Published online Sep 15, 2025. doi: 10.4251/wjgo.v17.i9.110166
Revised: July 5, 2025
Accepted: August 15, 2025
Published online: September 15, 2025
Processing time: 108 Days and 7.9 Hours
Irreversible electroporation (IRE) represents an innovative localized technique for tumor ablation, possessing the capacity to activate the immune response of the host. However, this method alone is inadequate to halt cancer progression, necessitating the integration of additional strategies to achieve effective immunotherapy.
To investigate the effects and underlying mechanisms of antitumor immunity derived from the synergistic application of IRE and anti-programmed cell death protein 1 (PD-1) therapy within a murine model of hepatocellular carcinoma.
C57BL-6 mice with tumor growth were divided into four separate cohorts: Control group; IRE group; Anti-PD-1 group; And IRE + anti-PD-1 group. The infiltration levels of T, B, and natural killer cells within the tumors, as well as the plasma concentrations of T helper type 1 cytokines (interleukin-2, interferon-γ, and tumor necrosis factor-β), were evaluated. Real-time polymerase chain reaction was utilized to quantify the expression of cluster of differentiation (CD) 8 (a marker indicative of CD8+ T cells) in the tumor specimens of the mice at various temporal intervals. Tumor growth trajectories were charted.
The results indicated that the IRE + anti-PD-1 group exhibited significantly heightened percentages of T lymphocyte infiltration, particularly CD4+ and CD8+ T cells, when compared to the control cohort. Additionally, this group displayed increased infiltration of natural killer and B cells, augmented cytokine levels, and elevated CD8 messenger RNA expression. A marked decrease in tumor volume was noted in the IRE + anti-PD-1 group, indicating enhanced therapeutic efficacy.
The combined application of IRE and checkpoint blockade elicits an antitumor immune response, leading to a more substantial reduction in tumor volume and improved therapeutic outcomes, thereby establishing a novel avenue for the ablation and immunotherapy of hepatocellular carcinoma.
Core Tip: This study highlights the combination of irreversible electroporation and anti-programmed cell death protein 1 therapy synergistically enhances antitumor immunity by significantly increasing tumor infiltration of T cells [cluster of differentiation (CD) 4+, CD8+], natural killer cells, and B cells, elevating Th1-associated cytokine levels (interleukin-2, interferon-γ, and tumor necrosis factor-β), and upregulating CD8 messenger RNA expression. This dual approach markedly reduces tumor volume compared to monotherapies, demonstrating potentiated therapeutic efficacy and providing a novel strategy for ablation-immunotherapy integration, with potential implications for hepatocellular carcinoma.
- Citation: Xing YL, Li HM, Pang XM, Zhang Y, Yang T, Li YH, Liu DC, Ma YY, Niu LZ. Irreversible electroporation combined with checkpoint blockade stimulates antitumor immune response in a hepatocellular carcinoma mouse model. World J Gastrointest Oncol 2025; 17(9): 110166
- URL: https://www.wjgnet.com/1948-5204/full/v17/i9/110166.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i9.110166
Hepatocellular carcinoma (HCC) is one of the most prevalent forms of liver cancer, accounting for approximately 75% of all primary liver cancer cases worldwide[1,2]. The incidence of HCC has been steadily increasing, particularly in regions with high rates of hepatitis B and C virus infections, leading to significant morbidity and mortality[3-5]. Current treatment options for HCC, such as surgical resection, liver transplantation, and systemic therapies like sorafenib, often have limited effectiveness, especially in advanced stages of the disease[6-9]. Furthermore, localized treatment techniques are being used more and more for treating HCC, such as percutaneous ablation, transarterial chemoembolization, and transarterial radioembolization, as well as emerging techniques like hepatic arterial infusion chemotherapy and external-beam radiation therapy[10]. Moreover, the heterogeneity of HCC tumors poses a significant challenge in achieving optimal therapeutic outcomes, as many patients either do not respond to existing treatments or experience rapid disease progression. Thus, there is an urgent need for innovative treatment approaches that can enhance antitumor immunity and improve patient prognosis in HCC.
Irreversible electroporation (IRE) is a non-thermal ablation technique that uses short, high-voltage electrical pulses to induce permanent nanopores in cell membranes, leading to cell death[11-15]. Allowing molecules like DNA, RNA, proteins, and drugs to enter cells. This process increases the cell membrane’s permeability, enabling substances to cross the membrane that would normally be unable to do. This method has gained attention for its ability to selectively target tumor cells while sparing surrounding healthy tissue[12,16]. The biological function of IRE is primarily attributed to its ability to induce immunogenic cell death, which can enhance the presentation of tumor antigens and stimulate an adaptive immune response[17-19].
