Published online Sep 15, 2025. doi: 10.4251/wjgo.v17.i9.110245
Revised: June 24, 2025
Accepted: July 24, 2025
Published online: September 15, 2025
Processing time: 105 Days and 2.9 Hours
Peritoneal lavage cytology-positive (CY1) gastric cancer (stage IV) has a poor prognosis, though some cases fare better. Therefore, identifying prognostic factors and an optimal treatment strategy is crucial.
To investigate prognostic factors in patients with gastric cancer who underwent gastrectomy with CY1, and to evaluate the optimal postoperative chemotherapy regimen.
This multicenter retrospective cohort study analyzed prognostic factors and postoperative chemotherapy in patients with CY1 gastric cancer who underwent gastrectomy, excluding those with macroscopic peritoneal dissemination. Data from 13 institutions (2015-2019) were reviewed.
Overall, 82 patients met the inclusion criteria. The median overall survival was 22.8 months, and diffuse-type histology and the absence of postoperative chemotherapy were identified as independent poor prognostic factors. The 5-year survival rate was 82.4% for those receiving fluoropyrimidine plus docetaxel/oxaliplatin vs 21.8% for those with S-1 monotherapy or a cisplatin-based regimen. Median overall survival was not reached in the fluoropyrimidine + docetaxel/oxaliplatin group but was 22.9 months in the S-1/cisplatin group. Chemotherapy regimen was an independent prognostic factor (hazard ratio = 5.47, P = 0.004). The fluoropyrimidine plus docetaxel/oxaliplatin group had an average relative dose intensity of 82.1%, with significantly more patients achieving a relative dose intensity ≥ 80% than in the S-1 monotherapy or cisplatin-based group (P = 0.001).
Diffuse-type histology and the absence of postoperative chemotherapy influence the prognosis of patients with CY1 gastric cancer. Combination therapy with oxaliplatin or docetaxel may enhance the treatment intensity and improve survival outcomes after gastrectomy.
Core Tip: This multicenter retrospective cohort study identifies key prognostic factors in cytology-positive gastric cancer patients undergoing gastrectomy. Diffuse-type histology and absence of postoperative chemotherapy were independent poor prognostic indicators. Critically, combination chemotherapy with fluoropyrimidine plus docetaxel or oxaliplatin significantly improved survival, with a 5-year survival rate of 82.4% vs 21.8% for S-1 or cisplatin-based regimens. The chemotherapy regimen was an independent predictor of overall survival. These findings highlight the importance of intensive postoperative chemotherapy in improving outcomes for this high-risk population.
- Citation: Sugiyama Y, Tanabe K, Yanagawa S, Tazawa H, Toyota K, Kano M, Misumi T, Shishida M, Okano K, Hotta R, Ota H, Imaoka Y, Fukuda T, Takahashi S, Ohdan H. Prognostic factors and efficacy of postoperative chemotherapy in patients with gastric cancer with positive peritoneal cytology after gastrectomy. World J Gastrointest Oncol 2025; 17(9): 110245
- URL: https://www.wjgnet.com/1948-5204/full/v17/i9/110245.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i9.110245
Gastric cancer is the fifth most common malignancy worldwide, accounting for 23% of all gastric cancers in Eastern Asia[1]. In Japan, although the incidence rate has been declining recently, it remains the third and fourth most common cancers in men and women, respectively. Additionally, its mortality rate ranked third. Therefore, developing effective gastric cancer treatment strategies is crucial[2]. Although the mortality rate has decreased recently[3], this can be attributed to advancements in endoscopic techniques, which have led to an increase in early diagnosis. The development of novel anticancer agents and immune checkpoint inhibitors has improved treatment outcomes for unresectable or recurrent advanced gastric cancer. However, the reported median survival periods remained relatively poor, ranging from 12.9 to 13.8 months in the Checkmate649, Keynote849, and Toga trials[4-6].
Peritoneal lavage cytology-positive (CY1) gastric cancer frequently results in peritoneal dissemination recurrence and is defined as M1 (metastasis to distant organs) in the major tumor-node-metastasis classifications (Japanese Classification of Gastric Carcinoma, Union for International Cancer Control, American Joint Committee on Cancer), categorizing it as a stage IV disease with a poor prognosis. The reported frequency of positive peritoneal cytology results during surgery ranges from 4% to 41%[3], with higher positivity rates observed in advanced tumors and approximately 10% in T3 or deeper tumors[4]. CY1 is a stronger prognostic factor than tumor depth or lymph node metastasis and has been reported to be the most significant prognostic determinant[5].
Palliative chemotherapy remains the primary treatment for stage IV gastric cancer in patients with peritoneal dissemination. However, the treatment strategies for CY1 gastric cancer differ significantly. Recently, when peritoneal dissemination is suspected based on imaging studies, diagnostic laparoscopy has been increasingly performed to confirm peritoneal dissemination or CY1 status. If either is confirmed, chemotherapy is initiated, and surgery is considered if therapeutic effects are achieved. However, when surgery is performed first, CY1 can be identified intraoperatively or postoperatively. In such cases, gastrectomy is frequently performed in clinical practice. This approach is weakly recommended in the Japanese “Gastric Cancer Treatment Guidelines, 5th Edition”, when no other non-curative factors are present and is combined with postoperative chemotherapy using S-1. In contrast to other stage IV gastric cancers, CY1 gastric cancer can achieve treatment outcomes comparable to those of R0-resected gastric cancer through gastrectomy; however, sufficient evidence supporting this approach has not yet been established. Despite the use of combination regimens incorporating S-1 with oxaliplatin or docetaxel as postoperative adjuvant therapy for stage III gastric cancer following curative resection, a discrepancy remains in current clinical guidelines. These guidelines recommend S-1 monotherapy for postoperative chemotherapy in CY1 gastric cancer, which is classified as stage IV. Although combination therapy with these agents is also expected to improve treatment outcomes in CY1 gastric cancer, there is currently no supporting evidence. This study aimed to investigate the prognostic factors in patients with gastric cancer who underwent gastrectomy with CY1 as the sole non-curative factor and evaluate the optimal postoperative chemotherapy regimen in these individuals. This multicenter retrospective cohort study was conducted by collecting data from institutions affiliated with the Hiroshima Surgical Study of Clinical Oncology (HiSCO).
We analyzed data from patients with gastric cancer with CY1 as the only non-curative factor who underwent gastrectomy at 13 HiSCO-affiliated institutions from 2015 to 2019. Among them, we targeted patients who met the following selection criteria: (1) Age ≥ 20 years; (2) Eastern Cooperative Oncology Group performance status of 0-2; (3) Histologically confirmed gastric adenocarcinoma and underwent standard surgery, including lymph node dissection, with D1+ dissection allowed; (4) CY1 status confirmed by peritoneal cytology; and (5) No evidence of distant metastasis. Although a central review of peritoneal lavage cytology was not conducted, but, all participating institutions evaluated the cytological specimens using Papanicolaou staining by the 15th edition of the Japanese Classification of Gastric Carcinoma. In addition, cases with suspected false-positive findings were uniformly categorized as CY0. Patients with localized peritoneal metastasis (P1a) who underwent complete macroscopic resection were excluded from this study.
