Published online Sep 15, 2025. doi: 10.4251/wjgo.v17.i9.110505
Revised: July 22, 2025
Accepted: August 20, 2025
Published online: September 15, 2025
Processing time: 99 Days and 22.2 Hours
Gastric oxyntic gland adenoma is a rare neoplasm often misdiagnosed as a hyperplastic or fundic gland polyp due to nonspecific endoscopic features and the limitations of superficial biopsies, leading to diagnostic delays.
In 2020, a gastroscopy of a 47-year-old man revealed a 0.5 cm lesion that was diagnosed as a hyperplastic polyp by superficial biopsy. By 2022, the lesion had enlarged to 1.0 cm exhibiting firmness, bleeding tendency, and disrupted sub
This case highlights the necessity of deep biopsy or complete resection for diagnosis. We recommend long-term endoscopic surveillance post-resection and heightened clinical vigilance to mitigate misdiagnosis risks.
Core Tip: This study presents the first systematic documentation of gastric oxyntic gland adenoma from misdiagnosis to definitive diagnosis. A 47-year-old male underwent two misdiagnosed biopsies before definitive histopathological confirmation via endoscopic submucosal dissection. We highlight gastric oxyntic gland adenoma’s nonspecific endoscopic presentation and the diagnostic pitfalls of superficial biopsies. Proposing a “deep biopsy or complete resection and early intervention” strategy, three-year follow-up demonstrated a favorable outcome without recurrence. These findings underscore the necessity of standardized sampling and timely management to mitigate diagnostic delays and potential malignant progression, offering critical evidence to refine clinical protocols for this under-recognized neoplasm.
- Citation: Xue RX, Lu XY, Deng P, Wang LH, Yun YF. Misdiagnosis of gastric oxyntic gland adenoma as hyperplastic polyp: A case report. World J Gastrointest Oncol 2025; 17(9): 110505
- URL: https://www.wjgnet.com/1948-5204/full/v17/i9/110505.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i9.110505
Gastric oxyntic gland tumor (GOGT) is a rare neoplasm of gastric glandular origin, typically composed of chief cells and often found in non-atrophic fundic mucosa. It may also occur in the context of chronic gastritis or familial adenomatous polyposis. According to the fifth edition of the World Health Organization (2019) classification of digestive system tumors, GOGT are classified into gastric oxyntic gland adenoma (GOGA) and gastric adenocarcinoma of fundic gland type (GA-FG). The difference between the two is whether the tumor infiltrates below the muscular layer of the mucosa or not. GOGA is confined to the mucosa and is a benign tumor, while GA-FG is slow-growing with a low rate of lymph node metastasis, and is regarded as a low-grade malignant tumor with a good prognosis[1]. Although GOGA is histologically benign, approximately 60% of cases exhibit submucosal invasion, which may predispose to progression toward GA-FG.
The prevalence of GOGA remains unclear, though a single-center study from Japan involving 7488 patients reported an incidence of 0.36%[2]. Clinical diagnosis of GOGA presents significant challenges: Endoscopic findings often reveal an intact mucosal surface without characteristic features; conventional endoscopic biopsies frequently fail to sample the deeper tumor tissue adequately[3-5].
As a result, accurate preoperative diagnosis is difficult, and many cases are confirmed only after endoscopic or surgical resection. Here, we report a case of GOGA that was initially misdiagnosed under endoscopy and subsequently confirmed through pathological examination, aiming to summarize its endoscopic and pathological features and discuss diagnostic and therapeutic implications, thereby enhancing clinicians’ awareness of this under-recognized entity.
A 47-year-old man from Beijing, was admitted to the Department of Gastrointestinal, Liver and Gallbladder in April 2022 with a submucosal gastric lesion detected over two years previously.
On admission, the patient had abdominal distension (occurring daily after meals, lasting 1-2 hours, self-rated severity 6/10 on a visual analog scale) and unintentional weight loss of 5 kg (from 75 kg to 70 kg, representing a 6.7% loss) over the past year.
