Published online Oct 26, 2025. doi: 10.4252/wjsc.v17.i10.109284
Revised: June 24, 2025
Accepted: August 27, 2025
Published online: October 26, 2025
Processing time: 171 Days and 16 Hours
Mesenchymal stromal cells (MSCs) are renowned for their immunosuppressive properties, which make them widely used in managing excessive inflammation. Although CD146+ and CD146- MSCs exhibit similar morphological traits and surface marker expression levels, the specific characteristics and differential regulatory mechanisms of these two subtypes remain poorly understood. This knowledge gap has limited the precise application of MSCs in targeted thera
To compare the functional differences between CD146+ and CD146- MSCs and investigate the underlying mechanisms.
In this study, magnetic beads were used to sort umbilical cord-derived MSCs into CD146+ and CD146- subsets. The pro-angiogenic factors (hepatocyte growth factor, prostaglandin E2, vascular endothelial growth factor, angiopoietin-1) production and immunomodulatory effects on T lymphocyte subsets were evaluated in vitro. The therapeutic efficacy was assessed in an acute respiratory distress syndrome (ARDS) mouse model via tail vein injection.
Cytokine secretion and angiogenesis: CD146+ MSCs significantly increased the production of hepatocyte growth factor, prostaglandin E2, vascular endothelial growth factor, and angiopoietin-1 and exhibited increased pro-angiogenic activity in vitro. Immunomodulatory effects: CD146+ MSCs potently inhibited the differentiation and proliferation of pro-inflammatory T helper type 1/T helper type 17 cells while promoting the expansion of regulatory T cells during T lymphocyte activation. ARDS therapy: In a mouse ARDS model, compared with CD146- MSCs, CD146+ MSCs demonstrated superior therapeutic efficacy, as evidenced by improved clinical scores. Mechanistically, CD146+ MSCs activated the nuclear factor kappa B pathway, upregulated cyclooxygenase 2 expression, and facilitated damaged epithelial cell repair.
CD146+ MSCs show stronger ARDS therapeutic potential than CD146- MSCs via pro-angiogenic/immunomodulatory traits. Nuclear factor kappa B/cyclooxygenase 2 activation aids epithelial repair, highlighting CD146+ MSCs as promising targets.
Core Tip: Mesenchymal stromal cells (MSCs) are recognized for their immunosuppressive properties and are widely used to control excessive inflammation. This study demonstrated that, compared with CD146- MSCs, CD146+ MSCs exhibit greater secretion of pro-angiogenic factors and enhanced anti-inflammatory and immunomodulatory capacities. CD146+ MSCs repair epithelial cells by activating the nuclear factor kappa B/cyclooxygenase 2 pathway, thereby exerting superior therapeutic efficacy in the treatment of acute respiratory distress syndrome.
- Citation: Zhang YL, Wen DK, Wang SN, Tan Y, Ma HR. Melanoma cell adhesion molecule-positive mesenchymal stromal cells alleviate acute respiratory distress syndrome via nuclear factor kappa-B-mediated paracrine regulation. World J Stem Cells 2025; 17(10): 109284
- URL: https://www.wjgnet.com/1948-0210/full/v17/i10/109284.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v17.i10.109284
Mesenchymal stem cells (MSCs) are increasingly utilized in biomedical applications. Since MSCs do not express histocompatibility complexes or immunostimulatory mole
Owing to their heterogeneous nature, MSCs express different surface markers and exhibit distinct biological properties. As a result, isolating and identifying specific MSC subsets from different sources to evaluate their therapeutic efficacy for particular disorders is a key focus for future precision stem cell therapies. Various studies have isolated MSC subpopulations, such as those expressing CD146, CD106, Stro-1, SSEA-4, and CD271, to investigate their functions and biological characteristics[7,8].
CD146, also referred to as melanoma cell adhesion molecule, is a membrane glycoprotein that belongs to the immunoglobulin gene superfamily and was first identified in human melanoma cells[9]. It is particularly useful for identifying authentic MSCs, as it is widely distributed across the vascular network and plays a key role in angiogenesis and endothelial cell activity[10-12]. Additionally, CD146 is highly expressed in various cell types, including smooth muscle and vascular endothelial cells[13]. In regenerative medicine, the CD146+ MSC subset has shown enhanced biological functionality and therapeutic potential[14]. CD146 is detectable in bone marrow derived MSCs (BM-MSCs) and MSCs derived from various sources, such as adipose tissue, synovium, umbilical cord (UC), UC blood, placenta, dermis, periodontal ligament, and intervertebral discs. Notably, CD146+ BM-MSCs exhibit stronger migratory and homing abilities[15,16], as well as enhanced immunosuppressive and cytokine secretion functions[17]. CD146+ MSCs from adipose tissue demonstrate superior proliferation and angiogenesis capabilities[18-20], whereas those from dental pulp tissue show increased osteogenic differentiation potential[21]. Additionally, CD146+ MSCs exhibit lower heterogeneity[22], indicating that CD146+ MSCs cultured in vitro possess greater stemness and primitive characteristics.
Despite its promise, the molecular pathways regulated by CD146 in MSCs remain insufficiently understood. In this study, we aimed to examine how CD146 influences MSC effects and to explore the underlying mechanisms thereof. Our findings indicate that CD146+ MSCs can repair damaged lung epithelial cells and more effectively treat acute respiratory distress syndrome (ARDS) in mice. Mechanistically, CD146+ MSCs increase cyclooxygenase 2 (COX2) expression by activating the nuclear factor kappa B (NF-κB) pathway. These results emphasize the potential of CD146+ MSCs for ARDS treatment and provide insights into strategies to improve the therapeutic potential of MSCs.
The BM-MSC serum-free medium used in cell cultures was supplied by Dakewe Biotech Co. in China (DY culture medium), while the BM-MSC serum-free medium was obtained from Shandong Qilu Cell Therapy Engineering Technology Co. Ltd in China (YF culture medium). Additionally, 3D FloTrix MSCs in serum-free medium were provided by Cytoniche in China (HK culture medium). Cell culture reagents (RPMI 1640 medium, DMEM and fetal bovine serum) were obtained from Gibco Co. (Grand Island, NY, United States).
The following antibodies were purchased from Cell Signaling Technology (MA, United States): Phospho-NF-κB (#3031), NF-κB (#8242), protein kinase A (PKA) (#4782), phospho-PKA (p-PKA) (#4781), cyclic-AMP response element-binding protein (CREB) (#9197), phospho-CREB (#9198), zonula occludens-1 (ZO-1) (#5406), COX2 (#4842) and VE-cadherin (#2158). The FITC Annexin V Apoptosis Detection Kit with propidium iodide (PI) (C1062M) was obtained from Beyotime in China. The anti-GAPDH antibody (#ab20272) was acquired from Abcam in United Kingdom. Caffeic acid phenethyl ester (CAPE, S7414) was purchased from Selleck (TX, United States).
Four-week-old male BALB/c mice were obtained from the Laboratory Animal Centre of Beijing Vital River Laboratory Animal Technology Co., Ltd. All the animal experiments were conducted in accordance with laboratory animal care guidelines, and the experimental protocols were approved by the Biomedical Research Ethics Committee of the Institute of Biophysics, Chinese Academy of Sciences (Beijing, China). All the mouse experiments were performed without blinding and were conducted in accordance with the Public Health Service Policy on Humane Care and Use of Laboratory Animals. The Experimental Animal Ethics Committee of Army Medical University approved the experiments, and the ethical approval number was No. AMUWEC20210830.
Human UC derived MSCs (UCMSCs) were obtained from Shandong Qilu Cell Therapy Engineering Technology Co. Ltd. The source, screening, and collection process of human UCs at Yantai Yuhuangding Hospital Affiliated with Qingdao University met the national ethical requirements, and the ethical approval number was No. [2021]003. Moreover, the participants signed an informed consent form before participation.
In brief, UCs were obtained from patients who consented to deliver a full-term infant via cesarean section. After the arteries and veins of the UC were removed, the UC was cut as finely as possible with sterile surgical scissors, preferably no more than 1 mm in size. The UC fragments were resuspended in MSC serum-free medium, and the suspension was subsequently transferred to cell culture flasks and incubated at 37 °C in a 5% CO2 environment. After the cells had climbed, the medium was changed every 2 days. When the cells reached 80% to 90% confluence, they were harvested and frozen at -80 °C until use in the experiments.
The pathogenesis of ARDS involves multiple factors, including inflammatory disorders, alveolar epithelial cell injury, pulmonary capillary endothelial cell injury, microcirculatory disorders, and cell apoptosis. One of the core pathological features of ARDS is the destruction of the alveolar-capillary barrier. Pulmonary microvascular endothelial cells are important components of the alveolar-capillary barrier, whereas type II alveolar epithelial cells are another key component of the alveolar barrier and are responsible for secreting surfactant, maintaining the alveolar fluid balance, and participating in epithelial repair[23-25]. To explore the repair mechanism, we selected alveolar epithelial cells and pulmonary capillary endothelial cells as experimental subjects. Human lung microvascular endothelial cells (HULEC-5a) and human type II alveolar epithelial cells (HPAEpic-II-SV40) were purchased from Zhejiang Meisen Cell Technology Co., Ltd., in 2024. Both cell lines were verified by suppliers. Following the experiments, polymerase chain reaction (PCR) was performed to ensure the absence of mycoplasma contamination. The cells were cultured in DMEM (Gibco Life Technologies, NY, United States) containing 10% fetal bovine serum and 1% penicillin-streptomycin (Gibco, NY, United States) at 37 °C and 5% CO2.
The EasySep™ Release Human PE Positive Selection Kit (catalog #17654, STEMCELL, Canada) was used to isolate and separate CD146+ cells from human UCMSCs. PE-conjugated anti-CD146 antibody (361006, Biolegend, CA, United States) was then used to sort CD146+ cells, with 12%-32% of the UCMSCs identified as CD146+. Cell separation followed the EasySep™ protocol. Briefly, the cells were incubated for 30 minutes with an anti-CD146 antibody at room temperature. The selection cocktail was then added, and the sample was incubated for 5 minutes at ambient temperature. RapidSpheres™ was added for an additional 5 minutes of incubation at room temperature, after which the sample was sorted via FasySen™ (catalog #18000, STEMCELL, Canada). The sorted cells were subsequently cultured in a growth medium as described. MSCs were collected at passages 5-6 for further analysis.