Immune checkpoint inhibitors (ICIs) are crucial in augmenting the immune system’s capacity to identify and eradicate tumors[20-22]. ICI therapies target proteins like programmed cell death protein 1 (PD-1) and cytotoxic T-lymphocyte-associated protein 4 to inhibit tumor pathways. This prevents tumors from evading immune detection. Recent studies have shown that IRE can enhance the efficacy of immune checkpoint blockade (ICB) by promoting the infiltration of immune cells into the tumor microenvironment and increasing the expression of major histocompatibility complex molecules. This process thereby facilitates T-cell activation[18,23,24].
Despite the promising results, there are still significant gaps in understanding the precise molecular mechanisms underlying the synergistic effects of IRE and ICB in HCC. Current research is limited by the variability in tumor responses and the need for further exploration of the optimal timing and sequencing of these therapies. Overall, the integration of IRE with checkpoint blockade represents a compelling area of research that holds potential for enhancing therapeutic outcomes in HCC.
This study aims to investigate the synergistic effects of IRE combined with checkpoint blockade therapy in enhancing antitumor immunity in HCC model. The primary objective is to elucidate the potential of this combination therapy to improve therapeutic outcomes in HCC, a malignancy known for its poor prognosis and limited treatment options. By employing IRE, a non-thermal ablation technique, alongside ICIs, we seek to explore how this dual approach can activate and amplify the immune response against tumor cells.
This research is important because it may provide a novel therapeutic strategy for HCC, which remains a major global health challenge. Understanding the mechanisms underlying the enhanced antitumor immunity resulting from this combination could pave the way for more effective treatment protocols and improve patient survival rates. Ultimately, this research aims to enhance the existing evidence that supports the combination of local ablation methods with immunotherapy for treating solid tumors.
H22 HCC cells were acquired from the BeNa Culture Collection (BNCC, Beijing, China). These H22 HCC cells were cultivated in a medium comprising 90% Dulbecco’s Modified Eagle’s Medium (Gibco) and 10% fetal bovine serum (Hyclone). The cells were subsequently maintained in a controlled environment at 37 °C with 5% carbon dioxide (CO2).
For the establishment of a mouse model possessing a normal immune system. Female C57BL/6 mice were housed in a specific pathogen-free facility (12-hour light/dark cycle, 22 ± 2 °C, 50% ± 10% humidity) with ad libitum access to food and water. All procedures strictly adhered to the ARRIVE guidelines and were approved by the laboratory animal welfare ethics committee of Jinan University. C57BL/6 mice were subcutaneously inoculated with 1 × 106 H22 HCC cells suspended in 100 μL of Hanks balanced salt solution, injected into the right flank of 6-week-old C57BL/6 mice. Mice meeting any endpoint criterion were euthanized via CO2 overdose followed by cervical dislocation.
Forty-eight female C57BL/6 mice were randomly allocated into four distinct groups: A control group receiving no treatment (n = 12), an IRE group (n = 12), an anti-PD-1 group (n = 12), and an IRE + anti-PD-1 group (n = 12). H22 HCC cells at a concentration of 1 × 106/mL were injected subcutaneously into the right flank of the mice, with tumor growth monitored every two days. The maximum (a) and minimum (b) diameters of the tumors were measured using vernier calipers, and tumor volume was computed using the formula a × b²/2. Upon reaching a maximum tumor diameter of 10 mm, IRE was performed, followed by the administration of an anti–programmed cell death ligand 1 (PD-L1) antibody (200 μg/mouse) via intraperitoneal injection immediately after the IRE procedure. Tumor tissues and peripheral blood samples were collected at various time points (1, 3, 7, 14 days) post-IRE. The tumor size was recorded every three days based on the length and width measurements.
The mice were secured on the surgical table, anesthetized with tribromoethanol administered intraperitoneally according to their body weight, and the surgical area was prepared, disinfected, and draped. Two electrode needles were introduced along the long axis of the tumors, we conducted a series of preliminary experiments to evaluate the efficacy and safety of various parameter combinations. Additionally, we reviewed relevant literature to identify optimal settings used in similar studies[25]. The ablation parameters are set as 1200 V/cm, 50 A, 70 pulses, 70 μs wavelength, and 100 ms intervals, while the spacing of the needles was adjusted based on tumor size. All in vivo interventional ablation of IRE was conducted solely by Niu LZ to ensure the surgical procedure was standardized and to reduce any potential discrepancies. The PD-1/PD-L1 inhibitor was procured from Aladdin. The anti-PD-1 treatment was administered intraperitoneally at a dose of 200 μg every three days for a total of four injections, with needle spacing modified according to tumor dimensions.
