Published online Sep 15, 2025. doi: 10.4251/wjgo.v17.i9.105937
Revised: March 31, 2025
Accepted: August 12, 2025
Published online: September 15, 2025
Processing time: 216 Days and 21 Hours
Immune checkpoint inhibitors (ICIs) are effective cancer treatments; however, a significant proportion of colorectal cancer (CRC) patients exhibit limited re
To examine the role of KAT6A in CRC progression and immune evasion.
The functional role of KAT6A was evaluated through genetic knockdown, pharmacological inhibition (WM-3835), and CRISPR/dCas9-mediated epigenetic editing in CRC cells. T cell-mediated apoptosis was assessed using co-culture models, and H3K23pr was measured via chromatin immunoprecipitation assays. PD-L1 expression at mRNA and protein levels was analyzed under KAT6A knockdown conditions.
KAT6A suppression reduced CRC cell proliferation, invasion, and migration. Pharmacological or epigenetic disruption of KAT6A phenocopied these effects, with dose-dependent reductions in H3K23pr (28.4% residual at 10 μM) and PD-L1 expression. KAT6A knockdown enhanced T cell-mediated apoptosis, evidenced by increased expression of granzyme B and perforin. Mechanistically, KAT6A loss decreased H3K23pr and reduced RNA polymerase II occupancy on the PD-L1 promoter, leading to suppressed PD-L1 transcription. CRISPR/dCas9-mediated H3K23pr editing at the PD-L1 promoter directly modulated immune evasion, confirming its causal role. Overexpression of PD-L1 mitigated the inhibitory effects of KAT6A knockdown on CRC progression and immune evasion.
KAT6A drives CRC progression and immune evasion by promoting histone H3 propionylation to epigenetically activate PD-L1 expression. Targeting KAT6A or its downstream H3K23pr-PD-L1 axis represents a promising therapeutic strategy to overcome ICI resistance in CRC.
Core Tip: KAT6A drives colorectal cancer (CRC) progression and immune evasion by promoting histone H3 propionylation to epigenetically activate PD-L1 expression. Targeting KAT6A or its downstream H3K23pr-PD-L1 axis represents a promising therapeutic strategy to overcome Immune checkpoint inhibitor resistance in CRC.
- Citation: Zhou ZD, Zhao JP, Zheng SC, Wang TT. Correlation between KAT6A and PD-L1 expression and role of KAT6A in colorectal cancer. World J Gastrointest Oncol 2025; 17(9): 105937
- URL: https://www.wjgnet.com/1948-5204/full/v17/i9/105937.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i9.105937
Recently, immune checkpoint inhibitors (ICIs), specifically PD-1, have greatly transformed the field of cancer treatment[1-3]. However, in colorectal cancer (CRC), which ranks among the three most prominent aggressive and common cancers, the therapeutic benefits of ICIs are predominantly observed in individuals with high microsatellite instability[4-6]. Notably, approximately 90% of CRC cases are microsatellite stable (MSS), where response rates to ICIs rarely exceed 5%-10%, highlighting an urgent need to decipher immune evasion mechanisms beyond mismatch repair deficiency[7,8]. The regONIVO trial recently demonstrated that combining Regorafenib with anti-PD-1 antibodies achieved a 33% response rate in MSS-CRC patients[9] suggesting epigenetic reprogramming of the tumor microenvironment may synergize with ICIs. Nevertheless, the molecular determinants underlying this synergy remain poorly characterized[10].
Epigenetic regulation, encompassing DNA/histone modifications and RNA post-transcriptional editing, profoundly influences oncogenesis and immune surveillance[11-15]. In CRC, DNA hypermethylation (e.g., MLH1 silencing in MSI-H tumors) and histone deacetylase-mediated PD-L1 suppression are well-established epigenetic mechanisms[16,17]. Recent studies further revealed that enhancer of EZH2-mediated H3K27me3 represses PD-L1 transcription in MSS-CRC[18], while DNA methyltransferase inhibitors upregulate PD-L1 through demethylation of its promoter[19]. These findings position epigenetic modifiers as pivotal regulators of immune checkpoint expression. However, compared to DNA methylation and canonical histone modifications (acetylation/methylation), the immunological implications of novel acylation marks remain underexplored.
Histone acylation, including propionylation, butyrylation, and crotonylation, represents an emerging layer of epigenetic regulation with distinct biological functions[20]. Unlike acetylation, crotonylation generates bulkier hydrophobic modifications that alter chromatin architecture and preferentially recruit readers like YEATS domains[21]. KAT6A, a MYST-family histone acetyltransferase paralog, uniquely catalyzes histone crotonylation in cancer cells[22,23]. In breast cancer, KAT6A-driven crotonylation facilitates SOX4 transcriptional activation to promote metastasis, while in glioblastoma, it stabilizes β-catenin to sustain stemness[24]. Notably, KAT6A amplification correlates with poor prognosis in multiple cancers, yet its immunological role, particularly in modulating PD-L1-mediated immune evasion, remains unknown. This knowledge gap is striking given that: (1) Histone crotonylation preferentially marks active enhancers of oncogenes; and (2) PD-L1 expression is regulated by enhancer remodeling in immunotherapy-resistant tumors[25].
Here, we interrogated the epigenetic-immune crosstalk in CRC by linking KAT6A-mediated histone acylation to PD-L1 regulation. We demonstrated that KAT6A orchestrates an immune-evasive microenvironment through crotonylation-dependent PD-L1 upregulation and identified combinatorial targeting strategies to overcome ICI resistance. Our work extends the paradigm of epigenetic control in cancer immunity by revealing a non-canonical acylation mechanism, providing therapeutic avenues for MSS-CRC patients refractory to current immunotherapies.
CRC cell lines were procured from Procell (Wuhan, China). Cells were cultured in DMEM (BI, Israel) supplemented with 10% fetal bovine serum (FBS, BI, Israel) and 1% penicillin-streptomycin (P-S, Sigma, United States) under standard conditions of 37 °C with 5% CO2. KAT6A-specific small interfering RNA (siRNA) and overexpression plasmid vectors were purchased from Gene Pharma (Shanghai, China). Transfections were carried out using Lipofectamine 2000 reagent (Thermo Fisher Scientific, United States) following the manufacturer’s protocol. Transfection efficiency was confirmed 48 hours post-transfection by quantitative PCR (qPCR) and Western blot analysis.
Cell counting kit-8 (CCK-8; SolarBio, China) was utilized to measure cell viability. CRC cells were seeded in 96-well plates at a density of 5 × 104 cells/well and incubated for 24 hours. After adding CCK-8 reagent, the cells were incubated for 2 hours at 37 °C, and absorbance at 450 nm was measured using a microplate reader. For the colony formation assay, 1 × 103 cells were seeded in 6-well plates and cultured for 10 days. Colonies were fixed with methanol, stained with 0.1% crystal violet, and photographed. Colony numbers were quantified manually.