Patients who did not undergo lymph node dissection or receive preoperative chemotherapy were excluded. Moreover, at the time of the data cut-off, no patients in this study had received immune checkpoint inhibitors as part of postoperative chemotherapy, nor had any received hyperthermia therapy. In the subgroup analysis of patients who underwent postoperative chemotherapy, individuals for whom an initial efficacy assessment could not be performed were excluded from this study. This study protocol was approved by the Ethics Committee of JA Hiroshima General Hospital, No. 23-27, and the Ethical Guidelines for Life Science and Medical Research Involving Human Subjects. Data were collected from the HiSCO database.
The regimens and durations of postoperative chemotherapy were determined according to the protocols established at each institution. For S-1 monotherapy, the dosage and schedule were determined based on the ACTS-GC trial. Similarly, for S1 + cisplatin therapy, the SPIRIT trial served as a reference. The new combination chemotherapy (NC) groups received S-1 plus oxaliplatin (SOX), capecitabine plus oxaliplatin, or S-1 plus docetaxel. In cases of human epidermal growth factor receptor 2 (HER2)-positive tumors, combination therapy with trastuzumab was permitted for any of the doublet therapies. Patients who could not continue the doublet therapy for at least two courses and switched to S-1 monotherapy were allocated to the S-1 monotherapy group. The administration period continued until disease progression, the occurrence of adverse events, or the patient requested to discontinue treatment.
We analyzed the overall survival (OS) and progression-free survival (PFS) based on the presence or absence of chemotherapy and conducted a subgroup analysis comparing the conventional chemotherapy (CC) and NC groups. OS was defined as the time from the date of surgery to death from any cause, and the last follow-up date was set as the cutoff. PFS was defined as the time from the date of surgery to the earlier occurrence of recurrence or death. Patients who experienced no recurrence and were still alive were censored at the last follow-up date. The data cutoff date was December 31, 2024. OS and PFS were calculated using the Kaplan-Meier method, and between-group differences were assessed using the log-rank test. The median observation periods and corresponding 95% confidence intervals (CI) were calculated using the inverse Kaplan-Meier method.
To minimize intergroup bias, survival rates were evaluated using multivariate analysis with the Cox proportional hazards regression model, and hazard ratios (HR) and 95%CIs were reported. The covariates for multivariate analysis were examined using factors that showed significant differences in the univariate analysis and those considered clinically relevant to prognosis. The variables included in the multivariate analysis were analyzed using Schoenfeld residual plots to assess the time-varying effects of covariates, confirming that the proportional hazards assumption was satisfied. Categorical data comparisons were performed using Fisher’s exact test or the χ2 test. To address potential confounding between treatment groups, propensity scores were estimated using a logistic regression model. Inverse probability of treatment weighting (IPTW) was applied to balance covariates across treatment groups, and stabilized weights were used to avoid extreme values. A Cox proportional hazards model weighted by IPTW was used to evaluate differences in recurrence-free and OS rates. Kaplan-Meier survival curves were also generated using IPTW weights. Propensity score analysis was performed using R version 4.3.1 (R Foundation for Statistical Computing, Vienna, Austria), and other statistical analyses were performed using IBM SPSS Statistics for Windows, version 26.0 (IBM Corp., Armonk, NY, United States).
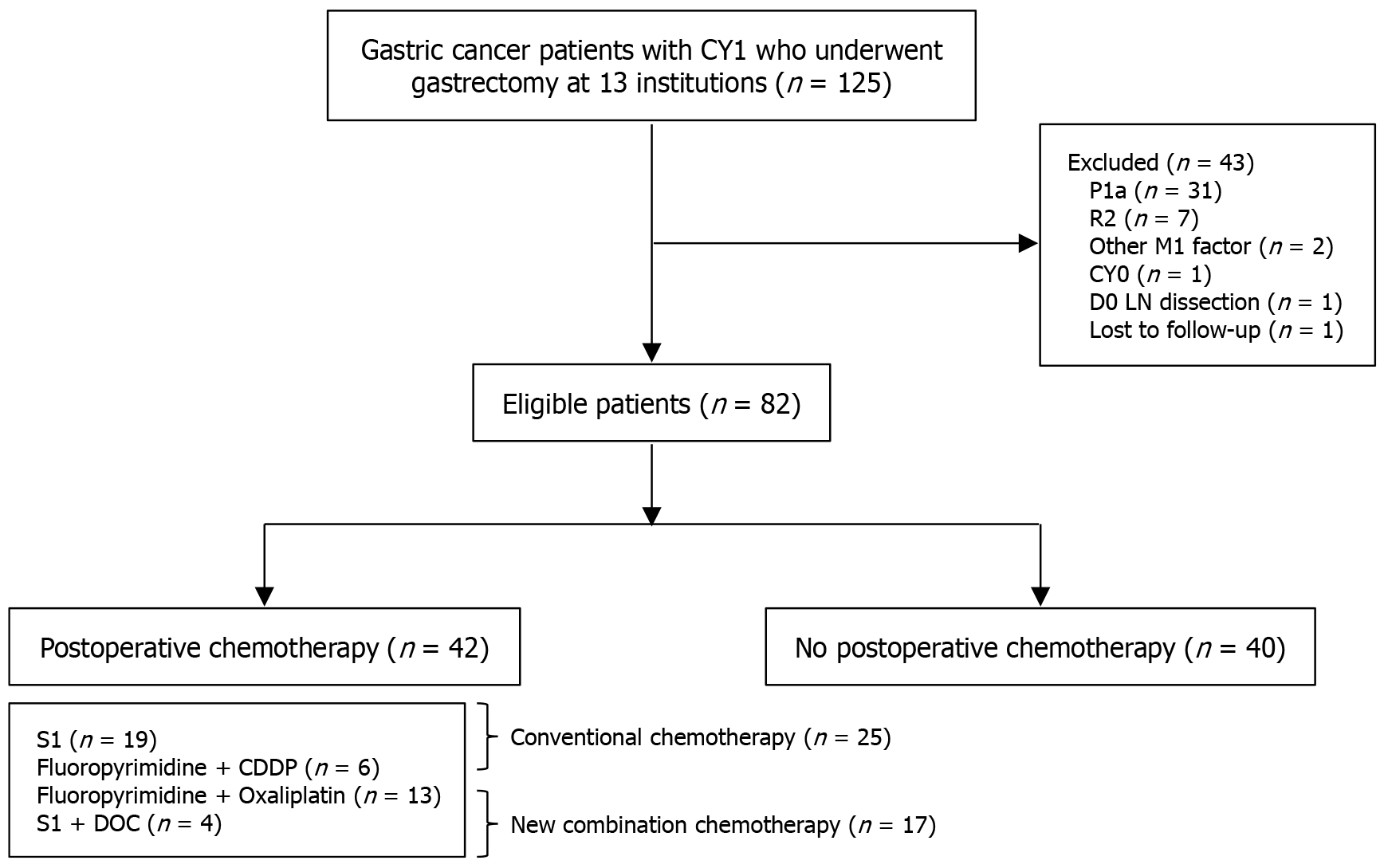
Overall, 125 cases were collected from 13 institutions in the Hiroshima Prefecture. Forty-three patients were excluded as they did not meet the eligibility criteria (macroscopically resected P1a cases, n = 31; R2 [residual tumor (gross/pathological) with peritoneal dissemination or positive margins in locally advanced disease] resection cases; macroscopic and/or pathological residual tumor due to peritoneal dissemination (P1a–c) or locally advanced disease with positive margins, n = 7; cases with other distant metastases, n = 2; CY0 cases, n = 1; cases without lymphadenectomy, n = 1; and cases without follow-up, n = 1). Among eligible patients, 42 underwent postoperative chemotherapy, whereas 40 did not. A CONSORT diagram, including the chemotherapy regimens, is shown in Figure 1.
