Published online Jan 26, 2026. doi: 10.4252/wjsc.v18.i1.115655
Revised: November 1, 2025
Accepted: December 5, 2025
Published online: January 26, 2026
Processing time: 90 Days and 16.2 Hours
We read with the great interest the study by Ababneh et al in which induced mesenchymal stem cell-derived exosomes were shown to exhibit a stronger and more sustained anti-proliferative effect by inducing a senescence-like state wi
Core Tip: Extracellular vesicles can promote or inhibit oncogenic progression by shaping the tumor microenvironment. The efficiency of extracellular vesicle delivery can be affected by the extracellular matrix and altered glycocalyx. The tumor extracellular matrix and cellular glycocalyx can create a permissive niche for invasion, immune evasion, and metastasis. These factors are also essential for predicting the effectiveness of targeted therapies and the responses of bystander cells. Understanding these factors is crucial for treating cancers that are resistant to immunotherapies.
- Citation: Klabukov ID, Kisel A, Yatsenko E, Sulina Y, Baranovskii DS. Cancer cell-dependent increase in senescence-like populations following exosome treatment: The role of extracellular matrix and cellular glycocalyx. World J Stem Cells 2026; 18(1): 115655
- URL: https://www.wjgnet.com/1948-0210/full/v18/i1/115655.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v18.i1.115655
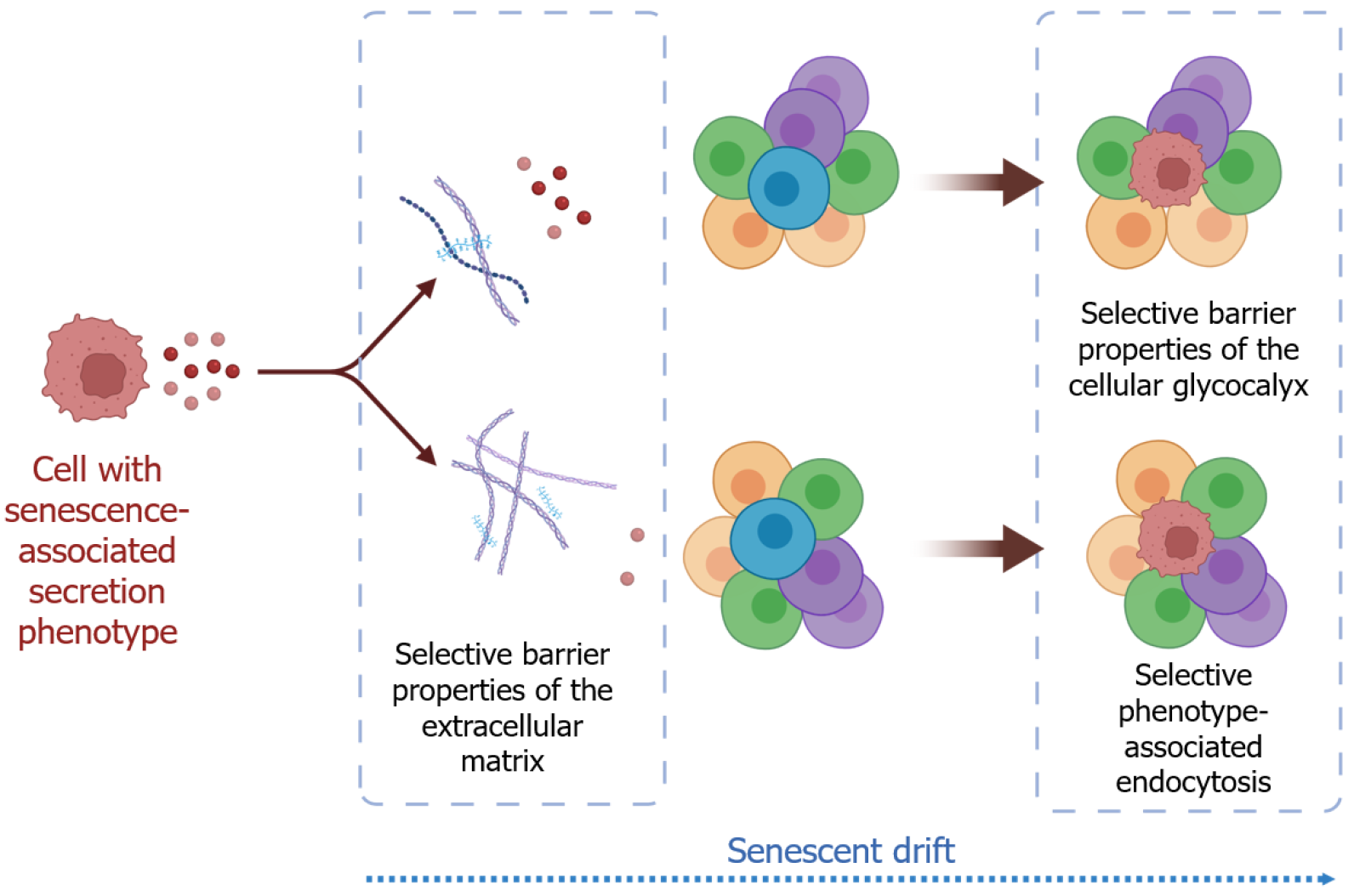
We read with special interest the study by Ababneh et al[1] in 2025, in which it was found that induced pluripotent stem cell-derived mesenchymal stem cell-derived exosomes exhibited a stronger anti-proliferative effect by inducing a senescence-like state without apoptosis. We would like to discuss an interesting phenomenon related to the differential effects of various cell cultures to exosomes derived from different sources. To explain this phenomenon, the approach of ‘senescent drift’ was determined to explain the targeted transfer of senescence-associated secretion phenotype (SASP)-associated exosomes, depending on tissue properties[2]. However, the underlying cause of this phenomenon and possible use-cases of them remains unclear.
