Published online Dec 21, 2025. doi: 10.3748/wjg.v31.i47.111900
Revised: October 7, 2025
Accepted: November 5, 2025
Published online: December 21, 2025
Processing time: 160 Days and 17.9 Hours
The incidence and prevalence of metabolic dysfunction-associated steatotic liver disease (MASLD) have continued to increase in recent years, making it one of the most common chronic liver diseases worldwide. MASLD is highly comorbid with obesity, type 2 diabetes, cardiovascular disease, and chronic kidney disease, po
Core Tip: Artificial intelligence (AI) is rapidly advancing the management of metabolic dysfunction-associated steatotic liver disease (MASLD). Because AI can integrate imaging, multiomics, and electronic health record data, the use of AI enables earlier diagnosis, more accurate risk stratification, and personalized treatment. This mini-review summarizes recent progress and future directions for applying AI to improve MASLD care and outcomes.
- Citation: Lou JJ, Zeng J. Artificial intelligence applications for managing metabolic dysfunction-associated steatotic liver disease: Current status and future prospects. World J Gastroenterol 2025; 31(47): 111900
- URL: https://www.wjgnet.com/1007-9327/full/v31/i47/111900.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i47.111900
Metabolic dysfunction-associated steatotic liver disease (MASLD), previously known as nonalcoholic fatty liver disease (NAFLD), is a chronic liver disorder closely linked to metabolic syndrome[1]. The diagnostic criteria for MASLD have shifted away from the traditional “exclusion-based” definition of NAFLD, which requires ruling out significant alcohol consumption or other specific liver diseases, to a “compatibility-based” standard centered on the evidence for metabolic dysfunction. This shift emphasizes the positive association between MASLD and metabolic abnormalities, such as obesity, type 2 diabetes (T2D), hypertension, and dyslipidemia[1]. The global increase in MASLD prevalence is linked to the increasing rates of obesity, T2D, hypertension, and dyslipidemia, especially in the context of unhealthy lifestyles and increasingly sedentary behavior[2-4]. Currently, approximately 38% of adults and 7%-14% of children and adolescents are affected by MASLD, and the prevalence in adults is projected to exceed 55% by 2040[5,6]. In China, the increasing pre
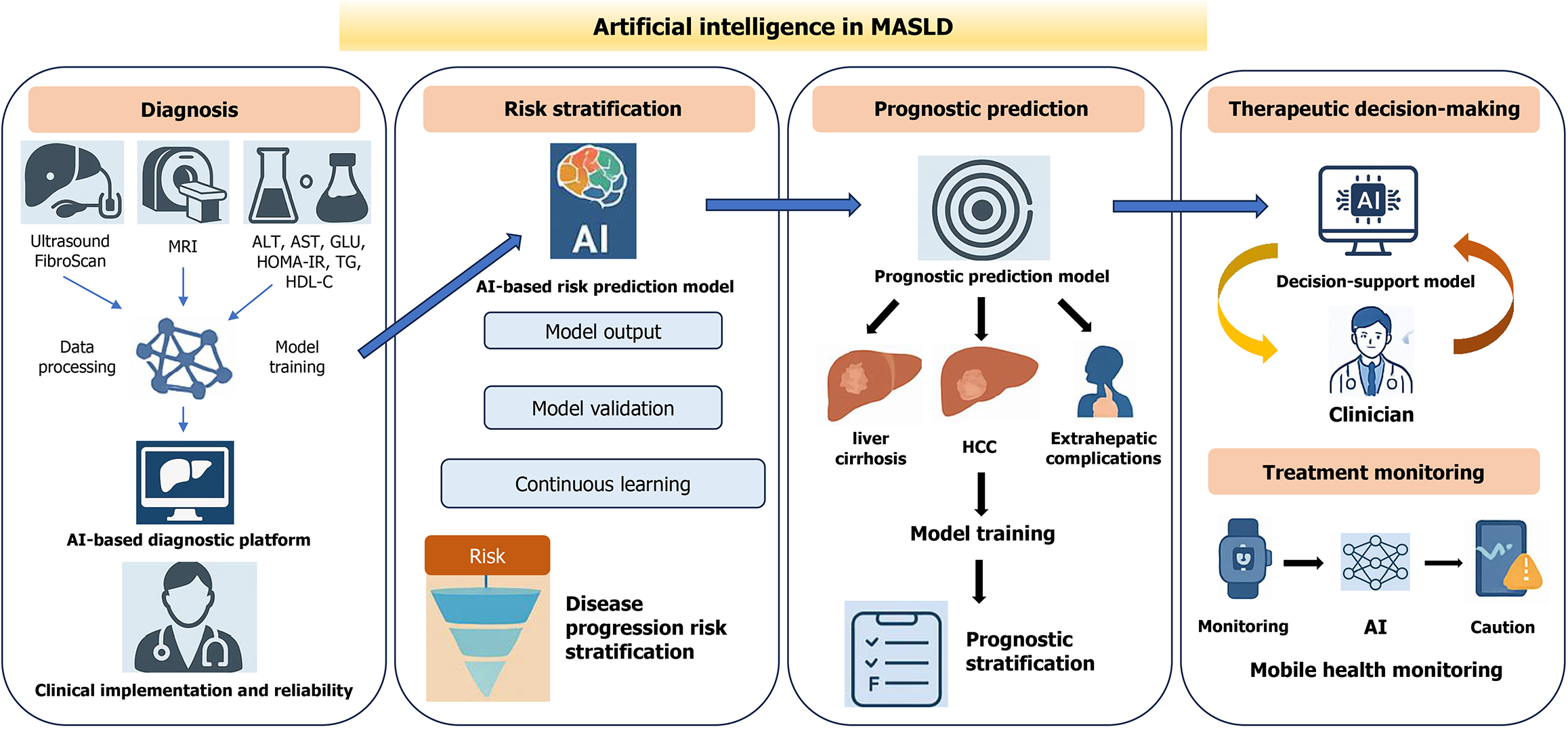
Despite its growing burden, management remains challenging given the highly variable clinical course and only partially understood mechanisms of progression to metabolic dysfunction-associated steatohepatitis (MASH), fibrosis, and cirrhosis[8,12]. Routine screening relies on serologic markers, risk scores, and imaging [e.g., ultrasound, computed tomography (CT)], which have limited sensitivity for mild steatosis and early fibrosis and are affected by operator and equipment variability[13-15]. Elastography (e.g., FibroScan) enables the quantification of steatosis and fibrosis, but its accuracy decreases in patients with obesity, ascites, or advanced fibrosis[16]. Magnetic resonance imaging (MRI) provides precise noninvasive quantification of liver fat but is hampered by high costs and limited accessibility[14,15]. Although liver biopsy remains the “gold standard”, its invasive nature, cost, and sampling variability make it unsuitable for large-scale screening[17]. Given the considerable heterogeneity in metabolic background, clinical features, and disease progression among MASLD patients, the traditional “one-size-fits-all” management approach is increasingly inadequate, underscoring the need for more precise stratification, dynamic risk prediction, and comprehensive intelligent manage
Artificial intelligence (AI) offers unique advantages for handling high-dimensional, heterogeneous data, uncovering latent patterns, and generating individualized predictions[18-20]. Its roles across hepatology, encompassing imaging, histopathology, noninvasive tests, and predictive modeling, have also been recognized and emphasized[21,22]. Beyond imaging and laboratory data, AI can leverage longitudinal weight trajectories, medication histories, and large-scale electronic health records to identify high-risk individuals who might otherwise be missed by conventional screening approaches. This provides innovative solutions to the challenges of high heterogeneity, slow progression, and fra
