić VP, Đikić D. Proinflammatory Microenvironment in Adenocarcinoma Tissue of Colorectal Carcinoma. Int J Mol Sci. 2024;25:10062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 30. | An AR, Kim KM, Park HS, Jang KY, Moon WS, Kang MJ, Lee YC, Kim JH, Chae HJ, Chung MJ. Association between Expression of 8-OHdG and Cigarette Smoking in Non-small Cell Lung Cancer. J Pathol Transl Med. 2019;53:217-224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 31. | Lee JD, Cai Q, Shu XO, Nechuta SJ. The Role of Biomarkers of Oxidative Stress in Breast Cancer Risk and Prognosis: A Systematic Review of the Epidemiologic Literature. J Womens Health (Larchmt). 2017;26:467-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 32. | Musarrat J, Arezina-Wilson J, Wani AA. Prognostic and aetiological relevance of 8-hydroxyguanosine in human breast carcinogenesis. Eur J Cancer. 1996;32 A:1209-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 153] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 33. | Okoh V, Deoraj A, Roy D. Estrogen-induced reactive oxygen species-mediated signalings contribute to breast cancer. Biochim Biophys Acta. 2011;1815:115-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 34. | Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact. 2006;160:1-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4052] [Cited by in RCA: 4150] [Article Influence: 207.5] [Reference Citation Analysis (0)] |
| 35. | Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655-1657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4101] [Cited by in RCA: 4329] [Article Influence: 180.4] [Reference Citation Analysis (0)] |
| 36. | Lee SR, Yang KS, Kwon J, Lee C, Jeong W, Rhee SG. Reversible inactivation of the tumor suppressor PTEN by H2O2. J Biol Chem. 2002;277:20336-20342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 785] [Cited by in RCA: 817] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 37. | Leslie NR, Bennett D, Lindsay YE, Stewart H, Gray A, Downes CP. Redox regulation of PI 3-kinase signalling via inactivation of PTEN. EMBO J. 2003;22:5501-5510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 470] [Cited by in RCA: 493] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 38. | Townsend AJ, Morrow CS, Sinha BK, Cowan KH. Selenium-dependent glutathione peroxidase expression is inversely related to estrogen receptor content of human breast cancer cells. Cancer Commun. 1991;3:265-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 39. | Clemons M, Goss P. Estrogen and the risk of breast cancer. N Engl J Med. 2001;344:276-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 722] [Cited by in RCA: 712] [Article Influence: 28.5] [Reference Citation Analysis (4)] |
| 40. | Yager JD, Liehr JG. Molecular mechanisms of estrogen carcinogenesis. Annu Rev Pharmacol Toxicol. 1996;36:203-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 425] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 41. | Felty Q, Xiong WC, Sun D, Sarkar S, Singh KP, Parkash J, Roy D. Estrogen-induced mitochondrial reactive oxygen species as signal-transducing messengers. Biochemistry. 2005;44:6900-6909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 151] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 42. | Parkash J, Felty Q, Roy D. Estrogen exerts a spatial and temporal influence on reactive oxygen species generation that precedes calcium uptake in high-capacity mitochondria: implications for rapid nongenomic signaling of cell growth. Biochemistry. 2006;45:2872-2881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 43. | Echtay KS. Mitochondrial uncoupling proteins--what is their physiological role? Free Radic Biol Med. 2007;43:1351-1371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 259] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 44. | Derdak Z, Mark NM, Beldi G, Robson SC, Wands JR, Baffy G. The mitochondrial uncoupling protein-2 promotes chemoresistance in cancer cells. Cancer Res. 2008;68:2813-2819. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 184] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 45. | Sastre-Serra J, Valle A, Company MM, Garau I, Oliver J, Roca P. Estrogen down-regulates uncoupling proteins and increases oxidative stress in breast cancer. Free Radic Biol Med. 2010;48:506-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 83] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 46. | Nogueira V, Park Y, Chen CC, Xu PZ, Chen ML, Tonic I, Unterman T, Hay N. Akt determines replicative senescence and oxidative or oncogenic premature senescence and sensitizes cells to oxidative apoptosis. Cancer Cell. 2008;14:458-470. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 651] [Cited by in RCA: 643] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 47. | Trachootham D, Zhou Y, Zhang H, Demizu Y, Chen Z, Pelicano H, Chiao PJ, Achanta G, Arlinghaus RB, Liu J, Huang P. Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by beta-phenylethyl isothiocyanate. Cancer Cell. 2006;10:241-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 870] [Cited by in RCA: 884] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 48. | Gorrini C, Harris IS, Mak TW. Modulation of oxidative stress as an anticancer strategy. Nat Rev Drug Discov. 2013;12:931-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2127] [Cited by in RCA: 2750] [Article Influence: 229.2] [Reference Citation Analysis (0)] |
| 49. | Knickle A, Fernando W, Greenshields AL, Rupasinghe HPV, Hoskin DW. Myricetin-induced apoptosis of triple-negative breast cancer cells is mediated by the iron-dependent generation of reactive oxygen species from hydrogen peroxide. Food Chem Toxicol. 2018;118:154-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 50. | Chang WH, Liu JJ, Chen CH, Huang TS, Lu FJ. Growth inhibition and induction of apoptosis in MCF-7 breast cancer cells by fermented soy milk. Nutr Cancer. 2002;43:214-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 51. | Alexandre J, Hu Y, Lu W, Pelicano H, Huang P. Novel action of paclitaxel against cancer cells: bystander effect mediated by reactive oxygen species. Cancer Res. 2007;67:3512-3517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 320] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 52. | Schafer ZT, Grassian AR, Song L, Jiang Z, Gerhart-Hines Z, Irie HY, Gao S, Puigserver P, Brugge JS. Antioxidant and oncogene rescue of metabolic defects caused by loss of matrix attachment. Nature. 2009;461:109-113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 890] [Cited by in RCA: 854] [Article Influence: 50.2] [Reference Citation Analysis (0)] |
| 53. | Das U. A radical approach to cancer. Med Sci Monit. 2002;8:RA79-RA92. [PubMed] |
| 54. | Iscan M, Coban T, Cok I, Bulbul D, Eke BC, Burgaz S. The organochlorine pesticide residues and antioxidant enzyme activities in human breast tumors: is there any association? Breast Cancer Res Treat. 2002;72:173-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 55. | Kumaraguruparan R, Balachandran C, Manohar BM, Nagini S. Altered oxidant-antioxidant profile in canine mammary tumours. Vet Res Commun. 2005;29:287-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 56. | Thomas PA, Oykutlu D, Pou B, Tyler D, Oberley LW, Robinson RA, Lenel JC. Immunohistochemical Characterization of Antioxidant Enzymes in Human Breast Cancer. Pathol Oncol Res. 1997;3:278-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 57. | Weydert CJ, Zhang Y, Sun W, Waugh TA, Teoh ML, Andringa KK, Aykin-Burns N, Spitz DR, Smith BJ, Oberley LW. Increased oxidative stress created by adenoviral MnSOD or CuZnSOD plus BCNU (1,3-bis(2-chloroethyl)-1-nitrosourea) inhibits breast cancer cell growth. Free Radic Biol Med. 2008;44:856-867. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 58. | Lushchak VI. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem Biol Interact. 2014;224:164-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 790] [Cited by in RCA: 1067] [Article Influence: 88.9] [Reference Citation Analysis (0)] |
| 59. | Slimen IB, Najar T, Ghram A, Dabbebi H, Ben Mrad M, Abdrabbah M. Reactive oxygen species, heat stress and oxidative-induced mitochondrial damage. A review. Int J Hyperthermia. 2014;30:513-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 590] [Article Influence: 53.6] [Reference Citation Analysis (0)] |
| 60. | Jelic MD, Mandic AD, Maricic SM, Srdjenovic BU. Oxidative stress and its role in cancer. J Cancer Res Ther. 2021;17:22-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 409] [Article Influence: 81.8] [Reference Citation Analysis (0)] |
| 61. | Trachootham D, Alexandre J, Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov. 2009;8:579-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3513] [Cited by in RCA: 4308] [Article Influence: 253.4] [Reference Citation Analysis (0)] |
| 62. | Abreu IA, Cabelli DE. Superoxide dismutases-a review of the metal-associated mechanistic variations. Biochim Biophys Acta. 2010;1804:263-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 386] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 63. | Negahdar M, Abtahi H, Sadeghi M, Aghvami T, Javadi E, Layegh H. Blood Superoxide Dismutase and Catalase Activities in Women Affected with Breast Cancer. Iran J Public Health. 2005;34:39-43. |
