Published online Sep 15, 2025. doi: 10.4251/wjgo.v17.i9.110376
Revised: June 15, 2025
Accepted: August 13, 2025
Published online: September 15, 2025
Processing time: 102 Days and 16.1 Hours
Pancreatic cystic neoplasms (PCNs) represent a spectrum of heterogeneous lesions with diverse biological behaviors and malignant potential. This category encompasses relatively common subtypes, such as intraductal papillary mucinous neoplasms, serous cystic neoplasms, and mucinous cystic neoplasms, alongside relatively rarer entities, including cystic degeneration of solid pancreatic tumors. The widespread use of cross-sectional imaging has led to increased incidental detection of PCNs, subsequently driving a surge in PCN-related medical consultations and interventions; thus, standardized management of PCNs demands heightened attention. Continuous advancements in endoscopic technologies, particularly endoscopic ultrasound (EUS) and EUS-guided procedures, now offer diversified diagnostic and therapeutic options, establishing EUS as a pivotal tool for diagnosing, surveillance, and treating PCNs. This review synthesizes current evidence and evolving clinical practices in the endoscopic management of PCNs, emphasizing optimizing preoperative diagnostic accuracy, standardizing endoscopic protocols, implementing subtype-specific risk stratification, promoting multidisciplinary team approaches, and addressing challenges in emerging technologies.
Core Tip: Pancreatic cystic neoplasms (PCNs) represent a broad spectrum of lesions with variable malignant potential, necessitating precise risk stratification and tailored therapeutic strategies. This review synthesizes current advancements in endoscopic management, emphasizing innovations in imaging-guided risk assessment, minimally invasive interventions, and persistent challenges in clinical decision-making. The continuous development in endoscopic techniques, including endoscopic retrograde cholangiopancreatography and endoscopic ultrasound, have enabled minimally invasive alternatives to traditional surgeries for certain PCNs. However, unresolved issues persist, including optimal surveillance intervals for diverse PCN subtypes, standardization of endoscopic ablation protocols, and long-term oncologic outcomes. Additionally, resource availability and operator expertise disparities limit the global adoption of advanced endoscopic modalities. This article underscores the need for multidisciplinary collaboration, artificial intelligence-enhanced surveillance protocols, and cost-effectiveness analyses to optimize patient-centric care. The global community can harmonize management and ensure evidence-based, individualized care for this heterogeneous patient population by integrating evolving technologies with evidence-based guidelines.
- Citation: Zeng Y, Zhang JW, Yang J. Endoscopic management of pancreatic cystic neoplasms. World J Gastrointest Oncol 2025; 17(9): 110376
- URL: https://www.wjgnet.com/1948-5204/full/v17/i9/110376.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i9.110376
Pancreatic cystic neoplasms (PCNs) are a heterogeneous group of fluid-filled lesions arising within the pancreas, classified into distinct subtypes based on histopathological and molecular features. The most common subtypes include intraductal papillary mucinous neoplasms (IPMNs), serous cystic neoplasms (SCNs), and mucinous cystic neoplasms (MCNs). Other relatively rare subtypes include cystic pancreatic neuroendocrine neoplasms (pNENs), solid pseudopapillary neoplasms, and pancreatic ductal adenocarcinomas (PDACs) exhibiting cystic degeneration or intertumoral hemorrhage in solid pancreatic tumors, among others[1-4]. In the population-based Study of Health in Pomerania, pancreatic cysts demonstrated a significant size increase with advancing age (P < 0.001)[5]. Study of Health in Pomerania participants with incidental cysts were approximately 9 years older (mean age 60.5 ± 11.6 years) compared to cyst-free individuals (mean age 51.7 ± 12.3 years). Concurrently, the same study revealed no significant association between cyst prevalence and sex (P = 0.220), with detection rates of 51.2% (260/556) in females and 46.8% (234/521) in males[5]. The incidence of PCNs has risen markedly in recent decades, attributed to enhanced awareness of pancreatic disease screening and corresponding increased utilization of cross-sectional imaging, with studies reporting a detection rate of 2.4%-49.1% in magnetic resonance tested individuals[3,6]. This epidemiological shift underscores the urgency of refining diagnostic and therapeutic strategies to mitigate the overtreatment of indolent lesions while preventing missed opportunities for potentially malignant lesions.
The clinical management of PCNs remains fraught with challenges due to their variable biological behavior and the limitations of non-invasive diagnostics. On the one hand, health-driven concerns over the malignant transformation of PCNs persist among both asymptomatic individuals undergoing screening and clinicians specializing in this field[7]. Given the influence of individualized risk factors and the dismal prognosis of pancreatic malignancies, neither patients nor physicians can afford to overlook the severe consequences of missed diagnoses[8]. On the other hand, accumulating evidence highlights the prevalence of surgical overtreatment. For instance, studies indicate that nearly half of patients undergoing resection for IPMNs may have undergone unnecessary surgery, while malignancy is detected in only 16% of resected MCNs[9]. Another study with a median follow-up of 5.7 years further reveals that pancreatic cancer-related mortality occurred in 2.4% of PCN patients (43/1800), and non-pancreatic cancer deaths accounted for 19.9% of the cohort (359/1800)[10]. Meanwhile, while conventional imaging modalities such as computed tomography (CT) and magnetic resonance imaging (MRI) provide excellent anatomical detail, they often fail to reliably differentiate benign from premalignant lesions or predict the progression risk of PCNs[11,12].
Driven by these clinical imperatives, advancements in endoscopic technologies have expanded the armamentarium for comprehensive PCN management by providing enhanced diagnostic insights and diversified therapeutic options. Endoscopic ultrasound (EUS) enables meticulous evaluation of intracystic structures and communication with the pancreatic ductal system, along with EUS-guided fine-needle aspiration (EUS-FNA), allowing targeted sampling of cyst contents for cytological examination, histological analysis, and carcinoembryonic antigen (CEA) quantification[13-15]. Compared to conventional imaging modalities, EUS-guided diagnostics significantly enhance diagnostic accuracy through direct morphological and biochemical interrogation[16,17]. This review synthesizes contemporary advancements in the endoscopic management of PCNs, integrating real-world clinical experiences from the authors’ tertiary referral center, aiming to inform optimized patient care, prognostication, and practice standardization across diverse clinical settings.
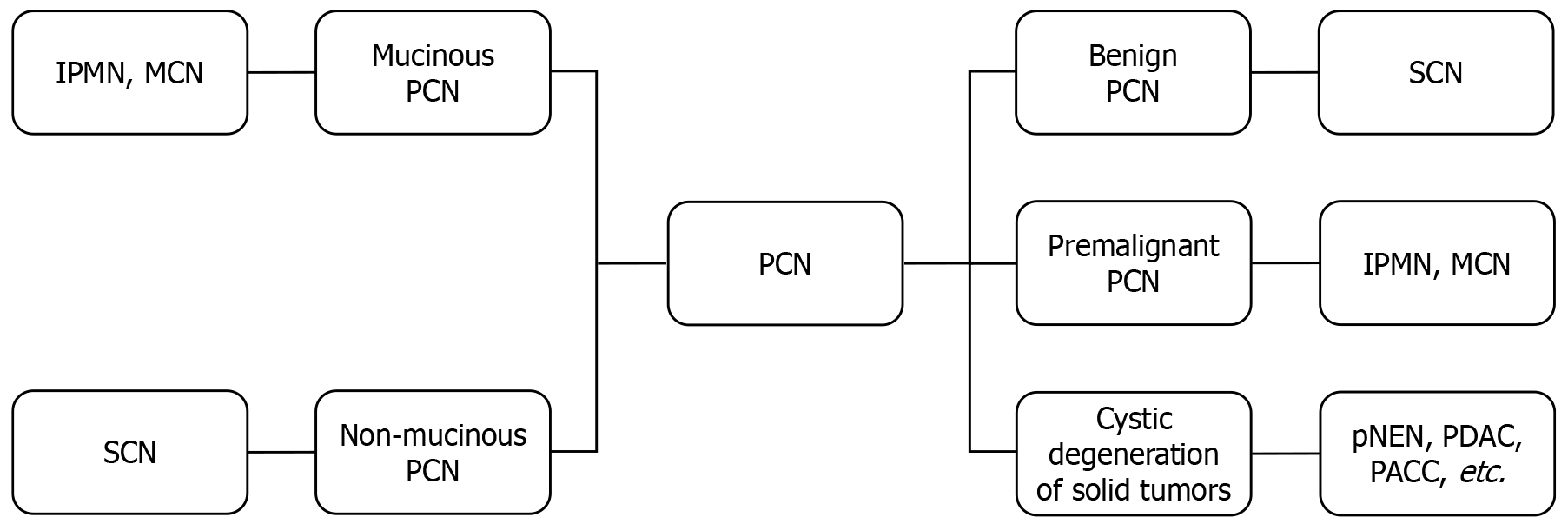
Pancreatic cystic lesions (PCLs) are broadly categorized into non-neoplastic cysts and neoplastic neoplasms. Neoplastic PCLs, termed PCNs, primarily comprise several common subtypes: IPMNs, SCNs, MCNs, and other relatively rare cystic neoplasms[1,18]. These major PCN subtypes are further classified based on mucin production into non-mucinous and mucinous variants (Figure 1)[19]. Despite several reports in the literature describing locally invasive behavior in rare patients, non-mucinous PCNs, such as SCNs, are generally considered benign and are thus managed through surveillance protocols involving periodic follow-up[20,21]. In contrast, mucinous PCNs such as IPMNs and MCNs are recognized as premalignant lesions with carcinogenic potential, necessitating more intensive surveillance and proactive intervention strategies to mitigate pancreatic cancer risk[22-24]. Screening-detected PCLs must be differentiated from cystic degeneration of solid pancreatic tumors, such as pNENs, PDACs, and pancreatic acinar cell carcinomas, which may mimic PCNs on initial imaging[25-28]. A pathologically validated analysis of 501 consecutive ordinary PDAC patients revealed that 10% of PDAC cases exhibited a diameter of pancreatic cysts ≥ 1 cm[29]. Therefore, this diagnostic challenge requires meticulous clinical, imaging, and pathological data integration to avoid misclassification and ensure accurate risk stratification.
