Published online Sep 15, 2025. doi: 10.4251/wjgo.v17.i9.110527
Revised: July 6, 2025
Accepted: August 6, 2025
Published online: September 15, 2025
Processing time: 99 Days and 3.5 Hours
Transcription factor 3 (TCF3) has a vital role in tumor occurrence and progression. However, the specific functions and underlying mechanisms of dysregulated TCF3 in hepatocellular carcinoma (HCC) have not been not thoroughly characterized. Thus, we explored the roles of TCF3 in HCC.
To explore the roles of TCF3 in HCC.
TCF3 knockdown and overexpression models were developed via lentiviral vectors in HCC cells. Transwell and in vivo metastasis experiments were per
TCF3 levels were markedly elevated in HCC samples and correlated with poor prognosis. Furthermore, overexpression of TCF3 promoted HCC cell invasion as well as migration, while TCF3 knockdown repressed HCC cell growth. In addition, TCF3 interacted with the promoter region of matrix metalloproteinase-11 (MMP11), facilitating the transcriptional activation of MMP11 mRNA, which consequently enhanced the expression of MMP11. MMP11 knockdown repressed TCF3-associated HCC cell migration and invasion, while its overexpression attenuated the TCF3 knockdown-mediated repression of HCC growth. In human-derived HCC samples, TCF3 was positively correlated with MMP11 expression levels.
TCF3 was significantly upregulated in HCC. TCF3 overexpression enhanced HCC cell invasion and metastasis through transactivation of MMP11 expression. TCF3 could be a prognostic biomarker and regulator of HCC metastasis.
Core Tip: Elevated transcription factor 3 (TCF3) levels were observed in hepatocellular carcinoma (HCC) samples and were associated with unfavorable outcomes. Additionally, overexpression of TCF3 facilitated the invasion and migration of HCC cells, while knocking down TCF3 suppressed their growth. In addition, TCF3 interacts with the promoter region of matrix metalloproteinase-11 (MMP11), facilitating the transcriptional activation of MMP11 mRNA, which consequently enhances the expression of MMP11. MMP11 knockdown repressed TCF3-associated HCC cell migration and invasion, while its overexpression attenuated the TCF3 knockdown-mediated repression of HCC growth. In human-derived HCC samples, TCF3 was positively correlated with MMP11 expression levels.
- Citation: Tian HP, Wu TH, Zhang GJ, Li SJ. Transcription factor 3 enhances hepatocellular carcinoma metastasis by upregulating matrix metalloproteinase-11. World J Gastrointest Oncol 2025; 17(9): 110527
- URL: https://www.wjgnet.com/1948-5204/full/v17/i9/110527.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i9.110527
Hepatocellular carcinoma (HCC) is a common cancer and the leading cause of cancer-related mortality worldwide[1]. After surgery, metastasis is a major factor in the long-term survival of HCC patients and is strongly correlated with high recurrence and death rates[2]. Therefore, understanding the signaling pathways that may be involved and identifying key regulators of HCC metastasis is crucial for gaining insight into its pathomechanisms and potential therapeutic targets.
Transcription factor 3 (TCF3), or Tcf7 L1, is a member of the Lef/Tcf family of transcription factors, characterized by a highly conserved high-mobility group domain that binds to a specific recognition sequence[3]. TCF3 can be activated when it interacts with β-catenin and inhibited when it associates with Groucho/TLE co-repressors[4,5]. Additionally, TCF3 serves as a transcription factor, promoting the transcription of multiple genes[6-8].
Several studies have highlighted the importance of TCF3 in tumors. TCF3 is up-regulated in colorectal[9], prostate[10,11], gastric[12], and renal[13] cancers. Previous study[14] identified that the HN1 L-mediated AP-2γ/METTL13/TCF3-ZEB1 transcriptional axis facilitates the growth and metastasis of HCC. Additionally, TCF3 may interact with microRNA-449a to preserve self-renewal in liver cancer stem-like cells[15]. However, the biological role and clinical importance of TCF3 in HCC are not completely understood.
In this study, we evaluated the expression of TCF3 in HCC cells and tissues to confirm its prognostic significance. Additionally, the effects of TCF3 on the migration and invasion of HCC cells, along with the underlying pathomechanisms, were investigated.
Normal liver hepatocytes (cell line HL-7702) were obtained from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). The 293T and human HCC cell lines (JHH-7, Huh-7, Hep3B, MHCC97H, HCCLM3, and HCCLM6) were procured from the American Type Culture Collection. They were seeded in Dulbecco's modified Eagle's medium with 10% fetal bovine serum (FBS) (both from Gibco; Thermo Fisher Scientific, Inc.), 100 μg/mL penicillin, and 100 μg/mL streptomycin. Cells were incubated in 5% CO2 at 37 °C. Cell identities had been verified via short tandem repeat profiling and were free from contamination by mycoplasma.
From September 2007 to December 2008, a total of 79 HCC tissues and their matching non-tumor samples (≥ 3 cm from the cancer margin) were obtained from patients undergoing surgical removal of tumors at The Affiliated Hospital of North Sichuan Medical College (Nanchong, Sichuan Province, China). Three cases with distant metastases were disqualified from this study. We included patients clinically diagnosed with primary liver cancer. Patients were not given any preoperative anticancer treatment before surgery. All patients underwent liver cancer resection. The patients agreed to take part in the study and signed the informed consent form. The exclusion criteria were as follows: (1) HCC patients with distant metastasis or other tumors; and (2) Withdrawal from the study group for various reasons. After removal, tissue samples were immediately placed in liquid nitrogen and kept at -80 °C for subsequent assays. The Medical Ethical Committee of The Affiliated Hospital of North Sichuan Medical College approved the application for ethics clearance [No. 2016ER(A)008]. Informed consent was acquired from all HCC patients in this study. Postoperative survival of all patients was monitored for 3-60 months.
After 12 hours of fixation in paraformaldehyde (4%) at room temperature (RT), paraffin-embedded HCC tissues were prepared and sectioned into 4 μm slices for the subsequent experiments. Then, the sections were incubated at 60 °C for 2 hours, after which deparaffinization was done using dimethylbenzene. An alcohol series was then used to rehydrate sections. After these steps, paraffin-embedded HCC sections were incubated in 0.3% hydrogen peroxide for 30 minutes, followed by blocking in 10% bovine serum albumin for 1 hour at ambient temperature (Sangon Biotech Co., Ltd.). Overnight incubation of treated sections at 4 °C was done with rabbit anti-TCF3 (1:500; No. ab69999; Abcam) and rabbit anti-matrix metalloproteinase-11 (MMP11) (1:500; No. Ab119284; Abcam). Next, they were hatched at 37 °C with an horseradish peroxidase (HRP)-conjugated secondary antibody [1:1000; goat anti-rabbit immunoglobulin G (IgG) H and L; No. ab205718; Abcam] for 2 hours. Afterward, tissue section staining was done for 1 hour with DAB (OriGene Technologies, Inc.) followed by counterstaining for 1 minute with hematoxylin at RT. Following dehydration and sealing, light microscopy (Carl Zeiss AG) was used for visual inspection and imaging. Incubations with rabbit IgG were regarded as negative controls (NCs). Scores for immunostaining were based on intensity and positively stained cell percentages: (1) Negative (-), 0%, intermediate (+); (2) 5%-10%, moderate (+++); (3) 10%-25%, strong (+++); and (4) ≥ 26%. Determination of final scores was done by multiplying positive cell percentage scores with staining intensity scores. The scoring was conducted independently by two pathologists.
