Published online Oct 26, 2021. doi: 10.12998/wjcc.v9.i30.9285
Peer-review started: June 3, 2021
First decision: June 25, 2021
Revised: June 30, 2021
Accepted: August 23, 2021
Article in press: August 23, 2021
Published online: October 26, 2021
Processing time: 140 Days and 1.5 Hours
Colon cancer is a common malignant disease of the gastrointestinal tract and usually occurs at the junction of the rectum and sigmoid colon. Lymphatic and hematogenous metastases occur frequently in colon cancer and the most common metastatic sites include the liver, lung, peritoneum, bone, and lymph nodes. As a manifestation of advanced tumor spread and metastasis, soft tissue metastasis, especially skeletal muscle metastasis with bone metaplasia caused by colon cancer, is rare, accounting for less than 1% of metastases.
A 43-year-old male patient developed skeletal muscle metastasis with bone metaplasia of the right proximal thigh 5 mo after colon cancer was diagnosed. The patient was admitted to the hospital because of pain caused by a local mass on his right thigh. Positron emission tomography-computed tomography showed many enlarged lymph nodes around the abdominal aorta but no signs of lung or liver metastases. Color ultrasound revealed a mass located in the skeletal muscle and the results of histological biopsy revealed a poorly differentiated adenocarcinoma suspected to be distant metastases from colon cancer. Immunohistochemistry showed small woven bone components that were considered to be ossified.
This case reminds us that for patients with advanced colorectal tumors, we should be alert to the possibility of unconventional metastasis.
Core Tip: In this article, we report a rare case of a 43-year-old male patient who developed skeletal muscle metastasis with bone metaplasia of the right proximal thigh 5 mo after colon cancer was diagnosed. This article discusses the possible mechanism of epithelial to mesenchymal transition from the perspective of BRAF mutation and tumor mutation burden. It prompts the need to guard against the occurrence of secondary malignant tumors with unusual metastasis pathways in patients with advanced colorectal cancer (CRC). It also reminds us to pay attention to unexplained masses and other discomforts in patients with advanced CRC.
- Citation: Guo Y, Wang S, Zhao ZY, Li JN, Shang A, Li DL, Wang M. Skeletal muscle metastasis with bone metaplasia from colon cancer: A case report and review of the literature. World J Clin Cases 2021; 9(30): 9285-9294
- URL: https://www.wjgnet.com/2307-8960/full/v9/i30/9285.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i30.9285
With the improvement of people’s living standards and changes in dietary structure, the incidence of colon cancer has increased year by year[1]. Colon cancer, as a kind of malignant tumor, can metastasize to many other parts of the body. Lymphatic and hematogenous metastases occur frequently in colon cancer and the most common metastatic sites include the liver, lung, peritoneum, bone, and lymph nodes[2]. In the case presented here, we demonstrated a solitary metastasis in the skeletal muscle of the thigh, with bone metaplasia, but no signs of metastases to common sites, such as the liver or lung. Reviewing the literature, there have been only 13 cases, including our case, of skeletal muscle metastasis (SMM) from colon carcinoma (Table 1).
| Ref. | Age/sex | Country | Primary carcinoma | Surgery | Metastasis site | Ossification | Interval (mo) | En bloc resection | Outcome |
| Laurence et al[12] | 70/F | Argentina | Caecum | Right colectomy | Right calf | N | 24 | Y | Died soon for generalized metastases |
| Laurence et al[12] | 51/M | Argentina | Transverse colon | Right colectomy | Right forearm | N | 0 | Y | Died soon for generalized metastases |
| Stulc et al[36] | 74/M | United States | Ascending colon | Right hemicolectomy | Left buttock | NS | 30 | Y | NS |
| Torosian et al[37] | 68/M | United States | Transverse colon | Right colectomy | Left thigh | N | 60 | Y | NS |
| Caskey et al[38] | 62/M | United States | Transverse colon | NS | Left gluteus | NS | 6 | NS | NS |
| Caskey et al[38] | 71/F | United States | Colon | NS | Right psoas | NS | NS | NS | NS |
| Araki et al[11] | 66/M | Japan | Colon | Colectomy | Right teres major | NS | 6 | NS | Died after 2 yr and 7 mo from carcinoma |
| Stabler et al[17] | 65/M | United Kingdom | Sigmoid cancer | Sigmoid colectomy | Left psoas | Y | 24 | NS | Died 2 yr after surgery |
| Avery et al[39] | 71/M | United Kingdom | Sigmoid cancer | Sigmoid colectomy | Left psoas | NS | 48 | NS | NS |
| Yoshikawaet al[18] | 54/M | Japan | Sigmoid cancer | Partialsigmoidcolectomy | Right buttock | Y | 24 | Y | Died after 8 mo from multiple metastases |
| Naik et al[40] | 56/M | Malay | Right colon | Right hemicolectomy | Recuts abdominis | Y | 60 | Y | NS |
| Takada et al[41] | 71/M | Japan | Sigmoid colon | Hartmann | Left iliopsoas | N | 60 | N | NS |
| Our present case | 43/M | China | Ascending colon | Laparoscopicextendedrighthemicolectomy | Right thigh | Y | 5 | N | Died 9 mo after surgery |
The possible mechanism of metastatic spread is considered to include hematogenous and lymphatics route, direct infiltration, and operative manipulation. In a comprehensive review on SMM about primary tumor type, prevalence, and radiological features, Surov et al[3] stated that the most commonly involved muscles by metastases from various primary tumor sites are paravertebral and pelvic musculature. In our case, we demonstrated the skeletal muscle of the thigh as the solitary metastases with bone metaplasia and with no signs of metastases to common sites, such as the liver or lung.
