Published online Oct 26, 2025. doi: 10.4252/wjsc.v17.i10.109369
Revised: June 24, 2025
Accepted: September 4, 2025
Published online: October 26, 2025
Processing time: 164 Days and 23.9 Hours
Osteoarthritis (OA) remains a challenging degenerative joint disease with limited therapeutic interventions.
To investigate the potential of synovial mesenchymal stem cell (SMSC)-derived exosomes (SMSCs-Exos) delivering GrpE-like 1 (GRPEL1) in promoting cartilage repair through phosphatase and tensin homolog-induced putative kinase 1 (PINK1)-mediated mitophagy activation.
A comprehensive research approach was employed, including bioinformatics analysis of gene expression datasets (GSE169077 and GSE114007), in vitro ex
Bioinformatics analysis revealed GRPEL1 as a critical mitophagy-related gene with significantly altered expression in OA. In vitro studies demonstrated that GRPEL1-loaded SMSCs-Exos effectively counteracted interleukin-1 beta-induced cellular damage by enhancing chondrocyte proliferation and migration, preserving extracellular matrix integrity. Mechanistic investigations confirmed direct interaction between GRPEL1 and PINK1, leading to enhanced mitophagy activation. In vivo rat models substantiated these findings, showing significantly reduced cartilage damage, restored proteoglycan content, and improved joint structure in groups receiving GRPEL1-overexpressing exosomes. Key molecular changes included decreased reactive oxygen species, improved mitochondrial membrane potential, and increased mitophagy markers.
This study provides compelling evidence that SMSCs-Exos delivering GRPEL1 can effectively activate PINK1-mediated mitophagy, offering a promising therapeutic strategy for cartilage repair in OA. The research unveils a novel molecular mechanism for targeting mitochondrial dysfunction and presents a potential disease-modifying approach beyond current symptomatic treatments.
Core Tip: This study demonstrates that synovial mesenchymal stem cell-derived exosomes engineered to deliver GrpE-like 1 effectively enhance phosphatase and tensin homolog-induced putative kinase 1-mediated mitophagy, promoting cartilage repair in osteoarthritis. By integrating bioinformatics, in vitro chondrocyte assays, and in vivo rat models, the findings reveal a novel mechanism by which GrpE-like 1 activates mitochondrial quality control and reduces oxidative stress. This work introduces a promising exosome-based therapeutic strategy that targets mitochondrial dysfunction, advancing the development of disease-modifying treatments beyond current symptomatic approaches for osteoarthritis.
- Citation: Xiang CH, Zou L, Huang ZG, Zhang GJ, Zeng HL, He ZX, Dai ZS. Synovial mesenchymal stem cell-derived exosomes delivering GRPEL1 activate PINK1-mediated mitophagy to promote cartilage repair in arthritis. World J Stem Cells 2025; 17(10): 109369
- URL: https://www.wjgnet.com/1948-0210/full/v17/i10/109369.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v17.i10.109369
Osteoarthritis (OA) is a common joint disorder, primarily marked by cartilage degeneration that progressively affects all joint tissues, significantly impairing daily function[1,2]. By 2021, over 22% of individuals aged 40 and above were affected by knee OA, impacting more than 500 million people worldwide and underscoring its significant societal and economic burden[3,4]. OA progression is influenced by multiple factors, including age, gender, obesity, genetic predisposition, and previous joint injuries[5-7]. Pathologically, it is defined by the degradation of articular cartilage and the formation of osteophytes, accompanied by key symptoms such as joint pain, swelling, and limited mobility. These symptoms not only disrupt daily activities but also contribute to psychological stress, exacerbating both personal and healthcare burdens[4,5,8,9]. Despite substantial research efforts, no curative treatments for OA have been developed. Joint replacement surgery remains the most effective intervention for advanced cases, while current pharmacological and non-pharmacological therapies primarily aim at symptom relief, with no treatments available to halt disease progression.
In recent years, mesenchymal stem cell (MSC)-based therapies have shown promise in OA treatment[10,11]. Initially, MSCs were studied for their ability to differentiate into chondrocytes, but it is now understood that their therapeutic benefits are largely mediated by paracrine factors, particularly exosomes (Exos)[12-15]. Exos, nanoscale (30-100 nm), single-membrane vesicles secreted by various cell types, have garnered attention for their role in transferring bioactive molecules, such as microRNAs (miRNAs), mRNAs, and tRNAs, to target cells[16,17]. MSC-derived Exos have demonstrated potential in promoting cartilage regeneration by enhancing cell proliferation, inhibiting apoptosis, and modulating inflammatory responses[18-20]. Notably, Exos derived from synovial MSCs (SMSC-Exos) have shown promise in reducing the severity of OA induced by interleukin (IL)-1β[21,22]. Despite these encouraging findings, further research is urgently needed to confirm the efficacy and clinical feasibility of Exo-based therapies.
Mitochondria are vital organelles that regulate key cellular functions, including energy metabolism, apoptosis, and calcium homeostasis[20]. In OA, chondrocytes frequently display mitochondrial dysfunction, marked by decreased electron transport chain complex activity and increased oxidative stress[21]. Impaired function of electron transport chain complexes I, II, and III results in energy deficiencies, compromising normal chondrocyte function and metabolism[22]. Additionally, heightened oxidative stress activates inflammatory pathways, promoting the expression and release of cytokines like tumor necrosis factor-alpha (TNF-α) and IL-1β, which further damage chondrocytes and degrade the cartilage matrix[23]. Studies show that mitophagy plays a critical role in modulating OA severity[24]. In OA chondrocytes, phosphatase and tensin homolog-induced putative kinase 1 (PINK1)-parkin-mediated mitophagy reduces reactive oxygen species (ROS) levels by clearing dysfunctional mitochondria[25]. Moreover, mitophagy pathways have been found to protect chondrocytes exposed to iodoacetic acid[26]. In summary, understanding the mechanisms of mitochondria-related genes in OA is crucial, offering new insights into potential therapeutic targets for the disease.
In this study, we utilized bioinformatics approaches to identify potential biomarkers linked to mitophagy processes in OA cartilage. Our comprehensive analysis of newly diagnosed OA patients focused on confirming the expression levels of GrpE-like 1 (GRPEL1), a mitophagy-related biomarker, and exploring its potential associations with inflammatory markers such as C-reactive protein (CRP), TNF-α, IL-6, as well as Kellgren-Lawrence (KL) grading. We hypothesized that SMSC-Exo could modulate OA progression via GRPEL1-mediated mechanisms. To investigate this, we examined whether SMSC-Exo carried GRPEL1 protein and its effects on knee OA through this pathway. We evaluated the ability of SMSC-Exo to inhibit chondrocyte death in vitro and alleviate OA symptoms in vivo, highlighting its mechanistic role as a potential therapeutic target. This research provides novel strategies for the clinical treatment of OA, laying a solid theoretical foundation for the development of innovative therapeutic agents.
We initially obtained two OA-related microarray datasets, GSE169077 and GSE114007, from the Gene Expression Omnibus database. The GSE169077 dataset contained gene chip sequencing data from cartilage samples of human knee OA patients, including 5 control samples and 6 OA samples. The GSE114007 dataset provided high-throughput sequencing data from the same patient cohort, consisting of 18 control and 20 OA samples. Using the “limma” package in R, we performed differential gene expression analysis on both dataset[27]. Differentially expressed genes (DEGs) were identified based on the following criteria: |logfold change| > 1 and P-value < 0.05. Heatmaps of the DEGs were generated using the “heatmap” package in R, and the intersection of DEGs across the datasets was determined using the Venn tool. To further explore gene expression variability, we visualized box plots of key gene expressions using the “ggpubr” package in R.
To assess immune cell infiltration in the cartilage samples, we applied cell type identification by estimating relative subsets of RNA transcripts, a robust deconvolution algorithm, to estimate the proportions of 22 distinct immune cell subpopulations. Differences between normal and OA tissues were evaluated using the Wilcoxon test. Additionally, Pearson correlation analysis was conducted to examine the associations between 24 long non-coding RNAs and immune cell subpopulations. A P-value of < 0.05 was considered statistically significant for all analyses[28]. Lastly, we performed protein-protein interaction (PPI) network analysis of key factors using the Genemania database, which provided the interacting proteins for further investigation. Functional enrichment analysis of these interacting proteins was carried out using the “ClusterProfiler” package in R[29]. Significantly enriched Kyoto Encyclopedia of Genes and Genomes (KEGG) and Gene Ontology (GO) pathways were identified using Fisher’s exact test, with P-values < 0.05 considered statistically significant.
According to the American College of Rheumatology criteria, 30 OA patients who underwent knee surgery at the Second Affiliated Hospital of Fujian Medical University were included in this study (OA group). Simultaneously, 20 individuals who underwent knee surgery due to acute trauma, without any history of joint diseases or systemic disorders, were recruited as the control group (normal group). Synovial fluid and cartilage tissue samples were collected from both groups, along with synovial tissue samples from healthy donors. Clinical information was recorded for all participants, including age, sex, body mass index, affected knee joint (left, right, or bilateral), serum concentrations of CRP, TNF-α, IL-6, and the severity of knee lesions based on the KL grading system (classified as grades 1 through 4). This study was conducted in full compliance with the guidelines of the Ethics Review Committee of the Second Affiliated Hospital of Fujian Medical University and was approved under a formally registered research protocol. Written informed consent was obtained from all participants before the study commenced.
Synovial MSCs (SMSCs) were isolated and characterized following a previously established protocol. Human synovial tissues obtained from surgical procedures were enzymatically digested overnight at 37 °C using 0.2% type I collagenase (Thermo Fisher Scientific, MA, United States). The resulting cell suspension was centrifuged, and the cells were seeded in high-glucose DMEM supplemented with 10% fetal bovine serum (FBS) (Thermo Fisher Scientific, MA, United States). After four days of incubation to allow cell attachment, the culture medium was changed every three days, and primary (P0) SMSCs were obtained by day 14. To characterize the surface markers of the SMSCs, cells were first blocked with BD Fc Block™ (Becton Dickinson, NJ, United States) before being stained with fluorescently labeled monoclonal antibodies, including anti-bodies against CD105, CD44, and SCA-1 (Becton Dickinson, NJ, United States)[30]. Furthermore, SMSCs underwent in vitro differentiation assays using specialized differentiation media (CytoTissue Inc., Wuxi, China) to evaluate their potential to differentiate into adipocytes and osteoblasts. These assays confirmed the multipotent differentiation capacity of the SMSCs. Adipogenesis and osteogenesis were verified by Oil Red O staining (mesenchymal adipogenesis kit; #SCR020; Millipore, MA, United States) and Alizarin Red S staining (#A5533; Sigma-Aldrich, MO, United States), respectively. For all subsequent experiments, passage 3 SMSCs were used.
SMSCs were randomly assigned to four experimental groups: The GRPEL1 overexpression group (GRPEL1 group), the empty vector control group (oeNC group), the GRPEL1 knockdown group (shGRPEL1 group), and the corresponding negative control group (shNC group). Each group was transfected using Lipofectamine 3000 reagent (Invitrogen, Carlsbad, CA, United States) according to the manufacturer’s instructions. Specifically, SMSCs in the GRPEL1 group received the pcDNA3.1-GRPEL1 plasmid, those in the oeNC group were transfected with the empty pcDNA3.1 vector, while the shGRPEL1 group was treated with GRPEL1-specific short hairpin RNA (shRNA), and the shNC group received non-targeting shRNA. Forty-eight hours post-transfection, we assessed the mRNA expression levels of GRPEL1 using quantitative real-time polymerase chain reaction (qRT-PCR) and evaluated protein expression through western blotting. These procedures aimed to confirm whether the overexpression or knockdown of GRPEL1 was successfully achieved in each experimental group.
Exos were isolated from the SMSC culture medium using the Ribo™ Exo isolation reagent (#C10130-1; Ribobio, Beijing, China). SMSCs were first cultured in Exo-depleted medium for 72 hours, after which the culture supernatant was collected and centrifuged at 2000 × g for 30 minutes at 4 °C to remove cellular debris and large protein aggregates. The supernatant was transferred to a fresh tube and mixed with an equal volume of Ribo™ Exo isolation reagent, followed by overnight incubation at 4 °C. After incubation, the Exo-containing mixture was centrifuged at 1500 × g for 30 minutes at
CHON-001 cells were cultured under standard conditions in DMEM supplemented with 10% FBS and 1% penicillin/streptomycin at 37 °C with 5% CO2. For the Exo treatment groups, CHON-001 cells were co-cultured with Exos derived from different sources as outlined below. The first experimental series (Exo intervention study) focused on Exo-mediated interventions under inflammatory conditions and included seven distinct groups: (1) Control group (untreated CHON-001 cells); (2) IL-1β group (treated with 10 ng/mL IL-1β for 48 hours); (3) IL-1β + Exo group (IL-1β treatment with Exos from untreated SMSCs); (4) IL-1β + Exo-oeNC group (IL-1β treatment with Exos from SMSCs transfected with the empty vector pcDNA3.1); (5) IL-1β + Exo-GRPEL1 group (IL-1β treatment with Exos from SMSCs transfected with the GRPEL1 overexpression vector); (6) IL-1β + Exo-shNC group (IL-1β treatment with Exos from SMSCs transfected with negative control shRNA); and (7) IL-1β + Exo-shGRPEL1 group (IL-1β treatment with Exos from SMSCs transfected with GRPEL1-specific shRNA). The second experimental series (gene manipulation study) aimed to investigate the functional roles of GRPEL1 and PINK1, comprising ten distinct groups: (1) oeNC group (CHON-001 cells transfected with the empty vector); (2) GRPEL1 group (CHON-001 cells overexpressing GRPEL1); (3) shNC group (CHON-001 cells transfected with negative control shRNA); (4) shGRPEL1 group (CHON-001 cells transfected with GRPEL1 shRNA); (5) shPINK1 group (CHON-001 cells transfected with PINK1 shRNA); (6) Control group (untreated CHON-001 cells); (7) IL-1β group (treated with 10 ng/mL IL-1β); (8) IL-1β + oeNC + shNC group (CHON-001 cells transfected with empty vector and negative shRNA, then treated with IL-1β); (9) IL-1β + GRPEL1 + shNC group (CHON-001 cells overexpressing GRPEL1 with negative shRNA, then treated with IL-1β); and (10) IL-1β + GRPEL1 + shPINK1 group (CHON-001 cells overexpressing GRPEL1 with PINK1 shRNA, then treated with IL-1β).
