Published online Dec 28, 2025. doi: 10.3748/wjg.v31.i48.112972
Revised: September 16, 2025
Accepted: November 12, 2025
Published online: December 28, 2025
Processing time: 137 Days and 21.4 Hours
The association between the serum uric acid-to-high-density lipoprotein cho
To investigate the relevance and dose-responsive relationship between UHR and 10-year CVD risk among Asian MASLD patients.
In this retrospective analysis, 3901 MASLD patients were enrolled based on esta
Multivariable-adjusted analyses revealed that elevated UHR levels were significantly associated with an increased likelihood of intermediate-to-high CVD risk. RCS modeling demonstrated a linear dose-response relationship between UHR and the Framingham risk score (P for nonlinearity = 0.114). Sex-stratified RCS analyses further indicated that this linear association persisted among males (P for nonlinearity = 0.167) but was not statistically significant in females (P for nonlinearity = 0.476). Further stratified analyses revealed that the association was particularly pronounced among younger individuals (< 50 years), males, and those with central obesity, whereas it was attenuated in older adults (≥ 50 years) and females. Receiver operating characteristic analysis demonstrated that UHR outperformed individual biomarkers in predicting 10-year CVD risk, showing an area under the curve of 0.655 (95% confidence interval: 0.635-0.674).
UHR functioned as an independent predictor of 10-year CVD risk in Asian patients with MASLD, demonstrating a linear dose-response association and superior discriminative performance relative to conventional biomarkers, especially among younger individuals, males, and those with central obesity.
Core Tip: This study demonstrated that an elevated uric acid-to-high-density lipoprotein cholesterol ratio (UHR) was independently associated with a higher predicted 10-year CVD risk, as estimated by the Framingham risk score, in a large cohort of Asian patients with metabolic dysfunction-associated steatotic liver disease. A distinct linear dose-response association between increasing UHR levels and CVD risk was observed. Notably, UHR exhibited superior predictive performance compared with conventional biomarkers (area under the curve = 0.655), with an optimal cutoff value of 292. Readily obtainable from routine biochemical testing, UHR thus represents a simple and cost-effective indicator for early CVD risk stratification in patients with metabolic dysfunction-associated steatotic liver disease.
- Citation: Wang Y, Ma GH, Qu MY, Xu QS, Huang HX. Serum uric acid-to-high-density lipoprotein cholesterol ratio and cardiovascular risk in Asian patients with metabolic dysfunction-associated steatotic liver disease. World J Gastroenterol 2025; 31(48): 112972
- URL: https://www.wjgnet.com/1007-9327/full/v31/i48/112972.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i48.112972
Metabolic dysfunction-associated steatotic liver disease (MASLD) is characterized by hepatic steatosis in the absence of harmful alcohol intake and the presence of at least one cardiometabolic risk factor[1]. Globally, MASLD has worked as one of the most common chronic liver disorders. Its swiftly increasing prevalence represents a growing public health cha
Recently, the uric acid (UA)-to-high-density lipoprotein cholesterol (HDL-C) ratio (UHR) has emerged as a potential biomarker, capturing both the detrimental metabolic impact of UA and the protective role of HDL-C[5]. Accumulating evidence has linked UHR to a range of chronic metabolic conditions[6,7]. For instance, a longitudinal study has reported that elevated UHR predicts accelerated renal function decline in hypertensive patients, suggesting its value in risk stratification for renal injury[8]. Similarly, a case-control research has proven a positive relationship between increased UHR and non-alcoholic fatty liver disease[9]. Moreover, UHR has been identified as a predictor of all-cause mortality and CVD[10,11]. The Framingham risk score (FRS) is an extensively used clinical tool for estimating cardiovascular risk and forecasting adverse cardiovascular outcomes[12]. However, whether UHR can improve CVD risk prediction using FRS in Asian MASLD populations has not been established. This study, therefore, represented the first to investigate the UHR-FRS dose-response relationship in this patient group, addressing an important gap in early risk stratification.