Apoptosis was analyzed using the Annexin V-FITC/PI Apoptosis Detection Kit (Beyotime Biotechnology, China). CRC cells were collected, washed with phosphate-buffered saline (PBS), and stained with Annexin V-FITC and PI following the manufacturer’s instructions. Stained cells were analyzed using flow cytometry (BD Biosciences, United States). Briefly, cells were stained with 5 μL Annexin V-FITC and 5 μL PI in the dark for 15 minutes, washed with PBS, and resuspended in 300 μL binding buffer. Flow cytometry was performed using a BD FACSCanto II system. FITC signals were detected with 488 nm excitation and 530 nm emission, while PI signals were detected with 561 nm excitation and 610 nm emission. Flow cytometry data were analyzed using FlowJo v10.8. Debris was excluded based on FSC-A/SSC-A gating. Early apoptotic cells were identified as Annexin V+/PI-, while late apoptotic cells were identified as Annexin V+/PI+.
Transwell migration and invasion assays were conducted using 24-well Transwell inserts (Costar, United States). For the migration assay, CRC cells were suspended in serum-free medium and seeded in the upper chamber. The lower chamber was filled with complete medium containing 10% FBS as a chemoattractant. After 24 hours, cells on the upper surface of the membrane were removed with a cotton swab, and migrated cells on the lower surface were stained with crystal violet. For the invasion assay, the upper chamber was pre-coated with a Matrigel/DMEM mixture (1:1, Corning, United States). Migrated and invaded cells were counted in five randomly selected fields under a light microscope (Leica, Germany).
Total protein lysates were extracted using radioimmunoprecipitation assay (RIPA) buffer supplemented with protease inhibitors. Protein concentrations were determined using a BCA protein assay kit (Thermo Fisher Scientific, United States). Equal amounts of protein samples were separated on SDS-PAGE gels and transferred to PVDF membranes (Millipore, United States). The membranes were blocked in 5% skimmed milk for 2 hours at room temperature, followed by overnight incubation at 4 °C with primary antibodies against E-cadherin, Vimentin, KAT6A, PD-L1, and β-actin (Abcam, United Kingdom). The membranes were then incubated with secondary antibodies for 1 hour at room temperature and visualized using an ECL reagent (Thermo Fisher Scientific).
Total RNA was isolated using TRIzol reagent (Qiagen, Hilden, Germany). RNA was reverse-transcribed using a reverse transcription kit (Qiagen), and cDNA was amplified using SYBR Green PCR Master Mix (Thermo Fisher Scientific). PCR amplification was performed in a StepOnePlus real-time PCR system (Applied Biosystems, United States). Gene expression was normalized to GAPDH as an internal control.
Peripheral blood mononuclear cells (PBMCs) were isolated from human blood samples using Ficoll density-gradient centrifugation. T cells were activated by incubating PBMCs with anti-CD3 and anti-CD28 antibodies (Gibco, United States) for 3 days. For co-culture experiments, CRC cells and T cells were seeded in separate compartments of a Transwell insert at a 10:1 ratio (T cells:CRC cells). After 72 hours, PBMCs were collected, stained with anti-CD4, anti-CD8, and anti-PD-1 antibodies (BioLegend, United States), and analyzed using flow cytometry.
