Published online Oct 26, 2025. doi: 10.4252/wjsc.v17.i10.109862
Revised: June 19, 2025
Accepted: September 8, 2025
Published online: October 26, 2025
Processing time: 154 Days and 13.8 Hours
Myocardial infarction (MI) is a significant global cause of chronic heart failure. In post-ischemic cardiac hypertrophy, multiple molecular targets and signals within the cardiac tissue are evident. Mesenchymal stem cell-derived exosomes (MSC-EXO) and exercise (EXE) showed promise in enhancing post-ischemic cardiac repair.
To investigate how the exosomes released by stem cells and/or EXE can promote cardiac repair and improve isoproterenol (ISO)-induced post-ischemic hypertrophy.
The enrolled animals were divided into 8 control rats and 32 experimental rats. Induction of MI was performed using ISO. Then, the experimental rats were divided into 4 groups: Rats subjected to 4 weeks of swimming EXE, rats treated with exosomes, and the combined treatment. Additionally, functional and interactional exploration of targeted proteins was conducted using Gene Ontology, Kyoto Encyclopedia of Genes and Genomes analysis, and STRING database, along with histological examination.
Both MSC-EXO or EXE significantly improved ISO induced elevation of cardiac enzymes, oxidative stress, and inflammatory markers, as well as the degenerative changes of the cardiac muscles, fibrosis, and apoptosis. Meanwhile, the combined treatment of EXE and MSC-EXO resulted in a significant improvement in cardiac function and structure as compared to all groups that synchronized with dual inhibition of extracellular signal-regulated kinase and protein kinase B/mammalian target of rapamycin (P < 0.01) signaling and modulation of matrix metalloproteinase 9 and sarcoplasmic endoplasmic reticulum calcium ATPase type 2a, with significant improved angiogenesis.
Functional and structural cardiac improvements are accompanied by reduced inflammation, oxidative stress, and apoptosis. Both MSC-EXO and EXE exert cardio-protection by upregulating sarcoplasmic endoplasmic reticulum calcium ATPase, the critical pump for normal calcium handling.
Core Tip: Myocardial infarction has been extensively validated in rats for reliably inducing post-infarction metabolic and histological changes. Combined therapy may target the extracellular signal-regulated kinase and protein kinase B/mammalian target of rapamycin signaling, both of which are central to the development of hypertrophic remodeling after myocardial infarction, along with the crosstalk between matrix metalloproteinase 9 and sarcoplasmic endoplasmic reticulum calcium ATPase type 2a, which maintains the balance between hypertrophic growth and contractile function. This could offer a new therapeutic regimen for treating early maladaptive left ventricular hypertrophy.
- Citation: ShamsEldeen AM, AbdElalim MM, Mohamed NS, AbdelRahman MM, Kamar SS, AbdelKader DH, Elsharkawy SH, Rashed LA, Faisal SS, Osman WA, Selmy AM. Mesenchymal stem-derived exosomes enhance therapeutic benefits of exercise in isoproterenol-induced myocardial ischemia: Targeting ERK and Akt/mTOR signaling. World J Stem Cells 2025; 17(10): 109862
- URL: https://www.wjgnet.com/1948-0210/full/v17/i10/109862.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v17.i10.109862
Myocardial infarction (MI) is a significant cardiovascular disease caused by an imbalance between coronary blood flow and myocardial tissue demand. Post-ischemic left ventricular hypertrophy (LVH) is a pathological consequence of MI characterized by adverse cardiac remodeling, fibrosis, impaired calcium handling, diastolic dysfunction, and heart failure with preserved ejection fraction (HFpEF)[1,2]. The key molecular players in this process include extracellular signal-regulated kinase (ERK), and protein kinase B (Akt)/mammalian target of rapamycin (mTOR) signaling[3,4]. The ERK and Akt/mTOR signaling pathways are beneficial when maintained within the physiological range, but their overstimulation can lead to pathological cardiac hypertrophy[5]. These signaling pathways are interconnected through the modulation of sarcoplasmic endoplasmic reticulum calcium ATPase type 2a (SERCA2a), a key Ca2+-handling protein involved in myocardial contractility, and matrix metalloproteinase 9 (MMP9), which targets and cleaves extracellular proteins. Therefore, both SERCA2a and MMP9 are useful markers for diagnosing and predicting the prognosis of LVH[6,7].
SERCA2a reuptakes calcium into the sarcoplasmic reticulum (SR), crucial for normal muscle contraction and relaxation. SERCA2a mRNA and protein levels are reduced in cardiac maladaptive hypertrophy, and reduced SERCA2a is thought to play an important role in both systolic and diastolic heart failure[7]. Persistent ERK activation in the post-ischemic period suppresses SERCA2a, worsening calcium imbalance that leads to concentric hypertrophy, fibrosis, and eventually heart failure[8]. MMP9 is involved in the extracellular matrix remodeling process, a crucial aspect of cardiac hypertrophy, fibrosis, and diastolic dysfunction, which is mediated by phosphorylation and subsequent ERK activation[9].
Enhanced Akt/mTOR pathway activation under chronic stress can modulate the expression of MMP9, linking cell growth signals with extracellular matrix remodeling and accelerating pathological remodeling, hypertrophy, and fibrosis[10]. Further research is needed to investigate antioxidative regimens that target the crosstalk between the ERK and Akt/mTOR signaling pathways and the interconnected proteins SERCA2a and MMP9, as they may have a potential protective role against unfavorable cardiac remodeling by restoring structural and functional disturbances.
It is well known that the Akt/mTOR signaling pathway is crucial in mediating stimuli that promote both maladaptive and adaptive ventricular hypertrophy[11]. Cardiac hypertrophy is followed by myocardial apoptosis within 24-48 hours, which exacerbates myocardial damage. Several studies have reported that Akt/mTOR signaling is triggered by various hypertrophic stimuli, including pressure overload, β-adrenergic stimulation, angiotensin II, and insulin-like growth factor 1[12]. Inhibition of the Akt/mTOR pathway can eliminate dysfunctional cytoplasmic organelles, which drive apoptosis by accumulating intracellular Ca2+, increased oxidative stress, and endoplasmic reticulum stress[13].
Exercise (EXE)-based cardiac rehabilitation is a crucial method for reducing high cardiovascular risk in MI by promoting beneficial changes that prevent the progression of cardiac ischemia through modulation of the MMP9 and SERCA2a interplay[14]. Under ischemic conditions, moderate-intensity swimming EXE enhances blood flow to the heart, promotes mitochondrial biogenesis, improves ATP availability, and reduces inflammation, oxidative stress, and subsequent structural damage[15]. While the exact mechanisms responsible for the beneficial antioxidative effects of EXE are not fully understood, they may involve modulation of the targeted ERK and Akt/mTOR pathways.
Mesenchymal stem cell-derived exosomes (MSC-EXO), the tiny vesicles released by stem cells, have emerged as a promising candidate for cell-based therapeutics, with proven efficacy in treating a wide range of diseases[16]. MSC-EXO has been shown to exert protective effects in MI by reducing inflammation, oxidative stress, apoptosis, and fibrosis while promoting angiogenesis[17]. Over the past decade, the relationship between exosomes and cardiac function has garnered significant interest, with exosomes being implicated in tissue repair and regeneration[18] as well as in the improvement of diabetic cardiomyopathy[19].
The present work aimed to study the therapeutic roles of MSC-EXO and EXE, either as monotherapy or combination therapy, in promoting cardiac repair following isoproterenol (ISO)-induced myocardial ischemia. We investigated the role of selected lines of intervention on the expression of SERCA2 and the fibrotic modulator MMP9. According to the functional and interactional exploration of targeted proteins using Gene Ontology (GO), Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis, and the STRING database, the expression levels of Akt/mTOR/MMP9 and the protein levels of ERK and Akt were investigated.
ISO hydrochloride powder from Sigma-Aldrich Co., St. Louis, MO, United States, was dissolved in normal saline. Xylazine HCl from Kepro Veterinary Solutions (Holland, Netherlands) was supplied in vials at a concentration of 20 mg/mL, and ketamine HCl from Sigma-Tec, Egypt, was supplied at a concentration of 50 mg/mL.
The study included 50 female albino Wistar rats obtained from the Animal House of Kasr Al-Ainy Faculty of Medicine, Cairo University. Ten rats aged 6-8 weeks were used for stem cell expansion and exosome preparation. Forty rats were included in the experimental group. The study protocol was approved by the Institutional Animal Care and Use Committee of Cairo University (Approval No. CU/III/F/59/20). The rats were housed in separate cages under standard conditions, with appropriate humidity (45% ± 5%) and temperature (24 ± 2 °C), and a 12-hour light/dark cycle. They were provided with standard chow and water ad libitum and acclimatized for 7 days before the study.
The study involved 40 adult female rats, randomly assigned to control rats (n = 8) and experimental rats (n = 32), which were subjected to MI induced by subcutaneous injections of ISO (85 mg/kg) for two consecutive days, administered at 24-hour intervals.
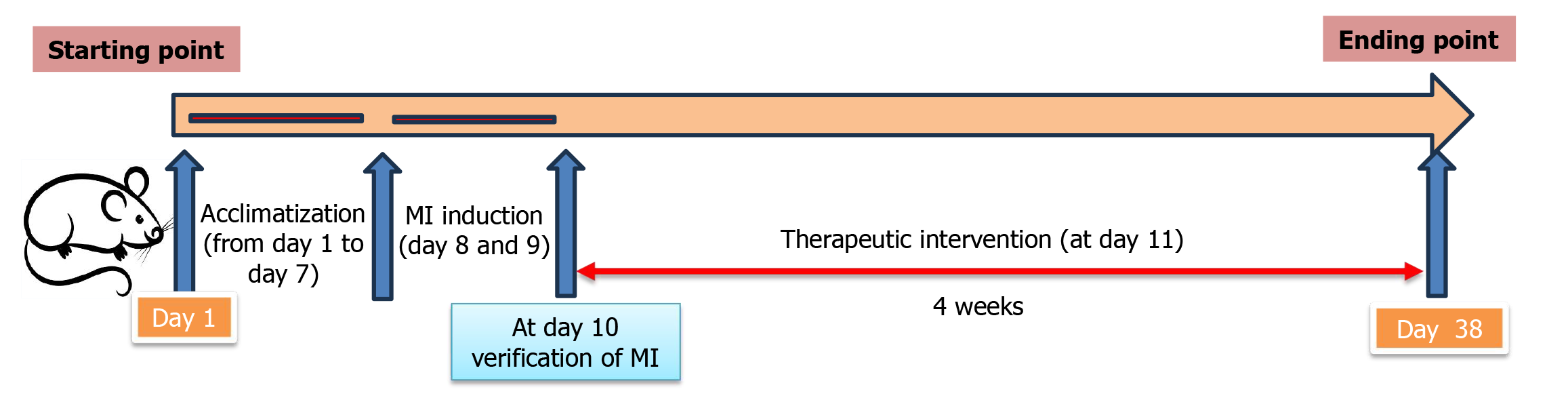
Electrocardiogram (ECG) and blood samples were collected on the third day after initiating the ISO injection (12 hours after the final ISO dose) to measure levels of creatine kinase MB (CK-MB) and lactate dehydrogenase (LDH). The MI rats exhibited ST-segment changes and significantly elevated CK-MB and LDH levels[20]. A timeline for all experimental interventions is presented in Figure 1. The rats were then subdivided into four groups (n = 8): (1) No intervention for four weeks (ISO + MI group); (2) Rats were subjected to swimming EXEs for four weeks (MI + EXE group)[21]; (3) Each rat received an intraperitoneal injection of MSC-EXO at a concentration of 0.5 mg after confirmation of the model (MI + MSC-EXO group)[22]; and (4) Rats received a combination of EXE and MSC-EXO treatment (MI + EXE + MSC-EXO group).
