©The Author(s) 2025.
World J Clin Oncol. Nov 24, 2025; 16(11): 111419
Published online Nov 24, 2025. doi: 10.5306/wjco.v16.i11.111419
Published online Nov 24, 2025. doi: 10.5306/wjco.v16.i11.111419
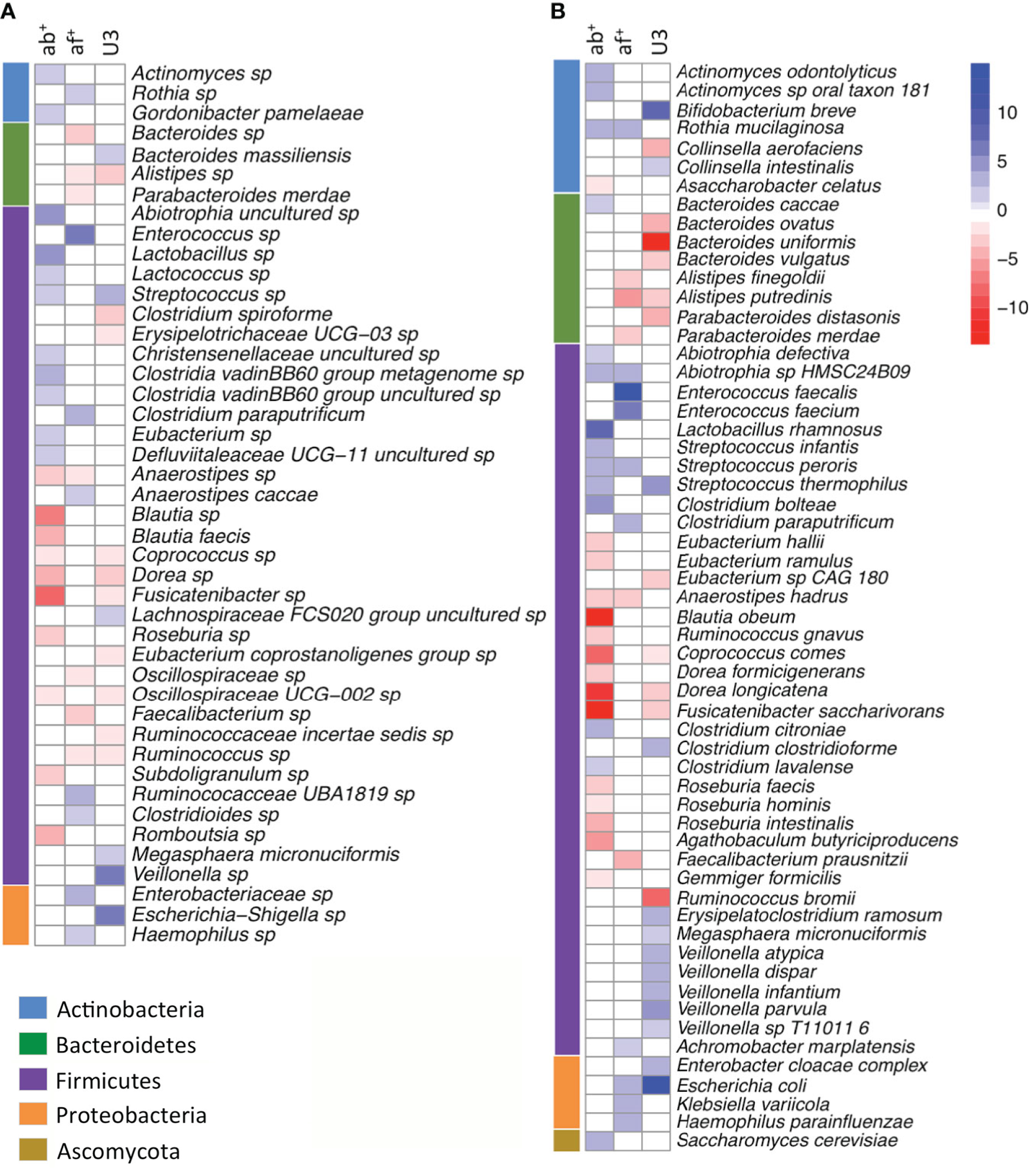
Figure 1 Multivariate analysis of gut microbiome changes associated with antimicrobial treatments[11].
A: 16S rRNA gene sequencing data; B: Whole shotgun metagenome sequencing data. Heatmaps showing bacterial taxa significantly associated with antibiotic use (ab+), antifungal use (af+), and age category (under 3 years of age, U3) using 16S rRNA gene sequencing data and whole shotgun metagenome sequencing data. Blue indicates taxa that increased with treatment or younger age; red indicates taxa that decreased with treatment or younger age. Taxa are organized by phylum, as indicated in the adjacent color bar. Note the consistent depletion of beneficial short-chain fatty acid-producing bacteria (Faecalibacterium, Anaerostipes, Dorea, Blautia) and the enrichment of opportunistic pathogens following antimicrobial treatment across both methodologies. Citation: Dunn KA, MacDonald T, Rodrigues GJ, Forbrigger Z, Bielawski JP, Langille MGI, Van Limbergen J, Kulkarni K. Antibiotic and antifungal use in pediatric leukemia and lymphoma patients are associated with increasing opportunistic pathogens and decreasing bacteria responsible for activities that enhance colonic defense. Front Cell Infect Microbiol 2022; 12: 924707. Copyright ©The Author(s) 2022. Published by Frontiers Media S.A (Supplementary material).