To construct lentiviral vectors with short hairpin (sh)RNA sequences, a pLKO.1-EGFP-PURO (No. FH1717; Hunan Fenghui Biotechnology Co., Ltd.) vector was used, with sequences designated as LV-shTCF3, LV-shMMP11, and LV-shcontrol. LV-shcontrol had a non-targeting shRNA control. The sequences for shRNA are listed in Supplementary Table 1. Lentiviral vectors containing human TCF3 and MMP11 gene sequences were developed in PLVX-EGFP-IRES-PURO and designated as LV-TCF3 and LV-MMP11, respectively. An empty vector was utilized as an NC and labeled as LV-control.
The pPACKH1 HIV Lentivector Packaging kit (Systems Bioscience, LLC) was used to generate stable cell lines. In brief, Lipofectamine® 3000 (Invitrogen; Thermo Fisher Scientific, Inc.) was utilized to transfect control plasmids (1.0 μg) and pPACKH1 packaging plasmid mix [5.0 μg; pPACKH1-REV (1.0 μg), pPACKH1-GAG (3.0 μg), and pVSV-G (1.0 μg) plasmids; third-generation packaging system] into 293T cells at an ambient temperature of 37 °C. After 24 hours, the culture medium was incubated overnight at 4 °C with 20% PEG-it reagent.
Pellets were washed in 1 × PBS, centrifuged at 72000 × g for 2 hours at 4 °C, and kept at -80 °C. Polybrene was used to transduce the viruses into Hep3B and HCCLM3 cells with 30-50 infection multiplicities for 24 hours at 37 °C. Subse
Shanghai SLAC Laboratory Animal Co., Ltd. provided 80 nude female BALB/C mice (6 weeks of age and weighing 20 g) for this study. All experimental animals were kept in a 60% humidified atmosphere with a 12-hour dark/Light cycle under a 60% humidified atmosphere at 24 °C. Pure drinking water and food were available. Ethical permission was acquired from The Animal Ethical Committee of The Affiliated Hospital of North Sichuan Medical College. For tail vein metastasis analysis in vivo, experimental mice (n = 10/group) were injected with 100 μL PBS containing 5 × 106 cells via the tail vein. Injected mice were divided into Hep3B (LV-TCF3 and LV-control), HCCLM3 (LV-shTCF3 and LV-shcontrol), Hep3B-TCF3 (LV-shMMP11 and LV-shcontrol), and HCCLM3-shTCF3 (LV-MMP11 and LV-Control) groups. Mouse survival was monitored daily for 9 weeks. The mice were sacrificed using CO2 (20% volume displacement/minute) asphyxiation and their lungs were removed. After collection of lung tissues, the specimens were embedded into paraffin, placed in paraformaldehyde (4%) overnight at RT, and sectioned into 4 μm slices via a microtome for further pathological elucidation. Finally, lung tumor metastases were measured via dissection microscopy (SZX7; Olympus Corporation).
After de-paraffinization with two xylene washes, the mouse lung tissue sections were rehydrated in gradient alcohol. Hematoxylin and Harris hematoxylin (Thermo Fisher Scientific, Inc.) were applied to the tissues after a brief wash with water. Slides were processed in acid alcohol (0.25%). Lithium carbonate was used for blueing while eosin solution (Thermo Fisher Scientific, Inc.) was used for counterstaining. Then, to dehydrate tissues, two washes in absolute and 95% ethanol were performed, followed by xylene clearing. A light microscope with a digital camera was used in conjunction with DPS-BSW v3.1 software (Olympus Corporation) to capture photomicrographs.
Chromatin immunoprecipitation (ChIP) analysis was done using the Magna ChIP G Assay kit (MilliporeSigma). In brief, cross-linking of transfected Hep3B cells for 10 minutes was done using 1% formaldehyde at 37 °C, after which they were quenched using glycine. At an ambient temperature of 4 °C, co-immunoprecipitations of bound DNA separated from sonicated cell lysates was done after incubation in the presence of primary antibodies against TCF3 (rabbit; 1:100; No. ab229605; Abcam) and normal IgG (rabbit; 1:100; No. 3900; Cell Signaling Technology, Inc.) for 12 hours. The corresponding promoter binding sites were amplified by PCR. The primer sequences for these PCR reactions are shown in Supplementary Table 1. A PCR system (Takara Biotechnology Co., Ltd.) and Taq DNA polymerase (No. EP0405; Thermo Fisher Scientific, Inc.) were used for PCR, with initial denaturation for 5 minutes at 94 °C; 35 cycles for 40 seconds at
Transwell inserts with polycarbonate membranes (pore size, 8.0 μm) were placed in 24-well plates. Before the invasion assays, the upper chamber was precoated with 50 μL Matrigel (Corning, Inc.) for 30 minutes at ambient temperature, with drying for 12 hours. Then, 1 × 104 and 1 × 105 experimental cells were placed into the top chamber for the subsequent invasion and migration analyses, respectively. Incubation was conducted for 24 hours with complete medium (600 μL) added to the lower chamber. Swabbing was carried out to remove the upper surface cells. The lower surface cells were fixed with 10% formalin at 25 °C for 20 minutes, and the mixture was then stained using crystal violet (0.1%) for 5 minutes at 25 °C. Inverted light microscopy was performed at × 10 (Olympus Corporation) to count the crystals.
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.) was used to extract RNA from HCC tissues and cells. Based on the manufacturer’s guidelines, the PrimeScript RT Reagent kit (Takara Bio, Inc.) was used for cDNA synthesis. SYBR Premix Ex Taq II (Takara Bio, Inc.) was used in conjunction with the ABI 7500 Real-Time PCR system to measure TCF3 mRNA expression. After RNA extraction and cDNA synthesis, an ABI PRISM7900 system (Applied Biosystems; Thermo Fisher Scientific, Inc.) and the Human Tumor Metastasis RT2 Profiler PCR array (SuperArray Bioscience) were utilized for RT-PCR. The qPCR thermocycling parameters were: (1) 10 minutes at 95 °C; (2) 40 cycles for 30 seconds at 95 °C; (3) 30 seconds at 60 °C; (4) 30 seconds at 72 °C; and (5) A final extension step for 2 minutes at 72 °C. Samples without a cDNA template served as the NCs. Analyses of amplification curves were conducted using SDS 1.9.1 software (Applied Biosystems; Thermo Fisher Scientific, Inc.). Expression levels of corresponding genes in cell lines or HCC samples were evaluated by the 2-ΔΔCq method[10] and the following formula: ΔCq = ΔCqtarget - ΔCqGAPDH, ΔΔCq = ΔCqexpression vector - ΔCqcontrol vector (in cells) and ΔΔCq = ΔCqtumor - ΔCqnontumor (HCC tissues). Normalized expression levels were compared to those of matched control cells or liver tissues, which were set to 1. The target gene primer sequences are shown in Supplementary Table 1.