Chromatin immunoprecipitation (ChIP) assays were performed using an EZ-ChIP Kit (Millipore, United States). CRC cells were fixed with 1% formaldehyde to cross-link proteins and DNA. Chromatin was fragmented into approximately 500 bp fragments by sonication. Immunoprecipitation was performed overnight at 4 °C using antibodies against H3K23pr, RNA polymerase II, or control IgG. DNA-protein complexes were purified using protein A/G magnetic beads. Enriched DNA was reverse cross-linked, treated with RNase A and proteinase K, and analyzed by qPCR with primers targeting the PD-L1 promoter region.
WM-3835 (MedChemExpress, HY-138487) was dissolved in DMSO to prepare a 10 mmol/L stock solution and stored at
The dCas9-p300 and dCas9-LSD1 plasmids were synthesized by Shanghai GeneChem Co., Ltd. The sgRNA targeting the PD-L1 promoter (sequence: 5’-GCGCTCCAGCTCCGACCTGA-3’) and the non-targeting control sgRNA (sequence: 5’-ACGGAGGCTAAGCGTCGCAA-3’) were cloned into the pLV-sgRNA vector. SW480 cells were seeded at a density of 1 × 106 cells per well and co-transfected with dCas9 plasmid (2 μg) and sgRNA plasmid (1 μg) using Lipofectamine 3000 (Thermo Fisher). After 48 hours, the medium was replaced with selection medium containing 2 μg/mL puromycin and incubated for 72 hours. For editing efficiency validation, H3K23pr enrichment was assessed by ChIP-qPCR, and PD-L1 mRNA levels were analyzed by qPCR.
Statistical analyses were conducted using SPSS 26.0 (IBM, United States) and GraphPad Prism 9.0 (GraphPad Software, United States). Results are expressed as the mean ± SD. Two-sided Student’s t-tests were applied for comparisons between two groups, and one-way ANOVA followed by Dunnett’s post-hoc test was used for multiple group compa
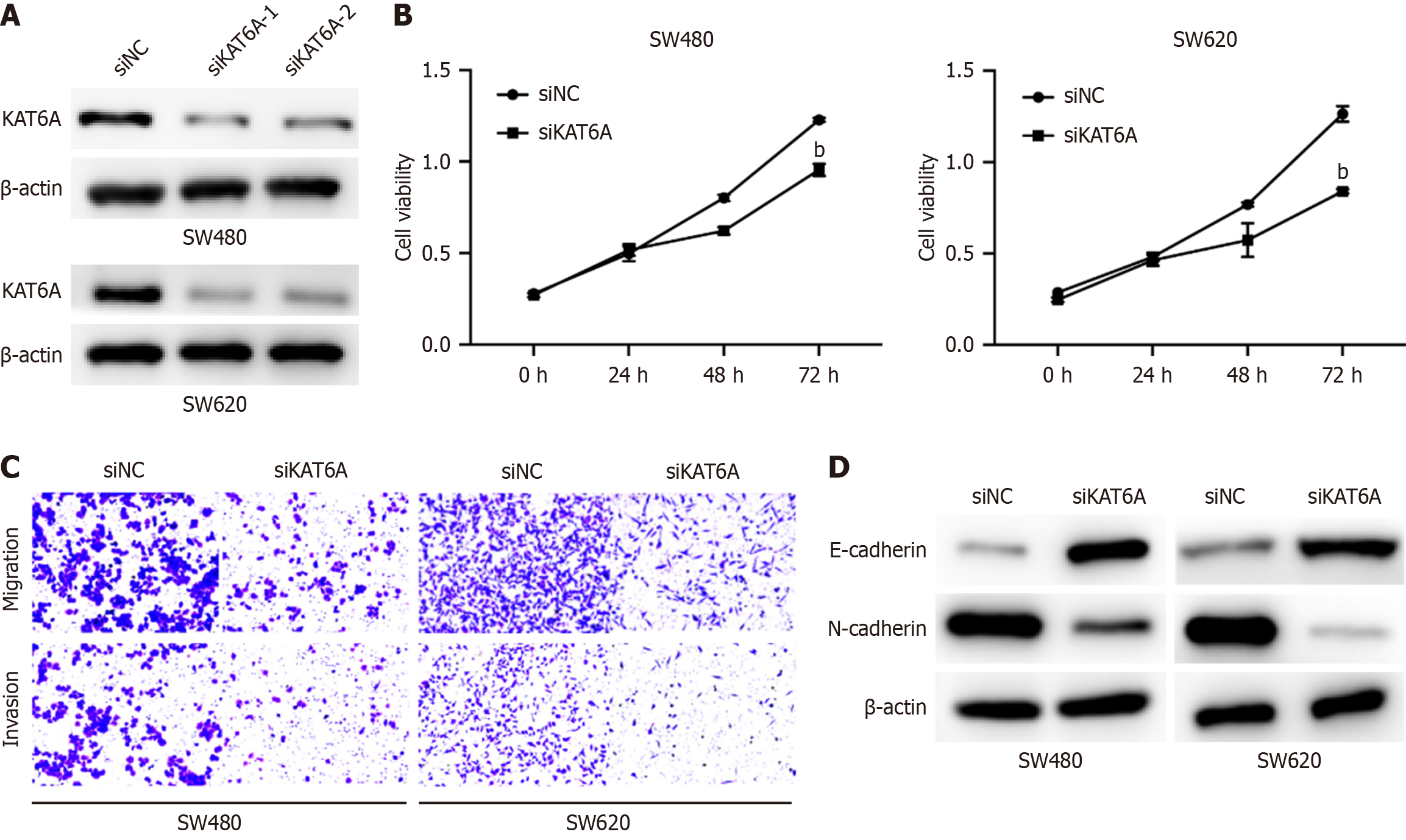
To investigate the role of KAT6A in CRC cell behavior, we transfected CRC cell lines with KAT6A siRNA to achieve its knockdown. Western blot analysis confirmed the efficient depletion of KAT6A expression in the cells (Figure 1A). CCK-8 assays revealed a significant reduction in cell proliferation following KAT6A knockdown, indicating its critical role in promoting CRC cell growth (Figure 1B). Furthermore, Transwell assays demonstrated a dramatic decrease in both migratory and invasive CRC cells after KAT6A depletion, highlighting its role in facilitating cell motility and invasion (Figure 1C). Western blot analysis also revealed molecular changes associated with epithelial-mesenchymal transition (EMT): KAT6A knockdown elevated E-cadherin levels (an epithelial marker) and reduced vimentin levels (a mesen
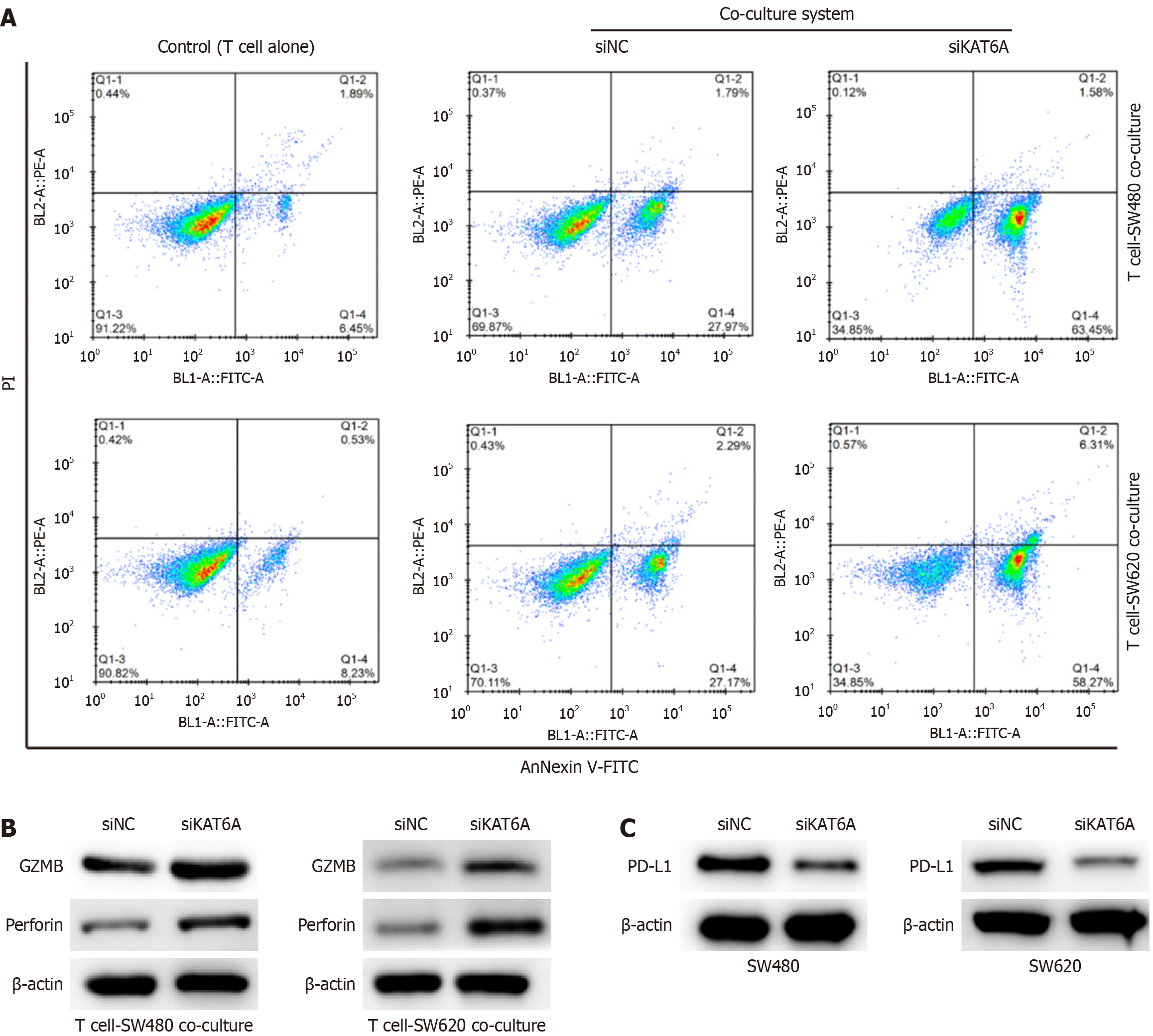
To examine the impact of KAT6A on immune evasion, we employed a co-culture system involving CRC cells and unstimulated or anti-CD3/CD28-activated T cells. Cell apoptosis assays showed that KAT6A-depleted CRC cells exhibited significantly increased apoptosis (SW480: 65.03% ± 3.99% vs siNC 29.76% ± 2.5%, P < 0.001; SW620: 64.58% ± 5.21% vs siNC 29.46% ± 1.69%, P < 0.001; Figure 2A). Additionally, depletion of KAT6A in the co-culture system elevated the expression levels of T-cell cytotoxic markers, GZMB and perforin, indicating enhanced T cell activity (Figure 2B). Notably, KAT6A knockdown also suppressed PD-L1 expression in CRC cells (Figure 2C), suggesting that KAT6A may promote immune evasion through upregulation of PD-L1. These findings indicate that KAT6A suppresses the immune response of T cells, enabling CRC cells to evade immune surveillance.
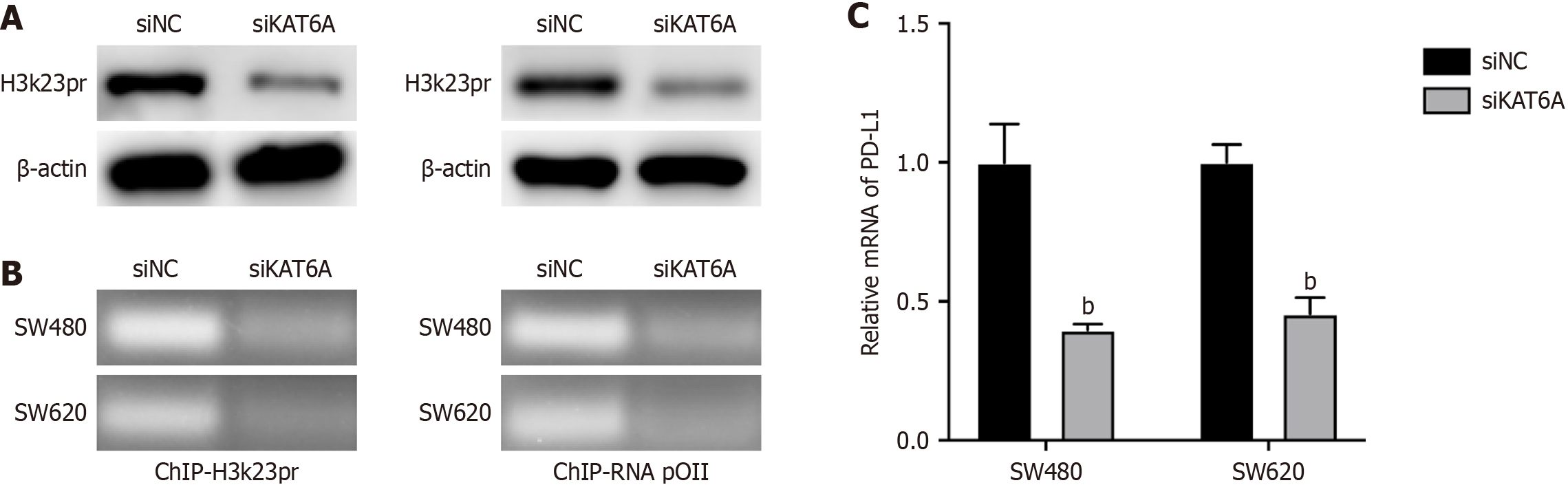
We further explored the molecular mechanism underlying KAT6A-mediated immune evasion. Western blot analysis demonstrated that KAT6A knockdown significantly reduced H3K23pr levels in CRC cells (Figure 3A). ChIP assays revealed a marked reduction in H3K23pr enrichment at the PD-L1 promoter following KAT6A knockdown, indicating that KAT6A-mediated histone modification plays a role in regulating PD-L1 transcription (Figure 3B). Consistently, qPCR analysis showed that PD-L1 mRNA levels were significantly reduced in CRC cells with depleted KAT6A expression (Figure 3C). These results suggest that KAT6A promotes PD-L1 expression by inducing H3K23pr modifications at the PD-L1 promoter, thereby facilitating immune evasion.
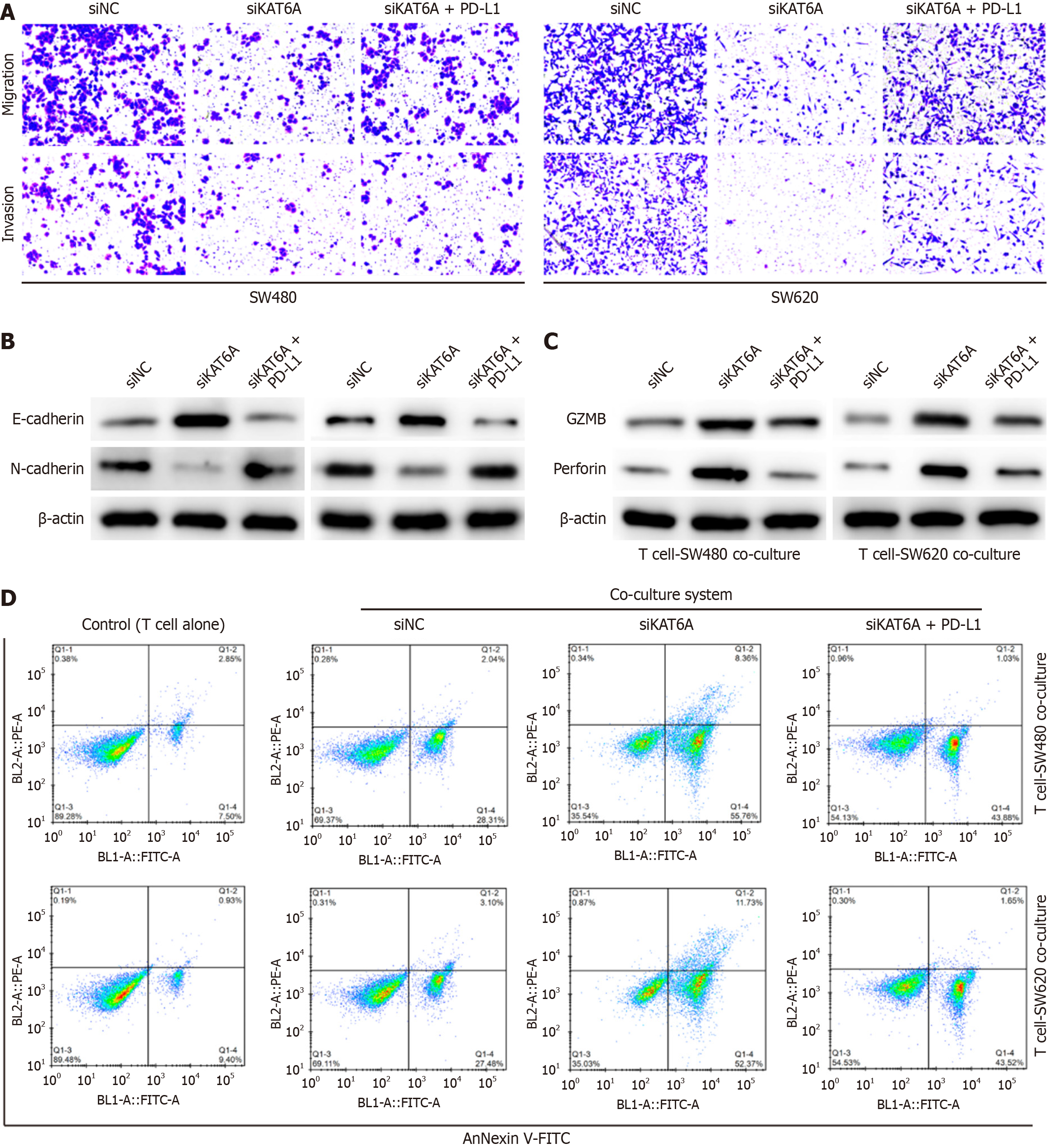
To establish the functional relationship between KAT6A and PD-L1 in CRC progression and immune evasion, we overexpressed PD-L1 in KAT6A-depleted cells. Overexpression of PD-L1 successfully restored the impaired invasion and migration capacities of KAT6A-depleted SW480 and SW620 CRC cells, as shown by Transwell assays (Figure 4A and B). In the co-culture system of CRC cells and T cells, PD-L1 overexpression also reversed the enhanced expression of GZMB and perforin caused by KAT6A knockdown, suggesting a suppression of T-cell cytotoxic activity (Figure 4C). Furthermore, PD-L1 overexpression abolished the increase in T cell-mediated killing activity observed in KAT6A-depleted CRC cells (Figure 4D). These findings indicate that KAT6A promotes CRC progression and immune evasion primarily through upregulation of PD-L1.