December 2020: Initial esophagogastroduodenoscopy at an outside hospital revealed a 0.5 cm × 0.5 cm Is-type submucosal lesion near the gastric cardia, with surface congestion. Endoscopic ultrasonography (EUS) indicated a hypoechoic submucosal lesion, and a neuroendocrine tumor was considered. Superficial biopsy suggested a hyperplastic fundic gland polyp.
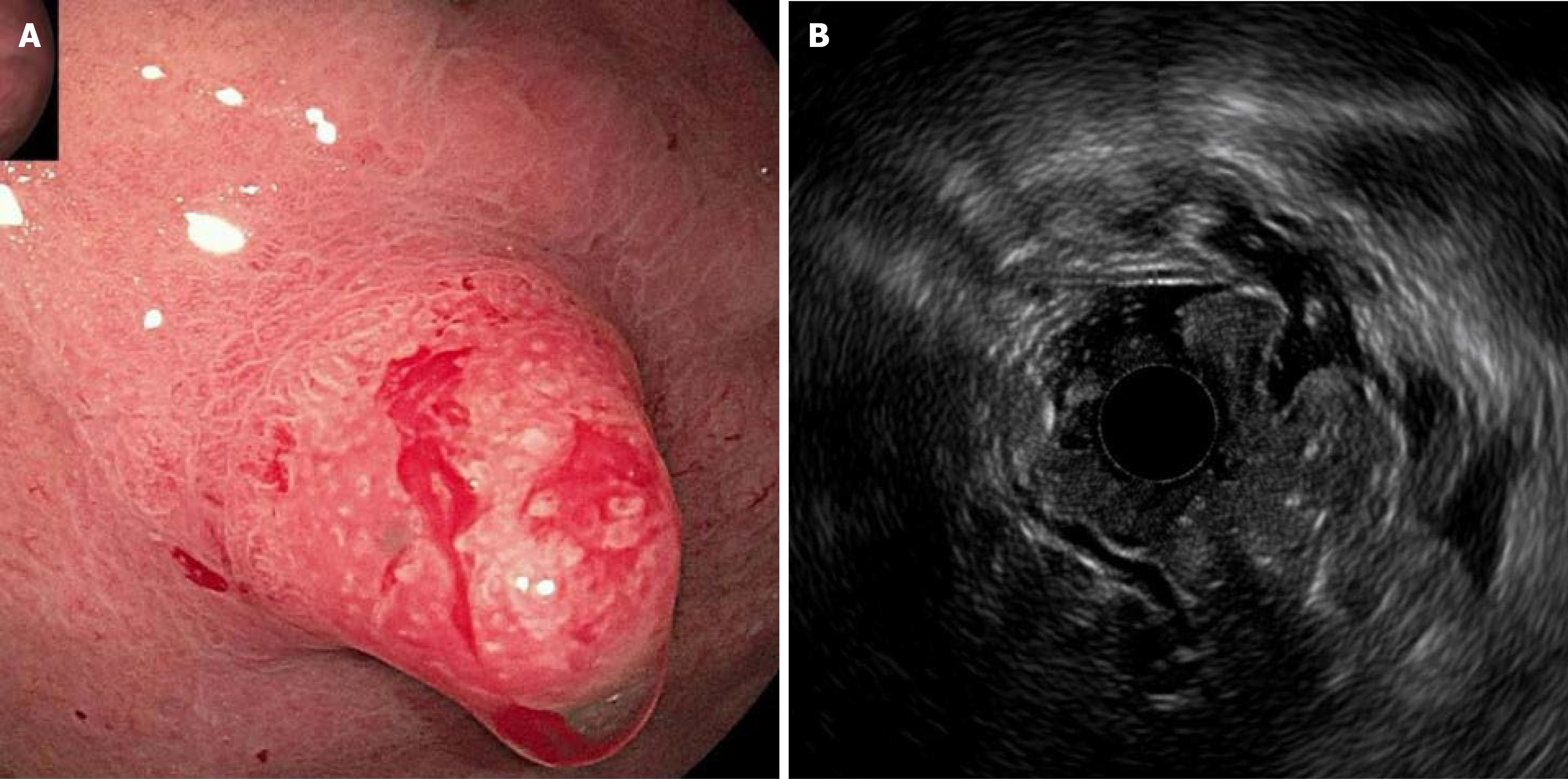
April 14, 2022: Repeat esophagogastroduodenoscopy at our hospital showed lesion enlargement to 1.0 cm × 0.8 cm, with a strawberry-like surface, firm texture, and bleeding tendency (Figure 1A). The surrounding mucosa was smooth, with no atrophy, intestinal epithelial hyperplasia, or Helicobacter pylori (H. pylori) infection. EUS demonstrated mucosal/submucosal thickening (1.95 cm to 2.50 cm), disruption of the muscularis mucosae, and indistinct layer boundaries (Figure 1B). Repeat biopsies were inconclusive, suggesting hyperplasia with moderate chronic inflammation.