| 64. | Quan F, Korneluk RG, Tropak MB, Gravel RA. Isolation and characterization of the human catalase gene. Nucleic Acids Res. 1986;14:5321-5335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 154] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 65. | Kirkman HN, Gaetani GF. Catalase: a tetrameric enzyme with four tightly bound molecules of NADPH. Proc Natl Acad Sci U S A. 1984;81:4343-4347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 297] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 66. | Nicholls P, Fita I, Loewen PC. Enzymology and structure of catalases. Adv Inorg Chem. 2000;51-106. [RCA] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 192] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 67. | Putnam CD, Arvai AS, Bourne Y, Tainer JA. Active and inhibited human catalase structures: ligand and NADPH binding and catalytic mechanism. J Mol Biol. 2000;296:295-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 351] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 68. | Glorieux C, Calderon PB. Catalase, a remarkable enzyme: targeting the oldest antioxidant enzyme to find a new cancer treatment approach. Biol Chem. 2017;398:1095-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 438] [Article Influence: 48.7] [Reference Citation Analysis (0)] |
| 69. | Jones P, Dunford HB. The mechanism of Compound I formation revisited. J Inorg Biochem. 2005;99:2292-2298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 57] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 70. | Bauer G. Increasing the endogenous NO level causes catalase inactivation and reactivation of intercellular apoptosis signaling specifically in tumor cells. Redox Biol. 2015;6:353-371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 71. | Kirkman HN, Rolfo M, Ferraris AM, Gaetani GF. Mechanisms of protection of catalase by NADPH. Kinetics and stoichiometry. J Biol Chem. 1999;274:13908-13914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 208] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 72. | Gabdoulline RR, Kummer U, Olsen LF, Wade RC. Concerted simulations reveal how peroxidase compound III formation results in cellular oscillations. Biophys J. 2003;85:1421-1428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 73. | Jiang S, Li H, Zhang L, Mu W, Zhang Y, Chen T, Wu J, Tang H, Zheng S, Liu Y, Wu Y, Luo X, Xie Y, Ren J. Generic Diagramming Platform (GDP): a comprehensive database of high-quality biomedical graphics. Nucleic Acids Res. 2025;53:D1670-D1676. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 507] [Article Influence: 507.0] [Reference Citation Analysis (0)] |
| 74. | Winternitz MC, Meloy CR. On the occurrence of catalase in human tissues and its variations in diseases. J Exp Med. 1908;10:759-781. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 75. | Chance B, Sies H, Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev. 1979;59:527-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4176] [Cited by in RCA: 3910] [Article Influence: 83.2] [Reference Citation Analysis (0)] |
| 76. | Middelkoop E, Wiemer EA, Schoenmaker DE, Strijland A, Tager JM. Topology of catalase assembly in human skin fibroblasts. Biochim Biophys Acta. 1993;1220:15-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 77. | Heinzelmann S, Bauer G. Multiple protective functions of catalase against intercellular apoptosis-inducing ROS signaling of human tumor cells. Biol Chem. 2010;391:675-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 78. | Böhm B, Heinzelmann S, Motz M, Bauer G. Extracellular localization of catalase is associated with the transformed state of malignant cells. Biol Chem. 2015;396:1339-1356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 79. | Deichman GI. Natural selection and early changes of phenotype of tumor cells in vivo: acquisition of new defense mechanisms. Biochemistry (Mosc). 2000;65:78-94. [PubMed] |