Peripheral blood (1 mL) was obtained from mice in 1, 3, 7, 14 days after IRE treatment for the detection of immune function by flow cytometry with a fluorescence activated cell sorting Canto II (BD Biosciences). Multitest 6-Color TBNK Reagent and Trucount tubes (337166, BD Biosciences) were used to detect the absolute numbers of cluster of differentiation (CD) 3+ T cells (CD3+), CD4+ T cells (CD3+ CD4+), CD8+ T cells (CD3+ CD8+), the ratio of CD4+ T cells (CD3+ CD4+) to CD8+ T cells, natural killer (NK) cells (CD16+ CD56+), and B cells (CD19). Cytokine Kit II (551809, BD Biosciences) for a cytometric bead array was used to detect the expression of interleukin-2 (IL-2), tumor necrosis factor (TNF)-β, and interferon-γ (IFN-γ). The tests were conducted according to the manufacturer’s instructions. Mice whose parameters fell within the manufacturer-defined reference range were considered to have normal immune function. Mice with one or more parameters below the normal range were considered to have immune dysfunction.
The expression levels of CD8 in murine tumor tissues were evaluated using SYBR Green staining, with glyceraldehyde 3-phosphate dehydrogenase serving as the internal reference gene. Tumor tissues were collected and sectioned into pieces measuring 0.3 cm × 0.3 cm × 0.3 cm, with total RNA extracted using TRIzol reagent. Complementary DNA synthesis was conducted according to the provided guidelines, and the reaction mixture (20 μL) was prepared based on reagent instructions before undergoing real-time polymerase chain reaction (RT-PCR) amplification and detection. The primer sequences for RT-PCR were: Β-actin (forward) GGGAAATCGTGCGTGAC, (reverse) AGGCTGGAA AAG-AGCCT; CD8 (forward) CAAACACGCTTTCGG CTCCTG, (reverse) CGGATTGGACTTCGCCTGTGA; results were analyzed using the 2-ΔΔCt method.
