Published online Sep 15, 2025. doi: 10.4251/wjgo.v17.i9.103816
Revised: June 29, 2025
Accepted: August 1, 2025
Published online: September 15, 2025
Processing time: 254 Days and 3.8 Hours
Chemotherapy, targeted therapy, and immunotherapy have all been shown to achieve some efficacy in treating intrahepatic cholangiocarcinoma (ICC). How
To evaluate the efficacy and safety of combining chemotherapy, targeted therapy, and immunotherapy, with or without TACE, in patients with ICC.
We recruited 83 patients with unresectable ICC from July 2021 to December 2023 at the Affiliated Hospital of Xuzhou Medical University. Forty-one patients received TACE combined with chemotherapy, tyrosine kinase inhibitors, and pro
The objective response rate in the experimental group was greater than that in the control group (39.0% vs 19.0%, P < 0.05). The disease control rate in the experimental group was significantly greater than that in the control group (75.6% vs 52.4%, P < 0.05). The median progression-free survival times were 14.3 months in the experimental group and 12.7 months in the control group (P < 0.05). All 41 patients in the experimental group developed postembolization syndrome. Among the symptoms, fever and pain were significantly more common in the experimental group than in the control group (85.4% vs 11.9%, P < 0.001 and 58.5% vs 9.5%, P < 0.001). No grade 4 or 5 treatment-related adverse events were observed in either group.
In patients with unresectable ICC, TACE combined with chemotherapy, tyrosine kinase inhibitors, and PD-1/PD-L1 inhibitors has good efficacy and high safety, indicating potential benefits for these patients.
Core Tip: This study retrospectively evaluated the efficacy and safety of transarterial chemoembolization combined with chemotherapy, tyrosine kinase inhibitors, and programmed death 1/programmed cell death ligand 1 inhibitors in patients with intrahepatic cholangiocarcinoma. In conclusion, the combination of transarterial chemoembolization with chemo
- Citation: Chen X, Sun XH, Xiao Y, Zhang D, Lu XY, Fu CL, Bi C, Wang X. Efficacy and safety of transarterial chemoembolization with chemotherapy, PD-1/PD-L1 inhibitors, and tyrosine kinase inhibitors in unresectable intrahepatic cholangiocarcinoma. World J Gastrointest Oncol 2025; 17(9): 103816
- URL: https://www.wjgnet.com/1948-5204/full/v17/i9/103816.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i9.103816
Intrahepatic cholangiocarcinoma (ICC) is the second most common primary liver cancer after hepatocellular carcinoma. It is characterized by an insidious onset, as early symptoms are often subtle or absent[1,2]. As a result, many patients are diagnosed at an advanced stage, missing the optimal surgical window. Thus, they must be treated with chemotherapy, immunotherapy, targeted therapy, or local interventional treatments to manage the disease[3-5]. Chemotherapy can effectively control disease progression[6], and given its well-established efficacy, it is widely used in clinical practice.
Transarterial chemoembolization (TACE) is a localized treatment for advanced ICC[5,7-9], that induces tumor ischemia and necrosis through embolization of the tumor-feeding artery while selectively delivering chemotherapeutic agents to the tumor site. For patients whose ICC has a high tumor burden and vascular invasion, complete embolization with TACE can be challenging and often requires repeated treatments. However, repeated TACE may stimulate increased ex
Rapid advancements in molecular biology and the increasing popularity of molecular targeting and immunotherapy have led to the relatively routine implementation of tyrosine kinase inhibitors (TKIs) for increasing treatment efficacy. For example, the TKI lenvatinib has been approved as a first-line treatment for advanced biliary malignant tumors[12-14]. However, few studies have evaluated the effectiveness of combining TACE with different systemic therapies in ICC. This study retrospectively assessed the efficacy and safety of TACE combined with chemotherapy, TKIs, and PD-1/PD-L1 inhibitors in patients with unresectable ICC.