To extract cellular total proteins, the RIPA lysis buffer with proteinase inhibitors was utilized (Beijing Solarbio Science and Technology Co., Ltd.). Then, the BCA assay was used to evaluate protein concentrations. Proteins (50 μg/Lane) in specimens were extracted through 10% gels sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes. Before overnight incubation at 4 °C with primary antibodies against TCF3 (rabbit; 1:1000; No. ab69999; Abcam), MMP11 (rabbit; 1:1000; No. 30615-1-AP; Proteintech), and the control GAPDH (rabbit; 1:500; No. Ab245355; Abcam), the membranes were blocked using 5% defatted milk at indoor temperature for 1.5 hours. Then, they were hatched with secondary antibodies (antirabbit, HRPconjugated; 1:5000; No. sc-2357; Santa Cruz Biotechnology, Inc.) for 1 hour at 37 °C. Electrochemiluminescence reagent (No. WBKLS0500; MilliporeSigma) was used for protein band visualization while the ImageJ software (version 1.8.0; National Institutes of Health) was utilized to semi-quantify protein levels. GAPDH was regarded as a loading control, and assays were performed for 3 biological repeats.
The plasmid vectors were developed in accordance with standard procedures and specific primers (Supplementary Table 1). To magnify the MMP11 promoter sequences (-1918/+189), which were separated from Hep3B cells by a genomic DNA extraction kit (No. ab156900; Abcam), PCR were conducted using a PCR system and Takara LA Taq polymerase (Takara Biotechnology Co., Ltd.). The thermocycling parameters were: (1) Initial denaturation for 5 minutes at 94 °C; (2) 35 cycles for 30 seconds at 94 °C; (3) 30 seconds at 60 °C; (4) 40 seconds at 72 °C; and (5) A final extension for 5 minutes at 72 °C. This sequence is located in the 5'-flanking region of the human MMP11 gene at transcriptional start site (-1918/+189). For vector construction, reverse and forward primers were respectively incorporated into the 3'-ends and 5'-ends of HindIII and KpnI sites. PCR products were inserted over the digested KpnI and HindIII sites in pGL3-Basic vector (Promega Corporation). In addition, mutants lacking a 5'-flanking region of the MMP11 promoter [(-1918/+189) MMP11, (-1655/+189) MMP11, (-1209/+189) MMP11, and (-1030/+189) MMP11] were created using the (-1918/+189) MMP11 vector. Our experiments used the QuikChange II Site-Directed Mutagenesis kit (Stratagene; Agilent Technologies, Inc.) to make mutations in the TCF3-binding sites of MMP11 promoter. Vector construction was affirmed via first DNA sequencing (Sangon Biotech Co., Ltd.).
To construct TCF3 expression plasmids, we cloned TCF3 DNA into the pCMV-tag2A vectors (Agilent Technologies, Inc.). Hep3B or HCCLM3 cells were cultivated in a twenty-four-well plate at 1 × 105 cells/well. After 12-24 hours incubation, co-transfections of expression plasmids [0.6 μg; pCMV-TCF3 or control (pCMV-Tag)], pRL-TK plasmids (0.02 μg) and reporter plasmids (0.18 μg) (Promega Corporation) into experimental cells was done by the Lipofectamine 3000 reagent. At 5-hour post-infection, cells were washed and put in fresh medium containing 1% FBS for 48 hours to recover. Then, cells were serum-starved for analysis. Based on manufacturer's guidelines, detection of luciferase activities was performed using a Dual-Luciferase Assay Kit (Promega Corporation). Lysed transfected cells were placed in Eppendorf microcentrifuge tubes centrifuged at 4 °C and 72000 × g for 120 minutes. Relative luciferase activities were determined by a ModulusTM TD20/20 Luminometer (Turner Designs). Luciferase activity was normalized to that of Renilla. Assays were performed in triplicate.
FireBrowse was used to identify the differential mRNA expression of the TCF3 gene in various cancers. To validate the TCF3 protein expression, immunohistochemical results for TCF3 were acquired from The Human Protein Atlas (THPA; proteinatlas.org). Furthermore, the expression of TCF3 was evaluated according to the presence of nodal metastases, cancer stage, and tumor grade in subgroups of HCC patients using The University of ALabama at Birmingham CANcer data analysis Portal (UALCAN), while Kaplan-Meier curves of overall survival (OS) were obtained from UALCAN. The University of California Santa Cruz (UCSC) Genome Browser was used to find the promoter sequence of MMP11, and all promoter sequences were analyzed using JASPAR website. The threshold for determining relative profile scores was set at 85%. After evaluation via the JASPAR website, data were downloaded. UCSC and cBioPortal databases were also utilized to assess the relationships between TCF3 and MMP11 expression levels in HCCs.
Continuous data are displayed as mean ± SD. The χ² test was used to analyze categorical data. Student’s t-tests and one-way analysis of variance followed by Tukey’s or Dunnett’s post hoc tests, were carried out for comparison of means between and among groups, respectively. Survival analyses were conducted following surgery using the Kaplan-Meier method, and a log-rank test was performed for comparison of survival outcomes. Spearman's rank correlation analyses were conducted to investigate associations between TCF3 and MMP11 Levels in HCC samples. Statistical Package for the Social Sciences software (version 19; IBM Corp.) was used for the analyses, while graphs were prepared using GraphPad Prism (version 9; GraphPad Software, Inc.), P < 0.05 denoted significance.