RNA was extracted from cells via a TRIzol kit (TaKaRa, Shiga, Japan). For cDNA synthesis, 1 μg of RNA was reverse transcribed via the PrimeScript RT reagent Kit (TaKaRa, Shiga, Japan). Quantitative PCR (qPCR) was subsequently performed to quantify cDNA via a SYBR Green real-time quantitative PCR kit (TaKaRa, Shiga, Japan), with GAPDH used as the endogenous reference. The following primers (GenScript, NJ, United States) were used for qPCR: COX2 forward: 5’-AGGACTCTGCTCACGAAGGA-3’ and reverse: 5’-TGACATGGATTGGAACAGCA-3’; GAPDH forward: 5’-ACCCTTAAGAGGGATGCTGC-3’ and reverse: 5’-CCCAATACGGCCAAATCCGT-3’. The short hairpin RNA (shRNA) NF-κB (shNF-κB) sequence (5’-3’) was as follows: 5’-GGACCTACGAGACCTTCAA-3’. Each sample was tested in triplicate. Gene expression was calculated using the 2-∆∆Ct method from three independent assays.
For western blotting, cell lysis was performed with RIPA buffer containing a protease inhibitor cocktail, as previously described. Protein concentrations were determined using Enhanced BCA Protein Assay Reagent (Beyotime, Jiangsu, China). The proteins were separated by 10% sodium-dodecyl sulfate gel electrophoresis and transferred to polyvinylidene fluoride membranes (Bio-Rad, 162-0177, CA, United States). The membranes were blocked with 5% nonfat dry milk and then incubated with the primary antibody overnight at 4 °C, followed by a 2-hour incubation with HRP-labeled secondary antibody at room temperature. The protein bands were detected via an enhanced chemiluminescence system (Perkin-Elmer Life Sciences, Boston, MA, United States). Band intensities were analyzed with ImageJ software, with GAPDH used as the internal control.
The 96-well plates were coated with growth factor-reduced Matrigel (BD Biosciences, CA, United States) and allowed to polymerize for 30 minutes at 37 °C. For coculture experiments, human umbilical vein endothelial cells (C2517A, Lonza, MD, United States) (3 × 104/well) and CD146+/- MSCs (1000/well) were seeded simultaneously onto polymerized Matrigel. After 5 hours of culture in DMEM, cord formation was monitored via microscopy by examining seven randomly chosen fields at 7 × magnification (IX71, Olympus, Japan). The analysis included measurements of cord length per field of view and branch points. Three replicate wells and images were used for each group.
For T-cell subset phenotype analysis, CD146+/- MSCs (2 × 105/well) were plated in 12-well plates with RPMI1640 containing 10% fetal bovine serum (1 mL per well) and three wells per group. After 24 hours of culture, 1 × 106 peripheral blood mononuclear cells were added to each well at a 5:1 ratio of peripheral blood mononuclear cells to MSCs, followed by overnight incubation for 18 hours. T helper type 1 (Th1) /T helper type 17 (Th17) cells were assessed by flow cytometry after treatment with PIB [PMA (50 ng/mL), Iono (1 μg/mL), or BFA (10 μg/mL)] for 6 hours. Regulatory T (Treg) cells were cocultured for 5 days without PIB supplementation. Flow cytometry was used to detect the proportions of Th1 (CD3+/CD8-/interferon-γ+), Th17 [CD3+/CD8-/interleukin (IL)-17A+], and Treg cells (CD4+CD25+/FoxP3+).
Flow cytometry was utilized to assess cell apoptosis through Annexin V-FITC and PI double-staining, following established protocols (Beyotime, Shanghai, China). Briefly, 5 μL of Annexin V-FITC and 10 μL of PI (50 μg/mL) in 1 × binding buffer were added to stain 1 × 106 cells in the dark for 15 minutes at room temperature. A FACScan flow cytometer was used to measure the number of apoptotic cells, and FlowJo software was used for analysis. The cells were considered apoptotic if they were stained with Annexin V-FITC and/or PI. Each experiment was conducted in triplicate.
Endothelial permeability was measured in 6-well plates with 24 transwell inserts following specific protocols. After resuspension, CD146+ MSCs and CD146+ MSCs + CAPE (5 μmol/L) were seeded at 4 × 105 cells per well, and transwell inserts (0.48 μm) were seeded at 2 × 105 cells per well. HULEC-5a/HPAEpic-II-SV40 cells were resuscitated, forming a dense monolayer after 24 hours of culture. HULEC-5a/HPAEpic-II-SV40 cells were cocultured with MSCs, and the medium was replaced with lipopolysaccharide (LPS) (50 μg/mL) and 2% fetal bovine serum. After 48 hours of culture, the medium in the transwell inserts was carefully removed. FluoroBrite DMEM containing 100 μg/mL FITC-dextran (MW 40000) was added to the top chamber, and 2.5 mL of FluoroBrite DMEM was added to the bottom chamber. After 1 hour of incubation, the fluorescence intensity of the transwell culture medium was measured via a multifunctional enzyme-labeling assay. The permeability assay was repeated three times for each time point with three samples. For dose conversion related to cell density, 5 μg of LPS corresponds to 1 × 106 cells (106 cells/mL).
mRNA sequencing was performed to examine differentially expressed genes (DEGs) in CD146+ MSCs and CD146- MSCs using BMKCloud (http://www.biocloud.net). The DESeq2 R package (1.26.0) was employed to identify significant DEGs in both groups, applying thresholds of adjusted P < 0.05 and |log2 (fold change)| > 1. Heatmap version 1.0.10 was used for the generation of the heatmap. Kyoto Encyclopedia of Genes and Genomes analysis was conducted to identify pathways enriched by DEGs using the clusterProfiler R package (v3.13.0), with a significance threshold of P < 0.05. Furthermore, gene set enrichment analysis was performed using gene set enrichment analysis software (v2.2.3, http://software.broadinstitute.org/gsea/downloads.jsp) and MSigDB-curated gene sets (c2.cp.kegg.v6.2.symbols.gmt).
CAPE was dissolved in DMSO to prepare a 10 mmol/L stock solution. For the experiments, the solution was diluted to a working concentration of 5 μM in serum-free medium, with a final DMSO concentration ≤ 0.1%. The control group was supplemented with an equal volume of DMSO simultaneously. The cells were pretreated with starvation medium for 1 hour, followed by incubation with CAPE for 24 hours, and control groups were established accordingly[26,27].
CD146+ MSCs were treated with caffeolate phenylethyl (CAPE) at a concentration of 5 μmol/L (Supplementary Figure 1). After 24 hours of CAPE treatment, COX2 mRNA expression was assessed via reverse transcription-qPCR, and COX2, p-NF-κB, and signal transducer and activator of transcription 5 protein levels were evaluated using western blotting. Additionally, the secretion levels of the cytokines hepatocyte growth factor (HGF)/prostaglandin E2 (PGE2)/angiopoietin-1 (Ang-1) and vascular endothelial growth factor (VEGF) by CD146+ MSCs were measured using enzyme-linked immunosorbent assay (ELISA). Each assay was performed in triplicate.
Lentiviral vectors encoding three different shRNAs, targeting human NF-κB {named as shNF-κB [shNF-κB(p65)]}, were purchased from Shanghai Genechem of China (Shanghai, China). CD146+ MSCs were transfected with the shSENP1 Lentivirus or empty lentiviral vector (named shCon) with polybrene (8 μg/mL, Sigma-Aldrich, MO, United States). The transfected cells were then subjected to selection with puromycin (3.0 μg/mL) for 4-5 days. Knockdown of NF-κB in stable cells was verified by reverse transcription-qPCR and western blotting.
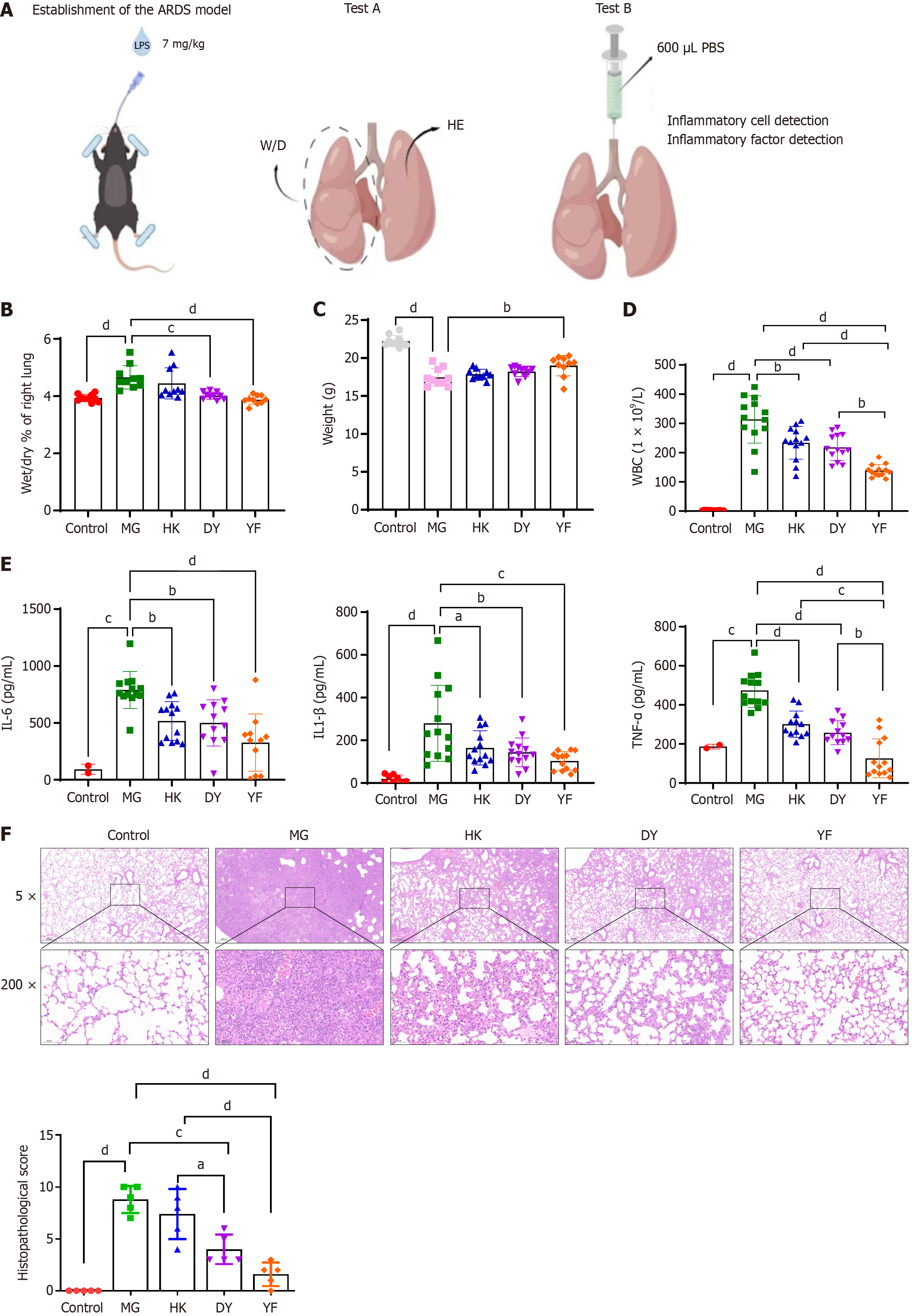
A total of 50 mice were selected for test A, and another 50 were selected for test B. The mice were randomly divided into 5 groups on the basis of body weight: Normal control and model control (MG). After the ARDS model was established, MSCs cultured in media from different brands were injected via the caudal vein to form different groups: The DY group, the YF group, and the HK group (Figure 1A and Supplementary Figure 2).