We recruited 83 patients with unresectable ICC treated at the Affiliated Hospital of Xuzhou Medical University between July 2021 and December 2023. Among them, 41 patients received TACE combined with chemotherapy, TKIs, and PD-1/PD-L1 inhibitors (experimental group), whereas 42 patients were treated with chemotherapy, TKIs, and PD-1/PD-L1 inhibitors (control group). Baseline data from patients with advanced-stage ICC in both groups were collected for comparative analysis. Follow-up assessments were conducted to evaluate the short-term efficacy, progression-free survival (PFS), and safety profiles of the treatments.
The inclusion criteria for this study were as follows: (1) A clinical diagnosis and/or histopathological confirmation of ICC; (2) Age between 18 and 75 years; (3) Child-Pugh class A or B; (4) Eastern Cooperative Oncology Group performance status (ECOG PS) score of 0 or 1; (5) Barcelona Clinic Liver Cancer stage B or C; and (6) Ability to provide informed consent for treatment. The exclusion criteria were as follows: (1) The presence of other malignant tumors; (2) Unmeasurable target lesions; (3) A history of prior surgery, radiotherapy, or organ transplantation; (4) Severe renal insufficiency, coagulation dysfunction, heart failure, myocardial infarction, or stroke; and (5) Serious complications such as hepatic encephalopathy or hepatorenal syndrome.
After the patient was placed under local anesthesia, the modified Seldinger technique was employed to puncture the right femoral artery. Under digital subtraction angiography guidance, a catheter was advanced into the celiac trunk and superior mesenteric arteries for angiography to identify the tumor’s arterial blood supply. After angiography, a 3F microcatheter was super selectively inserted into the tumor’s feeding artery. In accordance with the size of the tumor, 2-4 mg of raltitrexed, 20-40 mg of epirubicin, and 5-10 mL of ethiodized poppy seed oil were slowly injected. Gelatin sponge particles were used to embolize the tumor vessels, typically by occluding the hepatic artery on the tumor’s feeding side. Four to eight weeks after TACE, follow-up assessments, including enhanced abdominal computed tomography or magnetic resonance imaging, tumor marker measurements, liver and kidney function tests, and complete blood counts, were performed to determine whether additional TACE treatment was needed. If untreatable local progression was observed, TACE was discontinued.
Systemic chemotherapy was administered within one month following TACE treatment. The patient received a com
TKIs: TKIs plus PD-1/PD-L1 inhibitors were administered within one month after TACE. Oral lenvatinib was administered at 8 mg (2 capsules of 4 mg each) once daily for patients with a body weight < 60 kg or at 12 mg (3 capsules of 4 mg each) once daily for patients with a body weight ≥ 60 kg; alternatively, sorafenib (300 mg) was given once daily, or anlotinib (12 mg) was administered once daily from days 1-14 over a 3-week cycle.
PD-1/PD-L1 inhibitors: Patients were intravenously infused with camrelizumab every 3 weeks at a dose of 200 mg per infusion; with pembrolizumab every 3 weeks at a dose of 200 mg per infusion; or with toripalimab every 3 weeks at a dose of 240 mg per infusion. TKIs were suspended 3 days before and 3 days after subsequent TACE procedures. If the patient experienced disease progression or intolerable adverse events, the treating physician determined whether to discontinue or adjust the dosage.
Demographic characteristics (age and sex), ECOG PS score, history of hepatitis and liver cirrhosis, Child-Pugh classification, clinical features (number of tumors, maximum tumor size on imaging, major vascular invasion, extrahepatic metastasis), and tumor marker (alpha-fetoprotein and carbohydrate antigen 19-9) levels were obtained from the hospital’s electronic medical records system.
Patients were followed up until December 2024, during which data were collected from outpatient visits, telephone surveys, or in person (for hospitalized patients). Tumor response was evaluated based on the modified Response Evaluation Criteria in Solid Tumors (mRECIST) as follows[15-17]: (1) Complete response: Complete disappearance of all target lesions with arterial phase enhancement and a reduction of the short axes of all target lymph nodes to < 10 mm; (2) Partial response: A reduction of ≥ 30% in the sum of the maximum diameters of the target lesions (arterial phase enhancement) with respect to baseline; (3) Progressive disease: A ≥ 20% increase in the sum of the maximum diameters of the target lesions (arterial phase enhancement) with respect to the minimum value during the study, an absolute increase of at least 5 mm, or the appearance of new lesions; and (4) Stable disease: A decrease in the sum of the maximum dia
The objective response rate (ORR) was calculated as the total effective rate (%) = (complete response + partial response)/total number of patients × 100%. The disease control rate (DCR) was calculated as the percentage of patients who achieved disease control (%), that is, (complete response + partial response + stable disease)/total number of patients × 100%. PFS was defined as the time from treatment initiation to disease progression or death from any cause. Adverse events during the treatment process were assessed using the Common Terminology Criteria for Adverse Events version 5.0 to analyze the safety of the two treatment methods[18,19].