Rats in the MI + EXE and MI + EXE + MSC-EXO groups were trained by swimming in 36 °C water for 40 minutes per day, five days per week, for four weeks. The rats swam in a tank with a water depth of 50 cm and an average surface area of 200 cm2 per rat. After each training session, they were dried with a towel and placed under a lamp at 35 °C for 30 minutes[21].
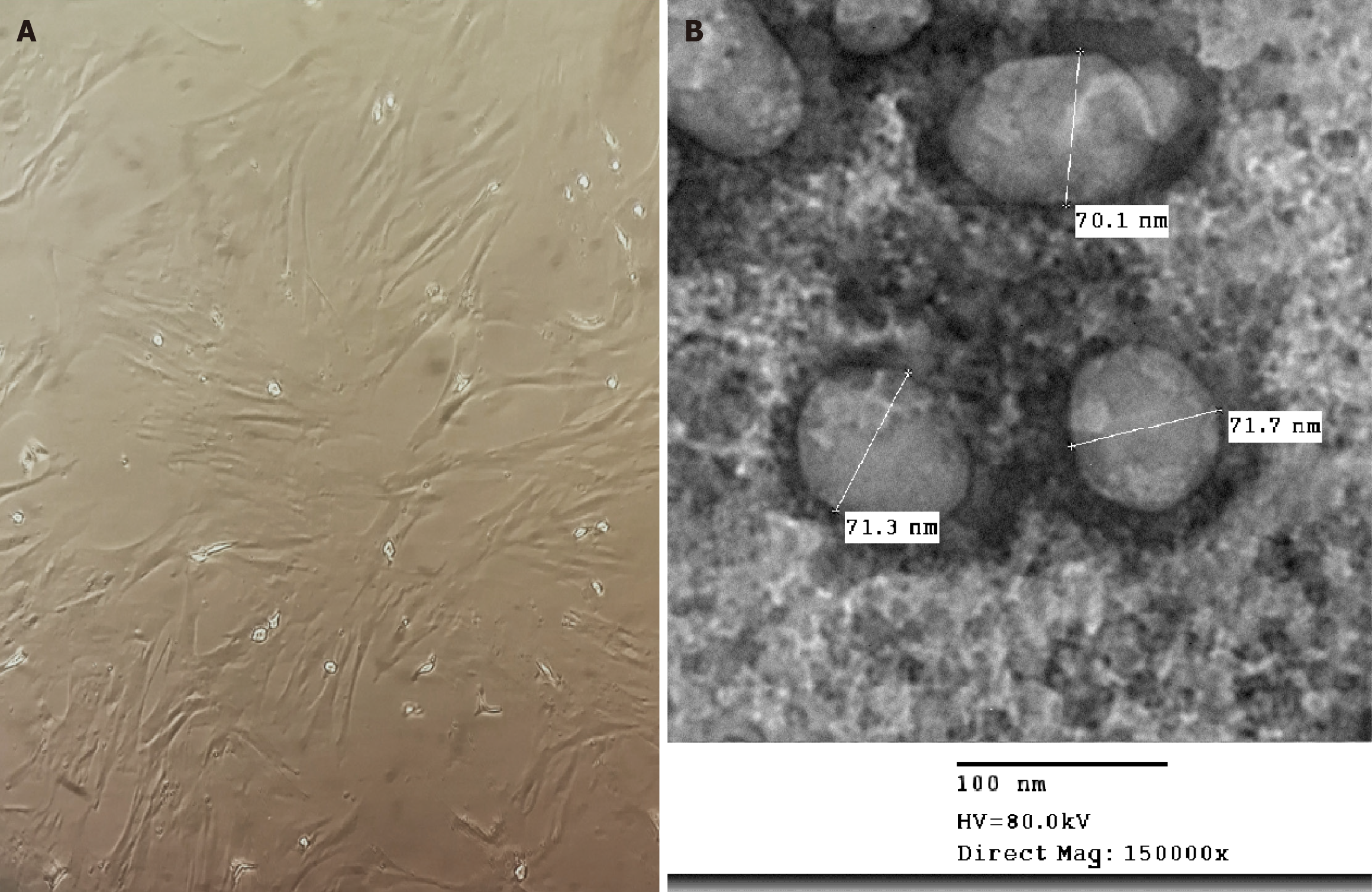
The femur and tibia were collected aseptically, and bone marrow samples were obtained by washing the marrow cavity with high-glucose Dulbecco’s modified Eagle medium. The collected suspension was centrifuged, and the supernatant was discarded. The pellet was then cultured in complete media containing 10% fetal bovine serum, 1% L-glutamic acid, and 1% double antibiotics. The cells were maintained in a 5% CO2 incubator at 37 °C[23]. Once approximately 85% confluence was reached, the cells were sub-cultured until passage 2 (P2), and bone marrow mesenchymal stem cells were characterized by their morphology (Figure 2A).
MSC-EXO was isolated from the media supernatant of the bone marrow mesenchymal stem cells. After thawing, the media were centrifuged at 2000 × g for 20 minutes, followed by ultracentrifugation at 100000 × g in an SW41 swing rotor (Beckman Coulter, Fullerton, CA, United States) for one hour at 4 °C. The exosomes were washed once with serum-free M199 (Sigma-Aldrich, MO, United States) containing 25 mmol/L 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (pH = 7.4) and subjected to a second ultracentrifugation under the same conditions[24]. MSC-EXO was stored at -80 °C until the experiment. Transmission electron microscopy analysis of the purified exosomes revealed their characteristic spheroid, electron-dense, membrane-bound structure with a diameter of less than 100 nm (Figure 2B).
The ECG was conducted at baseline and three days after ISO injection for all rats in the various groups under ketamine anesthesia (100 mg/kg) using a PowerLab 4/30 Model 866. Needle recording electrodes were used and connected to the animal BioAmp (ML136). ECG was recorded on channel 1 with a digital filter set at 30 Hz. A two-dimensional and M-mode recording at the level of the papillary muscles was performed using an 8-10 MHz linear transducer connected to an Aloka F31 machine with a 13-MHz phased array transducer. Measurements included left ventricular internal diameter during systole (LVIDS), and left ventricular internal diameter during diastole (LVIDD), interventricular septal diameter during systole (IVSS), and interventricular septal diameter during diastole (IVSD), lateral wall diameter during systole (LWS), and lateral wall diameter during diastole (LWD), stroke volume (SV), and ejection fraction (EF%)[25].
Blood samples were obtained from the tail veins shortly after MI induction and at the end of the study to assess the cardiac enzymes CK-MB and LDH in serum. The serum was then separated by centrifugation at 3000 rpm for ten minutes. The levels of CK-MB and LDH were determined using commercial kits from Stanbio Laboratory, Boerne, TX, United States.
Rats’ body weights were recorded before euthanasia using an overdose of intraperitoneal ketamine (100 mg/kg) and xylazine (10 mg/kg)[26]. The heart weight index (mg/g) was calculated by dividing left ventricular mass and heart weight by body weight (left ventricular/body weight and heart weight/body weight), serving as an index of hypertrophy[27]. For biochemical and histological studies, mid-ventricular heart specimens were divided into three sets for processing as tissue homogenates, paraffin blocks, and transmission electron microscopy capsules.
Cardiac tissue samples (30 mg) were homogenized in RIPA buffer containing phosphatase and protease inhibitors. After centrifugation at 12000 rpm for 20 minutes, the supernatants were collected. Tumor necrosis factor (TNF)-α, interleukin (IL)-6, and atrial natriuretic factor (ANF) concentrations in cardiac tissues were quantitatively measured using ELISA kits [TNF-α (Cat. No. RAB0480), IL-6 (Cat. No. RAB0312), and ANF (Cat. No. RAB0385) quantitative ELISA kits from Sigma-Aldrich, MO, United States] according to the manufacturer’s instructions.
The malondialdehyde (MDA) and glutathione (GSH) levels in cardiac tissue homogenate were measured using the Colorimetric Assay Kit (TBA method) from Elabscience® (Cat. No: E-BC-K025-S) and BioVision (Cat. No: K261-100), respectively.
The GO Consortium (http://geneontology.org/, accessed in February 2024) was selected as the gene set database for enrichment analysis due to its widespread use as a standard approach for annotating gene products. Pathway enrichment analysis was performed on the genes ERK or Ephb2, Akt, mTOR, MMP9, and Atp2a2 or SERCA2 using the KEGG database (https://www.kegg.jp/pathway/map05415). The analysis applied a significance threshold of P < 0.05 and a false discovery rate (FDR) < 0.05 to establish statistical significance. The findings were retrieved from the KEGG database in February 2024. A protein-protein interaction (PPI) network analysis was conducted using the STRING database (https://string-db.org/, accessed in February 2024) to investigate the relationships among the examined genes. This analysis provided insights into the interactions and connections among the genes based on extensive PPI data.