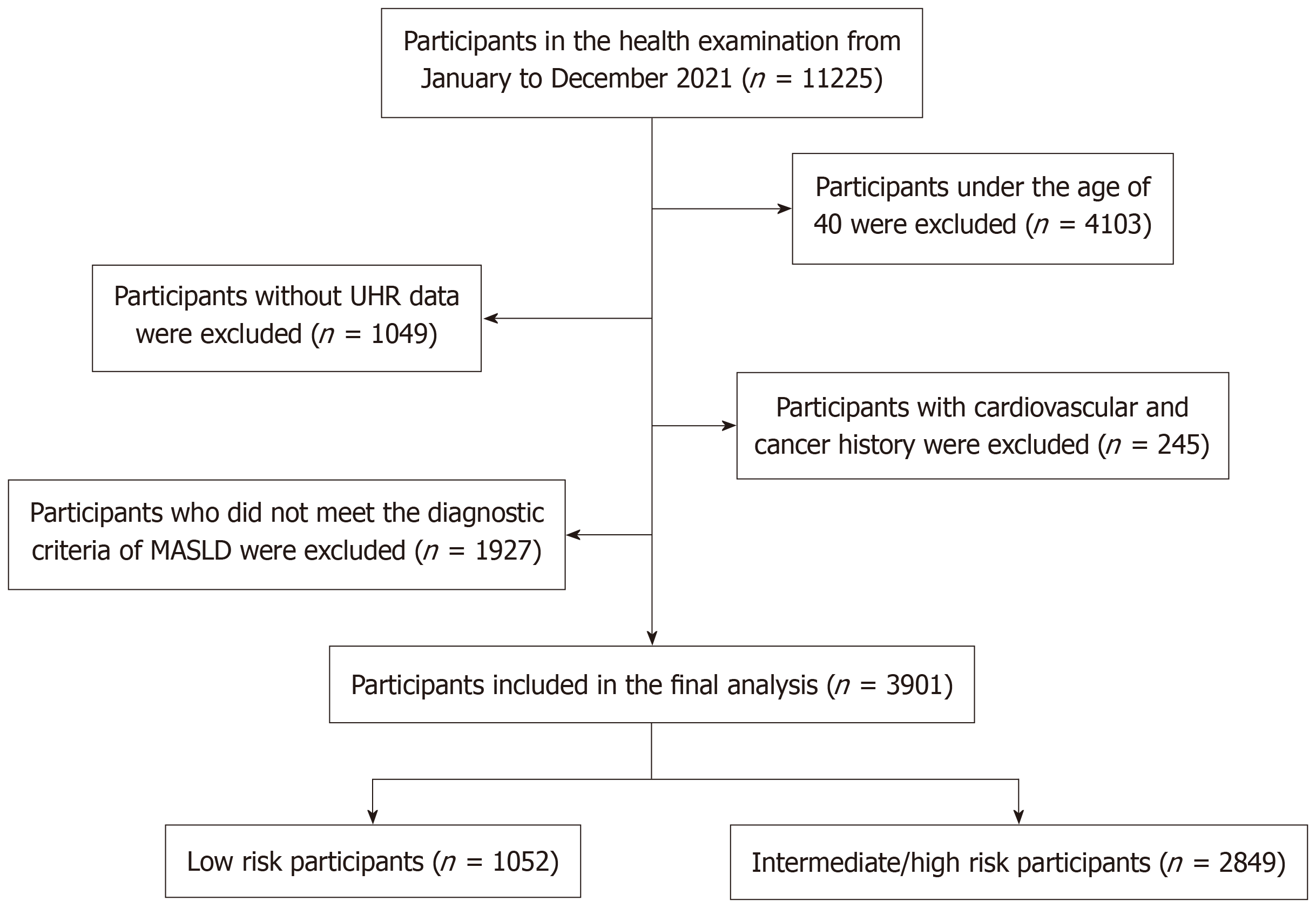
A retrospective study was implemented using consecutively collected data from adults undergoing health examinations at the Shanghai Health and Medical Center from January to December 2021. Data were obtained from the center’s electronic database. The predefined sampling frame included all individuals aged ≥ 40 years with complete abdominal ultrasonography and laboratory data. Participants were excluded if they had: (1) Incomplete UHR data (n = 1049); (2) Did not meet MASLD diagnostic criteria (n = 1927); (3) Were younger than 40 years (n = 4103); or (4) Had a prior history of CVD or malignancy (n = 245). The final analytic cohort comprised 3901 participants (Figure 1). The exclusion of individuals < 40 years corresponded to the 2019 American College of Cardiology/American Heart Association Guideline on the primary prevention of CVD, which suggests 10-year atherosclerotic CVD risk assessment for asymptomatic adults aged 40-75 years[13]. The Ethics Committee of Shanghai Health and Medical Center approved the study protocol. Given that the study was retrospective and relied on anonymized data, the necessity for written informed consent was waived in line with ethical regulations.
Demographic, anthropometric, and clinical information were extracted from the Shanghai Health and Medical Center electronic database. Collected variables included age, sex, systolic and diastolic blood pressure (SBP and DBP), presence of diabetes, hypertension, hyperuricemia, hyperlipidemia, metabolic syndrome (MetS), history of CVD, smoking status, as well as height, weight, body mass index (BMI), waist circumference (WC), and hip circumference. Height and weight were measured using an ultrasonic height-weight device (SK-L06B), and BMI was calculated as weight divided by height squared (kg/m2). Blood pressure was assessed with an Omron electronic sphygmomanometer, with SBP and DBP recorded separately. After a minimum of 12 hours of overnight fasting, venous blood samples were collected. Laboratory tests, ultrasonography, and anthropometric assessments were all conducted on the same day.
Biochemical measurements included fasting plasma glucose (FPG), glycated hemoglobin (HbA1c), serum UA (SUA), serum creatinine, blood urea nitrogen, total cholesterol (TC), triglycerides (TG), low-density lipoprotein cholesterol, and HDL-C. These parameters were quantified using a Roche C702 automated biochemical analyzer with Roche proprietary reagents.
Specific clinical definitions were standardized as follows. Smoking: Lifetime consumption of > 100 cigarettes; hyperuricemia: Fasting SUA > 420 μmol/L among males or > 360 μmol/L among females, confirmed on two separate tests; MetS, presence of ≥ 3 of the listed criteria: (1) Central obesity (WC ≥ 94 cm among males or ≥ 85 cm among females); (2) Hyperglycemia (FPG ≥ 6.1 mmol/L or 2-hour plasma glucose ≥ 7.8 mmol/L during a 75-g oral glucose tolerance test) or antidiabetic therapy; (3) Hypertension (SBP ≥ 130 mmHg or DBP ≥ 85 mmHg) or antihypertensive treatment; (4) High TG (≥ 1.70 mmol/L); and (5) Reduced HDL-C (< 1.04 mmol/L). The UHR was figured out as SUA (μmol/L) separated by HDL-C (mmol/L).
The 10-year risk of CVD was determined through the FRS, which incorporates seven established risk factors[14]: Age, sex, TC, HDL-C, SBP, smoking, and blood glucose. Based on FRS, participants were stratified into two categories: Low risk (10-year risk < 10%) and intermediate/high risk (10-year risk ≥ 10%).
MASLD was diagnosed through imaging-confirmed hepatic steatosis together with at least one cardiometabolic standard[1], including: BMI ≥ 25 kg/m2; WC > 94 cm in men or > 80 cm in women; FPG ≥ 5.6 mmol/L; 2-hour plasma glucose during oral glucose tolerance test ≥ 7.8 mmol/L; HbA1c ≥ 5.7% (39 mmol/mol); diagnosis or therapy of type 2 diabetes; blood pressure ≥ 130/85 mmHg or antihypertensive therapy; TG ≥ 1.70 mmol/L or lipid-lowering treatment for hypertriglyceridemia; and low HDL-C (≤ 1.0 mmol/L among males or ≤ 1.3 mmol/L among females) or lipid-lowering treatment for reduced HDL-C. The severity of MASLD was segregated as mild or medium-to-severe, according to ultrasonographic grading of fatty liver.