Statistical analysis of the data was performed using SPSS software. Categorical data are presented as frequencies (percentages), and comparisons of these data, as well as of adverse events, between the groups were conducted using the χ2 test or Fisher’s exact test. Median survival was determined with the Kaplan-Meier method, and the corresponding survival curves were plotted. The log-rank test was used to compare the survival time between treatment groups. A P value < 0.05 was considered to indicate statistical significance.
Among the 83 patients, 41 were in the experimental group, and 42 were in the control group. The baseline data, including age, sex, ECOG PS score, history of hepatitis, previous cirrhosis, Child-Pugh classification, number of tumors, maximum tumor size on imaging, macrovascular invasion, extrahepatic metastasis, and tumor marker levels, did not significantly differ between the groups, as shown in Table 1.
| Baseline data | Experimental group (n = 41) | Control group (n = 42) | P value |
| Sex | |||
| Male | 20 (48.8) | 27 (64.3) | 0.154 |
| Female | 21 (51.2) | 15 (35.7) | |
| Age | |||
| > 60 | 17 (41.5) | 23 (54.8) | 0.225 |
| ≤ 60 | 24 (58.5) | 19 (45.2) | |
| ECOG PS score | |||
| 0 | 24 (58.5) | 24 (57.1) | 0.898 |
| 1 | 17 (41.5) | 18 (42.9) | |
| HBV infection | |||
| No | 14 (34.1) | 12 (28.6) | 0.584 |
| Yes | 27 (65.9) | 30 (71.4) | |
| Cirrhosis | |||
| No | 14 (34.1) | 9 (21.4) | 0.196 |
| Yes | 27 (65.9) | 33 (78.6) | |
| Child-Pugh | |||
| A | 17 (41.5) | 18 (42.9) | 0.898 |
| B | 24 (58.5) | 24 (57.1) | |
| AFP (ng/mL) | |||
| < 400 | 34 (82.9) | 30 (71.4) | 0.213 |
| ≥ 400 | 7 (17.1) | 12 (28.6) | |
| CA19-9 (U/mL) | |||
| > 1000 | 7 (17.1) | 10 (23.8) | 0.447 |
| ≤ 1000 | 34 (82.9) | 32 (76.2) | |
| Max tumor size (mm) | |||
| > 50 | 20 (48.8) | 24 (57.1) | 0.445 |
| ≤ 50 | 21 (51.2) | 18 (42.9) | |
| Tumor number | |||
| One | 7 (17.1) | 6 (14.3) | 0.727 |
| More than one | 34 (82.9) | 36 (85.7) | |
| Large vessel invasion | |||
| No | 25 (61.0) | 32 (76.2) | 0.135 |
| Yes | 16 (39.0) | 10 (23.8) | |
| Extrahepatic metastasis | |||
| No | 25 (61.0) | 21 (50.0) | 0.315 |
| Yes | 16 (39.0) | 21 (50.0) | |
| TKIs | |||
| Lenvatinib | 26 (63.4) | 24 (57.1) | 0.121 |
| Anlotinib | 11 (26.8) | 7 (16.7) | |
| Sofantinib | 4 (9.8) | 11 (26.2) | |
| PD1/PD-L1 inhibitors | |||
| Camrelizumab | 19 (46.3) | 21 (50.0) | 0.815 |
| Toripalimab | 14 (34.2) | 15 (35.7) | |
| Pembrolizumab | 8 (19.5) | 6 (14.3) |
According to the mRECIST criteria, the ORR in the experimental group was greater than that in the control group (39.0% vs 19.0%, P < 0.05). The DCR significantly differed between the experimental group (75.6%) and the control group (52.4%) (P < 0.05), as shown in Table 2.