Total RNA was extracted from cardiac tissue homogenate using the Total RNA Isolation System (Promega, WI, United States). RNA concentration was determined. cDNA was synthesized from 1 μg of total RNA using the High-Capacity cDNA Reverse Transcription Kit (Fermentas, #K1621, Canada). Real-time quantitative polymerase chain reaction amplification with SYBR Green I was performed on an Applied Biosystems instrument with software version 3.1 (StepOne™). The quantitative polymerase chain reaction assay was optimized for annealing temperature. Duplicate samples were prepared for Akt, mTOR, MMP9, ERK1/2, and SERCA2 genes, with β-actin as the housekeeping gene and water as a non-template control to check for DNA contamination. Primer sequences for the investigated genes are listed in Table 1.
| Gene | Primer sequence (5’-3’) | |
| Akt | Forward | CACTTTCGGCAAGGTGATCC |
| Reverse | GTCCTTGGCCACGATGACTT | |
| mTOR | Forward | AGCATCGGATGCTTAGGAGTGG |
| Reverse | CAGCCAGTCATCTTTGGAGACC | |
| MMP9 | Forward | TATTTTTGT GTGGCGYCTGAGAA |
| Reverse | GAGGTGGTTTAGCCGGTGAA | |
| ERK | Forward | GAGCCCAGGGGAACTGCT |
| Reverse | TGGAAGCGGGCTGTCTC | |
| SERCA2 | Forward | CAGTTCATCCGCTACCTCATCTCC |
| Reverse | CGCAGTGGCAGGCAGACC | |
| β-actin | Forward | AGGTCGGAGTCAACGGATTTGGT |
| Reverse | CATGTGGGCCATAGGTCCACCAC |
The RIPA lysis buffer PL005 from Bio BASIC, Inc. (Canada) was used to lyse the cardiac tissue sample. Proteins were loaded onto a polyacrylamide gel for electrophoresis. Membranes were blocked, then incubated overnight with a primary antibody, followed by incubation with a secondary antibody for one hour. The primary antibodies used were anti-ERK1/2 (Thermo Fisher Scientific; rabbit monoclonal, 1:2000, MA, United States), anti-Akt (Sigma, MO, United States), and anti-β-actin (Calbiochem, Inc.; mouse monoclonal, 1:5000, CA, United States). An HRP-conjugated secondary antibody (Novus Biologicals; goat anti-rabbit immunoglobulin G goat mab, CO, United States) was used. The chemiluminescent substrate (Clarity™ Western ECL substrate - Bio-Rad; Cat. #170-5060, Canada) was applied to the blot. Chemiluminescent signals were captured. Image analysis software was used to quantify the band intensity of the target proteins normalized to β-actin.
For light microscopic examination, cardiac specimens were fixed in 10% formaldehyde for 48 hours and then trimmed at the mid-ventricular region. After dehydration in alcohol and clearing in xylene, the specimens were embedded in paraffin. Sections (4-5 μm thick) were stained with hematoxylin and eosin and Masson’s trichrome to illustrate morphological changes, the degree of collagen deposition, and the formation of fibrous tissue. For transmission electron microscopy examination, small cardiac specimens (approximately 1 mm3) were excised, washed in saline, and fixed in 2.5% glutaraldehyde. Ultrastructural changes in the myofibrils, intercalated discs, mitochondria, and SR tubules of myocardial tissue were examined.
To analyze apoptosis and angiogenesis, deparaffinized sections were immunostained with an anti-caspase-3 antibody (AHP2717, Bio-Rad Laboratories, CA, United States; 1:100 dilution) as a marker for apoptosis[28] and an anti-CD31 antibody (EPR17259, Abcam, MA, United States; 1:200 dilution), a marker for progenitor endothelial cells to assess angiogenesis[29]. The primary antibodies were applied to the sections overnight at 4 °C in a humidity chamber, followed by incubation with the secondary antibody for 45 minutes in the same chamber. Sections were then developed with diaminobenzidine for brown coloration and counterstained with Mayer’s hematoxylin.
Statistical analyses were conducted using “SPSS for Windows, Version 16.0 (SPSS Inc., Chicago, IL, United States)”. One-way ANOVA and the “Kruskal-Wallis test” were used to compare values between experimental groups for parametric and non-parametric data, respectively. Post hoc analysis was conducted to identify pairwise differences. Parametric data were presented as mean ± SEM, while non-parametric data were presented as median (range). Significance was set at P < 0.05. Pearson correlation analysis was used for correlation analysis.
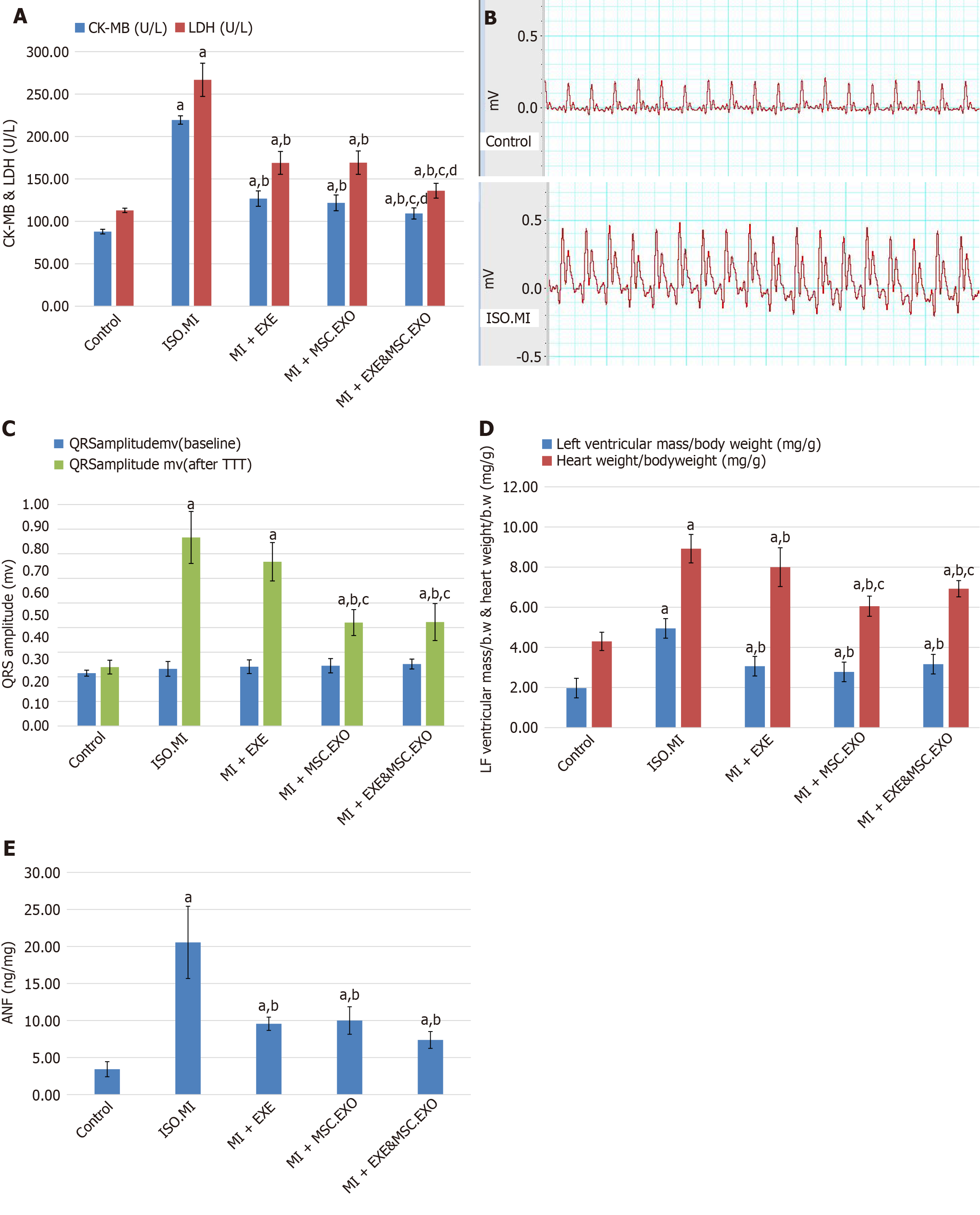
Cardiac injury was confirmed by an increase in serum CK-MB and LDH levels on the third day after initiating ISO injection. ECGs were initially normal in all groups; however, by the third day, dynamic changes with elevated ST segments indicative of MI were observed. Repeated measurements of serum CK-MB and LDH were not performed. Treatment with EXE alone and exosomes resulted in a notable decrease in CK-MB and LDH levels, as well as a reversal of ECG changes, compared to the ISO + MI group. However, the combination of EXE and MSC-EXO was significantly more effective than monotherapy (Figure 3A and B).
Cardiac injury induced by ISO was associated with ventricular hypertrophy, as indicated by increased QRS amplitude, left ventricular/body weight, heart weight/body weight indices, and elevated ANF levels, which are markers of hypertrophy and can be used to monitor treatment response. Treatment with EXE and/or MSC-EXO resulted in a notable improvement in signs of ventricular hypertrophy compared to the ISO + MI group, with the most significant improvement observed in QRS amplitude, left ventricular/body weight, and heart weight/body weight indices in the group that received combined therapy (Figure 3C-E).