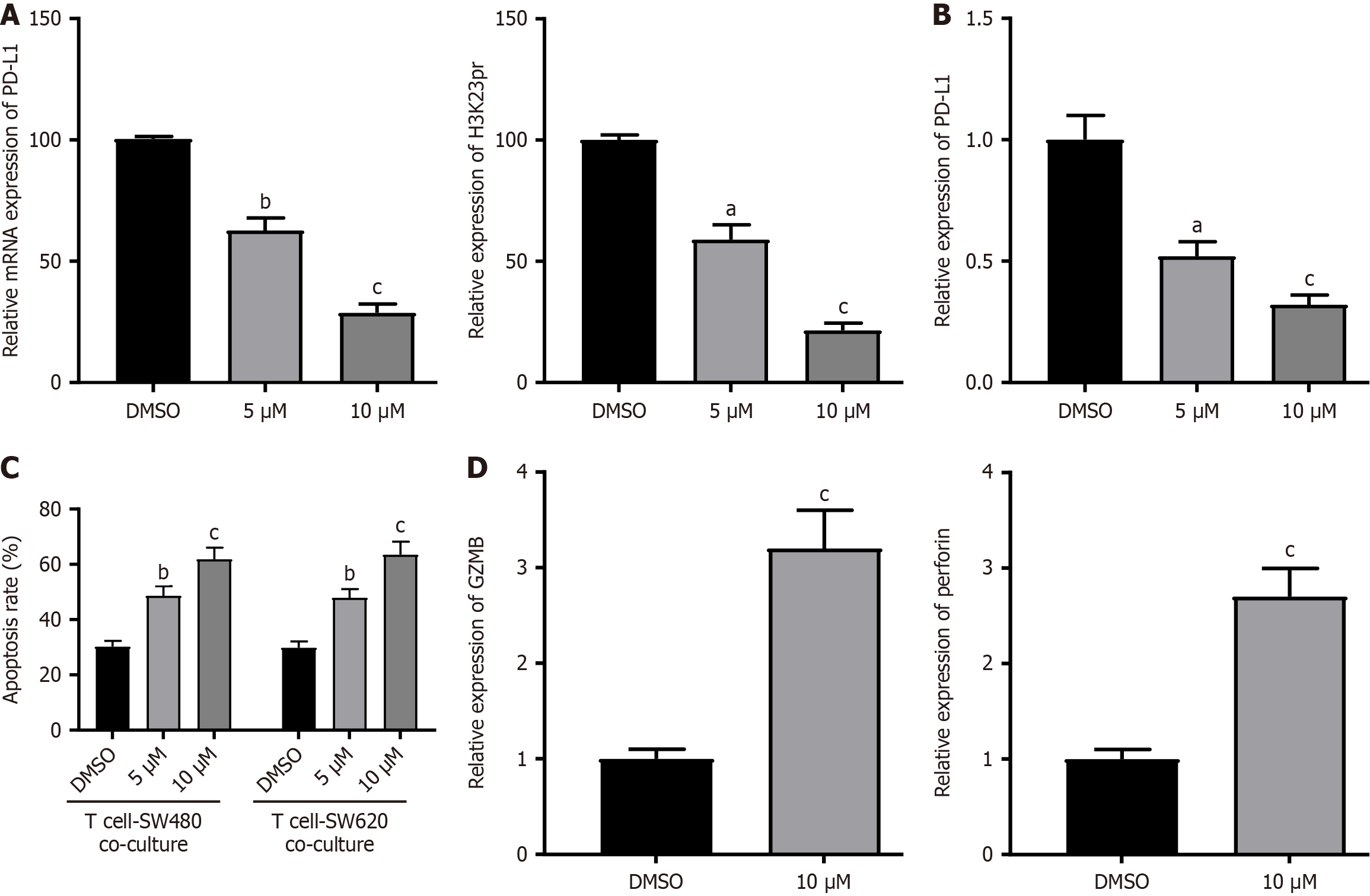
To pharmacologically validate KAT6A’s role, we treated CRC cells with WM-3835, a selective KAT6A inhibitor. Dose-dependent reductions in H3K23pr (28.4% residual at 10 μM, P < 0.001) and PD-L1 protein (21.5% residual, P < 0.001) were observed (Figure 5A), paralleled by 68% suppression of PD-L1 mRNA (P = 0.0032; Figure 5B). In the co-culture system of CRC cells and activated T cells, WM-3835 (10 μM) enhanced CRC cell apoptosis to 61.8% (vs DMSO 30.2%, P < 0.001; Figure 5C) and upregulated the T-cell cytotoxic markers GZMB (3.2-fold) and perforin (2.7-fold; Figure 5D). These results mirrored genetic KAT6A knockdown phenotypes, confirming its druggable potential.