The patient had a history of stable coronary artery disease (diagnosed 3 years prior, with no history of myocardial infarction or intervention), type 2 diabetes mellitus (glycated hemoglobin 7.2% at admission), and hyperlipidemia (low-density lipoprotein cholesterol 3.1 mmol/L at admission), managed with clopidogrel (75 mg daily), metformin (1000 mg twice daily), and atorvastatin (40 mg nightly). The patient denied a history of tuberculosis, typhoid fever, malaria, cerebrovascular disease, or mental illness. He had no history of trauma or surgery and denied food or drug allergies.
The patient had a 30-year smoking history, with an average of 20 cigarettes per day. He denied any significant history of heavy alcohol consumption, recent travel, surgery, or trauma history. He had no history of gastrointestinal malignancies or genetic disorders.
On physical examination, his vital signs were stable. The abdomen was soft, non-tender, with no palpable masses or organomegaly.
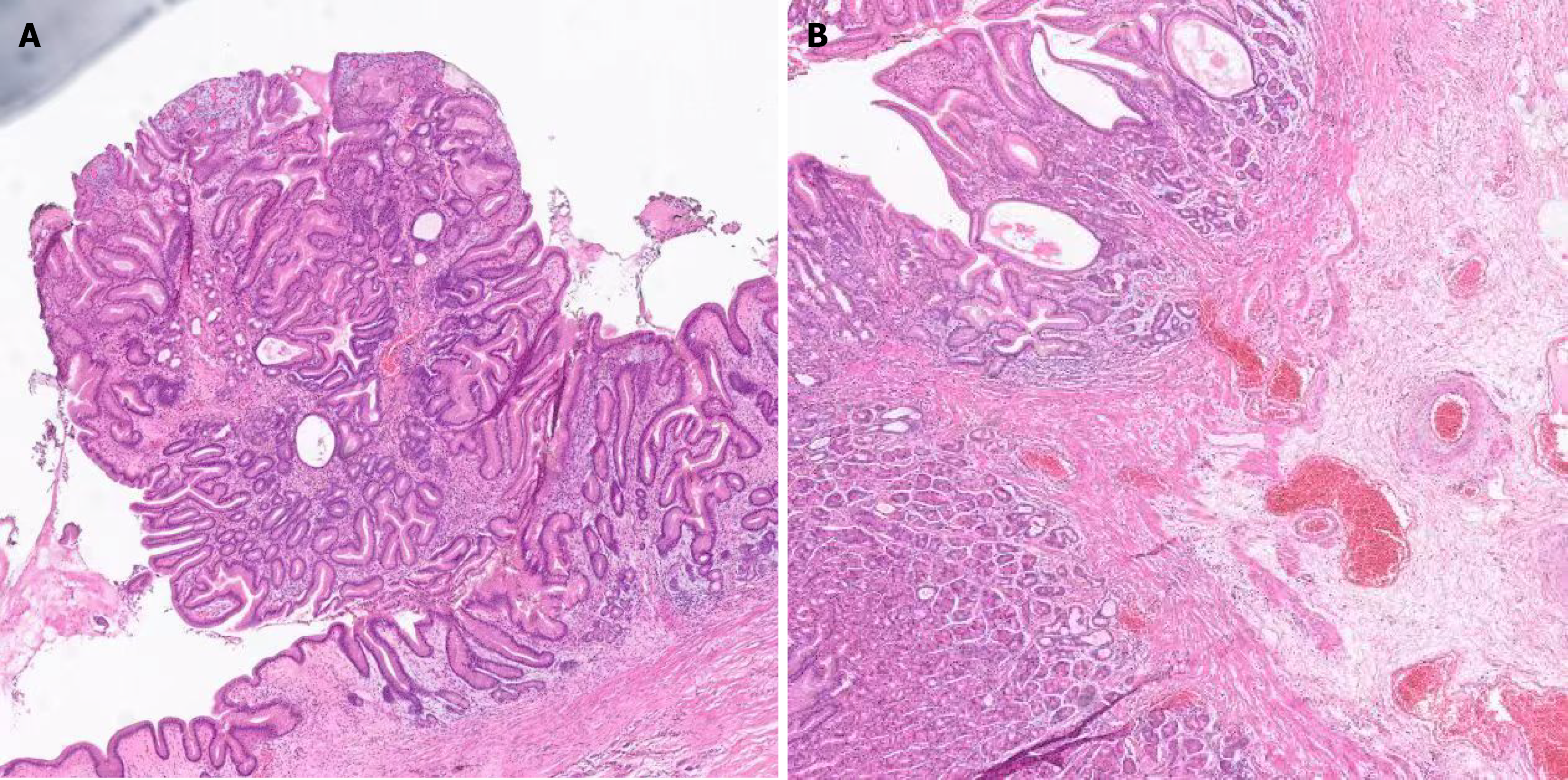
Endoscopic submucosal dissection (ESD) was performed on April 21, 2022. Histopathological examination showed that the lesion was confined to the mucosal layer, composed predominantly of parietal cell-differentiated glands with mild nuclear atypia and overlying normal foveolar epithelium (Figure 2). The immunohistochemical results indicated the following: Mucin 2 (+, small niche), mucin 5AC (+, small niche), mucin 6 (+), P53 (-), Ki67 (+, 3%), H. pylori (no significance), CD56 (+, small niche), salivary chromogranin A (+, small niche), and synuclein (+, small niche).
The patient’s postoperative abdominal computed tomography suggested only postoperative changes in the stomach, with no other abnormalities.
The final diagnosis was GOGA.
The patient underwent ESD and tissue specimens were examined pathologically.
Histopathological examination of the ESD specimen confirmed GOGA confined to the mucosa with negative deep margins. However, due to the lesion’s significant enlargement (0.5 cm to 1.0 cm), firm texture with bleeding tendency on endoscopy, and the EUS findings suggesting muscularis mucosae disruption and indistinct layer boundaries. A multidisciplinary team discussion recommended additional surgical resection to ensure the most definitive oncological clearance and obtain histologically confirmed negative lateral margins, given the theoretical risk of GA-FG progression associated with GOGA. The patient was then referred to a specialized surgical center for definitive oncological surgery. During the three-year follow-up, surveillance endoscopy demonstrated satisfactory healing with no signs of recurrence. However, localized mucosal thickening at the lesser curvature was observed on EUS (Figure 3). The patient remained asymptomatic and annual follow-up was recommended.