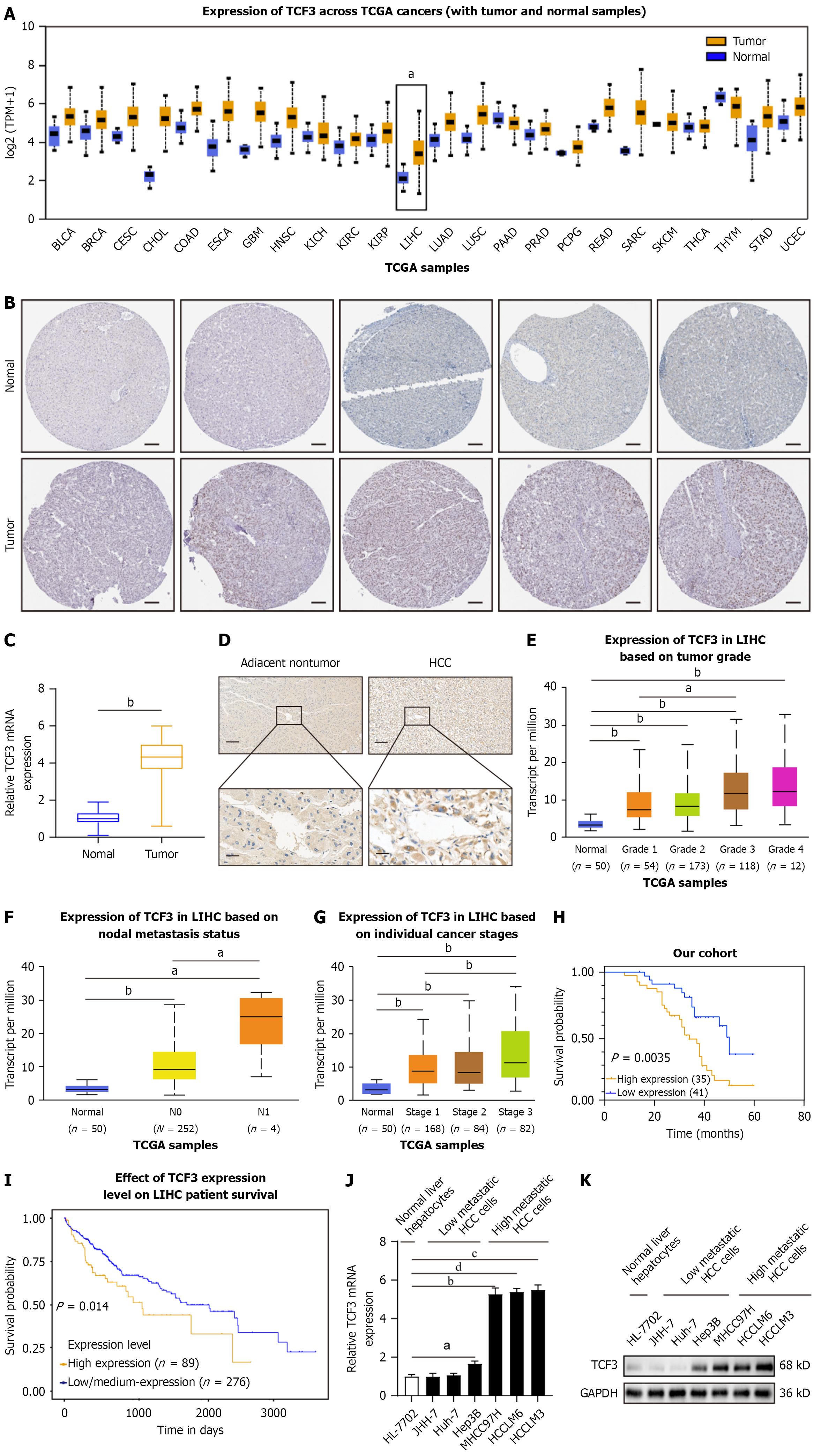
Differential expression of TCF3 in HCC tissues was verified using the FireBrowse database. TCF3 expression levels were higher in liver HCC (LIHC) samples than in the adjacent normal tissues (Figure 1A). Immunohistochemistry revealed that TCF3 expression was elevated in LIHC tissues according to the THPA database (Figure 1B). In addition, the levels of TCF3 protein and mRNA expression were evaluated in 76 pairs of newly resected HCC and normal samples to validate the bioinformatics analysis results. As illustrated in Figure 1C and D, TCF3 Levels were elevated in tumor samples compared to healthy specimens, consistent with the bioinformatics analysis.
We further studied TCF3 expression in patient groups categorized by clinical characteristics, utilizing the UALCAN tool. TCF3 Levels were significantly elevated in grade 3 specimens compared to grade 1 specimens, grade 2 specimens, and normal controls (Figure 1E). Based on cancer nodal metastasis, patients with LIHC classified as N1 showed higher TCF3 Levels than those classified as N0 and the corresponding normal controls (Figure 1F). Regarding tumor stage, stage 3 LIHC patients exhibited significant rises in TCF3 Levels (Figure 1G). Moreover, higher levels of TCF3 were linked to microvascular invasion (Table 1). The Kaplan-Meier analysis indicated that survival outcomes were poorer for patients with TCF3-positive tumors than for those with TCF3-negative tumors (Figure 1H), verified by Kaplan-Meier survival analyses of UALCAN data (Figure 1I). In summary, these results indicate that TCF3 Levels were increased in HCC and are associated with cancer progression and metastasis.
| Variables | TCF3 expression of low (n = 41) | TCF3 expression of high (n = 35) | P value |
| Age (years) | |||
| < 60 | 19 | 15 | 0.761 |
| ≥ 60 | 22 | 20 | |
| Gender | |||
| Male | 23 | 20 | 0.927 |
| Female | 18 | 15 | |