The patient backgrounds and clinical characteristics are summarized in Table 1. The mean age was 74.3 years, and six patients had a performance status of 2. Macroscopic type 4 was observed in 22 (26.8%) patients. All patients had tumor invasion of T3 or deeper, and all but one case had lymph node metastasis. The most common surgical procedure was distal gastrectomy (43 patients), followed by total gastrectomy (37 patients, 45.1%). Regarding lymph node dissection, 21 (25.6%) patients underwent reduced D2 dissection, whereas 13 (15.9%) underwent extended dissection. Postoperative complications occurred in 22 (24.4%) patients. Approximately half of the patients did not receive postoperative chemotherapy. While these patients underwent surgical resection, adjuvant chemotherapy was not initiated owing to various factors including poor performance status, presence of comorbidities, impaired organ function, or individual preference of the patients.
| Variables | All (n = 82) |
| Age | 74.3 (42-96) |
| Sex | |
| Male | 56 |
| Female | 26 |
| Performance status (ECOG) | |
| 0 | 56 |
| 1 | 20 |
| 2 | 6 |
| Macroscopic type | |
| Non-type 4 | 60 |
| Type 4 | 22 |
| Preoperative CEA | |
| > ULN | 21 |
| ≤ ULN | 60 |
| Unknown | 1 |
| Preoperative CA19-9 | 1 |
| > ULN | 25 |
| ≤ ULN | 55 |
| Unknown | 2 |
| Pathological T factor | |
| T3 | 16 |
| T4a | 59 |
| T4b | 7 |
| Pathological N factor | |
| N0 | 1 |
| N1-2 | 35 |
| N3 | 46 |
| Histology | |
| Intestinal | 31 |
| Diffuse | 51 |
| HER2 status | |
| Positive | 14 |
| Negative | 46 |
| Unknown | 22 |
| Surgery | |
| Distal gastrectomy | 43 |
| Total gastrectomy | 37 |
| Proximal gastrectomy | 2 |
| Lymph node dissection | |
| < D2 | 21 |
| D2 | 48 |
| D2+ | 13 |
| Postoperative complication1 | |
| Yes (grade III) | 22 (5) |
| No | 60 |
| Postoperative CTx | |
| Yes | 42 |
| No | 40 |
During the follow-up period until the analysis cutoff point, recurrence was observed in 50 (40%) patients. Peritoneal dissemination was the most common recurrence type, occurring in 33 (68.8%) patients, followed by hematogenous in nine patients and lymphatic metastases in seven patients, and other types in one patient. The median PFS was 13.6 months, with a 5-year PFS rate of 26.5%. The median observation period was 71.6 months. Based on the analysis cutoff, 51 deaths were observed, including 42 cancer-related and 9 from other causes. There were 31 survivors, with a median OS (MST) of 22.8 months and a 5-year survival rate of 31.2%.
Univariate analysis of OS, age (< 75 years vs ≥ 75 years), gender (male vs female), histology (intestinal vs diffuse), and the presence or absence of postoperative chemotherapy were significantly associated with prognosis. We conducted a multivariate analysis using these factors. The results showed that histology and the presence or absence of postoperative chemotherapy were independent prognostic factors (Table 2). Similar findings were observed in the PFS analysis (data not shown).
| Variables | n | Univariate analysis | Multivariate analysis | ||
| HR (95%CI) | P value | HR (95%CI) | P value | ||
| Age (years) | |||||
| ≤ 75 | 41 | Reference | Reference | ||
| > 75 | 41 | 2.20 (1.25-3.87) | 0.006 | 1.81 (0.98-3.34) | 0.057 |
| Gender | |||||
| Male | 56 | Reference | Reference | ||
| Female | 26 | 1.79 (1.01-3.21) | 0.048 | 1.45 (0.79-2.69) | 0.233 |
| Performance status (ECOG) | |||||
| 0, 1 | 76 | Reference | |||
| 2 | 6 | 1.03 (0.32-3.33) | 0.955 | ||
| BMI (kg/m2) | |||||
| < 25 | 73 | Reference | |||
| ≥ 25 | 8 | 1.06 (0.42-2.67) | 0.900 | ||
| Preoperative CEA | |||||
| ≤ ULN | 60 | Reference | |||
| > ULN | 21 | 1.50 (0.81-2.80) | 0.201 | ||
| Preoperative CA19-9 | |||||
| ≤ ULN | 55 | Reference | |||
| > ULN | 25 | 0.81 (0.42-1.56) | 0.527 | ||
| Surgery | |||||
| DG, PG | 45 | Reference | |||
| TG | 37 | 1.21 (0.70-2.10) | 0.504 | ||
| Lymph node dissection | |||||
| ≥ D2 | 61 | Reference | |||
| < D2 | 21 | 1.36 (0.71-2.62) | 0.354 | ||
| Postoperative complication | |||||
| No | 60 | Reference | |||
| Yes | 22 | 1.47 (0.82-2.63) | 0.200 | ||
| Macroscopic type | |||||
| Non-type 4 | 60 | Reference | |||
| Type 4 | 22 | 1.63 (0.90-2.95) | 0.107 | ||
| Histology | |||||
| Intestinal | 31 | Reference | Reference | ||
| Diffuse | 51 | 1.89 (1.05-3.39) | 0.034 | 2.65 (1.43-4.92) | 0.002 |
| pT | |||||
| T3 | 16 | Reference | |||
| T4 | 66 | 1.14 (0.53-2.42) | 0.743 | ||
| pN | |||||
| N0-2 | 36 | Reference | |||
| N3 | 46 | 1.08 (0.62-1.88) | 0.785 | ||
| Postoperative CTx | |||||
| Yes | 42 | Reference | Reference | ||
| No | 40 | 3.24 (1.82-5.78) | 0.001 | 3.26 (1.75-6.08) | 0.001 |
A subgroup analysis was conducted on 42 patients with CY1 gastric cancer who underwent gastrectomy followed by postoperative chemotherapy. The chemotherapy regimens administered included S-1 monotherapy, fluoropyrimidine plus cisplatin, fluoropyrimidine plus oxaliplatin, and S-1 plus docetaxel in 19, 6, 13, and 4 patients, respectively (Figure 1). The S-1 monotherapy and fluoropyrimidine plus cisplatin regimens were classified as the CC group, while the S-1 plus docetaxel and fluoropyrimidine plus oxaliplatin regimens were classified as the NC group. A comparative analysis was performed between the two groups, and their characteristics are summarized in Table 3. Although the mean age was ≤ 75 years in both groups, the CC group tended to be slightly older. No significant differences were observed between the two groups regarding clinical factors. Changes in chemotherapy regimens over time are shown in Figure 2. Initially, CC was predominantly used; however, from 2016 onward, NC was introduced, and since 2018, most patients have been treated with NC.