In June 2018, a 43-year-old male patient presented to our hospital with a right thigh mass of 4 cm × 4 cm with intolerable pain.
The lump on the patient's right thigh grew rapidly within 1 mo and was accompanied by unbearable pain.
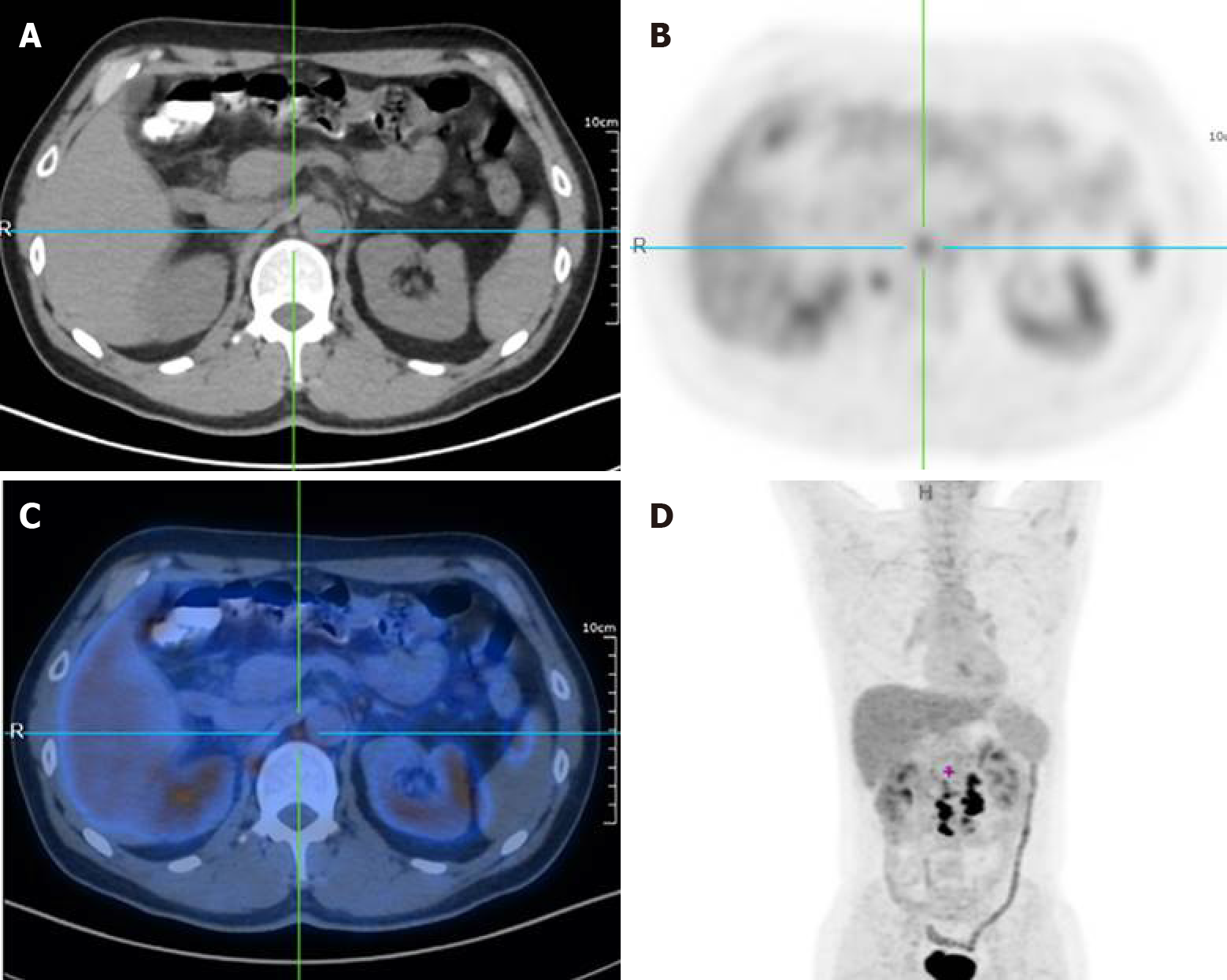
In January 2018, a 43-year-old male patient presented to our hospital with a right lower abdominal mass of 4 cm × 5 cm that had been present for 2 mo without any abdominal pain or other symptoms. We performed a radical resection of right colon cancer for this patient. To reduce the tumor recurrence and kill tumor cells throughout the body, the patient underwent four cycles of chemotherapy with the capecitabine and oxaliplatin regimen (CapeOX). The patient’s carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9) levels increased after receiving four cycles of chemotherapy, up to 184.14 ng/mL and 62.0 U/L, respectively. Positron emission tomography-computed tomography (PET-CT) was then performed, which showed multiple lymph nodes metastases around the abdominal aorta, without lung or liver metastases (Figure 1). The patient was recommended for radiotherapy with a total of 50 Gy in five regimens. However, after finishing the second radiotherapy regimen, the patient found a 4 cm × 4 cm mass in his right thigh that caused intolerable pain.