To evaluate the therapeutic potential of SMSC-Exo in repairing OA in adult male Sprague-Dawley rats, we designed an animal model experiment based on previously published methodologies[31-33]. The study included seven experimental groups: Control group (CTRL), OA model group (OA), Exo treatment group (OA + Exo), empty vector Exo group (OA + Exo-oeNC), GRPEL1 overexpression Exo group (OA + Exo-GRPEL1), empty shRNA Exo group (OA + Exo-shNC), and GRPEL1 knockdown Exo group (OA + Exo-shGRPEL1). All groups, except for the CTRL which underwent a sham surgery, were subjected to destabilization of the medial meniscus surgery to induce OA. Four weeks post-surgery, treatments commenced. The CTRL and OA received intra-articular injections of 100 μL PBS every three days, while the remaining groups were treated with 100 μL of SMSC-Exos (1011 particles/mL) injected intra-articularly every three days for four weeks. The Exos were derived from untreated SMSCs, SMSCs transfected with empty pcDNA3.1 vector, SMSCs overexpressing the GRPEL1 gene, SMSCs transfected with empty shRNA, or SMSCs with GRPEL1 knockdown via shRNA. All animal experiments were approved by the Institutional Animal Care and Use Committee and conducted in strict compliance with laboratory animal care guidelines.
Total RNA was extracted from chondrocytes and SMSCs using TRIzol reagent (Invitrogen, Carlsbad, CA, United States). For Exos, RNA and protein were isolated using the total Exo RNA and protein isolation kit (Invitrogen, Carlsbad, CA, United States). MiRNA was reverse transcribed into cDNA using the TaqMan™ advanced miRNA cDNA synthesis kit (Thermo Fisher Scientific, MA, United States), with RNU6B serving as the internal reference. For mRNA, reverse transcription was carried out using the TransScript® all-in-one first-strand cDNA synthesis SuperMix, with GAPDH as the internal control. SYBR Green master mix (Roche, Penzberg, Upper Bavaria, Germany) was employed for amplification detection. Primer sequences are provided in the Table 1.
| Gene | Primer sequences | |
| GRPEL1 | F | 5’-AAGTTGAACCCTGTCGGAGC-3’ |
| R | 5’-TGCAGCTTGTACCCCACTTT-3’ | |
| EFEMP1 | F | 5’-TGTGCTGTGCAAGGAACTCT-3’ |
| R | 5’-CAGTGCATTGCGTGTACGTG-3’ | |
| SERPINA5 | F | 5’-TGTGGCAAAGCAAACGAAGG-3’ |
| R | 5’-TCTTGGGTGCCTTTGTGGTT-3’ | |
| BNIP3 L | F | 5’-TGCAGTTGTTTCTGCTCCCA-3’ |
| R | 5’-TAGCTCCACCCAGGAACCTT-3’ |
Protein extraction and western blot analysis were conducted as previously described[34]. Antibodies targeting specific proteins, including GRPEL1, CD63, CD9, tumor susceptibility gene 101, collagen II, aggrecan, a disintegrin and metalloproteinase with thrombospondin motifs 5 (ADAMTS5), matrix metalloproteinase-13 (MMP-13), PINK1, parkin, P62, and light chain 3 (LC3) II/I (LC3-II/LC3-I), were utilized for the analysis.
Immunofluorescence staining was conducted as previously described[35] and visualized using a Nikon ECLIPSE Ni-U/DS-Ri2 fluorescence microscope (Nikon Co., Kanagawa, Japan). Staining was performed to detect SMSC markers, including CD105, CD44, and SCA-1, as well as to assess Exo uptake. Additionally, immunofluorescence was used to evaluate the expression and colocalization of key proteins in cartilage tissue, such as collagen II, aggrecan, MMP-13, translocase of the outer membrane 20 (TOM20), and LC3B.
The impact of IL-1β and SMSC-Exo on the viability of mouse chondrocytes was evaluated using the CCK-8 assay (K1018, Apexbio, Houston, TX, United States). Chondrocytes were seeded at a density of 1 × 103 cells per well in 96-well culture plates containing 100 μL of medium supplemented with 10% FBS. After 48 hours of incubation, 10 μL of CCK-8 solution was added to each well, followed by incubation at 37 °C for an additional 4 hours. Optical density was measured at 450 nm to assess cell viability. Absorbance values were recorded at 0, 12, 24, 48, and 72 hours. Each experiment included five replicate wells, and the entire procedure was repeated independently three times. The relative proliferation rates, compared to the CTRL, were used to generate cell growth curves. Statistical significance was determined using one-way analysis of variance.
Co-immunoprecipitation was performed to detect the interaction between GRPEL1 and PINK1. The procedure was carried out according to the manufacturer’s instructions using the Protein A/G magnetic beads IP kit (Beyotime, Shanghai, China). Briefly, the cell supernatant was incubated with the corresponding antibody for 12 hours at 4 °C. Protein A/G magnetic beads were then added to capture antigen-antibody complexes. The complexes were collected by centrifugation, and the beads were washed to remove non-specific binding. After washing, the antigen-antibody complexes were eluted by boiling the beads in sample buffer. The eluted samples were centrifuged to remove the beads, and the supernatant was subjected to sodium-dodecyl sulfate gel electrophoresis for protein expression analysis.
Intracellular ROS levels were measured using a commercial ROS detection kit (Cat. No. S0034S, Beyotime, Shanghai, China) according to the manufacturer’s instructions. Specifically, 2’,7’-dichlorodihydrofluorescein diacetate was used as the fluorescent probe to detect ROS. It is oxidized by intracellular ROS to generate the fluorescent compound DCF, which can be quantified by flow cytometry. Briefly, cells were plated in six-well plates and incubated with 2’,7’-dichlorodihydrofluorescein diacetate. The fluorescence intensity was then assessed using flow cytometry (BD FACSCelesta, Becton Dickinson, NJ, United States).
Mitochondrial membrane potential (ψm) was assessed using the JC-1 staining kit (Cat. No. C2006; Beyotime, Shanghai, China) following the manufacturer’s instructions. Cells were trypsinized, filtered through a sieve, and resuspended in 1 mL of JC-1 working solution. The cell suspension was incubated in the dark for 20 minutes. After incubation, cells were centrifuged at 4 °C and washed twice with JC-1 staining buffer for 3 minutes each. Finally, the cells were resuspended in fresh JC-1 staining buffer, and mitochondrial membrane potential was measured by flow cytometry.
Following euthanasia, mouse knee joints were collected and processed for hematoxylin and eosin (HE) staining. The severity of arthritis was quantitatively assessed using a standardized scoring system as described in previous protocols. Immunohistochemical analysis was performed with modifications from the established method. Tissue sections from the knee joints were incubated with primary antibodies against. Detection was carried out using a polymer horseradish peroxidase detection system and visualized with a 3,3’-diaminobenzidine peroxidase substrate kit.
Following the manufacturer’s protocol for the safranine O-fast green FCF cartilage stain kit (Solarbio, Wuhan, China), paraffin-embedded cartilage tissue sections were dewaxed and rehydrated in water. The sections were first stained with HE for 10-30 minutes, washed with running water for 15 minutes, and then differentiated in 1% hydrochloric acid ethanol solution for 2-10 seconds, until the sections turned red and the staining lightened. The sections were subsequently immersed in 50%, 70%, and 80% ethanol for 5 minutes each, followed by washing in 95% ethanol to remove excess red staining and then in absolute ethanol for 5 minutes. After dehydration and sealing, the sections were examined under a microscope. Images were captured and analyzed, with at least three random visual fields selected per sample. The Osteoarthritis Research Society International (OARSI) scoring was performed according to established guidelines[36].
SPSS 20.0 software was utilized for statistical analysis and visualization of experimental data. Comparisons among multiple groups were conducted using one-way analysis of variance, while differences between two groups were assessed using Student’s t-test. The experimental findings are presented as mean ± SD. P < 0.05 was deemed as a statistically significant distinction.
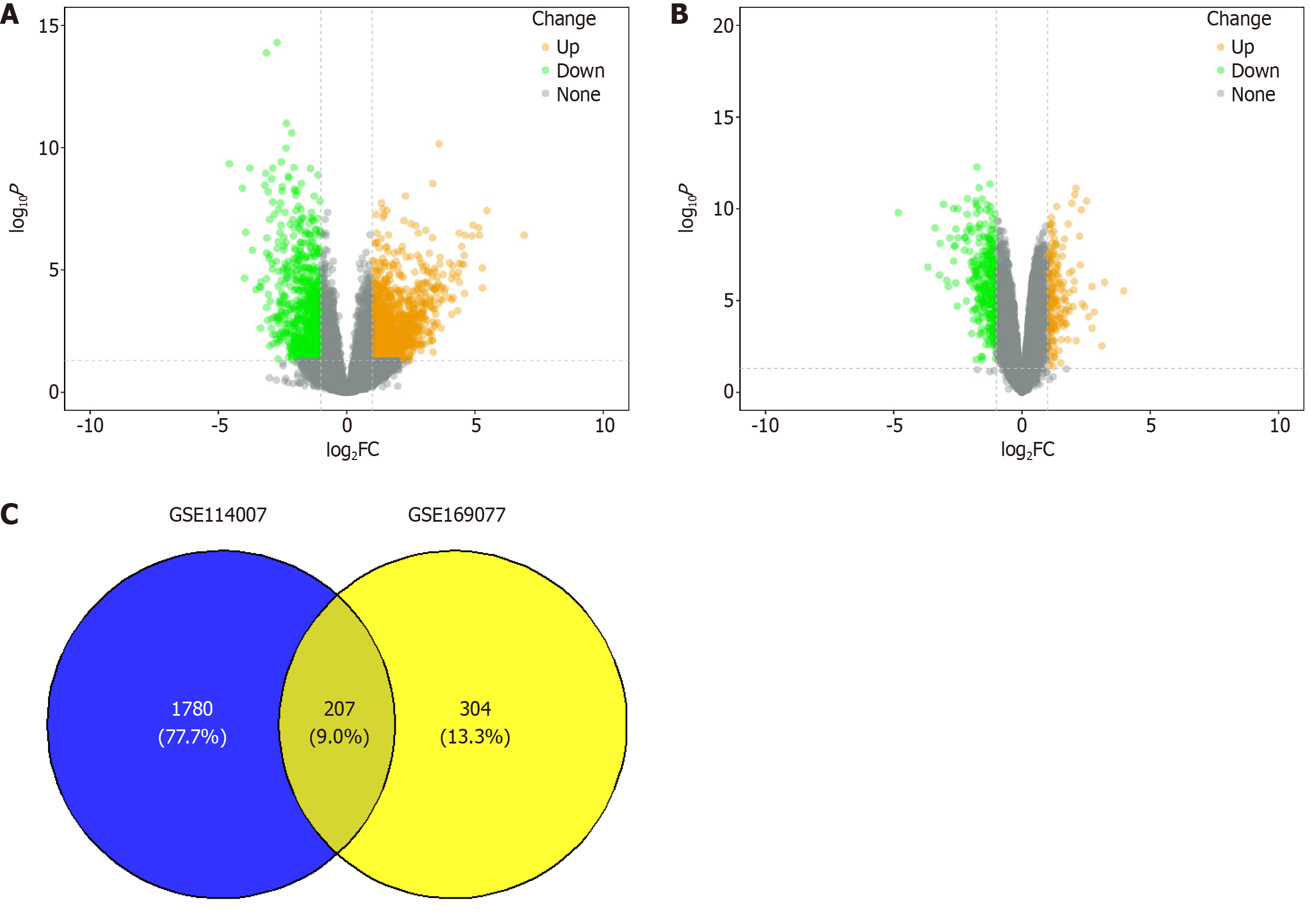
To deeply understand the genes that may play a key role in the pathological process of OA, we performed differential expression analysis on two publicly available gene expression datasets of human knee OA cartilage samples - GSE169077 and GSE114007. Through this analysis, we identified 180 upregulated genes and 331 downregulated genes in the GSE169077 dataset (Figure 1A), while in the GSE114007 dataset, we found 1175 upregulated genes and 812 downregulated genes (Figure 1B). There were 207 common DEGs between the two datasets (Figure 1C).
To further explore the biological significance of these DEGs in the OA pathological process, we performed GO and KEGG functional enrichment analyses. The GO enrichment analysis showed that many genes related to extracellular matrix (ECM) organization, ossification, and ECM structural constituents had altered expression, which may be associated with the observed cartilage degradation and abnormal bone formation in OA. Additionally, the upregulation of hypoxia response and response to acid chemical in OA patients suggested that the tissue may be subjected to the influence of multiple factors, such as inflammation and oxidative stress. Furthermore, changes in several collagen-related pathways reflected the alteration of the collagen structure in the cartilage matrix of OA (Supplementary Figure 1A-C). KEGG pathway analysis revealed abnormalities in carbohydrate, lipid, and amino acid metabolism pathways in OA patients, which may represent an adaptive response of the joint tissues to inflammation and oxidative stress. Notably, pathways related to mitochondrial metabolism, such as glycolysis/gluconeogenesis, fatty acid degradation, and valine, leucine and isoleucine degradation, showed significant differential enrichment (Supplementary Figure 1D).