| Efficacy evaluation | Experimental group (n = 41) | Control group (n = 42) | P value |
| Complete response | 2 (4.9) | 0 | |
| Partial response | 14 (34.1) | 8 (19.0) | |
| Stable disease | 15 (36.6) | 14 (33.3) | |
| Progressive disease | 10 (24.4) | 20 (47.6) | |
| ORR | 16 (39.0) | 8 (19.0) | 0.045 |
| DCR | 31 (75.6) | 22 (52.4) | 0.028 |
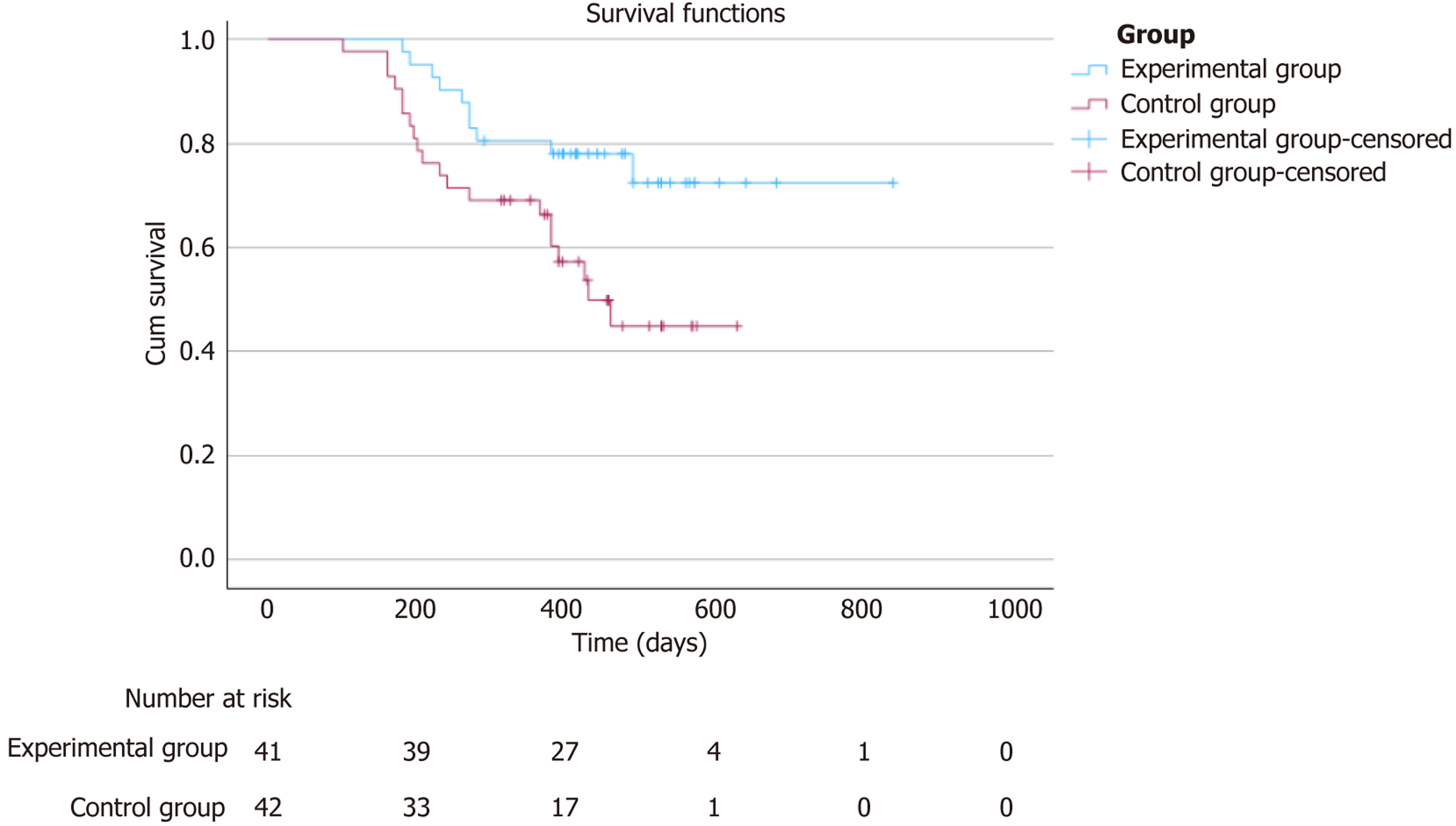
At the end of the follow-up period, a total of 98 TACE procedures were performed on the 41 patients in the experimental group. The median PFS (mPFS) in the experimental group was 14.3 months [95% confidence interval (CI): 13.1-16.0], which significantly differed from the 12.7 months (95%CI: 10.6-15.6) in the control group (P = 0.016), as shown in Figure 1.