The mice were fasted overnight but were allowed to drink water. All groups, except for the normal control group, received an LPS solution injected into the trachea to establish the ARDS model. Anesthesia was induced by intraperitoneal injection of tribromoethanol solution. After anesthesia, a tracheal intubation operating table was used to suspend the upper teeth of each mouse and extend its tongue. The glottic fissure was located with the aid of a lamp, and a tracheal drug delivery hose was inserted through it into the trachea. LPS solution (7 mg/kg) was administered via a pipette gun into the hose as the mouse breathed naturally[25,28,29]. Additionally, either 0.9% sodium chloride or MSCs (5 × 106 cells/kg) were administered once within 1 hour after modeling and again at 24 hours after tail vein injection (Figure 1A).
On the dissection day of experiment A, the mice in test A were weighed to determine the differences between the groups. At 48 hours post-administration, the test A mice were euthanized via the cervical dislocation method, followed by lung removal. The right lung weights were recorded initially, after which the lungs were dried in a 37 °C electric blast drying oven for 24 hours before being reweighed. This allowed for the calculation of the wet-dry weight ratios of the right lungs for each mouse in the experimental groups. ZO-1 and VE-cadherin expression in lung tissue was measured via immunohistochemistry, with 5 animals in each group. The experiments were performed without blinding and in compliance with the Public Health Service Policy on Humane Care and Use of Laboratory Animals.
Bronchoalveolar lavage fluid (BALF) was collected from test B mice 48 hours after administration. 500 μL/BALF was retrieved, and the white blood cell count was determined using an automatic blood cell analyzer. The remaining BALF was centrifuged at 1500 rpm for 5 minutes at 4 °C to separate the supernatants. The levels of IL-6, IL-1β, and tumor necrosis factor (TNF)-α in the supernatants were then measured using ELISA kits following the manufacturer’s instructions. A multifunctional enzyme marker was used to detect cytokine levels in the supernatants following centrifugation of the BALF.
After 48 hours of fixation in 10% formalin, the mouse lung tissues were subjected to gradient ethanol dehydration, wax impregnation, slicing, deparaffinization by baking, hematoxylin and eosin staining, and rubber sealing. The tissues were then examined and photographed under a microscope. The pathological changes in the lung tissue of each group were assessed. On the basis of the extent of lung tissue damage, including congestion, alveolar cavity destruction, thickening of the alveolar walls, and neutrophil infiltration, lung injury was classified into five levels: 0 (no injury), 1 (< 25% injury), 2 (25%-50% injury), 3 (50%-75% injury), and 4 (> 75% injury). The acute lung injury/ARDS pathological score was calculated by summing the individual lesion scores, with higher values indicating more severe injury.
Immunohistochemistry was performed to assess VE-cadherin and ZO-1 expression, as previously described[30]. Briefly, tissue samples were fixed in formalin, embedded in paraffin, and then sectioned into 4-μm slices, followed by deparaffinization with xylene and rehydration with alcohol. After antigen retrieval, the sections were incubated with anti-VE-cadherin and anti-ZO-1 primary antibodies at 4 °C for 24 hours. After rinsing with phosphate buffered saline, the sections were incubated for 1 hour at room temperature with peroxidase-labeled goat anti-human/mouse secondary antibodies (1:300). Five random fields per section were selected and observed under a light microscope (100X, BX51, Olympus, Japan) via a digital camera (DP72, Olympus, Japan). VE-cadherin and ZO-1 expression levels were quantified via the histochemistry score (H-score), which was calculated by multiplying the percentage of positive cells (PI) by the staining intensity (i+1).
Data normality was assessed using the Shapiro–Wilk test. Continuous outcomes were reported as mean ± SEM. For comparisons of gene expression levels, unpaired t tests were used. Differences in histological measurements across multiple groups were analyzed using one-way analysis of variance (ANOVA), followed by the Holm–Šidák method to correct for multiple comparisons and control the familywise type I error rate. Statistical significance was interpreted at four levels: P < 0.05, P < 0.01, P < 0.001, and P < 0.0001. All statistical analyses were conducted using GraphPad Prism software (version 9.0; GraphPad, Inc., La Jolla, CA, United States).
The right lungs of the mice in experiment A were dissected to determine the ratio of dry to wet weight of the right lung in each group. The ratio of the right lung in the MG group was significantly greater than that in the normal control group
Mouse weights were recorded on the necropsy day (Figure 1C). Compared with the normal control group, the MG group presented significant weight loss (dP < 0.0001), indicating that the LPS model caused weight reduction in the mice. In comparison, the YF treatment group presented a noticeable increase in weight compared with the MG group (bP < 0.01). Although weight increases were observed in the other treatment groups, no statistically significant differences were found.
Alveolar lavage fluid was collected from the mice in group B to examine changes in white blood cell counts. Compared with the normal control group, the MG group presented a significant increase in leukocyte number (cP < 0.001), whereas all the treatment groups presented a noticeable reduction in leukocyte count compared with the MG group (bP < 0.01, dP < 0.0001), with the YF treatment group showing the lowest leukocyte count (Figure 1D). These findings suggest that various MSC treatments can partially reduce the number of inflammatory cells in the BALF of model mice. Furthermore, the IL-1β, IL-6, and TNF-α levels in the BALF were significantly elevated compared with those in the control group (cP < 0.001, dP < 0.0001). Compared with those in the model group, the IL-1β levels were notably lower in the treatment groups, with the YF treatment group showing the lowest levels. These findings indicate that MSC treatments may help reduce the expression of inflammatory cytokines (Figure 1E).
Pathological analysis revealed that the control group had a well-preserved lung structure, clear alveolar cavities, and no signs of hyperemia, edema, or inflammatory cell infiltration in the alveolar walls. Conversely, the model group exhibited damaged alveolar structure, with hyperemia and edema in the alveolar walls, accompanied by inflammatory cell infiltration, indicating significant lung injury. Compared with that in the model group, lung tissue damage in the treatment groups was less severe (Figure 1F). The treatment effect in the YF group was the greatest. MG: Vehicle administered via caudal vein injection after establishing the ARDS model; DY: MSCs cultured in medium from Dakewe Biotech Co., administered via caudal vein injection after establishing the ARDS model; YF: MSCs cultured in medium from Shandong Qilu Cell Therapy Engineering Technology Co. Ltd., administered via caudal vein injection under the same model; HK: MSCs cultured in medium from Cytoniche, administered via caudal vein injection in the ARDS model.