| 80. | Sandstrom PA, Buttke TM. Autocrine production of extracellular catalase prevents apoptosis of the human CEM T-cell line in serum-free medium. Proc Natl Acad Sci U S A. 1993;90:4708-4712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 120] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 81. | Riethmüller M, Burger N, Bauer G. Singlet oxygen treatment of tumor cells triggers extracellular singlet oxygen generation, catalase inactivation and reactivation of intercellular apoptosis-inducing signaling. Redox Biol. 2015;6:157-168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 82. | Bauer G, Sersenová D, Graves DB, Machala Z. Cold Atmospheric Plasma and Plasma-Activated Medium Trigger RONS-Based Tumor Cell Apoptosis. Sci Rep. 2019;9:14210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 84] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 83. | Bauer G, Motz M. The Antitumor Effect of Single-domain Antibodies Directed Towards Membrane-associated Catalase and Superoxide Dismutase. Anticancer Res. 2016;36:5945-5956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 84. | Moran EC, Kamiguti AS, Cawley JC, Pettitt AR. Cytoprotective antioxidant activity of serum albumin and autocrine catalase in chronic lymphocytic leukaemia. Br J Haematol. 2002;116:316-328. [PubMed] [DOI] [Full Text] |
| 85. | Hirono A, Sasaya-Hamada F, Kanno H, Fujii H, Yoshida T, Miwa S. A novel human catalase mutation (358 T-->del) causing Japanese-type acatalasemia. Blood Cells Mol Dis. 1995;21:232-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 86. | TAKAHARA S. Progressive oral gangrene probably due to lack of catalase in the blood (acatalasaemia); report of nine cases. Lancet. 1952;2:1101-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 113] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 87. | Wen JK, Osumi T, Hashimoto T, Ogata M. Molecular analysis of human acatalasemia. Identification of a splicing mutation. J Mol Biol. 1990;211:383-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 88. | Zhou XF, Cui J, DeStefano AL, Chazaro I, Farrer LA, Manolis AJ, Gavras H, Baldwin CT. Polymorphisms in the promoter region of catalase gene and essential hypertension. Dis Markers. 2005;21:3-7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 89. | Góth L. Two cases of acatalasemia in Hungary. Clin Chim Acta. 1992;207:155-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 90. | Góth L, Eaton JW. Hereditary catalase deficiencies and increased risk of diabetes. Lancet. 2000;356:1820-1821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 133] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 91. | Park HH, Ha E, Uhm YK, Jin SY, Kim YJ, Chung JH, Lee MH. Association study between catalase gene polymorphisms and the susceptibility to vitiligo in Korean population. Exp Dermatol. 2006;15:377-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 92. | Kodydková J, Vávrová L, Kocík M, Žák A. Human catalase, its polymorphisms, regulation and changes of its activity in different diseases. Folia Biol (Praha). 2014;60:153-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 93. | Chistiakov DA, Savost'anov KV, Turakulov RI, Titovich EV, Zilberman LI, Kuraeva TL, Dedov II, Nosikov VV. A new type 1 diabetes susceptibility locus containing the catalase gene (chromosome 11p13) in a Russian population. Diabetes Metab Res Rev. 2004;20:219-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 94. | Ahn J, Gammon MD, Santella RM, Gaudet MM, Britton JA, Teitelbaum SL, Terry MB, Nowell S, Davis W, Garza C, Neugut AI, Ambrosone CB. Associations between breast cancer risk and the catalase genotype, fruit and vegetable consumption, and supplement use. Am J Epidemiol. 2005;162:943-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 102] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 95. | Watanabe Y, Metoki H, Ohkubo T, Katsuya T, Tabara Y, Kikuya M, Hirose T, Sugimoto K, Asayama K, Inoue R, Hara A, Obara T, Nakura J, Kohara K, Totsune K, Ogihara T, Rakugi H, Miki T, Imai Y. Accumulation of common polymorphisms is associated with development of hypertension: a 12-year follow-up from the Ohasama study. Hypertens Res. 2010;33:129-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 96. | Glorieux C, Buc Calderon P. Targeting catalase in cancer. Redox Biol. 2024;77:103404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 22] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 97. | Glorieux C, Zamocky M, Sandoval JM, Verrax J, Calderon PB. Regulation of catalase expression in healthy and cancerous cells. Free Radic Biol Med. 2015;87:84-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 196] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 98. | Glorieux C, Sandoval JM, Fattaccioli A, Dejeans N, Garbe JC, Dieu M, Verrax J, Renard P, Huang P, Calderon PB. Chromatin remodeling regulates catalase expression during cancer cells adaptation to chronic oxidative stress. Free Radic Biol Med. 2016;99:436-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 99. | Glorieux C, Sandoval JM, Dejeans N, Nonckreman S, Bahloula K, Poirel HA, Calderon PB. Evaluation of Potential Mechanisms Controlling the Catalase Expression in Breast Cancer Cells. Oxid Med Cell Longev. 