All continuous variables showed normal distribution, which was first evaluated through the Shapiro-Wilk test. Variables showing non-normal distributions were denoted as median (Q1, Q3), where Q1 and Q3 denote the 25th and 75th percentiles, respectively. Group differences were tested with the Mann-Whitney U test (Wilcoxon rank-sum test). Categorical variables were presented as counts and percentages. They were compared through the χ2 test.
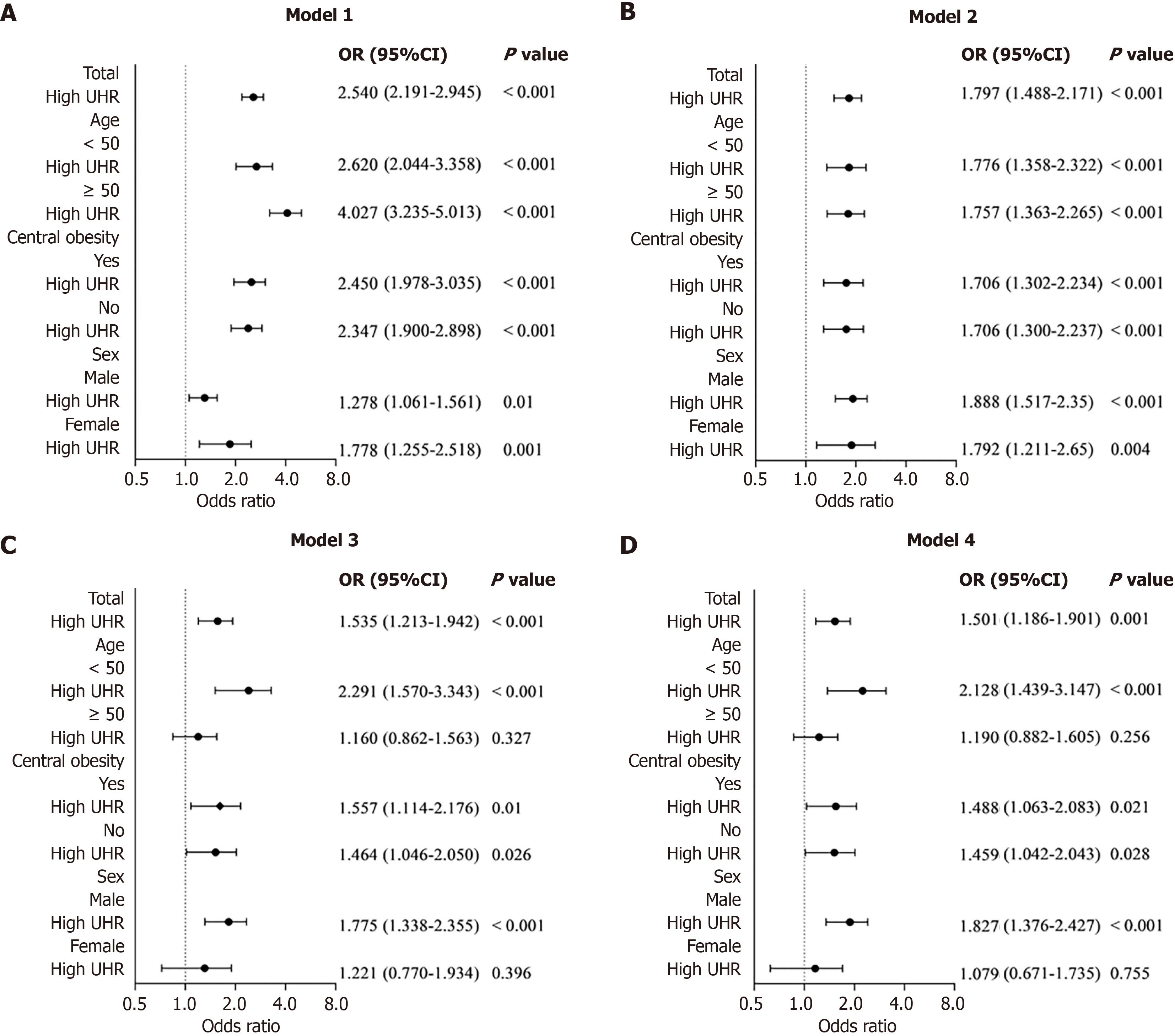
The association between UHR (dichotomized into high and low groups in light of the cohort median) and CVD risk categories was explored through binary logistic regression, showing odds ratios (ORs) and 95% confidence intervals (CIs). Multivariable models were constructed in a stepwise manner: Model 1, unadjusted; model 2, adjusted for age and sex; model 3, adjusted for model 2 covariates plus BMI, diabetes, smoking, hypertension, central obesity, hyperlipidemia, and MetS; and model 4, adjusted for model 3 covariates plus MASLD severity. Stratified analyses were further implemented based on prespecified subgroups: Age (< 50 years vs ≥ 50 years), central obesity (present vs absent), and sex (male vs female).
