Published online Oct 26, 2025. doi: 10.4252/wjsc.v17.i10.109942
Revised: July 28, 2025
Accepted: September 22, 2025
Published online: October 26, 2025
Processing time: 119 Days and 17.7 Hours
Congenital olfactory disorders (CODs) are rare but impactful conditions that impair the sense of smell from birth. These disorders can significantly affect a child’s appetite, nutrition, safety awareness, and overall quality of life. Despite their clinical importance, treatment options for CODs remain limited and largely ineffective, with no established therapies capable of restoring olfactory function in pediatric patients. Recent advances in regenerative medicine and stem cell therapy offer promising avenues for addressing sensory deficits. Nasal epithelial stem cells have emerged as a viable candidate for therapeutic intervention due to their accessibility and intrinsic ability to differentiate into olfactory sensory neurons. Preliminary studies suggest their potential in promoting the re
To evaluate the long-term efficacy and safety of autologous nasal epithelial stem cell transplantation for the treatment of CODs in children.
This prospective, single-center study enrolled 50 children aged 3-15 years with CODs. All patients underwent autologous nasal epithelial stem cell transplantation and were followed up for 3 years. The primary outcome measure was change in olfactory function, assessed using the Sniffin’ Sticks test and the University of Pennsylvania Smell Identification Test - Children’s Version. Secondary outcomes included quality of life (measured by the Pediatric Quality of Life Inventory™ and a custom olfaction-specific questionnaire), safety, endoscopic evaluation, and electro-olfactogram measurements. Data were analyzed using repeated measures analysis of variance, Friedman’s test, and multiple regression analysis.
The mean composite olfactory score increased from 8.3 ± 4.7 at baseline to 52.6 ± 18.9 at the 3-year follow-up (P < 0.001). Significant improvement (≥ 50% increase in score) was observed in 60% of patients, with 24% showing moderate improvement. Quality of life scores improved significantly across all domains (P < 0.001). No serious adverse events were reported. Minor complications occurred in 16% of patients, which resolved within 2 weeks. Endoscopic evaluation revealed normal-appearing olfactory epithelium in 84% of patients at 3 years, compared to 24% at baseline (P < 0.001). Electro-olfactogram amplitudes increased from 0.11 ± 0.08 mV to 0.67 ± 0.31 mV (P < 0.001). Age at intervention (β = 0.31, P = 0.02) and baseline residual olfactory function (β = 0.45, P < 0.001) were positively associated with treatment outcomes.
Autologous nasal epithelial stem cell transplantation demonstrates significant and sustained improvements in olfactory function and quality of life in children with CODs, with a favorable safety profile over a 3-year follow-up period. This approach represents a promising advancement in the treatment of pediatric sensory disorders.
Core Tip: This prospective study evaluated the long-term outcomes of autologous nasal epithelial stem cell transplantation in children with congenital olfactory disorders. Fifty pediatric patients were followed for 3 years, demonstrating significant improvements in olfactory function, electro-olfactogram readings, and quality of life scores. The treatment was well-tolerated, with only minor, self-limiting complications reported. Age at intervention and baseline residual function were positively associated with better outcomes. These findings offer promising evidence for a novel regenerative approach to pediatric anosmia, addressing a critical gap in current treatment options and paving the way for broader clinical application.
- Citation: Ni X, Shi J, Ning J, Tian XL. Long-term follow-up of autologous nasal epithelial stem cell transplantation for congenital olfactory disorders in children. World J Stem Cells 2025; 17(10): 109942
- URL: https://www.wjgnet.com/1948-0210/full/v17/i10/109942.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v17.i10.109942
Congenital olfactory disorders (CODs) in children represent a complex and often overlooked group of sensory impairments that can significantly affect a child’s quality of life, cognitive development, and overall well-being. These disorders encompass a range of conditions, including congenital anosmia (complete lack of smell), hyposmia (reduced sense of smell), and other olfactory dysfunctions present from birth. Recent epidemiological studies estimate the prevalence of CODs in children to be between 1 in 10000 and 1 in 20000 Live births, making them a rare yet noteworthy concern in pediatric healthcare[1].
The olfactory system plays an essential role in human life, extending beyond the simple perception of odors. It is intricately connected to the limbic system, influencing emotions, memory formation, and social behavior. In children, a properly functioning sense of smell is crucial for normal development, as it contributes to the formation of autobiographical memories, emotional responses, and even language acquisition[2]. Additionally, the olfactory system plays a crucial role in flavor perception, working in conjunction with the gustatory system to create a comprehensive sensory experience during eating. This sensory integration is essential for developing food preferences and maintaining proper nutrition, particularly during the formative years of childhood[3].