| Hepatitis B virus infection | |||
| Negative | 12 | 6 | 0.215 |
| Positive | 29 | 29 | |
| Cirrhosis | |||
| Absent | 13 | 10 | 0.767 |
| Present | 28 | 25 | |
| Alpha-fetoprotein | |||
| Elevatory | 26 | 26 | 0.310 |
| Normal | 15 | 9 | |
| Differentiation | |||
| Well | 16 | 8 | 0.0326 |
| Moderate | 20 | 14 | |
| Poor | 5 | 13 | |
| Tumor size | |||
| ≤ 5 cm | 25 | 16 | 0.183 |
| > 5 cm | 16 | 19 | |
| Number of tumors | |||
| Single | 33 | 22 | 0.087 |
| Multiple | 8 | 13 | |
| Lymph node metastasis | |||
| Absent | 29 | 11 | |
| Present | 12 | 24 | 0.001 |
| Microvascular invasion | |||
| Absent | 31 | 18 | 0.028 |
| Present | 10 | 17 | |
| American Joint Committee on Cancer stage | |||
| I | 15 | 3 | 0.0107 |
| II | 6 | 11 | |
| III | 20 | 21 |
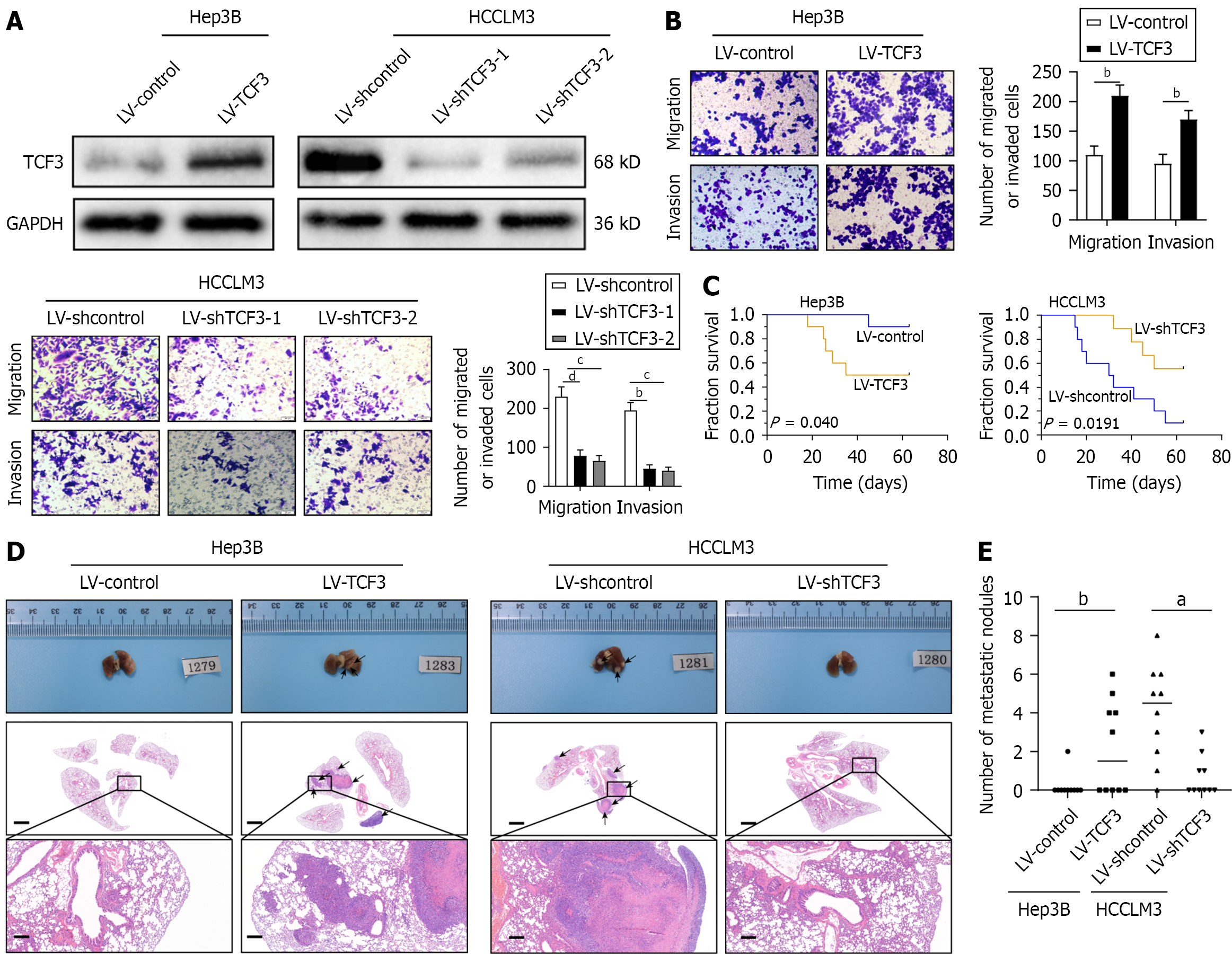
Compared to cell lines with low metastatic potential, those with high metastatic potential in HCC exhibited higher levels of TCF3 expression (Figure 1J and K, Supplementary Figure 1A). To investigate the role of TCF3 in HCC cell invasion and metastasis, we developed stable cell lines named Hep3B-TCF3 and HCCLM3-shTCF3 (Figure 2A, Supplementary Figure 1B). Transwell assays demonstrated that higher TCF3 expression notably boosted Hep3B cell migration and invasion, whereas reducing TCF3 decreased HCCLM3 cell migration and invasion (Figure 2B). Furthermore, in vivo studies on metastasis indicated that boosting TCF3 resulted in a higher frequency of lung metastases and an increase in the number of metastatic lung nodules, although the OS of the Hep3B-TCF3 group was reduced (Figure 2C-E, Table 2). When TCF3 Levels were lowered, there was a reduction in lung metastasis and nodules formed from metastatic lung tissue, along with decreased mortality in the HCCLM3-shTCF3 group (Figure 2C-E, Table 2). Therefore, TCF3 promotes both invasion and metastasis in HCC.
| Lung metastasis | |
| Hep3B-control | 1/10 |