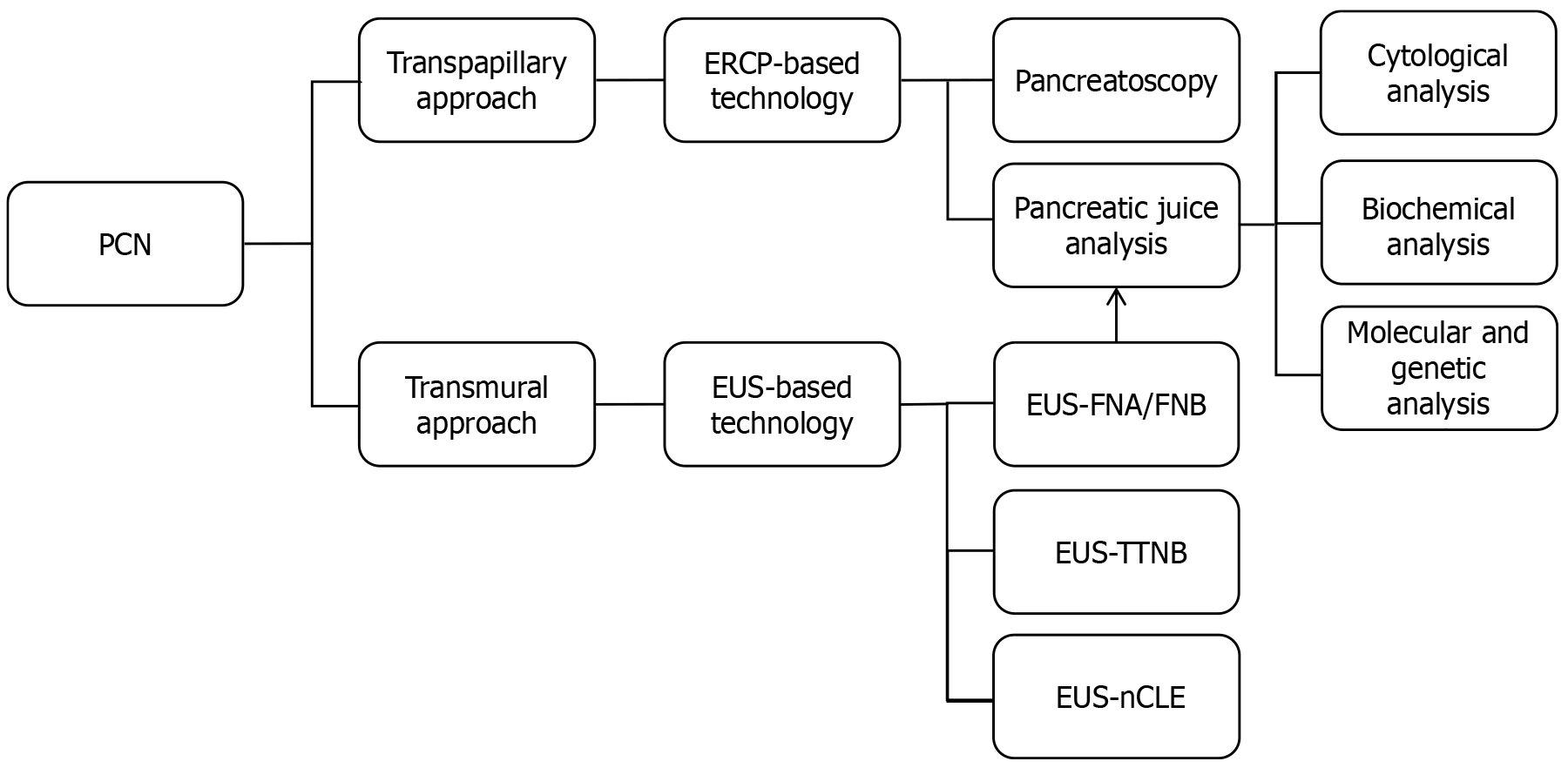
Meticulous characterization of PCN subtypes during the diagnostic phase is fundamental to formulating patient-tailored management strategies that balance clinical benefits with optimal resource utilization. However, the current preoperative diagnostic accuracy for PCNs remains suboptimal. A retrospective study demonstrated that while mucinous and non-mucinous PCNs could be differentiated in approximately 75% of cases, nearly 20% of pancreatectomies were performed in patients with benign PCNs lacking malignant potential[30]. Another study enrolling 672 presumed SCNs also highlighted critical concerns regarding current surgical practices of performing pancreatectomies on benign lesions[31]. Therefore, before formulating personalized therapeutic strategies for PCN patients, the imperative lies in enhancing diagnostic precision through continuous optimization of detection methods and diagnostic algorithms-a cornerstone of holistic PCN management that ensures risk-adapted interventions aligned with individual patient profiles. Research has recently focused on elevating PCN diagnostic accuracy via methodological refinements. For instance, multiple studies have indicated that MRI demonstrates superior sensitivity in PCN detection compared to CT during screening, especially 5.0T MRI[32,33]. Concurrently, another evidence positions 18F-fluorodeoxyglucose positron emission tomography-CT as the optimal preoperative imaging modality for distinguishing malignant from benign PCNs, with diagnostic performance surpassing both MRI and CT, thus warranting its integration into preoperative evaluation protocols[34]. Parallel to advancements in imaging technologies, rapid innovations in endoscopic techniques-including EUS, endoscopic retrograde cholangiopancreatography (ERCP), needle-based confocal laser endomicroscopy (nCLE), and pancreatoscopy-are expanding diagnostic possibilities to address current challenges in PCN characterization[35,36]. These endoscopic modalities for PCN diagnosis can be broadly categorized into transmural and transpapillary approaches (Figure 2). The transpapillary approach is exemplified by ERCP, which visualizes morphological alterations in the pancreatic duct (PD) and intraductal communication of presumed PCNs and enables biochemical, cytological, molecular, and genetic analyses of collected pancreatic juice[37]. These diagnostic capabilities establish ERCP as one of the pivotal adjunctive modalities for characterizing PCNs, particularly in differentiating mucinous lesions like IPMNs that exhibit direct ductal connectivity. However, ERCP is inherently limited by its inability to directly visualize intraluminal wall structures of PCNs, such as mural nodules (MNs) or septations, and its reduced diagnostic utility in PCNs with excessive mucin, which may prevent clear visualization of cystic lesions[38]. In recent years, the advent and clinical adoption of technologies such as SpyGlass™ pancreatoscopy have expanded the diagnostic potential of the transpapillary approach via ERCP, enabling real-time, direct visualization of PCNs communicating with PD during ERCP procedures[39,40]. The SpyGlass™ Digital System represents a miniaturized endoscopic platform that enables direct intraluminal visualization during endoscopic procedures, including ERCP and EUS. Unlike conventional ERCP, which relies on indirect fluoroscopic imaging, SpyGlass™ Digital System provides high-definition optical views of the biliary and pancreatic ductal system through a single-use fiberoptic probe, which allows for real-time differentiation of benign mucin deposits from malignant MNs, and targeted biopsies under direct vision (via integrated micro-forceps)[41].
On the other hand, EUS serves as the cornerstone of transmural approaches, providing significant diagnostic capabilities through direct transluminal access to cystic lesions[1,42]. EUS provides critical advantages in diagnosing PCNs, offering high spatial resolution, real-time, and continuous imaging capabilities[43]. These attributes are further enhanced by adjunctive EUS-based technologies such as contrast-enhanced EUS (CE-EUS), which elevates diagnostic precision. Previous studies have pointed out that the data analysis of a prospective database over a decade shows that EUS can help reduce the misdiagnosis rate[44]. Moreover, based on the final histological results, 31.5% of PCN patients (145/461) could be spared from pancreatectomy[44]. Another evidence indicates that EUS exhibits the highest sensitivity for detecting MNs in IPMNs[45]. In the same study above, CE-EUS demonstrates a diagnostic sensitivity of 60%-100% and specificity of 75%-92.9% for identifying malignant pancreatic cysts, underscoring its role in refining risk stratification[45,46]. CE-EUS also demonstrates a distinct advantage over contrast-enhanced multidetector CT and contrast-enhanced MRI in patients with renal insufficiency or iodinated contrast allergies, owing to the significantly lower incidence of adverse reactions associated with CE-EUS contrast agents (microbubble-based agents)[47]. This safety profile positions CE-EUS as a clinically viable option for PCN risk stratification in real-world practice, particularly among high-risk populations with contraindications to conventional contrast media. Like ERCP in the transpapillary approach, EUS within the transmural approach can also be combined with SpyGlass™ technology. However, studies suggest that while the SpyGlass™-assisted group demonstrates a numerically higher diagnostic accuracy for PCNs than EUS-FNA alone, no statistically significant difference was observed between the two modalities[48]. The integration of EUS with nCLE has also emerged as a promising research direction to enhance the diagnostic performance for PCNs[49,50]. Other studies have demonstrated that specific nCLE imaging patterns, such as dark aggregates of neoplastic cells, can achieve substantial diagnostic accuracy for malignant PCNs, with reported metrics of 94% accuracy, 75% sensitivity, and 100% specificity in select cohorts[51]. A recent meta-analysis revealed that the pooled sensitivity and specificity of EUS-FNA-based cytology for diagnosing malignant PCNs were 0.62 [95% confidence interval (CI): 0.42-0.78] and 0.96 (95%CI: 0.91-0.98), respectively. The positive and negative likelihood ratios for malignant PCNs were 16.3 (95%CI: 7.2-37.0) and 0.40 (95%CI: 0.25-0.64), highlighting its strong rule-in capability but limited utility in excluding malignancy[52].