All statistical evaluations were conducted and consolidated utilizing GraphPad Prism (version 8.01, GraphPad Software, San Diego, CA, United States) and SPSS software (version 20.0, SPSS Inc., Chicago, IL, United States). The data collected were represented as means ± SD. A t-test was employed to compare the two groups, while one-way analysis of variance was implemented for the comparison among multiple groups. A P value of less than 0.05 was deemed statistically significant.
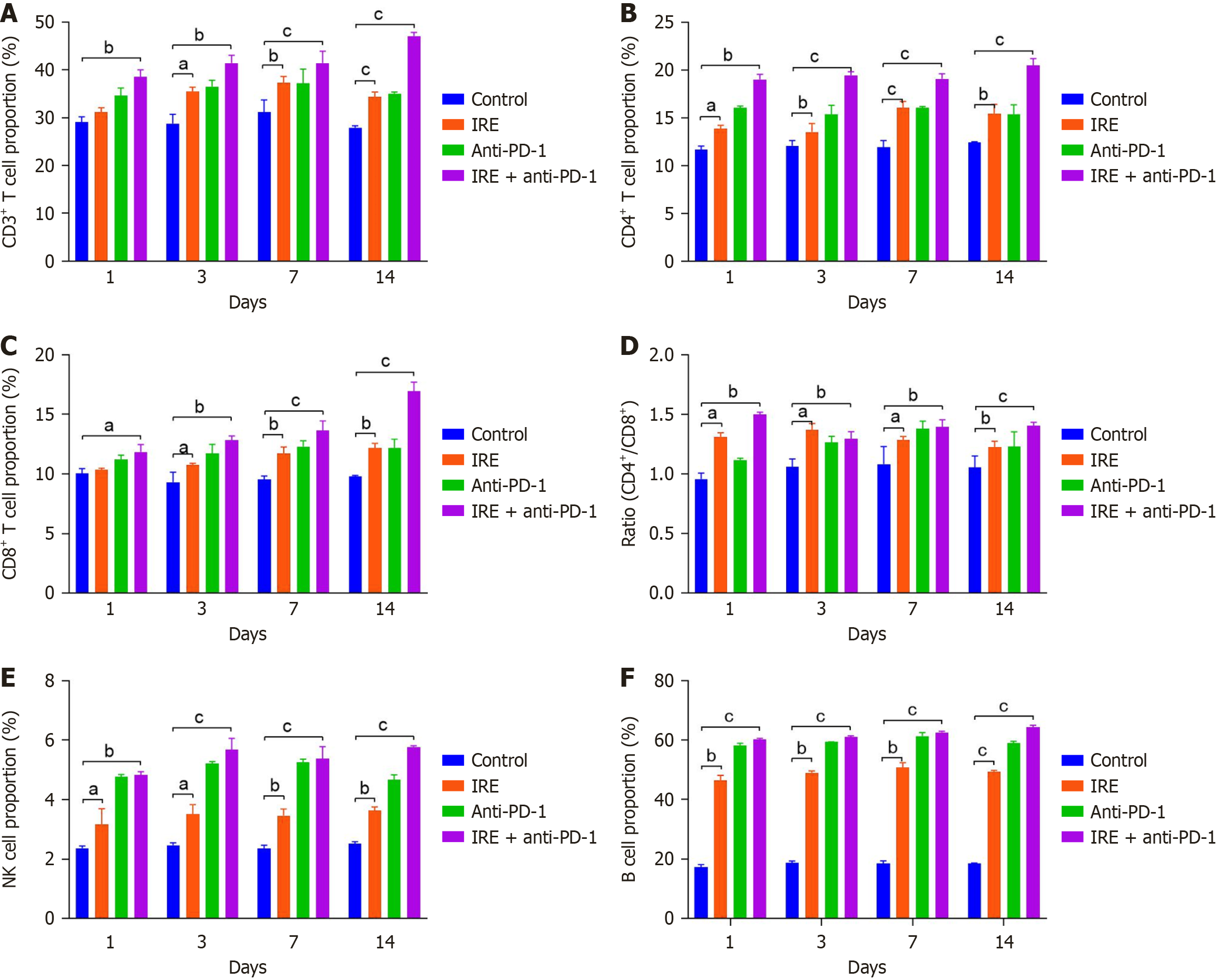
The dual application of IRE and anti-PD-1 therapy significantly enhanced T lymphocyte presence in peripheral circulation. When compared to untreated controls, the fraction of T lymphocytes rose markedly in mice treated with IRE alone (P < 0.01), anti-PD-1 monotherapy (P < 0.01), and the combined regimen (P < 0.001), with effects intensifying over time. CD4+ T cell proportions increased substantially in all intervention groups relative to controls (P < 0.001). Notably, tumors from the IRE plus anti-PD-1 cohort exhibited heightened CD8+ T cell recruitment (P < 0.001, Figure 1A-D). These findings imply that IRE-induced immune activation centers on CD8+ T cell recruitment, while combination therapy produces a stronger CD8+ T cell reaction.
Co-administration of IRE and anti-PD-1 augmented NK and B cell migration into peripheral blood. Percentages of NK and B cells were significantly elevated across the IRE-only, anti-PD-1-only, and combination groups vs controls. The integrated treatment yielded substantially greater cell influx than anti-PD-1 alone (P < 0.001, Figure 1E and F). This underscores that all strategies stimulated innate and humoral immunity, with IRE plus anti-PD-1 delivering superior outcomes.
Combining IRE with anti-PD-1 modified cytokine profiles in peripheral blood. IL-2, TNF-β, and IFN-γ concentrations increased significantly in all treatment arms relative to controls (IRE and anti-PD-1 groups: P < 0.01; combination group: P < 0.001). Importantly, IL-2 Levels in the dual-therapy mice showed considerable variation (P < 0.001, Figure 2), suggesting broader immune activation than monotherapies.