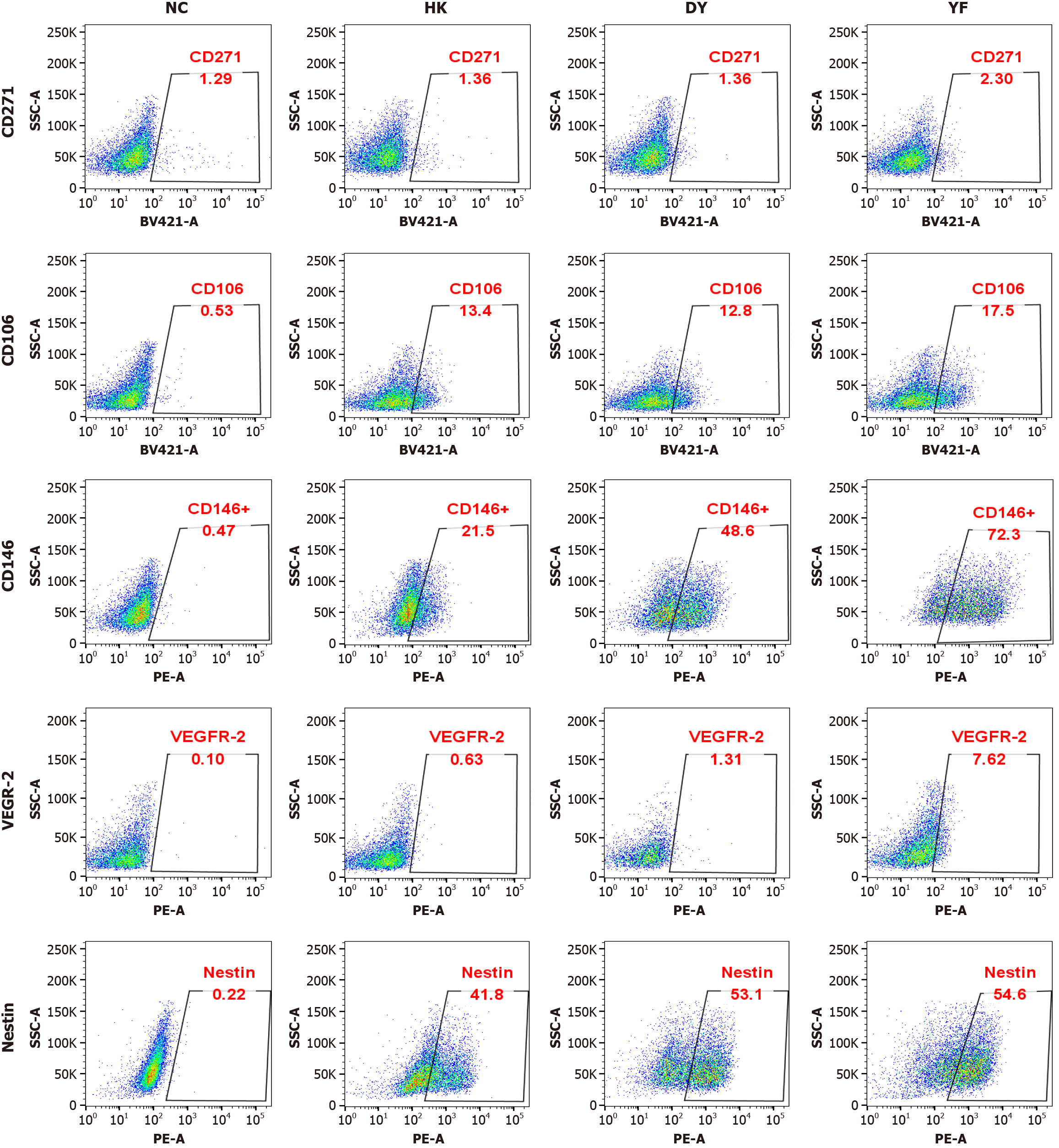
To determine which cell subsets in the YF culture system contribute to the improved effectiveness of MSCs in ARDS treatment, flow cytometry was employed to analyze protein changes. The findings revealed a significant increase in CD146 expression following culture in the YF system (Figure 2).
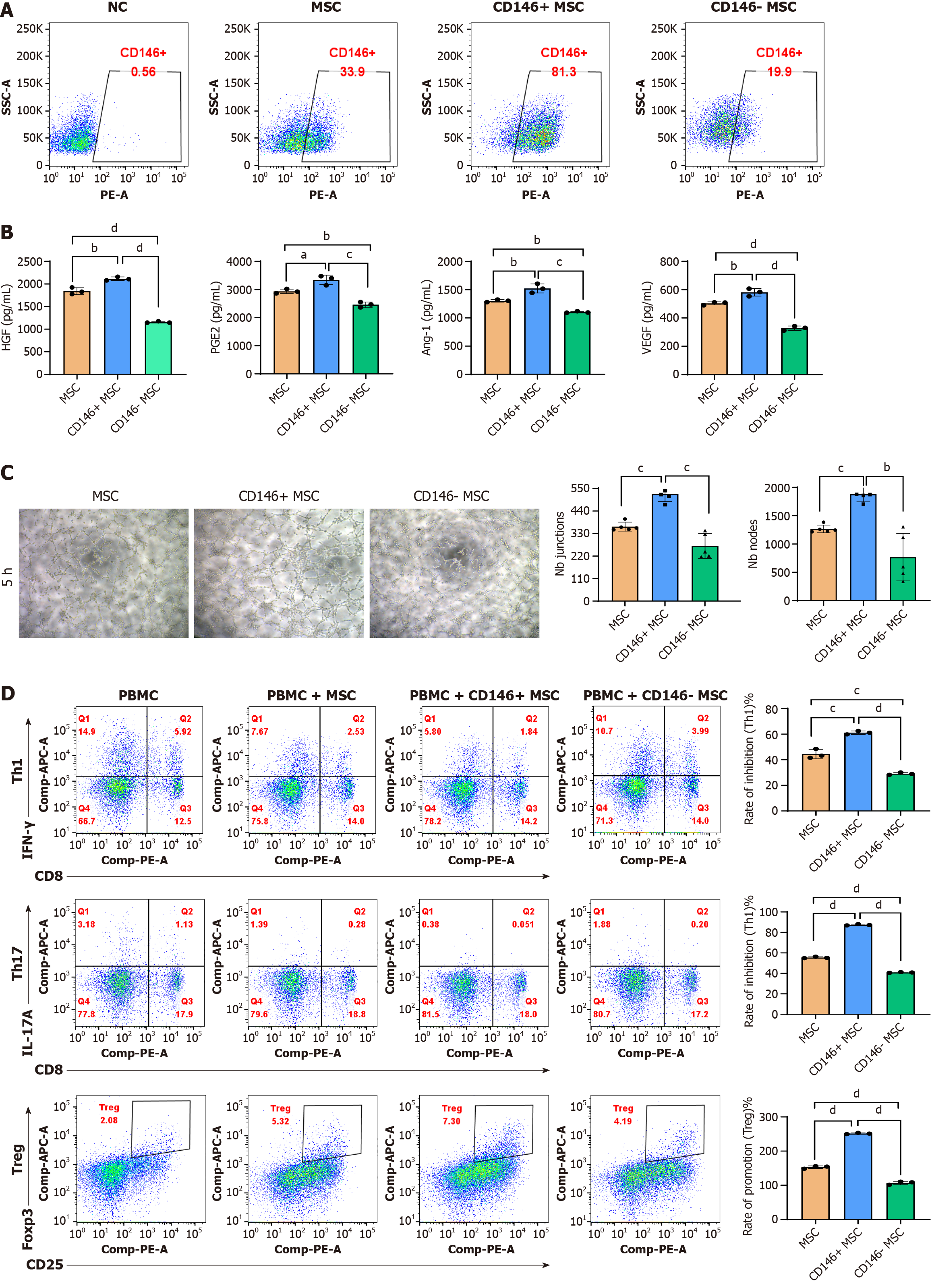
CD146+/- MSCs were separated via an immune magnetic bead system, and flow cytometry was performed to evaluate cell purity. Prior to separation, CD146+ MSCs typically represented 33.9%-81.3% of the population (Figure 3A). To assess immunomodulatory activity, BM-MSCs were inflammatory primed to initiate a response, followed by profiling of the secreted proteins and detection of transcriptional changes. The secretion levels of HGF, PGE-2, VEGF, and Ang-1 in both MSC subsets under different conditions were then measured via ELISA. The results indicated that the secretion of these factors was significantly greater in the CD146+ MSCs than in the CD146- MSCs (Figure 3B).
To compare the angiogenic ability of CD146+ MSCs and CD146- MSCs, the tubular structure of human UCMSCs was detected via Matrigel, and the results are shown in Figure 3. The morphology of the CD146- MSCs was spindle shaped and polygonal, and few cells formed processes. The connections between the cells were incomplete, and the CD146-MSCs could not form lumen-like structures. The other two groups of cells could form cell-cell connections and complete lumen-like structures. Among them, the combined induction group formed more lumen-like structures and cross-linked with each other to form a network structure, as shown in Figure 3C. ImageJ software was used to analyze the number of tubes and nodes formed by the cells, and the results revealed that the number in the CD146+ MSC group was significantly greater than that in the CD146- MSC group; moreover, the number in the CD146+ MSC group was significantly greater than that in the unsorted MSC group.
In the study of MSC immunoregulation, dynamic changes in T-cell subsets (such as Th1/Th17/Treg) are key indicators for evaluating immune balance[31-33]. MSCs exert immunomodulatory effects by inhibiting the differentiation of Th1 and Th17 cells (pro-inflammatory) and promoting the differentiation of Tregs (immunosuppressive). Therefore, in this study, the proportions of Th1 (CD3+/CD8-/interferon-γ+), Th17 (CD3+/CD8-/IL-17A+), and Treg (CD4+/CD25+/FoxP3+) cells were determined to verify the regulatory effect of CD146+/- MSCs on the direction of T-cell differentiation at the cellular phenotype level. Compared with the CD146- MSC coculture, the CD146+ MSC coculture more effectively inhibited Th1/Th17 cells and promoted Treg cells activation in vitro (dP < 0.0001) (Figure 3D).