Children with CODs may experience numerous challenges that can significantly impact their daily lives and long-term development. These include difficulty detecting environmental hazards such as smoke or gas leaks, reduced enjoyment of food - potentially leading to nutritional deficiencies - and impaired social interactions due to an inability to perceive body odors or other socially relevant scents[4]. Moreover, emerging research suggests a potential link between olfactory dysfunction and neurodevelopmental disorders, indicating that early olfactory impairment may serve as a marker for - or contribute to - broader neurological issues[5].
The etiology of CODs is diverse and often multifactorial. Genetic factors play a major role, with numerous genes implicated in the development and function of the olfactory system. Advances in genomic sequencing have identified mutations in genes such as cyclic nucleotide-gated channel alpha 2, prokineticin receptor 2, and fibroblast growth receptor 1 as causative in some cases of congenital anosmia[6]. Additionally, developmental abnormalities affecting the olfactory bulb, olfactory cortex, or the intricate network of neurons connecting these structures can result in congenital olfactory dysfunction. Environmental factors during fetal development, including exposure to certain toxins or infections, may also contribute to the onset of these disorders[7].
Diagnosing CODs in children presents unique challenges due to the subjective nature of smell perception and the limited ability of young children to articulate their sensory experiences. Current diagnostic approaches typically involve a combination of detailed medical history, physical examination, and specialized olfactory function tests adapted for pediatric use. These may include modified versions of the Sniffin’ Sticks test, the University of Pennsylvania Smell Identification Test (UPSIT), and other psychophysical assessments[8]. Advanced imaging techniques, such as high-resolution magnetic resonance imaging (MRI) of the olfactory structures, have also proven valuable in identifying structural abnormalities associated with CODs[9].
Traditional treatment approaches for CODs have been limited and largely ineffective. Conservative management typically involves olfactory training - a technique that uses repeated exposure to specific odors to stimulate the olfactory system. While this method has shown some promise in treating acquired olfactory dysfunction, particularly in adults, its efficacy in congenital cases remains limited[10]. Surgical interventions have also been explored in cases where structural abnormalities are identified, such as correction of nasal septal deviations or removal of obstructive lesions. However, these approaches are often inapplicable to the majority of congenital cases, where the underlying etiology is neurological or genetic[11].
In recent years, stem cell therapy has emerged as a promising avenue for treating various sensory disorders, including those affecting olfaction. The regenerative potential of stem cells, combined with the lifelong neurogenesis observed in the olfactory system, has sparked considerable interest in their application to olfactory disorders[12]. Autologous stem cell transplantation, in particular, has gained attention for its potential to regenerate damaged or underdeveloped tissues while minimizing the risk of immune rejection. Nasal epithelial stem cells, which are readily accessible and capable of differentiating into olfactory receptor neurons, offer a promising option for treating olfactory disorders[13].
Preliminary research and case reports have indicated the potential of autologous nasal epithelial stem cell transplantation in managing olfactory disorders. A pioneering study involving adult patients with post-traumatic anosmia reported partial restoration of olfactory function following stem cell transplantation, laying the groundwork for further investigation in congenital cases[14]. Additionally, recent animal studies have demonstrated the promise of olfactory ensheathing cells - a unique type of glial cell found in the olfactory system - in promoting neuroregeneration and functional recovery in models of olfactory injury[15].
Despite these encouraging preliminary findings, long-term data on the efficacy and safety of autologous nasal epithelial stem cell transplantation in pediatric populations with CODs remain scarce. The unique developmental characteristics of the pediatric olfactory system, along with the potential long-term implications of early intervention, underscore the need for comprehensive, longitudinal studies in this population. The present study aims to address this critical knowledge gap by presenting a comprehensive 3-year follow-up of 50 pediatric cases treated with autologous nasal epithelial stem cell transplantation for CODs.
This prospective single-center study was conducted in the Department of Otorhinolaryngology in Shanxi Children’s Hospital, Shanxi Maternal and Child Health Hospital between January 2020 and October 2024. The study protocol was approved by the institutional ethics committee, and all procedures were conducted in accordance with the Declaration of Helsinki and the guidelines of Good Clinical Practice. Patients were eligible for inclusion if they met the following criteria: (1) Children aged 3-15 years at the time of enrollment; (2) Confirmed diagnosis of a COD, including but not limited to congenital anosmia and severe hyposmia. The diagnosis of was based on documented absence or severe reduction of smell since birth, as reported by parents, absence of any identifiable postnatal causative events, and confirmation through age-appropriate olfactory testing; (3) No history of head trauma, sinonasal surgery, or other acquired causes of olfactory dysfunction; (4) Normal findings on neurological examination and brain MRI to exclude central nervous system pathologies; (5) Informed consent obtained from parents or legal guardians, and assent from children aged over 7 years; and (6) Failure of, or non-response to, at least 6 months of age-appropriate olfactory training. Exclusion criteria comprised: (1) Presence of acute or chronic sinonasal infections; (2) History of malignancy or ongoing chemotherapy/radiotherapy; (3) Severe cognitive impairment interfering with olfactory testing; (4) Known allergies to materials used in the transplantation procedure; and (5) Participation in other clinical trials within the past 6 months.