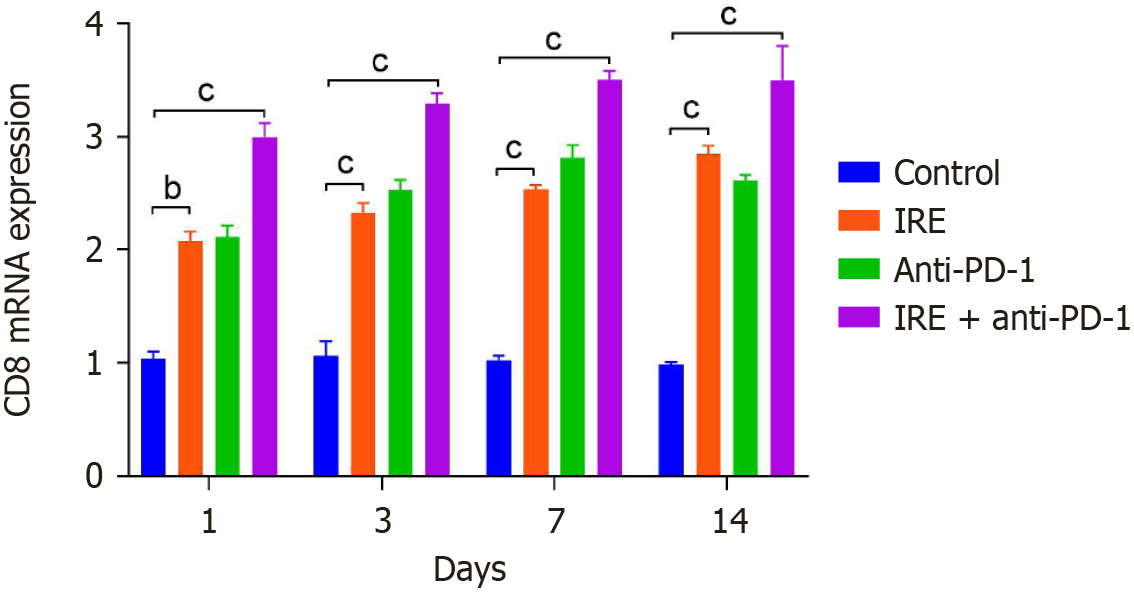
Joint IRE and anti-PD-1 treatment boosted CD8 expression at the messenger RNA (mRNA) level. Tumor tissues from IRE, anti-PD-1, and combination groups demonstrated notably higher CD8 mRNA expression than controls (P < 0.001). Moreover, CD8 mRNA in the combined cohort increased progressively over the observation duration (P < 0.001, Figure 3). These data indicate enhanced cytotoxic T cell function under combination therapy.
Co-application of IRE and anti-PD-1 diminished tumor burden. Tumor volumes decreased considerably in the IRE, anti-PD-1, and combination groups vs controls (P < 0.01 and P < 0.001). A pronounced time-dependent reduction occurred in the IRE plus anti-PD-1 cohort (Figure 4), affirming that combination therapy drove maximal tumor regression.
This study explores how integrated IRE and anti-PD-1 therapy activates antitumor immunity in a C57BL-6 murine model of HCC. Our evidence reveals that IRE combined with anti-PD-1 potently suppressed tumor growth relative to controls (P < 0.01). Flow cytometry detected elevated levels of CD4+ T cells, CD8+ T cells, NK cells, and B cells in treated mice serum. Rt-PCR demonstrated upregulated CD8 expression in excised tumor specimens, implying augmented cytotoxic T cell activity. Collectively, these outcomes confirm our hypothesis that pairing IRE with anti-PD-1 enhances tumor-directed immunity and therapeutic efficacy in HCC.
The principal innovation lies in comprehensively evaluating IRE and anti-PD-1 synergy within an HCC murine system, addressing gaps in mechanistic understanding. Prior research mostly examined standalone IRE or anti-PD-1 effects. For instance, IRE promotes immunogenic cell death and immune cell mobilization within the tumor niche[17,18]. Anti-PD-1 therapy reactivates exhausted T cells and bolsters antitumor defenses. Yet, combined mechanisms remain underexplored. Our work establishes that dual therapy fosters greater CD8+ T cell influx, amplifies cytokines (IL-2, TNF-β, IFN-γ), and causes deeper tumor shrinkage than monotherapy. This synergy suggests IRE primed the microenvironment, enhancing responsiveness to checkpoint inhibition-a foundation for novel HCC regimens.