Given that MASLD is often clinically silent yet progressive, early detection and accurate risk stratification are critical for preventing fibrosis, cirrhosis, and hepatocellular carcinoma (HCC)[3,13]. Biopsy and traditional diagnostic approaches face the well-known constraints of invasiveness, scalability, and variability[17,25,26]. The rise of AI offers new avenues for noninvasive, efficient, and standardized diagnostics, especially in the realms of image interpretation, serological modeling, and multiomics data integration[24]. Automated, AI-driven systems for MASLD diagnosis and staging have been validated across multiple studies and cohorts and often outperform conventional tools in terms of accuracy, sensitivity, and specificity, especially when clinical, imaging, and histopathological data[24] are integrated into multi
Deep learning algorithms, particularly convolutional neural networks (CNNs), can perform automatic segmentation, feature extraction, and quantitative analysis of both imaging and histological data, substantially increasing the standardization and efficiency of MASLD diagnostics[32]. On ultrasound, the use of AI-based texture analysis improves the de
In terms of pathology, AI systems can automatically segment and quantify steatosis, ballooning, inflammation, and fibrosis networks on biopsy slides and improve interobserver agreement[38]. Translational work, such as an AI-based measurement (AIM) tool for scoring MASH (formerly known as nonalcoholic steatohepatitis) histology, shows excellent reproducibility and alignment with expert consensus for histologic endpoints in MASH trials and yields continuous scores that correlate with outcomes[39].
Because single-modality inputs rarely capture disease complexity, multimodal integration has become central to robust AI diagnostics. Compared with single-modality approaches, contemporary models that combine MRI-PDFF, magnetic resonance elastography (MRE)-derived stiffness, ultrasound elastography, laboratory markers [e.g., alanine aminotransferase (ALT)/aspartate aminotransferase (AST)], and lifestyle/behavioral data achieve significantly higher areas under the curves (AUCs) for differentiating MASLD from MASH and predicting advanced fibrosis (≥ F2)[40-42]. Deep learning architectures such as ResNet and Transformer can simultaneously process both structured (e.g., laboratory results) and unstructured (e.g., imaging) data, paving the way for comprehensive, closed-loop AI diagnostic platforms that span data collection, processing, modeling, and clinical decision support, ultimately advancing diagnostic automation and standardization[43]. Future platforms will incorporate explainable AI (XAI) (e.g., Grad-CAM) and causal modeling frameworks to increase clinicians’ trust in model reasoning and accelerate clinical adoption[24]. For instance, AI-driven non-contrast MRI models using T1WI and T2FS sequences have demonstrated high accuracy in staging hepatic fibrosis[44], and in animal studies, the combination of second-harmonic generation/two-photon excitation fluorescence imaging with AI systems has enabled dynamic, automated assessment of steatosis patterns in MASLD models[45].
MASLD is characterized by marked clinical heterogeneity, with disease progression trajectories shaped by a complex interplay of metabolic background, inflammatory status, and genetic factors[3,46]. Consequently, the development of precise and dynamic risk stratification and prognostic prediction models is essential for enabling individualized treatment, optimal resource allocation, and improved long-term outcomes in MASLD patients[47,48]. AI can integrate heterogeneous signals from clinical, imaging, and multiomics sources without manually predefined hypotheses, enabling automated discovery and weighting of risk factors and improving risk assessment accuracy and efficiency[21,22,49].