2018;2018:5351967. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 100. | Glorieux C, Auquier J, Dejeans N, Sid B, Demoulin JB, Bertrand L, Verrax J, Calderon PB. Catalase expression in MCF-7 breast cancer cells is mainly controlled by PI3K/Akt/mTor signaling pathway. Biochem Pharmacol. 2014;89:217-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 101. | Dejeans N, Glorieux C, Guenin S, Beck R, Sid B, Rousseau R, Bisig B, Delvenne P, Buc Calderon P, Verrax J. Overexpression of GRP94 in breast cancer cells resistant to oxidative stress promotes high levels of cancer cell proliferation and migration: implications for tumor recurrence. Free Radic Biol Med. 2012;52:993-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 80] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 102. | Sen S, Kawahara B, Chaudhuri G. Maintenance of higher H₂O₂ levels, and its mechanism of action to induce growth in breast cancer cells: important roles of bioactive catalase and PP2A. Free Radic Biol Med. 2012;53:1541-1551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 103. | Thayer WS. Adriamycin stimulated superoxide formation in submitochondrial particles. Chem Biol Interact. 1977;19:265-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 198] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 104. | Akman SA, Forrest G, Chu FF, Doroshow JH. Resistance to hydrogen peroxide associated with altered catalase mRNA stability in MCF7 breast cancer cells. Biochim Biophys Acta. 1989;1009:70-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 105. | Aykin-Burns N, Ahmad IM, Zhu Y, Oberley LW, Spitz DR. Increased levels of superoxide and H2O2 mediate the differential susceptibility of cancer cells versus normal cells to glucose deprivation. Biochem J. 2009;418:29-37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 351] [Cited by in RCA: 348] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 106. | Abo Elsoud MR, Hewala TI. The clinical significance of serum oxidative stress biomarkers in breast cancer females. Med Res J. 2019;4:1-7. [DOI] [Full Text] |
| 107. | Maycotte P, Sarmiento-Salinas FL, García-Miranda A, Ovando-Ovando CI, Robledo-Cadena DX, Hernández-Esquivel L, Jasso-Chávez R, Marín-Hernández A. Metabolic and Oxidative Stress Management Heterogeneity in a Panel of Breast Cancer Cell Lines. Metabolites. 2024;14:435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 108. | Vafa O, Wade M, Kern S, Beeche M, Pandita TK, Hampton GM, Wahl GM. c-Myc can induce DNA damage, increase reactive oxygen species, and mitigate p53 function: a mechanism for oncogene-induced genetic instability. Mol Cell. 2002;9:1031-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 639] [Cited by in RCA: 696] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 109. | Pelicano H, Lu W, Zhou Y, Zhang W, Chen Z, Hu Y, Huang P. Mitochondrial dysfunction and reactive oxygen species imbalance promote breast cancer cell motility through a CXCL14-mediated mechanism. Cancer Res. 2009;69:2375-2383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 152] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 110. | Murphy MP, Smith RA. Targeting antioxidants to mitochondria by conjugation to lipophilic cations. Annu Rev Pharmacol Toxicol. 2007;47:629-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 981] [Cited by in RCA: 934] [Article Influence: 49.2] [Reference Citation Analysis (0)] |
| 111. | Fruehauf JP, Meyskens FL Jr. Reactive oxygen species: a breath of life or death? Clin Cancer Res. 2007;13:789-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 685] [Cited by in RCA: 701] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 112. | Wang Y, Zhou BP. Epithelial-mesenchymal transition in breast cancer progression and metastasis. Chin J Cancer. 2011;30:603-611. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 188] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 113. | Weil RJ, Palmieri DC, Bronder JL, Stark AM, Steeg PS. Breast cancer metastasis to the central nervous system. Am J Pathol. 2005;167:913-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 336] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 114. | Woodhouse EC, Chuaqui RF, Liotta LA. General mechanisms of metastasis. Cancer. 1997;80:1529-1537. [PubMed] [DOI] [Full Text] |
