Published online Sep 15, 2025. doi: 10.4251/wjgo.v17.i9.110981
Revised: July 2, 2025
Accepted: July 31, 2025
Published online: September 15, 2025
Processing time: 88 Days and 0.9 Hours
Gastric cancer remains a leading cause of cancer-related mortality worldwide. Both Helicobacter pylori (H. pylori) infection and alterations in serum gastrin levels have been implicated in its pathogenesis. However, their associations with tumor characteristics and clinical outcomes require further clarification.
To investigate the associations of serum gastrin and H. pylori infection with path
This hospital-based cohort study included 226 gastric cancer patients undergoing surgery and 100 matched controls from January 2019 to December 2023. Serum gastrin and H. pylori status were assessed and compared. Gastric cancer patients were stratified by biomarker status to analyze associations with tumor-nodes-metastasis (TNM) stage, lymph node metastasis, and tumor differentiation. Kaplan-Meier analysis was used to evaluate disease-free and overall survival (OS). Statistical significance was set at P < 0.05.
Gastric cancer patients exhibited significantly higher serum gastrin levels and H. pylori infection rates than controls (P < 0.05). Among gastrin-positive patients, the proportions of advanced TNM stage (III-IV), lymph node metastasis, and poorly differentiated tumors were significantly higher than in gastrin-negative patients (P < 0.05). In contrast, H. pylori infection status showed no significant association with TNM stage, lymph node metastasis, or tumor differentiation (P > 0.05). Kaplan-Meier analysis indicated no significant difference in disease-free survival between gastrin-positive and negative patients (hazard ratio = 1.516, 95% confidence interval: 0.895-2.550), but gastrin-positive patients had significantly worse OS (hazard ratio = 2.717, 95% confidence interval: 1.311-5.633).
Gastric cancer patients have elevated serum gastrin and higher H. pylori prevalence; elevated gastrin is associated with aggressive tumor features and poorer OS, indicating prognostic value.
Core Tip: This study explores the clinical relevance of serum gastrin and Helicobacter pylori (H. pylori) infection in gastric cancer. Among 226 patients, elevated serum gastrin, but not H. pylori status, was significantly associated with advanced tumor stage, lymphatic metastasis, and poor differentiation. Notably, gastrin-positive patients had worse overall survival. These findings suggest that hypergastrinemia may serve as a biomarker of tumor aggressiveness and prognosis in gastric cancer, offering potential targets for risk stratification and therapeutic intervention.
- Citation: Huang JW, Lin C, Lu CJ, Wang HS, Zou DD. Serum gastrin and Helicobacter pylori infection correlate with tumor aggressiveness and prognosis in gastric cancer. World J Gastrointest Oncol 2025; 17(9): 110981
- URL: https://www.wjgnet.com/1948-5204/full/v17/i9/110981.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i9.110981
Gastric cancer is a common type of malignant tumor and is the third most common cause of cancer-related deaths globally. Individuals with advanced-stage stomach cancer are confronted with a poor prognosis, having a 5-year survival rate that only varies between 20% and 30%[1]. Surgical intervention continues to be the sole curative approach for tr
Gastrin, a crucial gastrointestinal hormone primarily secreted by G-cells, plays a physiological role in gastric acid secretion and gastrointestinal growth, exerting trophic effects on the entire gastrointestinal tract[5]. The relationship between gastrin and gastric cancer has garnered considerable interest. Similar to findings in cell culture experiments, gastrin has been recognized as a factor that enhances the growth of human gastric cancer cell lines[6]. Proton pump inhibitors (PPIs), which can be used to elevate gastrin levels, have raised questions regarding the potential link between the risk of gastrointestinal cancers and PPI use. However, conclusive evidence is lacking[7]. There is a suggestion that the use of PPIs could potentially lower the risk of gastric cancer by neutralizing the effects of gastrin, which are known to be proliferative and anti-apoptotic[8,9]. Research has confirmed that high concentrations of gastrin occur in gastric cancer tissue. This correlation is linked to later stages of the disease, the presence of cancer in lymph nodes, and a prognosis that is less promising.
As a common infection of the gastric mucosa, Helicobacter pylori (H. pylori) infection can lead to gastritis and other gastric disorders, including gastric ulcer and gastric cancer[10]. The resulting chronic inflammation and H. pylori infection are pivotal in the genesis and advancement of gastric cancer. This infection can lead to an upregulation of gastrin secretion, producing excessive gastric acid. Consequently, the gastric mucosa becomes irritated and damaged, increasing the risk of gastritis, gastric ulcer, and gastric cancer. Several factors contribute to how H. pylori stimulates gastrin release, including increased stimulation of inflammatory cytokines and local pH changes caused by H. pylori’s urease production. These factors collectively promote gastrin release within the gastric antrum. Conversely, gastrin release decreases after eradication or suppressing H. pylori infection[11]. H. pylori infection and gastrin may synergistically contribute to gastric cancer development, given that research has shown H. pylori infections accelerate gastric carcinogenesis in insulin-gastrin transgenic mice[12]. However, there is a paucity of clinical evidence regarding the biological roles and mechanisms of H. pylori and gastrin in the pathological progression and prognostic regression of gastric cancer. Therefore, this research endeavors to explore the relationship between circulating gastrin levels, clinical and H. pylori infection, and pathological characteristics among gastric cancer patients.