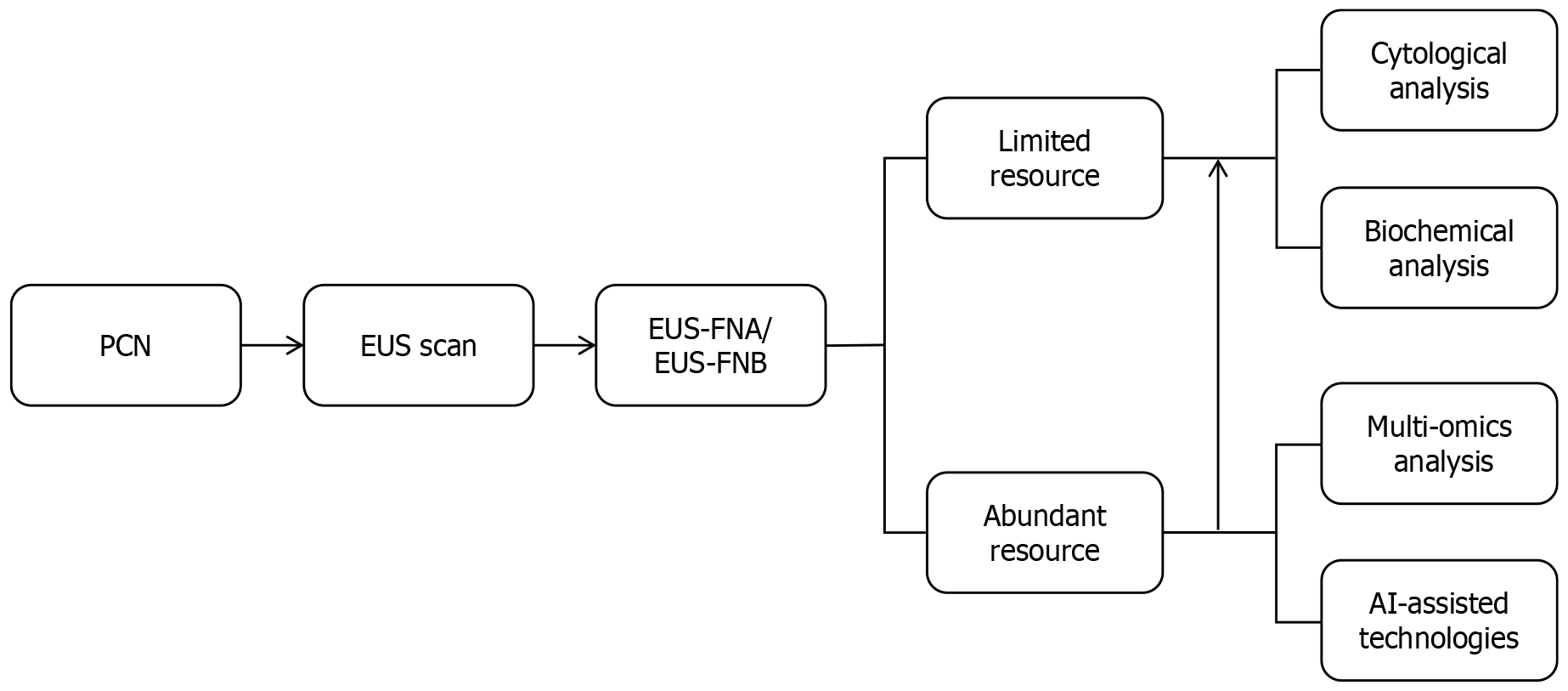
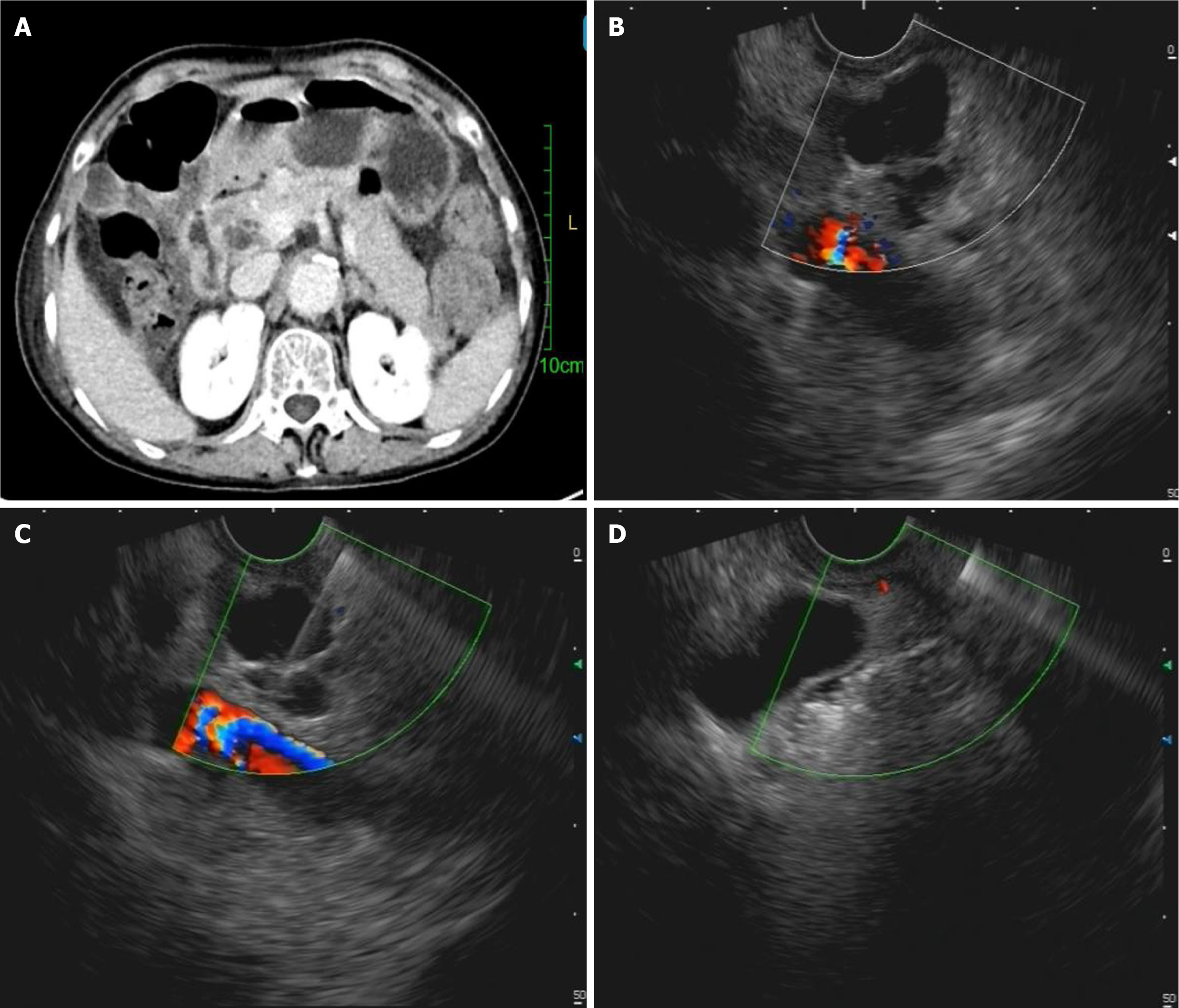
Beyond cyst size and location, EUS facilitates the detection of endosonographic morphologies-such as cyst wall thickness, microcystic count, central scars, mucin deposits, and PD communication-often undetectable by conventional imaging[53]. However, these features lack diagnostic specificity in differentiating PCNs. Although combining multiple EUS characteristics improves preoperative diagnostic accuracy in some studies, real-world data indicate that many patients still undergo pancreatectomy due to misinterpreted features like MNs masquerading as neoplastic nodules[54,55]. Additionally, some studies have pointed out that the aforementioned EUS characteristics may also lead to misclassification of SCNs as IPMNs, potentially triggering unnecessary aggressive surveillance or interventions[56]. The same study identified that the microcystic pattern is more reliable in distinguishing SCNs from IPMNs than other ambiguous EUS findings[56]. Beyond provoking overly aggressive interventions through risk overestimation, isolated EUS assessments may also underestimate the malignant potential of PCNs. For instance, a documented case revealed a multilocular cystic lesion with a central scar on EUS, initially suspected to be an SCN[28]. However, subsequent EUS-FNA ultimately diagnosed it as a retention cyst associated with PDAC, with the malignancy within the scar tissue itself-underscoring the critical limitations of morphological diagnosis alone[28]. Therefore, based on our clinical experience at a tertiary endoscopic referral center, we recommend that during the EUS evaluation of PCNs-particularly when determining subsequent therapeutic strategies-reliance solely on EUS morphological observations and its nonspecific acoustic features should be avoided. Instead, diagnostic approaches integrating EUS-FNA/EUS-guided fine-needle biopsy (EUS-FNB) with adjunctive techniques are imperative for accurate risk stratification[52]. EUS-based technologies, including EUS-FNA/EUS-FNB, EUS-guided through-the-needle biopsy (TTNB), nCLE, and CE-EUS, have demonstrated potential to augment diagnostic accuracy[15,57]. Previous studies have shown that a CEA level > 800 ng/mL has a specificity of 98% for mucinous cysts, and cyst fluid glucose (≤ 50 mg/dL) has a sensitivity of 92% and a specificity of 87% for MCNs[58,59]. A recent network meta-analysis showed that glucose assay exhibits high sensitivity but low specificity, whereas CEA analysis exhibits the opposite profile regarding pancreatic cyst fluid analysis[60]. Additionally, the same study concluded that EUS-guided TTNB emerged as the optimal modality for identifying malignant PCNs, and evidence supporting EUS-nCLE for diagnosing malignant pancreatic cysts remains insufficient[60]. However, limited accessibility to advanced tools like nCLE and TTNB restricts widespread adoption. To overcome the suboptimal sensitivity of single-session EUS-FNA in PCN characterization, CE-EUS-FNA of cyst fluid and MNs demonstrates significant promise, achieving sensitivities of 82.4% for mucinous cysts and 84.2% for malignancy with corresponding specificities of 92% and 100%, thereby supporting its routine application for PCNs exhibiting solid components[61]; concurrently, repeat EUS-FNA modifies clinical management in 20% of PCLs, primarily by preventing unwarranted surgeries through refined diagnostic accuracy and enabling risk-stratified management[54]. Other emerging studies also demonstrate that EUS-guided sulfur hexafluoride pancreatography, an adjunct to EUS-FNA, facilitates the identification of PD communication in PCNs[62]. This technique achieves diagnostic metrics of accuracy 96.6%, sensitivity 88.9%, specificity 100%, positive predictive value 100%, and negative predictive value 95.2%, positioning it as a reliable complementary alternative when conventional EUS or ERCP fails to delineate duct-cyst connectivity or is contraindicated[62]. In real-world practice, EUS-FNA/EUS-FNB remains the cornerstone of EUS-based diagnostics (Figure 3), offering both liquid-based cytological analysis and cyst fluid biomarker profiling (e.g., CEA, amylase) to resolve diagnostic ambiguities[63-66]. At other well-equipped institutions, multi-omics biomarker profiling (genomics, transcriptomics, epigenomics, proteomics, and metabolomics) of cyst fluid obtained via EUS-FNA/EUS-FNB should be pursued to enhance diagnostic sensitivity, supplemented by artificial intelligence technologies based on machine learning and deep learning models to optimize the differential diagnosis and risk stratification of PCNs (Figure 4)[67-71].