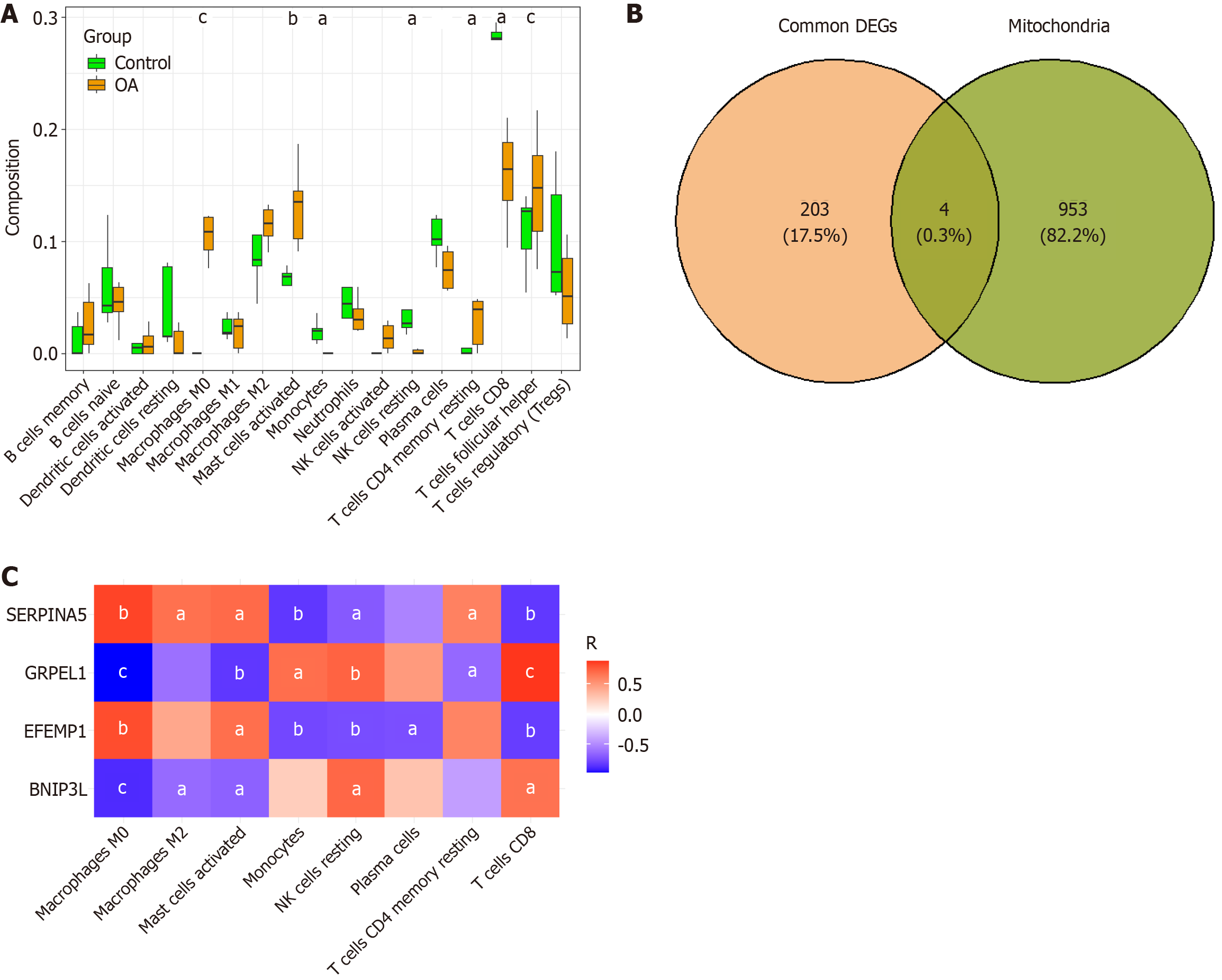
To further understand the interactions among the DEGs, we performed a PPI network analysis using the Genemania database, which revealed extensive interactions between these genes. Particularly, the PPI analysis of GRPEL1 unveiled its potential molecular mechanisms in OA (Supplementary Figure 2). To investigate the relationship between the DEGs and immune cells, we used the cell type identification by estimating relative subsets of RNA transcripts algorithm to analyze the association between gene expression levels and 22 immune cell types in the GSE169077 dataset, thereby obtaining the infiltration proportions of different immune cells in each sample. Through Wilcoxon rank-sum tests, we found that the infiltration levels of 8 immune cell types were significantly different between normal and OA tissues. Specifically, M0 macrophages, M2 macrophages, activated mast cells, and resting memory CD4 T cells showed significantly increased expression in OA tissues, while monocytes, resting natural killer cells, plasma cells, and CD8 T cells had higher expression levels in the CTRL (Figure 2A). Notably, we also identified 4 DEGs related to mitochondrial function, including the genes GRPEL1, epidermal growth factor-containing fibulin ECM protein 1 (EFEMP1), serine protease inhibitor 5 (SERPINA5), and Bcl2 interacting protein 3 Like (BNIP3 L) (Figure 2B). Further analysis revealed significant correlations between these 4 genes and the 8 immune cell types with significant differences (Figure 2C). In summary, our study results suggest that mitochondrial dysfunction plays an important role in the development of OA and may serve as a new therapeutic target for future interventions.
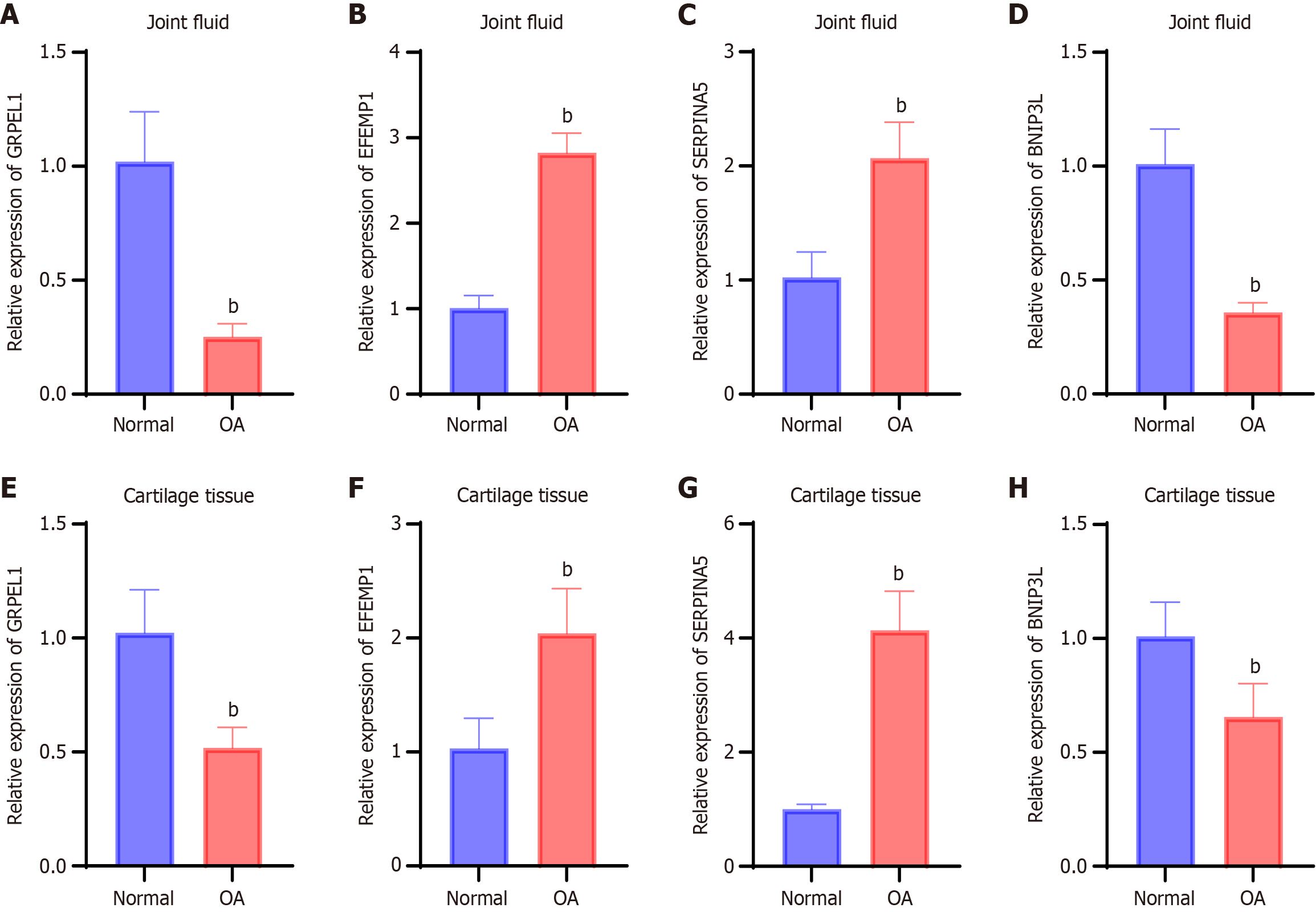
Bioinformatics analysis revealed that the genes GRPEL1, EFEMP1, SERPINA5, and BNIP3 L may be closely associated with the development of OA. We further explored the expression profiles of these genes in synovial fluid and cartilage tissue from OA patients. The results showed that, compared to healthy controls, the relative expression levels of GRPEL1 and BNIP3 L in the synovial fluid of OA patients were significantly decreased (P < 0.05), whereas the relative expression levels of EFEMP1 and SERPINA5 were significantly increased (Figure 3A-D). Similar expression patterns were also observed in cartilage tissue. Notably, the reduction in GRPEL1 and BNIP3 L gene expression in the synovial fluid was more pronounced than in cartilage tissue, while the overexpression of EFEMP1 was more prominent in cartilage tissue than in synovial fluid (Figure 3E-H). These findings suggest that abnormalities in biomarkers related to mitochondrial dysfunction may exist in the cellular tissues of the joint region in OA patients.
The correlation analysis revealed distinct relationships between the expression levels of GRPEL1, EFEMP1, SERPINA5, and BNIP3 L in synovial fluid and cartilage tissue of OA patients and clinical parameters, including CRP, TNF-α, IL-6 Levels, and KL grading. In synovial fluid (Supplementary Figure 3), GRPEL1 expression exhibited significant negative correlations with CRP (r = -0.482, P = 0.0169) and IL-6 (r = -0.4569, P = 0.0492) but no significant associations with TNF-α. EFEMP1 expression showed no statistically significant correlations with CRP, TNF-α, or IL-6 Levels. Conversely, SERPINA5 displayed a positive correlation with CRP (r = 0.488, P = 0.0156) but not with TNF-α or IL-6. BNIP3 L did not show any significant correlations with these inflammatory markers. Similarly, the analysis of cartilage tissue (Supplementary Figure 4) demonstrated that GRPEL1 expression negatively correlated with CRP (r = -0.5632, P = 0.0086) and IL-6 (r = -0.6751, P = 0.0014) but not TNF-α. EFEMP1 was positively correlated with CRP (r = 0.5154, P = 0.0100), while SERPINA5 exhibited significant positive correlations with CRP (r = 0.6109, P = 0.0015) and IL-6 (r = 0.5030, P = 0.0282). BNIP3 L showed no significant associations in either synovial fluid or cartilage. For KL grading, GRPEL1 expression was significantly reduced in higher KL grades in both synovial fluid and cartilage tissue. Therefore, we chose GRPEL1 for a subsequent series of experiments.
To ensure the successful isolation of human SMSCs (hSMSCs) from surgically obtained human synovial tissues, we conducted multiple characterization analyses. Initially, immunofluorescence staining revealed positive expression of CD105, CD44, and SCA-1 (Supplementary Figure 5A), confirming the MSC characteristics of the isolated cells. Subsequently, Alizarin Red S staining demonstrated calcium mineral deposition, verifying the osteogenic differentiation potential of hSMSCs (Supplementary Figure 5B). Additionally, Oil Red O staining detected small lipid droplets in the cytoplasm, further validating the adipogenic differentiation capacity of hSMSCs (Supplementary Figure 5C).
To investigate the function of GRPEL1 in hSMSCs, we employed qRT-PCR to evaluate GRPEL1 mRNA expression levels across different treatment groups (control non-coding overexpression group oeNC, GRPEL1 group, control non-coding knockdown group shNC, and shGRPEL1 group). Results demonstrated that GRPEL1 mRNA levels were significantly elevated in the GRPEL1 group compared to the oeNC group, while significantly reduced in the shGRPEL1 group compared to the shNC group, indicating effective modulation of GRPEL1 transcriptional activity through both overexpression and knockdown approaches (Supplementary Figure 5D). Furthermore, western blot analysis of GRPEL1 protein expression revealed significantly higher protein levels in the GRPEL1 group compared to the oeNC group, whereas protein levels were significantly lower in the shGRPEL1 group compared to the shNC group (Supplementary Figure 5E). In conclusion, these experiments not only validated the successful isolation of hSMSCs from human synovial tissue but also demonstrated their responsiveness to GRPEL1 regulation. These findings provide crucial experimental evidence for further investigation of GRPEL1’s role in hSMSC biological processes.