| Conventional chemotherapy1 (n = 25) | New combination chemotherapy2 (n = 17) | P value | |
| Age (years) | 72 (42-87) | 66.2 (45-76) | 0.073 |
| Gender | 0.589 | ||
| Male | 20 (80) | 14 (82) | |
| Female | 5 (20) | 3 (18) | |
| Performance status (ECOG) | 0.595 | ||
| 0, 1 | 24 (96) | 17 (100) | |
| 2 | 1 (4) | 0 (0) | |
| Preoperative BMI (kg/m2) | 21.1 (17.5-27.7) | 21.7 (17.3-27.5) | 0.531 |
| CEA | 0.437 | ||
| ≤ ULN | 20 (83) | 13 (76) | |
| > ULN | 4 (7) | 4 (24) | |
| CA19-9 | 0.565 | ||
| ≤ ULN | 19 (79) | 13 (76) | |
| > ULN | 5 (21) | 4 (24) | |
| Surgery | 0.175 | ||
| DG, PG | 17 (68) | 8 (47) | |
| TG | 8 (32) | 9 (53) | |
| Lymph node dissection | 0.397 | ||
| ≥ D2 | 20 (80) | 15 (88) | |
| < D2 | 5 (20) | 2 (12) | |
| Postoperative complication | 0.589 | ||
| No | 20 (80) | 14 (82) | |
| Yes | 5 (20) | 3 (18) | |
| Macroscopic type | 0.173 | ||
| Non-type 4 | 9 (36) | 3 (18) | |
| Type 4 | 16 (64) | 14 (82) | |
| Histology | 0.542 | ||
| Intestinal | 8 (32) | 7 (41) | |
| Diffuse | 17 (68) | 10 (59) | |
| pT | 0.463 | ||
| T3 | 6 (24) | 3 (18) | |
| T4 | 19 (76) | 14 (82) | |
| pN | 0.845 | ||
| N0-2 | 11 (44) | 8 (47) | |
| N3 | 14 (56) | 9 (53) | |
| HER2 status | 0.346 | ||
| Positive | 16 | 8 | |
| Negative | 6 | 4 | |
| Unknown | 3 | 5 | |
| BW decrease rate3 | 0.465 | ||
| < 10% | 18 (81) | 15 (88) | |
| ≥ 10% | 4 (19) | 2 (12) | |
| Pre-chemotherapy BMI (kg/m2) | 19.9 (15.1-26.3) | 20.7 (16.0-25.5) | 0.377 |
| Ccr (mL/minute) | 0.174 | ||
| < 60 | 14 (56) | 13 (82) | |
| ≥ 60 | 11 (44) | 4 (18) | |
| Pre-chemotherapy CEA | 0.534 | ||
| ≤ ULN | 21 (84) | 15 (88) | |
| > ULN | 4 (16) | 2 (12) | |
| Pre-chemotherapy CA19-9 | 0.630 | ||
| ≤ ULN | 22 (92) | 16 (94) | |
| > ULN | 2 (8) | 1 (6) |
The median observation period for PFS and OS was 77.5 and 68.0 months, respectively. During the observation period, 20 (80%) cases of recurrence were noted in the CC group, with 20 (80%) cases of peritoneal recurrence. In the NC group, there were five (29.4%) cases of recurrence, with three (17.6%) cases of peritoneal recurrence.
The 5-year PFS rate was 16.0% in the CC group, whereas it was 76.5% in the NC group. The median PFS was 10.8 months in the CC group and 90.4 months in the NC group, demonstrating a significant improved outcomes in the NC group (HR = 4.30, 95%CI: 1.71-10.82, P = 0.002; Figure 3A. Moreover, early postoperative recurrence within one year occurred in 15 of 25 patients in the CC group, compared to only 2 patients in the NC group. The MST in the CC group was 22.9 months with a 5-year survival rate of 21.8%. In contrast, in the NC group, the MST was not reached, and the 5-year survival rate was 82.4%, demonstrating significantly improved outcomes in the NC group (HR = 5.34, 95%CI: 1.77-16.14, P = 0.003; Figure 3B). Incorporating baseline covariates, including: Age at treatment initiation, performance status, presence of type 4 disease, and the year of postoperative treatment initiation, we estimated propensity scores using a logistic regression model. After IPTW adjustment, the new treatment group demonstrated significantly better PFS and OS rates than the conventional treatment group (HR = 0.10, 95%CI: 0.03-0.31, P < 0.001; HR = 0.09, 95%CI: 0.03-0.31, P < 0.002, respectively). The concordance index (C-index) of the IPTW-weighted model was 0.75, indicating its good discriminative ability. The weighted Kaplan-Meier curve also revealed a marked survival advantage in the new treatment group (Figure 3C and D). The concordance index of the IPTW-weighted Cox model was 0.75, indicating good predictive discrimination. In the multivariate analysis of OS, macroscopic type 4, presence of postoperative NC, and duration of postoperative chemotherapy were independent prognostic factors (Table 4).