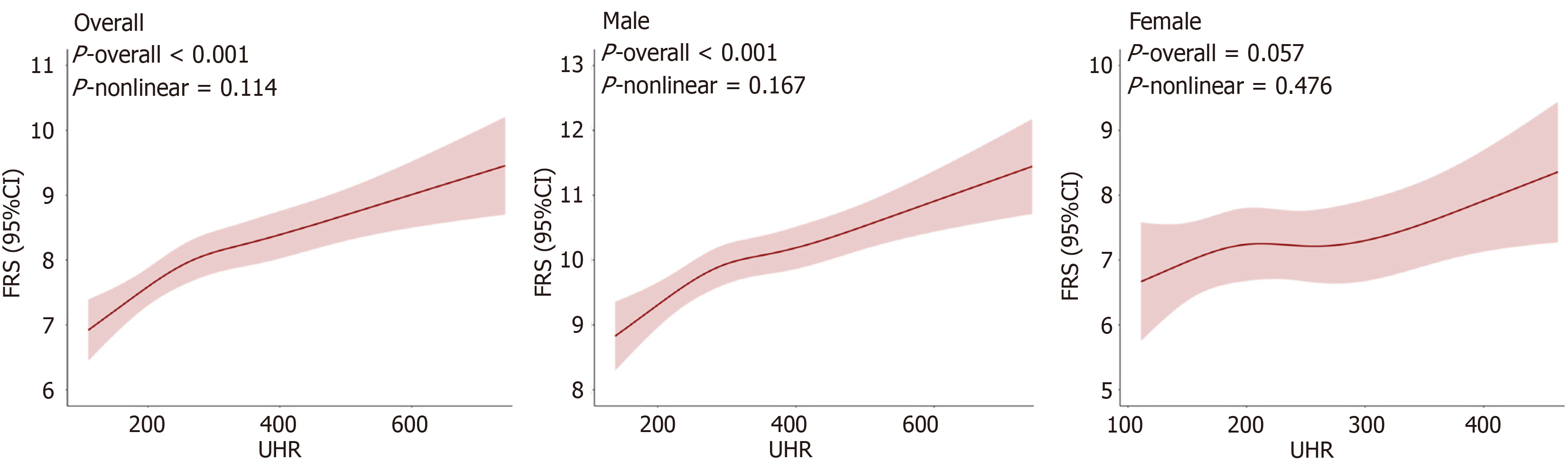
The dose-response relationship between UHR and the FRS was evaluated using restricted cubic spline (RCS) regression in the overall cohort and in sex-stratified subgroups. Four knots were positioned at the 5th, 35th, 65th, and 95th percentiles of the UHR distribution. Nonlinearity was assessed via a likelihood ratio test, comparing models including only the linear term with those incorporating both linear and spline terms. The predictive performance of UHR for CVD risk was further examined using receiver operating characteristic (ROC) curve analysis, with the area under the curve (AUC) and corresponding 95%CI reported. All analyses were executed with R (version 4.5.1; R Foundation for Statistical Computing, Vienna, Austria) and EasyR (version 2.0; Solutions, Inc., Shanghai, China; https://www.easyrdata.com). A two-sided P < 0.05 held statistical significance.
In total 3901 MASLD patients were included, showing a median age of 55 years and a median BMI of 26.21 kg/m2. The majority were male (76.9%, n = 2998), whereas females accounted for 23.1% (n = 903). Based on 10-year CVD risk, participants were classified into a low-risk group (n = 1052) and an intermediate/high-risk group (n = 2849). Compared with the low-risk group, individuals classified as intermediate/high-risk were more likely to be male, older, and exhibited higher BMI and elevated UHR levels (all P < 0.05). This group also demonstrated a significantly greater prevalence of hypertension, diabetes, central obesity, smoking, hyperlipidemia, MetS, and moderate-to-severe MASLD (all P < 0.05). Additionally, they presented with higher SBP and DBP, FPG, HbA1c, TG, blood urea nitrogen, SUA, and serum creatinine, while HDL-C levels were substantially lower (all P < 0.05). No significant differences were observed between groups for TC or low-density lipoprotein cholesterol (P > 0.05). Detailed baseline characteristics are summarized in Table 1.
| Variables | Total (n = 3901) | Low risk (n = 1052) | Intermediate/high risk (n = 2849) | P value |
| Gender | < 0.001 | |||
| Male | 2998 (76.9) | 504 (47.9) | 2494 (87.5) | |
| Female | 903 (23.1) | 548 (52.1) | 355 (12.5) | |
| Age (years) | 55 (49, 60) | 50 (44, 56) | 57 (51, 62) | < 0.001 |
| BMI (kg/m2) | 26.210 (24.530, 28.200) | 25.575 (23.970, 27.408) | 26.470 (24.770, 28.390) | < 0.001 |
| Central obesity | <0.001 | |||
| Yes | 2272 (58.2) | 444 (42.2) | 1828 (64.2) | |
| No | 1629 (41.8) | 608 (57.8) | 1021 (35.8) | |
| SBP (mmHg) | 129 (119, 139) | 121 (113, 130) | 131 (122, 141) | < 0.001 |
| DBP (mmHg) | 77 (71, 84) | 75 (69, 81) | 79 (72, 85) | < 0.001 |
| Smoking | < 0.001 | |||
| Yes | 1799 (46.1) | 111 (10.6) | 1688 (59.2) | |
| No | 2102 (53.9) | 941 (89.4) | 1161 (40.8) | |
| Hypertension | < 0.001 | |||
| Yes | 2450 (62.8) | 380 (36.1) | 2070 (72.7) | |
| No | 1451 (37.2) | 672 (63.9) | 779 (27.3) | |
| Diabetes | < 0.001 | |||
| Yes | 1271 (32.6) | 119 (11.3) | 1152 (40.4) | |
| No | 2630 (67.4) | 933 (88.7) | 1697 (62.7) | |
| Hyperlipidemia | < 0.001 | |||
| Yes | 2841 (72.8) | 706 (67.1) | 2135 (74.9) | |
| No | 1060 (27.2) | 346 (32.9) | 714 (25.1) | |
| MetS | < 0.001 | |||
| Yes | 1408 (36.1) | 137 (13.0) | 1271 (44.6) | |
| No | 2493 (63.9) | 915 (87.0) | 1578 (55.4) | |
| FBG (mmol/L) | 5.520 (5.120, 6.220) | 5.270 (4.970, 5.660) | 5.650 (5.190, 6.550) | < 0.001 |
| HbAlc | 5.800 (5.500, 6.200) | 5.700 (5.500, 5.900) | 5.900 (5.600, 6.300) | < 0.001 |
| TG (mmol/L) | 1.800 (1.280, 2.575) | 1.600 (1.140, 2.238) | 1.880 (1.340, 2.720) | < 0.001 |
| TC (mmol/L) | 4.970 (4.370, 5.640) | 4.980 (4.420, 5.670) | 4.970 (4.340, 5.640) | 0.351 |
| LDL-C (mmol/L) | 3.000 (2.560, 3.480) | 2.990 (2.600, 3.488) | 3.000 (2.550, 3.480) | 0.427 |