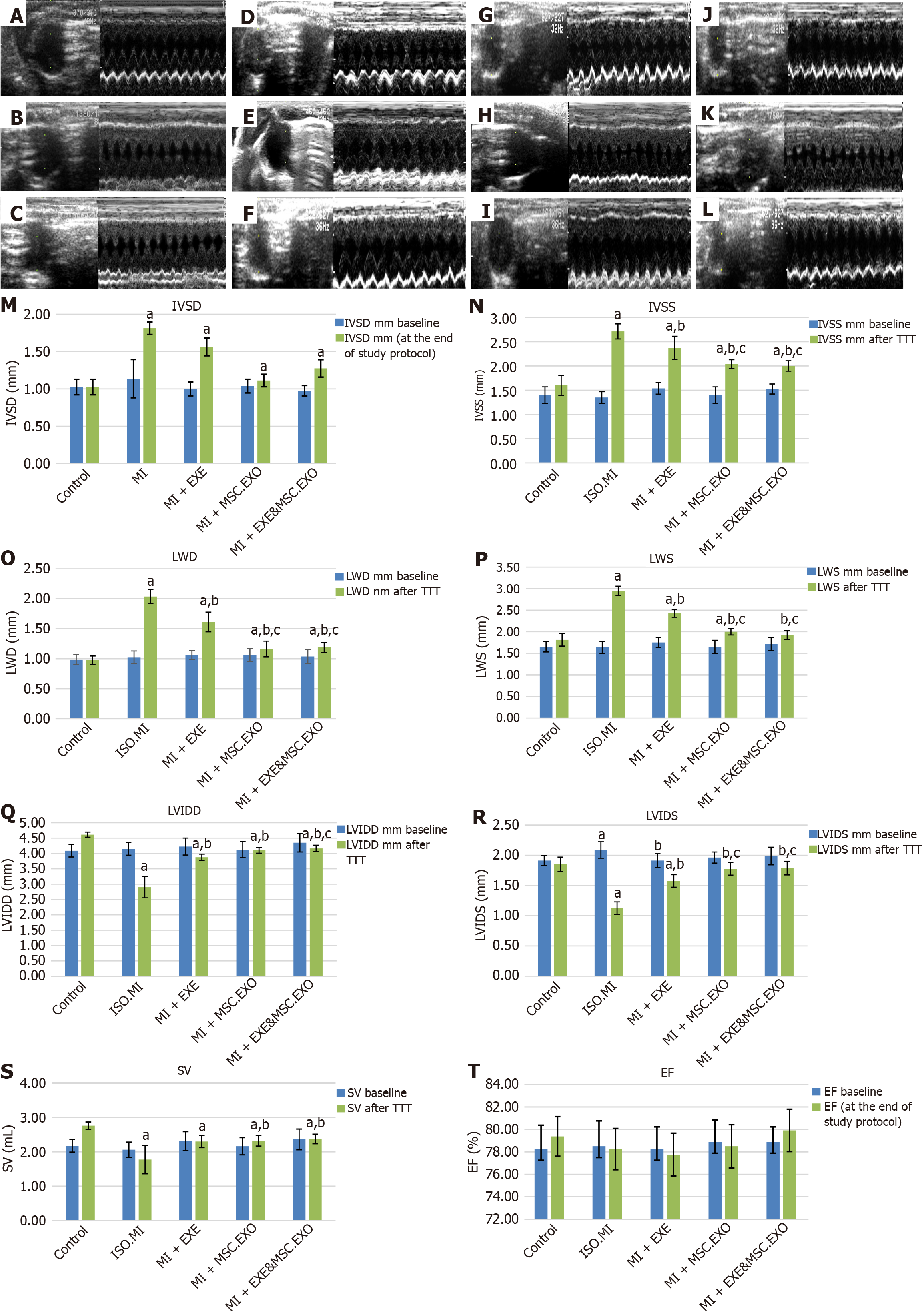
All groups exhibited similar baseline values during echocardiographic data recording (Figure 4A-L). MI induction by ISO led to septal and lateral wall hypertrophy, resulting in increased IVSD, IVSS, LWD, and LWS (Figure 4M-P), along with decreased LVIDD, LVIDS, and SV, indicating impaired ventricular filling and ejection (Figure 4Q-S). These changes were reversed by EXE therapy, with a significant improvement in wall indices observed when MSC-EXO therapy was added to the EXE training regimen. EF was not negatively affected by ISO or influenced by EXE and exosomes (Figure 4T), likely due to the modulation of SV, LVIDD, and LVIDS to a similar extent, maintaining a relatively constant EF ratio of EF = SV/EDV.
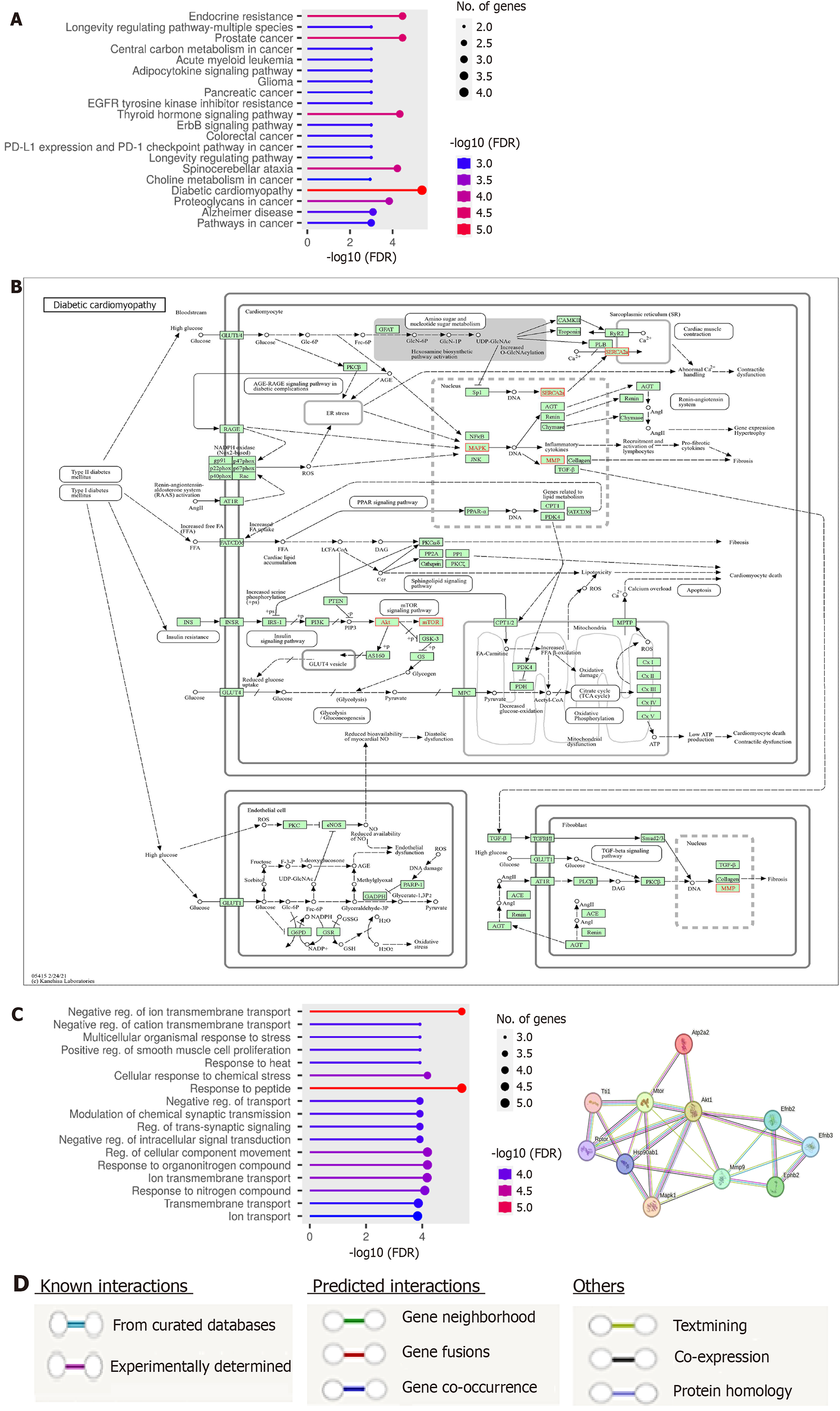
Using the KEGG pathway analysis, the identified target genes ERK, Akt, mTOR, SERCA2a, and MMP9 showed a strong association, particularly in diabetic cardiomyopathy, with a significant -log10FDR value of 5 (Figure 5A). Therefore, we have chosen these target genes for inclusion in our ISO + MI model, focusing on cardiovascular diseases. The analysis revealed that the identified genes are crucial in the diabetic cardiomyopathy pathway. The diagram below illustrates that SERCA2a is actively involved in this pathway, both within the nucleus and outside, supporting its connection to exosomes. This link facilitates its entry into the SR (Figure 5B).
The GO biological process analysis (Figure 5C) indicated significant changes in the biological processes of the predicted target genes. These changes are related to cellular transport systems similar to those in MSC-EXO functions. Key terms include negative regulation of ion transmembrane transport, regulation of cellular components transport, and ion transmembrane transport, with -log10FDR values of 5, 4, and 4, respectively. We utilized the STRING database to gain a deeper understanding of PPIs in our study targets. Each interaction is assigned a score based on seven evidence channels (Figure 5A), with colored lines representing different types of evidence. These include experimental data, database references, gene neighborhood relationships, fusion events, co-occurrence patterns, mentions in literature text, co-expression patterns, and protein homology. The network figure below demonstrates a significant level of interaction among the proteins studied, with a PPI enrichment P-value of 0.00686. Notably, SERCA2a directly interacts with Akt (confidence score 0.536) and mTOR (confidence score 0.401) with moderate confidence (Figure 5D). Akt strongly interacts with mTOR and MMP9, indicating their involvement in hypertrophy development.