| Hep3B-TCF3 | 5/10 |
| HCCLM3-shcontrol | 9/10 |
| HCCLM3-shTCF3 | 4/10 |
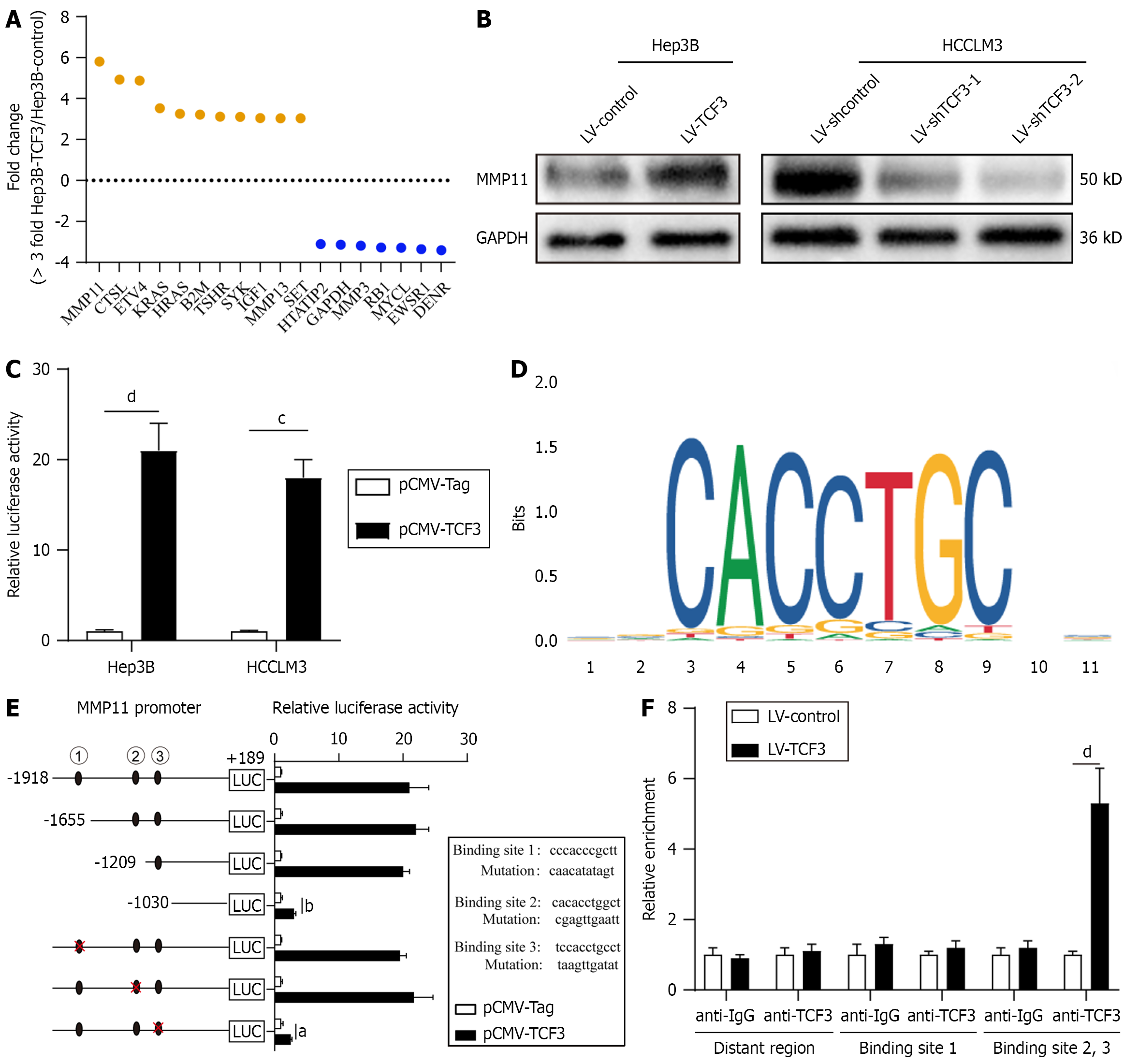
To clarify how TCF3 contributes to the progression of HCC, we examined changes in the transcriptome of Hep3B cells caused by TCF3 overexpression using the Metastasis RT Profiler PCR array[2]. With a cutoff value of a threefold change, 11 out of 89 metastasis-related genes were upregulated by TCF3 overexpression in Hep3B cells, 7 genes were downre
To determine whether TCF3 triggers MMP11 expression, we analyzed MMP11 protein levels in cells where TCF3 was either overexpressed or reduced. Elevated TCF3 expression led to higher MMP11 protein levels, while reduced TCF3 expression caused a decrease in MMP11 protein levels (Figure 3B, Supplementary Figure 1C). To investigate whether TCF3 activates MMP11 transcription, the MMP11 promoter constructs and pCMV-TCF3 were co-transfected. The luci
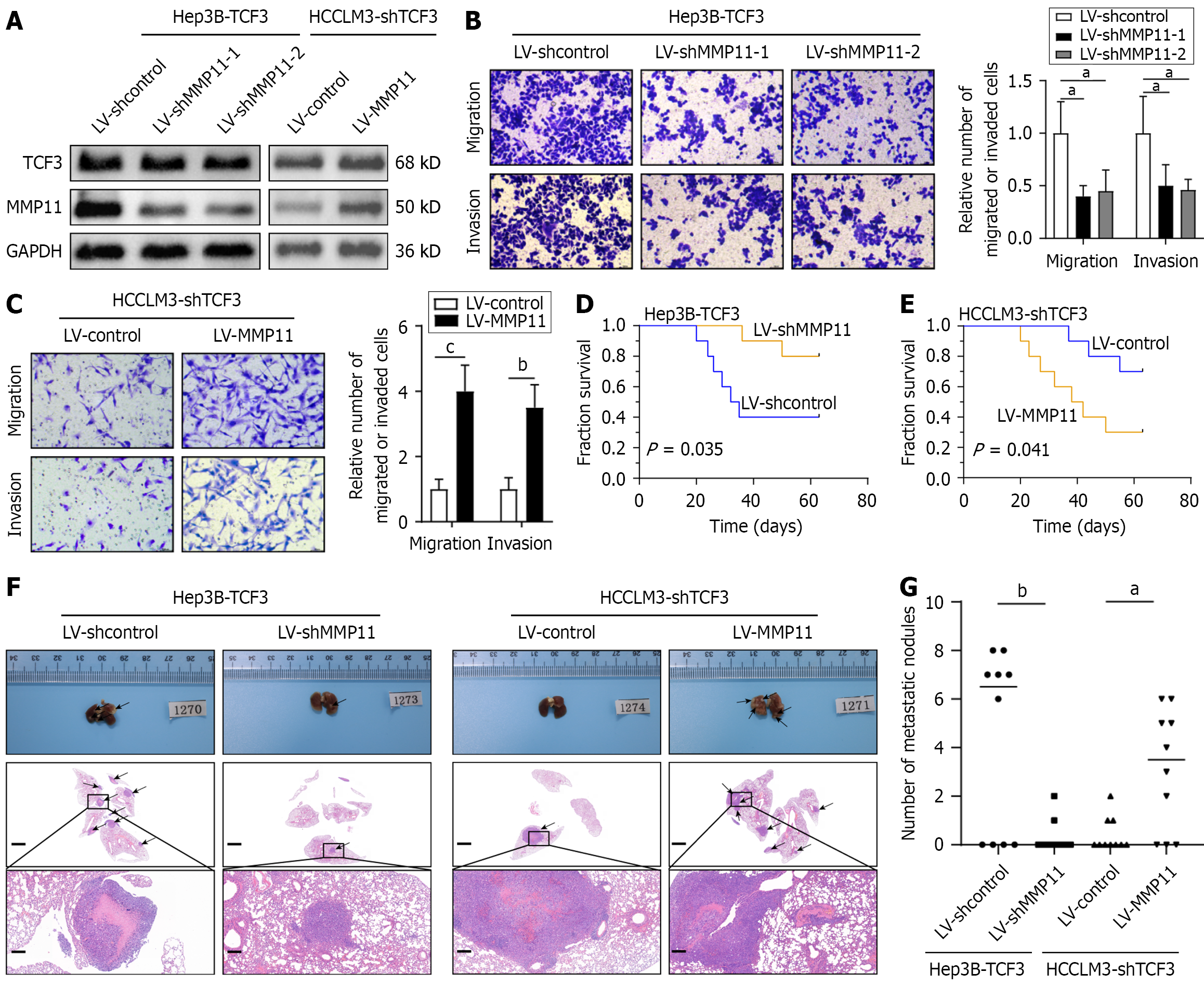
The effectiveness of MMP11 knockdown and overexpression was confirmed using Western blotting (Figure 4A, Supplementary Figure 1D). Lowering MMP11 expression significantly impeded TCF3-associated cell migration and invasion (Figure 4B); Conversely, elevated levels of MMP11 negated the decrease in migration and invasion capacities induced by TCF3 suppression (Figure 4C). In vivo, Hep3B cells with elevated TCF3 and suppressed MMP11 demonstrated poor meta
| Lung metastasis | |
| Hep3B-TCF3 + LV-shcontrol | 6/10 |
| Hep3B-TCF3 + LV-shMMP11 | 2/10 |
| HCCLM3-shTCF3 + LV-control | 3/10 |
| HCCLM3-shTCF3 + LV-MMP11 | 7/10 |
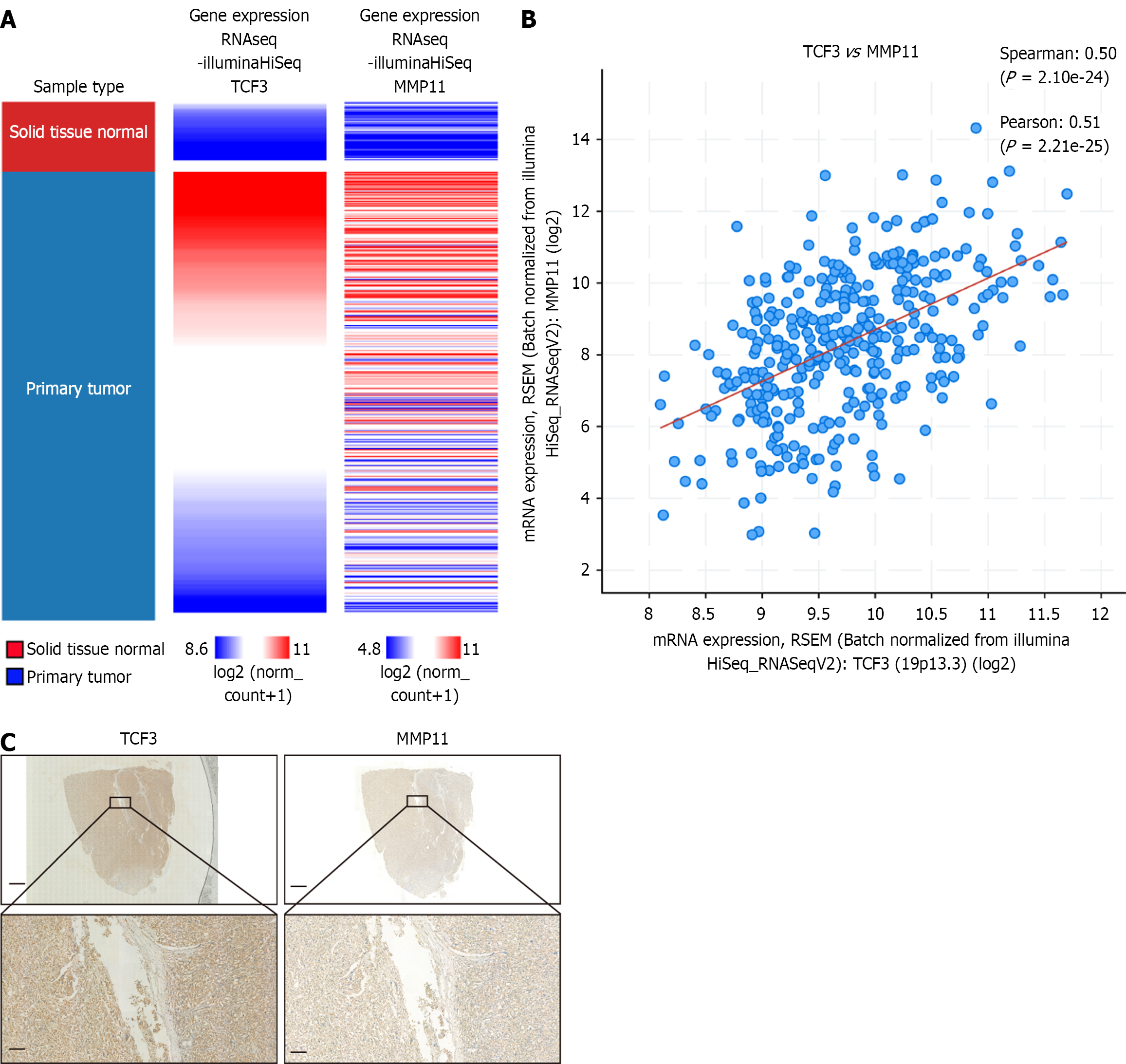
The University of California Santa Cruz Xena browser and cBioportal database were utilized to assess the correlation between TCF3 and MMP11 expression levels in HCC. We found that increased MMP11 mRNA expression was associated with elevated TCF3 mRNA expression (Figure 5A and B).