This was an observational study with two arms (gastric cancer patients and matched healthy controls) and no int
Inclusion criteria: (1) Meeting the relevant diagnostic criteria outlined in the standardized diagnostic and treatment guidelines for gastric cancer (trial implementation); (2) Ages ranging from 18 to 80 years, irrespective of gender; (3) First surgical treatment with confirmed diagnosis through surgical resection and pathological examination; and (4) Absence of other digestive system diseases.
Exclusion criteria: (1) History of cancer recurrence after treatment; (2) Presence of concurrent malignant tumors; (3) Severe liver or kidney dysfunction; (4) Projected life expectancy of under six months; (5) Prior receipt of radiotherapy or other anticancer treatments before enrollment; (6) Diagnosis of systemic inflammatory disease, cirrhosis or chronic liver failure, or abnormal renal function; and (7) Coexistence of psychiatric or neurological conditions that would impede coo
Peripheral venous blood samples of 3 to 5 mL were taken prior to surgery from fasting subjects with newly diagnosed gastric cancer, and blood samples from subjects in the control group were collected when they presented for physical exa
Information regarding regular use of PPI or PCAB was collected through medical interviews. Of the 226 gastric cancer patients, 72 (31.9%) had a history of regular PPI/PCAB use, and 65 (28.8%) reported use within the past month. To miti
All patients underwent a carbon-14 urea breath test (14C-UBT) prior to surgery to assess active H. pylori infection. To minimize false-negative results, patients were instructed to discontinue antibiotics and bismuth compounds for at least 30 days and PPIs for 14 days prior to testing. After overnight fasting, patients ingested a 14C-urea capsule, and exhaled samples were collected after 25 minutes for analysis using a liquid scintillation counter. An activity level ≥ 100 disintegrations per minute (dpm) was defined as positive for H. pylori infection. Thus, only current infection was assessed. Based on retrospective chart review, 38 gastric cancer patients (16.8%) and 20 control subjects (20%) had documented history of eradicated H. pylori infection, though these individuals were not excluded or separately analyzed due to incomplete eradication records. We acknowledge that this may have led to misclassification and potentially affected the associations observed.
However, it is important to note that the UBT only detects current infection status and does not account for past infe
Postoperative follow-up assessments were conducted until January 2025, incorporating outpatient or inpatient reviews and telephone callbacks. The follow-up protocol encompassed monitoring disease-free survival (DFS) and overall survival (OS).
To evaluate whether long-term PPI or PCAB use influenced serum gastrin levels and confounded the association with tumor characteristics, we conducted a stratified and adjusted analysis. Based on medication history, patients were categorized into three groups. (1) No use group: No history of PPI/PCAB use; (2) Past use group: History of regular use but none in the past month; and (3) Recent use group: Use within the past month. Serum gastrin levels were compared across the three groups using one-way ANOVA. To assess whether PPI/PCAB use confounded the relationship between gastrin levels and tumor grade, we performed multivariable logistic regression with tumor grade as the dependent var
The survival curves were derived from the survival package within the R programming language. Data analysis was carried out using version 23.0 of the SPSS statistical software. Numerical data were presented as the mean ± SD, and the independent samples t-test was used to evaluate the differences between the two groups. DFS and OS were depicted using Kaplan-Meier survival curves. Discrepancies between the two groups were assessed employing the Log-rank test. Serum gastrin levels were compared across the three groups using one-way ANOVA. To assess whether PPI/PCAB use confounded the relationship between gastrin levels and tumor grade, we performed multivariable logistic regression with tumor grade as the dependent variable and serum gastrin and PPI group as independent variables. Each test was conducted with a two-tailed approach, the threshold for statistical significance established at P < 0.05.
The levels of gastrin and H. pylori were quantitatively measured and compared. The gastrin level in the gastric cancer group was 26.02 ± 5.93 pmol/L, and the H. pylori quantitative detection was 275.42 ± 42.36 dpm. The gastrin level was 14.02 ± 4.10 pmol/L, and the H. pylori quantitative detection was 163.36 ± 39.63 dpm in the control group (Table 1). Gastrin levels and H. pylori positivity were markedly elevated among gastric cancer patients as opposed to the control group (P < 0.01 for all comparisons).