The primary mechanism by which IRE addresses HCC involves the initiation of apoptosis in tumor cells, which may lead to tumor-induced immune tolerance. Traditional perspectives indicate that the integration of IRE with immunotherapy may face challenges in achieving synergistic outcomes. Nevertheless, research by Shao et al[26] demonstrated that IRE outperforms both thermal and cryoablation techniques in fostering T-cell immunity, suggesting that a combination of IRE and immunotherapy could yield synergistic benefits. Dai et al[17] indicate that IRE significantly enhances antitumor immunity in HCC by promoting CD8+ T cell responses, resulting in tumor growth suppression and prevention of post-ablation progression, while concurrently mitigating immunosuppression through the reduction of regulatory T cells and PD-1+ T cells. Additionally, Shi et al[18] reported that the combination of IRE and immunotherapy has been shown to enhance tumor necrosis and immune responses not only within the IRE-treated tumors but also in adjacent lesions outside the targeted ablation field. Furthermore, a recent investigation indicated that IRE enhances the activation of T cells while concurrently diminishing regulatory T cell populations in the peripheral blood of patients, while findings from the murine model suggest that IRE promotes antitumor adaptive immunity through the infiltration of cytotoxic CD8+ T cells into tumors, accompanied by a reduction in Tregs[19]. Our results support these findings, showing IRE initiates innate immunity that fosters antigen presentation and adaptive responses.
The mechanisms underlying IRE combined anti-PD-1 treatment may encompass the following elements: (1) IRE facilitates the release of damage-related molecular patterns. Dendritic cells uptake these damage-related molecular patterns, migrate to the draining lymph nodes, and subsequently activate T cells that are specific to tumor antigens[27,28]. The activated T cells then navigate to the tumor sites to eradicate the residual tumor cells. Moreover, IRE enhances the extracellular matrix, augments the density and permeability of tumor vasculature, and promotes the infiltration of immune cells into the remaining tumor tissues; (2) IRE remodels the tumor microenvironment. Being a non-thermal modality, it preserves critical extracellular matrix structures and maintains the integrity of blood vessels within the tumor tissue, thereby encouraging the infiltration of subsequently primed effector T cells into the ablated tumor site[29,30]; and (3) IRE, functioning as an “electrical immunomodulatory therapy”, exhibits both synergistic and additive effects when used in conjunction with immunotherapy[31,32].
Our study demonstrates that combining IRE ablation with anti-PD-1 therapy synergistically enhances antitumor immunity in a murine HCC model. These findings suggest that IRE may overcome the immunosuppressive tumor microenvironment, thereby potentiating checkpoint blockade. Clinically, this strategy could offer a novel therapeutic method for HCC. Despite these promising results, several limitations must be acknowledged: First, our findings rely solely on a syngeneic mouse HCC model, which may not fully recapitulate human HCC heterogeneity, immune evasion mechanisms, or comorbidities. The absence of humanized models or spontaneous HCC models limits translational relevance. Second, IRE’s immunostimulatory effects could theoretically provoke off-target autoimmunity when combined with anti-PD-1, though this was not observed in our short-term study. Last, our study assessed outcomes only up to 14 days post-treatment. Long-term data are critical to determine whether immune activation translates to durable tumor control or risks immune exhaustion/T-cell dysfunction over time. To address these gaps, we validate findings in patient-derived xenograft models or immunocompetent rats with cirrhotic HCC to better mimic human disease. Future investigations should focus on optimizing treatment parameters, including both timing and dosage, while also exploring the long-term effects of this combination therapy to gain a comprehensive understanding of its clinical promise.
In conclusion, our study demonstrates that the combined use of IRE and anti-PD-1 therapy significantly boosts antitumor immunity in a murine model of HCC. Furthermore, this synergistic treatment not only mounts a direct offensive against tumor cells but also transforms the immune microenvironment to foster anti-tumor responses.
We wish to thank the staffs from Department of Surgery and Anesthesia of Guangzhou Fuda Cancer Hospital for their technical support.