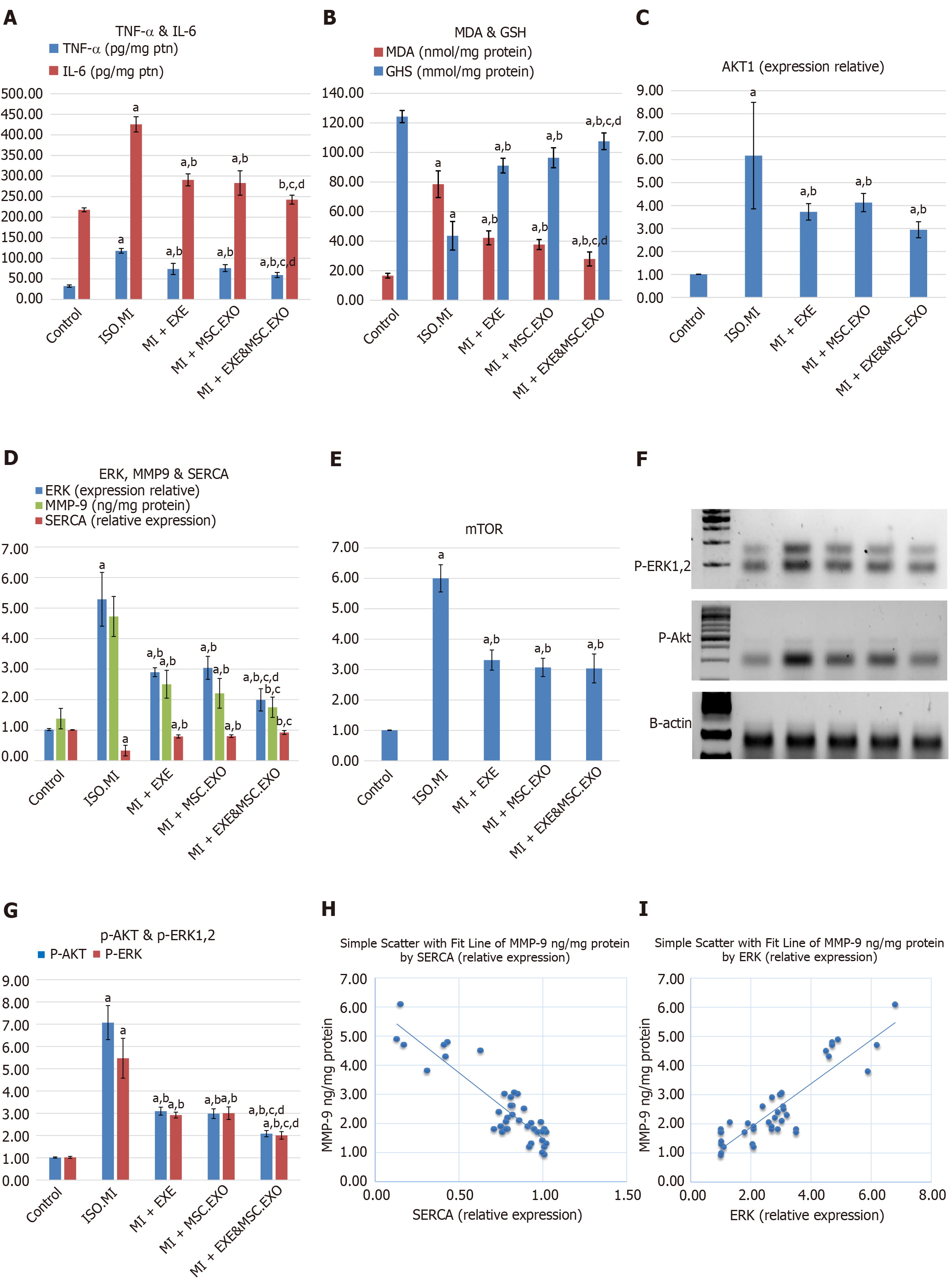
The ISO injection induced inflammation and oxidative stress in cardiac tissues, as evidenced by elevated levels of TNF-α, IL-6, and MDA, along with a reduction in antioxidative capacity (reduced GSH). However, EXE rehabilitation and MSC-EXO mitigated these effects by decreasing TNF-α, IL-6, and MDA levels and increasing GSH levels (Figure 6A and B). When EXE was combined with MSC-EXO, the anti-inflammatory and antioxidant effects of EXE were further enhanced, resulting in synergistic benefits.
Our study showed a significant increase in ERK and Akt/mTOR levels in the ISO + MI group. In contrast, all treated groups exhibited a significant decrease in their levels. Notably, there was a significant reduction in ERK levels in the MI + EXE + MSC-EXO combination group compared to each individual treatment group. Additionally, all treated groups showed a significant increase in SERCA2a levels and a decrease in MMP9 levels compared to rats in the ISO + MI group. Furthermore, the combined administration of MSC-EXO and EXE showed superior effects compared to the other treated groups, leading to the normalization of these levels relative to the control group (Figure 6C-E). Moreover, ERK and Akt proteins exhibited the same expression trend. These results suggest that MSC-EXO and EXE effectively diminish ERK and Akt protein levels in the cardiac tissue of post-MI rats (Figure 6F and G).
A strong positive correlation was found between ERK1/2 gene expression levels and TNF-α levels, supporting the role of post-ischemic cardiac inflammation as a key activator of the ERK hypertrophic pathway. Additionally, a strong negative correlation was observed between SERCA2a and MMP9, indicating a linkage between cardiac performance and the fibrotic state (Figure 6H and I).
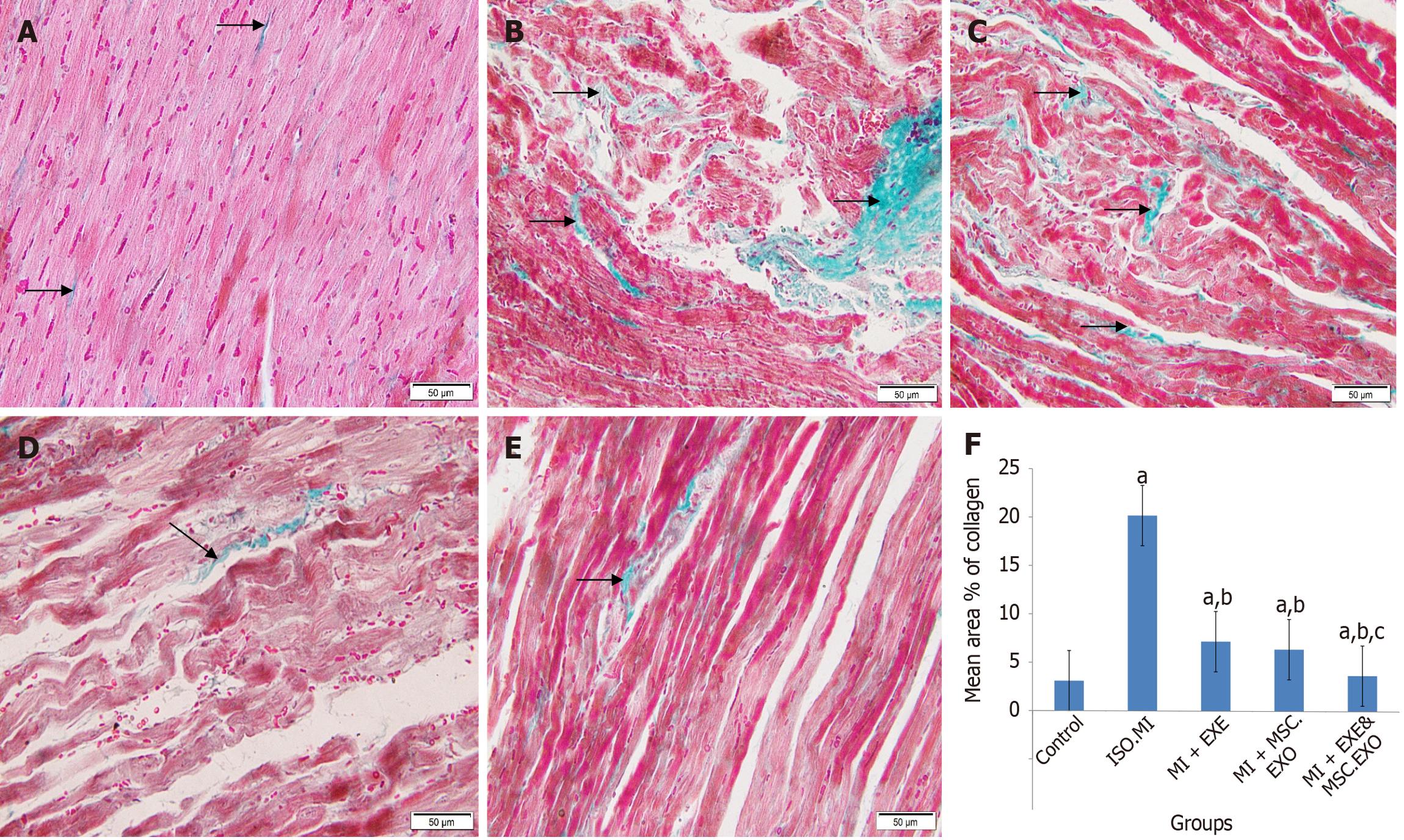
Heart sections from the control rats showed normal architecture with fine collagen deposition (Figures 7A, 7B and 8A). In the ISO + MI group (Figures 7C, 7D and 8B), a large focal area exhibited inflammatory cell infiltration, marked fibrosis, and intense degenerative changes. The surrounding muscle fibers showed increased diameter. The mean area% of collagen deposition was significantly higher than that of the control rats. The MI + EXE group (Figures 7E, 7F and 8C) exhibited a small focal area of degenerated cardiac myocytes with inflammatory cell infiltration and scattered coarse collagen deposition. Additionally, dilated blood vessels were observed. A significant decrease in the mean area% of collagen deposition was noted as compared to the control and MI groups. The MI + MSC-EXO group (Figures 7G, 7H and 8D) showed some muscle fibers with cytoplasmic vacuolation, loss of striation, and pyknotic nuclei, while others displayed a wavy appearance or thinning. Some apparently normal cardiomyocytes and small areas of collagen deposition were present. The mean area% of collagen deposition was significantly lower as compared to the ISO + MI and MI + EXE groups. In the MI + EXE + MSC-EXO group (Figures 7I, 7J and 8E), a small area displayed mildly separated fibers and a few thinned-out muscle fibers with pyknotic nuclei, surrounded by viable, closely arranged muscle fibers, dilated blood vessels, and minimal collagen deposition. The mean area% of collagen deposition was significantly decreased as compared to the ISO + MI, MI + EXE, and MI + MSC-EXO groups (Figure 8F).