The therapeutic mechanisms of cancer cell death are characterized by the exposure of damage-associated molecular patterns. These patterns are recognized by innate immune cells, which can then prime cell responses[3]. The effectiveness of targeted therapy depends on various factors related to molecular and cellular mechanisms and spatial-like patterns. For example, intratumor injection of therapeutics or exosomes is a more effective way for cancer treatment to be delivered than systemic delivery, which is related to the mechanical overcoming of the first physical barrier line in tumors[3,4]. It is well-known that the unique mechanical properties of the tumor extracellular matrix (ECM) differ from normal conditions and may hinder delivery of therapeutics both for tumor and bystander cells[5,6], as well as affect antigen presentation and subsequent T-cell activation[7]. In light of the promising principles of cancer immunotherapy, bystander cell reactions can be considered intermediate players in immune responses. This could explain why some drugs are effective even without targeted delivery mechanisms[8]. Recent studies have shown that alterations to the glycocalyx affect dendritic cells and prevent the development of therapeutic mechanisms. These alterations also affect the tumor cell ECM, which is composed of macromolecules and glycocalyx properties[9,10]. The phenomenon of targeting the tumor ECM and cellular glycocalyx should be recognized as an emerging frontier in cancer therapy. This unconventional feature should be considered to avoid misunderstanding the basic principles of targeted anti-cancer therapeutics, including monoclonal antibody conjugates and mRNA vaccines.
The SASP phenotype refers to the way senescent cells actively modify their environment by secreting various soluble factors and extracellular vesicles, i.e., senescent ‘poisoning’ of the extracellular environment. In contrast, senescent drift refers to the long-term spread or systemic integration of a senescence-like state in tissue caused by tissue-specific interactions. Figure 1 explains the differences between the SASP and senescent drift, and the ECM/glycocalyx-dependent transfer of the extracellular vesicles.
Tissue-specific ECM barrier functions can facilitate the design of drugs that effectively transport through target tissues, thereby improving their therapeutic efficacy[11,12]. Heparan sulfate proteoglycans function as receptors on the cell surface, with their heparan sulfate chains binding to exosomes, often due to the polyanionic nature of the heparan sulfate chains. This binding could trigger the internalization of the exosome into the target cell. Therefore, the glycocalyx imposes biophysical barriers that are also linked to inhibitory receptor signaling pathways in immune cells, which are related to cell reception and endocytosis[9,13-15].
The properties of tumor ECM and the cellular glycocalyx could regulate the effectiveness of targeted therapy through altered cellular endocytosis, as well as the responses of bystander cells. These bystander effects may include altered cytokine secretion, ECM remodeling, or immune cell infiltration modulation, all of which can impact treatment outcomes. Understanding and potentially manipulating the tumor ECM and glycocalyx’s biophysical and biochemical features are crucial for improving the precision and efficacy of targeted cancer therapies through modification of the structural biomolecules composition and organization.
The tumor ECM and cellular glycocalyx are key players in predicting the effectiveness of exosome-based targeted therapy, as well the responses of bystander cells. These two extracellular factors remain the final frontier in treating immunotherapy-resistant cancers. Future studies should focus on understanding the interactions between drugs and extracellular barriers in order to develop targeted delivery systems or combination therapies that can improve the effectiveness of immunotherapy in treating resistant cancers.