| 115. | Colleoni M, O'Neill A, Goldhirsch A, Gelber RD, Bonetti M, Thürlimann B, Price KN, Castiglione-Gertsch M, Coates AS, Lindtner J, Collins J, Senn HJ, Cavalli F, Forbes J, Gudgeon A, Simoncini E, Cortes-Funes H, Veronesi A, Fey M, Rudenstam CM. Identifying breast cancer patients at high risk for bone metastases. J Clin Oncol. 2000;18:3925-3935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 89] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 116. | Evans AJ, James JJ, Cornford EJ, Chan SY, Burrell HC, Pinder SE, Gutteridge E, Robertson JF, Hornbuckle J, Cheung KL. Brain metastases from breast cancer: identification of a high-risk group. Clin Oncol (R Coll Radiol). 2004;16:345-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 116] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 117. | Lei Y, Huang K, Gao C, Lau QC, Pan H, Xie K, Li J, Liu R, Zhang T, Xie N, Nai HS, Wu H, Dong Q, Zhao X, Nice EC, Huang C, Wei Y. Proteomics identification of ITGB3 as a key regulator in reactive oxygen species-induced migration and invasion of colorectal cancer cells. Mol Cell Proteomics. 2011;10:M110.005397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 106] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 118. | Schrijver WAME, Suijkerbuijk KPM, van Gils CH, van der Wall E, Moelans CB, van Diest PJ. Receptor Conversion in Distant Breast Cancer Metastases: A Systematic Review and Meta-analysis. J Natl Cancer Inst. 2018;110:568-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 219] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 119. | Yardley DA. Pharmacologic management of bone-related complications and bone metastases in postmenopausal women with hormone receptor-positive breast cancer. Breast Cancer (Dove Med Press). 2016;8:73-82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 120. | Cook KL, Clarke PA, Parmar J, Hu R, Schwartz-Roberts JL, Abu-Asab M, Wärri A, Baumann WT, Clarke R. Knockdown of estrogen receptor-α induces autophagy and inhibits antiestrogen-mediated unfolded protein response activation, promoting ROS-induced breast cancer cell death. FASEB J. 2014;28:3891-3905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 121. | Kim HM, Jung WH, Koo JS. Expression of reactive oxygen species-related proteins in metastatic breast cancer is dependent on the metastatic site. Int J Clin Exp Pathol. 2014;7:8802-8812. [PubMed] |
| 122. | Ambrosone CB, Ahn J, Singh KK, Rezaishiraz H, Furberg H, Sweeney C, Coles B, Trovato A. Polymorphisms in genes related to oxidative stress (MPO, MnSOD, CAT) and survival after treatment for breast cancer. Cancer Res. 2005;65:1105-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 123. | Petit E, Courtin A, Kloosterboer HJ, Rostène W, Forgez P, Gompel A. Progestins induce catalase activities in breast cancer cells through PRB isoform: correlation with cell growth inhibition. J Steroid Biochem Mol Biol. 2009;115:153-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 124. | Mobley JA, Brueggemeier RW. Estrogen receptor-mediated regulation of oxidative stress and DNA damage in breast cancer. Carcinogenesis. 2004;25:3-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 124] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 125. | Cavalieri E, Frenkel K, Liehr JG, Rogan E, Roy D. Estrogens as endogenous genotoxic agents--DNA adducts and mutations. J Natl Cancer Inst Monogr. 2000;75-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 370] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 126. | Forsberg L, Lyrenäs L, de Faire U, Morgenstern R. A common functional C-T substitution polymorphism in the promoter region of the human catalase gene influences transcription factor binding, reporter gene transcription and is correlated to blood catalase levels. Free Radic Biol Med. 2001;30:500-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 206] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 127. | Kim D, Koo JS, Lee S. Overexpression of reactive oxygen species scavenger enzymes is associated with a good prognosis in triple-negative breast cancer. Oncology. 2015;88:9-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 128. | Nourazarian AR, Kangari P, Salmaninejad A. Roles of oxidative stress in the development and progression of breast cancer. Asian Pac J Cancer Prev. 2014;15:4745-4751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 121] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 129. | Tas F, Hansel H, Belce A, Ilvan S, Argon A, Camlica H, Topuz E. Oxidative stress in breast cancer. Med Oncol. 