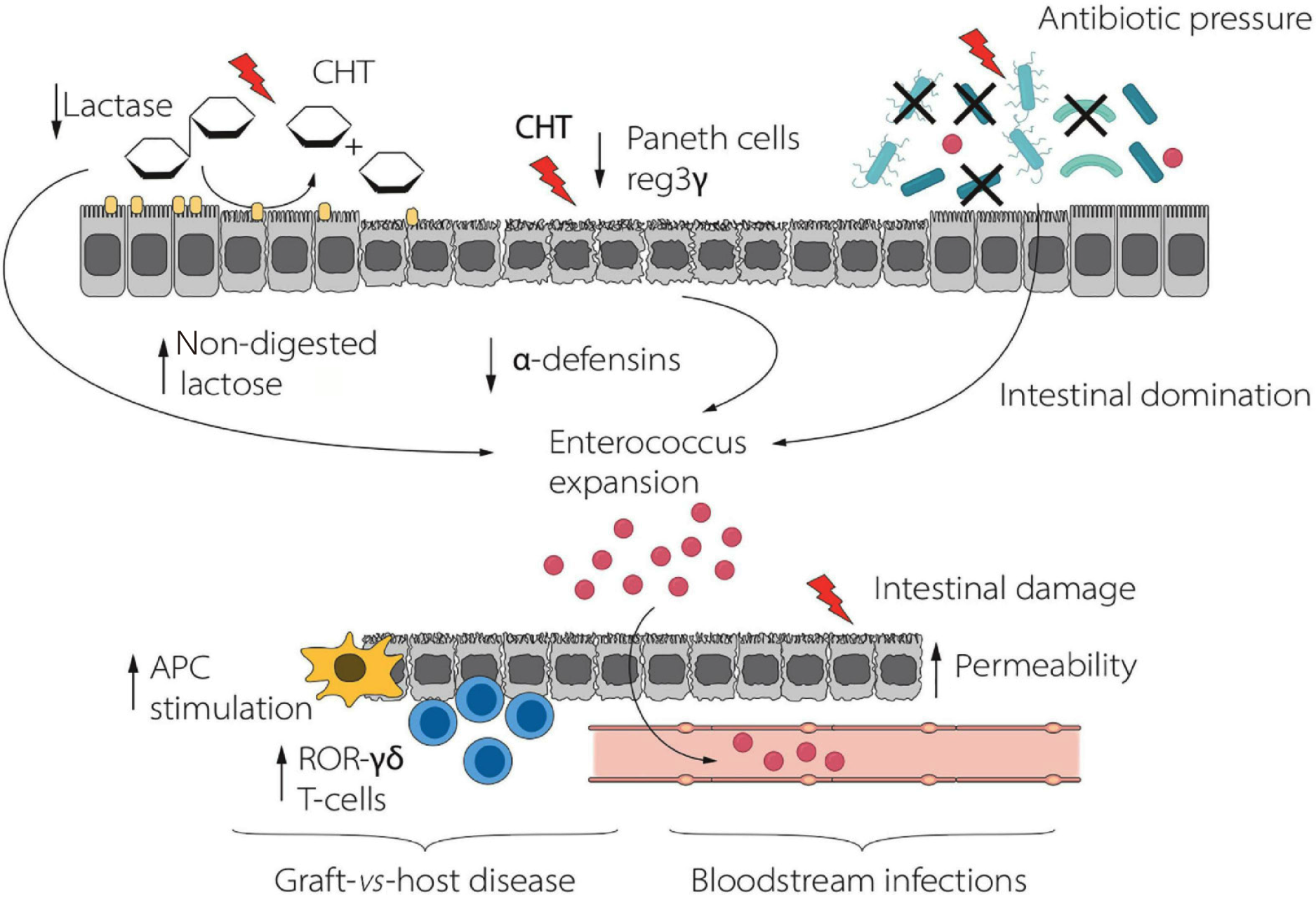
Figure 2 Lactose-driven enterococcus expansion in allogeneic hematopoietic stem cell transplantation recipients[19].