| 1. | Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 171] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 2. | Llovet JM, De Baere T, Kulik L, Haber PK, Greten TF, Meyer T, Lencioni R. Locoregional therapies in the era of molecular and immune treatments for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2021;18:293-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 692] [Article Influence: 138.4] [Reference Citation Analysis (0)] |
| 3. | Huang DQ, Mathurin P, Cortez-Pinto H, Loomba R. Global epidemiology of alcohol-associated cirrhosis and HCC: trends, projections and risk factors. Nat Rev Gastroenterol Hepatol. 2023;20:37-49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 148] [Cited by in RCA: 350] [Article Influence: 116.7] [Reference Citation Analysis (0)] |
| 4. | Brown ZJ, Tsilimigras DI, Ruff SM, Mohseni A, Kamel IR, Cloyd JM, Pawlik TM. Management of Hepatocellular Carcinoma: A Review. JAMA Surg. 2023;158:410-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 493] [Reference Citation Analysis (1)] |
| 5. | Ariizumi SI, Katagiri S, Kotera Y, Yamashita S, Omori A, Kato T, Shibuya G, Egawa H, Takasaki K, Yamamoto M. Improved Mortality, Morbidity, and Long-Term Outcome After Anatomical Hepatectomy With the Glissonean Pedicle Approach in Patients With Hepatocellular Carcinoma: 30 Years' Experience at a Single Institute. Ann Surg. 2022;275:947-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (1)] |
| 6. | Singal AG, Kanwal F, Llovet JM. Global trends in hepatocellular carcinoma epidemiology: implications for screening, prevention and therapy. Nat Rev Clin Oncol. 2023;20:864-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 577] [Cited by in RCA: 533] [Article Influence: 177.7] [Reference Citation Analysis (2)] |
| 7. | Di Benedetto F, Magistri P, Di Sandro S, Sposito C, Oberkofler C, Brandon E, Samstein B, Guidetti C, Papageorgiou A, Frassoni S, Bagnardi V, Clavien PA, Citterio D, Kato T, Petrowsky H, Halazun KJ, Mazzaferro V; Robotic HPB Study Group. Safety and Efficacy of Robotic vs Open Liver Resection for Hepatocellular Carcinoma. JAMA Surg. 2023;158:46-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 103] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 8. | Sapisochin G, Bruix J. Liver transplantation for hepatocellular carcinoma: outcomes and novel surgical approaches. Nat Rev Gastroenterol Hepatol. 2017;14:203-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 356] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 9. | Tang W, Chen Z, Zhang W, Cheng Y, Zhang B, Wu F, Wang Q, Wang S, Rong D, Reiter FP, De Toni EN, Wang X. The mechanisms of sorafenib resistance in hepatocellular carcinoma: theoretical basis and therapeutic aspects. Signal Transduct Target Ther. 2020;5:87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 790] [Article Influence: 131.7] [Reference Citation Analysis (1)] |
| 10. | Zhong BY, Fan W, Guan JJ, Peng Z, Jia Z, Jin H, Jin ZC, Chen JJ, Zhu HD, Teng GJ. Combination locoregional and systemic therapies in hepatocellular carcinoma. Lancet Gastroenterol Hepatol. 2025;10:369-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 34] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 11. | Gupta P, Maralakunte M, Sagar S, Kumar-M P, Bhujade H, Chaluvashetty SB, Kalra N. Efficacy and safety of irreversible electroporation for malignant liver tumors: a systematic review and meta-analysis. Eur Radiol. 2021;31:6511-6521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 12. | Frühling P, Stillström D, Holmquist F, Nilsson A, Freedman J. Irreversible electroporation of hepatocellular carcinoma and colorectal cancer liver metastases: A nationwide multicenter study with short- and long-term follow-up. Eur J Surg Oncol. 2023;49:107046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 13. | Ma Y, Chen Z, Liang B, Li R, Li J, Li Z, Lin M, Niu L. Irreversible Electroporation for Hepatocellular Carcinoma Abutting the Diaphragm: A Prospective Single-center Study. J Clin Transl Hepatol. 2022;10:190-196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Kalra N, Gupta P, Gorsi U, Bhujade H, Chaluvashetty SB, Duseja A, Singh V, Dhiman RK, Chawla YK, Khandelwal N. Irreversible Electroporation for Unresectable Hepatocellular Carcinoma: Initial Experience. Cardiovasc Intervent Radiol. 2019;42:584-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 15. | Cribbs KA, Baisley WT, Lahue BJ, Peddu P. Clinical and safety outcomes in unresectable, very early and early-stage hepatocellular carcinoma following Irreversible Electroporation (IRE) and Transarterial Chemoembolization (TACE): A systematic literature review and meta-analysis. PLoS One. 2025;20:e0322113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 16. | Partridge BR, O'Brien TJ, Lorenzo MF, Coutermarsh-Ott SL, Barry SL, Stadler K, Muro N, Meyerhoeffer M, Allen IC, Davalos RV, Dervisis NG. High-Frequency Irreversible Electroporation for Treatment of Primary Liver Cancer: A Proof-of-Principle Study in Canine Hepatocellular Carcinoma. J Vasc Interv Radiol. 