Before discussing the endoscopic management of PCNs, a pivotal preliminary step is diagnosing whether these lesions harbor definitive evidence of malignancy or high-grade dysplasia[1,72,73]. This foundational determination that whether surgical resection is oncologically indicated must precede any consideration of endoscopic therapies (Figure 5); simultaneously, indiscriminate pancreatectomy proves particularly detrimental, especially when considering the relatively low morbidity and mortality associated with the malignant transformation of PCNs[74]. One study, which included 211 patients with MCNs who underwent pancreatectomy, found that 16.1% were malignant, with an additional 13 cases showing high-grade dysplasia[75]; another study, which analyzed data from seven medical centers in India over a decade, showed that among 423 patients who underwent pancreatic surgery, only 9.2% were reported to have malignancy[76]. Only after confirming the absence of surgical necessity can rational endoscopic management be contemplated, a process that demands integration of lesion characteristics, clinical symptomatology, and individualized therapeutic preferences, rigorously safeguarded through multidisciplinary team (MDT) consensus to ensure decision-making quality and prevent unwarranted interventions[74,77-79]. Crucially, based on our tertiary center’s experience and contemporary evidence, neither the categorization as mucinous vs non-mucinous PCNs nor the presence of EUS-suspected MNs should independently justify pancreatectomy without histopathological confirmation, given that approximately 20% of resected lesions ultimately prove benign in registry data, representing not only a wasteful expenditure of finite healthcare resources but also inflicting irrevocable harm on patients through diabetes/exocrine insufficiency in up to 25% of unnecessary surgeries[30,56,80-82]. Consequently, irrespective of the intended endoscopic therapy, greater time investment must be allocated to the diagnostic phase to ensure that interventions target exclusively lesions with confirmed malignant potential, thereby aligning with the core oncologic principle of “first, do no harm” while optimizing resource utilization in PCN care pathways[83].
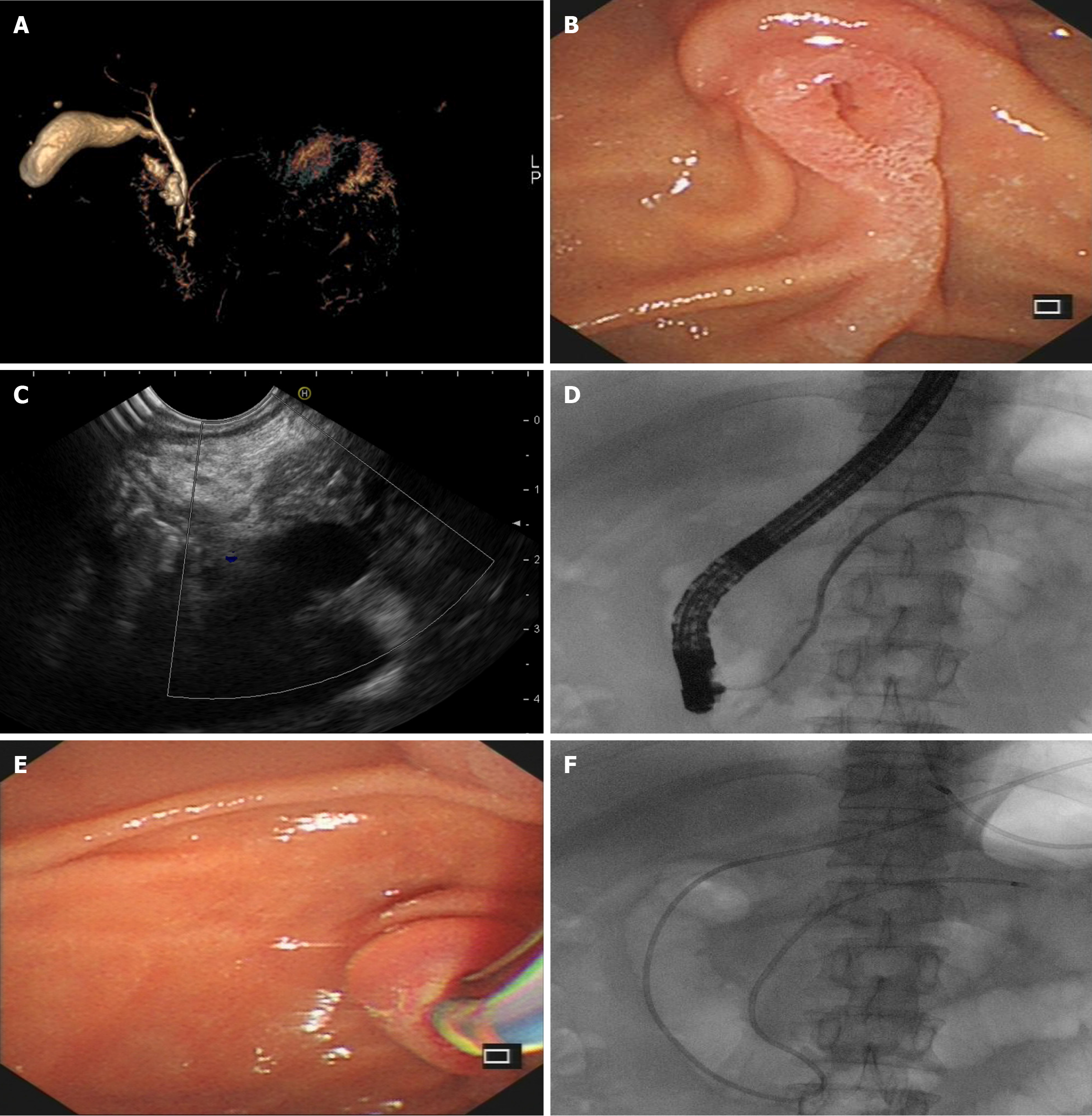
Indications for endoscopic intervention primarily target symptomatic PCNs, while contraindications align with general endoscopic restrictions such as severe coagulopathy or inability to tolerate endoscopy[84]. Furthermore, endotherapy represents an effective alternative for patients with definitive malignant diagnoses who are ineligible for or decline surgical resection[85]. Compared to pancreatectomies performed on benign or malignant PCNs, endoscopic therapies offer compelling advantages, including minimally invasive procedures, acceptable therapeutic outcomes, lower costs, shorter postoperative recovery, and preserved quality of life[85-87]. The two principal endoscopic approaches-transpapillary (ERCP-based) and transmural (EUS-guided)-provide distinct therapeutic pathways tailored to anatomical and clinical characteristics. The fundamental rationale for ERCP-based endoscopic management of PCNs centers on enhancing cyst drainage, primarily achieved through pancreatic sphincterotomy, endoscopic dilation or drainage procedures that reduce cyst size and alleviate clinical symptoms, with adjunctive radiofrequency ablation (RFA) integrated to prevent the progression of PCNs[40,88-90]. Before attempting ERCP-guided drainage, confirming the communication between the PCNs and PD is essential. Standard methods for this assessment include EUS, magnetic resonance cholangiopancreatography (MRCP), and endoscopic retrograde pancreatography. MRCP is recommended as the initial non-invasive approach due to zero radiation exposure. EUS evaluation of duct-cyst connectivity demands operator expertise and meticulous scanning techniques, with accuracy highly dependent on endoscopist experience and patience. Given its inherent radiation exposure, endoscopic retrograde pancreatography is reserved for cases where MRCP/EUS fails to delineate communication or as a confirmatory step immediately preceding pancreatic stenting/nasocystic drain placement[91]. Crucially, drainage efficacy differs significantly by IPMN subtype: Main-duct IPMNs respond differently with branch-duct IPMNs (BD-IPMN) to transpapillary drainage theoretically due to viscous mucin obstruction[92]. Consequently, IPMN subtyping is suggested to guide optimal, symptom-targeted endoscopic therapy before intervention. Based on our endoscopic center’s experience, pancreatic sphincterotomy alone yields suboptimal outcomes for PCNs and is thus typically combined with PD stenting or nasocystic drainage (Figure 6). During the procedure, meticulous attention must be paid to achieving a complete sphincter incision to avoid mechanical and thermal injury-induced edema and obstruction of the pancreatic fluid outflow, which may precipitate complications such as postoperative pancreatitis[93]. Additionally, the application of pancreatoscopy in PCN management is constrained by the degree of main PD (MPD) dilation; early-stage PCNs, including those with malignant potential, often preclude pancreatoscopy use due to insufficient ductal diameter. ERCP-based approaches apply not only to symptomatic benign PCNs but are also extensively utilized in malignancy-confirmed PDACs requiring palliative treatment[94].
Beyond ERCP-based techniques, an alternative endoscopic approach leverages EUS-guided minimally invasive interventions, whose primary advantages include eliminating radiation exposure for both endoscopists and patients and enabling transmural access via EUS-FNA technique, thus benefiting patients with gastric outlet or duodenal obstruction precluding ERCP[95]. Indications for EUS-guided PCN therapies also include symptomatic benign PCNs and malignant PCNs in surgically ineligible or refusal patients[85]. The cornerstone technique is EUS-guided pancreatic cyst ablation (EUS-PCA), involving cyst drainage followed by injection of ablative agents such as ethanol, sclerosants, or chemotherapeutic agents (e.g., paclitaxel, gemcitabine) (Figure 7). Studies indicate ethanol-paclitaxel EUS-PCA may eliminate mutant DNA within PCNs[96]; postoperative follow-up at 3 months in another study reveals morphological changes, including reduced septation in 24% of lesions, altered fluid viscosity in 48% and loss/reduction of cellular atypia in 15%[97]. These morphological and cytological changes may serve as indicators of the effectiveness of EUS-PCA. A recent study that included 620 patients with PCNs found that EUS-PCA showed superior long-term safety and pancreatic function preservation compared to pancreatectomy[98]. Another study with a median follow-up of 72 months in 164 pancreatic cyst patients revealed that 98.3% remained in remission at the 6-year postoperative mark[99]. Furthermore, unilocular cysts without septations (odds ratio = 7.12, 95%CI: 2.72-18.67) and cyst size < 35 mm (odds ratio = 2.39, 95%CI: 1.11-5.16) were identified as predictors of complete cyst resolution after EUS-PCA in the same study[99]. While ethanol offers cost and accessibility advantages, multiple studies associate it with complications, including pain, pancreatitis, peritonitis, pancreatic pseudocysts, intracystic/pericystic hemorrhage, and gastroduodenal wall cysts[100]. Consequently, recent research advocates replacing ethanol with alternative agents or combining ethanol with chemotherapy to improve outcomes[101]. Some evidence suggests ethanol omission in EUS-PCA reduces adverse events without compromising efficacy, noting that multi-drug chemoablation fails to increase complete ablation rates as expected[102]. Based on our tertiary center’s experience, we emphasize that before determining optimal regimens, standardization of EUS-PCA technical parameters demands more attention-particularly regarding PCN size, MPD communication, ethanol/paclitaxel/gemcitabine concentration, dosing, lavage cycles, and dwell time per cycle. A meta-analysis of 524 pancreatic lesions revealed that ethanol monotherapy and combined ethanol-paclitaxel therapy achieved comparable complete ablation rates in EUS-PCA[103]. Nevertheless, the combination group exhibited higher adverse event incidence[103]. Aligned with these findings, our endoscopic center recommends employing single ablative agents in EUS-PCA practice. Regarding optimal agent selection, the choice should be personalized through MDT discussion, integrating individual complication risk profiles, patient-specific therapeutic goals, and institutional expertise. EUS-guided thermal ablation constitutes another major category of EUS-based minimally invasive techniques. Its principle involves real-time EUS imaging-guided precise probe placement to reach deep-seated targets like pancreatic lesions for therapeutic intervention. Indications in pancreatic diseases include PDCAs, pNENs, and PCNs[104-106]. Diverse probe types enable multiple modalities: RFA, laser ablation, hybrid cryotherm ablation (combined radiofrequency and cryotechnology), and photodynamic therapy[107,108]. EUS-guided RFA has gained significant attention for its favorable safety-efficacy profile. RFA induces tissue coagulative necrosis and potentially triggers immunomodulation through the released intracellular antigens[105,109]. Prior studies confirm EUS-guided RFA’s safety in IPMNs and MCNs. However, efficacy is suboptimal for SCNs with multicystic honeycomb architecture-only 61.5% (8/13) achieved partial response at a median follow-up of 9.2 months[110,111]. Optimizing power and ablation duration based on cyst characteristics is suggested to prevent iatrogenic injuries (e.g., severe necrotizing pancreatitis, duodenal perforation) to adjacent critical structures[87]. Beyond benign and premalignant PCNs discussed previously, endoscopic minimally invasive therapies are similarly effective for palliative management of malignant pancreatic lesions exhibiting cystic degeneration, particularly in patients unsuitable for or declining pancreatectomy. Applicable EUS-guided techniques include EUS-guided injection therapy of drugs or viral and immunologic vectors, EUS-guided ablation therapies, and EUS-guided radiation therapy such as fiducial marker insertion[112]. When crafting endoscopic strategies for patients in palliative settings, it is essential to acknowledge that minimally invasive procedures do not preclude the risk of severe complications. Therefore, a thorough evaluation of individual tumor biology, anticipated survival, patient-centered therapeutic goals, and local institutional expertise is crucial. MDT collaboration should be utilized to avert unnecessary financial toxicity and perioperative quality of life deterioration-outcomes that contradict the core principles of palliative care[113,114].
Endoscopic surveillance constitutes a critical component of the comprehensive endoscopic management of PCNs, yet significant consensus gaps persist among major guidelines regarding surveillance duration, frequency, and endpoints[14,115]. Most scholars have widely accepted that different PCN subtypes exhibit distinct biological behaviors[115,116]. Based on this, previous guidelines have recommended lifelong follow-up for surgically fit IPMN patients[116]; another evidence indicates that cystic PNENs > 2 cm in diameter independently correlate with aggressive behavior, thereby supporting a watchful-waiting strategy for asymptomatic lesions < 2 cm[25]. Accordingly, our center advocates subtype-stratified surveillance protocols focused on the timely detection of alarm features, dynamically refined through MDT consensus based on diverse patients’ life expectancy, therapeutic goals, and local healthcare resources. Taking IPMNs, the more common mucinous PCNs, as an example, the 2024 International Evidence-Based Kyoto Guidelines for IPMN prioritize monitoring high-risk stigmata (MNs ≥ 5-10 mm, MPD ≥ 10 mm) and worrisome features (growth rate ≥ 2.5 mm/year), endorse distinct strategies for BD-IPMNs vs main-duct IPMNs (including potential discontinuation after 5 stable years for small BD-IPMNs despite acknowledging residual PDAC risk), and stratify follow-up intervals by BD-IPMN size (< 20 mm: 18 months; 20-30 mm: 12 months; ≥ 30 mm: 6 months)[117]. However, challenges in differentiating MNs from solid components and operator-dependent EUS/CE-EUS variability necessitate PCN subtype-specific risk models incorporating artificial intelligence-assisted imaging analysis (machine learning/deep learning for automated high-risk stigmata/worrisome features detection) alongside cost-effectiveness analyses to iteratively optimize regionally tailored surveillance protocols aligned with local disease profiles and economic constraints[42,70,118-120].
The diagnostic rate of PCNs has progressively increased with advancements in imaging technologies. Innovations in endoscopic techniques, particularly EUS and EUS-guided procedures, have expanded therapeutic options for PCN management. These developments enhance the diagnostic accuracy of conventional methods and offer quality of life-preserving alternatives for patients’ ineligible for or declining pancreatectomy. A pivotal focus of future research will be bridging existing discrepancies between guidelines, ultimately delivering personalized, cost-effective, and safe management strategies for broader patient populations.
| 1. | Gardner TB, Park WG, Allen PJ. Diagnosis and Management of Pancreatic Cysts. Gastroenterology. 2024;167:454-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 33] [Article Influence: 16.5] [Reference Citation Analysis (2)] |
| 2. | Lennon AM, Vege SS. Pancreatic Cyst Surveillance. Clin Gastroenterol Hepatol. 2022;20:1663-1667.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 3. | van Huijgevoort NCM, Del Chiaro M, Wolfgang CL, van Hooft JE, Besselink MG. Diagnosis and management of pancreatic cystic neoplasms: current evidence and guidelines. Nat Rev Gastroenterol Hepatol. 2019;16:676-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 167] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 4. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2554] [Cited by in RCA: 2748] [Article Influence: 458.0] [Reference Citation Analysis (3)] |
| 5. | Kromrey ML, Bülow R, Hübner J, Paperlein C, Lerch MM, Ittermann T, Völzke H, Mayerle J, Kühn JP. Prospective study on the incidence, prevalence and 5-year pancreatic-related mortality of pancreatic cysts in a population-based study. Gut. 2018;67:138-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 268] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 6. | Moris M, Bridges MD, Pooley RA, Raimondo M, Woodward TA, Stauffer JA, Asbun HJ, Wallace MB. Association Between Advances in High-Resolution Cross-Section Imaging Technologies and Increase in Prevalence of Pancreatic Cysts From 2005 to 2014. Clin Gastroenterol Hepatol. 2016;14:585-593.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 133] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 7. | Munigala S, Gelrud A, Agarwal B. Risk of pancreatic cancer in patients with pancreatic cyst. Gastrointest Endosc. 2016;84:81-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 8. | Stoffel EM, Brand RE, Goggins M. Pancreatic Cancer: Changing Epidemiology and New Approaches to Risk Assessment, Early Detection, and Prevention. Gastroenterology. 2023;164:752-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 323] [Reference Citation Analysis (1)] |
| 9. | De Stefano F, Pellegrini R, Marchegiani G, Crippa S. Reducing the burden of pancreatic cancer by surveilling mucinous cystic neoplasms: are we there yet? Best Pract Res Clin Gastroenterol. 2025;74:101998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 10. | Kwok K, Chang J, Duan L, Huang BZ, Wu BU. Competing Risks for Mortality in Patients With Asymptomatic Pancreatic Cystic Neoplasms: Implications for Clinical Management. Am J Gastroenterol. 2017;112:1330-1336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Aguilera Munoz L, Boros C, Bonvalet F, de Mestier L, Maire F, Lévy P, Cros J, Ronot M, Rebours V. Reappraising imaging features of pancreatic acinar cystic transformation: be aware of differential diagnoses. Eur Radiol. 2024;34:7650-7658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 12. | Scheiman JM, Hwang JH, Moayyedi P. American gastroenterological association technical review on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology. 2015;148:824-48.e22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 314] [Article Influence: 28.5] [Reference Citation Analysis (1)] |
| 13. | Vlăduţ C, Bilous D, Ciocîrlan M. Real-Life Management of Pancreatic Cysts: Simplified Review of Current Guidelines. J Clin Med. 2023;12:4020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (2)] |
| 14. | Aziz H, Acher AW, Krishna SG, Cloyd JM, Pawlik TM. Comparison of Society Guidelines for the Management and Surveillance of Pancreatic Cysts: A Review. JAMA Surg. 2022;157:723-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (1)] |
| 15. | Singh S, Chandan S, Vinayek R, Dhar J, Samanta J, Capurso G, Boskoski I, Spada C, Machicado JD, Crinò SF, Facciorusso A. Endoscopic techniques for the diagnosis of pancreatic cystic lesions. World J Gastroenterol. 2025;31:101082. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (2)] |
| 16. | Sun L, Huang H, Jin Z. Application of EUS-based techniques in the evaluation of pancreatic cystic neoplasms. Endosc Ultrasound. 2021;10:230-240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Blaszczak AM, Krishna SG. Endoscopic diagnosis of pancreatic cysts. Curr Opin Gastroenterol. 2019;35:448-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Bian Y, Jiang H, Zheng J, Shao C, Lu J. Basic Pancreatic Lesions: Radiologic-pathologic Correlation. J Transl Int Med. 2022;10:18-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 19. | Rossi G, Petrone MC, Tacelli M, Zaccari P, Crippa S, Belfiori G, Aleotti F, Locatelli M, Piemonti L, Doglioni C, Falconi M, Capurso G, Arcidiacono PG. Glucose and lactate levels are lower in EUS-aspirated cyst fluid of mucinous vs non-mucinous pancreatic cystic lesions. Dig Liver Dis. 2024;56:836-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (1)] |
| 20. | Papazarkadas X, Gialamas E, Hassan GM, Chautems R, Bornand A, Puppa G, Toso C. Degenerated Serous Cystic Tumor of the Pancreas: Case Report and Literature Review of an Aggressive Presentation of a Benign Tumor. Am J Case Rep. 2022;23:e936165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 21. | Chang JH, Perlmutter BC, Wehrle C, Naples R, Stackhouse K, McMichael J, Chao T, Naffouje S, Augustin T, Joyce D, Simon R, Walsh RM. Natural history and growth prediction model of pancreatic serous cystic neoplasms. Pancreatology. 2024;24:489-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 22. | Marchegiani G, Andrianello S, Crippa S, Pollini T, Belfiori G, Gozzini L, Cassalia F, Caravati A, Luchini C, Doglioni C, Bassi C, Falconi M, Salvia R. Actual malignancy risk of either operated or non-operated presumed mucinous cystic neoplasms of the pancreas under surveillance. Br J Surg. 2021;108:1097-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 23. | Servin-Rojas M, Fong ZV, Fernandez-Del Castillo C, Ferrone CR, Rocha-Castellanos DM, Roldan J, Zelga PJ, Warshaw AL, Lillemoe KD, Qadan M. Identification of high-risk features in mucinous cystic neoplasms of the pancreas. Surgery. 2023;173:1270-1274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 24. | Rift CV, Toxvaerd A, Kovacevic B, Klausen P, Hansen CP, Vilmann P, Hasselby JP. Pitfalls of histopathological evaluation of EUS-guided microbiopsies from pancreatic cystic neoplasms. Histopathology. 2020;76:630-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Maggino L, Schmidt A, Käding A, Westermark S, Ceppa EP, Falconi M, Javed AA, Landoni L, Pergolini I, Perinel J, Vollmer CM Jr, Sund M, Gaujoux S; on the behalf on the Pancreas 2000 research group. Reappraisal of a 2-Cm Cut-off Size for the Management of Cystic Pancreatic Neuroendocrine Neoplasms: A Multicenter International Study. Ann Surg. 2021;273:973-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 26. | Ikezawa K, Urabe M, Kai Y, Takada R, Akita H, Nagata S, Ohkawa K. Comprehensive review of pancreatic acinar cell carcinoma: epidemiology, diagnosis, molecular features and treatment. Jpn J Clin Oncol. 2024;54:271-281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 27] [Reference Citation Analysis (0)] |
| 27. | Shigaki T, Hisaka T, Fujita F, Kusano H, Naito Y, Midorikawa R, Ohchi T, Shiratsuchi I, Hidaka A, Tanaka T, Akagi Y. Mixed ductal-acinar cell carcinoma of the pancreas: A case report. Mol Clin Oncol. 2019;10:516-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 28. | Ogura T, Imanishi M, Okuda A, Yamada T, Higuchi K. Pancreatic ductal adenocarcinoma masquerading as a serous cystic tumor (with videos). Endosc Ultrasound. 2018;7:212-213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 29. | Muraki T, Jang KT, Reid MD, Pehlivanoglu B, Memis B, Basturk O, Mittal P, Kooby D, Maithel SK, Sarmiento JM, Christians K, Tsai S, Evans D, Adsay V. Pancreatic ductal adenocarcinomas associated with intraductal papillary mucinous neoplasms (IPMNs) versus pseudo-IPMNs: relative frequency, clinicopathologic characteristics and differential diagnosis. Mod Pathol. 2022;35:96-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 30. | Cho CS, Russ AJ, Loeffler AG, Rettammel RJ, Oudheusden G, Winslow ER, Weber SM. Preoperative classification of pancreatic cystic neoplasms: the clinical significance of diagnostic inaccuracy. Ann Surg Oncol. 2013;20:3112-3119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 31. | Marchegiani G, Caravati A, Andrianello S, Pollini T, Bernardi G, Biancotto M, Malleo G, Bassi C, Salvia R. Serous Cystic Neoplasms of the Pancreas Management in the Real-world: Still Operating on a Benign Entity. Ann Surg. 2022;276:e868-e875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 32. | Falqueto A, Pelandré GL, da Costa MZG, Nacif MS, Marchiori E. Prevalence of pancreatic cystic neoplasms on imaging exams: association with signs of malignancy risk. Radiol Bras. 2018;51:218-224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 33. | Zhao H, Xu Q, Gao R, Yin B, Sun G, Xue K, Yang Y, Li E, Zhu L, Feng F, Wu W. Clinical Feasibility of 5.0 T MRI/MRCP in Characterizing Pancreatic Cystic Lesions: Comparison with 3.0 T and MDCT. Diagnostics (Basel). 2024;14:2457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 34. | Lee SW, Shim SR, Jeong SY, Kim SJ. Comparison of Preoperative Imaging Modalities for the Assessment of Malignant Potential of Pancreatic Cystic Lesions: A Network Meta-analysis. Clin Nucl Med. 2022;47:849-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 35. | Okasha HH, Awad A, El-Meligui A, Ezzat R, Aboubakr A, AbouElenin S, El-Husseiny R, Alzamzamy A. Cystic pancreatic lesions, the endless dilemma. World J Gastroenterol. 2021;27:2664-2680. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 36. | Krishna SG, Hart PA, DeWitt JM, DiMaio CJ, Kongkam P, Napoleon B, Othman MO, Yew Tan DM, Strobel SG, Stanich PP, Patel A, Luthra AK, Chan MQ, Blaszczak AM, Lee D, El-Dika S, McCarthy ST, Walker JP, Arnold CA, Porter K, Conwell DL. EUS-guided confocal laser endomicroscopy: prediction of dysplasia in intraductal papillary mucinous neoplasms (with video). Gastrointest Endosc. 2020;91:551-563.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 37. | Hanada K, Shimizu A, Kurihara K, Ikeda M, Yamamoto T, Okuda Y, Tazuma S. Endoscopic approach in the diagnosis of high-grade pancreatic intraepithelial neoplasia. Dig Endosc. 2022;34:927-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 38. | Yamaguchi K, Tanaka M. Radiologic imagings of cystic neoplasms of the pancreas. Pancreatology. 2001;1:633-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 39. | Avila N, Tarnasky P, Kedia P. Use of direct cholangiopancreatoscopy to identify pancreaticobiliary fistula. Endoscopy. 2019;51:E380-E381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 40. | Zhao S, Xia T, Li ZS, Bai Y. Spyglass-guided pancreatic stent placement for intraductal papillary mucinous neoplasm with recurrent pancreatitis. Dig Liver Dis. 2018;50:513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 41. | De Luca L, Repici A, Koçollari A, Auriemma F, Bianchetti M, Mangiavillano B. Pancreatoscopy: An update. World J Gastrointest Endosc. 2019;11:22-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 42. | Keczer B, Benke M, Marjai T, Horváth M, Miheller P, Szücs Á, Harsányi L, Szijártó A, Hritz I. Quantitative Software Analysis of Endoscopic Ultrasound Images of Pancreatic Cystic Lesions. Diagnostics (Basel). 2022;12:2105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 43. | Ohno E, Hirooka Y, Kawashima H, Ishikawa T, Fujishiro M. Endoscopic ultrasonography for the evaluation of pancreatic cystic neoplasms. J Med Ultrason (2001). 2020;47:401-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 44. | Giannone F, Crippa S, Belfiori G, Partelli S, Tamburrino D, Pecorelli N, Longo E, Lena M, Palumbo D, Arcidiacono P, Falconi M. A Pancreatic Cystic Neoplasms Drama. Accuracy of Diagnostic Presumption and Surgical Indication on a Ten-year Experience. Pancreatology. 2021;21:S49. [DOI] [Full Text] |
| 45. | Kamata K, Kitano M. Endoscopic diagnosis of cystic lesions of the pancreas. Dig Endosc. 2019;31:5-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 46. | Lisotti A, Napoleon B, Facciorusso A, Cominardi A, Crinò SF, Brighi N, Gincul R, Kitano M, Yamashita Y, Marchegiani G, Fusaroli P. Contrast-enhanced EUS for the characterization of mural nodules within pancreatic cystic neoplasms: systematic review and meta-analysis. Gastrointest Endosc. 2021;94:881-889.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 47. | Yamashita Y, Shimokawa T, Ashida R, Napoléon B, Lisotti A, Fusaroli P, Gincul R, Dietrich CF, Omoto S, Kitano M. Comparison of endoscopic ultrasonography with and without contrast enhancement for characterization of pancreatic tumors: a meta-analysis. Endosc Int Open. 2022;10:E369-E377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 48. | Du C, Chai N, Linghu E, Li H, Feng X, Wang X, Tang P. Diagnostic value of SpyGlass for pancreatic cystic lesions: comparison of EUS-guided fine-needle aspiration and EUS-guided fine-needle aspiration combined with SpyGlass. Surg Endosc. 2022;36:904-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 49. | Krishna S, Abdelbaki A, Hart PA, Machicado JD. Endoscopic Ultrasound-Guided Needle-Based Confocal Endomicroscopy as a Diagnostic Imaging Biomarker for Intraductal Papillary Mucinous Neoplasms. Cancers (Basel). 2024;16:1238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 50. | Luthra AK, Arnold CA, Manilchuk AV, Krishna SG. The spectrum of confocal endomicroscopy findings in a cystic neuroendocrine tumor of the pancreas. Endoscopy. 2018;50:E323-E324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 51. | Feng Y, Chang X, Zhao Y, Wu D, Meng Z, Wu X, Guo T, Jiang Q, Zhang S, Wang Q, Yang A. A new needle-based confocal laser endomicroscopy pattern of malignant pancreatic mucinous cystic lesions (with video). Endosc Ultrasound. 2021;10:200-206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 52. | Ding C, Yang JF, Wang X, Zhou YF, Gu Y, Liu Q, Shen HZ, Zhang XF. Diagnostic yield of endoscopic ultrasound-guided fine-needle aspiration-based cytology for distinguishing malignant and benign pancreatic cystic lesions: A systematic review and meta-analysis. PLoS One. 2025;20:e0314825. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 53. | Yuan Z, Li J, Yang L, Shi Y, Luo Y. The differential diagnosis of pancreatic cystic neoplasms with conventional ultrasound and contrast-enhanced ultrasound. Quant Imaging Med Surg. 2024;14:4304-4318. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 54. | Faias S, Pereira L, Fonseca R, Chaves P, Dias Pereira A, Cravo M. A second endoscopic ultrasound with fine-needle aspiration for cytology identifies high-risk pancreatic cysts overlooked by current guidelines. Diagn Cytopathol. 2021;49:109-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 55. | Zhong L, Chai N, Linghu E, Li H, Yang J, Tang P. A prospective study on endoscopic ultrasound for the differential diagnosis of serous cystic neoplasms and mucinous cystic neoplasms. BMC Gastroenterol. 2019;19:127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 56. | Zhang XL, Chen K, He YP, Yang XJ, Liu JQ. Reassessment of EUS features in preoperative diagnosis of pancreatic serous cystic neoplasm: Lessons to avoid misdiagnosis. J Dig Dis. 2024;25:463-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 57. | Cheng B, Du C, He Z, Feng X, Li H, Wang Z, Gao F, Zhao Y, Chai N, Linghu E. Value of EUS-guided through-the-needle biopsy in the diagnosis of pancreatic cystic neoplasms: An 8-year experience. Endosc Ultrasound. 2024;13:345-350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 58. | Carr RA, Yip-Schneider MT, Simpson RE, Dolejs S, Schneider JG, Wu H, Ceppa EP, Park W, Schmidt CM. Pancreatic cyst fluid glucose: rapid, inexpensive, and accurate diagnosis of mucinous pancreatic cysts. Surgery. 2018;163:600-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 59. | van der Waaij LA, van Dullemen HM, Porte RJ. Cyst fluid analysis in the differential diagnosis of pancreatic cystic lesions: a pooled analysis. Gastrointest Endosc. 2005;62:383-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 456] [Cited by in RCA: 393] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 60. | Li SY, Wang ZJ, Pan CY, Wu C, Li ZS, Jin ZD, Wang KX. Comparative Performance of Endoscopic Ultrasound-Based Techniques in Patients With Pancreatic Cystic Lesions: A Network Meta-Analysis. Am J Gastroenterol. 2023;118:243-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 61. | Olar MP, Bolboacă SD, Pojoga C, Moșteanu O, Gheorghiu M, Seicean R, Rusu I, Sparchez Z, Al Hajjar N, Seicean A. Clinical Utility of the Contrast-Enhanced Endoscopic Ultrasound Guided Fine Needle Aspiration in the Diagnosis of Pancreatic Cyst. Diagnostics (Basel). 2022;12:2209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 62. | Li H, Feng X, Gao F, Chen Q, Linghu E. Diagnostic value of EUS-guided SF6 pancreatography for pancreatic cystic lesions on cyst communication with the pancreatic duct. Endosc Ultrasound. 2023;12:245-250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 63. | Du C, He Z, Gao F, Li L, Han K, Feng X, Wang X, Tang P, Chai N, Linghu E. Factors affecting the diagnostic value of liquid-based cytology by EUS-FNA in the diagnosis of pancreatic cystic neoplasms. Endosc Ultrasound. 2024;13:94-99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 64. | Vilas-Boas F, Ribeiro T, Macedo G, Dhar J, Samanta J, Sina S, Manfrin E, Facciorusso A, Conti Bellocchi MC, De Pretis N, Frulloni L, Crinò SF. Endoscopic Ultrasound-Guided Through-the-Needle Biopsy: A Narrative Review of the Technique and Its Emerging Role in Pancreatic Cyst Diagnosis. Diagnostics (Basel). 2024;14:1587. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 65. | Tacelli M, Celsa C, Magro B, Barchiesi M, Barresi L, Capurso G, Arcidiacono PG, Cammà C, Crinò SF. Diagnostic performance of endoscopic ultrasound through-the-needle microforceps biopsy of pancreatic cystic lesions: Systematic review with meta-analysis. Dig Endosc. 2020;32:1018-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 71] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 66. | Giannone F, Crippa S, Aleotti F, Palumbo D, Belfiori G, Partelli S, Schiavo Lena M, Capurso G, Petrone MC, De Cobelli F, Arcidiacono PG, Falconi M. Improving diagnostic accuracy and appropriate indications for surgery in pancreatic cystic neoplasms: the role of EUS. Gastrointest Endosc. 2022;96:648-656.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 67. | Kane LE, Mellotte GS, Conlon KC, Ryan BM, Maher SG. Multi-Omic Biomarkers as Potential Tools for the Characterisation of Pancreatic Cystic Lesions and Cancer: Innovative Patient Data Integration. Cancers (Basel). 2021;13:769. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 68. | Laquière AE, Lagarde A, Napoléon B, Bourdariat R, Atkinson A, Donatelli G, Pol B, Lecomte L, Curel L, Urena-Campos R, Helbert T, Valantin V, Mithieux F, Buono JP, Grandval P, Olschwang S. Genomic profile concordance between pancreatic cyst fluid and neoplastic tissue. World J Gastroenterol. 2019;25:5530-5542. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 69. | Singhi AD, McGrath K, Brand RE, Khalid A, Zeh HJ, Chennat JS, Fasanella KE, Papachristou GI, Slivka A, Bartlett DL, Dasyam AK, Hogg M, Lee KK, Marsh JW, Monaco SE, Ohori NP, Pingpank JF, Tsung A, Zureikat AH, Wald AI, Nikiforova MN. Preoperative next-generation sequencing of pancreatic cyst fluid is highly accurate in cyst classification and detection of advanced neoplasia. Gut. 2018;67:2131-2141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 210] [Cited by in RCA: 290] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 70. | Seyithanoglu D, Durak G, Keles E, Medetalibeyoglu A, Hong Z, Zhang Z, Taktak YB, Cebeci T, Tiwari P, Velichko YS, Yazici C, Tirkes T, Miller FH, Keswani RN, Spampinato C, Wallace MB, Bagci U. Advances for Managing Pancreatic Cystic Lesions: Integrating Imaging and AI Innovations. Cancers (Basel). 2024;16:4268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 71. | Jiang J, Chao WL, Cao T, Culp S, Napoléon B, El-Dika S, Machicado JD, Pannala R, Mok S, Luthra AK, Akshintala VS, Muniraj T, Krishna SG. Improving Pancreatic Cyst Management: Artificial Intelligence-Powered Prediction of Advanced Neoplasms through Endoscopic Ultrasound-Guided Confocal Endomicroscopy. Biomimetics (Basel). 2023;8:496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 72. | D'Haese JG, Werner J. Surgery of Cystic Tumors of the Pancreas - Why, When, and How? Visc Med. 2018;34:206-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 73. | Dhani H, Hinestrosa JP, Izaguirre-Carbonell J, Balcer HI, Kurzrock R, Billings PR. Case Report: Early detection of pancreatic pre-cancer lesion in multimodal approach with exosome liquid biopsy. Front Oncol. 2023;13:1170513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 74. | Salvia R, Marchegiani G. Evolving management of pancreatic cystic neoplasms. Br J Surg. 2020;107:1393-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 75. | Keane MG, Shamali A, Nilsson LN, Antila A, Millastre Bocos J, Marijinissen Van Zanten M, Verdejo Gil C, Maisonneuve P, Vaalavuo Y, Hoskins T, Robinson S, Ceyhan GO, Abu Hilal M, Pereira SP, Laukkarinen J, Del Chiaro M. Risk of malignancy in resected pancreatic mucinous cystic neoplasms. Br J Surg. 2018;105:439-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 76. | Chaudhari VA, Pradeep R, Ramesh H, Bhandare MS, Dhar P, Pal S, Palaniswamy S, Jeswanth S, Menon RN, Singh AN, Sabnis S, Rao GV, Shrikhande SV. Surgery for cystic tumors of pancreas: Report of high-volume, multicenter Indian experience over a decade. Surgery. 2019;166:1011-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 77. | Mortimer M, Kambal A, Shingler G, Al-Sarireh B. The Burden of Pancreatic Cystic Neoplasms on a Regional MDT. HPB. 2021;23:S899. [DOI] [Full Text] |
| 78. | Yoon JH, Cho IR, Chang W, Kim B, Jang S, Kim YY, Kim JW, Lee SH, Lee JM. Survey of Experts' Opinions on the Diagnosis and Management of Pancreatic Cystic Neoplasms. Korean J Radiol. 2024;25:1047-1060. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 79. | Abdelkader A, Hunt B, Hartley CP, Panarelli NC, Giorgadze T. Cystic Lesions of the Pancreas: Differential Diagnosis and Cytologic-Histologic Correlation. Arch Pathol Lab Med. 2020;144:47-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (1)] |
| 80. | Faias S, Pereira L, Luís Â, Chaves P, Cravo M. Genetic testing vs microforceps biopsy in pancreatic cysts: Systematic review and meta-analysis. World J Gastroenterol. 2019;25:3450-3467. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 81. | Yasrab M, Kwak SJ, Khoshpouri P, Fishman EK, Zaheer A. Misdiagnosis of pancreatic intraductal papillary mucinous neoplasms and the challenge of mimicking lesions: imaging diagnosis and differentiation strategies. Abdom Radiol (NY). 2025;50:2241-2257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 82. | Beger HG. Benign Tumors of the Pancreas-Radical Surgery Versus Parenchyma-Sparing Local Resection-the Challenge Facing Surgeons. J Gastrointest Surg. 2018;22:562-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 84. | Du C, Chai NL, Linghu EQ, Li HK, Feng XX. Endoscopic ultrasound-guided injective ablative treatment of pancreatic cystic neoplasms. World J Gastroenterol. 2020;26:3213-3224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 85. | Vargas A, Dutta P, Carpenter ES, Machicado JD. Endoscopic Ultrasound-Guided Ablation of Premalignant Pancreatic Cysts and Pancreatic Cancer. Diagnostics (Basel). 2024;14:564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 86. | Khoury T, Sbeit W, Napoléon B. Endoscopic ultrasound guided radiofrequency ablation for pancreatic tumors: A critical review focusing on safety, efficacy and controversies. World J Gastroenterol. 2023;29:157-170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 87. | Barthet M, Giovannini M, Lesavre N, Boustiere C, Napoleon B, Koch S, Gasmi M, Vanbiervliet G, Gonzalez JM. Endoscopic ultrasound-guided radiofrequency ablation for pancreatic neuroendocrine tumors and pancreatic cystic neoplasms: a prospective multicenter study. Endoscopy. 2019;51:836-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 148] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 88. | Schepis T, Tringali A, D'aversa F, Perri V, Familiari P, Boškoski I, Nista EC, Costamagna G. Endoscopic pancreatic sphincterotomy in patients with IPMN-related recurrent pancreatitis: A single center experience. Dig Liver Dis. 2023;55:107-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 89. | Qing Q, Deng X, Deng X, Mou T, Li B, Tan Y, Wu Q. A single-center study examining the safety and effectiveness of ERCP with pancreatoscopy and endoluminal radiofrequency ablation for main-duct IPMN treatment. Sci Rep. 2025;15:5420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 90. | Gonzalez JM, Lorenzo D, Ratone JP, Culetto A, Maire F, Levy P, Giovannini M, Barthet M. Pancreatic sphincterotomy improves pain symptoms due to branch-duct intrapapillary mucinous neoplasia without worrisome features: a multicenter study. Endosc Int Open. 2019;7:E1130-E1134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 91. | Ren X, Zhu CL, Qin XF, Jiang H, Xia T, Qu YP. Co-occurrence of IPMN and malignant IPNB complicated by a pancreatobiliary fistula: A case report and review of the literature. World J Clin Cases. 2019;7:102-108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 92. | Hipp J, Mohamed S, Pott J, Sick O, Makowiec F, Hopt UT, Fichtner-Feigl S, Wittel UA. Management and outcomes of intraductal papillary mucinous neoplasms. BJS Open. 2019;3:490-499. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 93. | Rogowska J, Semeradt J, Durko Ł, Małecka-Wojciesko E. Diagnostics and Management of Pancreatic Cystic Lesions-New Techniques and Guidelines. J Clin Med. 2024;13:4644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 94. | Shimizuguchi R, Kikuyama M, Kamisawa T, Kuruma S, Chiba K. Acute obstructive suppurative pancreatic ductitis in pancreatic malignancies. Endosc Int Open. 2020;8:E1765-E1768. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 95. | García-Alonso FJ, Peñas-Herrero I, Sanchez-Ocana R, Villarroel M, Cimavilla M, Bazaga S, De Benito Sanz M, Gil-Simon P, de la Serna-Higuera C, Perez-Miranda M. The role of endoscopic ultrasound guidance for biliary and pancreatic duct access and drainage to overcome the limitations of ERCP: a retrospective evaluation. Endoscopy. 2021;53:691-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 96. | DeWitt JM, Al-Haddad M, Sherman S, LeBlanc J, Schmidt CM, Sandrasegaran K, Finkelstein SD. Alterations in cyst fluid genetics following endoscopic ultrasound-guided pancreatic cyst ablation with ethanol and paclitaxel. Endoscopy. 2014;46:457-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 97. | Kim KH, McGreevy K, La Fortune K, Cramer H, DeWitt J. Sonographic and cyst fluid cytologic changes after EUS-guided pancreatic cyst ablation. Gastrointest Endosc. 2017;85:1233-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 98. | Cho SH, Seo DW, Oh D, Song TJ, Lee SK. Long-Term Outcomes of Endoscopic Ultrasound-Guided Ablation Vs Surgery for Pancreatic Cystic Tumors. Clin Gastroenterol Hepatol. 2024;22:1628-1636.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 99. | Choi JH, Seo DW, Song TJ, Park DH, Lee SS, Lee SK, Kim MH. Long-term outcomes after endoscopic ultrasound-guided ablation of pancreatic cysts. Endoscopy. 2017;49:866-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 69] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 100. | Papaefthymiou A, Johnson GJ, Maida M, Gkolfakis P, Ramai D, Facciorusso A, Arvanitakis M, Ney A, Fusai GK, Saftoiu A, Tabacelia D, Phillpotts S, Chapman MH, Webster GJ, Pereira SP. Performance and Safety of EUS Ablation Techniques for Pancreatic Cystic Lesions: A Systematic Review and Meta-Analysis. Cancers (Basel). 2023;15:2627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 101. | Ardeshna DR, Woods E, Tsung A, Krishna SG. An update on EUS-guided ablative techniques for pancreatic cystic lesions. Endosc Ultrasound. 2022;11:432-441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 102. | Moyer MT, Sharzehi S, Mathew A, Levenick JM, Headlee BD, Blandford JT, Heisey HD, Birkholz JH, Ancrile BB, Maranki JL, Gusani NJ, McGarrity TJ, Dye CE. The Safety and Efficacy of an Alcohol-Free Pancreatic Cyst Ablation Protocol. Gastroenterology. 2017;153:1295-1303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 103. | Saghir SM, Dhindsa BS, Daid SGS, Naga Y, Dhaliwal A, Mashiana HS, Bhogal N, Sayles H, Ramai D, Singh S, Bhat I, Rangray R, McDonough S, Adler DG. Safety and efficacy of EUS-guided ablation of pancreatic lesions with ethanol versus ethanol with paclitaxel: A systematic review and meta-analysis. Endosc Ultrasound. 2022;11:371-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 104. | Tacelli M, Partelli S, Falconi M, Arcidiacono PG, Capurso G. Pancreatic Neuroendocrine Neoplasms: Classification and Novel Role of Endoscopic Ultrasound in Diagnosis and Treatment Personalization. United European Gastroenterol J. 2025;13:34-43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 105. | Chavan R, Thosani N, Kothari S. Role of Endoscopic Ultrasound-Guided Radiofrequency Ablation in Pancreatic Lesions: Where Are We Now and What Does the Future Hold? Cancers (Basel). 2024;16:3662. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 106. | McCarty TR, Rustagi T. New Indications for Endoscopic Radiofrequency Ablation. Clin Gastroenterol Hepatol. 2018;16:1007-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 107. | Ardeshna DR, Leupold M, Cruz-Monserrate Z, Pawlik TM, Cloyd JM, Ejaz A, Shah H, Burlen J, Krishna SG. Advancements in Microwave Ablation Techniques for Managing Pancreatic Lesions. Life (Basel). 2023;13:2162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 108. | Testoni SGG, Healey AJ, Dietrich CF, Arcidiacono PG. Systematic review of endoscopy ultrasound-guided thermal ablation treatment for pancreatic cancer. Endosc Ultrasound. 2020;9:83-100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 109. | Wu J, Zhou Z, Huang Y, Deng X, Zheng S, He S, Huang G, Hu B, Shi M, Liao W, Huang N. Radiofrequency ablation: mechanisms and clinical applications. MedComm (2020). 2024;5:e746. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 110. | Oh D, Ko SW, Seo DW, Hong SM, Kim JH, Song TJ, Park DH, Lee SK, Kim MH. Endoscopic ultrasound-guided radiofrequency ablation of pancreatic microcystic serous cystic neoplasms: a retrospective study. Endoscopy. 2021;53:739-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 111. | Pai M, Habib N, Senturk H, Lakhtakia S, Reddy N, Cicinnati VR, Kaba I, Beckebaum S, Drymousis P, Kahaleh M, Brugge W. Endoscopic ultrasound guided radiofrequency ablation, for pancreatic cystic neoplasms and neuroendocrine tumors. World J Gastrointest Surg. 2015;7:52-59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 167] [Cited by in RCA: 173] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 112. | Hwang JS, Joo HD, Song TJ. Endoscopic Ultrasound-Guided Local Therapy for Pancreatic Neoplasms. Clin Endosc. 2020;53:535-540. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 113. | Kirkegård J, Aahlin EK, Al-Saiddi M, Bratlie SO, Coolsen M, de Haas RJ, den Dulk M, Fristrup C, Harrison EM, Mortensen MB, Nijkamp MW, Persson J, Søreide JA, Wigmore SJ, Wik T, Mortensen FV. Multicentre study of multidisciplinary team assessment of pancreatic cancer resectability and treatment allocation. Br J Surg. 2019;106:756-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 114. | Choi CC, Choi J, Houli N, Smith M, Usatoff V, Lipton L, Chan S. Evaluation of palliative treatments in unresectable pancreatic cancer. ANZ J Surg. 2021;91:915-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 115. | Alwahbi O, Ghumman Z, van der Pol CB, Patlas MN, Gopee-Ramanan P. Pancreatic Cystic Lesions: Review of the Current State of Diagnosis and Surveillance. Can Assoc Radiol J. 2023;74:557-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 116. | European Study Group on Cystic Tumours of the Pancreas. European evidence-based guidelines on pancreatic cystic neoplasms. Gut. 2018;67:789-804. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1144] [Cited by in RCA: 976] [Article Influence: 122.0] [Reference Citation Analysis (1)] |
| 117. | Ohtsuka T, Fernandez-Del Castillo C, Furukawa T, Hijioka S, Jang JY, Lennon AM, Miyasaka Y, Ohno E, Salvia R, Wolfgang CL, Wood LD. International evidence-based Kyoto guidelines for the management of intraductal papillary mucinous neoplasm of the pancreas. Pancreatology. 2024;24:255-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 246] [Article Influence: 123.0] [Reference Citation Analysis (1)] |
| 118. | Ardeshna DR, Cao T, Rodgers B, Onongaya C, Jones D, Chen W, Koay EJ, Krishna SG. Recent advances in the diagnostic evaluation of pancreatic cystic lesions. World J Gastroenterol. 2022;28:624-634. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (6)] |
| 119. | Cortesi PA, Tamburino D, Facchetti R, Micale M, Mantovani LG, Crippa S, Capurso G. Benefit and costs of surveillance on cancer risk patients: the example of pancreatic cystic tumors. Eur J Public Health. 2020;30. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 120. | Hu Y, Jones D, Esnakula AK, Krishna SG, Chen W. Molecular Pathology of Pancreatic Cystic Lesions with a Focus on Malignant Progression. Cancers (Basel). 2024;16:1183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/