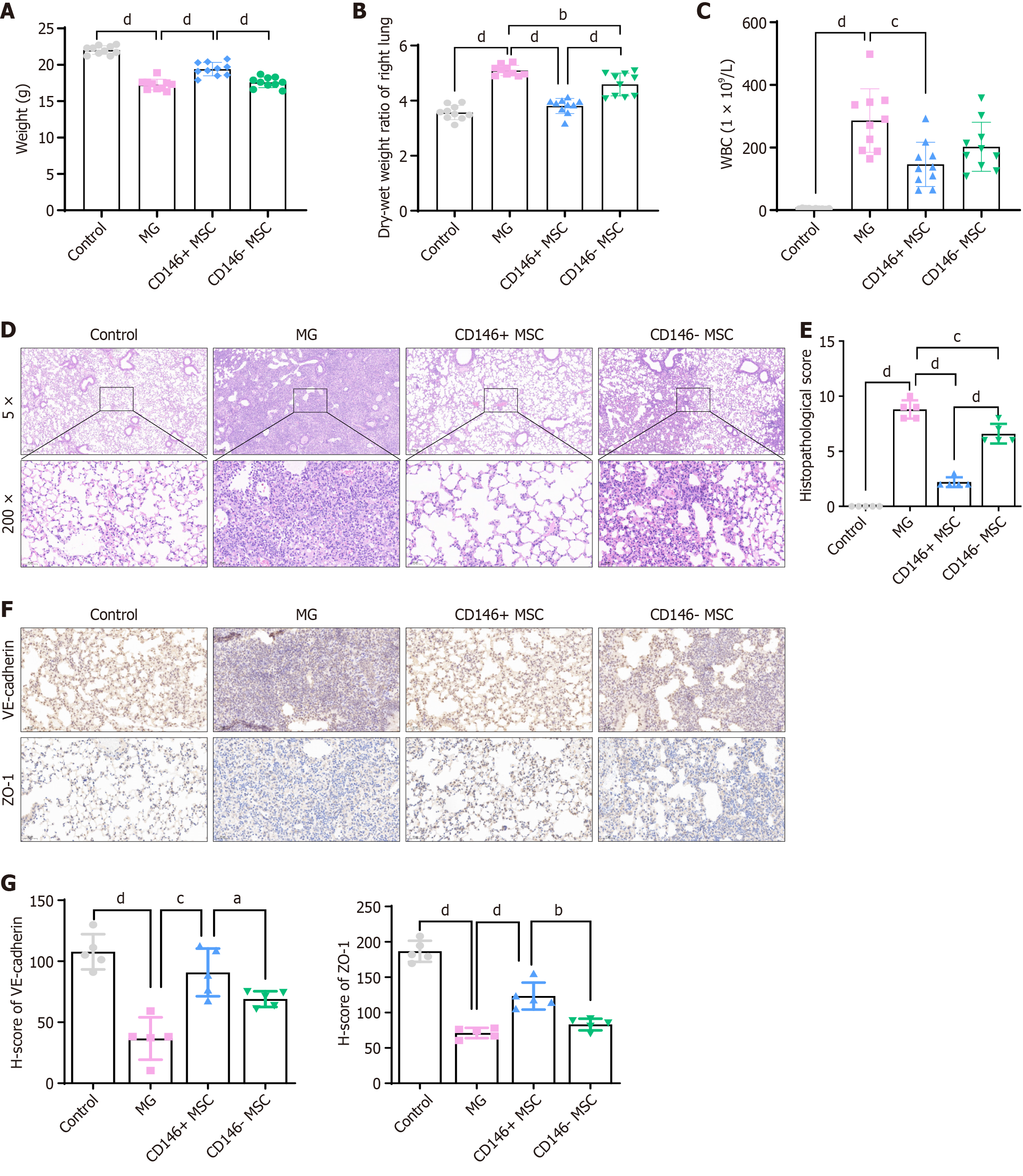
The therapeutic effect of CD146+ MSCs was further assessed in an LPS-induced ARDS model. Various cell subpopulations were injected into the mouse abdominal cavity, and after 48 hours, the CD146+ MSC group showed notable improvements in body weight, wet-dry weight ratios, and white blood cell count (Figure 4A-C). Histological staining (Figure 4D and E) revealed lung damage in ARDS mice treated with CD146+/- MSCs. The pathological scores were greater in the CD146- MSC group than in the saline or CD146+ MSC groups. Moreover, VE-cadherin and ZO-1 protein levels were significantly greater in the CD146+ MSC group than in the CD146- MSC group (Figure 4F and G), corroborating the results of the in vitro analyses.
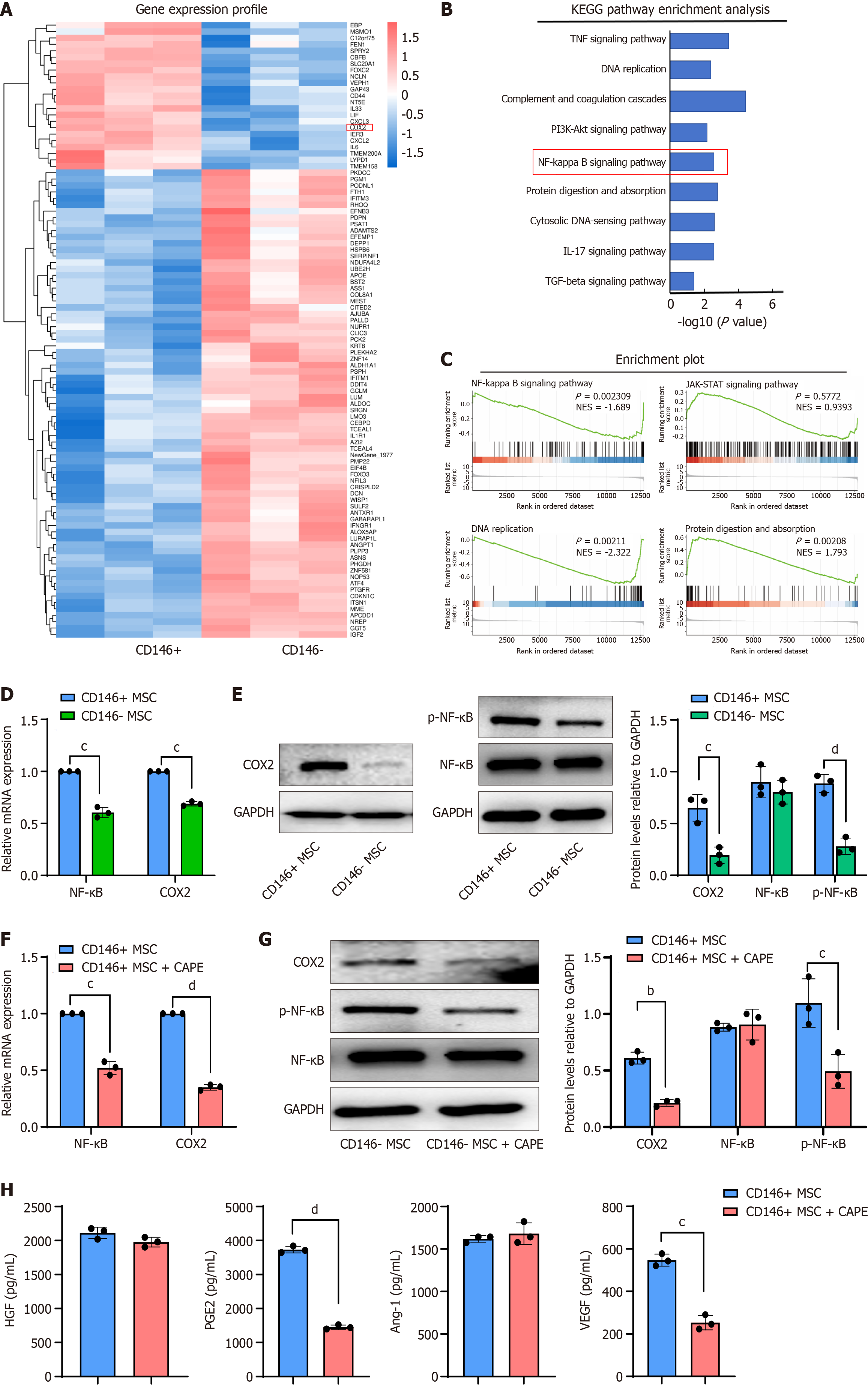
To explore the mechanism underlying the function of CD146 in MSCs, CD146+ MSCs and CD146- MSCs were subjected to mRNA sequencing after sorting. As depicted in Figure 5A, a significant number of DEGs were observed between the CD146+ MSC and CD146- MSC groups. Kyoto Encyclopedia of Genes and Genomes analysis revealed that these DEGs were enriched in multiple pathways, including the NF-κB pathway (Figure 5B).
Among the DEGs, COX2, an oxidase reductase, was found to be regulated through the NF-κB pathway. Our results indicated that COX2 mRNA expression was significantly lower in CD146- MSCs than in CD146+ MSCs on the basis of data from the Gene Expression Omnibus database (Figure 5C). Additionally, COX2 mRNA and protein levels were significantly increased in CD146+ MSCs (Figure 5D and E). CD146-MSCs also exhibited dysregulated p-NF-κB expression, with no significant effect on NF-κB levels (Figure 5E).
As a recognized NF-κB inhibitor, the mechanism of action of CAPE has been confirmed by multiple studies: It specifically inhibits the activity of the IkappaB kinase complex, blocks the phosphorylation and degradation of IkappaBα, and then inhibits the nuclear translocation and transcriptional activation of the NF-κB dimer[34]. Activated NF-κB (P65) needs to enter the nucleus to initiate target gene transcription[35]. CAPE directly inhibits the nuclear transport of p65 by interfering with the interaction between the nuclear localization signal of p65 and importin-α/β, thereby reducing the expression of its downstream genes (such as COX2)[36]. CAPE inhibits NF-κB phosphorylation[35,37], prompting us to treat CD146+ MSCs with CAPE and analyze NF-κB phosphorylation levels. The results indicated that CAPE inhibited NF-κB phosphorylation in CD146+ MSCs and reduced COX2 expression (Figure 5F and G). Additionally, the secretion of cytokines from CD146+ MSCs was evaluated, and CAPE was found to partially reverse the changes in cytokine secretion in CD146+ MSCs (Figure 5H).
To investigate the mechanism of action of NF-κB in CD146+ MSCs, we found that both the mRNA and protein levels of COX2 were significantly decreased after p65 knockdown (Supplementary Figure 3A and B). We also detected the factors secreted by these cell lines and found that the levels of HGF, PGE2, and VEGF were significantly decreased after p65 knockdown (Supplementary Figure 3C). Taken together, these results indicate that CD146+ MSCs promote COX2 expression by activating the NF-κB pathway. Overall, these findings suggest that CD146+ MSCs upregulate COX2 through the activation of the NF-κB pathway.
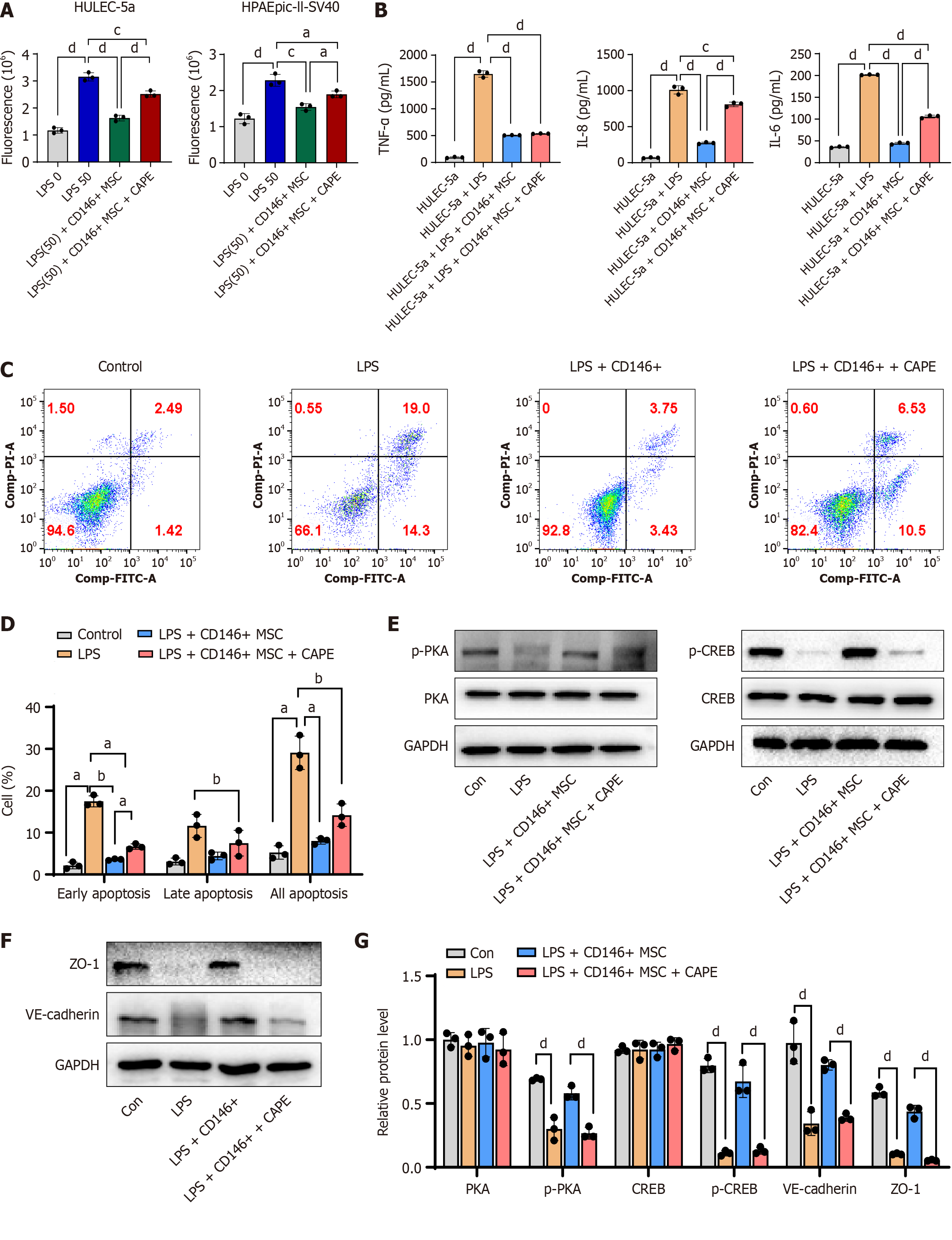
A cell permeability assay was used to evaluate the effect of MSCs on the barrier function of LPS-induced HULEC-5a cells. Specifically, by detecting the fluorescence intensity of 4 kDa FITC-dextran passing through the transwell membrane, the changes in permeability of the cell monolayer were quantified to verify whether MSCs improve endothelial barrier function by maintaining the integrity of tight junctions. An excessive inflammatory response is a core pathological feature of ARDS that can directly mediate endothelial cell injury, and this assay can verify the immunomodulatory effect of MSCs. Endothelial cell apoptosis assays revealed that CD146+ MSCs support the survival of endothelial cells.
We evaluated permeability, inflammatory factor secretion, and apoptosis in HULEC-5a cells and found that CD146+ MSCs reduced cell damage. Furthermore, CAPE partially restored the effects on HULEC-5a/HPAEpic-II-SV40 permeability, HULEC-5a inflammatory factor secretion, and apoptosis (Figure 6A-D). In addition, p-PKA, p-CREB, VE-cadherin, and ZO-1 Levels were significantly greater in the CD146+ MSC group than in the LPS group, with CAPE partially reversing these effects in CD146+ MSCs (Figure 6E-G). Overall, these results suggest that CD146+ MSCs promote lung epithelial cell repair through the activation of the NF-κB pathway.
ARDS is an acute, widespread lung injury caused by various intrapulmonary and extrapulmonary factors that may progress to acute respiratory failure. The primary pathological features include damage to pulmonary vascular endothelial cells, extensive lung inflammation, and osmotic pulmonary edema due to increased pulmonary microvascular permeability[38]. As a result, effectively reducing pulmonary inflammation and protecting endothelial barrier function in the pulmonary microvasculature are crucial approaches to lowering ARDS mortality. Vascular permeability serves as a selective mechanism for maintaining material exchange between blood vessels, tissues, and organs. Disruption of intercellular junctions and the formation of gaps between endothelial cells are key indicators of increased vascular permeability[39]. The functional status of endothelial cells is a major determinant of vascular permeability, and the integrity of endothelial cell junctions is regulated by proteins involved in tight and adherens junctions[38,40,41]. ZO-1 and VE-cadherin are essential components of tight and adherens junctions and are frequently used as indicators for assessing changes in endothelial cell permeability[42].
CD146, a membrane glycoprotein, is highly expressed in various cell types and plays a pivotal role in the activity of vascular endothelial cells and angiogenesis[11,12]. Moreover, CD146 is considered a valuable surface marker for identifying specific MSC subsets[43]. Studies on CD146+ stem cell subsets suggest that CD146 can be used as a quality marker for these MSC populations[44]. In regenerative medicine, the CD146+ MSC subpopulation has demonstrated remarkable biological and therapeutic potential[44,45]. MSCs secrete a variety of growth factors, with HGF and VEGF[46,47] being especially important in regulating vascular endothelial permeability[48,49]. Additionally, inflammatory factors and injury mediators, such as LPS, can induce the production of VEGF and HGF in MSCs[50]. As a result, the paracrine secretion of VEGF and HGF may play a crucial role in regulating vascular permeability during acute lung injury. Moreover, PGE2 alleviates vascular leakage and supports barrier function in human microvascular pulmonary endothelial cells[51,52]. PGE2 also protects against LPS-induced pneumonia in mouse models by decreasing TNF-α secretion and neutrophil recruitment[53]. However, the mechanisms underlying the enhanced functionality of CD146+ MSCs remain unclear.
In this study, we unexpectedly discovered that MSCs cultured in different media significantly differed in their ability to treat ARDS mice and that the YF group presented a greater advantage. Moreover, the expression of MSC CD146 in the YF group was significantly greater than that in the other groups and that CD146+ MSCs could regulate the expression of COX2. Therefore, we hypothesize that there is a potential signaling axis, the CD146/NF-κB/COX2 axis, in CD146+ MSCs. Activation of the NF-κB pathway and upregulation of COX2 contribute to the repair of damaged epithelial cells. Moreover, we observed enhanced pro-angiogenic and anti-inflammatory autocrine factor production: CD146+ MSCs expressed increased levels of HGF, PGE2, VEGF, and Ang-1, contributing to a stronger pro-angiogenic effect and anti-inflammatory effect.
COX, a key enzyme in arachidonic acid metabolism, has two isoforms: COX-1 and COX-2. COX-2 is an inducible enzyme not found under normal physiological conditions but is upregulated during inflammation and tumorigenesis. COX-2 expression is expressed in various inflammatory and traumatic diseases, as well as in multiple tumor types[54-57], and plays a significant role in the progression of osteoarthritis, particularly in cartilage inflammation and joint destruction. COX-2 has been implicated in both the initiation and resolution phases of inflammation, where it initiates the inflammatory response at the early stages and helps resolve inflammation during the recovery phase. PGE2, produced from arachidonic acid by COX-2, is involved in regulating blood pressure and protecting the gastrointestinal mucosa under normal conditions. Additionally, PGE2 acts as an immunosuppressive mediator, inhibiting pro-inflammatory factors in monocytes and promoting IL-10 expression. VEGF, a potent stimulator of osteogenesis, is crucial for the coupling of osteogenesis and angiogenesis[58]. COX-2, a rate-limiting enzyme in prostaglandin synthesis, is expressed under inflammatory, tumorigenic, and hypoxic conditions[59]. COX-2 activation increases the production of both PGE2 and VEGF in vitro, whereas inhibiting COX-2 reduces VEGF expression in vivo[60]. Moreover, MSC-derived conditioned media has been shown to support endothelial cell growth, promote tube formation, and reduce cell apoptosis, largely through the action of VEGF[61]. MSCs improve pulmonary endothelial barrier injury in ARDS through various pathways, including maintaining barrier integrity, inhibiting excessive inflammatory responses, and reducing endothelial cell apoptosis. Therefore, we designed three in vitro experiments to verify the therapeutic mechanisms of CD146+ MSCs from different pathological links.
Pulmonary endothelial cell permeability assay: This assay quantified the reparative effect of MSCs on LPS-induced endothelial barrier disruption. An excessive inflammatory response is a core pathological feature of ARDS that directly mediates endothelial cell injury. This study verified the immunomodulatory effect of CD146+ MSCs. Endothelial cell apoptosis assays clarified the support of CD146+ MSCs for the survival of endothelial cells. Together, these three experiments constitute a complete logical chain of “preventing injury/repairing structure/maintaining function”, demonstrating that CD146+ MSCs have sufficient advantages in improving pulmonary endothelial barrier injury in ARDS. These observations were confirmed in an in vitro model.
In summary, we found that, compared with CD146- MSCs, CD146+ MSCs demonstrated superior immunomodulatory abilities and cytokine secretion. This may be attributed to the activation of the NF-κB pathway and increased COX2 expression. Therefore, CD146 expression plays a pivotal role in influencing the stem cell properties of MSCs, likely contributing to the enhancement of MSC-mediated regulatory mechanisms.
This study demonstrated that, compared with CD146- MSCs, CD146- MSCs exhibit superior therapeutic efficacy in ARDS, primarily due to their increased secretory capacity and immunomodulatory properties. Specifically, CD146+ MSCs effectively promoted alveolar vascular regeneration and mitigated excessive inflammatory responses, which are crucial for lung tissue repair, in ARDS models. Mechanistically, the activation of the NF-κB/COX2 signaling pathway was identified as a key molecular event underlying the epithelial reparative functions of CD146+ MSCs; this pathway regulated the secretion of PGE2 and angiogenic factors, thereby facilitating alveolar epithelial cell proliferation and barrier restoration. These findings not only reveal the subtype-specific functional divergence in MSCs and highlight CD146 as a pivotal marker for isolating MSCs with enhanced therapeutic potential but also establish CD146+ MSCs as a promising cell subtype for targeted ARDS therapy. Moreover, the identification of the NF-κB/COX2 axis as a regulatory hub for MSC-mediated repair provides novel insights into the molecular mechanisms governing MSC heterogeneity, potentially informing the development of precision cell therapies and pharmacologic strategies to optimize therapeutic outcomes in ARDS and other inflammatory diseases.
| 1. | Han Y, Li X, Zhang Y, Han Y, Chang F, Ding J. Mesenchymal Stem Cells for Regenerative Medicine. Cells. 2019;8:886. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 591] [Cited by in RCA: 775] [Article Influence: 110.7] [Reference Citation Analysis (0)] |
| 2. | Van Nguyen TT, Vu NB, Van Pham P. Mesenchymal Stem Cell Transplantation for Ischemic Diseases: Mechanisms and Challenges. Tissue Eng Regen Med. 2021;18:587-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 3. | Burnham AJ, Daley-Bauer LP, Horwitz EM. Mesenchymal stromal cells in hematopoietic cell transplantation. Blood Adv. 2020;4:5877-5887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 4. | Zheng W, Li H, Hu K, Li L, Bei M. Chondromalacia patellae: current options and emerging cell therapies. Stem Cell Res Ther. 2021;12:412. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 5. | Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11055] [Cited by in RCA: 13019] [Article Influence: 685.2] [Reference Citation Analysis (12)] |
| 6. | Costa LA, Eiro N, Fraile M, Gonzalez LO, Saá J, Garcia-Portabella P, Vega B, Schneider J, Vizoso FJ. Functional heterogeneity of mesenchymal stem cells from natural niches to culture conditions: implications for further clinical uses. Cell Mol Life Sci. 2021;78:447-467. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 226] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 7. | Lv FJ, Tuan RS, Cheung KM, Leung VY. Concise review: the surface markers and identity of human mesenchymal stem cells. Stem Cells. 2014;32:1408-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 644] [Cited by in RCA: 759] [Article Influence: 69.0] [Reference Citation Analysis (0)] |
| 8. | Bikorimana JP, Saad W, Abusarah J, Lahrichi M, Talbot S, Shammaa R, Rafei M. CD146 Defines a Mesenchymal Stromal Cell Subpopulation with Enhanced Suppressive Properties. Cells. 2022;11:2263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 9. | Lehmann JM, Holzmann B, Breitbart EW, Schmiegelow P, Riethmüller G, Johnson JP. Discrimination between benign and malignant cells of melanocytic lineage by two novel antigens, a glycoprotein with a molecular weight of 113,000 and a protein with a molecular weight of 76,000. Cancer Res. 1987;47:841-845. [PubMed] |
| 10. | Lehmann JM, Riethmüller G, Johnson JP. MUC18, a marker of tumor progression in human melanoma, shows sequence similarity to the neural cell adhesion molecules of the immunoglobulin superfamily. Proc Natl Acad Sci U S A. 1989;86:9891-9895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 336] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 11. | Lerman DA, Diaz M, Peault B. Changes in coexpression of pericytes and endogenous cardiac progenitor cells from heart development to disease state. Eur Heart J. 2018;39:P1850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Chen J, Luo Y, Hui H, Cai T, Huang H, Yang F, Feng J, Zhang J, Yan X. CD146 coordinates brain endothelial cell-pericyte communication for blood-brain barrier development. Proc Natl Acad Sci U S A. 2017;114:E7622-E7631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 161] [Cited by in RCA: 156] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 13. | Wang Z, Yan X. CD146, a multi-functional molecule beyond adhesion. Cancer Lett. 2013;330:150-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 231] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 14. | Wu CC, Liu FL, Sytwu HK, Tsai CY, Chang DM. CD146+ mesenchymal stem cells display greater therapeutic potential than CD146- cells for treating collagen-induced arthritis in mice. Stem Cell Res Ther. 2016;7:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 83] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 15. | Harkness L, Zaher W, Ditzel N, Isa A, Kassem M. CD146/MCAM defines functionality of human bone marrow stromal stem cell populations. Stem Cell Res Ther. 2016;7:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 74] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 16. | Wangler S, Menzel U, Li Z, Ma J, Hoppe S, Benneker LM, Alini M, Grad S, Peroglio M. CD146/MCAM distinguishes stem cell subpopulations with distinct migration and regenerative potential in degenerative intervertebral discs. Osteoarthritis Cartilage. 2019;27:1094-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 17. | Bowles AC, Kouroupis D, Willman MA, Perucca Orfei C, Agarwal A, Correa D. Signature quality attributes of CD146(+) mesenchymal stem/stromal cells correlate with high therapeutic and secretory potency. Stem Cells. 2020;38:1034-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 72] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 18. | Lee NE, Kim SJ, Yang SJ, Joo SY, Park H, Lee KW, Yang HM, Park JB. Comparative characterization of mesenchymal stromal cells from multiple abdominal adipose tissues and enrichment of angiogenic ability via CD146 molecule. Cytotherapy. 2017;19:170-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 19. | Lauvrud AT, Kelk P, Wiberg M, Kingham PJ. Characterization of human adipose tissue-derived stem cells with enhanced angiogenic and adipogenic properties. J Tissue Eng Regen Med. 2017;11:2490-2502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 20. | Gomes JP, Coatti GC, Valadares MC, Assoni AF, Pelatti MV, Secco M, Zatz M. Human Adipose-Derived CD146(+) Stem Cells Increase Life Span of a Muscular Dystrophy Mouse Model More Efficiently than Mesenchymal Stromal Cells. DNA Cell Biol. 2018;37:798-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Ulrich C, Abruzzese T, Maerz JK, Ruh M, Amend B, Benz K, Rolauffs B, Abele H, Hart ML, Aicher WK. Human Placenta-Derived CD146-Positive Mesenchymal Stromal Cells Display a Distinct Osteogenic Differentiation Potential. Stem Cells Dev. 2015;24:1558-1569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 22. | Wu YX, Jing XZ, Sun Y, Ye YP, Guo JC, Huang JM, Xiang W, Zhang JM, Guo FJ. CD146+ skeletal stem cells from growth plate exhibit specific chondrogenic differentiation capacity in vitro. Mol Med Rep. 2017;16:8019-8028. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Allen RJ, Guillen-Guio B, Oldham JM, Ma SF, Dressen A, Paynton ML, Kraven LM, Obeidat M, Li X, Ng M, Braybrooke R, Molina-Molina M, Hobbs BD, Putman RK, Sakornsakolpat P, Booth HL, Fahy WA, Hart SP, Hill MR, Hirani N, Hubbard RB, McAnulty RJ, Millar AB, Navaratnam V, Oballa E, Parfrey H, Saini G, Whyte MKB, Zhang Y, Kaminski N, Adegunsoye A, Strek ME, Neighbors M, Sheng XR, Gudmundsson G, Gudnason V, Hatabu H, Lederer DJ, Manichaikul A, Newell JD Jr, O'Connor GT, Ortega VE, Xu H, Fingerlin TE, Bossé Y, Hao K, Joubert P, Nickle DC, Sin DD, Timens W, Furniss D, Morris AP, Zondervan KT, Hall IP, Sayers I, Tobin MD, Maher TM, Cho MH, Hunninghake GM, Schwartz DA, Yaspan BL, Molyneaux PL, Flores C, Noth I, Jenkins RG, Wain LV. Genome-Wide Association Study of Susceptibility to Idiopathic Pulmonary Fibrosis. Am J Respir Crit Care Med. 2020;201:564-574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 184] [Cited by in RCA: 244] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 24. | Silva JD, Krasnodembskaya AD. Investigation of the MSC Paracrine Effects on Alveolar-Capillary Barrier Integrity in the In Vitro Models of ARDS. Methods Mol Biol. 2021;2269:63-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Dutra Silva J, Su Y, Calfee CS, Delucchi KL, Weiss D, McAuley DF, O'Kane C, Krasnodembskaya AD. Mesenchymal stromal cell extracellular vesicles rescue mitochondrial dysfunction and improve barrier integrity in clinically relevant models of ARDS. Eur Respir J. 2021;58:2002978. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 183] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 26. | Natarajan K, Singh S, Burke TR Jr, Grunberger D, Aggarwal BB. Caffeic acid phenethyl ester is a potent and specific inhibitor of activation of nuclear transcription factor NF-kappa B. Proc Natl Acad Sci U S A. 1996;93:9090-9095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 880] [Cited by in RCA: 930] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 27. | Onori P, DeMorrow S, Gaudio E, Franchitto A, Mancinelli R, Venter J, Kopriva S, Ueno Y, Alvaro D, Savage J, Alpini G, Francis H. Caffeic acid phenethyl ester decreases cholangiocarcinoma growth by inhibition of NF-kappaB and induction of apoptosis. Int J Cancer. 2009;125:565-576. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 75] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 28. | D'Alessio FR. Mouse Models of Acute Lung Injury and ARDS. Methods Mol Biol. 2018;1809:341-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 85] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 29. | Chen R, Xie F, Zhao J, Yue B. Suppressed nuclear factor-kappa B alleviates lipopolysaccharide-induced acute lung injury through downregulation of CXCR4 mediated by microRNA-194. Respir Res. 2020;21:144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 30. | Zhang Y, Ma Y, Wu G, Xie M, Luo C, Huang X, Tian F, Chen J, Li X. SENP1 promotes MCL pathogenesis through regulating JAK-STAT5 pathway and SOCS2 expression. Cell Death Discov. 