No treatment-related deaths occurred in either group. A comparison of adverse event rates between the two groups is shown in Table 3. All 41 patients in the experimental group developed postembolization syndrome after TACE, classified as grade 1 or 2. Fever and pain were significantly more common in the experimental group than in the control group (85.4% vs 11.9%, P < 0.001 and 58.5% vs 9.5%, P < 0.001). No grade 4 or 5 treatment-related adverse events were observed in either group.
| Treatment-related adverse events | Experimental group (n = 41) | Control group (n = 42) | P value |
| Nausea and vomiting | 20 (48.8) | 14 (33.3) | 0.152 |
| Fever | 35 (85.4) | 5 (11.9) | < 0.001a |
| Pain | 24 (58.5) | 4 (9.5) | < 0.001a |
| Weakness | 13 (31.7) | 18 (42.9) | 0.294 |
| Diarrhea | 9 (22.0) | 8 (19.0) | 0.743 |
| Constipation | 17 (41.5) | 19 (45.2) | 0.729 |
| Trachyphonia | 4 (9.8) | 2 (4.8) | 0.326 |
| Gingival hemorrhage | 7 (17.1) | 3 (7.1) | 0.146 |
| Hypertension | 5 (12.2) | 12 (28.6) | 0.065 |
| Hand-foot syndrome | 15 (36.6) | 17 (40.5) | 0.716 |
| Hypothyroidism | 8 (19.5) | 7 (16.7) | 0.736 |
| Rash | 6 (14.6) | 9 (21.4) | 0.421 |
| Proteinuria | 7 (17.1) | 8 (19.0) | 0.815 |
Currently, systemic chemotherapy remains the first-line treatment for unresectable ICC. However, the survival benefit of chemotherapy alone is limited. Therefore, combining systemic chemotherapy with other therapeutic approaches is ess
Although chemotherapy, targeted therapy, and immunotherapy have been shown to achieve some efficacy in ICC, systemic treatments have not provided optimal results for some patients. Therefore, the combination of TACE with hepatic artery infusion chemotherapy or other local interventional therapy methods is being considered for use in the treatment of liver tumors, demonstrating therapeutic benefits in certain circumstances[5]. In the study by Xia et al[22], the mOS and mPFS in the TACE plus lenvatinib group were significantly longer than those in the lenvatinib monotherapy group (mOS: 15.9 months vs 8.6 months, P = 0.002; mPFS: 8.6 months vs 4.4 months, P < 0.001), suggesting that TACE was effective in prolonging patient survival over systemic therapy alone.
In this study, we retrospectively analyzed the efficacy and safety of TACE combined with chemotherapy, TKIs, and PD-1/PD-L1 inhibitors in treating unresectable ICC. The results revealed that the mPFS of the experimental group was significantly longer than that of the control group (P < 0.05). Moreover, the ORR (according to the mRECIST criteria) in the experimental group was greater than that in the control group (39.0% vs 19.0%, P < 0.05). Similarly, the DCR in the experimental group (75.6%) was significantly greater than that in the control group (52.4%) (P < 0.05). These findings suggest that the combination of chemotherapy with targeted therapy and immunotherapy, along with TACE as a local treatment, is more effective than systemic therapy alone. Wang et al[23] reported that the combination of TACE, lenvatinib and PD-1 inhibitors significantly improved overall survival, PFS, ORR, and DCR without significantly increasing the risk of adverse events of any grade, which is consistent with the findings of this study.