| HDL-C (mmol/L) | 1.190 (1.050, 1.380) | 1.280 (1.110, 1.450) | 1.160 (1.030, 1.340) | < 0.001 |
| BUN (mmol/L) | 5.490 (4.770, 6.360) | 5.265 (4.590, 6.110) | 5.590 (4.840, 6.450) | < 0.001 |
| SUA (μmol/L) | 366.000 (312.950, 421.550) | 338.100 (287.550, 396.575) | 374.200 (324.950, 430.750) | < 0.001 |
| Cr (μmol/L) | 74.900 (65.000, 84.200) | 68.400 (57.325, 79.275) | 76.700 (68.300, 85.300) | < 0.001 |
| UHR (μmol/L/ mmol/L) | 304.646 (240.567, 379.099) | 264.235 (209.028, 332.826) | 320.263 (254.204, 395.221) | < 0.001 |
| MASLD | 0.002 | |||
| Mild | 3217 (82.5) | 901 (85.6) | 2316 (81.3) | |
| Moderate/severe | 684 (17.5) | 151 (14.4) | 533 (18.7) |
In the overall cohort, unadjusted analysis (model 1) demonstrated that participants with elevated UHR had significantly greater odds of being categorized as intermediate/high CVD risk relative to those with low UHR (OR = 2.540, 95%CI: 2.191-2.945, P < 0.001). This relevance was still robust after sequential adjustment: Model 2 (adjusted for age and sex: OR = 1.797, 95%CI: 1.488-2.171, P < 0.001); model 3 (deeply adjusted for diabetes, hypertension, BMI, central obesity, hyperlipidemia, smoking, and MetS: OR = 1.535, 95%CI: 1.213-1.942, P < 0.001); and model 4 (additionally adjusted for MASLD severity: OR = 1.501, 95%CI: 1.186-1.901, P = 0.001).
Figure 2 presents stratified analyses by sex, age, and central obesity. Among participants aged < 50 years (n = 1091), high UHR consistently conferred significantly elevated odds of intermediate/high risk across all four models (P < 0.05). In contrast, among those aged ≥ 50 years (n = 2810), high UHR strongly related to heightened odds in model 1 (OR = 4.027, 95%CI: 3.235-5.013, P < 0.001) and model 2 (OR = 1.757, 95%CI: 1.363-2.265, P < 0.001), yet the relevance was attenuated and lost significance after full adjustment in models 3 and 4 (both P > 0.05). In participants with central obesity, high UHR was related to significantly higher odds across all models, with the association persisting in the fully adjusted model 4 (OR = 1.488, 95%CI: 1.063-2.083, P = 0.021). A significant relationship was also observed among participants with no central obesity in model 4 (OR = 1.459, 95%CI: 1.042-2.043, P = 0.028). Marked sex-specific differences were evident. In males, high UHR demonstrated a robust and consistent positive relationship with CVD risk throughout all models (all P < 0.05). By contrast, in females, the association was evident only in unadjusted and partially adjusted analyses (models 1-2), but was abolished following further adjustment in models 3 and 4.
In the fully adjusted RCS analysis (adjusted for sex, age, smoking, hypertension, central obesity, hyperlipidemia, BMI, diabetes, MetS, and MASLD severity), a remarkable linear dose-responsive relationship between UHR and FRS was visualized in the overall cohort (n = 3901; P for overall < 0.001; P for nonlinearity = 0.114). Notably, this relationship was strongly sex-dependent. Among males (n = 2998), the relationship remained robust and linear (P for overall < 0.001; P for nonlinearity = 0.167). By contrast, among females (n = 903), the association was only marginal (P for overall = 0.057) and showed no evidence of nonlinearity (P for nonlinearity = 0.476; Figure 3).
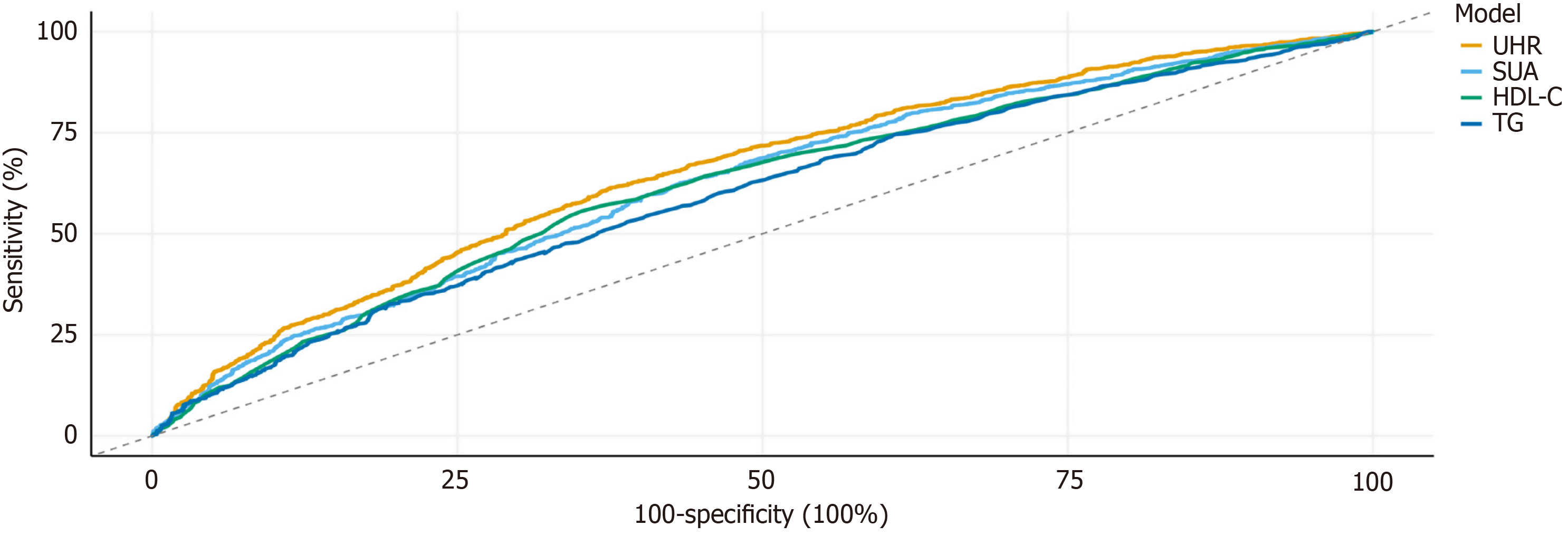
ROC analysis was executed to contrast the discriminative performance of UHR against conventional biomarkers to forecast intermediate/high CVD risk as defined by FRS. UHR demonstrated superior predictive ability relative to SUA, HDL-C, and TG, with an AUC of 0.655 (95%CI: 0.635-0.674). The optimum cutoff value for UHR reached 291.74, corresponding to a sensitivity of 61.4% and specificity of 62.5% (Figure 4; Table 2).