| Variables | n | Univariate analysis | Multivariate analysis | ||
| HR (95%CI) | P value | HR (95%CI) | P value | ||
| Age (years) | |||||
| ≤ 75 | 29 | Reference | |||
| > 75 | 13 | 1.39 (0.58-3.33) | 0.460 | ||
| Gender | |||||
| Male | 34 | Reference | |||
| Female | 8 | 1.55 (0.57-4.21) | 0.395 | ||
| Performance status (ECOG) | |||||
| 0, 1 | 41 | Reference | |||
| 2 | 1 | 1.82 (0.24-13.69) | 0.562 | ||
| Pre-chemotherapy BMI (kg/m2) | |||||
| ≥ 25 | 2 | Reference | |||
| < 25 | 37 | 1.21 (0.16-9.04) | 0.854 | ||
| Pre-chemotherapy CEA | |||||
| ≤ ULN | 36 | Reference | |||
| > ULN | 6 | 1.46 (0.49-4.30) | 0.498 | ||
| Pre-chemotherapy CA19-9 | |||||
| ≤ ULN | 38 | Reference | |||
| > ULN | 3 | 1.53 (0.36-6.60) | 0.566 | ||
| Surgery | |||||
| DG, PG | 25 | Reference | |||
| TG | 17 | 1.21 (0.52-2.82) | 0.667 | ||
| Lymph node dissection | |||||
| ≥ D2 | 35 | Reference | |||
| < D2 | 7 | 1.09(0.37-3.22) | 0.883 | ||
| Postoperative complication | |||||
| No | 34 | Reference | |||
| Yes | 8 | 1.18 (0.43-3.21) | 0.746 | ||
| Macroscopic type | |||||
| Non-type 4 | 14 | Reference | Reference | ||
| Type 4 | 28 | 2.17 (0.92-5.09) | 0.076 | 5.00 (1.76-14.25) | 0.003 |
| Histology | |||||
| Intestinal | 15 | Reference | |||
| Diffuse | 27 | 1.78 (0.72-4.38) | 0.210 | ||
| pT | |||||
| T3 | 9 | Reference | |||
| T4 | 33 | 1.86 (0.55-6.33) | 0.318 | ||
| pN | |||||
| N0-2 | 19 | Reference | |||
| N3 | 23 | 0.98 (0.42-2.27) | 0.964 | ||
| HER2 status | |||||
| Positive | 10 | Reference | |||
| Negative | 24 | 2.09 (0.69-6.39) | 0.194 | ||
| Postoperative CTx | |||||
| New combination chemotherapy | 17 | Reference | Reference | ||
| Conventional chemotherapy | 25 | 5.34 (1.77-16.14) | 0.003 | 9.53 (2.54-35.79) | < 0.001 |
| Duration of postoperative CTx | |||||
| ≥ 1 year | 16 | Reference | Reference | ||
| < 1 year | 26 | 12.75 (2.98-55.55) | < 0.001 | 18.98 (3.68-97.93) | < 0.001 |
| Ccr (mL/minute) | |||||
| ≥ 60 | 27 | Reference | |||
| < 60 | 15 | 1.08 (0.45-2.61) | 0.862 | ||
| Grade III adverse event1 | |||||
| No | 22 | Reference | |||
| Yes | 20 | 0.64 (0.27-1.55) | 0.326 | ||
The details of chemotherapy are outlined in Table 5. The average time from surgery to chemotherapy initiation was within 6 weeks in both groups. The time to treatment failure was longer in the NC group (17.0 months) than in the CC group (12.6 months), although this difference was not statistically significant. Regarding adverse events, grade III or higher events were observed significantly more frequently in the NC group (71%) than in the CC group (32%). The reasons for discontinuing chemotherapy in the CC group included recurrence and adverse events in 15 and 4 patients, respectively. In the NC group, recurrence was the reason for discontinuation in four patients and adverse events in another four. Post-recurrence treatments were administered to 10 of the 15 patients in the CC group and two of the four patients in the NC group, with no significant difference between the groups. Regarding the relative dose intensity (RDI) of the infusions, the NC group maintained a higher intensity (82.1%) than the CC group (74.5%), with an approximately 8% difference. Furthermore, the NC group had a significantly higher proportion of patients maintaining over 80% RDI (58.8%) than the CC group (33.3%).
| Conventional chemotherapy (n = 25) | New combination chemotherapy (n = 17) | P value | |
| TI until the postoperative CTx (week)1 | 5.1 (1.9-9.1) | 5.1 (1.4-10.6) | 0.908 |
| TTF (months) | |||
| Oral drug | 12.6(1.6-49.0) | 17.0 (3.0-45.8) | 0.129 |
| Intravenous | 5.3 (1.2-8.6) | 5.4 (2.1-11.4) | 0.946 |
| Course: Median (range) | |||
| Intravenous | 3.6 (1-5) | 6.2 (2-15) | 0.098 |
| RDI: Median (%) | |||
| Intravenous | 74.5 (54.5-100) | 82.1 (60.9-100) | 0.276 |
| < 80%: > 80% | 4:2 | 7:10 | 0.001 |
| Adverse event (grade III)2 | 8 (32%) | 17 (71%) | 0.014 |
| Reason for CTx discontinuation | |||
| Recurrence: Adverse events, etc.3 | 15:4:6 | 4:4:9 | 0.051 |
| Subsequent CTx after recurrence | 10/15 (67%) | 2/4 (50%) | 0.475 |
In our analysis of prognostic factors in patients with gastric cancer who underwent gastrectomy, where CY1 was the only non-curative factor, histological type, and the presence or absence of postoperative chemotherapy were identified as independent prognostic determinants. Furthermore, in patients receiving postoperative chemotherapy, the addition of oxaliplatin or docetaxel to fluoropyrimidine, may enhance treatment intensity and improve survival outcomes after gastrectomy; however, prospective validation is needed.
CY1 is an independent non-curative factor and is classified as stage IV gastric cancer. However, in real-world clinical practice, gastrectomy is frequently performed, even when CY1 is detected intraoperatively. The MST in patients undergoing gastrectomy alone is only 12.1 months; however, the administration of postoperative S-1 monotherapy has been reported to improve the 5-year OS and PFS rates to 26% and 21%, respectively, demonstrating more favorable outcomes than other stage IV gastric cancers. Based on these findings, postoperative chemotherapy following gastrectomy for CY1 gastric cancer may yield treatment outcomes comparable to those for R0-resected gastric cancer. Therefore, gastrectomy, followed by postoperative chemotherapy, is currently considered the standard treatment. However, patients with concomitant peritoneal dissemination or a high number of cancer cell clusters in the peritoneal fluid have been reported to have a poor prognosis, similar to those with R2 resection[7]. Consequently, we excluded macroscopically resected P1a cases and focused on those where CY1 was the sole non-curative factor.
Independent prognostic factors for CY1 gastric cancer after gastrectomy have been reported to include signet ring cell carcinoma, lymph node metastasis, type 4 tumors, and the presence or absence of postoperative chemotherapy[7,8]. In our study, the histological subtype and receipt of postoperative chemotherapy were identified as prognostic factors, whereas type 4 tumors and lymph node metastasis were not.
No significant difference was observed in lymph node metastasis, as there was only one N0 case, and the treatment outcomes were comparable between N1 and N3 cases. Although type 4 tumors showed no significant difference, the MST was 12.9 and 24.4 months for type 4 and non-type 4 tumors, respectively, indicating a favorable trend for non-type 4 tumors. Moreover, in patients who underwent postoperative chemotherapy, non-type 4 cases demonstrated significantly better outcomes, indicating a potentially greater prognostic benefit of postoperative chemotherapy in this subgroup.