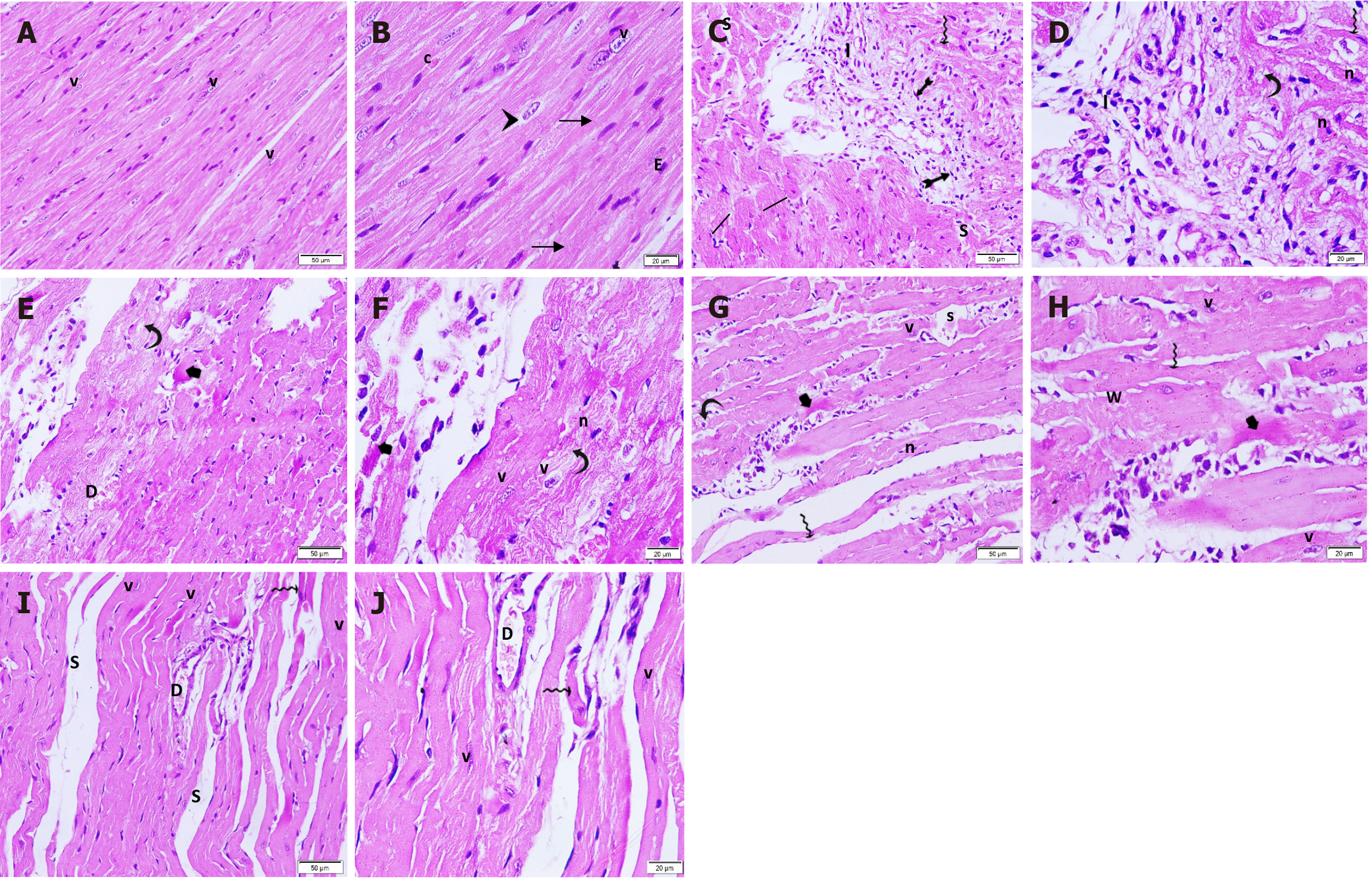
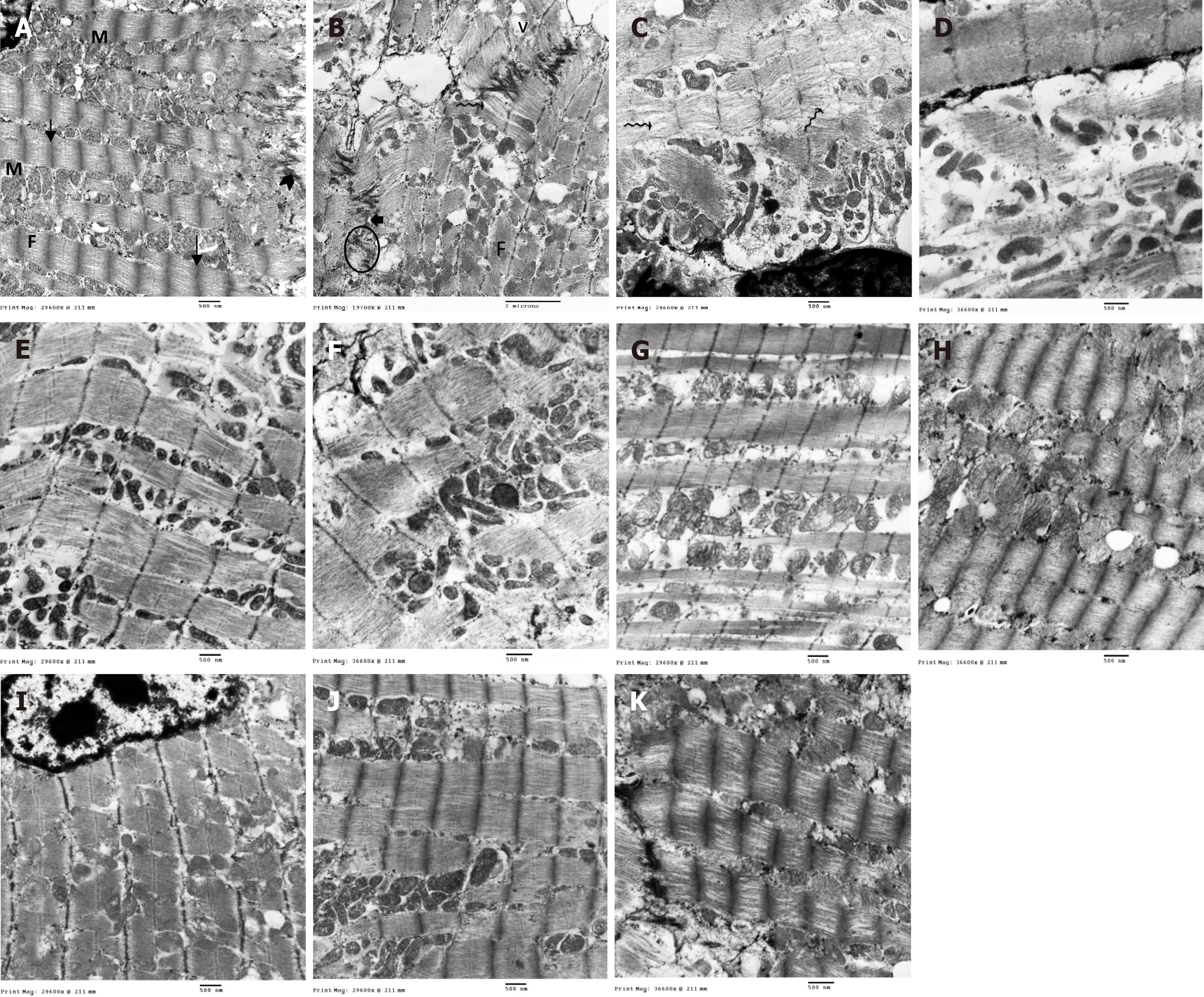
Ultrathin sections of cardiac muscle fibers from the control rats showed normal ultrastructure, with numerous mitochondria alongside the myofibrils, myofibrils exhibiting regular sarcomeres separated by Z-lines, and attachment to the transverse portion of the intercalated disc (Figure 9A). In contrast, the ISO + MI group exhibited disrupted myofibrils with separated myofilaments, irregularly shaped mitochondria with disrupted cristae, cytoplasmic vacuolations, dilated SR tubules, pale desmosomes, widened adherent junction gaps, and clumping of nuclear chromatin (Figure 9B-D). The MI + EXE group exhibited closely packed myofibrils with regular sarcomeres, and mitochondria were arranged between them. Some of these mitochondria had disrupted cristae and irregular shapes, accompanied by subsarcolemmal widening and focal loss of myofilaments (Figure 9E and F). The MI + MSC-EXO group displayed closely packed myofibrils with regular sarcomeres, although some swollen mitochondria and dilated SR cisternae were observed (Figure 9G and H). In the MI + EXE + MSC-EXO group, closely packed myofibrils with regular sarcomeres were seen, attached to the transverse portion of the intercalated disc, along with numerous mitochondria filled with intact cristae and abundant glycogen granules (Figure 9I-K).
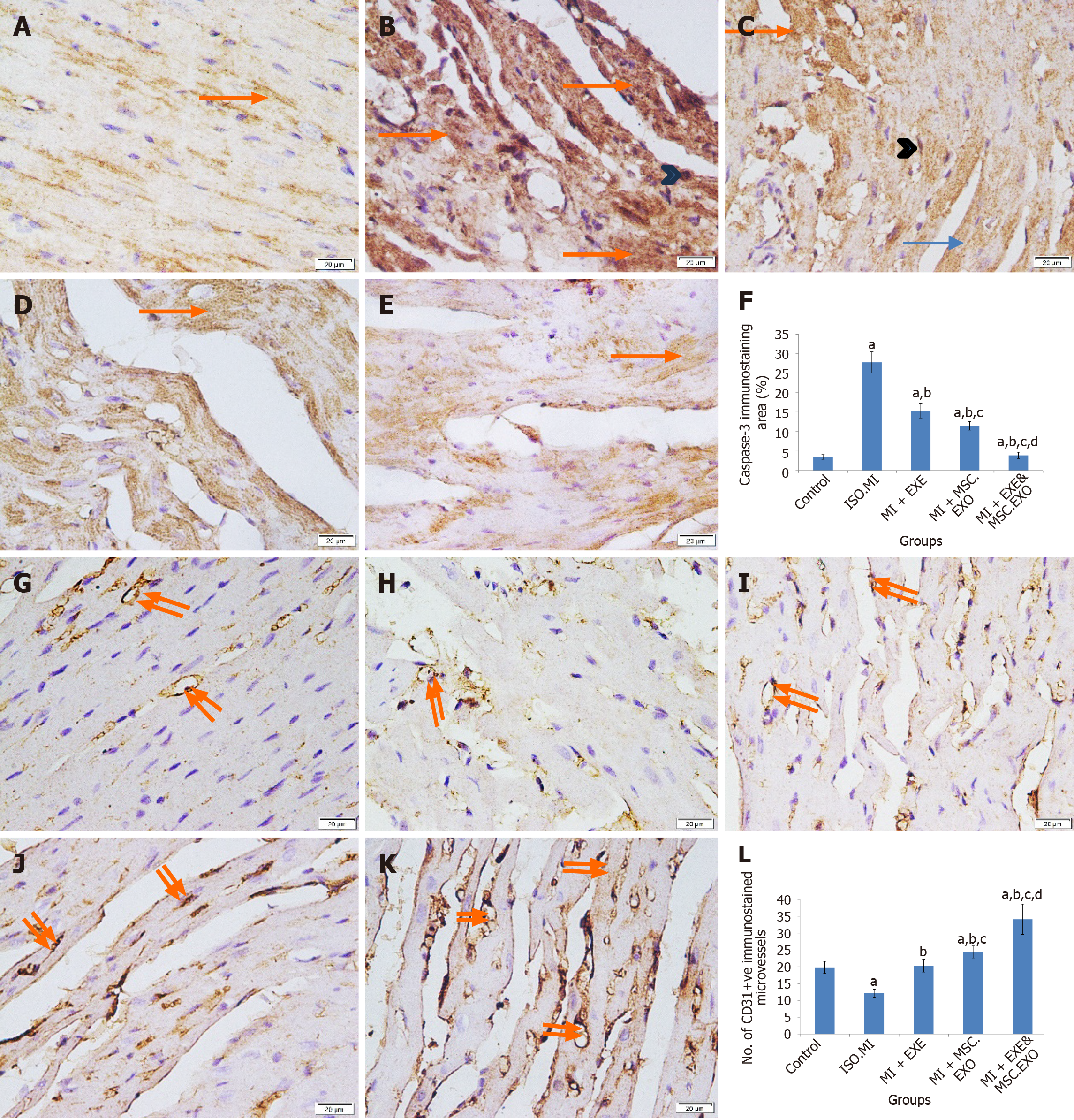
The ISO + MI group showed a significant increase in caspase-3 expression, which was notably reduced in cardiac tissue by either EXE or MSC-EXO treatment alone, with greater reductions observed in the combined therapy compared to monotherapy (Figure 10A-F). Furthermore, there was a significant decrease in CD31-positive immunostained microvessels in the ISO + MI group as compared to the control group, indicating attenuated microvasculature in the myocardial tissue. Conversely, the MI + EXE, MI + MSC-EXO, and MI + EXE + MSC-EXO groups showed significant increases in CD31-positive immunostained microvessels compared to both the control and ISO + MI groups, with the highest vascular density in the MI + EXE + MSC-EXO group, suggesting substantial promotion of angiogenesis (Figure 10G-L).