Dietary lactose that is unabsorbed due to conditioning-induced lactase loss accumulates in the gut lumen and serves as a substrate for Enterococcus species, leading to intestinal domination, enhanced mucosal barrier disruption, and an increased risk of acute graft-versus-host disease and bloodstream infections. Lactose avoidance or enzymatic supplementation restores community balance and reduces transplant-related complications. APC: Antigen presenting cell; CHT: Chemotherapy. Citation: Muratore E, Leardini D, Baccelli F, Venturelli F, Prete A, Masetti R. Nutritional modulation of the gut microbiome in allogeneic hematopoietic stem cell transplantation recipients. Front Nutr 2022; 9: 993668. Copyright ©The Author(s) 2022. Published by Frontiers Media S.A (Supplementary material).
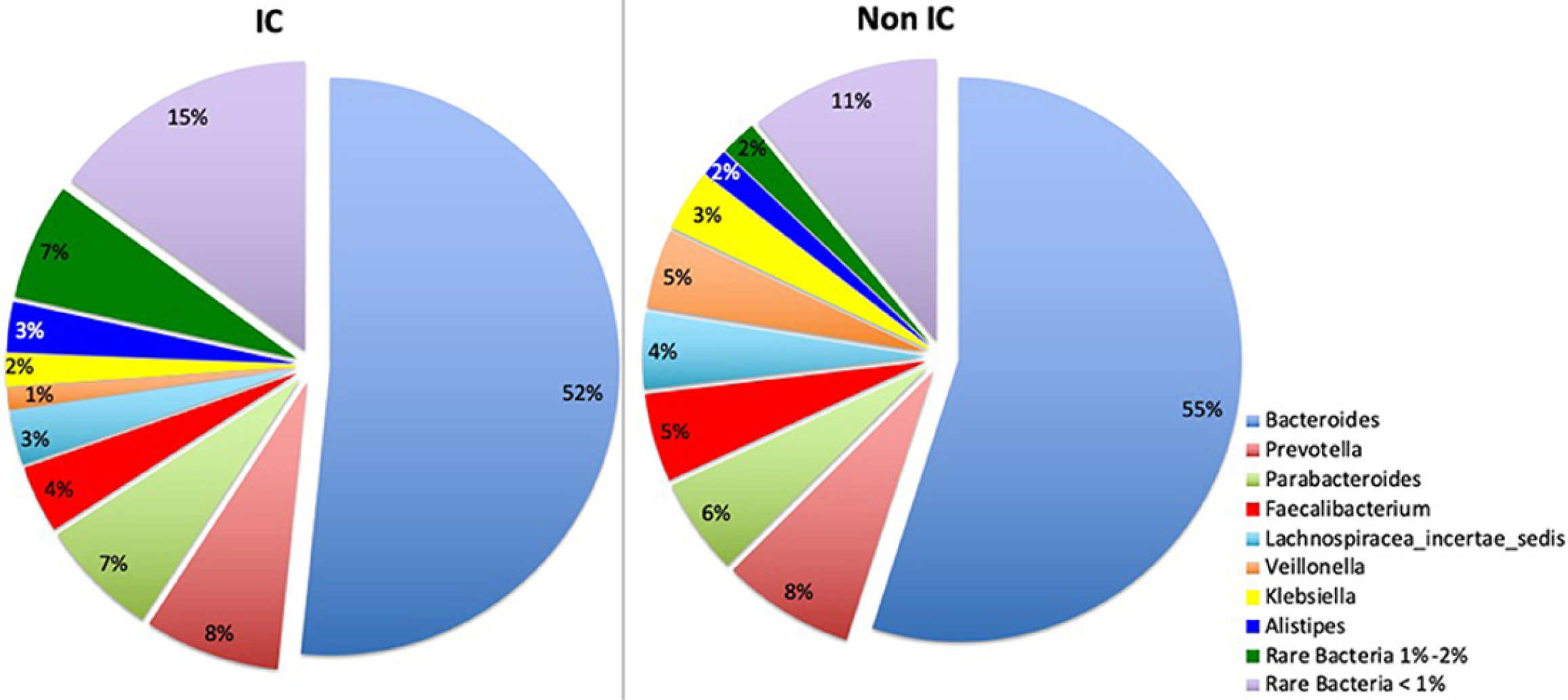
Figure 3 Impact of antibiotics, proton pump inhibitors, and Clostridium difficile colonization on gut microbial diversity in hospitalized children[23].
Heatmaps of α-diversity metrics comparing species richness (left) and Shannon index (right) across the entire cohort. Each point represents an individual patient; boxplots denote the median, interquartile range, and full range. IC: Immunocompromised. Citation: Mohandas S, Soma VL, Tran TDB, Sodergren E, Ambooken T, Goldman DL, Weinstock G, Herold BC. Differences in gut microbiome in hospitalized immunocompetent vs. immunocompromised children, including those with sickle cell disease. Front Pediatr 2020; 8: 583446. Copyright ©The Author(s) 2020. Published by Frontiers Media S.A (Supplementary material).
- Citation: Roganovic J, Radosevic M, Dordevic A. Role of the gut microbiome in the development and prognosis of pediatric leukemia. World J Clin Oncol 2025; 16(11): 111419
- URL: https://www.wjgnet.com/2218-4333/full/v16/i11/111419.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i11.111419