| 1. | Ababneh NA, Nashwan S, AlDiqs R, Ismail MA, Abdulelah AA, Abu-Humaidan AHA, AlQirem L, Al-Abdallat K, Al-Qaisi T, Saleh T, Awidi A. Cancer cell-dependent increase in senescence-like populations following exosome treatment from bone marrow and induced pluripotent stem cell-derived mesenchymal stem cells. World J Stem Cells. 2025;17:110381. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Smirnova A, Yatsenko E, Baranovskii D, Klabukov I. Mesenchymal stem cell-derived extracellular vesicles in skin wound healing: the risk of senescent drift induction in secretome-based therapeutics. Mil Med Res. 2023;10:60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 3. | Van Hoecke L, Saelens X. Therapeutic anti-tumor immunity directed against neo-epitopes by intratumor delivery of mRNA encoding MLKL. Cell Stress. 2018;2:279-281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Muñoz NM, Williams M, Dixon K, Dupuis C, McWatters A, Avritscher R, Manrique SZ, McHugh K, Murthy R, Tam A, Naing A, Patel SP, Leach D, Hartgerink JD, Young S, Prakash P, Hwu P, Sheth RA. Influence of injection technique, drug formulation and tumor microenvironment on intratumoral immunotherapy delivery and efficacy. J Immunother Cancer. 2021;9:e001800. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 91] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 5. | Klabukov I, Smirnova A, Yakimova A, Kabakov AE, Atiakshin D, Petrenko D, Shestakova VA, Sulina Y, Yatsenko E, Stepanenko VN, Ignatyuk M, Evstratova E, Krasheninnikov M, Sosin D, Baranovskii D, Ivanov S, Shegay P, Kaprin AD. Oncomatrix: Molecular Composition and Biomechanical Properties of the Extracellular Matrix in Human Tumors. J Mol Pathol. 2024;5:437-453. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 18] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 6. | Piperigkou Z, Mangani S, Koletsis NE, Koutsakis C, Mastronikolis NS, Franchi M, Karamanos NK. Principal mechanisms of extracellular matrix-mediated cell-cell communication in physiological and tumor microenvironments. FEBS J. 2025. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 7. | Buffone A, Weaver VM. Don't sugarcoat it: How glycocalyx composition influences cancer progression. J Cell Biol. 2020;219:e201910070. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 68] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 8. | Wu Z, Li T. Nanoparticle-Mediated Cytoplasmic Delivery of Messenger RNA Vaccines: Challenges and Future Perspectives. Pharm Res. 2021;38:473-478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 68] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 9. | Ghasempour S, Freeman SA. The glycocalyx and immune evasion in cancer. FEBS J. 2023;290:55-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 10. | Mitchell MJ, Jain RK, Langer R. Engineering and physical sciences in oncology: challenges and opportunities. Nat Rev Cancer. 2017;17:659-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 183] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 11. | American Association of Neurological Surgeons (AANS); American Society of Neuroradiology (ASNR), Cardiovascular and Interventional Radiology Society of Europe (CIRSE); Canadian Interventional Radiology Association (CIRA); Congress of Neurological Surgeons (CNS), European Society of Minimally Invasive Neurological Therapy (ESMINT), European Society of Neuroradiology (ESNR); European Stroke Organization (ESO); Society for Cardiovascular Angiography and Interventions (SCAI), Society of Interventional Radiology (SIR), Society of NeuroInterventional Surgery (SNIS); World Stroke Organization (WSO); Sacks D, Baxter B, Campbell BCV, Carpenter JS, Cognard C, Dippel D, Eesa M, Fischer U, Hausegger K, Hirsch JA, Shazam Hussain M, Jansen O, Jayaraman MV, Khalessi AA, Kluck BW, Lavine S, Meyers PM, Ramee S, Rüfenacht DA, Schirmer CM, Vorwerk D. Multisociety Consensus Quality Improvement Revised Consensus Statement for Endovascular Therapy of Acute Ischemic Stroke. Int J Stroke. 2018;13:612-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 407] [Reference Citation Analysis (0)] |
| 12. | Cahn D, Stern A, Buckenmeyer M, Wolf M, Duncan GA. Extracellular Matrix Limits Nanoparticle Diffusion and Cellular Uptake in a Tissue-Specific Manner. ACS Nano. 2024;18:32045-32055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 13. | Joshi BS, Zuhorn IS. Heparan sulfate proteoglycan-mediated dynamin-dependent transport of neural stem cell exosomes in an in vitro blood-brain barrier model. Eur J Neurosci. 2021;53:706-719. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 68] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 14. | Christianson HC, Svensson KJ, van Kuppevelt TH, Li JP, Belting M. Cancer cell exosomes depend on cell-surface heparan sulfate proteoglycans for their internalization and functional activity. Proc Natl Acad Sci U S A. 2013;110:17380-17385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 551] [Cited by in RCA: 717] [Article Influence: 55.2] [Reference Citation Analysis (0)] |
| 15. | Cerezo-Magaña M, Bång-Rudenstam A, Belting M. The pleiotropic role of proteoglycans in extracellular vesicle mediated communication in the tumor microenvironment. Semin Cancer Biol. 2020;62:99-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/