A total of 73 children were initially screened for eligibility, of whom 50 met the inclusion criteria and were enrolled in the study. The enrolled patients underwent a comprehensive pre-treatment evaluation, which included a detailed medical history, physical examination, olfactory function testing using age-appropriate methods, quality of life assessment using validated pediatric questionnaires, sinonasal endoscopy, and imaging studies [computed tomography (CT) and MRI] to assess nasal anatomy and exclude other pathologies. Additionally, genetic testing was performed to identify potential genetic causes of olfactory dysfunction.
Autologous nasal epithelial stem cells were harvested from each patient under general anesthesia. The procedure was performed by experienced otolaryngologists following a standardized protocol. After preparing the nasal cavity with topical decongestants and local anesthetic agents, a small biopsy (approximately 2-3 mm2) of nasal epithelium was obtained from the superior turbinate under endoscopic guidance. The biopsy specimen was immediately transferred to a sterile transport medium and delivered to the on-site Good Manufacturing Practice-compliant cell processing facility.
At the facility, the specimen underwent enzymatic dissociation to obtain a single-cell suspension. Nasal epithelial stem cells were isolated using fluorescence-activated cell sorting based on the expression of specific surface markers, including CD44 and nerve growth factor receptor. The isolated cells were expanded in culture to passages 3-4 using a defined, serum-free medium supplemented with growth factors known to support the proliferation and maintenance of nasal epithelial stem cells. The target dose for implantation was (1-2) × 106 cells/mL, with a minimum required viability of > 95%. Quality control measures were implemented at every step, including sterility testing, cell viability assessment, and phenotypic characterization of the expanded cell population. The final stem cell product was cryopreserved until the day of transplantation.
The stem cell transplantation procedure was performed approximately 4-6 weeks after the initial biopsy to allow sufficient time for cell expansion. It was conducted under general anesthesia and involved several key steps. Pre-operative CT-guided planning was first used to identify optimal sites for cell delivery. The olfactory cleft and surrounding areas were visualized endoscopically, and the recipient site was prepared through gentle debridement and the application of topical agents to promote cell adherence.
The cryopreserved stem cell suspension was thawed and prepared following standardized protocols. Using a custom-designed microcatheter system, the cell suspension was delivered evenly across the target area of the olfactory epithelium. A biodegradable hydrogel matrix was applied to retain the transplanted cells in place and provide a supportive microenvironment. Post-procedure care included nasal packing and the administration of prophylactic antibiotics.
Patients were followed up for a period of 3 years post-transplantation. Follow-up visits were scheduled at weeks 1, 2, and 4 post-transplantation, at months 3, 6, 9, and 12, and then every 6 months thereafter until the 3-year mark. At each follow-up visit, a comprehensive assessment was conducted, including clinical examination, nasal endoscopy, olfactory function testing, and quality of life assessment. Any adverse events or complications were carefully monitored and documented. Additional investigations, such as imaging studies, were performed as clinically indicated.
The primary outcome measure was the change in olfactory function from baseline to the 3-year follow-up. Olfactory function was assessed using a combination of standardized tests adapted for pediatric use. These included the Sniffin’ Sticks test, which provides a comprehensive evaluation of odor threshold, discrimination, and identification. Age-appropriate modifications were made to ensure suitability for the pediatric population. Additionally, the UPSIT - Children’s Version was employed to provide a quantitative measure of olfactory function. For younger participants, a simplified, child-friendly tool specifically developed for this study - the Pediatric Smell Wheel - was used to assess odor identification.
Secondary outcome measures included quality of life, evaluated using validated instruments such as the Pediatric Quality of Life Inventory™ and the Child Health Questionnaire. A custom, olfaction-specific quality of life questionnaire was also developed to capture aspects unique to olfactory dysfunction. Safety and complications were carefully monitored and recorded throughout the study period.
Endoscopic evaluation of the olfactory epithelium was performed using a standardized grading system to assess changes in tissue appearance and health. In a subset of cooperative older children, an electro-olfactogram (EOG) was performed to provide an objective measure of olfactory function. Due to technical requirements, EOG was limited to cooperative older children; younger participants were assessed using validated behavioral methods, including the Pediatric Smell Wheel.