Previous studies have also demonstrated a synergistic effect among TACE, TKIs and PD-1 inhibitors[24-27]. The tumor cell necrosis generated via TACE induces the release of antigens and proinflammatory cytokines, which increases the efficacy of PD-1 inhibitors[28]. Additionally, the hypoxic microenvironment produced with TACE increases the ex
There were several limitations in this study. First, the sample size for this study was small. Second, the patients included in the study were treated with different types of TKIs and PD-1 inhibitors, leading to some inconsistency in the treatment protocols. Additionally, we employed PFS as the primary endpoint, but sufficient data on overall survival were unavailable, limiting the ability to assess the long-term efficacy and safety of the treatment.
The combination of TACE with chemotherapy, TKIs, and PD-1/PD-L1 inhibitors has good efficacy and a favorable safety profile, offering potential benefits for patients with ICC. However, given the limitations of the current study, large-scale, randomized controlled trials are needed to further validate the efficacy and safety of the combination of TACE with chemotherapy, TKIs, and PD-1/PD-L1 inhibitors in patients with ICC in the future.
In patients with unresectable ICC, TACE combined with chemotherapy, TKIs, and PD-1/PD-L1 inhibitors demonstrated good efficacy and high safety, indicating potential benefits for these patients.
The authors gratefully acknowledge the support and contributions of colleagues and friends who assisted in the preparation of this manuscript. We also extend our heartfelt thanks to all the patients at the Affiliated Hospital of Xuzhou Medical University whose participation was essential to the completion of this study.
| 1. | Moris D, Palta M, Kim C, Allen PJ, Morse MA, Lidsky ME. Advances in the treatment of intrahepatic cholangiocarcinoma: An overview of the current and future therapeutic landscape for clinicians. CA Cancer J Clin. 2023;73:198-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 314] [Article Influence: 104.7] [Reference Citation Analysis (0)] |
| 2. | Clements O, Eliahoo J, Kim JU, Taylor-Robinson SD, Khan SA. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: A systematic review and meta-analysis. J Hepatol. 2020;72:95-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 394] [Article Influence: 65.7] [Reference Citation Analysis (1)] |
| 3. | Valle JW, Kelley RK, Nervi B, Oh DY, Zhu AX. Biliary tract cancer. Lancet. 2021;397:428-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 728] [Article Influence: 145.6] [Reference Citation Analysis (3)] |
| 4. | Ilyas SI, Affo S, Goyal L, Lamarca A, Sapisochin G, Yang JD, Gores GJ. Cholangiocarcinoma - novel biological insights and therapeutic strategies. Nat Rev Clin Oncol. 2023;20:470-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 139] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 5. | Renzulli M, Ramai D, Singh J, Sinha S, Brandi N, Ierardi AM, Albertini E, Sacco R, Facciorusso A, Golfieri R. Locoregional Treatments in Cholangiocarcinoma and Combined Hepatocellular Cholangiocarcinoma. Cancers (Basel). 2021;13:3336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 6. | Le Roy B, Gelli M, Pittau G, Allard MA, Pereira B, Serji B, Vibert E, Castaing D, Adam R, Cherqui D, Sa Cunha A. Neoadjuvant chemotherapy for initially unresectable intrahepatic cholangiocarcinoma. Br J Surg. 2018;105:839-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 187] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 7. | Martin RCG 2nd, Simo KA, Hansen P, Rocha F, Philips P, McMasters KM, Tatum CM, Kelly LR, Driscoll M, Sharma VR, Crocenzi TS, Scoggins CR. Drug-Eluting Bead, Irinotecan Therapy of Unresectable Intrahepatic Cholangiocarcinoma (DELTIC) with Concomitant Systemic Gemcitabine and Cisplatin. Ann Surg Oncol. 2022;29:5462-5473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Krieg S, Essing T, Krieg A, Roderburg C, Luedde T, Loosen SH. Recent Trends and In-Hospital Mortality of Transarterial Chemoembolization (TACE) in Germany: A Systematic Analysis of Hospital Discharge Data between 2010 and 2019. Cancers (Basel). 2022;14:2088. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 9. | Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37:429-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2207] [Cited by in RCA: 2303] [Article Influence: 100.1] [Reference Citation Analysis (1)] |