GOGT is an epithelial neoplasm originating from the oxyntic mucosa of the gastric body and fundus, and encompasses two major subtypes: GOGA and GA-FG[6]. These tumors are primarily composed of chief and parietal cells and typically arise in non-atrophic fundic gland mucosa, exhibiting varying degrees of oxyntic gland differentiation. To date, most studies on fundic gland-type tumors have been reported from Japan, likely due to the widespread use of endoscopic screening. Müller-Höcker and Rellecke[7] and Matsukawa et al[8] first described cases of fundic gland polyps characterized by predominant chief cell differentiation, architectural distortion, and mild cytological atypia. Currently, significant controversy persists in the classification and management of GOGA. Ueyama et al[9] first proposed the concept of GA-FG based on a series of ten cases, classified it as a low-grade malignant tumor with unique clinicopathological features. Later studies suggested that GOGA may represent an early, intramucosal stage of GA-FG and advocated early resection to prevent potential malignant progression[10]. Singhi et al[11] argued that these lesions lack overt malignant features and recommended reclassifying them as oxyntic gland polyps or adenomas based on observations of biological behavior, emphasizing that routine surveillance without aggressive intervention may be sufficient. More recently, Ushiku et al[12] introduced the entity of gastric adenocarcinoma of fundic gland mucosa type, characterized by additional differentiation toward foveolar and mucous neck cells, with a higher malignant potential. The divergence in current perspectives may be attributed to the histopathological features of GOGA, which demonstrate only minimal cytological atypia and architectural patterns that closely resemble normal fundic glands. In addition, most reported cases exhibit indolent clinical behavior, with a lack of long-term follow-up data confirming malignant progression[11]. Generally, the prevailing classification system currently categorizes oxyntic gland neoplasms into three subtypes: GOGA, GA-FG (low-grade), and gastric adenocarcinoma of fundic gland mucosa type (high-grade)[2,11].