Data analysis was performed using SPSS version 26.0 (IBM Corp., Armonk, NY, United States). Descriptive statistics were used to summarize patient demographics and baseline characteristics. Continuous variables were expressed as means ± SDs or medians with interquartile ranges, as appropriate. Categorical variables were presented as frequencies and percentages. Changes in olfactory function scores and quality of life measures over time were analyzed using repeated measures analysis of variance or Friedman’s test, as appropriate. Post-hoc analyses were conducted using Bonferroni-corrected pairwise comparisons. Potential factors influencing treatment outcomes - such as age at intervention, specific diagnosis, genetic factors, and baseline olfactory function - were evaluated using multiple regression analysis. Safety data were summarized descriptively, and the incidence of adverse events was calculated. Kaplan-Meier analysis was used to estimate the cumulative incidence of complications over the 3-year follow-up period. A P-value < 0.05 was considered statistically significant for all analyses.
A total of 50 children (28 males, 22 females) with CODs were enrolled in the study and underwent autologous nasal epithelial stem cell transplantation. The mean age of the participants at the time of transplantation was 8.7 ± 3.2 years (range: 3-15 years). The cohort included 32 patients (64%) with congenital anosmia, 15 patients (30%) with severe congenital hyposmia, and three patients (6%) with Kallmann syndrome. Genetic testing identified pathogenic variants in genes associated with olfactory development in 18 patients (36%), including mutations in cyclic nucleotide-gated channel alpha 2 (n = 7), prokineticin receptor 2 (n = 5), and fibroblast growth receptor 1 (n = 6). The remaining patients had no identifiable genetic cause for their olfactory dysfunction. Baseline olfactory function testing showed that 32 patients (64%) had no measurable olfactory function (functional anosmia), while 18 patients (36%) exhibited severely reduced olfactory function. The mean baseline composite olfactory score - derived from the Sniffin’ Sticks and UPSIT tests - was 8.3 ± 4.7 out of a possible 100 points. Table 1 summarizes the baseline characteristics of the study participants.
| Characteristic | Value |
| Total patients, n | 50 |
| Age, mean ± SD (range), years | 8.7 ± 3.2 (3-15) |
| Sex | |
| Male | 28 (56) |
| Female | 22 (44) |
| Diagnosis | |
| Congenital anosmia | 32 (64) |
| Severe congenital hyposmia | 15 (30) |
| Kallmann syndrome | 3 (6) |
| Genetic mutations identified | 18 (36) |
| Baseline composite olfactory score, mean ± SD | 8.3 ± 4.7 |
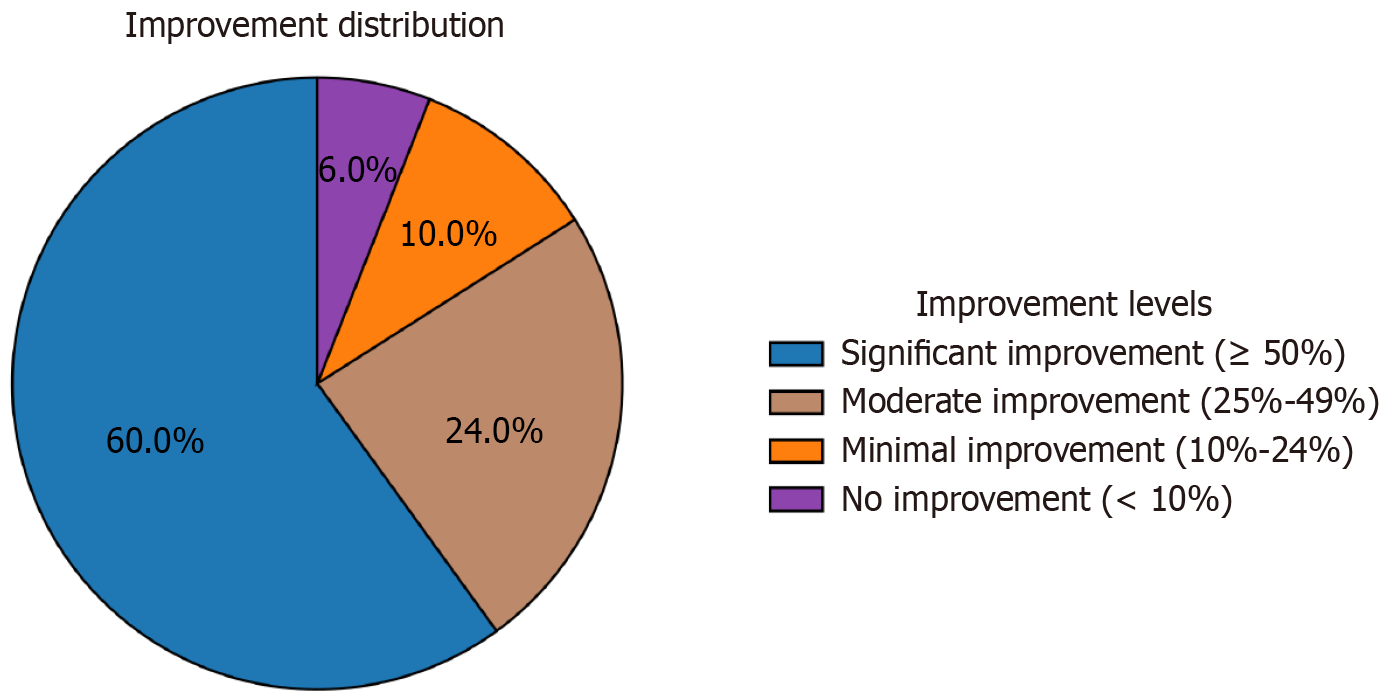
At the 3-year follow-up, significant improvements in olfactory function were observed in the majority of patients. The mean composite olfactory score increased from 8.3 ± 4.7 at baseline to 52.6 ± 18.9 at the 3-year follow-up (P < 0.001). This improvement was evident across all components of olfactory function (Table 2). Based on predefined criteria for improvement, 30 patients (60%) exhibited significant improvement (≥ 50% increase in composite olfactory score), 12 patients (24%) showed moderate improvement (25%-49% increase), five patients (10%) had minimal improvement (10%-24% increase), and three patients (6%) showed no improvement (< 10% change). Figure 1 illustrates the distribution of improvement levels among patients at the 3-year follow-up. Subgroup analysis revealed that patients with identifiable genetic mutations tended to show greater improvement compared to those without (mean score increase of 48.5 ± 16.7 vs 41.2 ± 19.3, P = 0.09), although this difference did not reach statistical significance.