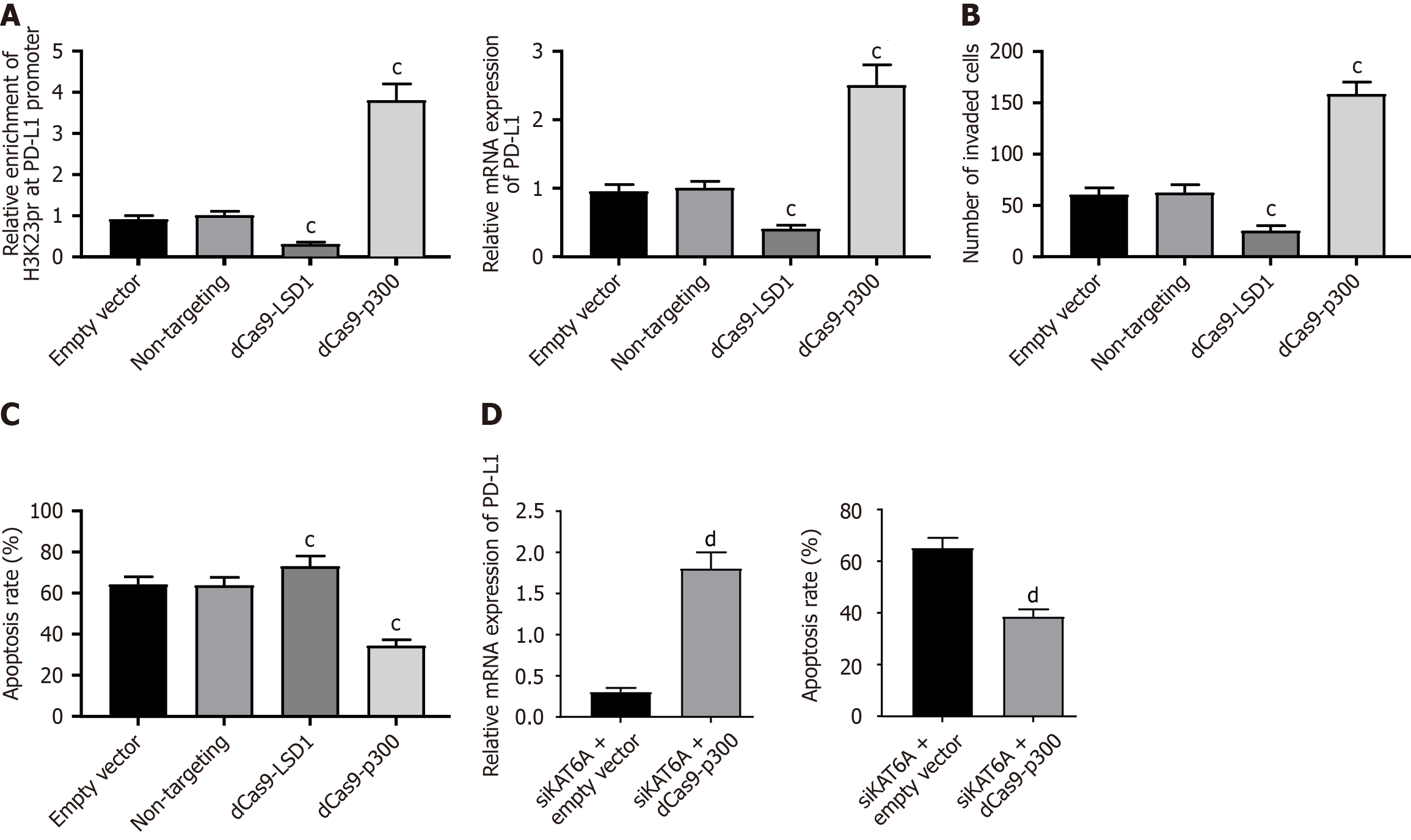
To establish a direct causal link between H3K23pr and PD-L1, we employed CRISPR/dCas9-based epigenetic editing. Targeting dCas9-p300 to the PD-L1 promoter (-154 bp) specifically enhanced H3K23pr enrichment by 3.8-fold (ChIP-qPCR, P < 0.001; Figure 6A), concomitant with 2.5-fold upregulation of PD-L1 mRNA (P = 0.0076; Figure 6B). Conversely, dCas9-LSD1 reduced both H3K23pr (70% decrease) and PD-L1 expression (P < 0.001). Functionally, dCas9-p300-edited cells exhibited enhanced invasion (158 vs 62 cells/field, P < 0.001) and resistance to T cell killing (34.2% vs 63.5% apoptosis, P < 0.001; Figure 6C). Importantly, dCas9-p300 rescued PD-L1 expression and immune evasion even in KAT6A-depleted cells (mRNA 1.8-fold, apoptosis 38.5% vs 65.0%, P = 0.0062; Figure 6D), confirming H3K23pr as the dominant downstream mechanism.
Overall, our data demonstrate that KAT6A enhances CRC cell proliferation, migration, invasion, and immune evasion. Mechanistically, KAT6A-mediated H3K23pr epigenetically activates PD-L1 expression, promoting tumor progression and suppressing T cell activity. Targeting KAT6A may therefore represent a potential therapeutic strategy for CRC.
CRC is a life-threatening malignancy characterized by poor prognosis and limited effective therapeutic options. Despite advancements in treatment strategies, the molecular mechanisms underlying CRC progression and immune evasion remain poorly understood, highlighting the urgent need for novel therapeutic targets. In this study, we identified lysine KAT6A as a key regulator of CRC progression and immune evasion, offering new insights into its role as a potential therapeutic target. Our findings reveal a previously unrecognized mechanism by which KAT6A drives PD-L1 expression through H3K23pr-mediated epigenetic remodeling, and further demonstrate the therapeutic potential of KAT6A inhibition in combination with immune checkpoint blockade.
KAT6A has been implicated in various biological processes in cancer, including cell growth, metastasis, drug resi
Furthermore, KAT6A has been shown to interact with diverse signaling pathways in cancer due to its acetyltransferase activity. For instance, KAT6A-mediated acetylation enhances PIK3CA transcription, activating the PI3K/AKT signaling pathway in glioblastoma, thereby promoting tumor growth and metastasis[29]. Similarly, KAT6A acetylates YAP in hepatocellular carcinoma, contributing to sorafenib resistance and cancer progression[30]. In our study, we identified a novel axis wherein KAT6A regulates H3K23pr, which epigenetically activates PD-L1 transcription. CRISPR/dCas9-mediated editing of H3K23pr at the PD-L1 promoter (-154 bp) directly modulated PD-L1 expression (3.8-fold increase with dCas9-p300 vs 70% decrease with dCas9-LSD1) and immune evasion phenotypes (Figure 6), confirming the causal role of this modification. This mechanism highlights the ability of KAT6A to drive immune evasion by modulating chromatin accessibility at immune checkpoint loci.
PD-L1 plays a pivotal role in tumor immune escape by binding to PD-1 on T cells, thereby suppressing T cell activation and cytotoxicity[31]. This interaction creates an immunosuppressive tumor microenvironment, enabling cancer cells to evade immune surveillance[32,33]. Our findings revealed that KAT6A knockdown significantly reduced H3K23pr and RNA polymerase II enrichment at the PD-L1 promoter, resulting in decreased PD-L1 expression at both the mRNA and protein levels. Consequently, T cell-mediated killing of CRC cells was enhanced following KAT6A knockdown. Notably, forced H3K23pr enrichment via dCas9-p300 restored PD-L1 expression and immune evasion even in KAT6A-depleted cells (Figure 6D), underscoring the centrality of this epigenetic mark in mediating KAT6A’s effects. Importantly, overexpression of PD-L1 reversed the inhibitory effects of KAT6A depletion on CRC progression and immune evasion, further confirming that KAT6A exerts its pro-tumorigenic effects through PD-L1 regulation.