Clinically, GOGA predominantly occurs in middle-aged men, with approximately 80% of lesions located in the upper third of the stomach, 18% in the middle third, and 1% in the lower third[13]. Most cases are discovered incidentally during endoscopic examinations performed for other indications, although some patients may present with gastroesophageal reflux-like symptoms. Endoscopically, GOGA typically appears as a solitary polypoid or submucosal lesion, measuring 3 mm to 10 mm, with mucosal color similar to or slightly different from the surrounding mucosa (yellowish, whitish, reddish, or pale yellow), often accompanied by surface vascular dilation[1,13]. Histopathological evaluation remains essential for definitive diagnosis, characterized by glands resembling normal oxyntic mucosa with mild nuclear atypia and subtle architectural irregularities. Recent studies recommend immunohistochemical markers such as mucin 6 positivity, mucin 5AC negativity, diffuse muscle intestine and stomach expression 1 expression, and reduced alpha1,4-linked N-acetylglucosamine glycosylation of mucin 6 as effective adjuncts for differentiation from benign lesions[14-16]. In terms of treatment, ESD is considered the first-line approach for GOGA due to its indolent biological behavior. Even in cases with superficial submucosal invasion (SM1 or SM2), if the tumor retains chief cell predominance without high-grade cytological atypia, the prognosis remains favorable[17].
In the present case, the patient’s clinical course and endoscopic findings were consistent with previous reports. The initial lesion, detected at an outside hospital, was small (0.5 cm) with surface hyperemia, and superficial biopsy only yielded a diagnosis of hyperplastic polyp. Two years later, the lesion had enlarged to 1.0 cm, with a firm texture and easy bleeding upon contact. EUS suggested disruption of the muscularis mucosae; however, repeated superficial biopsies again failed to yield a definitive diagnosis. Ultimately, complete resection via ESD provided adequate tissue for histological analysis, confirming GOGA. Microscopic examination revealed numerous well-differentiated glands composed of cells with weakly basophilic cytoplasm and mild nuclear atypia, interspersed with parietal-like cells characterized by eosinophilic granular cytoplasm and centrally located nuclei. No evidence of submucosal invasion was observed, and resection margins were negative. While GOGA is typically asymptomatic, the patient’s postprandial bloating and weight loss likely arose from the lesion’s mechanical effects on gastric motility and mucosal integrity. Tumor enlargement near the cardia may have impaired gastric accommodation, while chronic inflammation and micro-bleeding contributed to dyspeptic symptoms and subclinical nutritional deficits. Symptom resolution post-resection further corroborates the causal relationship, emphasizing that GOGA, though rare, should be considered in symptomatic patients with submucosal gastric lesions. In the present case, considering the lesion size and indistinct layer demarcation on EUS, additional surgical resection was performed following ESD to ensure negative margins. The patient has remained recurrence-free during the three-year follow-up period, although localized mucosal thickening at the lesser curvature was noted (Figure 3), which likely represents fibrotic changes secondary to ESD and subsequent surgical resection rather than residual or recurrent GOGA. Similar fibrotic changes have been documented following endoscopic resection of other gastric lesions, emphasizing the necessity of distinguishing procedural changes from true recurrence during ongoing surveillance.