Our cohort examined the connection between TCF3 and MMP11 Levels in HCC tissues. The positive relationship between TCF3 and MMP11 expression was further validated by IHC staining and correlation analyses (Figure 5C, Table 4).
| Transcription factor 3 | ||||
| Positive (n = 35) | Negative (n = 41) | |||
| Matrix metalloproteinase-11 | Positive (n = 30) | 20 | 10 | P = 0.004 |
| Negative (n = 46) | 15 | 31 | ||
The major cause of HCC's high recurrence and mortality is tumor metastasis. Several reports have indicated that serum amyloid A, CircANTXR1, silencer of death domains, and apoptosis-linked gene 2 enhance the metastasis of HCC[16-18]. The underlying molecular mechanisms of HCC metastasis and potential prognostic biomarkers have yet to be estab
Few investigations have been conducted on the clinical importance of TCF3 for tumors. Li et al[19] revealed that recurrent colorectal cancer tissues had much higher TCF3 Levels than tissues without recurrence. High expression of TCF3 promotes proliferation and metastasis of gastric cancer cells[20]. Furthermore, higher TCF3 Levels were linked to unfavorable prognostic outcomes in Chinese patients with nasopharyngeal carcinoma[21]. Recent studies[22,23] have shown that TCF3 promotes the proliferation and metastasis of HCC. However, no further exploration of the mechanism has been conducted. Therefore, this study aimed to address the shortcomings of previous research. Our findings indicated that TCF3 Levels were markedly elevated in HCC tissues and correlated with more advanced clinical stages, microvascular invasion, and lymph node metastasis. Compared to patients with negative TCF3 expression, those with positive expression had shorter OS times in HCC cases. Consistent with these results, four HCC cell lines showed significant upregulation of TCF3 expression compared to the normal human hepatocyte cell line. TCF3 Levels were found to be elevated in metastatic HCC cells (MHCC97H, HCCLM6, and HCCLM3) compared to non-metastatic ones (JHH-7, Huh-7, and Hep3B) among the six HCC cell lines. These results indicate that increased expression of TCF3 could be involved in the metastasis of HCC.
In poorly differentiated human breast cancers, the transcription factor TCF3 is overexpressed, enhancing sphere formation in cells[24]. TCF3 promotes glioma progression by activating DNMT1 transcription, which inhibits SFRP1 expression[25]. Furthermore, TCF3 promotes cell growth and provides resistance against the apoptosis caused by doxorubicin in prostate cancer[11]. We created two stable cell lines with either increased or decreased TCF3 expression to investigate its biological functions in HCC. Increased TCF3 expression significantly boosted the invasion and metastasis of HCC cells, whereas suppressing TCF3 had the opposite effect. The variation in TCF3's biological roles across various cancers might stem from differences in cellular genetic background factors, such as viral infections and genetic mutations.
MMPs play a role in the invasion and metastasis of tumor cells[26]. In various cancers, MMP11 functions as an oncogene. Inhibiting MMP11 can, for instance, reduce the metastasis of gastric cancer[27]. In HCC[28], MMP11 influences proliferation and metastasis, and it also serves as a key driver gene in lung adenocarcinoma[29]. Suppressing MMP11 hindered the advancement and growth of lung adenocarcinoma tumors[30]. MMP11 genetic variations might be markers for the advancement of HCC[31]. MMP11 is mechanistically linked to HCC proliferation and metastasis, functioning as a target gene for miR-125a[32]. Therefore, MMP11 is an essential oncogene in HCC; however, the mechanisms underlying its dysregulation in HCC remain unidentified. We found that TCF3 directly targets MMP11 and increases its expression by binding to its promoter. Suppressing MMP11 significantly reduced TCF3-driven HCC cell migration, invasion, and lung metastasis, whereas increasing MMP11 Levels counteracted the decrease in malignancy caused by TCF3 knockdown.
Biologically, there is a positive correlation between TCF3 and MMP11 expression, which leads to enhanced HCC metastasis due to TCF3's activation of MMP11.
TCF3 is markedly elevated in HCC, and its overexpression promotes HCC cell invasion and metastasis by transactivating MMP11 expressions. Overall, TCF3 could be considered a potential marker for prognosis and a target for therapy in HCC.