These results align with previous studies suggesting that epigenetic regulation of PD-L1 is a critical mechanism by which tumors evade immune destruction. KAT6A’s role as a chromatin modifier positions it as a key player in this process, and its dual involvement in promoting tumor progression and immune suppression makes it an attractive therapeutic target. While our data show that KAT6A inhibition reduces PD-L1 expression, the potential synergy with ICIs may arise from complementary mechanisms. For example, KAT6A inhibition could simultaneously downregulate PD-L1 and destabilize other immunosuppressive pathways (e.g., enhancing antigen presentation or reducing myeloid-derived suppressor cell infiltration), thereby creating a more immunogenic tumor microenvironment. This hypothesis is supported by our observation that WM-3835-treated cells exhibited elevated T cell cytotoxic markers (GZMB and perforin; Figure 5D) independent of PD-L1 modulation, suggesting broader immunostimulatory effects. Future studies exploring combination therapies with KAT6A inhibitors and anti-PD-1 antibodies are warranted to validate this paradigm.
While our study provides compelling evidence for the role of KAT6A in CRC, there are limitations that warrant further investigation. For example, the precise upstream regulators of KAT6A in CRC remain unclear, and additional studies are needed to elucidate how KAT6A expression is modulated in the tumor microenvironment. Moreover, the translational relevance of our findings should be tested in vivo, particularly in MSS-CRC models resistant to current ICIs. Such experiments could clarify whether KAT6A inhibition synergizes with PD-1/PD-L1 blockade by overcoming epigenetic-driven immune tolerance.
In conclusion, we found that the acetyltransferase KAT6A contributes to the progression and immune evasion of CRC cells by inducing histone H3 propionylation to epigenetically activate PD-L1 expression. Pharmacological or epigenetic disruption of the KAT6A-H3K23pr-PD-L1 axis restores T cell cytotoxicity and suppresses tumor aggressiveness, positioning KAT6A as a dual therapeutic target for CRC. Our work provides a rationale for combining KAT6A inhibitors with immunotherapies to counteract resistance mechanisms in MSS-CRC.
| 1. | Liu C, Liu R, Wang B, Lian J, Yao Y, Sun H, Zhang C, Fang L, Guan X, Shi J, Han S, Zhan F, Luo S, Yao Y, Zheng T, Zhang Y. Blocking IL-17A enhances tumor response to anti-PD-1 immunotherapy in microsatellite stable colorectal cancer. J Immunother Cancer. 2021;9:e001895. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 188] [Article Influence: 37.6] [Reference Citation Analysis (1)] |
| 2. | Bagchi S, Yuan R, Engleman EG. Immune Checkpoint Inhibitors for the Treatment of Cancer: Clinical Impact and Mechanisms of Response and Resistance. Annu Rev Pathol. 2021;16:223-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 1518] [Article Influence: 253.0] [Reference Citation Analysis (0)] |
| 3. | Doroshow DB, Bhalla S, Beasley MB, Sholl LM, Kerr KM, Gnjatic S, Wistuba II, Rimm DL, Tsao MS, Hirsch FR. PD-L1 as a biomarker of response to immune-checkpoint inhibitors. Nat Rev Clin Oncol. 2021;18:345-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 1069] [Article Influence: 213.8] [Reference Citation Analysis (0)] |
| 4. | Vilar E, Gruber SB. Microsatellite instability in colorectal cancer-the stable evidence. Nat Rev Clin Oncol. 2010;7:153-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 660] [Cited by in RCA: 772] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 5. | Picard E, Verschoor CP, Ma GW, Pawelec G. Relationships Between Immune Landscapes, Genetic Subtypes and Responses to Immunotherapy in Colorectal Cancer. Front Immunol. 2020;11:369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 382] [Article Influence: 63.7] [Reference Citation Analysis (1)] |
| 6. | Lin A, Zhang J, Luo P. Crosstalk Between the MSI Status and Tumor Microenvironment in Colorectal Cancer. Front Immunol. 2020;11:2039. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 273] [Article Influence: 45.5] [Reference Citation Analysis (0)] |
| 7. | Müller MF, Ibrahim AE, Arends MJ. Molecular pathological classification of colorectal cancer. Virchows Arch. 2016;469:125-134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 289] [Cited by in RCA: 283] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 8. | Parikh AR, Szabolcs A, Allen JN, Clark JW, Wo JY, Raabe M, Thel H, Hoyos D, Mehta A, Arshad S, Lieb DJ, Drapek LC, Blaszkowsky LS, Giantonio BJ, Weekes CD, Zhu AX, Goyal L, Nipp RD, Dubois JS, Van Seventer EE, Foreman BE, Matlack LE, Ly L, Meurer JA, Hacohen N, Ryan DP, Yeap BY, Corcoran RB, Greenbaum BD, Ting DT, Hong TS. Radiation therapy enhances immunotherapy response in microsatellite stable colorectal and pancreatic adenocarcinoma in a phase II trial. Nat Cancer. 2021;2:1124-1135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 214] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 9. | Fukuoka S, Hara H, Takahashi N, Kojima T, Kawazoe A, Asayama M, Yoshii T, Kotani D, Tamura H, Mikamoto Y, Hirano N, Wakabayashi M, Nomura S, Sato A, Kuwata T, Togashi Y, Nishikawa H, Shitara K. Regorafenib Plus Nivolumab in Patients With Advanced Gastric or Colorectal Cancer: An Open-Label, Dose-Escalation, and Dose-Expansion Phase Ib Trial (REGONIVO, EPOC1603). J Clin Oncol. 2020;38:2053-2061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 589] [Article Influence: 98.2] [Reference Citation Analysis (0)] |
| 10. | Srikrishna D. Does Physical Activity Play a Role in the Efficacy of RegoNivo for MSS-mCRC? Clin Colorectal Cancer. 2022;21:e76-e77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 11. | Li Y, Chen X, Lu C. The interplay between DNA and histone methylation: molecular mechanisms and disease implications. EMBO Rep. 2021;22:e51803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 165] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 12. | Tessarz P, Kouzarides T. Histone core modifications regulating nucleosome structure and dynamics. Nat Rev Mol Cell Biol. 2014;15:703-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 722] [Cited by in RCA: 745] [Article Influence: 62.1] [Reference Citation Analysis (0)] |
| 13. | Hogg SJ, Beavis PA, Dawson MA, Johnstone RW. Targeting the epigenetic regulation of antitumour immunity. Nat Rev Drug Discov. 2020;19:776-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 470] [Article Influence: 78.3] [Reference Citation Analysis (0)] |