The following are two approaches to postoperative chemotherapy for CY1 gastric cancer after gastrectomy: One considering it as adjuvant chemotherapy for recurrence prevention and the first-line chemotherapy for unresectable or recurrent gastric cancer. One year of postoperative adjuvant S-1 monotherapy has long been the standard treatment for stage II and III gastric cancer in Japan[9]. However, comparative trials between combination therapies, including oxaliplatin or docetaxel, and S-1 monotherapy in patients with pStage III have demonstrated improved prognosis[10,11]. Therefore, oxaliplatin- or docetaxel-based combination therapies are currently recommended as adjuvant therapies for stage III gastric cancer. In contrast, the SPIRIT trial previously established S-1 plus cisplatin as the standard regimen for first-line treatment of unresectable or recurrent gastric cancer[12]. However, the non-inferiority of SOX to S-1 plus cisplatin has been demonstrated. Furthermore, owing to the lower incidence of adverse events, such as myelosuppression and renal dysfunction, as well as the advantages of administration methods, oxaliplatin has become the preferred platinum-based agent. Additionally, subgroup analysis of the G-SOX trial reported favorable therapeutic effects against peritoneal dissemination and a high disease control rate[13]. Consequently, SOX is currently considered one of the most promising first-line regimens for gastric cancer.
Based on the development of these treatment strategies in clinical practice, postoperative chemotherapy for CY1 gastric cancer after gastrectomy generally comprises mainly S1 monotherapy or combination therapy with fluoropyrimidine and cisplatin. However, combination regimens incorporating oxaliplatin or docetaxel have become more prevalent recently. Nevertheless, the Japanese guidelines weakly recommend postoperative chemotherapy with S-1 monotherapy for CY1 gastric cancer[14], with reported treatment outcomes including a 5-year survival rate and MST of 26%-27.1% and 23.2-29.5 months, respectively[15,16]. In contrast, combination therapy with cisplatin demonstrated no additional survival benefit, as the MST remained at 24.4 months, similar to that of S-1 monotherapy. One potential contributing factor is the high incidence of adverse events associated with cisplatin administration after gastrectomy, which prevents the maintenance of an adequate RDI. Although no definitive threshold for effective RDI in cisplatin combination therapy has been established, the SPIRIT trial reported that maintaining a cisplatin RDI of ≥ 90% resulted in an additional survival benefit over S1 monotherapy[12]. In chemotherapy for gastric cancer, an RDI of approximately 80% is generally considered the cutoff value for efficacy[17]. In that study, tumor shrinkage was observed in patients maintaining an RDI of ≥ 80% compared to those with a RDI of < 80%; however, no significant contribution to OS was observed. This lack of an OS benefit may be attributed to factors such as sample size limitations and the ability to adjust the dosage to reduce adverse events in patients with unresectable gastric cancer, which differs in intent from adjuvant therapy for CY1 gastric cancer after gastrectomy.
Regarding RDI in adjuvant chemotherapy, studies in breast cancer have reported better PFS and OS when an RDI of ≥ 85% was maintained[18]. In postoperative adjuvant chemotherapy for gastric cancer, an analysis using an RDI cutoff of 76% for S-1 showed that patients maintaining an RDI of ≥ 76% had significantly better PFS, identifying RDI as an independent prognostic factor[19]. Since the 5-year survival rate for CY1 gastric cancer is better than that for other stage IV gastric cancers, some cases may achieve curative outcomes. Therefore, maintaining a high RDI could contribute to achieving curative intent in this population. In this study, the RDI of cisplatin was approximately 72%. Similarly, Nakayama et al[20] reported that the RDI for cisplatin in combination with S1 for CY1 gastric cancer after gastrectomy was approximately 70%; however, no additional benefit from combination therapy was observed. Given that these two studies reported nearly identical RDIs, the real-world postoperative RDI of cisplatin for CY1 gastric cancer was estimated to be approximately 70%. At this RDI level, no significant advantage over S-1 monotherapy was observed.
In contrast, regarding combination therapy with oxaliplatin or docetaxel, the ARTIST2 trial reported a high completion rate of 85% for the planned eight cycles, with no significant differences in adverse events compared to S-1 monotherapy, except for an increase in grade 3 peripheral neuropathy[11]. In the JACCRO GC-07 trial, the completion rate for six planned cycles of docetaxel was 71%, with an increase in grade 3 Leukopenia and neutropenia; however, these toxicities were reported to be manageable[10]. Similarly, in our study, the mean intravenous RDI in the NC group exceeded 80%, indicating that chemotherapy intensity was well maintained. Moreover, while 15 cases of recurrence within 1 year were observed in the CC group, early recurrence within 1 year occurred in only approximately 10% of the cases in the NC group. Therefore, maintaining high-intensity chemotherapy during the early postoperative period likely contributes to the prevention of early recurrence, ultimately leading to improved PFS and OS. However, caution is warranted given the high incidence (70%) of grade III or higher adverse events in the NC group. Such severe events may necessitate further dose reductions or discontinuation of treatment. To sustain an RDI of ≥ 80%, it is important to limit dose reductions to a single step, which requires appropriate management of treatment-related toxicities.
Other treatment strategies for CY1 gastric cancer include neoadjuvant and intraperitoneal chemotherapy. Recently, staging laparoscopy has enabled preoperative identification of P1a or CY1 cases when peritoneal dissemination is suspected on imaging. A multicenter retrospective study conducted by the JCOG compared the outcomes of patients with P0CY1 or P1a disease who received neoadjuvant chemotherapy and those who underwent upfront surgery. The 5-year survival rates were 22.3% and 21.5%, respectively, with no significant difference between the two groups[21]. However, more than 90% of the chemotherapy regimens used in this study were cisplatin-based, with only a few patients receiving oxaliplatin- or docetaxel-based regimens. Notably, 25% of the patients received a triplet regimen that included a taxane-based agent, which demonstrated a high rate of CY0 conversion and improved prognosis. These findings suggest that intensifying chemotherapy with taxane-based regimens improves the treatment outcomes for CY1 gastric cancer.
Furthermore, in cases where chemotherapy induced CY1, conversion surgery resulted in remarkably favorable survival outcomes, with an MST of 42.4-108.5 months[22,23]. A particularly noteworthy finding was that the rate of peritoneal recurrence postoperatively was approximately 20%, which was significantly lower than the 55% rate reported in patients with CY1 gastric cancer who received S-1 monotherapy after gastrectomy. This suggests that maintaining sufficient chemotherapy intensity, even after gastrectomy, helps suppress peritoneal recurrence. The chemotherapy regimens most commonly used in conversion surgery cases were doublet or triplet regimens, indicating that perioperative administration of surgery combined with high-intensity chemotherapy involving at least two agents may contribute to an improved prognosis in CY1 gastric cancer.
Regarding intraperitoneal chemotherapy, Kuramoto et al[24] reported that peritoneal lavage combined with intraperitoneal cisplatin administration achieved a 5-year survival rate of 43.8%. However, peritoneal lavage alone yielded negative results in previous studies[25,26], highlighting the importance of intraperitoneal chemotherapy. Currently, a phase III randomized controlled trial is underway to compare standard systemic chemotherapy alone with systemic chemotherapy combined with intraperitoneal chemotherapy for CY1 type 4 gastric cancer diagnosed via staging laparoscopy[27]. These treatments are designed to directly reduce or eradicate free-floating cancer cells in the peritoneal cavity, aiming to suppress peritoneal recurrence.
This study had some limitations. First, as this was a retrospective cohort study with a small sample size, the results should be carefully interpreted owing to potential issues such as selection bias, random error, and reduced statistical power. Second, the postoperative chemotherapy regimens varied across institutions because they were selected at the discretion of individual clinicians at each facility. Some institutions adhered strictly to these guidelines and administered S-1 monotherapy; thus, potential selection bias due to patient background characteristics could not be fully eliminated. Third, the postoperative chemotherapy regimens were influenced by temporal trends. Since the efficacy of combination therapy for adjuvant treatment of stage III gastric cancer has been demonstrated, its use has increased. Consequently, the selection of the TC and NC groups was influenced by their different historical backgrounds. Fourth, data on HER2 status were missing for some patients. In the analysis of cases with available HER2 status, no significant impact on prognosis was observed in the present study. However, given that HER2 overexpression can influence prognosis and guide the use of targeted therapies such as trastuzumab; the absence of this information may have introduced unmeasured confounding. Fifth, the sample size was small, and multiple comparison corrections were not performed in subgroup analysis; thus, the interpretation of statistical significance should be approached with caution. As previously mentioned, recently, staging laparoscopy has been increasingly performed in cases suspected of peritoneal dissemination or malignant ascites, thereby substantially limiting the number of cases that met the criteria for this study. Therefore, drawing definitive conclusions from this study is difficult. To increase the sample size, aggregating data through the utilization of clinical registries or conducting a meta-analysis is essential.
In conclusion, prognostic factors for gastric cancer with CY1 after gastrectomy include host-related factors such as diffuse-type histology, which is associated with poor prognosis. Additionally, the introduction of postoperative chemotherapy may have contributed to improved prognosis. Combination regimens involving oxaliplatin or docetaxel are potentially particularly effective, as they are thought to help maintain treatment intensity even after gastrectomy.
We would like to express our gratitude to all patients who participated in this study and to the co-investigators affiliated with Hiroshima Surgical Study of Clinical Oncology for their valuable contributions.
| 1. | Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5690] [Cited by in RCA: 12706] [Article Influence: 6353.0] [Reference Citation Analysis (6)] |
| 2. | Sekiguchi M, Oda I, Matsuda T, Saito Y. Epidemiological Trends and Future Perspectives of Gastric Cancer in Eastern Asia. Digestion. 2022;103:22-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 90] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 3. | Higashi T, Kurokawa Y. Incidence, mortality, survival, and treatment statistics of cancers in digestive organs-Japanese cancer statistics 2024. Ann Gastroenterol Surg. 2024;8:958-965. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 4. | Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, Wyrwicz L, Yamaguchi K, Skoczylas T, Campos Bragagnoli A, Liu T, Schenker M, Yanez P, Tehfe M, Kowalyszyn R, Karamouzis MV, Bruges R, Zander T, Pazo-Cid R, Hitre E, Feeney K, Cleary JM, Poulart V, Cullen D, Lei M, Xiao H, Kondo K, Li M, Ajani JA. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021;398:27-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1962] [Cited by in RCA: 2228] [Article Influence: 445.6] [Reference Citation Analysis (1)] |
| 5. | Rha SY, Oh DY, Yañez P, Bai Y, Ryu MH, Lee J, Rivera F, Alves GV, Garrido M, Shiu KK, Fernández MG, Li J, Lowery MA, Çil T, Cruz FM, Qin S, Luo S, Pan H, Wainberg ZA, Yin L, Bordia S, Bhagia P, Wyrwicz LS; KEYNOTE-859 investigators. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for HER2-negative advanced gastric cancer (KEYNOTE-859): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2023;24:1181-1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 422] [Article Influence: 140.7] [Reference Citation Analysis (0)] |
| 6. | Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, Kulikov E, Hill J, Lehle M, Rüschoff J, Kang YK; ToGA Trial Investigators. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5823] [Cited by in RCA: 5531] [Article Influence: 345.7] [Reference Citation Analysis (3)] |
| 7. | Higaki E, Yanagi S, Gotohda N, Kinoshita T, Kuwata T, Nagino M, Ochiai A, Fujii S. Intraoperative peritoneal lavage cytology offers prognostic significance for gastric cancer patients with curative resection. Cancer Sci. 2017;108:978-986. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | Endo S, Nishikawa K, Ikenaga M, Fujitani K, Kawada J, Yamatsuji T, Kubota H, Higashida M, Fujiwara Y, Ueno T. Prognostic factors for cytology-positive gastric cancer: a multicenter retrospective analysis. Int J Clin Oncol. 2021;26:858-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, Furukawa H, Nakajima T, Ohashi Y, Imamura H, Higashino M, Yamamura Y, Kurita A, Arai K; ACTS-GC Group. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357:1810-1820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1771] [Cited by in RCA: 1979] [Article Influence: 104.2] [Reference Citation Analysis (0)] |
| 10. | Yoshida K, Kodera Y, Kochi M, Ichikawa W, Kakeji Y, Sano T, Nagao N, Takahashi M, Takagane A, Watanabe T, Kaji M, Okitsu H, Nomura T, Matsui T, Yoshikawa T, Matsuyama J, Yamada M, Ito S, Takeuchi M, Fujii M. Addition of Docetaxel to Oral Fluoropyrimidine Improves Efficacy in Patients With Stage III Gastric Cancer: Interim Analysis of JACCRO GC-07, a Randomized Controlled Trial. J Clin Oncol. 2019;37:1296-1304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 305] [Cited by in RCA: 296] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 11. | Park SH, Lim DH, Sohn TS, Lee J, Zang DY, Kim ST, Kang JH, Oh SY, Hwang IG, Ji JH, Shin DB, Yu JI, Kim KM, An JY, Choi MG, Lee JH, Kim S, Hong JY, Park JO, Park YS, Lim HY, Bae JM, Kang WK; ARTIST 2 investigators. A randomized phase III trial comparing adjuvant single-agent S1, S-1 with oxaliplatin, and postoperative chemoradiation with S-1 and oxaliplatin in patients with node-positive gastric cancer after D2 resection: the ARTIST 2 trial(☆). Ann Oncol. 2021;32:368-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 203] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 12. | Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama W, Toh Y, Nagaie T, Takagi S, Yamamura Y, Yanaoka K, Orita H, Takeuchi M. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008;9:215-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1320] [Cited by in RCA: 1435] [Article Influence: 79.7] [Reference Citation Analysis (0)] |
| 13. | Takahari D, Hamaguchi T, Yoshimura K, Katai H, Ito S, Fuse N, Kinoshita T, Yasui H, Terashima M, Goto M, Tanigawa N, Shirao K, Sano T, Sasako M. Feasibility study of adjuvant chemotherapy with S-1 plus cisplatin for gastric cancer. Cancer Chemother Pharmacol. 2011;67:1423-1428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 14. | Japanese Gastric Cancer Association. Japanese Gastric Cancer Treatment Guidelines 2021 (6th edition). Gastric Cancer. 2023;26:1-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 199] [Cited by in RCA: 841] [Article Influence: 280.3] [Reference Citation Analysis (2)] |
| 15. | Kodera Y, Ito S, Mochizuki Y, Kondo K, Koshikawa K, Suzuki N, Kojima H, Kojima T, Matsui T, Takase T, Tsuboi K, Fujiwara M, Nakao A; Chubu Clinical Oncology Group. A phase II study of radical surgery followed by postoperative chemotherapy with S-1 for gastric carcinoma with free cancer cells in the peritoneal cavity (CCOG0301 study). Eur J Surg Oncol. 2009;35:1158-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Yamaguchi T, Takashima A, Nagashima K, Makuuchi R, Aizawa M, Ohashi M, Tashiro K, Yamada T, Kinoshita T, Hata H, Kawachi Y, Kawabata R, Tsuji T, Hihara J, Sakamoto T, Fukagawa T, Katai H, Higuchi K, Boku N. Efficacy of Postoperative Chemotherapy After Resection that Leaves No Macroscopically Visible Disease of Gastric Cancer with Positive Peritoneal Lavage Cytology (CY1) or Localized Peritoneum Metastasis (P1a): A Multicenter Retrospective Study. Ann Surg Oncol. 2020;27:284-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 17. | Kitagawa M, Shimura T, Yamada T, Ebi M, Hirata Y, Mizoshita T, Tanida S, Kataoka H, Kamiya T, Joh T. The relationship between antitumor effects and relative dose intensity of S-1 plus cisplatin treatment for metastatic gastric cancer. Anticancer Res. 2012;32:1763-1768. [PubMed] |
| 18. | Bonadonna G, Valagussa P, Moliterni A, Zambetti M, Brambilla C. Adjuvant cyclophosphamide, methotrexate, and fluorouracil in node-positive breast cancer: the results of 20 years of follow-up. N Engl J Med. 1995;332:901-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 748] [Cited by in RCA: 703] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 19. | Fujita S, Sakuramoto S, Matsui K, Ebara G, Nishibeppu K, Oya S, Fujihata S, Lee S, Miyawaki Y, Sugita H, Sato H, Yamashita K. Relative dose intensity and 1-year psoas muscle index reduction rate as prognostic factors in gastric cancer patients with postoperative adjuvant chemotherapy. Int J Clin Oncol. 2023;28:110-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 20. | Nakayama I, Chin K, Matsushima T, Takahari D, Ogura M, Shinozaki E, Suenaga M, Ozaka M, Wakatsuki T, Ichimura T, Hiroki O, Yamaguchi K. Retrospective comparison of S-1 plus cisplatin versus S-1 monotherapy for the treatment of advanced gastric cancer patients with positive peritoneal cytology but without gross peritoneal metastasis. Int J Clin Oncol. 2017;22:1060-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Yamaguchi T, Takashima A, Nagashima K, Terashima M, Aizawa M, Ohashi M, Tanaka R, Yamada T, Kinoshita T, Matsushita H, Ishiyama K, Hosoda K, Yuasa Y, Haruta S, Kakihara N, Nishikawa K, Yunome G, Satoh T, Fukagawa T, Katai H, Boku N. Impact of preoperative chemotherapy as initial treatment for advanced gastric cancer with peritoneal metastasis limited to positive peritoneal lavage cytology (CY1) or localized peritoneal metastasis (P1a): a multi-institutional retrospective study. Gastric Cancer. 2021;24:701-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 22. | Yasufuku I, Nunobe S, Ida S, Kumagai K, Ohashi M, Hiki N, Sano T. Conversion therapy for peritoneal lavage cytology-positive type 4 and large type 3 gastric cancer patients selected as candidates for R0 resection by diagnostic staging laparoscopy. Gastric Cancer. 2020;23:319-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 23. | Yoshida K, Yasufuku I, Terashima M, Young Rha S, Moon Bae J, Li G, Katai H, Watanabe M, Seto Y, Hoon Noh S, Kwang Yang H, Ji J, Baba H, Kitagawa Y, Morita S, Nishiyama M, Kodera Y; CONVO‐GC‐1 Study Group, Federation of Asian Clinical Oncology (FACO). International Retrospective Cohort Study of Conversion Therapy for Stage IV Gastric Cancer 1 (CONVO-GC-1). Ann Gastroenterol Surg. 2022;6:227-240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 106] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 24. | Kuramoto M, Shimada S, Ikeshima S, Matsuo A, Yagi Y, Matsuda M, Yonemura Y, Baba H. Extensive intraoperative peritoneal lavage as a standard prophylactic strategy for peritoneal recurrence in patients with gastric carcinoma. Ann Surg. 2009;250:242-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 192] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 25. | Yang HK, Ji J, Han SU, Terashima M, Li G, Kim HH, Law S, Shabbir A, Song KY, Hyung WJ, Kosai NR, Kono K, Misawa K, Yabusaki H, Kinoshita T, Lau PC, Kim YW, Rao JR, Ng E, Yamada T, Yoshida K, Park DJ, Tai BC, So JBY; EXPEL study group. Extensive peritoneal lavage with saline after curative gastrectomy for gastric cancer (EXPEL): a multicentre randomised controlled trial. Lancet Gastroenterol Hepatol. 2021;6:120-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 26. | Misawa K, Mochizuki Y, Sakai M, Teramoto H, Morimoto D, Nakayama H, Tanaka N, Matsui T, Ito Y, Ito S, Tanaka K, Uemura K, Morita S, Kodera Y; Chubu Clinical Oncology Group. Randomized clinical trial of extensive intraoperative peritoneal lavage versus standard treatment for resectable advanced gastric cancer (CCOG 1102 trial). Br J Surg. 2019;106:1602-1610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 27. | Ishigami H, Tsuji Y, Shinohara H, Kodera Y, Kanda M, Yabusaki H, Ito S, Imano M, Yamashita H, Hidemura A, Yamaguchi H, Fukagawa T, Oba K, Kitayama J, Seto Y. Intraperitoneal Chemotherapy as Adjuvant or Perioperative Chemotherapy for Patients with Type 4 Scirrhous Gastric Cancer: PHOENIX-GC2 Trial. J Clin Med. 2021;10:5666. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/