| 1. | Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74:12-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2279] [Cited by in RCA: 6068] [Article Influence: 3034.0] [Reference Citation Analysis (4)] |
| 2. | El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2881] [Cited by in RCA: 3129] [Article Influence: 208.6] [Reference Citation Analysis (0)] |
| 3. | Miao Q, Ku AT, Nishino Y, Howard JM, Rao AS, Shaver TM, Garcia GE, Le DN, Karlin KL, Westbrook TF, Poli V, Nguyen H. Tcf3 promotes cell migration and wound repair through regulation of lipocalin 2. Nat Commun. 2014;5:4088. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 4. | Arce L, Yokoyama NN, Waterman ML. Diversity of LEF/TCF action in development and disease. Oncogene. 2006;25:7492-7504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 333] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 5. | MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17:9-26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4613] [Cited by in RCA: 4635] [Article Influence: 272.6] [Reference Citation Analysis (1)] |
| 6. | Jia H, Wu D, Zhang Z, Li S. TCF3-activated FAM201A enhances cell proliferation and invasion via miR-186-5p/TNKS1BP1 axis in triple-negative breast cancer. Bioorg Chem. 2020;104:104301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Su D, Ju Y, Han W, Yang Y, Wang F, Wang T, Tang J. Tcf3-activated lncRNA Gas5 regulates newborn mouse cardiomyocyte apoptosis in diabetic cardiomyopathy. J Cell Biochem. 2020;121:4337-4346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Zhou D, Fan J, Liu Z, Tang R, Wang X, Bo H, Zhu F, Zhao X, Huang Z, Xing L, Tao K, Zhang H, Nie H, Zhang H, Zhu W, He Z, Fan L. TCF3 Regulates the Proliferation and Apoptosis of Human Spermatogonial Stem Cells by Targeting PODXL. Front Cell Dev Biol. 2021;9:695545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 9. | Kijanka G, Hector S, Kay EW, Murray F, Cummins R, Murphy D, MacCraith BD, Prehn JH, Kenny D. Human IgG antibody profiles differentiate between symptomatic patients with and without colorectal cancer. Gut. 2010;59:69-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 10. | Asirvatham AJ, Carey JP, Chaudhary J. ID1-, ID2-, and ID3-regulated gene expression in E2A positive or negative prostate cancer cells. Prostate. 2007;67:1411-1420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Patel D, Chaudhary J. Increased expression of bHLH transcription factor E2A (TCF3) in prostate cancer promotes proliferation and confers resistance to doxorubicin induced apoptosis. Biochem Biophys Res Commun. 2012;422:146-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Sagara N, Katoh M. Mitomycin C resistance induced by TCF-3 overexpression in gastric cancer cell line MKN28 is associated with DT-diaphorase down-regulation. Cancer Res. 2000;60:5959-5962. [PubMed] |
| 13. | Krishnamachary B, Zagzag D, Nagasawa H, Rainey K, Okuyama H, Baek JH, Semenza GL. Hypoxia-inducible factor-1-dependent repression of E-cadherin in von Hippel-Lindau tumor suppressor-null renal cell carcinoma mediated by TCF3, ZFHX1A, and ZFHX1B. Cancer Res. 2006;66:2725-2731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 339] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 14. | Li L, Zheng YL, Jiang C, Fang S, Zeng TT, Zhu YH, Li Y, Xie D, Guan XY. HN1L-mediated transcriptional axis AP-2γ/METTL13/TCF3-ZEB1 drives tumor growth and metastasis in hepatocellular carcinoma. Cell Death Differ. 2019;26:2268-2283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 15. | Zhang Q, Yang Z, Shan J, Liu L, Liu C, Shen J, Chen X, Xu Y, Chen J, Ma Q, Yang L, Qian C. MicroRNA-449a maintains self-renewal in liver cancer stem-like cells by targeting Tcf3. Oncotarget. 2017;8:110187-110200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Huang C, Yu W, Wang Q, Huang T, Ding Y. CircANTXR1 Contributes to the Malignant Progression of Hepatocellular Carcinoma by Promoting Proliferation and Metastasis. J Hepatocell Carcinoma. 2021;8:1339-1353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 17. | Li G, Shen Q, Xu H, Zhou Y, Li C, Li Y, He M. SAA1 identified as a potential prediction biomarker for metastasis of hepatocellular carcinoma via multi-omics approaches. Front Oncol. 2023;13:1138995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 18. | Wang S, Gao F, Sun Y, Yu X, Bai Q, Tang J, Wang X. Expression Characteristics of SODD and ALG-2 as Possible Biomarkers for Evaluating Lymphatic Metastasis Potential of Hepatocarcinoma in a Mouse Model. Cell Mol Biol (Noisy-le-grand). 2022;67:167-173. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 19. | Li C, Cai S, Wang X, Jiang Z. Hypomethylation-associated up-regulation of TCF3 expression and recurrence in stage II and III colorectal cancer. PLoS One. 2014;9:e112005. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Mohammadi M, Salehzadeh A, Talesh Sasani S, Tarang A. The miR526b-5p-Related Single Nucleotide Polymorphisms, rs72618599, Located in 3'-UTR of TCF3 Gene, is Associated with the Risk of Breast and Gastric Cancers. Iran Biomed J. 2022;26:53-63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 21. | Shen X, Yuan J, Zhang M, Li W, Ni B, Wu Y, Jiang L, Fan W, Tian Z. The increased expression of TCF3 is correlated with poor prognosis in Chinese patients with nasopharyngeal carcinoma. Clin Otolaryngol. 2017;42:824-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Feng W, Liu Y, Chen J, Qiao C, Xia L, Wu K. [T cell factor 3 (TCF3) is overexpressed in hepatocellular carcinoma and promotes their invasion and metastasis]. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2022;38:66-73. [PubMed] |
| 23. | Pu XY, Zheng DF, Lv T, Zhou YJ, Yang JY, Jiang L. Overexpression of transcription factor 3 drives hepatocarcinoma development by enhancing cell proliferation via activating Wnt signaling pathway. Hepatobiliary Pancreat Dis Int. 2022;21:378-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | Slyper M, Shahar A, Bar-Ziv A, Granit RZ, Hamburger T, Maly B, Peretz T, Ben-Porath I. Control of breast cancer growth and initiation by the stem cell-associated transcription factor TCF3. Cancer Res. 2012;72:5613-5624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 70] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 25. | Zeng W, Jiang H, Wang Y, Wang C, Yu B. TCF3 Induces DNMT1 Expression to Regulate Wnt Signaling Pathway in Glioma. Neurotox Res. 2022;40:721-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Shay G, Lynch CC, Fingleton B. Moving targets: Emerging roles for MMPs in cancer progression and metastasis. Matrix Biol. 2015;44-46:200-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 338] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 27. | Xu G, Zhang B, Ye J, Cao S, Shi J, Zhao Y, Wang Y, Sang J, Yao Y, Guan W, Tao J, Feng M, Zhang W. Exosomal miRNA-139 in cancer-associated fibroblasts inhibits gastric cancer progression by repressing MMP11 expression. Int J Biol Sci. 2019;15:2320-2329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 132] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 28. | 28 Yang L, Si H, Ma M, Fang Y, Jiang Y, Wang J, Zhang C, Xiao H. LINC00221 silencing prevents the progression of hepatocellular carcinoma through let-7a-5p-targeted inhibition of MMP11. Cancer Cell Int. 2021;21:202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 29. | Dali-Youcef N, Hnia K, Blaise S, Messaddeq N, Blanc S, Postic C, Valet P, Tomasetto C, Rio MC. Matrix metalloproteinase 11 protects from diabesity and promotes metabolic switch. Sci Rep. 2016;6:25140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 30. | Yang H, Jiang P, Liu D, Wang HQ, Deng Q, Niu X, Lu L, Dai H, Wang H, Yang W. Matrix Metalloproteinase 11 Is a Potential Therapeutic Target in Lung Adenocarcinoma. Mol Ther Oncolytics. 2019;14:82-93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 31. | Wang B, Hsu CJ, Lee HL, Chou CH, Su CM, Yang SF, Tang CH. Impact of matrix metalloproteinase-11 gene polymorphisms upon the development and progression of hepatocellular carcinoma. Int J Med Sci. 2018;15:653-658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 32. | Bi Q, Tang S, Xia L, Du R, Fan R, Gao L, Jin J, Liang S, Chen Z, Xu G, Nie Y, Wu K, Liu J, Shi Y, Ding J, Fan D. Ectopic expression of MiR-125a inhibits the proliferation and metastasis of hepatocellular carcinoma by targeting MMP11 and VEGF. PLoS One. 2012;7:e40169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 164] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/