| Component | Baseline | 3-year follow-up | P value |
| Odor threshold | 1.2 ± 0.8 | 6.8 ± 2.3 | < 0.001 |
| Odor discrimination | 3.1 ± 1.5 | 10.4 ± 3.1 | < 0.001 |
| Odor identification | 4.0 ± 2.4 | 35.4 ± 13.5 | < 0.001 |
Quality of life assessments demonstrated substantial improvements across multiple domains. The mean total Pediatric Quality of Life Inventory™ score increased from 62.3 ± 14.5 at baseline to 83.7 ± 10.2 at the 3-year follow-up (P < 0.001). Significant improvements were observed across all subscales, with the most pronounced changes in social functioning (from 58.9 ± 16.7 to 85.3 ± 11.4, P < 0.001) and emotional well-being (from 60.7 ± 15.3 to 82.1 ± 12.6, P < 0.001). The olfaction-specific quality of life questionnaire developed for this study showed a mean improvement of 62.4% (95% confidence interval: 54.7%-70.1%) from baseline to the 3-year follow-up. Notably, marked improvements were reported in food enjoyment (71.3% increase, P < 0.001) and social confidence (68.9% increase, P < 0.001).
The autologous nasal epithelial stem cell transplantation procedure was generally well-tolerated. No serious adverse events related to the stem cell harvesting or transplantation procedures were reported throughout the study period. Minor complications were observed in eight patients (16%), including transient nasal congestion (n = 5), mild epistaxis (n = 2), and local infection at the biopsy site (n = 1). All complications resolved with conservative management within 2 weeks of onset. No systemic infections, neoplastic transformations, or other major safety concerns were observed during the 3-year follow-up period.
Endoscopic evaluation of the olfactory epithelium revealed progressive improvements in tissue health and appearance over the follow-up period. At 3 years post-transplantation, 42 patients (84%) exhibited normal-appearing olfactory epithelium, compared to only 12 patients (24%) at baseline (P < 0.001). EOG measurements, performed in a subset of 28 cooperative older children, showed a significant increase in response amplitudes from baseline to the 3-year follow-up. The mean EOG amplitude increased from 0.11 ± 0.08 mV at baseline to 0.67 ± 0.31 mV at 3 years (P < 0.001), indicating improved electrophysiological function of the olfactory epithelium.
Multiple regression analysis identified several factors associated with better treatment outcomes. Age at intervention was positively correlated with improvement in olfactory function (β = 0.31, P = 0.02), suggesting that older children within the cohort tended to exhibit greater improvements. The presence of residual olfactory function at baseline was also a significant positive predictor of outcome (β = 0.45, P < 0.001). Notably, the specific genetic mutations identified were not significantly associated with treatment outcomes (P = 0.14), although the small sample size within each genetic subgroup limits the statistical power of this analysis.