Regulating protein levels of ERK and Akt/mTOR signaling is crucial for hypertrophy development after MI, and the crosstalk between MMP9 and SERCA2a controls the balance between post-ischemic heart failure and maintenance of contractile function[11]. Thus, identifying therapeutic modalities that offer balanced crosstalk is crucial for treating early-stage maladaptive LVH.
Once left ventricular systolic function becomes decompensated, it is too late to restore normal function[30]. Early incorporation of EXE training and/or MSC-EXO in the current study proved highly beneficial, protecting the heart from apoptosis, fibrosis, and further progression to heart failure. The present study found that high-dose ISO injection resulted in increased cardiac enzymes and ANF levels, induced cardiac hypertrophy, impaired diastolic filling function, and compensated left ventricular contractile function with no change in EF compared to controls, indicating HFpEF. At the tissue level, degenerative myocardial changes, apoptosis, inflammation, and fibrosis surrounded hypertrophied muscle fibers.
This work was designed to investigate the possible beneficial effects of MSC-EXO and EXE training, alone or combined, on halting post-MI cardiac hypertrophy. Combined EXE and exosome treatment showed greater benefits than monotherapy, leading to a significant decrease in ANF levels and hypertrophic wall indices (LWD, LWS, IVSD, IVSS, left ventricular/body weight, and heart weight/body weight), as well as improved diastolic filling function indicated by increased LVIDD and LVIDS compared to the MI + EXE group. Echocardiography findings align with histological results, showing well-organized muscle fibers, dense myofibrils, abundant mitochondria, and minimal fibrosis. Novaković et al[31] demonstrated that EXE training reduced ventricular wall thickness post-MI in rats. Exosome injection before reperfusion in mice also limited fibrotic scar formation and promoted neovascularization[32].
The pathogenesis of diastolic dysfunction following MI-induced cardiac hypertrophy is complex following MI, ATP depletion triggers ischemic injury pathways, resulting in oxidative stress, inflammation, apoptosis, and fibrosis. Our results showed myocardial inflammatory cell infiltration and increased levels of TNF-α and IL-6 after the ISO insult. Treatment with EXE or MSC-EXO prevented ISO-induced cardiomyocyte oxidative stress, as evidenced by decreased MDA levels, increased antioxidant capacity, and elevated GSH levels. Combined therapy provided greater protection against cardiomyocyte oxidative damage and apoptosis, as indicated by a significant decrease in caspase-3 immunostaining. Consistent with previous studies, EXE significantly reduced the expression of TNF-α, IL-1β, and IL-6 in a rat MI model compared to the MI group[33].
Functional and interactional exploration of PPIs using GO, KEGG analysis, and the STRING database predicted core targets and pathways associated with ischemic heart disease and post-MI cardiac hypertrophy. Accordingly, we investigated PPIs considering the primary targets predicted by network analysis. ERK and Akt/mTOR pathways contribute to post-MI cardiac hypertrophy, increasing cardiac dysfunction and fibrosis. Inhibiting these pathways reduces the expression of inflammatory factors, such as IL-1β, IL-6, TNF-α, and MMP9[3,34,35]. The current study showed downregulation of ERK and Akt following the selected therapeutic interventions. It highlights the combined inhibitory effects of EXE and exosomes on the ERK and Akt/mTOR pathways, reducing inflammation, oxidative damage, and apoptosis in cardiomyocytes after ISO exposure.
PI3K/Akt/mTOR signaling is a key regulator of ventricular diastolic function, as it controls SERCA2a, the modulator of intracytoplasmic Ca2+ levels[3]. Dysregulation of this pathway is common in many cardiovascular diseases and can initiate a vicious cycle of tissue damage and impaired function, resulting in cardiac apoptosis, fibrosis, pathological hypertrophy, stiffness, and impaired relaxation. Over time, this leads to the initiation and progression of cardiac remodeling following myocardial insult[14,36]. Our experimental model showed increased ERK and Akt/mTOR signaling, MMP9 overexpression, decreased SERCA2a gene expression, and elevated caspase-3 staining in the MI group compared to controls, indicating pathological remodeling. Previous studies have highlighted the role of MMP9 in calcium homeostasis in diabetic hearts[14,37]. They found that diabetic hearts exhibited downregulated SERCA2a, leading to impaired cardiomyocyte contractility, while MMP9 expression and activity were increased[14]. MMP9 can regulate SERCA2a intracellularly or through β-adrenergic receptors extracellularly[38]. Although pharmacological agents targeting MMP inhibition, such as CP-471,474 (a broad-spectrum MMP inhibitor)[39], and agents targeting SERCA2a, such as istaroxime[40], have shown positive effects in animal models, their clinical application is limited by side effects and low bioavailability[41]. Therefore, the challenge of pharmacologically regulating MMP9 and SERCA2a gene expression can be circumvented by using alternative non-pharmacological agents such as EXE training or MSC-EXO.
Our study demonstrated that both MSC-EXO and EXE mitigated cardiac damage, promoted angiogenesis, and improved cardiac function. Moreover, combining EXE with exosomal administration in post-ischemic cardiac hypertrophy normalized MMP9 gene expression and SERCA2a levels, resulting in improved cardiac function and structure compared to the ISO + MI group. The MI + EXE + MSC-EXO group showed the most significant improvement in echocardiographic measurements compared to either EXE or MSC-EXO monotherapy. This suggests that combining cell-free therapy with lifestyle interventions, such as EXE, may enhance recovery and functional outcomes in patients with heart disease. The findings of this study offer valuable insights into alternative therapeutic approaches for enhancing human cardiac health. Similar mechanisms of cardiac stress, inflammation, and remodeling in humans occur following ischemic events[42]. Moreover, modulation of signaling pathways, such as ERK and Akt/mTOR, indicates potential targets for pharmacological intervention. Understanding these mechanisms could enable the development of strategies that leverage these pathways to improve heart health in individuals at risk of ischemic heart disease.
This study demonstrated that functional and structural cardiac improvements were accompanied by reduced inflammation, oxidative stress, and apoptosis. Both MSC-EXO and EXE demonstrated potential therapeutic benefits in HFpEF by targeting the ERK and Akt/mTOR signaling pathways, as well as the connection between SERCA2a and MMP9. The therapeutic modalities confirmed the cardioprotective benefits afforded by the upregulation of SERCA2a, which enhances the ability of cardiomyocytes to contract and relax rapidly and regularly. Additionally, a significant improvement in SERCA2a levels, along with an increase in CD31-positive immunostained microvessels in cardiac tissues, explains the cardiac improvement observed with combined treatment.