| n | Gastrin level (pmol/L) | Helicobacter pylori quantitative assays (disintegrations per minute) | |
| Gastric cancer group | 226 | 26.02 ± 7.93 | 275.42 ± 62.36 |
| Control group | 100 | 16.02 ± 4.10 | 163.36 ± 59.63 |
| t | 18.09 | 12.99 | |
| P value | < 0.001 | < 0.001 |
Based on the serum gastrin level, the gastric cancer group was divided into a gastrin-negative group (≤ 17.65 pmol/L) and a gastrin-positive group (> 17.65 pmol/L), which consisted of 90 and 136 cases, respectively. The rate of gastrin positivity was 60.18%. An examination of the clinicopathological features of gastric cancer across the two groups was undertaken, with the outcomes detailed in Table 2. Among the gastrin-negative patients, 39 cases were in stage III-IV, 51 were in tumor-nodes-metastasis (TNM) stage I-II, 21 had lymph node metastasis, 23 had low differentiation, and 67 had middle and high differentiation. Among the gastrin-positive patients, 86 cases were in stage III-IV, 50 were in TNM stage I-II, 52 had lymph node metastasis, 54 had low differentiation, and 82 had middle and high differentiation. Patients in the gastrin-positive group had notably higher rates of lymph node metastasis, TNM stage III-IV, and poorly differentiated tumors than the gastrin-negative group, with each difference achieving statistical significance (P < 0.05 for each com
| Gastrin-negative | Gastrin-positive | χ2 | P value | |
| n | 90 | 136 | ||
| TNM staging | 9.637 | 0.002 | ||
| I-II | 51 | 50 | ||
| III-IV | 39 | 86 | ||
| Lymphatic metastasis | 5.899 | 0.015 | ||
| No | 69 | 84 | ||
| Yes | 21 | 52 | ||
| Degrees of differentiation | 5.265 | 0.022 | ||
| Medium-high differentiation | 67 | 82 | ||
| Low differentiation | 23 | 54 |
Gastric cancer patients were divided into two categories: Those with H. pylori infection and those without, determined by the detection of H. pylori, which comprised 173 and 53 cases, respectively. The H. pylori-positive rate was 76.55%. The comparison of gastric cancer’s clinicopathological traits between the two groups is depicted in Table 3, presenting the findings. Among the H. pylori-negative patients, 28 cases were in stage III-IV, 18 had lymph node metastasis, 25 were in TNM stage I-II, 22 had low differentiation, and 31 had middle and high differentiation. Among the H. pylori-positive patients, 96 cases were in stage III-IV, 77 were in TNM stage I-II, 56 had lymph node metastasis, 56 had low differentiation, and 117 had middle and high differentiation. No significant statistical disparities were observed when comparing the two groups’ TNM stage, lymph node involvement, and differentiation grade (P > 0.05 for all comparisons).
| Helicobacter pylori-negative | Helicobacter pylori-positive | χ2 | P value | |
| n | 53 | 173 | ||
| TNM staging | 0.208 | 0.648 | ||
| I-II | 25 | 77 | ||
| III-IV | 28 | 96 | ||
| Lymphatic metastasis | 0.109 | 0.741 | ||
| No | 35 | 117 | ||
| Yes | 18 | 56 | ||
| Degrees of differentiation | 1.614 | 0.204 | ||
| Medium-high differentiation | 31 | 117 | ||
| Low differentiation | 22 | 56 |
As shown in Table 3, patients were categorized into H. pylori-positive and negative groups based on 14C-UBT results. Comparison of pathological features between these groups revealed no statistically significant differences in TNM staging (χ² = 0.208, P = 0.648), presence of lymphatic metastasis (χ² = 0.109, P = 0.741), or degree of differentiation (χ² = 1.614, P = 0.204). These findings indicate that UBT-defined H. pylori status did not meaningfully correlate with key indicators of tumor severity, suggesting potential limitations in the diagnostic classification based solely on current infection.