| 14. | Ilango S, Paital B, Jayachandran P, Padma PR, Nirmaladevi R. Epigenetic alterations in cancer. Front Biosci (Landmark Ed). 2020;25:1058-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 162] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 15. | Sun L, Zhang H, Gao P. Metabolic reprogramming and epigenetic modifications on the path to cancer. Protein Cell. 2022;13:877-919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 512] [Article Influence: 102.4] [Reference Citation Analysis (0)] |
| 16. | Jin ML, Jeong KW. Histone modifications in drug-resistant cancers: From a cancer stem cell and immune evasion perspective. Exp Mol Med. 2023;55:1333-1347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 36] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 17. | Jung G, Hernández-Illán E, Moreira L, Balaguer F, Goel A. Epigenetics of colorectal cancer: biomarker and therapeutic potential. Nat Rev Gastroenterol Hepatol. 2020;17:111-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 542] [Cited by in RCA: 569] [Article Influence: 94.8] [Reference Citation Analysis (5)] |
| 18. | Neganova ME, Klochkov SG, Aleksandrova YR, Aliev G. Histone modifications in epigenetic regulation of cancer: Perspectives and achieved progress. Semin Cancer Biol. 2022;83:452-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 133] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 19. | Yang J, Ren B, Ren J, Yang G, Fang Y, Wang X, Zhou F, You L, Zhao Y. Epigenetic reprogramming-induced guanidinoacetic acid synthesis promotes pancreatic cancer metastasis and transcription-activating histone modifications. J Exp Clin Cancer Res. 2023;42:155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 20. | Zhu C, Zhang Y, Li YE, Lucero J, Behrens MM, Ren B. Joint profiling of histone modifications and transcriptome in single cells from mouse brain. Nat Methods. 2021;18:283-292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 201] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 21. | Yan K, Rousseau J, Machol K, Cross LA, Agre KE, Gibson CF, Goverde A, Engleman KL, Verdin H, De Baere E, Potocki L, Zhou D, Cadieux-Dion M, Bellus GA, Wagner MD, Hale RJ, Esber N, Riley AF, Solomon BD, Cho MT, McWalter K, Eyal R, Hainlen MK, Mendelsohn BA, Porter HM, Lanpher BC, Lewis AM, Savatt J, Thiffault I, Callewaert B, Campeau PM, Yang XJ. Deficient histone H3 propionylation by BRPF1-KAT6 complexes in neurodevelopmental disorders and cancer. Sci Adv. 2020;6:eaax0021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 81] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 22. | Yu B, Luo F, Sun B, Liu W, Shi Q, Cheng SY, Chen C, Chen G, Li Y, Feng H. KAT6A Acetylation of SMAD3 Regulates Myeloid-Derived Suppressor Cell Recruitment, Metastasis, and Immunotherapy in Triple-Negative Breast Cancer. Adv Sci (Weinh). 2021;8:e2100014. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 23. | Mendoza-Reinoso V, Schnepp PM, Baek DY, Rubin JR, Schipani E, Keller ET, McCauley LK, Roca H. Bone Marrow Macrophages Induce Inflammation by Efferocytosis of Apoptotic Prostate Cancer Cells via HIF-1α Stabilization. Cells. 2022;11:3712. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 24. | Baell JB, Leaver DJ, Hermans SJ, Kelly GL, Brennan MS, Downer NL, Nguyen N, Wichmann J, McRae HM, Yang Y, Cleary B, Lagiakos HR, Mieruszynski S, Pacini G, Vanyai HK, Bergamasco MI, May RE, Davey BK, Morgan KJ, Sealey AJ, Wang B, Zamudio N, Wilcox S, Garnham AL, Sheikh BN, Aubrey BJ, Doggett K, Chung MC, de Silva M, Bentley J, Pilling P, Hattarki M, Dolezal O, Dennis ML, Falk H, Ren B, Charman SA, White KL, Rautela J, Newbold A, Hawkins ED, Johnstone RW, Huntington ND, Peat TS, Heath JK, Strasser A, Parker MW, Smyth GK, Street IP, Monahan BJ, Voss AK, Thomas T. Inhibitors of histone acetyltransferases KAT6A/B induce senescence and arrest tumour growth. Nature. 2018;560:253-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 231] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 25. | Liu W, Zhan Z, Zhang M, Sun B, Shi Q, Luo F, Zhang M, Zhang W, Hou Y, Xiao X, Li Y, Feng H. KAT6A, a novel regulator of β-catenin, promotes tumorigenicity and chemoresistance in ovarian cancer by acetylating COP1. Theranostics. 2021;11:6278-6292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 26. | Partynska A, Piotrowska A, Pawelczyk K, Rzechonek A, Ratajczak-Wielgomas K, Podhorska-Okolow M, Dziegiel P. The Expression of Histone Acetyltransferase KAT6A in Non-small Cell Lung Cancer. Anticancer Res. 2022;42:5731-5741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 27. | Saglam O, Tang Z, Tang G, Medeiros LJ, Toruner GA. KAT6A amplifications are associated with shorter progression-free survival and overall survival in patients with endometrial serous carcinoma. PLoS One. 2020;15:e0238477. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Turner-Ivey B, Guest ST, Irish JC, Kappler CS, Garrett-Mayer E, Wilson RC, Ethier SP. KAT6A, a chromatin modifier from the 8p11-p12 amplicon is a candidate oncogene in luminal breast cancer. Neoplasia. 2014;16:644-655. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 29. | Lv D, Jia F, Hou Y, Sang Y, Alvarez AA, Zhang W, Gao WQ, Hu B, Cheng SY, Ge J, Li Y, Feng H. Histone Acetyltransferase KAT6A Upregulates PI3K/AKT Signaling through TRIM24 Binding. Cancer Res. 2017;77:6190-6201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 99] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 30. | Jin Y, Yang R, Ding J, Zhu F, Zhu C, Xu Q, Cai J. KAT6A is associated with sorafenib resistance and contributes to progression of hepatocellular carcinoma by targeting YAP. Biochem Biophys Res Commun. 2021;585:185-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 31. | Kim W, Chu TH, Nienhüser H, Jiang Z, Del Portillo A, Remotti HE, White RA, Hayakawa Y, Tomita H, Fox JG, Drake CG, Wang TC. PD-1 Signaling Promotes Tumor-Infiltrating Myeloid-Derived Suppressor Cells and Gastric Tumorigenesis in Mice. Gastroenterology. 2021;160:781-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 114] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 32. | Shen X, Zhao B. Efficacy of PD-1 or PD-L1 inhibitors and PD-L1 expression status in cancer: meta-analysis. BMJ. 2018;362:k3529. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 239] [Cited by in RCA: 419] [Article Influence: 52.4] [Reference Citation Analysis (0)] |
| 33. | Wu Q, Jiang L, Li SC, He QJ, Yang B, Cao J. Small molecule inhibitors targeting the PD-1/PD-L1 signaling pathway. Acta Pharmacol Sin. 2021;42:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 249] [Article Influence: 49.8] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/