| 1. | Naing P, Forrester D, Kangaharan N, Muthumala A, Mon Myint S, Playford D. Heart failure with preserved ejection fraction: A growing global epidemic. Aust J Gen Pract. 2019;48:465-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 2. | Simmonds SJ, Cuijpers I, Heymans S, Jones EAV. Cellular and Molecular Differences between HFpEF and HFrEF: A Step Ahead in an Improved Pathological Understanding. Cells. 2020;9:242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 267] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 3. | Shimada BK, Yorichika N, Higa JK, Baba Y, Kobayashi M, Aoyagi T, Suhara T, Matsui T. mTOR-mediated calcium transients affect cardiac function in ex vivo ischemia-reperfusion injury. Physiol Rep. 2021;9:e14807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Wen J, Liu G, Liu M, Wang H, Wan Y, Yao Z, Gao N, Sun Y, Zhu L. Transforming growth factor-β and bone morphogenetic protein signaling pathways in pathological cardiac hypertrophy. Cell Cycle. 2023;22:2467-2484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 5. | Zhang Q, Wang L, Wang S, Cheng H, Xu L, Pei G, Wang Y, Fu C, Jiang Y, He C, Wei Q. Signaling pathways and targeted therapy for myocardial infarction. Signal Transduct Target Ther. 2022;7:78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 514] [Article Influence: 128.5] [Reference Citation Analysis (0)] |
| 6. | Mishra PK, Chavali V, Metreveli N, Tyagi SC. Ablation of MMP9 induces survival and differentiation of cardiac stem cells into cardiomyocytes in the heart of diabetics: a role of extracellular matrix. Can J Physiol Pharmacol. 2012;90:353-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 7. | Nogami K, Maruyama Y, Sakai-Takemura F, Motohashi N, Elhussieny A, Imamura M, Miyashita S, Ogawa M, Noguchi S, Tamura Y, Kira JI, Aoki Y, Takeda S, Miyagoe-Suzuki Y. Pharmacological activation of SERCA ameliorates dystrophic phenotypes in dystrophin-deficient mdx mice. Hum Mol Genet. 2021;30:1006-1019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 8. | Huang H, Joseph LC, Gurin MI, Thorp EB, Morrow JP. Extracellular signal-regulated kinase activation during cardiac hypertrophy reduces sarcoplasmic/endoplasmic reticulum calcium ATPase 2 (SERCA2) transcription. J Mol Cell Cardiol. 2014;75:58-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 9. | Tan Y, Li H, Cao G, Xin J, Yan D, Liu Y, Li P, Zhang Y, Shi L, Zhang B, Yi W, Sun Y. N-terminal domain of CTRP9 promotes cardiac fibroblast activation in myocardial infarction via Rap1/Mek/Erk pathway. J Transl Med. 2025;23:300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 10. | Wingard MC, Dalal S, Shook PL, Ramirez P, Raza MU, Johnson P, Connelly BA, Thewke DP, Singh M, Singh K. Deficiency of ataxia-telangiectasia mutated kinase attenuates Western-type diet-induced cardiac dysfunction in female mice. Physiol Rep. 2022;10:e15434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Sciarretta S, Forte M, Frati G, Sadoshima J. New Insights Into the Role of mTOR Signaling in the Cardiovascular System. Circ Res. 2018;122:489-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 375] [Article Influence: 53.6] [Reference Citation Analysis (0)] |
| 12. | Wang Y, Liao H, Wang Y, Zhou J, Wang F, Xie Y, Zhao K, Gao W. KLK11 promotes the activation of mTOR and protein synthesis to facilitate cardiac hypertrophy. BMC Cardiovasc Disord. 2021;21:266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Packer M. Longevity genes, cardiac ageing, and the pathogenesis of cardiomyopathy: implications for understanding the effects of current and future treatments for heart failure. Eur Heart J. 2020;41:3856-3861. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 14. | Prathipati P, Metreveli N, Nandi SS, Tyagi SC, Mishra PK. Ablation of Matrix Metalloproteinase-9 Prevents Cardiomyocytes Contractile Dysfunction in Diabetics. Front Physiol. 2016;7:93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Vargas-Mendoza N, Angeles-Valencia M, Morales-González Á, Madrigal-Santillán EO, Morales-Martínez M, Madrigal-Bujaidar E, Álvarez-González I, Gutiérrez-Salinas J, Esquivel-Chirino C, Chamorro-Cevallos G, Cristóbal-Luna JM, Morales-González JA. Oxidative Stress, Mitochondrial Function and Adaptation to Exercise: New Perspectives in Nutrition. Life (Basel). 2021;11:1269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 16. | Zarà M, Amadio P, Campodonico J, Sandrini L, Barbieri SS. Exosomes in Cardiovascular Diseases. Diagnostics (Basel). 2020;10:943. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 17. | Zhang Y, Bi J, Huang J, Tang Y, Du S, Li P. Exosome: A Review of Its Classification, Isolation Techniques, Storage, Diagnostic and Targeted Therapy Applications. Int J Nanomedicine. 2020;15:6917-6934. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 566] [Cited by in RCA: 937] [Article Influence: 156.2] [Reference Citation Analysis (0)] |
| 18. | Ebrahim N, Al Saihati HA, Mostafa O, Hassouna A, Abdulsamea S, Abd El Aziz M El Gebaly E, Abo-Rayah NH, Sabry D, El-Sherbiny M, Madboly AG, Hussien NI, Saadani REH, Ebrahim HA, Badr OAM, Elsherbiny NM, Salim RF. Prophylactic Evidence of MSCs-Derived Exosomes in Doxorubicin/Trastuzumab-Induced Cardiotoxicity: Beyond Mechanistic Target of NRG-1/Erb Signaling Pathway. Int J Mol Sci. 2022;23:5967. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 19. | Sanganalmath SK, Dubey S, Veeranki S, Narisetty K, Krishnamurthy P. The interplay of inflammation, exosomes and Ca(2+) dynamics in diabetic cardiomyopathy. Cardiovasc Diabetol. 2023;22:37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 29] [Reference Citation Analysis (10)] |
| 20. | Zhu C, Li W, Wang X, Xue J, Zhao L, Song Y, Zhou T, Zhang M. Phloroglucinol averts isoprenaline hydrochloride induced myocardial infarction in rats. Drug Dev Res. 2019;80:453-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Wang Y, Zhang L, Jia L, Liu J, Liu K, Feng Q, Wang Q. Calcitonin gene-related peptide in aerobic exercise induces collateral circulation development in rat ischemia myocardium. Biomed Pharmacother. 2016;82:561-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Helal O, Mansy A, Aly O, Salama N. Histological and Immunohistochemical Study of the Possible Therapeutic Effect of Mesenchymal Stem Cells, Their Exosomes and Vitamin E on Experimentally Induced Cardio-Toxicity in Adult Male Albino Rats. Benha J Appl Sci. 2020;5:1-11. [DOI] [Full Text] |
| 23. | Zaki SM, Algaleel WA, Imam RA, Abdelmoaty MM. Mesenchymal stem cells pretreated with platelet-rich plasma modulate doxorubicin-induced cardiotoxicity. Hum Exp Toxicol. 2019;38:857-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | González-Cubero E, González-Fernández ML, Gutiérrez-Velasco L, Navarro-Ramírez E, Villar-Suárez V. Isolation and characterization of exosomes from adipose tissue-derived mesenchymal stem cells. J Anat. 2021;238:1203-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 25. | Akhtar MS, Pillai KK, Hassan MQ, Dhyani N, Ismail MV, Najmi AK. Levosimendan reduces myocardial damage and improves cardiodynamics in streptozotocin induced diabetic cardiomyopathy via SERCA2a/NCX1 pathway. Life Sci. 2016;153:55-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Navarro KL, Huss M, Smith JC, Sharp P, Marx JO, Pacharinsak C. Mouse Anesthesia: The Art and Science. ILAR J. 2021;62:238-273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 106] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 27. | Wang QW, Yu XF, Xu HL, Zhao XZ, Sui DY. Ginsenoside Re Improves Isoproterenol-Induced Myocardial Fibrosis and Heart Failure in Rats. Evid Based Complement Alternat Med. 2019;2019:3714508. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 28. | Eskandari E, Eaves CJ. Paradoxical roles of caspase-3 in regulating cell survival, proliferation, and tumorigenesis. J Cell Biol. 2022;221:e202201159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 291] [Reference Citation Analysis (0)] |
| 29. | Hu H, Chen Y, Zou Z, Li L, Wei F, Liu C, Ling Z, Zou X. Panax Notoginseng Saponins Prevent Bone Loss by Promoting Angiogenesis in an Osteoporotic Mouse Model. Biomed Res Int. 2020;2020:8412468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 30. | Savarese G, Stolfo D, Sinagra G, Lund LH. Heart failure with mid-range or mildly reduced ejection fraction. Nat Rev Cardiol. 2022;19:100-116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 269] [Article Influence: 67.3] [Reference Citation Analysis (0)] |
| 31. | Novaković M, Novak T, Vižintin Cuderman T, Krevel B, Tasič J, Rajkovič U, Fras Z, Jug B. Exercise capacity improvement after cardiac rehabilitation following myocardial infarction and its association with long-term cardiovascular events. Eur J Cardiovasc Nurs. 2022;21:76-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 32. | Lai RC, Arslan F, Lee MM, Sze NS, Choo A, Chen TS, Salto-Tellez M, Timmers L, Lee CN, El Oakley RM, Pasterkamp G, de Kleijn DP, Lim SK. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4:214-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1435] [Cited by in RCA: 1728] [Article Influence: 108.0] [Reference Citation Analysis (0)] |
| 33. | Shahi HA, Kadoguchi T, Shimada K, Fukao K, Matsushita S, Aikawa T, Ouchi S, Shiozawa T, Takahashi S, Sato-Okabayashi Y, Akita K, Isoda K, Miyazaki T, Daida H. Voluntary exercise and cardiac remodeling in a myocardial infarction model. Open Med (Wars). 2020;15:545-555. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 34. | Su F, Zhao L, Zhang S, Wang J, Chen N, Gong Q, Tang J, Wang H, Yao J, Wang Q, Zhong M, Yan J. Cardioprotection by PI3K-mediated signaling is required for anti-arrhythmia and myocardial repair in response to ischemic preconditioning in infarcted pig hearts. Lab Invest. 2015;95:860-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 35. | Ruan Y, Jin Q, Zeng J, Ren F, Xie Z, Ji K, Wu L, Wu J, Li L. Grape Seed Proanthocyanidin Extract Ameliorates Cardiac Remodelling After Myocardial Infarction Through PI3K/AKT Pathway in Mice. Front Pharmacol. 2020;11:585984. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 36. | Palabiyik O, Tastekin E, Doganlar ZB, Tayfur P, Dogan A, Vardar SA. Alteration in cardiac PI3K/Akt/mTOR and ERK signaling pathways with the use of growth hormone and swimming, and the roles of miR21 and miR133. Biomed Rep. 2019;0:1-10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 37. | Moshal KS, Tipparaju SM, Vacek TP, Kumar M, Singh M, Frank IE, Patibandla PK, Tyagi N, Rai J, Metreveli N, Rodriguez WE, Tseng MT, Tyagi SC. Mitochondrial matrix metalloproteinase activation decreases myocyte contractility in hyperhomocysteinemia. Am J Physiol Heart Circ Physiol. 2008;295:H890-H897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 81] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 38. | Dillmann WH. Diabetic Cardiomyopathy. Circ Res. 2019;124:1160-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 518] [Article Influence: 86.3] [Reference Citation Analysis (0)] |
| 39. | Lindsey ML, Gannon J, Aikawa M, Schoen FJ, Rabkin E, Lopresti-Morrow L, Crawford J, Black S, Libby P, Mitchell PG, Lee RT. Selective matrix metalloproteinase inhibition reduces left ventricular remodeling but does not inhibit angiogenesis after myocardial infarction. Circulation. 2002;105:753-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 127] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 40. | Newbury D, Frishman W. Istaroxime: A Novel Therapeutic Agent for Acute Heart Failure. Cardiol Rev. 2025;33:187-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 41. | Cao Z, Liao Q, Su M, Huang K, Jin J, Cao D. AKT and ERK dual inhibitors: The way forward? Cancer Lett. 2019;459:30-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 166] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 42. | Azevedo PS, Polegato BF, Minicucci MF, Paiva SA, Zornoff LA. Cardiac Remodeling: Concepts, Clinical Impact, Pathophysiological Mechanisms and Pharmacologic Treatment. Arq Bras Cardiol. 2016;106:62-69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 212] [Article Influence: 19.3] [Reference Citation Analysis (0)] |