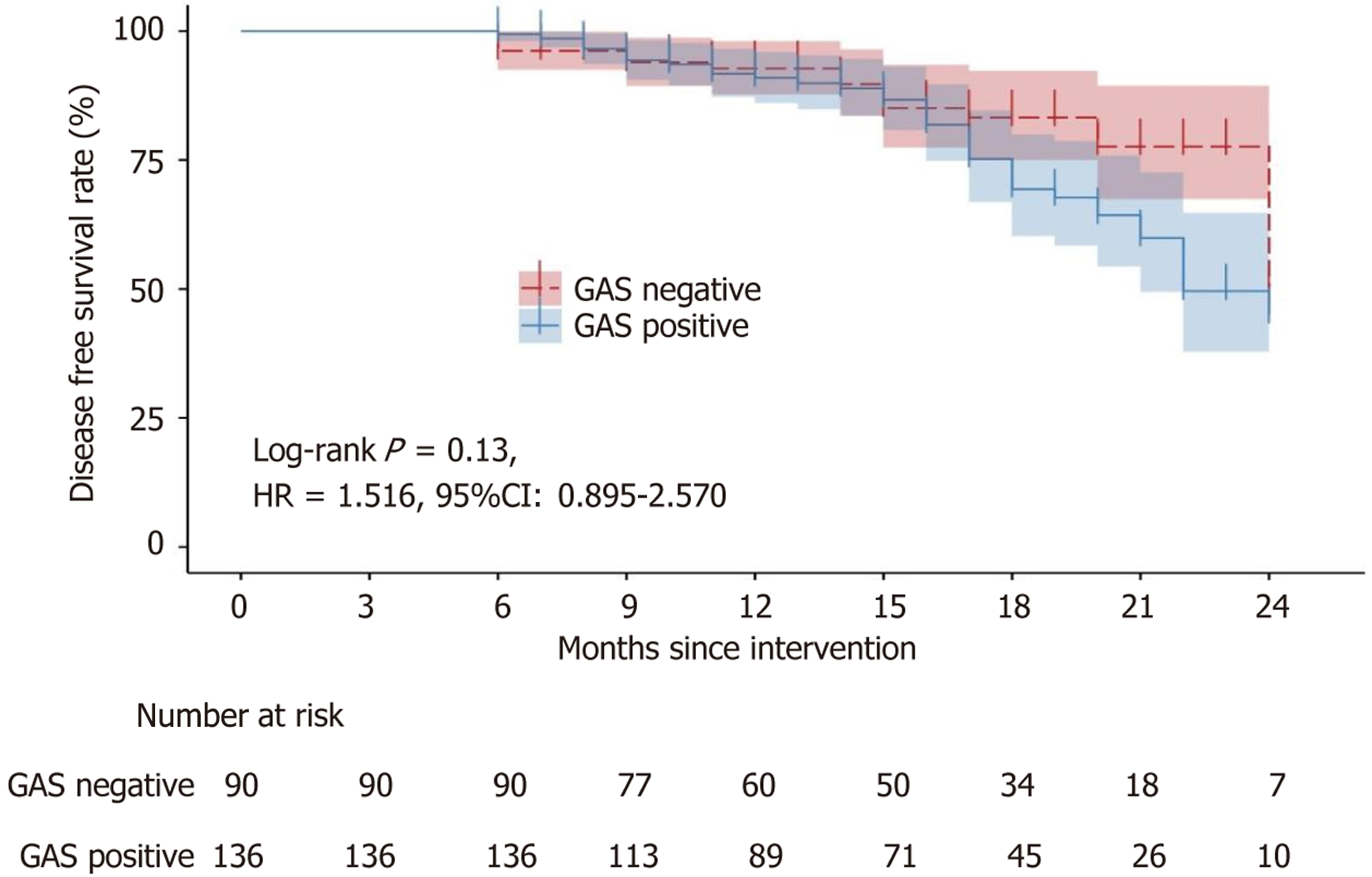
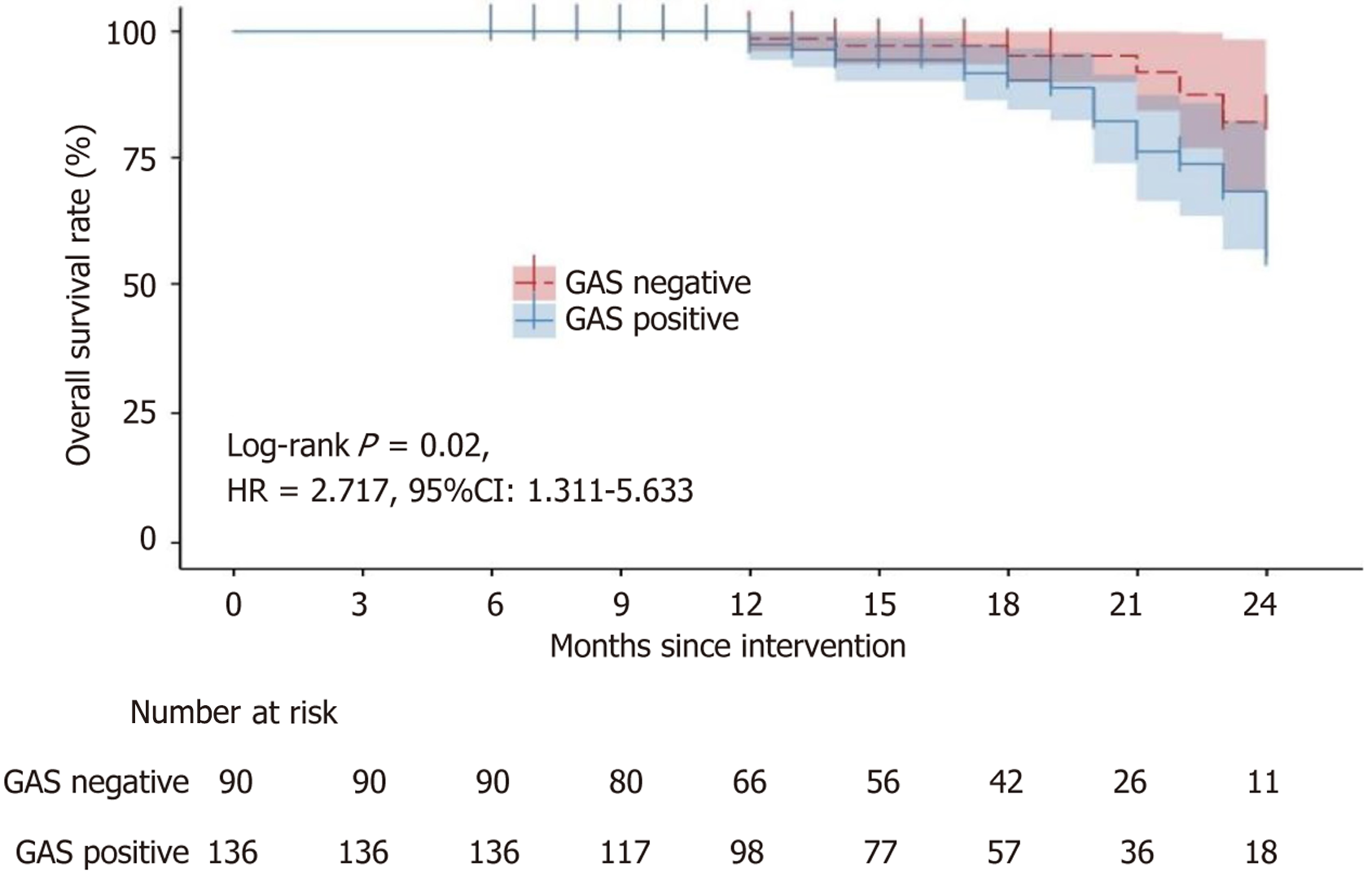
Post-discharge surveillance of patients within the gastric cancer cohort was conducted to document DFS and OS. Follow-up periods ranged from a minimum of 6 months to a maximum of 24 months, with a median follow-up time of 16 months. The DFS was illustrated with the Kaplan-Meier survival analysis, depicted in Figure 1. Throughout the follow-up, 18 disease progressions were noted among gastrin-negative patients, and 39 among gastrin-positive patients. Kaplan-Meier survival analysis showed no substantial difference in DFS between individuals with positive and negative gastrin status [hazard ratio = 1.516, 95% confidence interval (CI): 0.895-2.570]. The OS was described using Kaplan-Meier survival curves, as shown in Figure 2. During the follow-up period, six deaths occurred in the gastrin-negative patients, and 24 deaths occurred in the gastrin-positive patients. Kaplan-Meier analysis showed that the OS of gastrin-positive patients was inferior to that of gastrin-negative patients (hazard ratio = 2.717, 95%CI: 1.311-5.633).
PPI use was independently associated with elevated gastrin levels (Table 4). No statistically significant association between histologic type and tumor location.
| Predictor | Coefficient (β) | P value | 95%CI (lower, upper) | Interpretation |
| PPI use (yes) | 1.567 | 0.037 | (0.095, 3.039) | PPI use significantly increases odds of high gastrin |
| Helicobacter pylori (positive) | –0.401 | 0.39 | (-1.317, 0.514) | Not statistically significant |
| Tumor location (body) | 0.291 | 0.562 | (-0.694, 1.276) | Not significant |
| Tumor location (esophagogastric junction) | 0.286 | 0.569 | (-0.697, 1.268) | Not significant |
| TNM stage (III-IV) | –0.143 | 0.738 | (-0.983, 0.696) | Not significant |
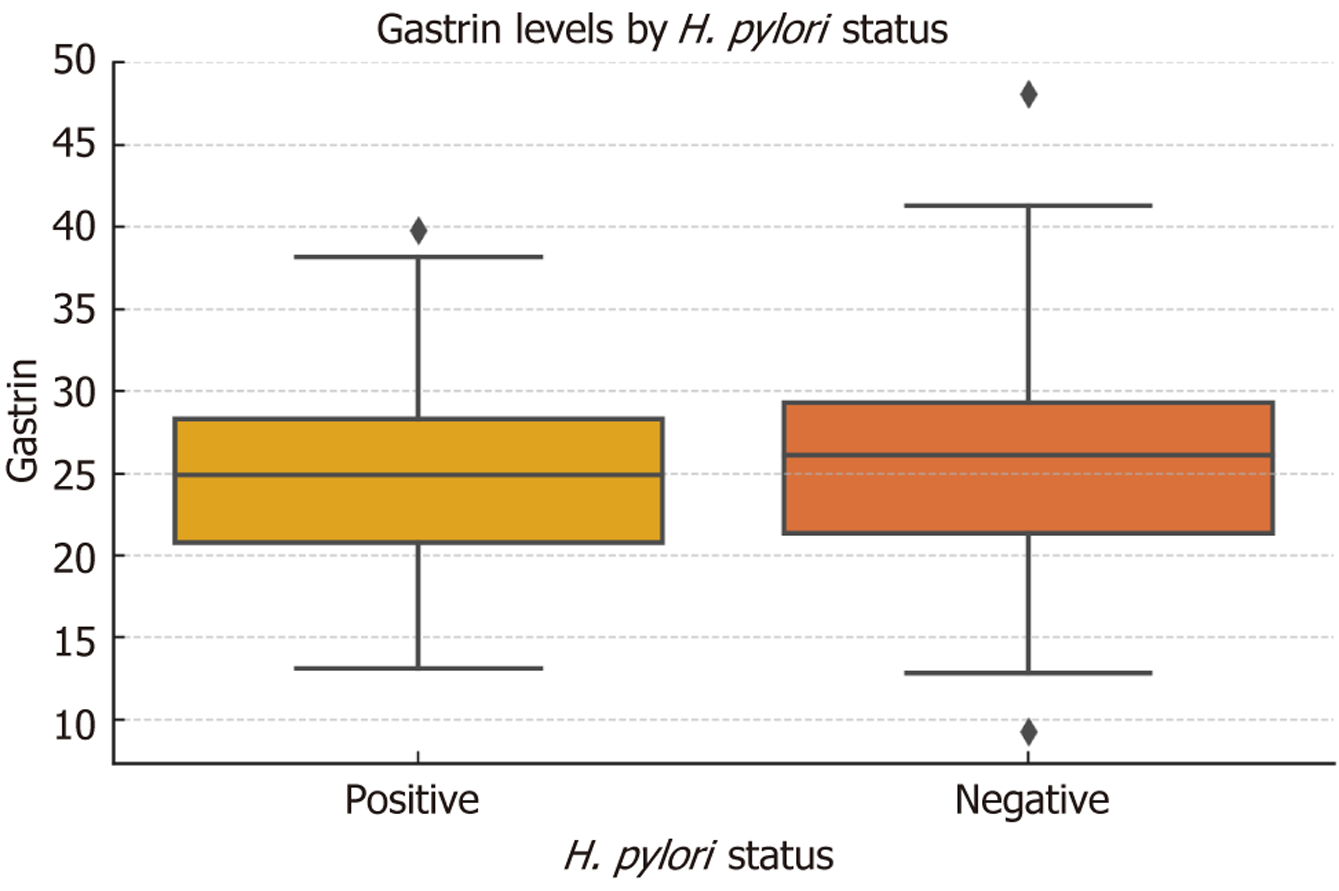