| 10. | Llovet JM, De Baere T, Kulik L, Haber PK, Greten TF, Meyer T, Lencioni R. Locoregional therapies in the era of molecular and immune treatments for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2021;18:293-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 692] [Article Influence: 138.4] [Reference Citation Analysis (0)] |
| 11. | Xing R, Gao J, Cui Q, Wang Q. Strategies to Improve the Antitumor Effect of Immunotherapy for Hepatocellular Carcinoma. Front Immunol. 2021;12:783236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 137] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 12. | Shi GM, Huang XY, Wu D, Sun HC, Liang F, Ji Y, Chen Y, Yang GH, Lu JC, Meng XL, Wang XY, Sun L, Ge NL, Huang XW, Qiu SJ, Yang XR, Gao Q, He YF, Xu Y, Sun J, Ren ZG, Fan J, Zhou J. Toripalimab combined with lenvatinib and GEMOX is a promising regimen as first-line treatment for advanced intrahepatic cholangiocarcinoma: a single-center, single-arm, phase 2 study. Signal Transduct Target Ther. 2023;8:106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 116] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 13. | Ding X, Sun W, Li W, Shen Y, Guo X, Teng Y, Liu X, Zheng L, Li W, Chen J. Transarterial chemoembolization plus lenvatinib versus transarterial chemoembolization plus sorafenib as first-line treatment for hepatocellular carcinoma with portal vein tumor thrombus: A prospective randomized study. Cancer. 2021;127:3782-3793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 102] [Article Influence: 20.4] [Reference Citation Analysis (1)] |
| 14. | Spahn S, Kleinhenz F, Shevchenko E, Stahl A, Rasen Y, Geisler C, Ruhm K, Klaumuenzer M, Kronenberger T, Laufer SA, Sundberg-Malek H, Bui KC, Horger M, Biskup S, Schulze-Osthoff K, Templin M, Malek NP, Poso A, Bitzer M. The molecular interaction pattern of lenvatinib enables inhibition of wild-type or kinase-mutated FGFR2-driven cholangiocarcinoma. Nat Commun. 2024;15:1287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 15. | Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3353] [Cited by in RCA: 3439] [Article Influence: 214.9] [Reference Citation Analysis (43)] |
| 16. | Llovet JM, Lencioni R. mRECIST for HCC: Performance and novel refinements. J Hepatol. 2020;72:288-306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 503] [Cited by in RCA: 495] [Article Influence: 82.5] [Reference Citation Analysis (0)] |
| 17. | Finn RS, Ikeda M, Zhu AX, Sung MW, Baron AD, Kudo M, Okusaka T, Kobayashi M, Kumada H, Kaneko S, Pracht M, Mamontov K, Meyer T, Kubota T, Dutcus CE, Saito K, Siegel AB, Dubrovsky L, Mody K, Llovet JM. Phase Ib Study of Lenvatinib Plus Pembrolizumab in Patients With Unresectable Hepatocellular Carcinoma. J Clin Oncol. 2020;38:2960-2970. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 661] [Cited by in RCA: 924] [Article Influence: 154.0] [Reference Citation Analysis (0)] |
| 18. | Trotti A, Colevas AD, Setser A, Basch E. Patient-reported outcomes and the evolution of adverse event reporting in oncology. J Clin Oncol. 2007;25:5121-5127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 258] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 19. | Kawaguchi T, Azuma K, Sano M, Kim S, Kawahara Y, Sano Y, Shimodaira T, Ishibashi K, Miyaji T, Basch E, Yamaguchi T. The Japanese version of the National Cancer Institute's patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE): psychometric validation and discordance between clinician and patient assessments of adverse events. J Patient Rep Outcomes. 2017;2:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 20. | Kelley RK, Ueno M, Yoo C, Finn RS, Furuse J, Ren Z, Yau T, Klümpen HJ, Chan SL, Ozaka M, Verslype C, Bouattour M, Park JO, Barajas O, Pelzer U, Valle JW, Yu L, Malhotra U, Siegel AB, Edeline J, Vogel A; KEYNOTE-966 Investigators. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2023;401:1853-1865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 669] [Article Influence: 223.0] [Reference Citation Analysis (0)] |