2021;7:192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 31. | Galipeau J, Krampera M, Barrett J, Dazzi F, Deans RJ, DeBruijn J, Dominici M, Fibbe WE, Gee AP, Gimble JM, Hematti P, Koh MB, LeBlanc K, Martin I, McNiece IK, Mendicino M, Oh S, Ortiz L, Phinney DG, Planat V, Shi Y, Stroncek DF, Viswanathan S, Weiss DJ, Sensebe L. International Society for Cellular Therapy perspective on immune functional assays for mesenchymal stromal cells as potency release criterion for advanced phase clinical trials. Cytotherapy. 2016;18:151-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 389] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 32. | Rozenberg A, Rezk A, Boivin MN, Darlington PJ, Nyirenda M, Li R, Jalili F, Winer R, Artsy EA, Uccelli A, Reese JS, Planchon SM, Cohen JA, Bar-Or A. Human Mesenchymal Stem Cells Impact Th17 and Th1 Responses Through a Prostaglandin E2 and Myeloid-Dependent Mechanism. Stem Cells Transl Med. 2016;5:1506-1514. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 78] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 33. | Heidari M, Pouya S, Baghaei K, Aghdaei HA, Namaki S, Zali MR, Hashemi SM. The immunomodulatory effects of adipose-derived mesenchymal stem cells and mesenchymal stem cells-conditioned medium in chronic colitis. J Cell Physiol. 2018;233:8754-8766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 34. | Darwish SS, Chen PJ, Hamed MM, Wagdy RA, Chen SH, Abadi AH, Abdel-Halim M, Hwang TL, Engel M. Development of (4-Phenylamino)quinazoline Alkylthiourea Derivatives as Novel NF-κB Inhibitors. Pharmaceuticals (Basel). 2022;15:778. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 35. | Liu Q, Du J, Yu X, Xu J, Huang F, Li X, Zhang C, Li X, Chang J, Shang D, Zhao Y, Tian M, Lu H, Xu J, Li C, Zhu H, Jin N, Jiang C. miRNA-200c-3p is crucial in acute respiratory distress syndrome. Cell Discov. 2017;3:17021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 95] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 36. | Kuo HC, Kuo WH, Lee YJ, Wang CJ, Tseng TH. Enhancement of caffeic acid phenethyl ester on all-trans retinoic acid-induced differentiation in human leukemia HL-60 cells. Toxicol Appl Pharmacol. 2006;216:80-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 37. | Kuang L, Wu J, Su N, Qi H, Chen H, Zhou S, Xiong Y, Du X, Tan Q, Yang J, Jin M, Luo F, Ouyang J, Zhang B, Wang Z, Jiang W, Chen L, Chen S, Wang Z, Liu P, Yin L, Guo F, Deng C, Chen D, Liu C, Xie Y, Ni Z, Chen L. FGFR3 deficiency enhances CXCL12-dependent chemotaxis of macrophages via upregulating CXCR7 and aggravates joint destruction in mice. Ann Rheum Dis. 2020;79:112-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 38. | Jiang J, Huang K, Xu S, Garcia JGN, Wang C, Cai H. Targeting NOX4 alleviates sepsis-induced acute lung injury via attenuation of redox-sensitive activation of CaMKII/ERK1/2/MLCK and endothelial cell barrier dysfunction. Redox Biol. 2020;36:101638. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 190] [Article Influence: 31.7] [Reference Citation Analysis (7)] |
| 39. | Adil MS, Somanath PR. Endothelial Permeability Assays In Vitro. Methods Mol Biol. 2021;2367:177-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 40. | Yu WK, McNeil JB, Wickersham NE, Shaver CM, Bastarache JA, Ware LB. Vascular endothelial cadherin shedding is more severe in sepsis patients with severe acute kidney injury. Crit Care. 2019;23:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 41. | Li L, Liu Q, Shang T, Song W, Xu D, Allen TD, Wang X, Jeong J, Lobe CG, Liu J. Aberrant Activation of Notch1 Signaling in Glomerular Endothelium Induces Albuminuria. Circ Res. 2021;128:602-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 42. | Wang T, Liu C, Pan LH, Liu Z, Li CL, Lin JY, He Y, Xiao JY, Wu S, Qin Y, Li Z, Lin F. Inhibition of p38 MAPK Mitigates Lung Ischemia Reperfusion Injury by Reducing Blood-Air Barrier Hyperpermeability. Front Pharmacol. 2020;11:569251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 43. | Covas DT, Panepucci RA, Fontes AM, Silva WA Jr, Orellana MD, Freitas MC, Neder L, Santos AR, Peres LC, Jamur MC, Zago MA. Multipotent mesenchymal stromal cells obtained from diverse human tissues share functional properties and gene-expression profile with CD146+ perivascular cells and fibroblasts. Exp Hematol. 2008;36:642-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 450] [Cited by in RCA: 453] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 44. | Jin HJ, Kwon JH, Kim M, Bae YK, Choi SJ, Oh W, Yang YS, Jeon HB. Downregulation of Melanoma Cell Adhesion Molecule (MCAM/CD146) Accelerates Cellular Senescence in Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells. Stem Cells Transl Med. 2016;5:427-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 45. | Pavlidis P, Tsakmaki A, Pantazi E, Li K, Cozzetto D, Digby-Bell J, Yang F, Lo JW, Alberts E, Sa ACC, Niazi U, Friedman J, Long AK, Ding Y, Carey CD, Lamb C, Saqi M, Madgwick M, Gul L, Treveil A, Korcsmaros T, Macdonald TT, Lord GM, Bewick G, Powell N. Interleukin-22 regulates neutrophil recruitment in ulcerative colitis and is associated with resistance to ustekinumab therapy. Nat Commun. 2022;13:5820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 91] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 46. | Crisostomo PR, Wang Y, Markel TA, Wang M, Lahm T, Meldrum DR. Human mesenchymal stem cells stimulated by TNF-alpha, LPS, or hypoxia produce growth factors by an NF kappa B- but not JNK-dependent mechanism. Am J Physiol Cell Physiol. 2008;294:C675-C682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 375] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 47. | Kinnaird T, Stabile E, Burnett MS, Shou M, Lee CW, Barr S, Fuchs S, Epstein SE. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004;109:1543-1549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 979] [Cited by in RCA: 989] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 48. | Liu XL, Sato S, Dai W, Yamanaka N. The protective effect of hepatocyte growth-promoting factor (pHGF) against hydrogen peroxide-induced acute lung injury in rats. Med Electron Microsc. 2001;34:92-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 49. | Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, Noiseux N, Zhang L, Pratt RE, Ingwall JS, Dzau VJ. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005;11:367-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1249] [Cited by in RCA: 1280] [Article Influence: 61.0] [Reference Citation Analysis (0)] |
| 50. | Lee HJ, Ko JH, Kim HJ, Jeong HJ, Oh JY. Mesenchymal stromal cells induce distinct myeloid-derived suppressor cells in inflammation. JCI Insight. 2020;5:e136059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 51. | Meng SS, Guo FM, Huang LL, Huang YZ, Xie JF, Yang CS, Qiu HB, Yang Y. mTORC2 Activation Mediated by Mesenchymal Stem Cell-Secreted Hepatocyte Growth Factors for the Recovery of Lipopolysaccharide-Induced Vascular Endothelial Barrier. Stem Cells Int. 2021;2021:9981589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 52. | Birukova AA, Zagranichnaya T, Fu P, Alekseeva E, Chen W, Jacobson JR, Birukov KG. Prostaglandins PGE(2) and PGI(2) promote endothelial barrier enhancement via PKA- and Epac1/Rap1-dependent Rac activation. Exp Cell Res. 2007;313:2504-2520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 245] [Cited by in RCA: 238] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 53. | Hezam K, Wang C, Fu E, Zhou M, Liu Y, Wang H, Zhu L, Han Z, Han ZC, Chang Y, Li Z. Superior protective effects of PGE2 priming mesenchymal stem cells against LPS-induced acute lung injury (ALI) through macrophage immunomodulation. Stem Cell Res Ther. 2023;14:48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 37] [Reference Citation Analysis (0)] |
| 54. | Debnath T, Kim DH, Lim BO. Natural products as a source of anti-inflammatory agents associated with inflammatory bowel disease. Molecules. 2013;18:7253-7270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 114] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 55. | Li ZG, Wang XY, Chang JL, Xie WB, Liu TF, Zhang QL, Deng YJ, Ding YQ. The establishment of supramolecular immunobead real-time PCR and the identification of Cox-2 as a metastasis-related marker in colorectal carcinoma. Oncol Rep. 2012;28:977-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 56. | Wakefield AP, Ogborn MR, Ibrahim N, Aukema HM. A dietary conjugated linoleic acid treatment that slows renal disease progression alters renal cyclooxygenase-2-derived prostanoids in the Han: SPRD-cy rat. J Nutr Biochem. 2012;23:908-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 57. | Reverte V, Tapia A, Moreno JM, Rodríguez L, Salazar F, Llinás MT, Salazar FJ. Renal effects of prolonged high protein intake and COX2 inhibition on hypertensive rats with altered renal development. Am J Physiol Renal Physiol. 2011;301:F327-F333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 58. | Majewska A, Wilkus K, Brodaczewska K, Kieda C. Endothelial Cells as Tools to Model Tissue Microenvironment in Hypoxia-Dependent Pathologies. Int J Mol Sci. 2021;22:520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (1)] |
| 59. | Ern C, Krump-Konvalinkova V, Docheva D, Schindler S, Rossmann O, Böcker W, Mutschler W, Schieker M. Interactions of human endothelial and multipotent mesenchymal stem cells in cocultures. Open Biomed Eng J. 2010;4:190-198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 60. | Schott NG, Friend NE, Stegemann JP. Coupling Osteogenesis and Vasculogenesis in Engineered Orthopedic Tissues. Tissue Eng Part B Rev. 2021;27:199-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 61. | Wong BW, Marsch E, Treps L, Baes M, Carmeliet P. Endothelial cell metabolism in health and disease: impact of hypoxia. EMBO J. 2017;36:2187-2203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 213] [Article Influence: 23.7] [Reference Citation Analysis (0)] |