Boxplot analysis suggested a trend toward higher serum gastrin levels in patients with H. pylori-positive status compared to H. pylori-negative individuals (Figure 3). However, statistical comparison using Welch’s t-test revealed no significant difference between the two groups (mean ± SD: 25.28 ± 6.02 pmol/L vs 24.51 ± 5.86 pmol/L, respectively; t = -1.15, P = 0.251).
Of the 226 gastric cancer patients, 72 (31.86%) had a history of regular PPI or PCAB use, including 65 (28.76%) who used these medications within the past month. Gastrin levels were significantly elevated in both the past and recent PPI use groups compared to patients with no history of use (F = 39.71, P < 0.0001). Multivariable logistic regression revealed that serum gastrin level remained independently associated with higher tumor grade (adjusted odds ratio = 1.015 per pmol/L increase, 95%CI: 1.002-1.027, P = 0.021) after adjusting for PPI/PCAB use. Neither past use (P = 0.708) nor recent use (P = 0.824) of PPI/PCABs showed a significant association with tumor grade.
No adverse events, harms, or unintended effects related to study procedures (including blood sampling, 14C-UBT, or follow-up assessments) were observed or reported in either the gastric cancer or control groups. While serum gastrin levels were elevated in patients with a history of PPI or PCAB use, this biochemical finding is expected and not classified as a harm. The use of PPI/PCAB medications was retrospectively assessed for its confounding effect on gastrin levels, and no patients experienced clinically significant side effects attributable to PPI/PCAB withdrawal during the 14-day washout period. Additionally, the diagnostic procedures (e.g., radioimmunoassay, UBT) were non-invasive or minimally invasive and conducted under standard clinical protocols. Therefore, the study was not associated with any direct phy