This study presents the first long-term follow-up of autologous nasal epithelial stem cell transplantation for the treatment of CODs in children. Our findings demonstrate significant and sustained improvements in olfactory function and quality of life over a 3-year period following the intervention. These results offer promising insights into the potential of stem cell-based therapies to address previously intractable sensory deficits in pediatric populations.
The observed improvements in olfactory function were substantial, with 60% of patients showing significant improvement and an additional 24% demonstrating moderate improvement. These findings are particularly noteworthy given the congenital nature of the olfactory disorders in our cohort, which have traditionally been considered untreatable. The magnitude of improvement surpasses that reported in previous studies of olfactory training or other conservative management approaches for congenital anosmia[16]. Furthermore, the natural history of congenital anosmia indicates virtually no spontaneous recovery, making significant placebo effects unlikely. The sustained nature of the improvements over the 3-year follow-up period further suggests that the intervention may lead to long-term restoration of olfactory function.
The mechanisms underlying the observed improvements likely involve the regenerative capacity of the transplanted nasal epithelial stem cells. Recent research has highlighted the unique properties of these cells, including their ability to differentiate into olfactory receptor neurons and supporting cells[17]. The primary mechanism of action appears to be local regeneration of the olfactory epithelium through stem cell differentiation, as supported by our endoscopic and electrophysiological findings. However, central pathway effects cannot be ruled out. The improved appearance of the olfactory epithelium and increased EOG amplitudes observed in our study provide additional evidence for the functional integration of the transplanted cells within the olfactory system.
Interestingly, our results suggest that older children within the cohort tended to show greater improvements in olfactory function. This finding contrasts with the general principle in neurodevelopmental disorders, which suggests that earlier intervention typically yields better outcomes[18,19]. A potential explanation for this observation is that older children may possess more advanced cognitive and perceptual abilities, enabling them to engage more effectively with and benefit from the newly acquired sensory input. Additionally, the more mature nasal anatomy in older children might provide a more favorable environment for the engraftment and differentiation of transplanted stem cells[20-22]. This observation is consistent with neurodevelopmental principles, which suggest that sensory-cognitive integration improves with age; however, further mechanistic studies are needed to confirm this hypothesis[23].
The presence of residual olfactory function at baseline was also identified as a positive predictor of treatment outcome. This finding aligns with previous research on acquired olfactory disorders, where partial anosmia has been associated with a better prognosis compared to complete anosmia[24-29]. Residual function may indicate the presence of intact neural pathways or supporting structures within the olfactory system, which could facilitate the integration and functionality of the transplanted cells.
The improvements in quality of life observed in our study highlight the profound impact of olfactory function on various aspects of children’s well-being. The marked enhancements in social functioning and emotional well-being are particularly noteworthy, as they highlight the often-overlooked role of olfaction in social interactions and emotional processing during childhood and adolescence. These findings are consistent with recent research demonstrating the intricate links between olfactory function and social-emotional development in children[30-32].
The safety profile of the autologous nasal epithelial stem cell transplantation in our study was favorable, with only minor and transient complications observed. This observation is a crucial consideration, particularly in pediatric populations, where long-term safety is of paramount importance. The absence of serious adverse events or safety concerns over the 3-year follow-up period offers reassurance regarding the safety of this approach. However, continued long-term monitoring remains essential to ensure the sustained safety of the intervention.
The absence of a statistically significant association between specific genetic mutations and treatment outcomes in our study may be due to the limited sample size in each genetic subgroup. The trend toward improved outcomes in patients with identifiable genetic mutations is intriguing and warrants further investigation. Recent advances in understanding the genetic basis of CODs have revealed a complex interplay among multiple genes involved in olfactory development and function[33]. Future studies with larger cohorts may provide more definitive insights into the role of genetic factors in treatment response.
The successful application of stem cell therapy in this study opens up new possibilities for addressing other sensory deficits in pediatric populations. The approach used - autologous cell harvesting, ex vivo expansion, and targeted transplantation - could potentially be adapted for other sensory systems or neurological conditions where tissue regeneration is a therapeutic goal[34,35].
This study has some limitations that should be acknowledged. The single-center design and the absence of a control group limit the generalizability of our findings. Additionally, while our 3-year follow-up provides valuable long-term data, even more extended observation periods would be beneficial to assess the true durability of the improvements and to monitor for any potential late-onset complications. Although no tumorigenic complications were observed during the 3-year follow-up, extended monitoring will be necessary to fully establish the long-term safety profile of stem cell therapy. Future multi-center collaborations are underway to validate these findings across diverse populations and clinical settings.