| 21. | Zhu C, Xue J, Wang Y, Wang S, Zhang N, Wang Y, Zhang L, Yang X, Long J, Yang X, Sang X, Zhao H. Efficacy and safety of lenvatinib combined with PD-1/PD-L1 inhibitors plus Gemox chemotherapy in advanced biliary tract cancer. Front Immunol. 2023;14:1109292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 29] [Reference Citation Analysis (0)] |
| 22. | Xia D, Bai W, Wang E, Li J, Chen X, Wang Z, Huang M, Huang M, Sun J, Yang W, Lin Z, Wu J, Li Z, Yang S, Zhu X, Chen Z, Zhang Y, Fan W, Mai Q, Ding R, Nie C, Feng L, Li X, Huang W, Sun J, Wang Q, Lv Y, Li X, Luo B, Wang Z, Yuan J, Guo W, Li K, Li B, Li R, Yin Z, Xia J, Han G. Lenvatinib with or without Concurrent Drug-Eluting Beads Transarterial Chemoembolization in Patients with Unresectable, Advanced Hepatocellular Carcinoma: A Real-World, Multicenter, Retrospective Study. Liver Cancer. 2022;11:368-382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 23. | Wang L, Lin L, Zhou W. Efficacy and safety of transarterial chemoembolization combined with lenvatinib and PD-1 inhibitor in the treatment of advanced hepatocellular carcinoma: A meta-analysis. Pharmacol Ther. 2024;257:108634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 24. | Chang Y, Jeong SW, Young Jang J, Jae Kim Y. Recent Updates of Transarterial Chemoembolilzation in Hepatocellular Carcinoma. Int J Mol Sci. 2020;21:8165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 246] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 25. | Vogl TJ, Naguib NN, Nour-Eldin NE, Bechstein WO, Zeuzem S, Trojan J, Gruber-Rouh T. Transarterial chemoembolization in the treatment of patients with unresectable cholangiocarcinoma: Results and prognostic factors governing treatment success. Int J Cancer. 2012;131:733-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 94] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 26. | Gadaleta CD, Ranieri G. Trans-arterial chemoembolization as a therapy for liver tumours: New clinical developments and suggestions for combination with angiogenesis inhibitors. Crit Rev Oncol Hematol. 2011;80:40-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 61] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 27. | Kudo M, Ueshima K, Ikeda M, Torimura T, Tanabe N, Aikata H, Izumi N, Yamasaki T, Nojiri S, Hino K, Tsumura H, Kuzuya T, Isoda N, Yasui K, Aino H, Ido A, Kawabe N, Nakao K, Wada Y, Yokosuka O, Yoshimura K, Okusaka T, Furuse J, Kokudo N, Okita K, Johnson PJ, Arai Y; TACTICS study group. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut. 2020;69:1492-1501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 221] [Cited by in RCA: 543] [Article Influence: 90.5] [Reference Citation Analysis (0)] |
| 28. | Llovet JM, Montal R, Sia D, Finn RS. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat Rev Clin Oncol. 2018;15:599-616. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1487] [Cited by in RCA: 1462] [Article Influence: 182.8] [Reference Citation Analysis (0)] |
| 29. | Palmer DH, Malagari K, Kulik LM. Role of locoregional therapies in the wake of systemic therapy. J Hepatol. 2020;72:277-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 72] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 30. | Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2800] [Cited by in RCA: 4363] [Article Influence: 545.4] [Reference Citation Analysis (6)] |
| 31. | Tajiri K, Futsukaichi Y, Kobayashi S, Nagata K, Yasumura S, Takahara T, Minemura M, Yasuda I. Efficacy of on-demand intrahepatic arterial therapy in combination with sorafenib for advanced hepatocellular carcinoma. Onco Targets Ther. 2019;12:2205-2214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/