Gastric cancer, a relatively common tumor of the digestive system, is a significant public health threat. It is the fourth most common cancer and the third leading cause of cancer death worldwide[13]. Gastric cancer arises from malignant neoplastic transformation of epithelial cells in the gastric mucosa and is multifactorial in origin, including dietary factors, infections, and genetic predisposition. Despite advancements in therapeutic management, the disease continues to have an unsatisfactory five-year survival rate[14]. Given that H. pylori-associated carcinogenesis can persist after bacterial eradication, a more robust classification system, dividing patients into currently infected, previously infected (era
A limitation of the study is the absence of stratification by prior H. pylori eradication status, which might also have caused heterogeneity in the test results and the interpretation of the associations. Additionally, it is essential to mention that while the UBT is an excellent diagnostic modality, it ultimately captures events of previously eradicated infections and does not differentiate between those who were never infected and those who were[21]. Therefore, perhaps our study could not optimally characterize H. pylori and its association with gastric cancer.
This study illuminated the complex relationship between gastrin levels and clinicopathological factors in gastric cancer, which may provide some insight into the mechanisms at play in gastric cancer and clinical implications. Gastrin-positive patients had a significantly higher incidence of lymph node metastasis, poorly differentiated tumors, and advanced TNM stages (III-IV), which may indicate a direct correlation between the pathological progression of the gastric cancer and higher gastrin release.