This study demonstrates that autologous nasal epithelial stem cell transplantation is a promising therapeutic approach for children with CODs, resulting in significant and sustained improvements in both olfactory function and quality of life. The procedure exhibited a favorable safety profile over the 3-year follow-up period. These findings represent a substantial advancement in the treatment of pediatric sensory disorders and underscore the potential of regenerative medicine in addressing previously intractable conditions.
| 1. | Jafari A, Holbrook EH. Therapies for Olfactory Dysfunction - an Update. Curr Allergy Asthma Rep. 2022;22:21-28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 2. | Pellegrino R, Cooper KW, Di Pizio A, Joseph PV, Bhutani S, Parma V. Corona Viruses and the Chemical Senses: Past, Present, and Future. Chem Senses. 2020;bjaa031. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 3. | Saltagi AK, Saltagi MZ, Nag AK, Wu AW, Higgins TS, Knisely A, Ting JY, Illing EA. Diagnosis of Anosmia and Hyposmia: A Systematic Review. Allergy Rhinol (Providence). 2021;12:21526567211026568. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 4. | Boesveldt S, Postma EM, Boak D, Welge-Luessen A, Schöpf V, Mainland JD, Martens J, Ngai J, Duffy VB. Anosmia-A Clinical Review. Chem Senses. 2017;42:513-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 205] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 5. | Mainland JD, Barlow LA, Munger SD, Millar SE, Vergara MN, Jiang P, Schwob JE, Goldstein BJ, Boye SE, Martens JR, Leopold DA, Bartoshuk LM, Doty RL, Hummel T, Pinto JM, Trimmer C, Kelly C, Pribitkin EA, Reed DR. Identifying Treatments for Taste and Smell Disorders: Gaps and Opportunities. Chem Senses. 2020;45:493-502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 6. | Trang H, Samuels M, Ceccherini I, Frerick M, Garcia-Teresa MA, Peters J, Schoeber J, Migdal M, Markstrom A, Ottonello G, Piumelli R, Estevao MH, Senecic-Cala I, Gnidovec-Strazisar B, Pfleger A, Porto-Abal R, Katz-Salamon M. Guidelines for diagnosis and management of congenital central hypoventilation syndrome. Orphanet J Rare Dis. 2020;15:252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 84] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 7. | Fletcher RB, Das D, Gadye L, Street KN, Baudhuin A, Wagner A, Cole MB, Flores Q, Choi YG, Yosef N, Purdom E, Dudoit S, Risso D, Ngai J. Deconstructing Olfactory Stem Cell Trajectories at Single-Cell Resolution. Cell Stem Cell. 2017;20:817-830.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 154] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 8. | Pekala K, Chandra RK, Turner JH. Efficacy of olfactory training in patients with olfactory loss: a systematic review and meta-analysis. Int Forum Allergy Rhinol. 2016;6:299-307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 155] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 9. | Hummel T, Whitcroft KL, Rueter G, Haehner A. Intranasal vitamin A is beneficial in post-infectious olfactory loss. Eur Arch Otorhinolaryngol. 2017;274:2819-2825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 10. | Hummel T, Liu DT, Müller CA, Stuck BA, Welge-Lüssen A, Hähner A. Olfactory Dysfunction: Etiology, Diagnosis, and Treatment. Dtsch Arztebl Int. 2023;120:146-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 11. | Likhar A, Baghel P, Patil M. Early Childhood Development and Social Determinants. Cureus. 2022;14:e29500. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 12. | Schwob JE, Jang W, Holbrook EH, Lin B, Herrick DB, Peterson JN, Hewitt Coleman J. Stem and progenitor cells of the mammalian olfactory epithelium: Taking poietic license. J Comp Neurol. 2017;525:1034-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 201] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 13. | Reshamwala R, Shah M, St John J, Ekberg J. Survival and Integration of Transplanted Olfactory Ensheathing Cells are Crucial for Spinal Cord Injury Repair: Insights from the Last 10 Years of Animal Model Studies. Cell Transplant. 2019;28:132S-159S. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 14. | Yoo SH, Kim HW, Lee JH. Restoration of olfactory dysfunctions by nanomaterials and stem cells-based therapies: Current status and future perspectives. J Tissue Eng. 2022;13:20417314221083414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Gilmour AD, Reshamwala R, Wright AA, Ekberg JAK, St John JA. Optimizing Olfactory Ensheathing Cell Transplantation for Spinal Cord Injury Repair. J Neurotrauma. 2020;37:817-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 16. | Liu DT, Sabha M, Damm M, Philpott C, Oleszkiewicz A, Hähner A, Hummel T. Parosmia is Associated with Relevant Olfactory Recovery After Olfactory Training. Laryngoscope. 2021;131:618-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 17. | Hummel T, Rissom K, Reden J, Hähner A, Weidenbecher M, Hüttenbrink KB. Effects of olfactory training in patients with olfactory loss. Laryngoscope. 