| Variables | AUC | 95%CI | Sensitivity | Specificity | Optimal cutoff | P value |
| UHR | 0.655 | (0.635, 0.674) | 61.4 | 62.5 | 291.739 | < 0.0001 |
| SUA | 0.626 | (0.607, 0.646) | 62.6 | 56.7 | 350.650 | < 0.0001 |
| HDL-C | 0.617 | (0.598, 0.637) | 55.6 | 64.8 | 1.195 | < 0.0001 |
| TG | 0.597 | (0.577, 0.617) | 52.9 | 61.1 | 1.815 | < 0.0001 |
Our research comprehensively examined the relation between the UHR and 10-year CVD risk, estimated using the FRS, in a large Asian cohort with MASLD and no prior CVD history. Three main findings emerged. First, high UHR was independently connected to significantly higher odds of intermediate/high 10-year CVD risk, even after established cardiovascular risk factors and MASLD severity were rigorously adjusted. Second, a clear linear dose-responsive relationship was visualized, indicating that increased UHR corresponded proportionally to escalating predicted CVD risk. Third, UHR demonstrated superior discriminative performance compared with conventional single biomarkers, including SUA, HDL-C, and TG, highlighting its potential clinical utility. Notably, stratified analyses revealed that the correlation was exceedingly pronounced among younger peoples (< 50 years), males, and those showing central obesity, while attenuated in older adults and females. As far as we know, this is the first study to systematically assess the relevance, dose-response pattern, and predictive value of UHR for CVD risk in an Asian MASLD population.
MASLD is increasingly identified as a disease closely linked with cardiometabolic disorders[15], sharing patho
Wang et al[30] have reported a UHR-FRS association in a Western MAFLD cohort. Our study extended these findings to a large, well-characterized Asian population diagnosed according to the 2023 international consensus criteria. Unlike the nonlinear association reported by Wang et al[30], our research observed a linear dose-responsive relationship (P for overall < 0.001; P for nonlinearity = 0.114), potentially reflecting population-specific differences in genetic background, dietary habits, and obesity phenotypes[31]. Specifically, polymorphisms in UA metabolism enzymes (e.g., solute carrier family 2 member 9, ATP binding cassette subfamily G member 2), lower dairy intake, higher purine consumption, and central adiposity prevalence in Asians may modulate UA metabolism and its relationship with CVD risk, producing a linear pattern in our cohort. Further functional and genetic studies are warranted to confirm these mechanisms.
Stratified analyses demonstrated that age, central obesity, and sex significantly modified the UHR-CVD association. Logistic regression and RCS analyses confirmed that UHR was a robust predictor in males (P for overall < 0.001), whereas the association in females was attenuated (P for overall = 0.057), possibly reflecting testosterone’s inhibitory effect on UA metabolism[30]. Age-stratified analyses indicated a stronger association in individuals < 50 years, likely because age-related renal decline elevated baseline UA, reducing the discriminatory capacity of UHR. Among participants with central obesity, the correlation between UHR and CVD risk was intensified, corresponding to the impact of visceral fat accumulation on metabolic abnormalities and impaired UA excretion[32,33].
UHR’s predictive performance remained significant after adjustment for MASLD severity, as confirmed by ROC analysis (AUC = 0.655) and identification of a clinically related cutoff (292) for early risk classification. These discoveries indicated that UHR might work as a practical biomarker for identifying early CVD risk, particularly among younger individuals, males, and patients with central obesity. Calculated from routine laboratory parameters obtained during standard health assessments, UHR can be integrated seamlessly into existing CVD risk evaluation frameworks without additional cost or burden, making it advantageous in resource-limited settings. Nevertheless, large-scale prospective studies and randomized controlled trials across diverse populations are necessary to validate its clinical utility and optimize risk-stratification thresholds.
Our research has some remarkable strengths. These include a large, well-characterized Asian MASLD cohort, contemporary diagnostic criteria, comprehensive adjustment for confounders, and rigorous evaluation of dose-response relationships and predictive performance relative to conventional biomarkers. However, certain restrictions warrant cautious consideration. First, the cross-sectional design intrinsically prevents any inference of causality. Second, cardiovascular risk was estimated using the FRS, a model derived from Western populations that may not be optimally calibrated for Asian cohorts. This miscalibration could lead to an overestimation of absolute risk and thereby distort the strength of the relevance between UHR and CVD risk. Furthermore, because the FRS primarily emphasizes coronary heart disease, it may insufficiently account for stroke, which constitutes a major burden of CVD in Asia. Although contemporary region-specific models such as China-PAR (Prediction for Atherosclerotic Cardiovascular Disease Risk in China) may offer superior calibration, their application was precluded by the retrospective design and incomplete data availability in our study. Third, hepatic steatosis was evaluated by ultrasonography, a method that, while widely adopted, is inherently subject to operator dependency. Fourth, selection bias cannot be excluded, as participants were recruited from a single tertiary center and might represent individuals with relatively higher socioeconomic status, thereby restricting the external generalizability of our results. Fifth, residual confounding from unmeasured or inadequately captured factors, like dietary patterns, lifestyle behaviors, or genetic predispositions, remains possible and could have influenced the observed associations. Finally, although UHR outperformed traditional biomarkers, its moderate discriminatory ability (AUC = 0.655) limits its application for individual-level decision-making. Therefore, UHR should complement, rather than replace, comprehensive CVD risk assessment. Future multicenter, prospective studies using region-specific risk scores and adjudicated clinical outcomes are essential to validate and refine UHR’s clinical application across diverse populations.
In conclusion, this study provided robust evidence that, among Asian MASLD patients, elevated UHR was independently associated with higher predicted 10-year CVD risk, exhibited a linear dose-response relationship with the FRS, and outperformed traditional single biomarkers (SUA, HDL-C, and TG) in predictive accuracy. This association was particularly pronounced in individuals with central obesity, those under 50 years of age, and males. The simplicity and cost-effectiveness of calculating UHR from routine laboratory tests render it a practical and readily implementable adjunctive tool for early recognition of MASLD patients at high risk of CVD, especially in younger adults and those with central obesity. In the future, large-scale yet multicenter prospective studies will be warranted to verify UHR’s predictive value for hard CVD endpoints, establish causality, refine optimal cutoff values across diverse ethnicities and age groups, and determine its incremental utility when integrated with established risk scores and emerging biomarkers.
| 1. | European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). J Hepatol. 2024;81:492-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 983] [Article Influence: 491.5] [Reference Citation Analysis (1)] |
| 2. | Miao L, Targher G, Byrne CD, Cao YY, Zheng MH. Current status and future trends of the global burden of MASLD. Trends Endocrinol Metab. 2024;35:697-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 428] [Article Influence: 214.0] [Reference Citation Analysis (0)] |
| 3. | Stefan N, Yki-Järvinen H, Neuschwander-Tetri BA. Metabolic dysfunction-associated steatotic liver disease: heterogeneous pathomechanisms and effectiveness of metabolism-based treatment. Lancet Diabetes Endocrinol. 2025;13:134-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 144] [Article Influence: 144.0] [Reference Citation Analysis (0)] |
| 4. | Targher G, Byrne CD, Tilg H. MASLD: a systemic metabolic disorder with cardiovascular and malignant complications. Gut. 2024;73:691-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 281] [Article Influence: 140.5] [Reference Citation Analysis (1)] |
| 5. | Li Y, Bai L. Association between uric acid to high-density lipoprotein cholesterol ratio and abdominal aortic calcification: A cross-sectional study. J Clin Lipidol. 2025;19:899-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Yang Y, Zhang J, Jia L, Su J, Ma M, Lin X. Uric acid to high-density lipoprotein cholesterol ratio predicts adverse cardiovascular events in patients with coronary chronic total occlusion. Nutr Metab Cardiovasc Dis. 2023;33:2471-2478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 7. | Zhou X, Xu J. Association between serum uric acid-to-high-density lipoprotein cholesterol ratio and insulin resistance in an American population: A population-based analysis. J Diabetes Investig. 2024;15:762-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 39] [Article Influence: 19.5] [Reference Citation Analysis (1)] |
| 8. | Liu P, Li J, Yang L, Zhang Z, Zhao H, Zhao N, Ou W, Zhang Y, Chen S, Wang G, Zhang X, Wu S, Yang X. Association between cumulative uric acid to high-density lipoprotein cholesterol ratio and the incidence and progression of chronic kidney disease. Front Endocrinol (Lausanne). 2023;14:1269580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 9. | Zhao H, Qiu X, Li HZ, Cui JJ, Sun YY. Association between Serum Uric Acid to HDL-Cholesterol Ratio and Nonalcoholic Fatty Liver Disease Risk among Chinese Adults. Biomed Environ Sci. 2023;36:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (1)] |
| 10. | Li Z, Liu Q, Yao Z. The serum uric acid-to-high-density lipoprotein cholesterol ratio is a predictor for all-cause and cardiovascular disease mortality: a cross-sectional study. Front Endocrinol (Lausanne). 2024;15:1417485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 11. | Cui Y, Zhang W. Long-term cardiovascular risk and mortality associated with uric acid to HDL-C ratio: a 20-year cohort study in adults over 40. Sci Rep. 2025;15:14242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 12. | Svenšek A, Lorber M, Gosak L, Verbert K, Klemenc-Ketis Z, Stiglic G. The Role of Visualization in Estimating Cardiovascular Disease Risk: Scoping Review. JMIR Public Health Surveill. 2024;10:e60128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 13. | Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd-Jones D, McEvoy JW, Michos ED, Miedema MD, Muñoz D, Smith SC Jr, Virani SS, Williams KA Sr, Yeboah J, Ziaeian B. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e596-e646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1000] [Cited by in RCA: 2022] [Article Influence: 288.9] [Reference Citation Analysis (0)] |
| 14. | Rehman H, Ang TFA, Tao Q, Au R, Farrer LA, Qiu WQ, Zhang X; Alzheimer's Disease Neuroimaging Initiative. Plasma protein risk scores for mild cognitive impairment and Alzheimer's disease in the Framingham heart study. Alzheimers Dement. 2025;21:e70066. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | Hakeem A, van Berlo J, Revelo XS. The Cardiohepatic Axis in Metabolic Disease: Liver to Heart. JACC Basic Transl Sci. 2025;10:101309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 16. | Hagström H, Shang Y, Hegmar H, Nasr P. Natural history and progression of metabolic dysfunction-associated steatotic liver disease. Lancet Gastroenterol Hepatol. 2024;9:944-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 139] [Reference Citation Analysis (0)] |
| 17. | Byrne CD, Armandi A, Pellegrinelli V, Vidal-Puig A, Bugianesi E. Μetabolic dysfunction-associated steatotic liver disease: a condition of heterogeneous metabolic risk factors, mechanisms and comorbidities requiring holistic treatment. Nat Rev Gastroenterol Hepatol. 2025;22:314-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 18. | Endo Y, Fujita M, Ikewaki K. HDL Functions-Current Status and Future Perspectives. Biomolecules. 2023;13:105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 42] [Reference Citation Analysis (0)] |
| 19. | Chen JX, Li Y, Zhang YB, Wang Y, Zhou YF, Geng T, Liu G, Pan A, Liao YF. Nonlinear relationship between high-density lipoprotein cholesterol and cardiovascular disease: an observational and Mendelian randomization analysis. Metabolism. 2024;154:155817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 38] [Reference Citation Analysis (0)] |
| 20. | He L, Qiu K, Zheng W, Kong W, Zeng T. Uric acid may serve as the sixth cardiometabolic criterion for defining MASLD. J Hepatol. 2024;80:e152-e153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 21. | Yang S, Ye Z, Liu M, Zhang Y, Wu Q, Zhou C, Zhang Z, He P, Zhang Y, Li H, Liu C, Qin X. Association of serum uric acid with all-cause and cardiovascular mortality among adults with nonalcoholic fatty liver disease. Clin Endocrinol (Oxf). 2023;98:49-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 22. | Park B, Jung DH, Lee YJ. Predictive Value of Serum Uric Acid to HDL Cholesterol Ratio for Incident Ischemic Heart Disease in Non-Diabetic Koreans. Biomedicines. 2022;10:1422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 42] [Reference Citation Analysis (0)] |
| 23. | Zhu W, Liang A, Shi P, Yuan S, Zhu Y, Fu J, Zheng T, Wen Z, Wu X. Higher serum uric acid to HDL-cholesterol ratio is associated with onset of non-alcoholic fatty liver disease in a non-obese Chinese population with normal blood lipid levels. BMC Gastroenterol. 2022;22:196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 39] [Reference Citation Analysis (0)] |
| 24. | Li XM, Liu SL, He YJ, Shu JC. Using new indices to predict metabolism dysfunction-associated fatty liver disease (MAFLD): analysis of the national health and nutrition examination survey database. BMC Gastroenterol. 2024;24:109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 17] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 25. | Lai X, Chen T. Association of serum uric acid to high-density lipoprotein cholesterol ratio with all-cause and cardiovascular mortality in patients with diabetes or prediabetes: a prospective cohort study. Front Endocrinol (Lausanne). 2024;15:1476336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 26. | Yi Y, Luo Q, Chen J, Chen Z, Aydemir HA, Chen P, Tang J, Luo F, Fang Z. Association between the uric acid-to-HDL-cholesterol ratio (UHR) and the risk of cardiovascular disease and dyslipidemia: a population-based study. Lipids Health Dis. 2025;24:143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 27. | Yu X, Sun F, Ming J, Liang S, Zhang W, Wang L, Li Q, Xu Q, Wang L, Shi L, Gao B, Ji Q. Serum uric acid to high-density lipoprotein cholesterol ratio is a promising marker for identifying metabolic syndrome in nondiabetic Chinese men. Postgrad Med. 2023;135:741-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 28. | Ding L, Guo H, Zhang C, Jiang B, Zhang S, Zhang J, Sui X. Serum uric acid to high-density lipoprotein cholesterol ratio is a predictor for all-cause and cardiovascular disease mortality in patients with diabetes: Evidence from NHANES 2005-2018. Nutr Metab Cardiovasc Dis. 2024;34:2480-2488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 29. | Ding L, Guo H, Zhang C, Liang X, Liu Y. Association between serum uric acid to high-density lipoprotein cholesterol ratio and all-cause and cardiovascular disease mortality after stroke: A cross-sectional study from 2005 to 2018. Nutr Metab Cardiovasc Dis. 2025;35:103909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 30. | Wang X, Shi Y, Zi Y, Long J, Shi R. Association of serum uric acid-to-high-density lipoprotein cholesterol ratio with cardiovascular disease risk in patients with metabolic dysfunction-associated fatty liver disease: a cross-sectional NHANES analysis. Front Nutr. 2025;12:1561594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 31. | Du L, Zong Y, Li H, Wang Q, Xie L, Yang B, Pang Y, Zhang C, Zhong Z, Gao J. Hyperuricemia and its related diseases: mechanisms and advances in therapy. Signal Transduct Target Ther. 2024;9:212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 266] [Article Influence: 133.0] [Reference Citation Analysis (0)] |
| 32. | Mina T, Xie W, Low DY, Wang X, Lam BCC, Sadhu N, Ng HK, Aziz NA, Tong TYY, Kerk SK, Choo WL, Low GL, Ibrahim H, Lim L, Tai ES, Wansaicheong G, Dalan R, Yew YW, Elliott P, Riboli E, Loh M, Ngeow J, Lee ES, Lee J, Best J, Chambers J. Adiposity and metabolic health in Asian populations: an epidemiological study using dual-energy x-ray absorptiometry in Singapore. Lancet Diabetes Endocrinol. 2024;12:704-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 33. | Panlu K, Zhou Z, Huang L, Ge L, Wen C, Lv H. Associations between obesity and hyperuricemia combing mendelian randomization with network pharmacology. Heliyon. 2024;10:e27074. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/