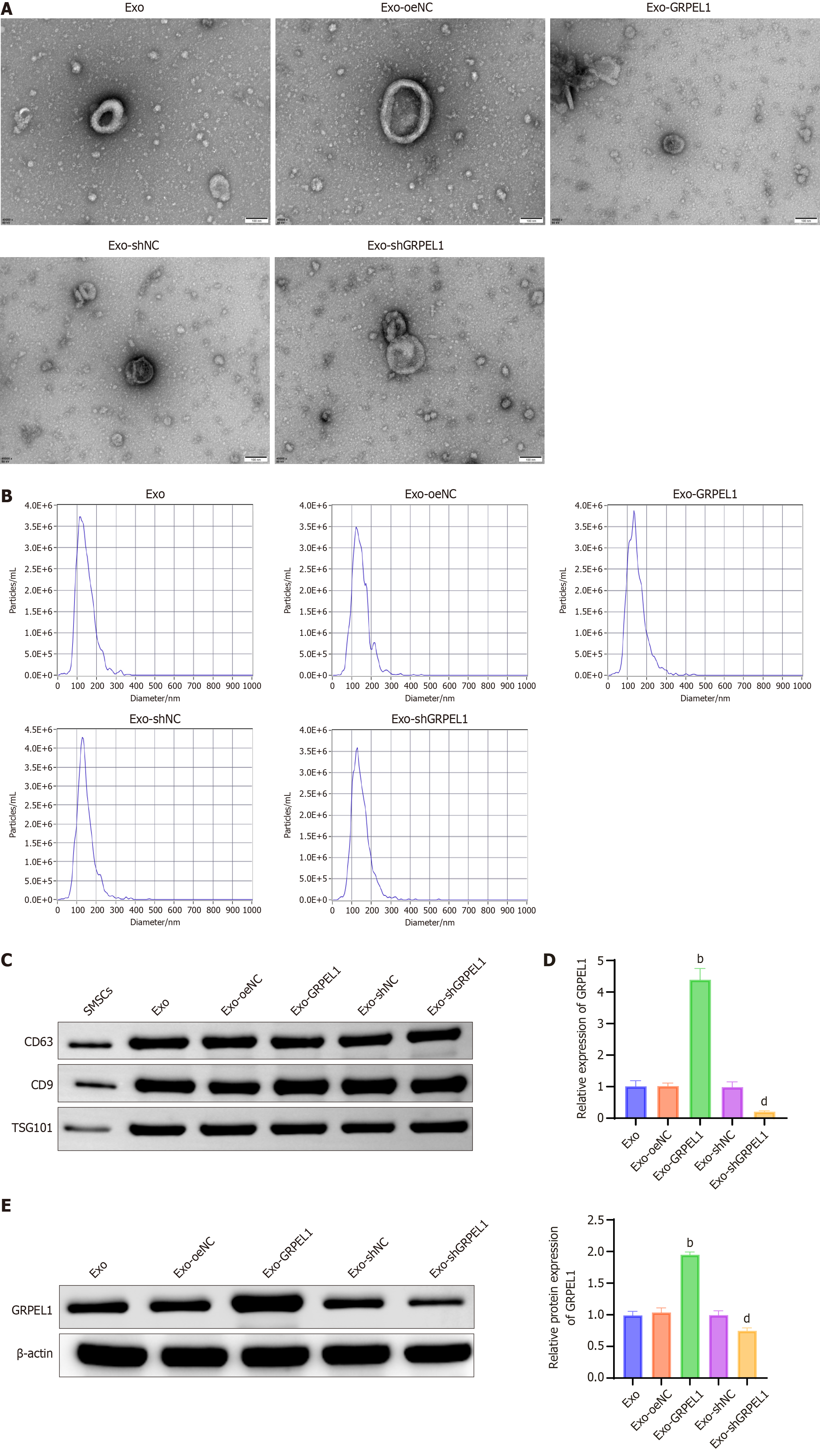
To identify and characterize Exos derived from hSMSCs, we employed transmission electron microscopy for morphological analysis, NTA for size distribution measurement, and western blot for the detection of exosomal marker proteins. Results revealed that the Exos exhibited typical morphological structures, characterized by double membrane-bound vesicles (Figure 4A), with diameters ranging from 30 to 150 nm (Figure 4B). Western blot analysis further demonstrated that the relative protein expression levels of exosomal markers (CD63, CD9, and tumor susceptibility gene 101) remained consistently high across all groups (Figure 4C). These findings collectively confirmed the successful isolation of hSMSCs-derived Exos, with no significant differences observed between groups, suggesting that genetic manipulation did not affect the expression levels of these key exosomal markers. qRT-PCR analysis (Figure 4D) and western blot (Figure 4E) further verified GRPEL1 expression in Exos. Results showed significantly higher GRPEL1 mRNA and protein expression levels in the Exo-GRPEL1 group compared to other groups, while expression levels were significantly reduced in the Exo-shGRPEL1 group. In conclusion, through comprehensive analysis of the morphological characteristics, size distribution, marker protein levels, and specific gene expression levels of hSMSCs-derived Exos, this study confirms that genetic engineering techniques can effectively regulate GRPEL1 expression in hSMSCs-derived Exos.
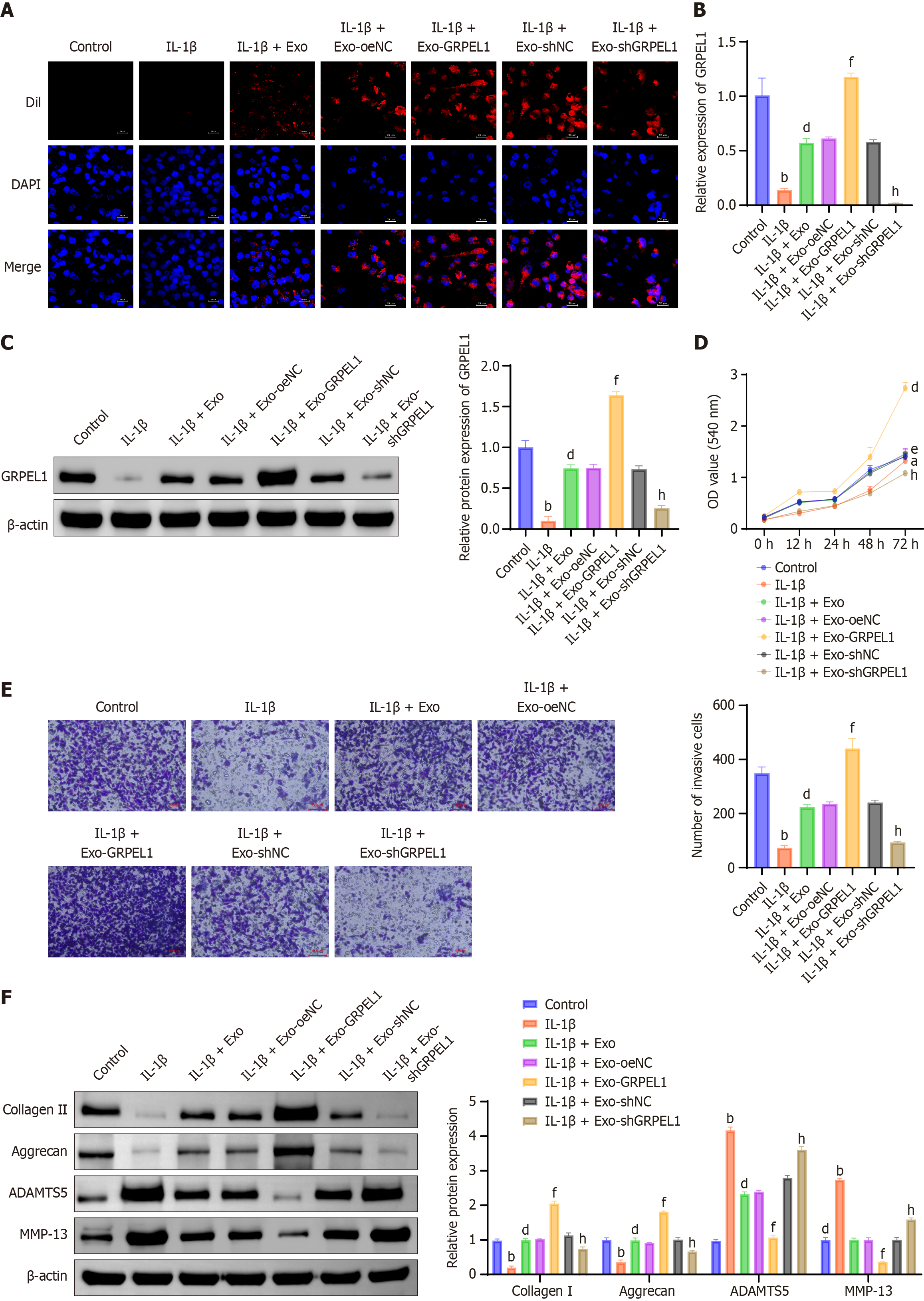
In our preliminary bioinformatics analysis, we identified significant correlations between GRPEL1 and immune cells. To further explore GRPEL1’s role in immune regulation, we investigated the effects of SMSC-Exos on IL-1β-induced CHON-001 cells. Initially, immunofluorescence staining using Dil-labeled Exos demonstrated that SMSCs-Exos were effectively internalized by IL-1β-treated CHON-001 cells (Figure 5A). qRT-PCR (Figure 5B) and western blot (Figure 5C) analyses revealed that IL-1β stimulation significantly decreased both mRNA and protein expression levels of GRPEL1 in CHON-001 cells compared to untreated controls. However, treatment with GRPEL1-containing Exos significantly restored these levels, with the most pronounced effect observed in the GRPEL1-overexpressing Exo treatment group. This indicates that SMSCs-Exos effectively deliver GRPEL1 to target cells, thereby influencing gene expression. CCK-8 assay results demonstrated that IL-1β treatment significantly inhibited CHON-001 cell growth. However, cell viability was significantly enhanced following treatment with GRPEL1-containing Exos, with the most notable improvement observed in the GRPEL1-overexpressing Exo treatment group (Figure 5D). Transwell assay results showed that IL-1β treatment significantly reduced CHON-001 cell migration capacity. However, treatment with GRPEL1-containing Exos significantly enhanced migration ability, particularly in the GRPEL1-overexpressing Exo treatment group (Figure 5E). Furthermore, western blot analysis of ECM-related proteins revealed that IL-1β treatment significantly downregulated collagen II and aggrecan expression levels while upregulating ADAMTS5 and MMP-13 expression levels. Conversely, treatment with GRPEL1-containing Exos restored normal expression patterns of these proteins, increasing collagen II and aggrecan expression levels while reducing ADAMTS5 and MMP-13 expression levels (Figure 5F). In conclusion, these experimental data collectively demonstrate that SMSCs-Exos can alleviate IL-1β-induced anti-proliferation, anti-migration, and ECM degradation in CHON-001 cells through the delivery of GRPEL1.
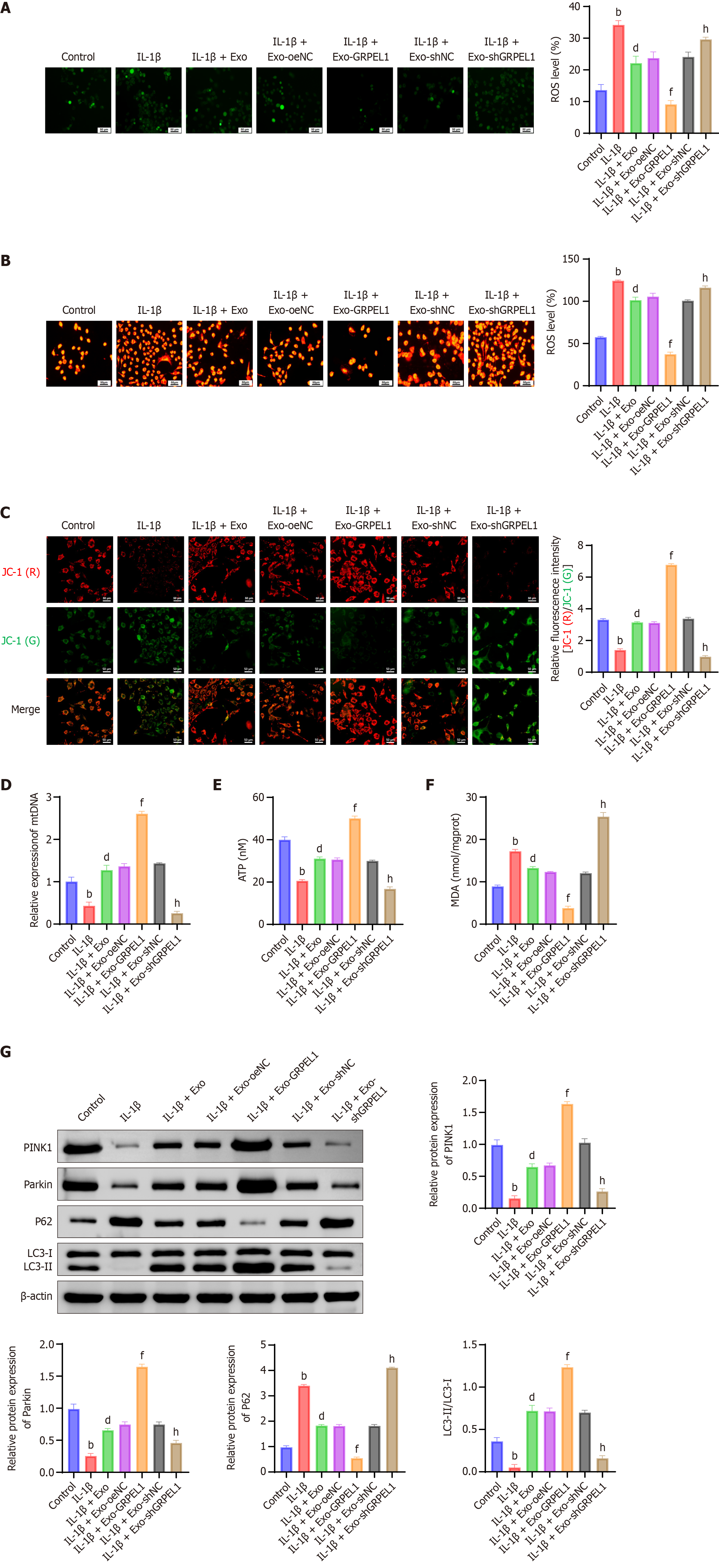
To further investigate the effects of SMSC-Exos on IL-1β-induced mitochondrial function in CHON-001 cells, we conducted multiple experiments. Results showed that IL-1β-induced CHON-001 cells exhibited significantly increased levels of both total ROS and mitochondrial ROS. However, the IL-1β + Exo and IL-1β + Exo-GRPEL1 groups demonstrated significantly reduced levels of both total and mitochondrial ROS, indicating that SMSCs-Exos and their carried GRPEL1 effectively decreased IL-1β-induced ROS generation (Figure 6A and B).
Furthermore, we observed changes in mitochondrial membrane potential. Results revealed that the IL-1β group showed significantly decreased mitochondrial membrane potential, suggesting mitochondrial dysfunction. However, the IL-1β + Exo and IL-1β + Exo-GRPEL1 groups exhibited significant improvement in mitochondrial membrane potential (Figure 6C). Similarly, mitochondrial DNA content (Figure 6D) and cellular ATP production (Figure 6E) demonstrated comparable trends, potentially attributed to the protective effects of SMSCs-Exos and GRPEL1 on maintaining mitochondrial integrity and function. Subsequently, we investigated malondialdehyde (MDA) levels in IL-1β-induced CHON-001 cells. Results showed significantly increased MDA content in the IL-1β group, reflecting enhanced lipid peroxidation. However, MDA levels were significantly reduced in both IL-1β + Exo and IL-1β + Exo-GRPEL1 groups (Figure 6F).
To further examine changes in mitophagy, we detected protein expression levels of PINK1, parkin, P62, and the LC3-II/LC3-I ratio. Results demonstrated that the IL-1β group exhibited lower levels of PINK1 and parkin proteins and LC3-II/LC3-I ratio, while showing higher P62 protein levels, all indicating decreased mitophagy activity. In contrast, the IL-1β + Exo and IL-1β + Exo-GRPEL1 groups showed significantly increased levels of PINK1 and parkin proteins and LC3-II/LC3-I ratio, while demonstrating significantly decreased P62 protein levels, collectively indicating enhanced mitophagy activity (Figure 6G). In conclusion, these experimental results demonstrate that SMSCs-Exos delivering GRPEL1 effectively alleviates IL-1β-induced mitochondrial dysfunction and enhances mitophagy in CHON-001 cells. These findings provide important experimental evidence for further investigation of GRPEL1’s role in cellular protection and mitochondrial function regulation.
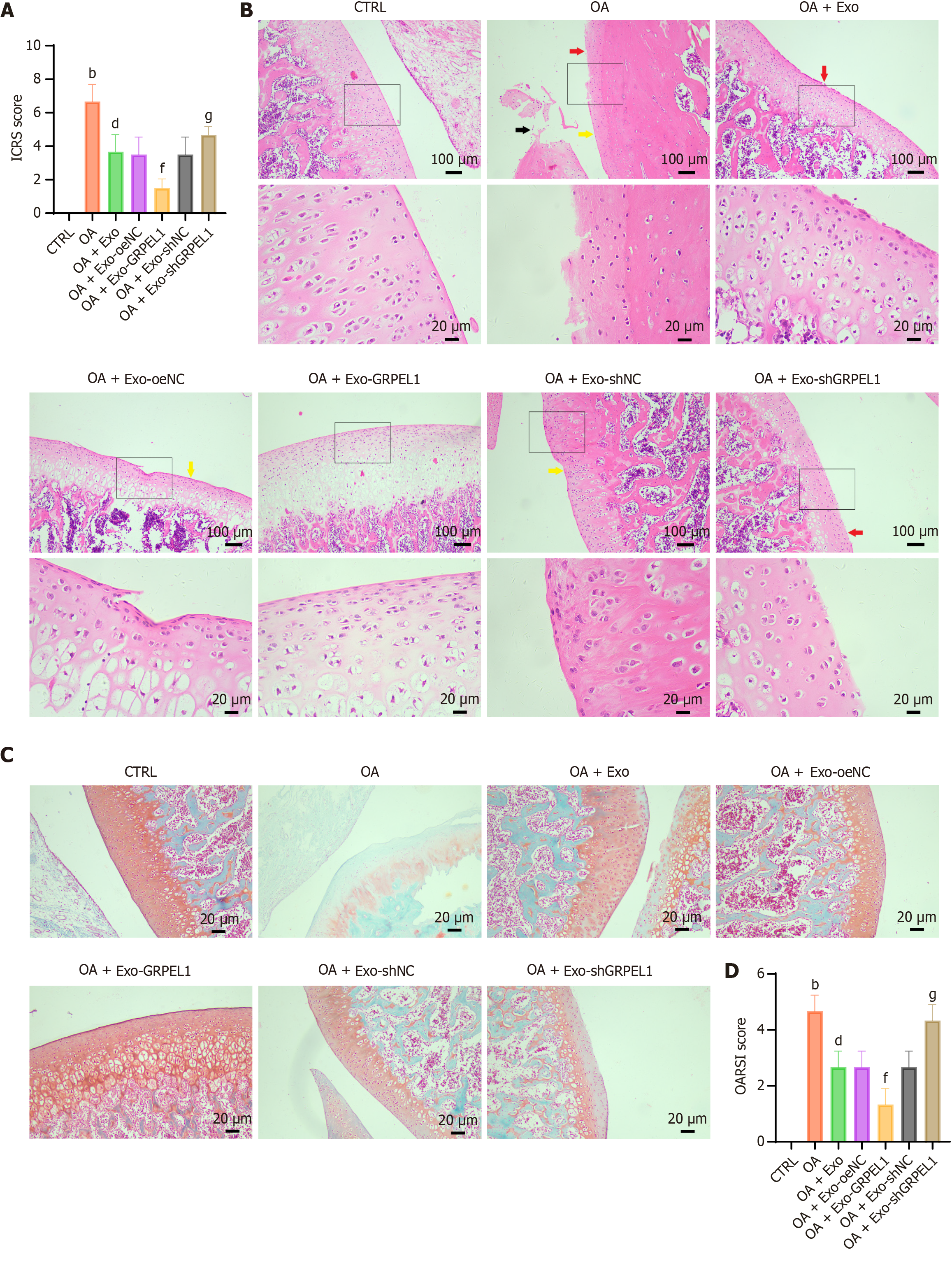
To investigate the protective effects of GRPEL1 on OA in vivo, we established a rat OA model. According to the predetermined protocol, OA rats received intraperitoneal injections of genetically modified SMSCs-Exos from different groups. Initially, International Cartilage Repair Society macroscopic scoring was performed on the knee joints of all groups. Results showed that the OA group exhibited significantly higher knee joint scores compared to other groups, indicating evident knee joint lesions. Following SMSCs-Exos injection, International Cartilage Repair Society scores decreased significantly, with the OA + Exo-GRPEL1 group showing the lowest scores, suggesting potentially optimal therapeutic effects (Figure 7A). To further evaluate articular cartilage damage, we performed HE staining and Safranin-O-Fast-Green staining. HE staining results showed smooth and intact articular cartilage surfaces in the CTRL (Figure 7B). The OA group exhibited notably thinned cartilage layers, irregular cell arrangement, and discontinuous cartilage. Following SMSCs-Exos injection, cell arrangement appeared more regular and smoother compared to the OA group, particularly in the OA + Exo-GRPEL1 group, which demonstrated clearer layered structures and fewer disruptions in articular cartilage. Safranin-O-Fast-Green staining results revealed extensive proteoglycan loss in the OA group, which was notably reversed following SMSCs-Exos injection, especially in the OA + Exo-GRPEL1 group, where collagen fiber arrangement appeared more organized and increased in quantity (Figure 7C). Finally, we employed the OARSI scoring system to comprehensively reflect the degree of joint lesions. OARSI scoring results were consistent with previous experimental findings, with the OA group showing the highest OARSI scores, indicating the most severe joint damage. Following SMSCs-Exos treatment, OARSI scores decreased significantly, with the OA + Exo-GRPEL1 group displaying the lowest scores, further supporting the efficacy of enhanced GRPEL1 expression in alleviating joint damage (Figure 7D). These experimental results demonstrate that SMSCs-Exos delivering GRPEL1 effectively ameliorates cartilage damage in rat knee OA.
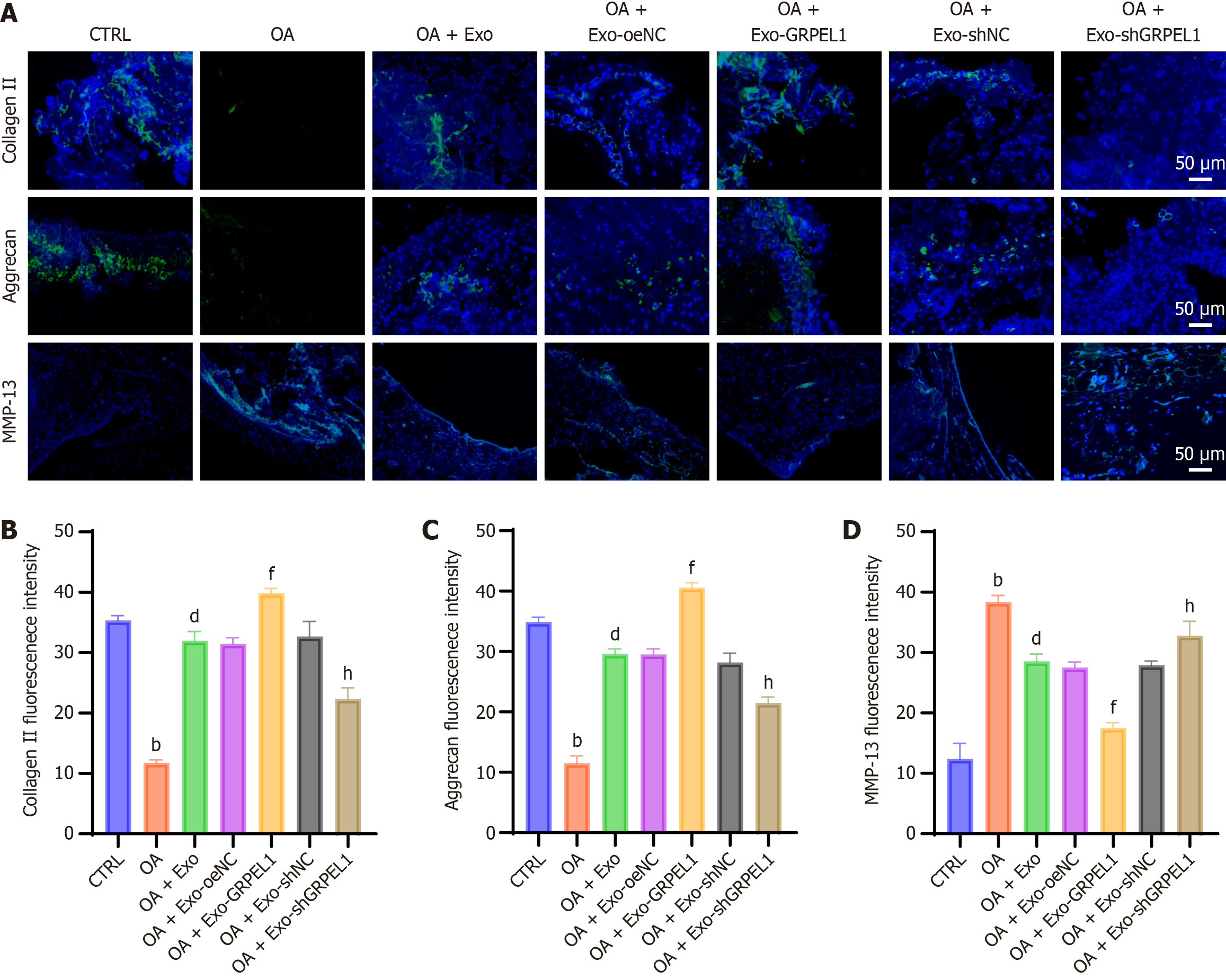
To further investigate the effects of GRPEL1 on cartilage degeneration in rat knee OA, we examined the levels of collagen II, aggrecan, and MMP-13 in cartilage tissue. Through immunofluorescence imaging and quantitative analysis, we compared the expression levels of these proteins in cartilage tissues across different groups (Figure 8A). Results demonstrated that the fluorescence intensities of collagen II and aggrecan were significantly lower in the OA group compared to the CTRL, indicating OA-induced degradation of collagen II and aggrecan. Following Exo treatment, particularly in the OA + Exo-GRPEL1 group, the fluorescence intensities of collagen II and aggrecan significantly increased, approaching levels comparable to the CTRL. Regarding MMP-13, the CTRL exhibited low fluorescence intensity, while the OA group showed high fluorescence intensity, reflecting increased MMP-13 Levels under OA conditions. In the OA + Exo-GRPEL1 group, MMP-13 fluorescence intensity was significantly reduced, approximating CTRL levels, indicating that GRPEL1 could effectively reduce MMP-13 production (Figure 8B-D). In conclusion, these data demonstrate that GRPEL1 delivered via SMSCs-Exos effectively inhibits cartilage degeneration in rat knee OA, specifically manifested by enhanced expression of collagen II and aggrecan, and reduced production of MMP-13.
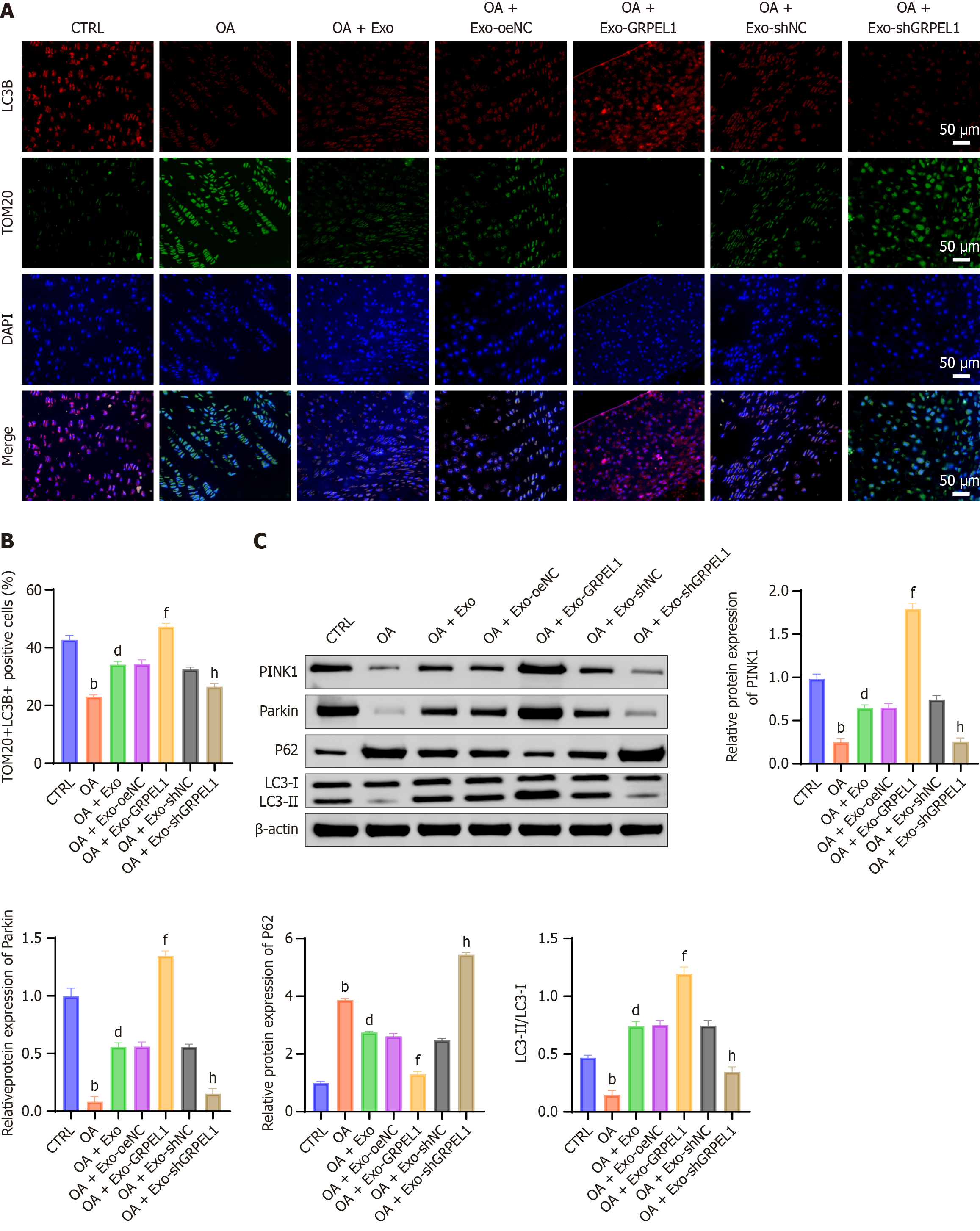
To investigate the activating effects of GRPEL1 on mitophagy, we examined mitophagy markers in cartilage tissues across different groups. Results showed that in the OA group, fluorescence intensities of collagen II and aggrecan were significantly lower compared to the CTRL, indicating OA-induced degradation of these proteins. Following Exo treatment, particularly in the OA + Exo-GRPEL1 group, fluorescence intensities of collagen II and aggrecan significantly increased, approaching levels comparable to the CTRL. Regarding MMP-13, the CTRL exhibited low fluorescence intensity, while the OA group showed high fluorescence intensity, demonstrating increased MMP-13 Levels under OA conditions. Immunofluorescence staining revealed reduced co-localization of TOM20 and LC3B in the OA group compared to the control, indicating impaired mitophagy. Following Exo treatment, especially in the OA + Exo-GRPEL1 group, TOM20 and LC3B co-localization was markedly enhanced, suggesting GRPEL1-induced activation of mitophagy (Figure 9A). We employed double immunofluorescence staining to examine TOM20 (mitochondrial marker) and LC3B (autophagy marker). Results revealed that the co-localization coefficient of TOM20 and LC3B was significantly lower in the OA group compared to the CTRL. Following Exo treatment, the co-localization coefficient increased, with the OA + Exo-GRPEL1 group showing significantly higher coefficients compared to the OA group (Figure 9B). Western blot analysis of mitochondria-related proteins in cartilage revealed that compared to the CTRL, the OA group exhibited significantly decreased levels of PINK1, parkin, and LC3-II/LC3-I ratio, while showing increased P62 expression. These changes indicate reduced autophagy levels, potentially leading to accumulation of damaged mitochondria. However, Exo treatment reversed these changes, with particularly pronounced effects in the OA + Exo-GRPEL1 group (Figure 9C). These data demonstrate that SMSCs-Exos delivering GRPEL1 can effectively activate mitophagy in cartilage cells of rat knee OA.
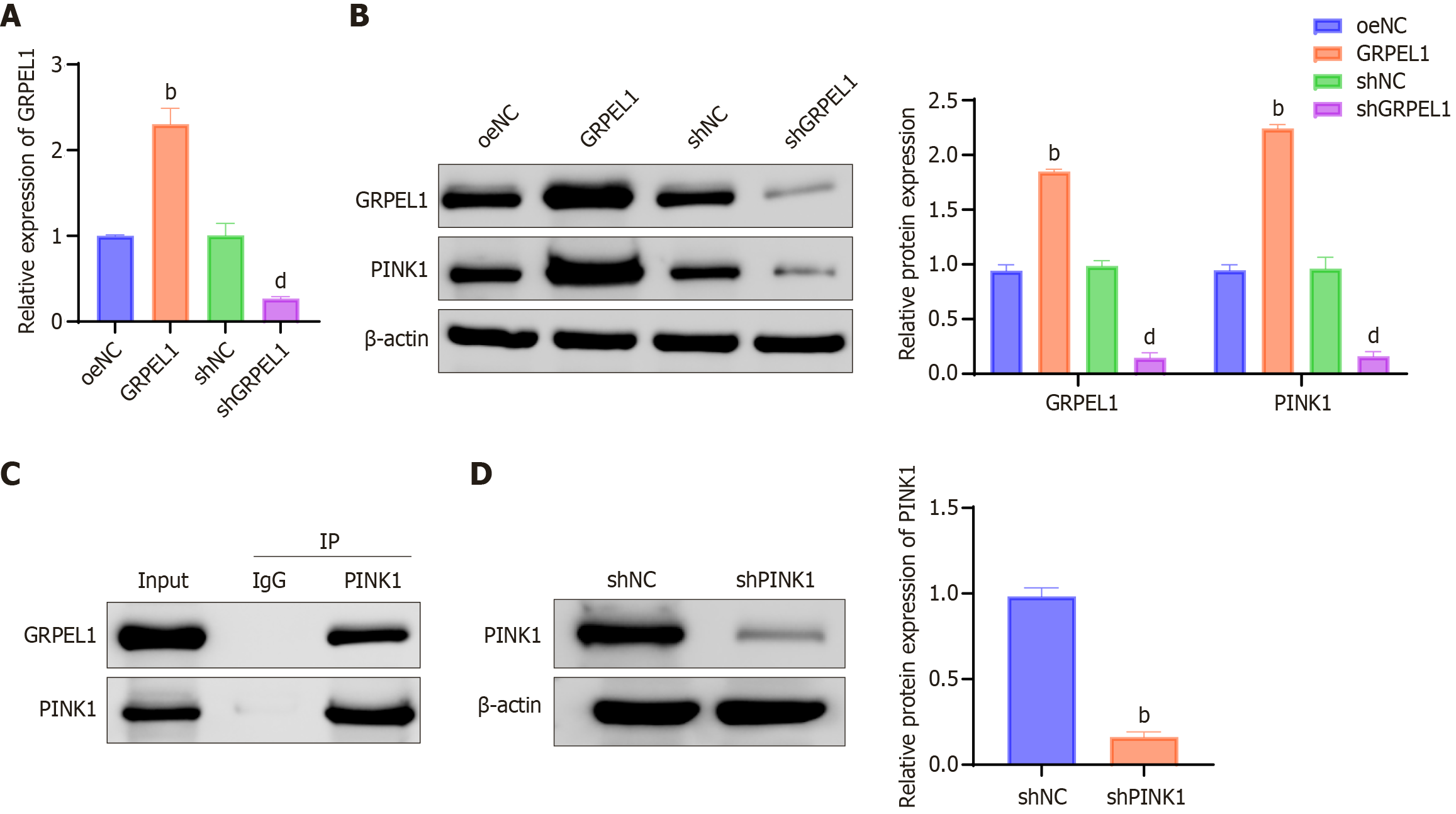
To confirm the interaction between GRPEL1 and PINK1, we employed qRT-PCR and western blot techniques to monitor expression changes of GRPEL1 and PINK1 across different cell lines. Results demonstrated that GRPEL1 Levels were significantly higher in the GRPEL1 group compared to other groups, while significantly lower in the shGRPEL1 group (Figure 10A and B). Through co-immunoprecipitation experiments, we detected the presence of PINK1 protein in samples from the GRPEL1 group, while PINK1 signals were weak or indistinct in samples from the shGRPEL1 group (Figure 10C). Additionally, we observed significantly reduced PINK1 protein levels in the PINK1 knockdown group (shPINK1 knockdown group) compared to the shNC control group (Figure 10D). These data collectively reveal the crucial role of GRPEL1 in regulating PINK1 function.
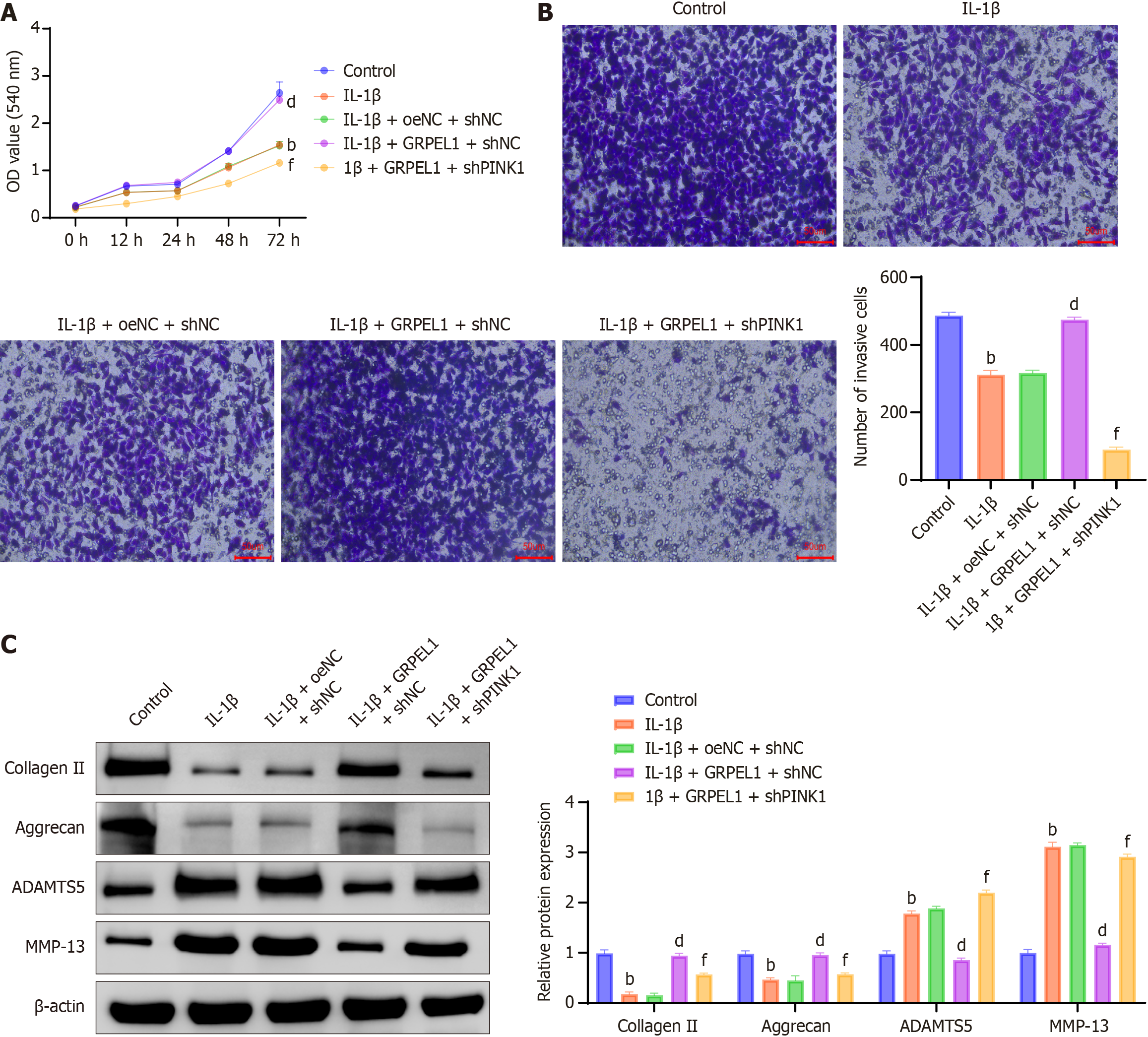
To investigate the potential impact of PINK1 knockdown on GRPEL1’s beneficial effects, we first observed changes in CHON-001 cell viability under IL-1β stimulation using the CCK-8 assay. Results demonstrated significantly decreased cell viability under IL-1β treatment. When GRPEL1 was overexpressed, it inhibited the action of IL-1β. However, when PINK1 was simultaneously knocked down, the cell viability-enhancing effects induced by GRPEL1 were partially inhibited (Figure 11A). Through transwell migration assays, we observed that IL-1β treatment reduced CHON-001 cell migration capacity, while GRPEL1 overexpression inhibited this effect. However, when PINK1 was knocked down, the increased migration capacity induced by GRPEL1 was significantly inhibited (Figure 11B).
Western blot analysis revealed that following IL-1β treatment, CHON-001 cells exhibited decreased expression of collagen II and aggrecan, while showing increased expression of matrix MMP-13 and ADAMTS5 (Figure 11C). GRPEL1 overexpression reversed these changes, restoring levels to those of the CTRL, but PINK1 knockdown inhibited GRPEL1’s beneficial effects. Specifically, following PINK1 knockdown, expression levels of collagen II and aggrecan were lower than those in the GRPEL1 group, while ADAMTS5 and MMP-13 expression levels were higher than those in the GRPEL1 group. These findings indicate that PINK1 knockdown attenuates GRPEL1’s effects on IL-1β-induced CHON-001 cell proliferation, migration, and ECM degradation.
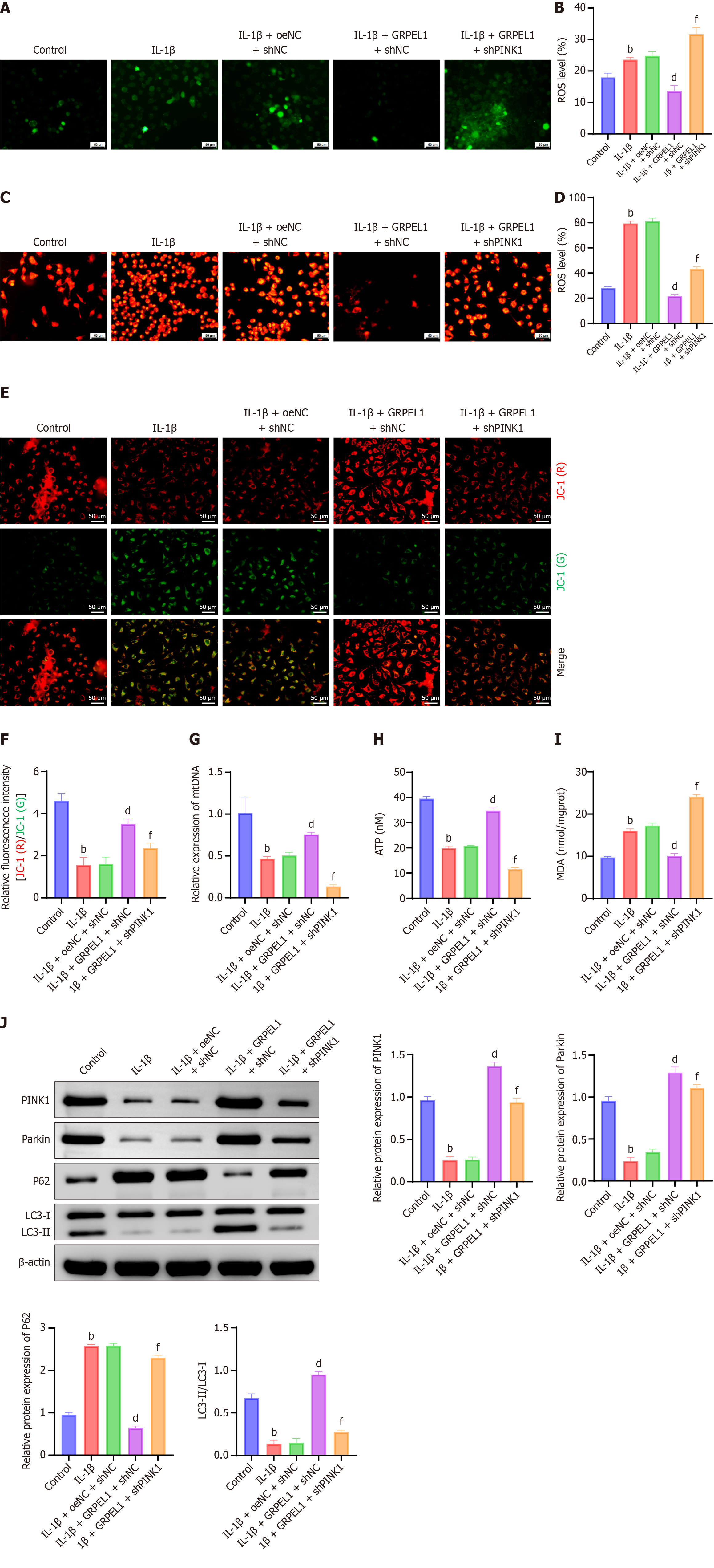
To further investigate the impact of PINK1 knockdown on the protective effect of GRPEL1 against IL-1β-induced mitochondrial dysfunction in CHON-001 cells, we conducted a series of experiments. IL-1β treatment significantly increased both total ROS levels and mitochondrial ROS levels in CHON-001 cells. While GRPEL1 overexpression reduced these elevated ROS levels, PINK1 knockdown led to a marked increase in both total ROS and mitochondrial ROS levels, exceeding those observed in the GRPEL1 group (Figure 12A-D). Additionally, IL-1β treatment caused a significant reduction in mitochondrial membrane potential, which was partially restored by GRPEL1 overexpression. However, simultaneous PINK1 knockdown resulted in a further decrease in mitochondrial membrane potential, to levels even lower than those observed in the GRPEL1 group (Figure 12E and F).
Changes in mitochondrial DNA content (Figure 12G) and intracellular ATP production (Figure 12H) supported these findings, indicating that PINK1 knockdown diminished GRPEL1’s protective effect on maintaining mitochondrial integrity and function. Further examination of MDA levels, a marker of lipid peroxidation, revealed that IL-1β treatment significantly increased MDA levels, reflecting exacerbated oxidative damage. GRPEL1 overexpression reduced MDA levels, but this protective effect was abolished upon PINK1 knockdown, leading to a significant increase in MDA levels (Figure 12I).
Analysis of protein expression levels of PINK1, parkin, P62, and LC3-II/LC3-I provided additional insights (Figure 12J). In the IL-1β-treated group, PINK1 and parkin protein levels, as well as the LC3-II/LC3-I ratio, were decreased, while P62 protein levels were elevated, indicating reduced mitophagic activity. In contrast, GRPEL1 overexpression increased PINK1 and parkin protein levels and the LC3-II/LC3-I ratio while decreasing P62 protein levels. However, PINK1 knockdown reversed these changes, leading to reduced PINK1 and parkin protein levels, a decreased LC3-II/LC3-I ratio, and elevated P62 Levels compared to the GRPEL1 group. These findings collectively demonstrate that PINK1 knockdown mitigates the protective effects of GRPEL1 against IL-1β-induced mitochondrial dysfunction and impaired mitophagy in CHON-001 cells.
OA is a prevalent chronic degenerative joint disease that significantly impacts the quality of life for elderly populations while imposing a substantial socio-economic burden[4,37,38]. This study investigates the potential therapeutic mechanisms of SMSC-Exos in OA treatment. SMSCs are particularly promising due to their exceptional multi-lineage differentiation potential and self-renewal capacity, making them an ideal stem cell source for managing diseases like OA[39,40]. Moreover, Exo-based therapies derived from MSCs have demonstrated remarkable potential in OA management[41]. Exos derived from bone morphogenetic protein-7-modified SMSCs have been shown to alleviate inflammation and cartilage damage in knee OA by promoting macrophage M2 polarization[33]. In the context of cartilage formation, Exos can reduce chondrocyte apoptosis and mitigate OA progression, as seen in SMSCs-Exos expressing miR-212-5p[42] and miR-486-5p[43].
In this study, we designed both in vitro and in vivo models to explore the therapeutic potential of SMSC-Exo in OA management, providing unprecedented insights into their underlying mechanisms. We identified GRPEL1 as a key molecular target for intervention. Preliminary bioinformatics analyses revealed that GRPEL1 plays a critical role in SMSC-Exo’s effects on OA, highlighting the complex molecular landscape of OA pathogenesis[44-46]. By identifying GRPEL1 as a DEG, we uncovered a potential mechanism linking mitochondrial dysfunction to cartilage degradation. The significant reduction of GRPEL1 in synovial fluid and cartilage tissues suggests that it may serve as a critical biomarker for disease progression.
In vitro experiments using CHON-001 chondrocytes confirmed the multifaceted protective effects of GRPEL1-loaded Exos. Under IL-1β-induced inflammatory conditions, these Exos effectively countered cell damage by enhancing proliferation, migration, and ECM preservation. Furthermore, we demonstrated that SMSC-Exo delivering GRPEL1 alleviated IL-1β-induced mitochondrial dysfunction and promoted mitochondrial autophagy in CHON-001 cells. This mechanism appears closely linked to the activation of mitochondrial autophagy, particularly via the PINK1 pathway, which plays a crucial role in maintaining cellular homeostasis. Co-immunoprecipitation experiments revealed a direct interaction between GRPEL1 and PINK1, providing molecular evidence for the observed therapeutic effects. This interaction seems fundamental for regulating mitochondrial quality control, reducing oxidative stress, and preventing premature chondrocyte dysfunction.
Enhancing autophagy is a potential therapeutic strategy for OA, with interventions targeting mitochondrial dysfunction and improving mitochondrial health playing a key role in regulating autophagy and promoting chondrocyte survival[44,46,47]. Metformin, for example, regulates mitochondrial autophagy via the sirtuin 3/PINK1/PRKN signaling pathway, counteracting IL-1β-induced oxidative stress and metabolic imbalances in chondrocytes, highlighting the potential of metformin in preventing and treating OA through modulation of mitochondrial autophagy[9,25,48]. In OA chondrocytes, PINK1/parkin-mediated mitochondrial autophagy reduces ROS levels by clearing dysfunctional mitochondria[49,50]. Our in vivo model further validated the impact of GRPEL1 on OA, showing that GRPEL1-enriched SMSC-Exo significantly promoted cartilage repair, mitochondrial autophagy activation, and cell function in an OA context.
The PINK1/parkin pathway is a classic route for activating mitophagy. Typically, PINK1 is continuously imported into the mitochondrial matrix, where it is degraded by the mitochondrial rhomboid protease PARL[51]. However, when damaged mitochondria lose their ψm, the translocation and degradation of PINK1 are disrupted, leading to its accumulation on the outer mitochondrial membrane[52]. PINK1 then activates ubiquitin (Ub) and parkin to form the parkin-Ub complex, which marks the damaged mitochondria for degradation. The adapter protein P62 connects the parkin-Ub complex with LC3B, enabling the autophagosome to engulf the damaged mitochondria[53]. Recent studies have shown that Exos can activate mitochondrial autophagy through various pathways, including PINK1-parkin and phosphoinositide 3-kinase/protein kinase B/mammalian target of rapamycin signaling[25,49,50]. Although mitophagy is generally triggered by a loss of mitochondrial membrane potential (ψm)[54,55], our findings demonstrated that treatment with GRPEL1-loaded Exos led to both an increase in ψm and a concurrent upregulation of mitophagy markers. This seemingly paradoxical observation may reflect a two-step process, in which GRPEL1 first restores mitochondrial function in IL-1β-damaged chondrocytes, thereby increasing ψm, while simultaneously promoting the clearance of previously damaged mitochondria through PINK1/parkin-mediated mitophagy. Thus, in this context, elevated ψm serves not as a trigger but as an indicator of mitochondrial recovery, and mitophagy functions as a complementary mechanism to maintain mitochondrial quality. Our results also support the role of PINK1 in GRPEL1-mediated effects. PINK1 knockdown weakened the beneficial effects of GRPEL1 on IL-1β-induced cell proliferation, migration, and ECM degradation, suggesting that PINK1 is crucial for the full manifestation of GRPEL1’s protective effects, particularly in regulating key cellular processes like proliferation and ECM synthesis, which are essential for cartilage repair.
While this study presents important findings, some research gaps remain. Future studies should focus on the specific stages of mitochondrial autophagy activation by GRPEL1-delivering SMSC-Exo, including the identification of damaged mitochondria, autophagosome formation, lysosomal fusion, and extracellular clearance. In addition, due to the limited clinical documentation and the retrospective nature of sample collection, we were unable to correlate GRPEL1 expression with clinical outcomes. In the future prospective studies, we plan to include larger patient cohorts along with more detailed clinical data to explore potential correlations between GRPEL1 expression and disease severity or key clinical parameters. In summary, we have successfully demonstrated that SMSC-Exo-GRPEL1 injection reduces inflammation and alleviates cartilage damage. By promoting mitochondrial autophagy, reducing cartilage degradation, and enhancing chondrocyte function, GRPEL1 helps restore cartilage homeostasis in OA. The GRPEL1-PINK1 interaction is the key mechanism underlying these effects, and PINK1 is essential for the full protective potential of GRPEL1 in chondrocytes.