2009;119:496-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 475] [Cited by in RCA: 405] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 18. | Baumgartner LS, Moore E, Shook D, Messina S, Day MC, Green J, Nandy R, Seidman M, Baumgartner JE. Safety of Autologous Umbilical Cord Blood Therapy for Acquired Sensorineural Hearing Loss in Children. J Audiol Otol. 2018;22:209-222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Zhang P, Yang J, Shu Y, Cheng M, Zhao X, Wang K, Lu L, Xing Q, Niu G, Meng L, Wang X, Zhou L, Zhang X. The value of synthetic MRI in detecting the brain changes and hearing impairment of children with sensorineural hearing loss. Front Neurosci. 2024;18:1365141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 20. | Sun X, Yang J. [Research advances on umbilical cord blood derived mesenchymal stem cell in therapy of sensorineural hearing loss]. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2020;34:1149-1152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 21. | Ronner EA, Benchetrit L, Levesque P, Basonbul RA, Cohen MS. Quality of Life in Children with Sensorineural Hearing Loss. Otolaryngol Head Neck Surg. 2020;162:129-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 22. | Rezaeyan A, Asadi S, Kamrava SK, Khoei S, Zare-Sadeghi A. Reorganizing brain structure through olfactory training in post-traumatic smell impairment: An MRI study. J Neuroradiol. 2022;49:333-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 23. | Genetzaki S, Nikolaidis V, Markou K, Konstantinidis I. Olfactory training with four and eight odors: comparison with clinical testing and olfactory bulb volumetrics. Eur Arch Otorhinolaryngol. 2024;281:497-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 24. | Altundag A, Saatci O, Kandemirli SG, Sanli DET, Duz OA, Sanli AN, Yildirim D. Imaging Features to Predict Response to Olfactory Training in Post-Traumatic Olfactory Dysfunction. Laryngoscope. 2021;131:E2243-E2250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 25. | Chen B, Espin M, Haussmann R, Matthes C, Donix M, Hummel T, Haehner A. The Effect of Olfactory Training on Olfaction, Cognition, and Brain Function in Patients with Mild Cognitive Impairment. J Alzheimers Dis. 2022;85:745-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 26. | Lebedeva GV, Svistushkin MV, Selezneva LV, Muzychenko YN, Suvorov AY, Khutornoi IV, Pedder AV, Pedder VV, Kudryavtseva VA, Pogosyan KK. [Development and validation of Russian olfactory test]. Vestn Otorinolaringol. 2024;89:41-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 27. | Pellegrino R, Han P, Reither N, Hummel T. Effectiveness of olfactory training on different severities of posttraumatic loss of smell. Laryngoscope. 2019;129:1737-1743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 28. | Rozenkrantz L, Zachor D, Heller I, Plotkin A, Weissbrod A, Snitz K, Secundo L, Sobel N. A Mechanistic Link between Olfaction and Autism Spectrum Disorder. Curr Biol. 2015;25:1904-1910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 29. | Okumura T, Kumazaki H, Singh AK, Touhara K, Okamoto M. Individuals With Autism Spectrum Disorder Show Altered Event-Related Potentials in the Late Stages of Olfactory Processing. Chem Senses. 2020;45:37-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 30. | Sweigert JR, St John T, Begay KK, Davis GE, Munson J, Shankland E, Estes A, Dager SR, Kleinhans NM. Characterizing Olfactory Function in Children with Autism Spectrum Disorder and Children with Sensory Processing Dysfunction. Brain Sci. 2020;10:362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 31. | Yang R, Zhang G, Shen Y, Ou J, Liu Y, Huang L, Zeng Y, Lin J, Liu R, Wu R, Xia K, Zhang F, Zhao J. Odor identification impairment in autism spectrum disorder might be associated with mitochondrial dysfunction. Asian J Psychiatr. 2022;72:103072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 32. | Barros F, Soares SC. Giving meaning to the social world in autism spectrum disorders: Olfaction as a missing piece of the puzzle? Neurosci Biobehav Rev. 2020;116:239-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 33. | Kurtenbach S, Goss GM, Goncalves S, Choi R, Hare JM, Chaudhari N, Goldstein BJ. Cell-Based Therapy Restores Olfactory Function in an Inducible Model of Hyposmia. Stem Cell Reports. 2019;12:1354-1365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 34. | Xie C, Martens JR. Potential Therapeutic Targets for Olfactory Dysfunction in Ciliopathies Beyond Single-Gene Replacement. Chem Senses. 2021;46:bjab010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 35. | Uytingco CR, Green WW, Martens JR. Olfactory Loss and Dysfunction in Ciliopathies: Molecular Mechanisms and Potential Therapies. Curr Med Chem. 2019;26:3103-3119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |