Published online Dec 21, 2025. doi: 10.3748/wjg.v31.i47.112705
Revised: September 28, 2025
Accepted: November 4, 2025
Published online: December 21, 2025
Processing time: 123 Days and 2.5 Hours
Indolent T-cell lymphoma of the gastrointestinal tract (iTCL-GI) is a rare mature T-cell lymphoma that has been formally recognized as a distinct entity in the 5th Edition World Health Organization Classification of Tumours of Haematolymphoid Tumours. However, the coexistence of iTCL-GI with epithelial malig
A 65-year-old female presented with a 5-month history of lower abdominal pain, bloating, and vomiting. An abdominal computed tomography scan revealed irregular thickening of the gastric wall. Endoscopy revealed diffuse mucosal edema and rigid mucosa along the lesser curvature of the gastric body. There was a 1.5 cm mucosal protrusion on the greater curvature. Biopsy revealed that the lamina propria was expanded by a dense, nondestructive infiltrate of small lym
Clinicians and pathologists must integrate assessment of these rare cases to prevent misdiagnosis and guide clinical practice.
Core Tip: This article is the first to present a rare case of indolent T-cell lymphoma of the gastrointestinal tract coexisting with gastric signet-ring cell carcinoma. The immunohistochemistry results revealed that CD3, CD8, and TIA-1 expression was positive, whereas the Ki-67 score was low. Clonal T-cell receptor rearrangement was detected. The main differential diagnoses were aggressive T-cell lymphomas and inflammatory issues. This rare case emphasizes that clinicians and patho
- Citation: Chen X, Bo JQ, Gao XX, Zhang SX, Li J, Wang H, Yang MY, Guo QQ, Xiu B, Zeng Y. Indolent T-cell lymphoma of the gastrointestinal tract coexisting with gastric signet-ring cell carcinoma: A case report and review of literature. World J Gastroenterol 2025; 31(47): 112705
- URL: https://www.wjgnet.com/1007-9327/full/v31/i47/112705.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i47.112705
Indolent T-cell lymphoma of the gastrointestinal tract (iTCL-GI) is a clonal T-cell proliferation that has been formally recognized as a distinct entity in the 5th Edition World Health Organization Classification of Tumours of Haematolymphoid Tumours[1]. However, the coexistence of iTCL-GI with primary epithelial malignancies has not been reported in clinical practice. We present the first case of iTCL-GI and gastric signet-ring cell carcinoma (SRCC), with a comprehensive analysis of its clinicopathological characteristics and treatment. We aim to increase diagnostic awareness among clinicians and pathologists regarding the coexistence of multiple primary tumors.
A 65-year-old female presented with persistent chronic abdominal pain, bloating and vomiting.
The patient underwent right hemicolectomy for mid-ascending colon perforation, with histopathology confirming the perforation and surrounding acute inflammatory changes (no malignancy identified). Postoperatively, the patient developed persistent chronic abdominal pain, bloating and vomiting.
The patient had a history of systemic lupus erythematosus (SLE), which was managed with long-term immunosuppressive therapy consisting of 2.5 mg per day of prednisone and 100 mg per day of hydroxychloroquine.
There was mild pressing pain in the lower abdomen region, and other physical examinations were normal.
Blood carbohydrate antigen (CA) 242 mass concentration, A242: 60.90 U/mL (reference range: < 20 U/mL), blood CA 19-9 mass concentration > 1000.00 U/mL (exceeding the upper limit of detection), and blood CA 50 mass concentration > 500.00 U/mL (exceeding the upper limit of the assay). No abnormalities were detected in routine blood or urine analyses.
T-cell receptor gene rearrangement analysis: The sample was analyzed for T-cell receptor (TCR) gene rearrangements using a TCR Gene Rearrangement Assay Kit (capillary electrophoresis) (Suzhou Yuntai Biomedical Technology Co., Ltd.). The targeted regions were TCRB-A (νβ + Јβ), TCRB-B (νβ + Јβ), TCRB-C (Dβ-Јβ), TCRG-A (νγ1-8, νγ10 + Јγ), TCRG-B (νγ9, νγ11 + Јγ) and TCRD (vδ + Dδ + Јδ). The cycling parameters were as follows: (1) Activation for 7 minutes at 95 °C; (2) 40 cycles of denaturation (95 °C for 45 seconds, 60 °C for 45 seconds, and 72 °C for 90 seconds); and (3) A final 10 minutes extension at 72 °C. After amplification, the polymerase chain reaction (PCR) product, quality control PCR product and GeneScan™ 500 LIZ™ Size Standard (Thermo Fisher Scientific, United States) were mixed with Hi-Di™ Formamide (Thermo Fisher Scientific, United States) to induce denaturation at 95 °C for 3 minutes. The products were subsequently separated through a polymer capillary electrophoresis system and automatically detected by fluorescence reading in Applied Biosystems 3500 and 3500 Dx Series Genetic Analyzers (Thermo Fisher Scientific, United States). Both blank controls and positive controls were used simultaneously (Table 1).
| Mastermix | TCRB tube-A | TCRB tube-B | TCRB tube-C | TCRG tube-A | TCRG tube-B | TCRD |
| Target | νβ-Јβ | νβ-Јβ | Dβ-Јβ | νγ1-8, νγ10 + Јγ | νγ9, νγ11 + Јγ | νδ + Dδ + Јδ |
| Valid detection range (bp) | 240-285 | 240-285 | 170-210, 285-325 | 145-255 | 80-140, 160-220 | 120-280 |
| Highest peak/third highest peak ratio | > 3-5 | > 3-5 | > 3-5 | > 5-7 | > 5-7 | > 5-7 |
| Result | + | + | - | - | - | - |
Next-generation sequencing: For the formalin-fixed paraffin-embedded samples, five 10 µm tumor slices were used for DNA extraction using the FFPE Nucleic Acid Extraction Kit (Geneseeq Technology Inc.) following the manufacturer’s instructions. DNA quality was assessed by spectrophotometry at absorbances of 230 nm, 260 nm, and 280 nm and quantified by a Qubit 2.0.
Libraries were prepared as reported previously[2]. Hybridization-based target enrichment was carried out with a panel of 571 leukemia-related and lymphoma-related genes (Geneseeq Technology, Inc.). Libraries captured by Dynabeads M-270 (Life Technologies) were amplified in KAPA HiFi HotStart ReadyMix (KAPA Biosystems). The target-enriched libraries were sequenced on the DNBSEQ-T7 platform with 2 × 150 bp pair-end reads.
Sequencing data were demultiplexed by bcl2fastq (v 2.19), analyzed by Trimmomatic[3] to remove low-quality (quality < 15) or N bases, and mapped to the reference hg19 genome (Human Genome version 19) using the Burrows-Wheeler Aligner[4]. Germline mutation analysis was performed using a single-sample variant caller DNBSEQ-T7. Single nucleotide variants and indels were retained if they had a variant allele fraction ≥ 2% and at least three unique reads on different strands with good quality scores after filtering for common single-nucleotide polymorphisms. Copy number variants (CNVs) with fold changes ≥ 1.6 and ≤ 0.6 were identified as CNV amplification and deletion, respectively.
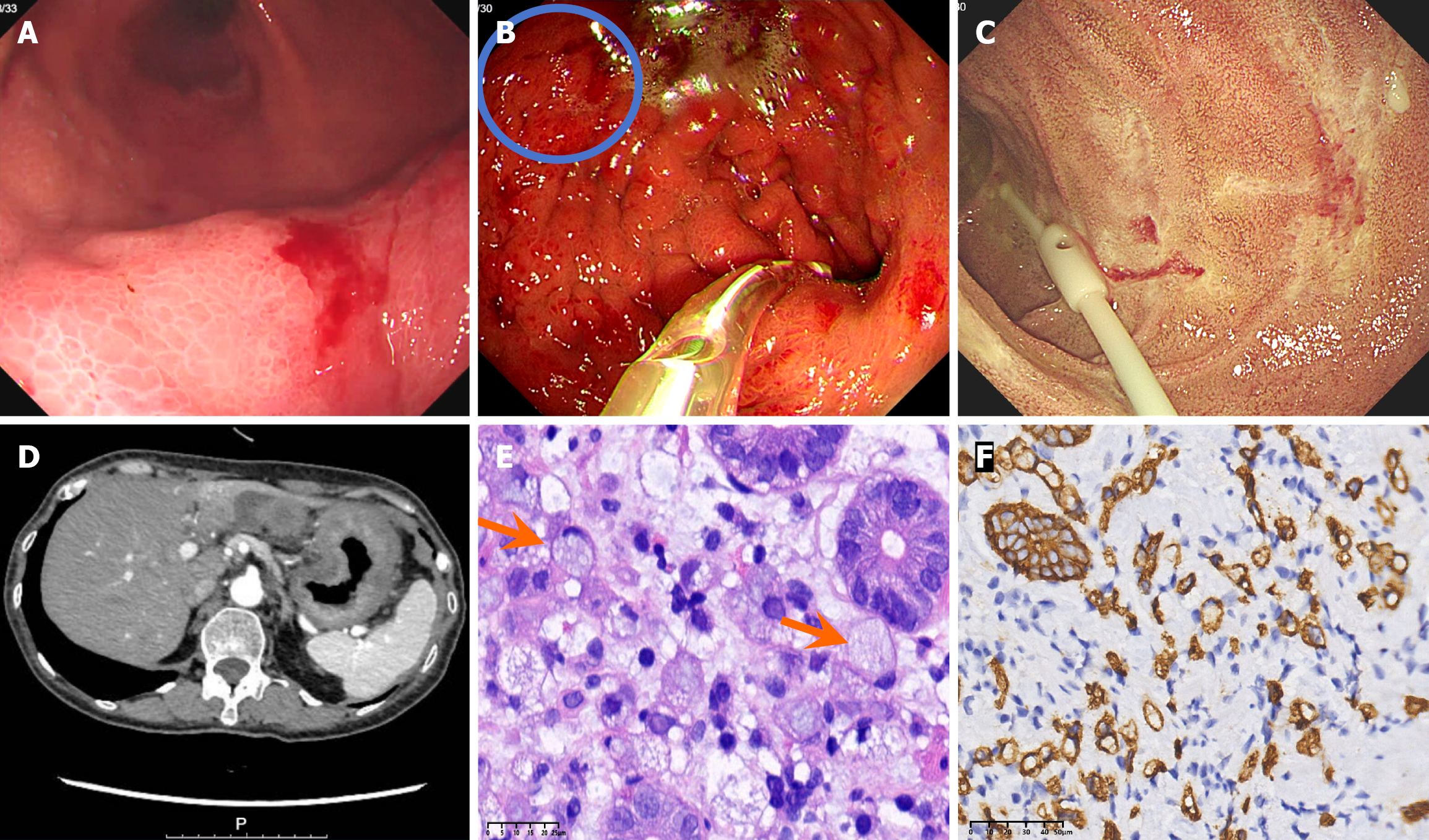
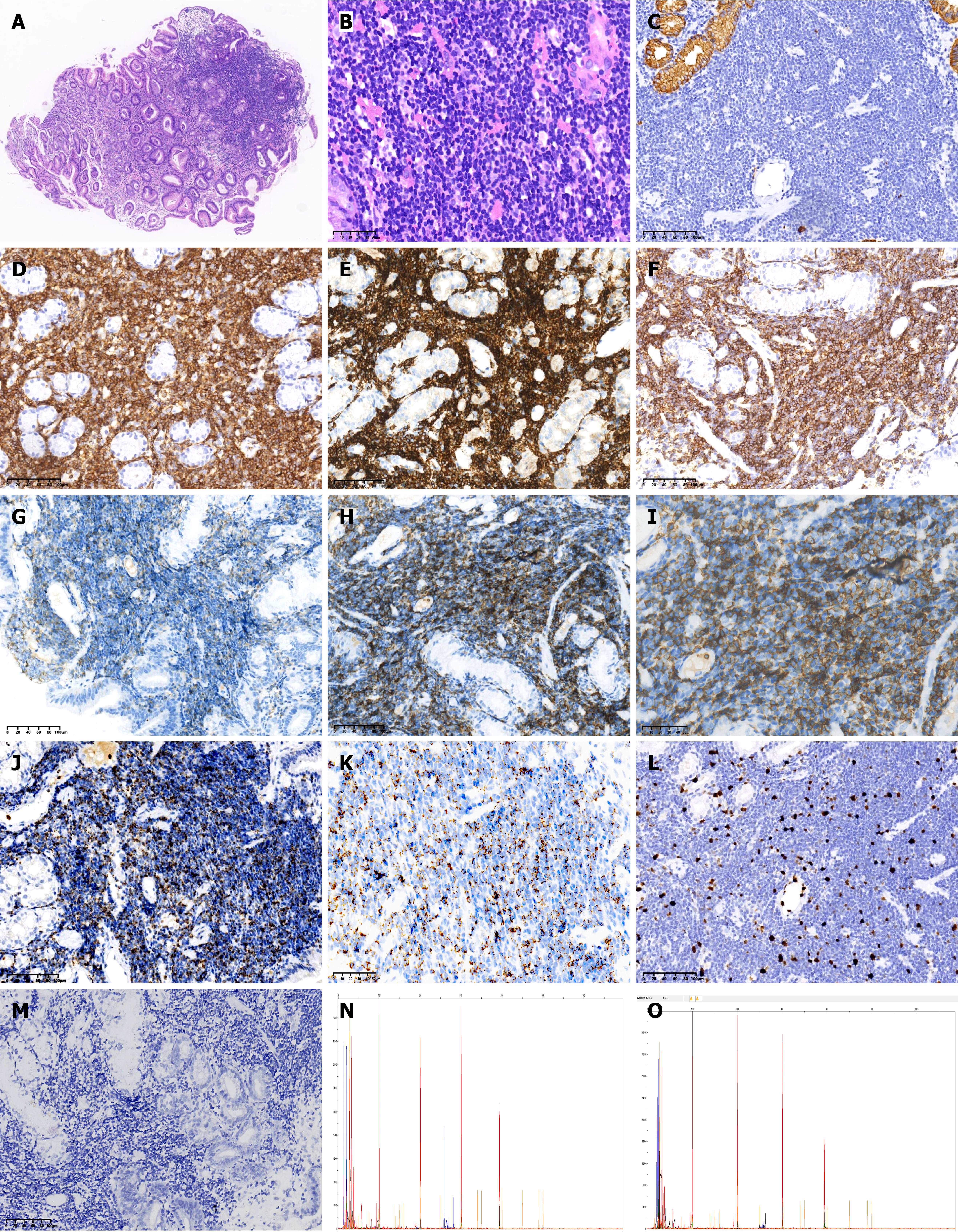
Pathological characteristics: (1) Gastric mucosal lesions in the lesser curvature of the gastric body: Histopathological examination revealed diffuse infiltration of signet-ring cells within the lamina propria. Immunohistochemical studies confirmed that these tumor cells were positive for the cytokeratins AE1/AE3 and CK8 (Figure 1); and (2) Mucosal lesion of the greater curvature of the stomach: Histopathological examination revealed that the lamina propria had a dense infiltrate of small lymphocytes, with round or oval nuclei, inconspicuous nucleoli, and scant cytoplasm. Immunohistochemical examination revealed that these lymphocytes expressed CD3, CD8, CD5, CD7 and TIA-1 but were negative for CD4, CD56, ALK, and CD30. The Ki-67 index was < 10%. Molecular studies revealed that TCR gene rearrangement was positive (TCRB νβ-Јβ1/2 and TCRB νβ-Јβ2 were positive, but TCRB Dβ-Јβ-1/2, TCRD νδ + Dδ + Јδ, TCRG νγ1-8, νγ10 + Јγ, and TCRG νγ9, νγ11 + Јγ were negative). Epstein-Barr virus encoded RNA's (EBER) was negative (Figure 2). Next-generation sequencing revealed 2 somatic variants: (1) Succinate dehydrogenase subunit-A gene (SDHA) (missense mutation, c.634_636delinsACC, p. D212T, exon 6, 15.39%); and (2) Ten-eleven translocation 2 (TET2) (missense mutation, c.286C>G, p. R96G, exon 3, 2.66%). No gene mutations in isocitrate dehydrogenase 2 (IDH2), Ras homolog gene family, member A, or SET domain-containing 2 (SETD2) were detected. The SDHA variant has not been previously reported in iTCL-GI, and its pathogenic significance in this context is uncertain.
A computed tomography (CT) scan revealed irregular wall thickening in the gastric body and antrum with moderate enhancement on an enhanced scan. There were no enlarged lymph nodes in the abdominal cavity. Endoscopy revealed diffuse mucosal edema and erythema throughout the gastric fundus, body, and antrum with poor expansion of the gastric cavity after air insufflation. Mucosal rigidity on the lesser curvature and a 1.5 cm mucosal protrusion on the greater curvature was observed. Multiple superficial erosions in the descending duodenum with a fine granular pattern were observed (Figure 1).
The iTCL-GI coexisting with SRCC.
Treatment plans focused on the SRCC with chemotherapy, including oxaliplatin (200 mg) and fluorouracil (3 g); the treatment plan did not target the iTCL-GI. All immunosuppressive medications were stopped before chemotherapy.
After three months of follow-up, the patient had completed four cycles of chemotherapy and experienced a weight loss of 5 kg. Although the CT scan revealed persistent irregular thickening of the gastric wall, the symptoms of abdominal pain markedly improved. Laboratory examinations revealed a CA 50 (243.40 U/mL) (lower than the first time), while the levels of the other CAs were within normal limits.
The iTCL-GI primarily affects adults. The small intestine is the most frequently involved site, followed by the colon. Dissemination to extragastrointestinal locations, such as the lymph nodes and bone marrow, is uncommon in the initial state but can occur during disease progression or transformation. Clinical symptoms and signs are not specific and include chronic diarrhea, abdominal pain, vomiting, dyspepsia, and weight loss. Endoscopic evaluation also reveals nonspecific inflammatory changes, including mucosal hyperplasia or ulcers (without deep ulceration). In some cases, it may present as a small polypoid. Histopathology shows the lamina propria is expanded by a dense, nondestructive infiltrate of small lymphocytes. These lymphocytes typically exhibit a mature T-cell phenotype and CD2, CD3, CD4 and/or CD8 expression, but may lose CD5 and CD7 expression in some cases. However, granzyme B expression is infrequent. It was observed in our patient that the CD8+ cells were TIA-1+. These cells were negative for CD30 and CD56 and had a low proliferation index (Ki-67 < 10%)[1].
Four distinct CD4/CD8 expression patterns were identified: (1) CD4-/CD8+; (2) CD4+/CD8-; (3) CD4-/CD8-; and (4) CD4+/CD8+. The CD8-positive subgroup (CD4-/CD8+) is the most common, and it frequently expresses TIA-1[5-8]. The CD4-positive subgroup (CD4+/CD 8-) is the second most frequent pattern. The other two subgroups are rarely observed. The immunophenotype of our patient (CD3+, CD8+, CD4- and TIA-1+) is consistent with that of the most common subgroup reported in the literature. In addition, cases with abnormal expression of CD20 must be distinguished from B-cell lymphoma[9,10].
Structural alterations in the IL2 gene are observed in CD8+ patients, while CD4+ patients frequently have mutations in Janus kinase (JAK)-signal transducer and activator of transcription (STAT) pathway genes (e.g., STAT3 and SOCS1) and epigenetic regulatory genes (e.g., TET2, DNMT3A and KMT2D)[7]. These differences in gene expression provide a theo
This case had 2 somatic variants, namely, TET2 (missense mutation, c.286C>G, p.R96G) and SDHA (missense mutation, c.634_636delinsACC, p.D212T). TET2 mutations have been previously reported in CD4+ iTCL-GI; two of these mutations were c.2457T>G (p.Y819*), and the other mutation was c.2725C>T (p.Q909*)[7]. The mutation site of TET2 in our patient (CD8+ iTCL-GI) was different from that reported in the CD4+ iTCL-GI patient. The SDHA variant has not been reported in iTCL-GI. Mutations in aggressive T-cell lymphomas, such as enteropathy-associated T-cell lymphoma (EATL) and monomorphic epitheliotropic intestinal T-cell lymphoma (MEITL) (SETD2 mutation) and angioimmunoblastic T-cell lymphoma (IDH2 mutation), were not identified in our case, which is consistent with the results reported by Soderquist et al[7].
The relationship between SLE and non-Hodgkin lymphoma (NHL) remains unclear. Some studies have reported that these patients have a high risk of developing NHL. The most commonly reported NHL subtypes are diffuse large B-cell lymphoma (DLBCL) and marginal zone lymphoma, whereas T-cell lymphomas are rare[12-14]. Another study revealed that SLE is associated with a 2.7-fold overall increase in NHL risk, with the highest risk concentrated within the first 2-5 years following SLE diagnosis[15]. Although B-cells and autoantibody production are central to SLE pathogenesis, their activation and functionality are strongly dependent on T-cell-mediated costimulatory signals. Thus, T-cells also play a key role in the immunopathology of SLE and have become important therapeutic targets[16,17]. It has been reported that the tumor suppressor gene PTEN may provide a link between SLE and B-cell lymphoma via a defective apoptosis pathway[18]. While this mechanism is described for B-cell lymphomas, similar dysregulation of apoptotic pathways in T-cells could theoretically contribute to the development of iTCL-GI in the setting of SLE, though this remains speculative and requires further investigation. In addition, some immunosuppressive therapies may increase the risk of NHL[19,20].
Inflammatory diseases: Histologically, inflammatory disorders usually involve mixed inflammatory cell infiltrates, and a granulomatous structure is seen in some cases[1]. TCR gene rearrangement is negative in these cases, and no abnormal T-cell populations are detected by flow cytometry. Notably, tumour necrosis factor-alpha (TNF-α) inhibitor therapy for Crohn's disease (CD) may induce high infiltration of T-cells into the lesion site. These cells are positive for CD8, TIA-1 and TCR-βF1, which may regress after drug withdrawal. However, after 2 years of withdrawal, the same monoclonal T-cell infiltration reappeared at the active inflammatory site, suggesting that there may be a potential correlation between active inflammation and iTCL-GI. Moreover, TNF-α inhibitors can be used to treat CD or affect the evolution of iTCL-GI[21].
MEITL: MEITL arises most commonly in the small intestine. It is more common in Asia, where it is not associated with celiac disease. The tumor often appears as a large, ulcerated mass and has a more aggressive clinical course. Histopathologically, the lymphocytes are small to medium-sized cells with irregular nuclei. Necrosis and an inflammatory back
| Feature | Indolent T-cell lymphoma of the gastrointestinal tract | Enteropathy-associated T-cell lymphoma | Monomorphic epitheliotropic intestinal T-cell lymphoma |
| Epidemiology | Often chronic and relapsing, but with a favorable long-term prognosis. The etiology is unknown | More common in Europe and United States. Genetic background of CD, refractory celiac disease | More common in Asia. There is no association with celiac disease (CD) |
| Major clinical presentation | Abdominal symptoms (such as chronic diarrhea, pain, vomiting dyspepsia) | Abdominal symptoms (such as pain, diarrhea) and weight loss; common bowel perforation or obstruction | Abdominal symptoms (such as pain, bleeding) and weight loss; common bowel perforation or obstruction |
| Commonest localization in the gastrointestinal tract | Small bowel or colon | Small intestine | Small intestine |
| Lesional involvement of gastrointestinal tract | Superficial (mucosal or submucosal) | Circumferentially oriented ulcers or ulcerated nodules | Ulcerated mass |
| Histopathology | Small lymphoid cells with minimal nuclear atypia. The lamina propria is usually expanded. Intraepithelial lymphocytes are not increased | Medium-sized to large lymphoid cells. A variable inflammatory background. Angioinvasion and angiodestruction | Small to medium-sized cells. Lacking necrosis and an inflammatory background. Epitheliotropism is common |
| Immunophenotype | CD3+, CD8+ with TIA-1+ and/or CD4+, Ki 67 < 10% | CD3+, CD7+, CD103+; positive for TIA1, granzyme B, and perforin | CD2+, CD3+, CD7+, CD8+, CD56+, CD5-, CD4-, TIA-1+ |
| T-cell receptor expression | TCRαβ+ | Usually, negative | Usually, TCRγδ+ or TCRαβ+ |
| Molecular genetics | JAK2: STAT3 fusion; mutations of JAK/STAT pathway genes and epigenetic modifier genes | Gains of 9q34; loss of 16q12; mutations of JAK/STAT pathway genes (commonly JAK1 and STAT3) | Gains of 9q34; loss of 16q12; mutations of JAK/STAT pathway genes (commonly JAK3, STAT5B) and SET domain-containing 2 |
| Clinical course | Indolent, often chronic persistent or relapsing | Aggressive | Aggressive |
EATL: EATL is an aggressive T-cell lymphoma that predominantly arises in the small intestine. It is more common in Europe and the United States. All patients have a history of refractory celiac disease. Intestinal tumors can manifest as circumferentially oriented ulcers, and histopathological examination reveals pleomorphic large or medium-sized lymphoid cells. These tumors may have a pronounced inflammatory background, and neovascularization and angiodestruction are common. Immunophenotypically, lymphocytes express CD3, CD7, CD103 and cytotoxic markers (TIA-1, granzyme B, perforin), with a high Ki-67 index (> 50%). EBER is typically negative. The presence of JAK1 and/or STAT3 SH2 domain hotspot mutations is helpful in differentiation (Table 2).
Gastrointestinal T-cell lymphoma, not otherwise specified: Gastrointestinal T-cell lymphoma (GITCL), not otherwise specified is an aggressive T-cell lymphoma that affects mainly the colon and small intestine, with regional lymph node involvement in some cases. Histopathology reveals mucosal infiltration by pleomorphic medium-to-large lymphocytes, often with ulceration. The tumor cells usually exhibit a CD4+ or CD4-/CD8-, TIA-1+ and rarely Epstein-Barr virus positive (8%) phenotype. Diagnosis requires the exclusion of other T-cell and natural killer (NK)-cell lymphomas.
Secondary GITCL: Patients usually have a history of a primary tumor and constitutional B symptoms. Histopathological examination reveals more aggressive features than primary GITCLs, with large, atypical lymphoid cells and high mitotic activity (> 15/10 high power field) and P53 overexpression. In addition, necrosis is common. The immunophenotype is the same as that of the primary disease.
Indolent NK-cell lymphoproliferative disorder of the gastrointestinal tract: Indolent NK-cell lymphoproliferative disorder is rare and predominantly involves the stomach. Patients are usually asymptomatic or have vague gastroin
The iTCL-GI tends to grow slowly, whereas SRCC is more aggressive. Therefore, treatments should mainly target the more aggressive cancer. For locally advanced gastric SRCC, the standard treatment often involves perioperative chemo
The patient received oxaliplatin, calcium folinate, and fluorouracil, with a focus on SRCC, because iTCL-GI has a poor response to conventional chemotherapy. Immunosuppressants have been reported to effectively alleviate gastrointestinal symptoms associated with iTCL-GI, although these lymphocytes could not be eradicated[26-28]. The patient had a history of SLE, and all medications for SLE had been discontinued because the SLE was assessed as stable, following evaluation by a rheumatology specialist.
Despite its generally indolent behavior, iTCL-GI may develop into aggressive T-cell lymphoma, which results in a poor prognosis[6,29]. Some cases have been reported in which the afflicted areas are outside the stomach, such as the bone marrow[10] or the lung[27]. Other rare cases of concurrent DLBCL have been reported[9,30]. One of the patients died from coronavirus disease 2019 during a three-month follow-up without receiving chemotherapy[9], while another achieved stable disease following eight cycles of C/EBP homologous protein plus rituximab, with no evidence of metastasis on six-month positron emission tomography (PET)-CT[30].
It is important to perform regular endoscopic reexaminations because PET-CT often lacks specificity. Endoscopic findings such as nodularity, erythema, or erosions may indicate either persistent lymphoma or carcinoma; the final diagnosis depends on rebiopsy. The iTCL-GI has a long overall survival, with slow progression and a tendency for recurrence. The long-term prognosis depends mainly on how the stomach cancer progresses. However, the possibility of iTCL-GI transformation into aggressive lymphoma remains a concern and requires long-term endoscopic and clinical monitoring for both malignancies. We recommend performing endoscopy every 3-6 months during the first year, with subsequent individualized adjustments.
The iTCL-GI is a rare type of T-cell lymphoma. Its clinical symptoms and signs are nonspecific. The pathological features include small lymphocytes confined to the mucosal and submucosal layers without the formation of ulcerations; CD2, CD3, CD4 and/or CD8 positivity; low proliferative activity; negative EBER expression; and clonal TCR gene rearran
We gratefully acknowledge Professor Li XQ (Department of Pathology, Fudan University Shanghai Cancer Center) for histopathological diagnosis and Dr. Wei R (Department of Molecular Pathology Laboratory, Fudan University Shanghai Cancer Center) for her help with the interpretation of the bioinformatics results.
| 1. | World Health Organization Classification of Tumours Editorial Board. WHO Classification of Tumours Haematolymphoid Tumours. 5th ed. Lyon (France): International Agency for Research on Cancer, 2024: 712-714. |
| 2. | Yang Z, Yang N, Ou Q, Xiang Y, Jiang T, Wu X, Bao H, Tong X, Wang X, Shao YW, Liu Y, Wang Y, Zhou C. Investigating Novel Resistance Mechanisms to Third-Generation EGFR Tyrosine Kinase Inhibitor Osimertinib in Non-Small Cell Lung Cancer Patients. Clin Cancer Res. 2018;24:3097-3107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 413] [Article Influence: 51.6] [Reference Citation Analysis (2)] |
| 3. | Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114-2120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30322] [Cited by in RCA: 44562] [Article Influence: 3713.5] [Reference Citation Analysis (2)] |
| 4. | Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754-1760. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29052] [Cited by in RCA: 36485] [Article Influence: 2146.2] [Reference Citation Analysis (0)] |
| 5. | Perry AM, Warnke RA, Hu Q, Gaulard P, Copie-Bergman C, Alkan S, Wang HY, Cheng JX, Bacon CM, Delabie J, Ranheim E, Kucuk C, Hu X, Weisenburger DD, Jaffe ES, Chan WC. Indolent T-cell lymphoproliferative disease of the gastrointestinal tract. Blood. 2013;122:3599-3606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 144] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 6. | Fan W, Niu L, He H, Yuan J, Yuan F, Shi X, Wang Y, Chen M, Huang M, Zhou F, Xu J, Chen Q. Indolent T-cell lymphoproliferative disorder of gastrointestinal tract with unusual clinical courses: report of 6 cases and literature review. Virchows Arch. 2023;482:729-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 7. | Soderquist CR, Patel N, Murty VV, Betman S, Aggarwal N, Young KH, Xerri L, Leeman-Neill R, Lewis SK, Green PH, Hsiao S, Mansukhani MM, Hsi ED, de Leval L, Alobeid B, Bhagat G. Genetic and phenotypic characterization of indolent T-cell lymphoproliferative disorders of the gastrointestinal tract. Haematologica. 2020;105:1895-1906. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 8. | Shao SH, Gu HY, Lin DL, Shi HL, Zhang YJ, Li YJ. [Clinicopathological features of indolent T-cell lymphoproliferative disorder of the gastrointestinal tract: a report of five cases]. Zhonghua Bing Li Xue Za Zhi. 2019;48:762-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 9. | Chen LJ, Chu X, Li BB, Li L, Li WS. [Indolent T-cell lymphoma of the gastrointestinal tract with synchronous diffuse large B-cell lymphoma: report of a case]. Zhonghua Bing Li Xue Za Zhi. 2024;53:870-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Wang X, Ng CS, Chen C, Yu G, Yin W. An unusual case report of indolent T-cell lymphoproliferative disorder with aberrant CD20 expression involving the gastrointestinal tract and bone marrow. Diagn Pathol. 2018;13:82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Cao X, Mulroney C, Wang HY. Treatment of an Indolent T-Cell Lymphoma of the Gastrointestinal Tract Harboring STAT3::JAK2 With Jakafi (Ruxolitinib) With Significant Clinical Improvements. EJHaem. 2025;6:e70047. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Bernatsky S, Ramsey-Goldman R, Rajan R, Boivin JF, Joseph L, Lachance S, Cournoyer D, Zoma A, Manzi S, Ginzler E, Urowitz M, Gladman D, Fortin PR, Edworthy S, Barr S, Gordon C, Bae SC, Sibley J, Steinsson K, Nived O, Sturfelt G, St Pierre Y, Clarke A. Non-Hodgkin's lymphoma in systemic lupus erythematosus. Ann Rheum Dis. 2005;64:1507-1509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 92] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 13. | Bernatsky S, Ramsey-Goldman R, Labrecque J, Joseph L, Boivin JF, Petri M, Zoma A, Manzi S, Urowitz MB, Gladman D, Fortin PR, Ginzler E, Yelin E, Bae SC, Wallace DJ, Edworthy S, Jacobsen S, Gordon C, Dooley MA, Peschken CA, Hanly JG, Alarcón GS, Nived O, Ruiz-Irastorza G, Isenberg D, Rahman A, Witte T, Aranow C, Kamen DL, Steinsson K, Askanase A, Barr S, Criswell LA, Sturfelt G, Patel NM, Senécal JL, Zummer M, Pope JE, Ensworth S, El-Gabalawy H, McCarthy T, Dreyer L, Sibley J, St Pierre Y, Clarke AE. Cancer risk in systemic lupus: an updated international multi-centre cohort study. J Autoimmun. 2013;42:130-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 226] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 14. | Knight JS, Blayney DW, Somers EC. Patients with systemic lupus erythematosus and haematological malignancy at a tertiary care centre: timing, histopathology and therapy. Lupus Sci Med. 2014;1:e000051. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Ekström Smedby K, Vajdic CM, Falster M, Engels EA, Martínez-Maza O, Turner J, Hjalgrim H, Vineis P, Seniori Costantini A, Bracci PM, Holly EA, Willett E, Spinelli JJ, La Vecchia C, Zheng T, Becker N, De Sanjosé S, Chiu BC, Dal Maso L, Cocco P, Maynadié M, Foretova L, Staines A, Brennan P, Davis S, Severson R, Cerhan JR, Breen EC, Birmann B, Grulich AE, Cozen W. Autoimmune disorders and risk of non-Hodgkin lymphoma subtypes: a pooled analysis within the InterLymph Consortium. Blood. 2008;111:4029-4038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 445] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 16. | Tenbrock K, Rauen T. T cell dysregulation in SLE. Clin Immunol. 2022;239:109031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 55] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 17. | Muñoz-Urbano M, Quintero-González DC, Vasquez G. T cell metabolism and possible therapeutic targets in systemic lupus erythematosus: a narrative review. Immunopharmacol Immunotoxicol. 2022;44:457-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 18. | Xu Y, Wiernik PH. Systemic lupus erythematosus and B-cell hematologic neoplasm. Lupus. 2001;10:841-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Bernatsky S, Joseph L, Boivin JF, Gordon C, Urowitz M, Gladman D, Fortin PR, Ginzler E, Bae SC, Barr S, Edworthy S, Isenberg D, Rahman A, Petri M, Alarcón GS, Aranow C, Dooley MA, Rajan R, Sénécal JL, Zummer M, Manzi S, Ramsey-Goldman R, Clarke AE. The relationship between cancer and medication exposures in systemic lupus erythaematosus: a case-cohort study. Ann Rheum Dis. 2008;67:74-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 114] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 20. | Lanjewar S, McFarlane IM, Parker KN, Saad H, Haddadin M, Hirsch E, Benyaminov F, Kecelli M, Lazaro D, Bukhari Z, Gupta R, Haseeb MA. Long-term immunosuppression and multiple transplants predispose systemic lupus erythematosus patients with cytopenias to hematologic malignancies. Medicine (Baltimore). 2021;100:e25985. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Edison N, Belhanes-Peled H, Eitan Y, Guthmann Y, Yeremenko Y, Raffeld M, Elmalah I, Trougouboff P. Indolent T-cell lymphoproliferative disease of the gastrointestinal tract after treatment with adalimumab in resistant Crohn's colitis. Hum Pathol. 2016;57:45-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 22. | Shitara K, Rha SY, Wyrwicz LS, Oshima T, Karaseva N, Osipov M, Yasui H, Yabusaki H, Afanasyev S, Park YK, Al-Batran SE, Yoshikawa T, Yanez P, Dib Bartolomeo M, Lonardi S, Tabernero J, Van Cutsem E, Janjigian YY, Oh DY, Xu J, Fang X, Shih CS, Bhagia P, Bang YJ; KEYNOTE-585 investigators. Neoadjuvant and adjuvant pembrolizumab plus chemotherapy in locally advanced gastric or gastro-oesophageal cancer (KEYNOTE-585): an interim analysis of the multicentre, double-blind, randomised phase 3 study. Lancet Oncol. 2024;25:212-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 223] [Article Influence: 111.5] [Reference Citation Analysis (0)] |
| 23. | Zhang X, Liang H, Li Z, Xue Y, Wang Y, Zhou Z, Yu J, Bu Z, Chen L, Du Y, Wang X, Wu A, Li G, Su X, Xiao G, Cui M, Wu D, Chen L, Wu X, Zhou Y, Zhang L, Dang C, He Y, Zhang Z, Sun Y, Li Y, Chen H, Bai Y, Wang Y, Yu P, Zhu G, Suo J, Jia B, Li L, Huang C, Li F, Ye Y, Xu H, Wang X, Yuan Y, E J, Ying X, Yao C, Shen L, Ji J. Perioperative or postoperative adjuvant oxaliplatin with S-1 versus adjuvant oxaliplatin with capecitabine in patients with locally advanced gastric or gastro-oesophageal junction adenocarcinoma undergoing D2 gastrectomy (RESOLVE): final report of a randomised, open-label, phase 3 trial. Lancet Oncol. 2025;26:312-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 25] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 24. | Liu M, Feng B, He N, Yan R, Qin J. Efficacy of fluorouracil combined with paclitaxel and oxaliplatin for the treatment of advanced gastric signet ring cell carcinoma. World J Gastrointest Surg. 2025;17:94286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 25. | Kang YK, Yook JH, Park YK, Lee JS, Kim YW, Kim JY, Ryu MH, Rha SY, Chung IJ, Kim IH, Oh SC, Park YS, Son T, Jung MR, Heo MH, Kim HK, Park C, Yoo CH, Choi JH, Zang DY, Jang YJ, Sul JY, Kim JG, Kim BS, Beom SH, Cho SH, Ryu SW, Kook MC, Ryoo BY, Kim HK, Yoo MW, Lee NS, Lee SH, Kim G, Lee Y, Lee JH, Noh SH. PRODIGY: A Phase III Study of Neoadjuvant Docetaxel, Oxaliplatin, and S-1 Plus Surgery and Adjuvant S-1 Versus Surgery and Adjuvant S-1 for Resectable Advanced Gastric Cancer. J Clin Oncol. 2021;39:2903-2913. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 270] [Article Influence: 54.0] [Reference Citation Analysis (1)] |
| 26. | Wang L, Koh E, Kumar B, Low MSY. Indolent T Cell Lymphoproliferation of the Gastrointestinal Tract: An Evolving Disease Entity. Hematol Rep. 2024;16:179-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 27. | David R, Mishra K, Gilbert ER, Mirza KM, Hendler S. Indolent T-Cell Lymphoproliferative Disease: A Rare Case of a Benign Lymphoma of the Gastrointestinal Tract With Extra-Gastrointestinal Involvement. ACG Case Rep J. 2022;9:e00879. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 28. | Liu H, Cao L, Zhao X, Miao Y, Wu W, Shi X, Zhang X, Yin H, Zhu H, Xu W, Li J, Fan L. Metronomic chemotherapy for indolent T-cell lymphoproliferative disorder of the gastrointestinal tract. Cancer Sci. 2023;114:3793-3796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 29. | Perry AM, Bailey NG, Bonnett M, Jaffe ES, Chan WC. Disease Progression in a Patient With Indolent T-Cell Lymphoproliferative Disease of the Gastrointestinal Tract. Int J Surg Pathol. 2019;27:102-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 30. | Guo L, Wen Z, Su X, Xiao S, Wang Y. Indolent T-cell lymphoproliferative disease with synchronous diffuse large B-cell lymphoma: A case report. Medicine (Baltimore). 2019;98:e15323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/