In the construction and validation of risk stratification models, AI typically employs feature selection and preprocessing [e.g., ALT, AST, platelet count, body mass index (BMI), homeostatic model assessment of insulin resistance, liver stiffness] with demographic and comorbidity information. Algorithms such as random forest, XGBoost, support vector machines, and neural networks are widely utilized for model training[50,51]. Among these, XGBoost has achieved the highest AUCs for MASLD subtyping and MASH prediction[23,50]. However, high AUCs do not guarantee robustness or transportability. For example, in the construction of the LiverRisk score for long-term liver outcomes, multiple algorithms (including XGBoost) were evaluated, yet a simpler linear model was ultimately selected because of its superior calibration and reproducibility[52,53], underscoring the risks of overfitting and dataset dependence and the need for external validation and calibration assessment[21,22]. Notably, NASHMap, a machine learning model based on 14 parameters, achieved areas under the receiver operating characteristic curve of 0.82 and 0.76 in the NIDDK and Optum datasets, respectively, and identified a substantial number of NASH cases that were missed in routine clinical practice[54].
For prognostic prediction, deep learning models using MRI-PDFF, T1 mapping, and elastography can automatically extract subtle hepatic features, enabling earlier risk identification than traditional visual assessment does[44,55]. Time series neural networks such as T-LSTM and Transformer-XL have further advanced the field by overcoming the limi
The management of MASLD is inherently long-term, dynamic, and highly individualized. Traditional treatment relies largely on lifestyle interventions and empiric pharmacotherapy, but the significant heterogeneity in treatment response and the lag in conventional monitoring often impede precise management[9]. The advent of AI has introduced new opportunities and tools for personalized therapeutic decision-making, outcome prediction, dynamic monitoring, and long-term disease management in MASLD[61-63].
Multimodal models that combine liver stiffness, MRI-PDFF, glycemia, lipids, BMI, and comorbidities can generate individualized response predictions and quantify how fibrosis modulates expected benefit, thereby informing choices that pertain to exercise, diet, and pharmacotherapy[27,64]. AI can also analyze pharmacogenomic data, such as CYP450 genotypes, to predict drug metabolism and adverse reactions, enabling dose individualization and a more accurate selection of suitable patients for novel therapeutics[65]. For dynamic management, AI is used not only to optimize the initial plan but also to adapt therapy over time. Data from wearable devices (steps, heart rate, sleep, etc.), when combined with digital dietary logs, enable the dynamic detection of physical inactivity or glycemic excursions, further streng
Deep CNNs and other AI models can detect subtle changes in hepatic fat distribution and fibrosis on imaging, enabling the early prediction of treatment response; these models have been successfully applied in MRE and PDFF analyses for pharmacological efficacy evaluation. AI can also integrate longitudinal data on blood GLU, weight, and liver function for daily tracking of therapeutic efficacy, automatically generating clinical recommendations on the basis of trends in body weight and transaminases. By analyzing historical drug response data and genomic data, AI is also able to predict the risk of adverse drug reactions and refine monitoring priorities. Because MASLD patients often require long-term or lifelong follow-up, AI systems offer particular advantages in terms of remote monitoring and early warning. AI can generate adaptive follow-up schedules based on a patient’s therapeutic response, metabolic control, and lifestyle factors, shortening intervals for high-risk individuals and extending them for low-risk individuals, thus optimizing resource utilization[68]. AI-enabled remote health monitoring platforms now allow comprehensive tracking of weight, activity, diet, and mood, with abnormal trends triggering automated follow-up reminders or physician alerts, supporting a seamless “data collection – dynamic evaluation – AI feedback – remote intervention” closed-loop model. The incor
AI-driven therapeutic decision-making and outcome monitoring are transforming MASLD management from passive, experience-based approaches to active, intelligent systems, laying a robust technical foundation for comprehensive, lifelong liver disease care. By bridging advanced predictive modeling with pragmatic decision-support tools, AI not only holds promise as a frontier technology but also as a practical clinical aid ready for near-term translation, laying the groundwork for comprehensive, lifelong, and multidisciplinary liver disease care for MASLD.
Despite the tremendous promise of AI applications in MASLD – demonstrated by advances in early diagnosis, risk stratification, individualized therapeutic decision-making, and long-term follow-up – there remain significant barriers to widespread clinical implementation. Foremost among these is the persistent challenge of data quality and heterogeneity. Clinical records for MASLD patients frequently suffer from missing information, inconsistent labeling, and non-standardized data formats; differences in imaging equipment and protocols, variability in laboratory units, and gaps in follow-up data can directly compromise model training and performance[71,72]. In addition, limited model interpre
In conclusion, the use of AI is driving the transformation of MASLD management from traditional experience-based approaches to a data-driven and intelligent precision medicine paradigm. At present, AI has significantly increased the efficiency and scientific rigor of key aspects of MASLD care, including early noninvasive screening, disease classification, risk prediction, individualized therapy, and longitudinal monitoring. The integration of multimodal data, real-time monitoring with wearable devices, and federated learning is laying a solid foundation for a closed-loop, intelligent management system that encompasses the entire disease course. However, the large-scale clinical adoption of AI in MASLD still faces challenges related to data heterogeneity, model interpretability, and ethical and privacy issues. Moving forward, it will be essential to build high-quality, multicenter, standardized data platforms, increase model transparency and explainability, establish comprehensive compliance frameworks, and ensure secure, collaborative data sharing across regions. Only then can AI progress beyond algorithmic proof-of-concept to achieve meaningful clinical application. As AI technologies continue to merge with MASLD research, they are positioned to become an indispensable engine for precision subtyping, adaptive disease management, and remote health interventions, ultimately facilitating the deve
| 1. | Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, Romero D, Abdelmalek MF, Anstee QM, Arab JP, Arrese M, Bataller R, Beuers U, Boursier J, Bugianesi E, Byrne CD, Castro Narro GE, Chowdhury A, Cortez-Pinto H, Cryer DR, Cusi K, El-Kassas M, Klein S, Eskridge W, Fan J, Gawrieh S, Guy CD, Harrison SA, Kim SU, Koot BG, Korenjak M, Kowdley KV, Lacaille F, Loomba R, Mitchell-Thain R, Morgan TR, Powell EE, Roden M, Romero-Gómez M, Silva M, Singh SP, Sookoian SC, Spearman CW, Tiniakos D, Valenti L, Vos MB, Wong VW, Xanthakos S, Yilmaz Y, Younossi Z, Hobbs A, Villota-Rivas M, Newsome PN; NAFLD Nomenclature consensus group. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J Hepatol. 2023;79:1542-1556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1676] [Cited by in RCA: 1800] [Article Influence: 600.0] [Reference Citation Analysis (2)] |
| 2. | Goldberg D, Ditah IC, Saeian K, Lalehzari M, Aronsohn A, Gorospe EC, Charlton M. Changes in the Prevalence of Hepatitis C Virus Infection, Nonalcoholic Steatohepatitis, and Alcoholic Liver Disease Among Patients With Cirrhosis or Liver Failure on the Waitlist for Liver Transplantation. Gastroenterology. 2017;152:1090-1099.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 459] [Cited by in RCA: 480] [Article Influence: 53.3] [Reference Citation Analysis (0)] |
| 3. | European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). J Hepatol. 2024;81:492-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 952] [Article Influence: 476.0] [Reference Citation Analysis (1)] |
| 4. | Fan JG, Xu XY, Yang RX, Nan YM, Wei L, Jia JD, Zhuang H, Shi JP, Li XY, Sun C, Li J, Wong VW, Duan ZP; Chinese Society of Hepatology, Chinese Medical Association. Guideline for the Prevention and Treatment of Metabolic Dysfunction-associated Fatty Liver Disease (Version 2024). J Clin Transl Hepatol. 2024;12:955-974. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 33] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 5. | Younossi ZM, Golabi P, Paik JM, Henry A, Van Dongen C, Henry L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): a systematic review. Hepatology. 2023;77:1335-1347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 270] [Cited by in RCA: 1990] [Article Influence: 663.3] [Reference Citation Analysis (3)] |
| 6. | Younossi ZM, Kalligeros M, Henry L. Epidemiology of metabolic dysfunction-associated steatotic liver disease. Clin Mol Hepatol. 2025;31:S32-S50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 328] [Article Influence: 328.0] [Reference Citation Analysis (3)] |
| 7. | Yip TC, Fan JG, Wong VW. China's Fatty Liver Crisis: A Looming Public Health Emergency. Gastroenterology. 2023;165:825-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 8. | Miao L, Targher G, Byrne CD, Cao YY, Zheng MH. Current status and future trends of the global burden of MASLD. Trends Endocrinol Metab. 2024;35:697-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 421] [Article Influence: 210.5] [Reference Citation Analysis (0)] |
| 9. | Zeng J, Fan JG, Francque SM. Therapeutic management of metabolic dysfunction associated steatotic liver disease. United European Gastroenterol J. 2024;12:177-186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 74] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 10. | Lee HH, Lee HA, Kim EJ, Kim HY, Kim HC, Ahn SH, Lee H, Kim SU. Metabolic dysfunction-associated steatotic liver disease and risk of cardiovascular disease. Gut. 2024;73:533-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 86] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 11. | Targher G, Byrne CD, Tilg H. MASLD: a systemic metabolic disorder with cardiovascular and malignant complications. Gut. 2024;73:691-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 266] [Article Influence: 133.0] [Reference Citation Analysis (1)] |
| 12. | Huang CX, Zhou XD, Pan CQ, Zheng MH. Screening for metabolic dysfunction-associated fatty liver disease: Time to discard the emperor's clothes of normal liver enzymes? World J Gastroenterol. 2024;30:2839-2842. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (2)] |
| 13. | Rinella ME, Neuschwander-Tetri BA, Siddiqui MS, Abdelmalek MF, Caldwell S, Barb D, Kleiner DE, Loomba R. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology. 2023;77:1797-1835. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1465] [Cited by in RCA: 1570] [Article Influence: 523.3] [Reference Citation Analysis (1)] |
| 14. | De Robertis R, Spoto F, Autelitano D, Guagenti D, Olivieri A, Zanutto P, Incarbone G, D'Onofrio M. Ultrasound-derived fat fraction for detection of hepatic steatosis and quantification of liver fat content. Radiol Med. 2023;128:1174-1180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 15. | Starekova J, Hernando D, Pickhardt PJ, Reeder SB. Quantification of Liver Fat Content with CT and MRI: State of the Art. Radiology. 2021;301:250-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 205] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 16. | Eddowes PJ, Sasso M, Allison M, Tsochatzis E, Anstee QM, Sheridan D, Guha IN, Cobbold JF, Deeks JJ, Paradis V, Bedossa P, Newsome PN. Accuracy of FibroScan Controlled Attenuation Parameter and Liver Stiffness Measurement in Assessing Steatosis and Fibrosis in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2019;156:1717-1730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 509] [Cited by in RCA: 1061] [Article Influence: 151.6] [Reference Citation Analysis (1)] |
| 17. | Piccinino F, Sagnelli E, Pasquale G, Giusti G. Complications following percutaneous liver biopsy. A multicentre retrospective study on 68,276 biopsies. J Hepatol. 1986;2:165-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 850] [Cited by in RCA: 808] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 18. | Beam AL, Drazen JM, Kohane IS, Leong TY, Manrai AK, Rubin EJ. Artificial Intelligence in Medicine. N Engl J Med. 2023;388:1220-1221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 142] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 19. | Kalapala R, Rughwani H, Reddy DN. Artificial Intelligence in Hepatology- Ready for the Primetime. J Clin Exp Hepatol. 2023;13:149-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 20. | Colapietro F, Piovani D, Pugliese N, Aghemo A, Ronca V, Lleo A. Is ChatGPT-4 a Reliable Tool in Autoimmune Hepatitis? Am J Gastroenterol. 2025;120:914-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 21. | Schattenberg JM, Chalasani N, Alkhouri N. Artificial Intelligence Applications in Hepatology. Clin Gastroenterol Hepatol. 2023;21:2015-2025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 34] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 22. | Spann A, Strauss AT, Davis SE, Bhat M. The Role of Artificial Intelligence in Chronic Liver Diseases and Liver Transplantation. Gastroenterology. 2025;169:456-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 23. | Dinani AM, Kowdley KV, Noureddin M. Application of Artificial Intelligence for Diagnosis and Risk Stratification in NAFLD and NASH: The State of the Art. Hepatology. 2021;74:2233-2240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 24. | Zamanian H, Shalbaf A, Zali MR, Khalaj AR, Dehghan P, Tabesh M, Hatami B, Alizadehsani R, Tan RS, Acharya UR. Application of artificial intelligence techniques for non-alcoholic fatty liver disease diagnosis: A systematic review (2005-2023). Comput Methods Programs Biomed. 2024;244:107932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 25. | Lee YT, Fujiwara N, Yang JD, Hoshida Y. Risk stratification and early detection biomarkers for precision HCC screening. Hepatology. 2023;78:319-362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 84] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 26. | Theodossi A, Skene AM, Portmann B, Knill-Jones RP, Patrick RS, Tate RA, Kealey W, Jarvis KJ, O'Brian DJ, Williams R. Observer variation in assessment of liver biopsies including analysis by kappa statistics. Gastroenterology. 1980;79:232-241. [PubMed] |
| 27. | Gao B, Duan W. The current status and future directions of artificial intelligence in the prediction, diagnosis, and treatment of liver diseases. Digit Health. 2025;11:20552076251325418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 28. | Marti-Aguado D, Rodríguez-Ortega A, Mestre-Alagarda C, Bauza M, Valero-Pérez E, Alfaro-Cervello C, Benlloch S, Pérez-Rojas J, Ferrández A, Alemany-Monraval P, Escudero-García D, Monton C, Aguilera V, Alberich-Bayarri Á, Serra MÁ, Marti-Bonmati L. Digital pathology: accurate technique for quantitative assessment of histological features in metabolic-associated fatty liver disease. Aliment Pharmacol Ther. 2021;53:160-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 29. | Broggi G, Maniaci A, Lentini M, Palicelli A, Zanelli M, Zizzo M, Koufopoulos N, Salzano S, Mazzucchelli M, Caltabiano R. Artificial Intelligence in Head and Neck Cancer Diagnosis: A Comprehensive Review with Emphasis on Radiomics, Histopathological, and Molecular Applications. Cancers (Basel). 2024;16:3623. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 30. | Zarella MD, McClintock DS, Batra H, Gullapalli RR, Valante M, Tan VO, Dayal S, Oh KS, Lara H, Garcia CA, Abels E. Artificial intelligence and digital pathology: clinical promise and deployment considerations. J Med Imaging (Bellingham). 2023;10:051802. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 31. | Shafi S, Parwani AV. Artificial intelligence in diagnostic pathology. Diagn Pathol. 2023;18:109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 136] [Reference Citation Analysis (0)] |
| 32. | Ajmera P, Dillard R, Kline T, Missert A, Korfiatis P, Khandelwal A. FDA-approved artificial intelligence products in abdominal imaging: A comprehensive review. Curr Probl Diagn Radiol. 2025;S0363-0188(25)00082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 33. | Cao LL, Peng M, Xie X, Chen GQ, Huang SY, Wang JY, Jiang F, Cui XW, Dietrich CF. Artificial intelligence in liver ultrasound. World J Gastroenterol. 2022;28:3398-3409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (2)] |
| 34. | Liu JQ, Ren JY, Xu XL, Xiong LY, Peng YX, Pan XF, Dietrich CF, Cui XW. Ultrasound-based artificial intelligence in gastroenterology and hepatology. World J Gastroenterol. 2022;28:5530-5546. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (1)] |
| 35. | Pickhardt PJ, Blake GM, Kimmel Y, Weinstock E, Shaanan K, Hassid S, Abbas A, Fox MA. Detection of Moderate Hepatic Steatosis on Portal Venous Phase Contrast-Enhanced CT: Evaluation Using an Automated Artificial Intelligence Tool. AJR Am J Roentgenol. 2023;221:748-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 36. | Fujii I, Matsumoto N, Ogawa M, Konishi A, Kaneko M, Watanabe Y, Masuzaki R, Kogure H, Koizumi N, Sugitani M. Artificial Intelligence and Image Analysis-Assisted Diagnosis for Fibrosis Stage of Metabolic Dysfunction-Associated Steatotic Liver Disease Using Ultrasonography: A Pilot Study. Diagnostics (Basel). 2024;14:2585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (5)] |
| 37. | Gómez-Gavara C, Bilbao I, Piella G, Vazquez-Corral J, Benet-Cugat B, Pando E, Molino JA, Salcedo MT, Dalmau M, Vidal L, Esono D, Cordobés MÁ, Bilbao Á, Prats J, Moya M, Dopazo C, Mazo C, Caralt M, Hidalgo E, Charco R. Enhanced Artificial Intelligence Methods for Liver Steatosis Assessment Using Machine Learning and Color Image Processing: Liver Color Project. Clin Transplant. 2024;38:e15465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 38. | Ratziu V, Hompesch M, Petitjean M, Serdjebi C, Iyer JS, Parwani AV, Tai D, Bugianesi E, Cusi K, Friedman SL, Lawitz E, Romero-Gómez M, Schuppan D, Loomba R, Paradis V, Behling C, Sanyal AJ. Artificial intelligence-assisted digital pathology for non-alcoholic steatohepatitis: current status and future directions. J Hepatol. 2024;80:335-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 63] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 39. | Iyer JS, Juyal D, Le Q, Shanis Z, Pokkalla H, Pouryahya M, Pedawi A, Stanford-Moore SA, Biddle-Snead C, Carrasco-Zevallos O, Lin M, Egger R, Hoffman S, Elliott H, Leidal K, Myers RP, Chung C, Billin AN, Watkins TR, Patterson SD, Resnick M, Wack K, Glickman J, Burt AD, Loomba R, Sanyal AJ, Glass B, Montalto MC, Taylor-Weiner A, Wapinski I, Beck AH. AI-based automation of enrollment criteria and endpoint assessment in clinical trials in liver diseases. Nat Med. 2024;30:2914-2923. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 27] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 40. | Noureddin M, Truong E, Gornbein JA, Saouaf R, Guindi M, Todo T, Noureddin N, Yang JD, Harrison SA, Alkhouri N. MRI-based (MAST) score accurately identifies patients with NASH and significant fibrosis. J Hepatol. 2022;76:781-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 144] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 41. | Jung J, Loomba RR, Imajo K, Madamba E, Gandhi S, Bettencourt R, Singh S, Hernandez C, Valasek MA, Behling C, Richards L, Fowler K, Sirlin CB, Nakajima A, Loomba R. MRE combined with FIB-4 (MEFIB) index in detection of candidates for pharmacological treatment of NASH-related fibrosis. Gut. 2021;70:1946-1953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 116] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 42. | Sanyal AJ, Foucquier J, Younossi ZM, Harrison SA, Newsome PN, Chan WK, Yilmaz Y, De Ledinghen V, Costentin C, Zheng MH, Wai-Sun Wong V, Elkhashab M, Huss RS, Myers RP, Roux M, Labourdette A, Destro M, Fournier-Poizat C, Miette V, Sandrin L, Boursier J. Enhanced diagnosis of advanced fibrosis and cirrhosis in individuals with NAFLD using FibroScan-based Agile scores. J Hepatol. 2023;78:247-259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 151] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 43. | Wu W, Guo Y, Li Q, Jia C. Exploring the potential of large language models in identifying metabolic dysfunction-associated steatotic liver disease: A comparative study of non-invasive tests and artificial intelligence-generated responses. Liver Int. 2025;45:e16112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 44. | Li C, Wang Y, Bai R, Zhao Z, Li W, Zhang Q, Zhang C, Yang W, Liu Q, Su N, Lu Y, Yin X, Wang F, Gu C, Yang A, Luo B, Zhou M, Shen L, Pan C, Wang Z, Wu Q, Yin J, Hou Y, Shi Y. Development of fully automated models for staging liver fibrosis using non-contrast MRI and artificial intelligence: a retrospective multicenter study. EClinicalMedicine. 2024;77:102881. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 45. | Wang XX, Song YY, Jin R, Wang ZL, Li XH, Yang Q, Teng X, Liu FF, Wu N, Xie YD, Rao HY, Liu F. Hepatic Steatosis Analysis in Metabolic Dysfunction-Associated Steatotic Liver Disease Based on Artificial Intelligence. Diagnostics (Basel). 2024;14:2889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 46. | Raverdy V, Tavaglione F, Chatelain E, Lassailly G, De Vincentis A, Vespasiani-Gentilucci U, Qadri SF, Caiazzo R, Verkindt H, Saponaro C, Kerr-Conte J, Baud G, Marciniak C, Chetboun M, Oukhouya-Daoud N, Blanck S, Vandel J, Olsson L, Chakaroun R, Gnemmi V, Leteurtre E, Lefebvre P, Haas JT, Yki-Järvinen H, Francque S, Staels B, Le Roux CW, Tremaroli V, Mathurin P, Marot G, Romeo S, Pattou F. Data-driven cluster analysis identifies distinct types of metabolic dysfunction-associated steatotic liver disease. Nat Med. 2024;30:3624-3633. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 63] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 47. | Lin H, Lee HW, Yip TC, Tsochatzis E, Petta S, Bugianesi E, Yoneda M, Zheng MH, Hagström H, Boursier J, Calleja JL, Goh GB, Chan WK, Gallego-Durán R, Sanyal AJ, de Lédinghen V, Newsome PN, Fan JG, Castéra L, Lai M, Harrison SA, Fournier-Poizat C, Wong GL, Pennisi G, Armandi A, Nakajima A, Liu WY, Shang Y, de Saint-Loup M, Llop E, Teh KK, Lara-Romero C, Asgharpour A, Mahgoub S, Chan MS, Canivet CM, Romero-Gomez M, Kim SU, Wong VW; VCTE-Prognosis Study Group. Vibration-Controlled Transient Elastography Scores to Predict Liver-Related Events in Steatotic Liver Disease. JAMA. 2024;331:1287-1297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 140] [Article Influence: 70.0] [Reference Citation Analysis (0)] |
| 48. | Loomba R, Huang DQ, Sanyal AJ, Anstee QM, Trauner M, Lawitz EJ, Ding D, Ma L, Jia C, Billin A, Huss RS, Chung C, Goodman Z, Wong VW, Okanoue T, Romero-Gómez M, Abdelmalek MF, Muir A, Afdhal N, Bosch J, Harrison S, Younossi ZM, Myers RP. Liver stiffness thresholds to predict disease progression and clinical outcomes in bridging fibrosis and cirrhosis. Gut. 2023;72:581-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 75] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 49. | Bakrania A, Joshi N, Zhao X, Zheng G, Bhat M. Artificial intelligence in liver cancers: Decoding the impact of machine learning models in clinical diagnosis of primary liver cancers and liver cancer metastases. Pharmacol Res. 2023;189:106706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 38] [Reference Citation Analysis (0)] |
| 50. | Fialoke S, Malarstig A, Miller MR, Dumitriu A. Application of Machine Learning Methods to Predict Non-Alcoholic Steatohepatitis (NASH) in Non-Alcoholic Fatty Liver (NAFL) Patients. AMIA Annu Symp Proc. 2018;2018:430-439. [PubMed] |
| 51. | Pugliese N, Bertazzoni A, Hassan C, Schattenberg JM, Aghemo A. Revolutionizing MASLD: How Artificial Intelligence Is Shaping the Future of Liver Care. Cancers (Basel). 2025;17:722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 52. | Serra-Burriel M, Juanola A, Serra-Burriel F, Thiele M, Graupera I, Pose E, Pera G, Grgurevic I, Caballeria L, Piano S, van Kleef L, Reichert M, Roulot D, Pericàs JM, Schattenberg JM, Tsochatztis EA, Guha IN, Garcia-Retortillo M, Hernández R, Hoyo J, Fuentes M, Expósito C, Martínez A, Such P, Madir A, Detlefsen S, Tonon M, Martini A, Ma AT, Pich J, Bonfill E, Juan M, Soria A, Carol M, Gratacós-Ginès J, Morillas RM, Toran P, Navarrete JM, Torrejón A, Fournier C, Llorca A, Arslanow A, de Koning HJ, Cucchietti F, Manns M, Newsome PN, Hernáez R, Allen A, Angeli P, de Knegt RJ, Karlsen TH, Galle P, Wong VW, Fabrellas N, Castera L, Krag A, Lammert F, Kamath PS, Ginès P; LiverScreen Consortium Investigators. Development, validation, and prognostic evaluation of a risk score for long-term liver-related outcomes in the general population: a multicohort study. Lancet. 2023;402:988-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 101] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 53. | Liu S, Chen X, Jiang X, Yin X, Fekadu G, Liu C, He Y, Chen H, Ni W, Wang R, Zeng QL, Chen Y, Yang L, Shi R, Ju SH, Shen J, Gao J, Zhao L, Ming WK, Zhong VW, Teng GJ, Qi X. LiverRisk score: An accurate, cost-effective tool to predict fibrosis, liver-related, and diabetes-related mortality in the general population. Med. 2024;5:570-582.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 54. | Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3544] [Cited by in RCA: 5211] [Article Influence: 651.4] [Reference Citation Analysis (9)] |
| 55. | Jackson E, Dennis A, Alkhouri N, Samala N, Vuppalanchi R, Sanyal AJ, Muthiah M, Banerjee R, Banerjee A. Cardiac and liver impairment on multiorgan MRI and risk of major adverse cardiovascular and liver events. Nat Med. 2025;31:2289-2296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 56. | Wu T, Simonetto DA, Halamka JD, Shah VH. The digital transformation of hepatology: The patient is logged in. Hepatology. 2022;75:724-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 57. | Li Z, Lan L, Zhou Y, Li R, Chavin KD, Xu H, Li L, Shih DJH, Jim Zheng W. Developing deep learning-based strategies to predict the risk of hepatocellular carcinoma among patients with nonalcoholic fatty liver disease from electronic health records. J Biomed Inform. 2024;152:104626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 58. | Ghosh S, Zhao X, Alim M, Brudno M, Bhat M. Artificial intelligence applied to 'omics data in liver disease: towards a personalised approach for diagnosis, prognosis and treatment. Gut. 2025;74:295-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 46] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 59. | Kendall TJ, Jimenez-Ramos M, Turner F, Ramachandran P, Minnier J, McColgan MD, Alam M, Ellis H, Dunbar DR, Kohnen G, Konanahalli P, Oien KA, Bandiera L, Menolascina F, Juncker-Jensen A, Alexander D, Mayor C, Guha IN, Fallowfield JA. An integrated gene-to-outcome multimodal database for metabolic dysfunction-associated steatotic liver disease. Nat Med. 2023;29:2939-2953. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 49] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 60. | Wang CH, Ji XY, Ji N, Yan QF, Xu HQ, Wang XQ, Chen XF, Lu CF. Association between glucose time-in-range and the severity of metabolic dysfunction-associated steatotic liver disease in Chinese adults with type 2 diabetes mellitus. BMC Endocr Disord. 2025;25:113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 61. | Zeng Q, Klein C, Caruso S, Maille P, Allende DS, Mínguez B, Iavarone M, Ningarhari M, Casadei-Gardini A, Pedica F, Rimini M, Perbellini R, Boulagnon-Rombi C, Heurgué A, Maggioni M, Rela M, Vij M, Baulande S, Legoix P, Lameiras S; HCC-AI study group, Bruges L, Gnemmi V, Nault JC, Campani C, Rhee H, Park YN, Iñarrairaegui M, Garcia-Porrero G, Argemi J, Sangro B, D'Alessio A, Scheiner B, Pinato DJ, Pinter M, Paradis V, Beaufrère A, Peter S, Rimassa L, Di Tommaso L, Vogel A, Michalak S, Boursier J, Loménie N, Ziol M, Calderaro J. Artificial intelligence-based pathology as a biomarker of sensitivity to atezolizumab-bevacizumab in patients with hepatocellular carcinoma: a multicentre retrospective study. Lancet Oncol. 2023;24:1411-1422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 66] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 62. | Dana J, Venkatasamy A, Saviano A, Lupberger J, Hoshida Y, Vilgrain V, Nahon P, Reinhold C, Gallix B, Baumert TF. Conventional and artificial intelligence-based imaging for biomarker discovery in chronic liver disease. Hepatol Int. 2022;16:509-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 63. | Valenzuela-Vallejo L, Sanoudou D, Mantzoros CS. Precision Medicine in Fatty Liver Disease/Non-Alcoholic Fatty Liver Disease. J Pers Med. 2023;13:830. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 64. | Subramanian M, Wojtusciszyn A, Favre L, Boughorbel S, Shan J, Letaief KB, Pitteloud N, Chouchane L. Precision medicine in the era of artificial intelligence: implications in chronic disease management. J Transl Med. 2020;18:472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 139] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 65. | Niu H, Alvarez-Alvarez I, Chen M. Artificial Intelligence: An Emerging Tool for Studying Drug-Induced Liver Injury. Liver Int. 2025;45:e70038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 66. | Liu M, Ye Z, Zhang Y, He P, Zhou C, Yang S, Zhang Y, Gan X, Qin X. Accelerometer-derived moderate-to-vigorous physical activity and incident nonalcoholic fatty liver disease. BMC Med. 2024;22:398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 67. | Nam D, Chapiro J, Paradis V, Seraphin TP, Kather JN. Artificial intelligence in liver diseases: Improving diagnostics, prognostics and response prediction. JHEP Rep. 2022;4:100443. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 111] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 68. | Stine JG, Rivas G, Hummer B, Duarte-Rojo A, May CN, Geyer N, Chinchilli VM, Conroy DE, Mitchell ES, McCallum M, Michealides A, Schmitz KH. Mobile health lifestyle intervention program leads to clinically significant loss of body weight in patients with NASH. Hepatol Commun. 2023;7:e0052. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 69. | Pugliese N, Polverini D, Lombardi R, Pennisi G, Ravaioli F, Armandi A, Buzzetti E, Dalbeni A, Liguori A, Mantovani A, Villani R, Gardini I, Hassan C, Valenti L, Miele L, Petta S, Sebastiani G, Aghemo A; Nafld Expert Chatbot Working Group. Evaluation of ChatGPT as a Counselling Tool for Italian-Speaking MASLD Patients: Assessment of Accuracy, Completeness and Comprehensibility. J Pers Med. 2024;14:568. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 70. | Pugliese N, Wai-Sun Wong V, Schattenberg JM, Romero-Gomez M, Sebastiani G; NAFLD Expert Chatbot Working Group, Aghemo A. Accuracy, Reliability, and Comprehensibility of ChatGPT-Generated Medical Responses for Patients With Nonalcoholic Fatty Liver Disease. Clin Gastroenterol Hepatol. 2024;22:886-889.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 59] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 71. | Su TH, Wu CH, Kao JH. Artificial intelligence in precision medicine in hepatology. J Gastroenterol Hepatol. 2021;36:569-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 72. | Rajpurkar P, Lungren MP. The Current and Future State of AI Interpretation of Medical Images. N Engl J Med. 2023;388:1981-1990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 233] [Reference Citation Analysis (0)] |
| 73. | Hunter DJ, Holmes C. Where Medical Statistics Meets Artificial Intelligence. N Engl J Med. 2023;389:1211-1219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 77] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 74. | Alowais SA, Alghamdi SS, Alsuhebany N, Alqahtani T, Alshaya AI, Almohareb SN, Aldairem A, Alrashed M, Bin Saleh K, Badreldin HA, Al Yami MS, Al Harbi S, Albekairy AM. Revolutionizing healthcare: the role of artificial intelligence in clinical practice. BMC Med Educ. 2023;23:689. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 1041] [Article Influence: 347.0] [Reference Citation Analysis (1)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/