In conclusion, this study elucidates a novel therapeutic approach for OA by demonstrating that synovial MSC-derived Exos delivering GRPEL1 can effectively activate PINK1-mediated mitophagy, thereby promoting cartilage repair. Our comprehensive investigation, spanning bioinformatics analysis, in vitro cell studies, and in vivo rat models, reveals that GRPEL1-loaded Exos significantly mitigate inflammatory-induced cellular damage, restore ECM integrity, and enhance mitochondrial function. By targeting mitochondrial dysfunction through precise molecular mechanisms, this research provides critical insights into potential disease-modifying strategies for OA, transcending current symptomatic treatments and offering a promising foundation for future therapeutic interventions.
| 1. | Barnett R. Osteoarthritis. Lancet. 2018;391:1985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 143] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 2. | Glyn-Jones S, Palmer AJ, Agricola R, Price AJ, Vincent TL, Weinans H, Carr AJ. Osteoarthritis. Lancet. 2015;386:376-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1429] [Cited by in RCA: 2141] [Article Influence: 194.6] [Reference Citation Analysis (0)] |
| 3. | Vincent TL. Mechanoflammation in osteoarthritis pathogenesis. Semin Arthritis Rheum. 2019;49:S36-S38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 103] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 4. | Abramoff B, Caldera FE. Osteoarthritis: Pathology, Diagnosis, and Treatment Options. Med Clin North Am. 2020;104:293-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 764] [Article Influence: 127.3] [Reference Citation Analysis (0)] |
| 5. | Katz JN, Arant KR, Loeser RF. Diagnosis and Treatment of Hip and Knee Osteoarthritis: A Review. JAMA. 2021;325:568-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 634] [Cited by in RCA: 1474] [Article Influence: 294.8] [Reference Citation Analysis (3)] |
| 6. | Fayet M, Hagen M. Pain characteristics and biomarkers in treatment approaches for osteoarthritis pain. Pain Manag. 2021;11:59-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 7. | Hwang JJ, Rim YA, Nam Y, Ju JH. Recent Developments in Clinical Applications of Mesenchymal Stem Cells in the Treatment of Rheumatoid Arthritis and Osteoarthritis. Front Immunol. 2021;12:631291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 102] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 8. | Nasiri N, Nateghi R, Zarei F, Hosseini S, Eslaminejad MB. Mesenchymal Stem Cell Therapy for Osteoarthritis: Practice and Possible Promises. Adv Exp Med Biol. 2022;1387:107-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 9. | Dezawa M. Muse Cells Provide the Pluripotency of Mesenchymal Stem Cells: Direct Contribution of Muse Cells to Tissue Regeneration. Cell Transplant. 2016;25:849-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 85] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 10. | Ju Y, Yi L, Li C, Wang T, Zhang W, Chai W, Yin X, Weng T. Comparison of biological characteristics of human adipose- and umbilical cord- derived mesenchymal stem cells and their effects on delaying the progression of osteoarthritis in a rat model. Acta Histochem. 2022;124:151911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 11. | He L, He T, Xing J, Zhou Q, Fan L, Liu C, Chen Y, Wu D, Tian Z, Liu B, Rong L. Bone marrow mesenchymal stem cell-derived exosomes protect cartilage damage and relieve knee osteoarthritis pain in a rat model of osteoarthritis. Stem Cell Res Ther. 2020;11:276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 298] [Article Influence: 49.7] [Reference Citation Analysis (0)] |
| 12. | Zhang S, Teo KYW, Chuah SJ, Lai RC, Lim SK, Toh WS. MSC exosomes alleviate temporomandibular joint osteoarthritis by attenuating inflammation and restoring matrix homeostasis. Biomaterials. 2019;200:35-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 440] [Article Influence: 62.9] [Reference Citation Analysis (0)] |
| 13. | Zhang D, Lee H, Zhu Z, Minhas JK, Jin Y. Enrichment of selective miRNAs in exosomes and delivery of exosomal miRNAs in vitro and in vivo. Am J Physiol Lung Cell Mol Physiol. 2017;312:L110-L121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 255] [Article Influence: 28.3] [Reference Citation Analysis (1)] |
| 14. | Matsuzaka Y, Yashiro R. Extracellular Vesicles as Novel Drug-Delivery Systems through Intracellular Communications. Membranes (Basel). 2022;12:550. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 15. | Witkin SS, Linhares IM, Bongiovanni AM, Herway C, Skupski D. Unique alterations in infection-induced immune activation during pregnancy. BJOG. 2011;118:145-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 16. | Bian D, Wu Y, Song G, Azizi R, Zamani A. The application of mesenchymal stromal cells (MSCs) and their derivative exosome in skin wound healing: a comprehensive review. Stem Cell Res Ther. 2022;13:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 237] [Article Influence: 59.3] [Reference Citation Analysis (1)] |
| 17. | Duan L, Xu L, Xu X, Qin Z, Zhou X, Xiao Y, Liang Y, Xia J. Exosome-mediated delivery of gene vectors for gene therapy. Nanoscale. 2021;13:1387-1397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 179] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 18. | Wang K, Li F, Yuan Y, Shan L, Cui Y, Qu J, Lian F. Synovial Mesenchymal Stem Cell-Derived EV-Packaged miR-31 Downregulates Histone Demethylase KDM2A to Prevent Knee Osteoarthritis. Mol Ther Nucleic Acids. 2020;22:1078-1091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 19. | Qiu M, Liu D, Fu Q. MiR-129-5p shuttled by human synovial mesenchymal stem cell-derived exosomes relieves IL-1β induced osteoarthritis via targeting HMGB1. Life Sci. 2021;269:118987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 77] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 20. | Annesley SJ, Fisher PR. Mitochondria in Health and Disease. Cells. 2019;8:680. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 412] [Article Influence: 58.9] [Reference Citation Analysis (0)] |
| 21. | Mao X, Fu P, Wang L, Xiang C. Mitochondria: Potential Targets for Osteoarthritis. Front Med (Lausanne). 2020;7:581402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 22. | Blanco FJ, Rego I, Ruiz-Romero C. The role of mitochondria in osteoarthritis. Nat Rev Rheumatol. 2011;7:161-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 400] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 23. | Liao CR, Wang SN, Zhu SY, Wang YQ, Li ZZ, Liu ZY, Jiang WS, Chen JT, Wu Q. Advanced oxidation protein products increase TNF-α and IL-1β expression in chondrocytes via NADPH oxidase 4 and accelerate cartilage degeneration in osteoarthritis progression. Redox Biol. 2020;28:101306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 107] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 24. | Ansari MY, Khan NM, Ahmad I, Haqqi TM. Parkin clearance of dysfunctional mitochondria regulates ROS levels and increases survival of human chondrocytes. Osteoarthritis Cartilage. 2018;26:1087-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 191] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 25. | Wang C, Yang Y, Zhang Y, Liu J, Yao Z, Zhang C. Protective effects of metformin against osteoarthritis through upregulation of SIRT3-mediated PINK1/Parkin-dependent mitophagy in primary chondrocytes. Biosci Trends. 2019;12:605-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 131] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 26. | Huang LW, Huang TC, Hu YC, Hsieh BS, Chiu PR, Cheng HL, Chang KL. Zinc protects chondrocytes from monosodium iodoacetate-induced damage by enhancing ATP and mitophagy. Biochem Biophys Res Commun. 2020;521:50-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 27. | Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16184] [Cited by in RCA: 28059] [Article Influence: 2550.8] [Reference Citation Analysis (0)] |
| 28. | Tay JK, Narasimhan B, Hastie T. Elastic Net Regularization Paths for All Generalized Linear Models. J Stat Softw. 2023;106:1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 268] [Article Influence: 89.3] [Reference Citation Analysis (0)] |
| 29. | Wu T, Hu E, Xu S, Chen M, Guo P, Dai Z, Feng T, Zhou L, Tang W, Zhan L, Fu X, Liu S, Bo X, Yu G. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation (Camb). 2021;2:100141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 522] [Cited by in RCA: 5815] [Article Influence: 1163.0] [Reference Citation Analysis (0)] |
| 30. | Liao HJ, Yang YP, Liu YH, Tseng HC, Huo TI, Chiou SH, Chang CH. Harnessing the potential of mesenchymal stem cells-derived exosomes in degenerative diseases. Regen Ther. 2024;26:599-610. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 31. | Lou Y, Song F, Kang Y, Xu Y. Periodic Mechanical Stress Inhibits the Development of Osteoarthritis via Regulating ATF3-Akt Axis. J Inflamm Res. 2023;16:5613-5628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 32. | Kong R, Ji L, Pang Y, Zhao D, Gao J. Exosomes from osteoarthritic fibroblast-like synoviocytes promote cartilage ferroptosis and damage via delivering microRNA-19b-3p to target SLC7A11 in osteoarthritis. Front Immunol. 2023;14:1181156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 34] [Reference Citation Analysis (0)] |
| 33. | Sun W, Qu S, Ji M, Sun Y, Hu B. BMP-7 modified exosomes derived from synovial mesenchymal stem cells attenuate osteoarthritis by M2 polarization of macrophages. Heliyon. 2023;9:e19934. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 34. | Chu M, Zhang C. Inhibition of angiogenesis by leflunomide via targeting the soluble ephrin-A1/EphA2 system in bladder cancer. Sci Rep. 2018;8:1539. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 35. | Wang Z, Zhou F, Dou Y, Tian X, Liu C, Li H, Shen H, Chen G. Melatonin Alleviates Intracerebral Hemorrhage-Induced Secondary Brain Injury in Rats via Suppressing Apoptosis, Inflammation, Oxidative Stress, DNA Damage, and Mitochondria Injury. Transl Stroke Res. 2018;9:74-91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 145] [Cited by in RCA: 253] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 36. | Glasson SS, Chambers MG, Van Den Berg WB, Little CB. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthritis Cartilage. 2010;18 Suppl 3:S17-S23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 890] [Cited by in RCA: 1317] [Article Influence: 82.3] [Reference Citation Analysis (0)] |
| 37. | Yao Q, Wu X, Tao C, Gong W, Chen M, Qu M, Zhong Y, He T, Chen S, Xiao G. Osteoarthritis: pathogenic signaling pathways and therapeutic targets. Signal Transduct Target Ther. 2023;8:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 790] [Cited by in RCA: 717] [Article Influence: 239.0] [Reference Citation Analysis (0)] |
| 38. | Felson DT, Lawrence RC, Dieppe PA, Hirsch R, Helmick CG, Jordan JM, Kington RS, Lane NE, Nevitt MC, Zhang Y, Sowers M, McAlindon T, Spector TD, Poole AR, Yanovski SZ, Ateshian G, Sharma L, Buckwalter JA, Brandt KD, Fries JF. Osteoarthritis: new insights. Part 1: the disease and its risk factors. Ann Intern Med. 2000;133:635-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1555] [Cited by in RCA: 1656] [Article Influence: 63.7] [Reference Citation Analysis (0)] |
| 39. | Sakaguchi Y, Sekiya I, Yagishita K, Muneta T. Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum. 2005;52:2521-2529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1084] [Cited by in RCA: 1098] [Article Influence: 52.3] [Reference Citation Analysis (0)] |
| 40. | Nakashima H, Uchida S, Hatakeyama A, Murata Y, Yamanaka Y, Tsukamoto M, Sekiya I, Sakai A. Isolation and Characterization of Synovial Mesenchymal Stem Cells Derived From Patients With Chronic Lateral Ankle Instability: A Comparative Analysis of Synovial Fluid, Adipose Synovium, and Fibrous Synovium of the Ankle Joint. Orthop J Sports Med. 2022;10:23259671221094615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 41. | Toh WS, Lai RC, Hui JHP, Lim SK. MSC exosome as a cell-free MSC therapy for cartilage regeneration: Implications for osteoarthritis treatment. Semin Cell Dev Biol. 2017;67:56-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 388] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 42. | Zheng T, Li Y, Zhang X, Xu J, Luo M. Exosomes Derived From miR-212-5p Overexpressed Human Synovial Mesenchymal Stem Cells Suppress Chondrocyte Degeneration and Inflammation by Targeting ELF3. Front Bioeng Biotechnol. 2022;10:816209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 43. | Wang Y, Fan A, Lu L, Pan Z, Ma M, Luo S, Liu Z, Yang L, Cai J, Yin F. Exosome modification to better alleviates endoplasmic reticulum stress induced chondrocyte apoptosis and osteoarthritis. Biochem Pharmacol. 2022;206:115343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 44. | Xiao SQ, Cheng M, Wang L, Cao J, Fang L, Zhou XP, He XJ, Hu YF. The role of apoptosis in the pathogenesis of osteoarthritis. Int Orthop. 2023;47:1895-1919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 45. | Zhang M, Wu J, Cai K, Liu Y, Lu B, Zhang J, Xu J, Gu C, Chen T. From dysfunction to healing: advances in mitochondrial therapy for Osteoarthritis. J Transl Med. 2024;22:1013. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 46. | Qi Z, Zhu J, Cai W, Lou C, Li Z. The role and intervention of mitochondrial metabolism in osteoarthritis. Mol Cell Biochem. 2024;479:1513-1524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 32] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 47. | Sun K, Wu Y, Zeng Y, Xu J, Wu L, Li M, Shen B. The role of the sirtuin family in cartilage and osteoarthritis: molecular mechanisms and therapeutic targets. Arthritis Res Ther. 2022;24:286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 48. | Fukuchi Y, Nakajima H, Sugiyama D, Hirose I, Kitamura T, Tsuji K. Human placenta-derived cells have mesenchymal stem/progenitor cell potential. Stem Cells. 2004;22:649-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 451] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 49. | Zhang Y, Xi X, Mei Y, Zhao X, Zhou L, Ma M, Liu S, Zha X, Yang Y. High-glucose induces retinal pigment epithelium mitochondrial pathways of apoptosis and inhibits mitophagy by regulating ROS/PINK1/Parkin signal pathway. Biomed Pharmacother. 2019;111:1315-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 133] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 50. | Jin Z, Chang B, Wei Y, Yang Y, Zhang H, Liu J, Piao L, Bai L. Curcumin exerts chondroprotective effects against osteoarthritis by promoting AMPK/PINK1/Parkin-mediated mitophagy. Biomed Pharmacother. 2022;151:113092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 123] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 51. | Chin LS, Li L. Ubiquitin phosphorylation in Parkinson's disease: Implications for pathogenesis and treatment. Transl Neurodegener. 2016;5:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 52. | Aerts L, Craessaerts K, De Strooper B, Morais VA. PINK1 kinase catalytic activity is regulated by phosphorylation on serines 228 and 402. J Biol Chem. 2015;290:2798-2811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 100] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 53. | Lan J, Tang L, Wu S, Huang R, Zhong G, Jiang X, Tang Z, Hu L. Curcumin alleviates arsenic-induced injury in duck skeletal muscle via regulating the PINK1/Parkin pathway and protecting mitochondrial function. Toxicol Appl Pharmacol. 2022;434:115820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 54. | Zorova LD, Popkov VA, Plotnikov EY, Silachev DN, Pevzner IB, Jankauskas SS, Babenko VA, Zorov SD, Balakireva AV, Juhaszova M, Sollott SJ, Zorov DB. Mitochondrial membrane potential. Anal Biochem. 2018;552:50-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1004] [Cited by in RCA: 1471] [Article Influence: 183.9] [Reference Citation Analysis (0)] |
| 55. | Jin SM, Lazarou M, Wang C, Kane LA, Narendra DP, Youle RJ. Mitochondrial membrane potential regulates PINK1 import and proteolytic destabilization by PARL. J Cell Biol. 2010;191:933-942. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 867] [Cited by in RCA: 1080] [Article Influence: 72.0] [Reference Citation Analysis (0)] |