In this case, we found that under endoscopy, GOGA exhibits distinct differentiating characteristics compared to hyperplastic polyps. Hyperplastic polyps are relatively soft and elastic in texture, usually smooth in surface, seldom show obvious abnormal vascular features, and have a low bleeding tendency; on the other hand, GOGA lesions are firm in texture, can be felt as resistance when examined, have a strawberry-like rough appearance, are associated with abnormal vasodilatation and an obvious bleeding tendency, and have a twisted, dendritic vascular pattern; GOGA lesions, on the other hand, have a hard texture, with palpable resistance during examination, presenting a characteristic strawberry-like rough appearance, accompanied by abnormal vascular dilation and a notable tendency for bleeding. The background mucosa of hyperplastic polyps typically shows changes of chronic inflammation, often accompanied by varying degrees of gastric mucosal atrophy and intestinal metaplasia, with some cases possibly coexisting with H. pylori infection; the boundaries of GOGA lesions are clearly defined, and the surrounding mucosa generally shows no significant atrophy or signs of intestinal metaplasia. Clinical diagnosis of GOGA remains challenging primarily due to nonspecific endoscopic features, making differentiation from benign polyps difficult. Typical lesions present as solitary submucosal nodules with color similar to the surrounding mucosa, often accompanied by vascular dilation and bleeding propensity. Moreover, superficial biopsies frequently fail to obtain deep glandular tissue, resulting in misdiagnosis as hyperplastic or fundic gland polyps. Our case notably underscores these limitations, as repeated superficial biopsies failed to yield a definitive diagnosis. Given these diagnostic challenges and potential for malignant progression, clinical vigilance towards GOGA should be heightened, and a standardized follow-up monitoring strategy should be established. We recommend the “deep biopsy or complete resection and early intervention” strategy for suspicious cases. For lesions less than 1.0 cm without alarming features (soft texture, minimal vascularity), annual monitoring by endoscopy is sufficient. For lesions measuring ≥ 1.0 cm or exhibiting firmness, marked vascular changes, or bleeding tendency, prompt ESD is advised for histological evaluation and definitive treatment. Post-resection, annual endoscopic surveillance for a minimum of three years is essential to identify recurrence early and distinguish procedural fibrosis from recurrent or residual disease, thus better controlling the risk of progression and improving the safety and effectiveness of diagnosis and treatment.
GOGA is a rare and under-recognized submucosal tumor. Its nonspecific endoscopic presentation and the limitations of superficial biopsy frequently result in misdiagnosis and underdiagnosis, suggesting that its true prevalence may be underestimated. Deep biopsy or ESD followed by annual endoscopic surveillance is recommended as a standardized clinical protocol. Currently, reports on GOGA are limited. This case systematically records the entire diagnostic process from initial misdiagnosis to completion of endoscopic resection and pathological diagnosis, emphasizing the importance of improving endoscopic diagnostic vigilance, implementing deep biopsy or complete resection, thereby enhancing clinical awareness and optimizing diagnostic and management strategies. As a single-case report, this study has inherent limitations regarding the generalizability of its findings and observations. The single-case design cannot provide robust epidemiological data on the incidence, natural history, or characteristics of GOGA across different populations. Further studies are needed to validate the long-term outcomes of early interventions in larger cohorts.
We would like to express our sincere gratitude to the clinical team for their excellent care of the patient and to the Department of Pathology for their expert diagnostic support. Special thanks to the patients for their informed consent and contribution to medical knowledge.