2005;22:11-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 84] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 130. | Theriault RL, Carlson RW, Allred C, Anderson BO, Burstein HJ, Edge SB, Farrar WB, Forero A, Giordano SH, Goldstein LJ, Gradishar WJ, Hayes DF, Hudis CA, Isakoff SJ, Ljung BM, Mankoff DA, Marcom PK, Mayer IA, McCormick B, Pierce LJ, Reed EC, Schwartzberg LS, Smith ML, Soliman H, Somlo G, Ward JH, Wolff AC, Zellars R, Shead DA, Kumar R; National Comprehensive Cancer Network. Breast cancer, version 3.2013: featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2013;11:753-60; quiz 761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 126] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 131. | Kaufmann SH, Earnshaw WC. Induction of apoptosis by cancer chemotherapy. Exp Cell Res. 2000;256:42-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 845] [Cited by in RCA: 892] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 132. | Florea AM, Büsselberg D. Cisplatin as an anti-tumor drug: cellular mechanisms of activity, drug resistance and induced side effects. Cancers (Basel). 2011;3:1351-1371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1005] [Cited by in RCA: 1282] [Article Influence: 85.5] [Reference Citation Analysis (0)] |
| 133. | Simůnek T, Stérba M, Popelová O, Adamcová M, Hrdina R, Gersl V. Anthracycline-induced cardiotoxicity: overview of studies examining the roles of oxidative stress and free cellular iron. Pharmacol Rep. 2009;61:154-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 524] [Cited by in RCA: 565] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 134. | Koeberle SC, Kipp AP, Stuppner H, Koeberle A. Ferroptosis-modulating small molecules for targeting drug-resistant cancer: Challenges and opportunities in manipulating redox signaling. Med Res Rev. 2023;43:614-682. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 66] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 135. | Cao Y, Zhang H, Tang J, Wang R. Ferulic Acid Mitigates Growth and Invasion of Esophageal Squamous Cell Carcinoma through Inducing Ferroptotic Cell Death. Dis Markers. 2022;2022:4607966. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 136. | Cos P, Ying L, Calomme M, Hu JP, Cimanga K, Van Poel B, Pieters L, Vlietinck AJ, Vanden Berghe D. Structure-activity relationship and classification of flavonoids as inhibitors of xanthine oxidase and superoxide scavengers. J Nat Prod. 1998;61:71-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 707] [Cited by in RCA: 676] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 137. | Pal S, Saha C. A review on structure-affinity relationship of dietary flavonoids with serum albumins. J Biomol Struct Dyn. 2014;32:1132-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 138. | Majumder D, Das A, Saha C. Catalase inhibition an anti cancer property of flavonoids: A kinetic and structural evaluation. Int J Biol Macromol. 2017;104:929-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 139. | Yang YJ, Nam SJ, Kong G, Kim MK. A case-control study on seaweed consumption and the risk of breast cancer. Br J Nutr. 2010;103:1345-1353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 140. | Settacomkul R, Sangpairoj K, Phuagkhaopong S, Meemon K, Niamnont N, Sobhon P, Vivithanaporn P. Ethanolic extract of Halymenia durvillei induced G2/M arrest and altered the levels of cell cycle regulatory proteins of MDA-MB-231 triple-negative breast cancer cells. Res Pharm Sci. 2023;18:279-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 141. | Feinstein RN, Fry RJ, Staffeldt EF. Carcinogenic and antitumor effects of aminotriazole on acatalasemic and normal catalase mice. J Natl Cancer Inst. 1978;60:1113-1116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 142. | Dong J, Yu Y, Pei Y, Pei Z. pH-responsive aminotriazole doped metal organic frameworks nanoplatform enables self-boosting reactive oxygen species generation through regulating the activity of catalase for targeted chemo/chemodynamic combination therapy. J Colloid Interface Sci. 2022;607:1651-1660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 143. | Mertani HC, Zhu T, Goh EL, Lee KO, Morel G, Lobie PE. Autocrine human growth hormone (hGH) regulation of human mammary carcinoma cell gene expression. Identification of CHOP as a mediator of hGH-stimulated human mammary carcinoma cell survival. J Biol Chem. 2001;276:21464-21475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 144. | Zhu Z, Mukhina S, Zhu T, Mertani HC, Lee KO, Lobie PE. p44/42 MAP kinase-dependent regulation of catalase by autocrine human growth hormone protects human mammary carcinoma cells from oxidative stress-induced apoptosis. Oncogene. 