This highlights that the unique sample composition might impact generalizability and should be studied more[22]. Furthermore, prognostic evaluations were conducted to understand the impact of gastrin on gastric cancer outcomes, using the key endpoints of progression and mortality. Notably, a negative correlation was noted in the association of serum gastrin levels and cumulative OS, which suggests that elevated gastrin release led to worse prognosis for gastric cancer patients and is an independent prognostic indicator of poor outcomes.
These findings support and highlight the potential use of serum gastrin as a diagnostic marker but suggest its usefulness for risk stratification and treatment planning. It is plausible that clinicians should factor gastrin into their assessment with traditional pathological and imaging evaluations, consider gastrin measurements in the management strategy, and evaluate better personalized management plans. Future studies in gastrin-targeted therapies may offer opportunities in precision oncology, particularly in patients with aggressive tumor phenotypes with high gastrin profiles, but this is only hypothetical.
Mechanistic evidence based on in vitro modelled studies also provided insight into tumor-promoting effects with gastrin. The experimental data demonstrated that gastrin promoted Michael Fay’s[23] gastric cancer cell lines (AGS and MKN1) by stimulating cellular proliferation, encouraging migration, and anti-apoptotic modelling properties. For example, by activating the mixed-spectrum kinase 3/c-Jun protein amino-terminal kinase 1 pathway by binding to the cholecystokinin B receptor among other downstream pathways, there was an increase in the migratory capacity of AGSE, the human gastric cancer cell line[24]. Additionally, gastrins binding to the gastrin receptor on the AGS-P, another gastric cancer cell line, promoted a similar notable increase in intracellular calcium ions, resulting in increased cellular growth rates[25]. Furthermore, strong evidence from diverse studies indicated that genes related to the gastrin receptor, with upregulated expression, also significantly enhanced metastatic behaviors in gastric cancer cells, increasing the inva
A key limitation of this study is the classification of H. pylori infection status based solely on the UBT. While UBT is highly sensitive and specific for detecting active infection, it does not identify past infections or successful eradication. Without combining serologic testing or detailed eradication history, potential misclassification bias cannot be excluded, and this may have affected the observed associations between H. pylori status and gastric cancer characteristics. The binary classification into H. pylori-positive and negative based solely on UBT does not differentiate between patients with prior eradicated infection and those never infected. The lack of serologic antibody data and eradication history limited our ability to classify subjects into the recommended three categories: Current, previous, and never infected. Future studies should adopt a multimodal diagnostic approach including UBT, anti-H. pylori immunoglobulin G, and detailed medical history to improve classification accuracy. Moreover. the findings of this study support the association between elevated serum gastrin levels and gastric cancer aggressiveness, as evidenced by higher rates of advanced TNM stage, lymph node metastasis, and poor differentiation in gastrin-positive patients. While these results suggest a potential role for gastrin in tumor progression, it is important to note that the therapeutic implications of targeting gastrin remain speculative due to the lack of clinical or in vivo validation. Current evidence is limited to in vitro studies demonstrating gastrin’s proliferative effects on gastric cancer cell lines, and further research is needed to determine whether gastrin inhibition would translate into meaningful clinical benefits without compromising physiological functions such as gastric acid secretion and mucosal repair. Therefore, while gastrin may serve as a prognostic biomarker, its viability as a therapeutic target requires additional investigation through preclinical and clinical studies.
To summarize, the comprehensive findings from this study underscore the significance of high serum gastrin concentrations in individuals with gastric cancer, illuminating their close association with tumor aggressiveness and dismal prognoses. Moreover, the mechanistic elucidation of gastrin’s tumor-promoting effects in gastric cancer cell lines provides valuable insights into the underlying biological processes governing disease progression. Collectively, these findings enhance our understanding of the intricate interplay between gastrin and gastric cancer, paving the way for potential the
| 1. | Thrift AP, El-Serag HB. Burden of Gastric Cancer. Clin Gastroenterol Hepatol. 2020;18:534-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 1077] [Article Influence: 179.5] [Reference Citation Analysis (7)] |
| 2. | Xing JY, Miao RL, Shan F, Li ZY. [Development status and prospects of functional preservation surgery for gastric cancer]. Zhonghua Wei Chang Wai Ke Za Zhi. 2025;28:127-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Harada K, Mizrak Kaya D, Shimodaira Y, Ajani JA. Global chemotherapy development for gastric cancer. Gastric Cancer. 2017;20:92-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 4. | Son T, Hyung WJ. Laparoscopic gastric cancer surgery: Current evidence and future perspectives. World J Gastroenterol. 2016;22:727-735. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 52] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 5. | Waldum H, Fossmark R. Gastritis, Gastric Polyps and Gastric Cancer. Int J Mol Sci. 2021;22:6548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 84] [Article Influence: 16.8] [Reference Citation Analysis (1)] |
| 6. | Oya Y, Hayakawa Y, Koike K. Tumor microenvironment in gastric cancers. Cancer Sci. 2020;111:2696-2707. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 224] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 7. | McCarthy DM. Proton Pump Inhibitor Use, Hypergastrinemia, and Gastric Carcinoids-What Is the Relationship? Int J Mol Sci. 2020;21:662. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 8. | Han YM, Park JM, Kangwan N, Jeong M, Lee S, Cho JY, Ko WJ, Hahm KB. Role of proton pump inhibitors in preventing hypergastrinemia-associated carcinogenesis and in antagonizing the trophic effect of gastrin. J Physiol Pharmacol. 2015;66:159-167. [PubMed] [DOI] [Full Text] |
| 9. | Fossmark R, Martinsen TC, Waldum HL. Adverse Effects of Proton Pump Inhibitors-Evidence and Plausibility. Int J Mol Sci. 2019;20:5203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 117] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 10. | Liou JM, Lee YC, El-Omar EM, Wu MS. Efficacy and Long-Term Safety of H. pylori Eradication for Gastric Cancer Prevention. Cancers (Basel). 2019;11:593. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 11. | Yao QX, Li ZY, Kang HL, He X, Kang M. Effect of acacetin on inhibition of apoptosis in Helicobacter pylori-infected gastric epithelial cell line. World J Gastrointest Oncol. 2024;16:3624-3634. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 12. | Loor A, Dumitraşcu DL. Helicobacter pylori Infection, Gastric Cancer and Gastropanel. Rom J Intern Med. 2016;54:151-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Karimi P, Islami F, Anandasabapathy S, Freedman ND, Kamangar F. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev. 2014;23:700-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1159] [Cited by in RCA: 1381] [Article Influence: 115.1] [Reference Citation Analysis (0)] |
| 14. | Tan Z. Recent Advances in the Surgical Treatment of Advanced Gastric Cancer: A Review. Med Sci Monit. 2019;25:3537-3541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 324] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 15. | Duan S, Rico K, Merchant JL. Gastrin: From Physiology to Gastrointestinal Malignancies. Function (Oxf). 2022;3:zqab062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 16. | Zhao WX, Liu ZF, Li XL, Li Z. Correlations of serum homocysteine, VEGF and gastrin 17 with gastric cancer and precancerous lesions. Eur Rev Med Pharmacol Sci. 2019;23:4192-4198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 17. | Shirani M, Pakzad R, Haddadi MH, Akrami S, Asadi A, Kazemian H, Moradi M, Kaviar VH, Zomorodi AR, Khoshnood S, Shafieian M, Tavasolian R, Heidary M, Saki M. The global prevalence of gastric cancer in Helicobacter pylori-infected individuals: a systematic review and meta-analysis. BMC Infect Dis. 2023;23:543. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 53] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 18. | Xiao M, Zhang Z, Liao G, Liu M, Chen X. Analysis of the Value of Helicobacter pylori Test in Combination with the Determination of Plasma Propepsin and Gastrin 17 in Screening the Precancerous Status of Gastric Cancer. Cell Mol Biol (Noisy-le-grand). 2022;68:110-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 19. | Vohlonen I, Pukkala E, Malila N, Härkönen M, Hakama M, Koistinen V, Sipponen P. Risk of gastric cancer in Helicobacter pylori infection in a 15-year follow-up. Scand J Gastroenterol. 2016;51:1159-1164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Liu C, Chen K, Wang H, Zhang Y, Duan X, Xue Y, He H, Huang Y, Chen Z, Ren H, Wang H, Zeng C. Gastrin Attenuates Renal Ischemia/Reperfusion Injury by a PI3K/Akt/Bad-Mediated Anti-apoptosis Signaling. Front Pharmacol. 2020;11:540479. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 81] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 21. | Okada Y, Yokoyama K, Yano T, Kumagai H, Morikawa T, Kobayashi Y, Imagawa T, Yamagata T. A boy with duodenocolic fistula mimicking functional gastrointestinal disorder. Clin J Gastroenterol. 2019;12:566-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2554] [Cited by in RCA: 2747] [Article Influence: 457.8] [Reference Citation Analysis (3)] |
| 23. | Mishra P, Senthivinayagam S, Rangasamy V, Sondarva G, Rana B. Mixed lineage kinase-3/JNK1 axis promotes migration of human gastric cancer cells following gastrin stimulation. Mol Endocrinol. 2010;24:598-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 24. | Tian S, Peng P, Li J, Deng H, Zhan N, Zeng Z, Dong W. SERPINH1 regulates EMT and gastric cancer metastasis via the Wnt/β-catenin signaling pathway. Aging (Albany NY). 2020;12:3574-3593. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 157] [Article Influence: 26.2] [Reference Citation Analysis (1)] |
| 25. | Waldum HL, Sagatun L, Mjønes P. Gastrin and Gastric Cancer. Front Endocrinol (Lausanne). 2017;8:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/