| 1. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2554] [Cited by in RCA: 2758] [Article Influence: 459.7] [Reference Citation Analysis (3)] |
| 2. | Asahara H, Takao T, Asahara Y, Asahara M, Motomura D, Sakaguchi H, Yoshizaki T, Ikezawa N, Takao M, Morita Y, Toyonaga T, Komatsu M, Kushima R, Kodama Y. Clinicopathological Features and the Prevalence of Oxyntic Gland Neoplasm: A Single-center Retrospective Study. Intern Med. 2023;62:2763-2774. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 3. | He MJ, Liu XY, Xu JX, Xu XY, Li QL, Chen WF, Zhou PH. Endoscopic submucosal dissection for gastric adenocarcinoma of the fundic gland type (chief cell predominate type): Four years’ experience from a tertiary hospital. J Dig Dis. 2022;23:620-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 4. | Benedict MA, Lauwers GY, Jain D. Gastric Adenocarcinoma of the Fundic Gland Type: Update and Literature Review. Am J Clin Pathol. 2018;149:461-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 5. | Miyazawa M, Matsuda M, Yano M, Hara Y, Arihara F, Horita Y, Matsuda K, Sakai A, Noda Y. Gastric adenocarcinoma of the fundic gland (chief cell-predominant type): A review of endoscopic and clinicopathological features. World J Gastroenterol. 2016;22:10523-10531. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 6. | Ueyama H, Matsumoto K, Nagahara A, Hayashi T, Yao T, Watanabe S. Gastric adenocarcinoma of the fundic gland type (chief cell predominant type). Endoscopy. 2014;46:153-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 7. | Müller-Höcker J, Rellecke P. Chief cell proliferation of the gastric mucosa mimicking early gastric cancer: an unusual variant of fundic gland polyp. Virchows Arch. 2003;442:496-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Matsukawa A, Kurano R, Takemoto T, Kagayama M, Ito T. Chief cell hyperplasia with structural and nuclear atypia: a variant of fundic gland polyp. Pathol Res Pract. 2005;200:817-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Ueyama H, Yao T, Nakashima Y, Hirakawa K, Oshiro Y, Hirahashi M, Iwashita A, Watanabe S. Gastric adenocarcinoma of fundic gland type (chief cell predominant type): proposal for a new entity of gastric adenocarcinoma. Am J Surg Pathol. 2010;34:609-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 168] [Article Influence: 10.5] [Reference Citation Analysis (1)] |
| 10. | Yamada S, Yamanoi K, Sato Y, Nakayama J. Diffuse MIST1 expression and decreased α1,4-linked N-acetylglucosamine (αGlcNAc) glycosylation on MUC6 are distinct hallmarks for gastric neoplasms showing oxyntic gland differentiation. Histopathology. 2020;77:413-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Singhi AD, Lazenby AJ, Montgomery EA. Gastric adenocarcinoma with chief cell differentiation: a proposal for reclassification as oxyntic gland polyp/adenoma. Am J Surg Pathol. 2012;36:1030-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 79] [Article Influence: 5.6] [Reference Citation Analysis (1)] |
| 12. | Ushiku T, Kunita A, Kuroda R, Shinozaki-Ushiku A, Yamazawa S, Tsuji Y, Fujishiro M, Fukayama M. Oxyntic gland neoplasm of the stomach: expanding the spectrum and proposal of terminology. Mod Pathol. 2020;33:206-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 13. | Krooks J, Thaker H, Qiu S, Reep G, He J. Oxyntic Gland Adenoma in a Patient With Refractory Reflux. Cureus. 2023;15:e38577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 14. | Iwamuro M, Kusumoto C, Nakagawa M, Kobayashi S, Yoshioka M, Inaba T, Toyokawa T, Hori S, Tanaka S, Matsueda K, Tanaka T, Okada H. Endoscopic resection is a suitable initial treatment strategy for oxyntic gland adenoma or gastric adenocarcinoma of the fundic gland type. Sci Rep. 2021;11:7375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 15. | Imamura K, Yao K, Nimura S, Tanabe H, Kanemitsu T, Miyaoka M, Ono Y, Ueki T, Iwashita A. Characteristic endoscopic findings of gastric adenocarcinoma of fundic-gland mucosa type. Gastric Cancer. 2021;24:1307-1319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 16. | Imamura K, Yao K, Nimura S, Tanabe H, Kanemitsu T, Miyaoka M. Numerous lesions of gastric adenocarcinoma of fundic-gland and fundic gland-mucosa type in a patient. Gastric Cancer. 2023;26:1069-1073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Chiba T, Kato K, Masuda T, Ohara S, Iwama N, Shimada T, Shibuya D. Clinicopathological features of gastric adenocarcinoma of the fundic gland (chief cell predominant type) by retrospective and prospective analyses of endoscopic findings. Dig Endosc. 2016;28:722-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/