2020;31:482-491.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 17. | Dai Z, Wang Z, Lei K, Liao J, Peng Z, Lin M, Liang P, Yu J, Peng S, Chen S, Kuang M. Irreversible electroporation induces CD8(+) T cell immune response against post-ablation hepatocellular carcinoma growth. Cancer Lett. 2021;503:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 18. | Shi X, O'Neill C, Wang X, Chen Y, Yu Y, Tan M, Lv G, Li Y, Martin RC. Irreversible electroporation enhances immunotherapeutic effect in the off-target tumor in a murine model of orthotopic HCC. Am J Cancer Res. 2021;11:3304-3319. [PubMed] |
| 19. | Guo X, Du F, Liu Q, Guo Y, Wang Q, Huang W, Wang Z, Ding X, Wu Z. Immunological effect of irreversible electroporation on hepatocellular carcinoma. BMC Cancer. 2021;21:443. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 20. | Llovet JM, Castet F, Heikenwalder M, Maini MK, Mazzaferro V, Pinato DJ, Pikarsky E, Zhu AX, Finn RS. Immunotherapies for hepatocellular carcinoma. Nat Rev Clin Oncol. 2022;19:151-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 1229] [Article Influence: 307.3] [Reference Citation Analysis (3)] |
| 21. | Pinter M, Scheiner B, Pinato DJ. Immune checkpoint inhibitors in hepatocellular carcinoma: emerging challenges in clinical practice. Lancet Gastroenterol Hepatol. 2023;8:760-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 85] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 22. | Li Q, Han J, Yang Y, Chen Y. PD-1/PD-L1 checkpoint inhibitors in advanced hepatocellular carcinoma immunotherapy. Front Immunol. 2022;13:1070961. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 173] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 23. | Chen S, Zeng X, Su T, Xiao H, Lin M, Peng Z, Peng S, Kuang M. Combinatory local ablation and immunotherapies for hepatocellular carcinoma: Rationale, efficacy, and perspective. Front Immunol. 2022;13:1033000. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 24. | De Re V, Rossetto A, Rosignoli A, Muraro E, Racanelli V, Tornesello ML, Zompicchiatti A, Uzzau A. Hepatocellular Carcinoma Intrinsic Cell Death Regulates Immune Response and Prognosis. Front Oncol. 2022;12:897703. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Wu Z, Shan Q, Jiang Y, Huang W, Wang Z, Zhuang Y, Liu J, Li T, Yang Z, Li C, Wei T, Wen C, Cui W, Qiu Z, Liu X, Wang Z. Irreversible electroporation combined with PD-L1/IL-6 dual blockade promotes anti-tumor immunity via cDC2/CD4(+)T cell axis in MHC-I deficient pancreatic cancer. Cancer Lett. 2025;617:217620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 26. | Shao Q, O'Flanagan S, Lam T, Roy P, Pelaez F, Burbach BJ, Azarin SM, Shimizu Y, Bischof JC. Engineering T cell response to cancer antigens by choice of focal therapeutic conditions. Int J Hyperthermia. 2019;36:130-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 92] [Article Influence: 13.1] [Reference Citation Analysis (1)] |
| 27. | Polajzer T, Jarm T, Miklavcic D. Analysis of damage-associated molecular pattern molecules due to electroporation of cells in vitro. Radiol Oncol. 2020;54:317-328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 28. | He C, Sun S, Zhang Y, Xie F, Li S. The role of irreversible electroporation in promoting M1 macrophage polarization via regulating the HMGB1-RAGE-MAPK axis in pancreatic cancer. Oncoimmunology. 2021;10:1897295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 29. | Rubinsky B, Onik G, Mikus P. Irreversible electroporation: a new ablation modality--clinical implications. Technol Cancer Res Treat. 2007;6:37-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 498] [Cited by in RCA: 547] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 30. | Bulvik BE, Rozenblum N, Gourevich S, Ahmed M, Andriyanov AV, Galun E, Goldberg SN. Irreversible Electroporation versus Radiofrequency Ablation: A Comparison of Local and Systemic Effects in a Small-Animal Model. Radiology. 2016;280:413-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 98] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 31. | Narayanan JSS, Ray P, Hayashi T, Whisenant TC, Vicente D, Carson DA, Miller AM, Schoenberger SP, White RR. Irreversible Electroporation Combined with Checkpoint Blockade and TLR7 Stimulation Induces Antitumor Immunity in a Murine Pancreatic Cancer Model. Cancer Immunol Res. 2019;7:1714-1726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 92] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 32. | O'Neill C, Hayat T, Hamm J, Healey M, Zheng Q, Li Y, Martin RCG 2nd. A phase 1b trial of concurrent immunotherapy and irreversible electroporation in the treatment of locally advanced pancreatic adenocarcinoma. Surgery. 2020;168:610-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/