2005;24:3774-3785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 145. | Khan MA, Chen HC, Wan XX, Tania M, Xu AH, Chen FZ, Zhang DZ. Regulatory effects of resveratrol on antioxidant enzymes: a mechanism of growth inhibition and apoptosis induction in cancer cells. Mol Cells. 2013;35:219-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 100] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 146. | Dai J, Liu M, Ai Q, Lin L, Wu K, Deng X, Jing Y, Jia M, Wan J, Zhang L. Involvement of catalase in the protective benefits of metformin in mice with oxidative liver injury. Chem Biol Interact. 2014;216:34-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 147. | Cheng T, Wang C, Lu Q, Cao Y, Yu W, Li W, Liu B, Gao X, Lü J, Pan X. Metformin inhibits the tumor-promoting effect of low-dose resveratrol, and enhances the anti-tumor activity of high-dose resveratrol by increasing its reducibility in triple negative breast cancer. Free Radic Biol Med. 2022;180:108-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 148. | He L, He T, Farrar S, Ji L, Liu T, Ma X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell Physiol Biochem. 2017;44:532-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 728] [Cited by in RCA: 1375] [Article Influence: 152.8] [Reference Citation Analysis (0)] |
| 149. | Pongsavee M. Effects of ERCC5 rs751402 Polymorphism on Oxidative Stress and the Impact of Curcumin on Catalase Activity in Breast Carcinogenesis. Asian Pac J Cancer Prev. 2022;23:2065-2070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 150. | Alhasawi MAI, Aatif M, Muteeb G, Alam MW, Oirdi ME, Farhan M. Curcumin and Its Derivatives Induce Apoptosis in Human Cancer Cells by Mobilizing and Redox Cycling Genomic Copper Ions. Molecules. 2022;27:7410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 151. | Oberley TD, Oberley LW. Antioxidant enzyme levels in cancer. Histol Histopathol. 1997;12:525-535. [PubMed] |
| 152. | Uetaki M, Tabata S, Nakasuka F, Soga T, Tomita M. Metabolomic alterations in human cancer cells by vitamin C-induced oxidative stress. Sci Rep. 2015;5:13896. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 117] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 153. | Glorieux C, Calderon PB. Catalase down-regulation in cancer cells exposed to arsenic trioxide is involved in their increased sensitivity to a pro-oxidant treatment. Cancer Cell Int. 2018;18:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 154. | Zhang L, Qi Y, Min H, Ni C, Wang F, Wang B, Qin H, Zhang Y, Liu G, Qin Y, Duan X, Li F, Han X, Tao N, Zhang L, Qin Z, Zhao Y, Nie G. Cooperatively Responsive Peptide Nanotherapeutic that Regulates Angiopoietin Receptor Tie2 Activity in Tumor Microenvironment To Prevent Breast Tumor Relapse after Chemotherapy. ACS Nano. 2019;13:5091-5102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 155. | Huang G, Chen H, Dong Y, Luo X, Yu H, Moore Z, Bey EA, Boothman DA, Gao J. Superparamagnetic iron oxide nanoparticles: amplifying ROS stress to improve anticancer drug efficacy. Theranostics. 2013;3:116-126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 231] [Cited by in RCA: 225] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 156. | Liu Y, Liu Y, Bu W, Xiao Q, Sun Y, Zhao K, Fan W, Liu J, Shi J. Radiation-/hypoxia-induced solid tumor metastasis and regrowth inhibited by hypoxia-specific upconversion nanoradiosensitizer. Biomaterials. 2015;49:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 108] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 157. | Abbasi AZ, Gordijo CR, Amini MA, Maeda A, Rauth AM, DaCosta RS, Wu XY. Hybrid Manganese Dioxide Nanoparticles Potentiate Radiation Therapy by Modulating Tumor Hypoxia. Cancer Res. 2016;76:6643-6656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 85] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 158. | Zhang R, Song X, Liang C, Yi X, Song G, Chao Y, Yang Y, Yang K, Feng L, Liu Z. Catalase-loaded cisplatin-prodrug-constructed liposomes to overcome tumor hypoxia for enhanced chemo-radiotherapy of cancer. Biomaterials. 2017;138:13-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 197] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 159. | Chehelgerdi M, Chehelgerdi M, Allela OQB, Pecho RDC, Jayasankar N, Rao DP, Thamaraikani T, Vasanthan M, Viktor P, Lakshmaiya N, Saadh MJ, Amajd A, Abo-Zaid MA, Castillo-Acobo RY, Ismail AH, Amin AH, Akhavan-Sigari R. Progressing nanotechnology to improve targeted cancer treatment: overcoming hurdles in its clinical implementation. Mol Cancer. 2023;22:169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 451] [Article Influence: 150.3] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/