The patient had no family history related to tumor.
Before surgery, a mass about 4 cm × 5 cm in size in the patient's right lower abdomen can be palpated, which was hard and poor in mobility, and had no tenderness. After surgery, a mass of about 4 cm × 4 cm in size can be palpable on the right thigh, which was hard in nature and unclear with the surrounding tissues, and had obvious tenderness and normal skin temperature.
Before surgery, the levels of tumor biomarkers CEA and CA19-9 were 10.18 ng/mL (normal range: < 3 ng/mL) and 289.24 U/L (normal range: < 37 U/L), respectively. After surgery, to reduce the tumor recurrence and kill tumor cells throughout the body, the patient underwent four cycles of chemotherapy with the CapeOX regimen. The patient’s CEA and CA19-9 levels increased after receiving four cycles of chemotherapy, up to 184.14 ng/mL and 62.0 U/L, respectively.
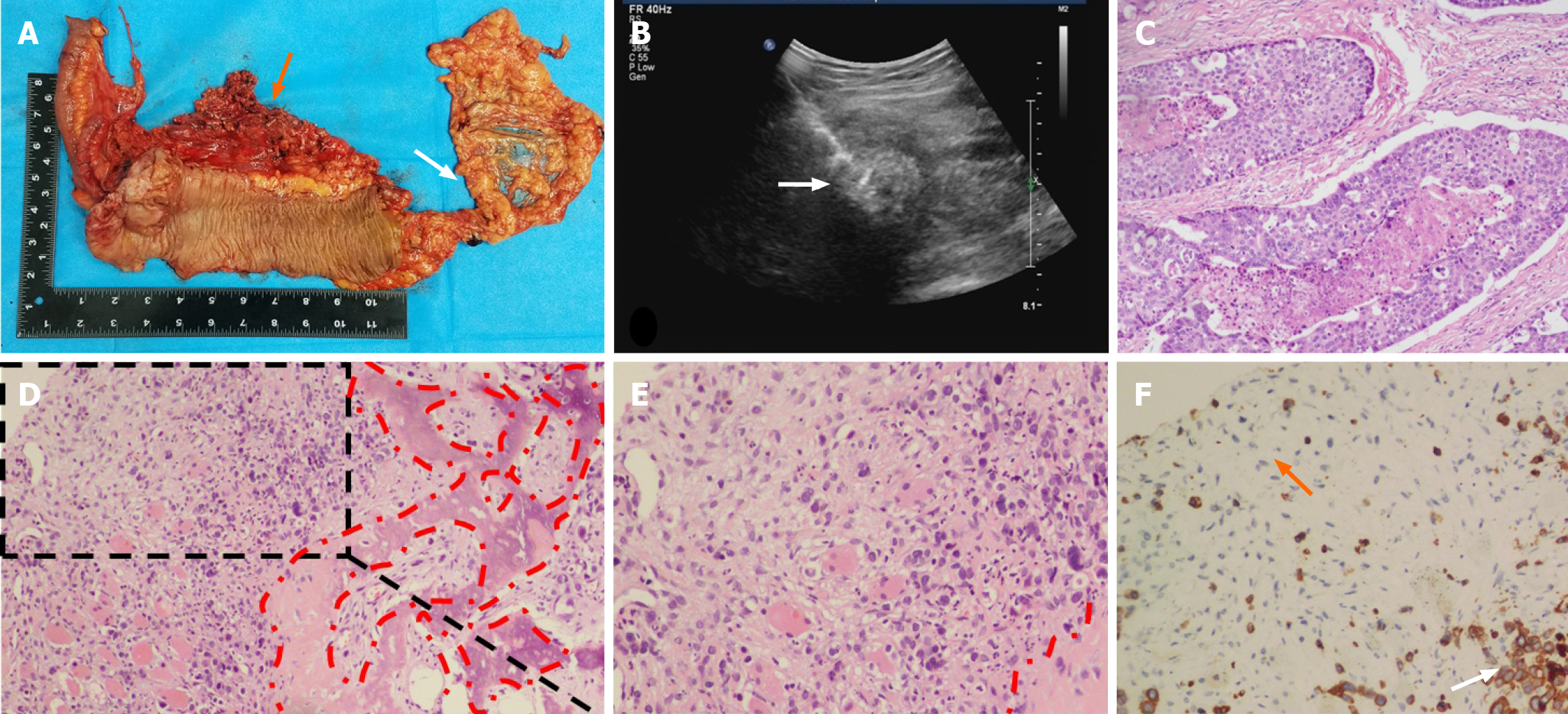
Before surgery, colonoscopy revealed a mass in the ascending colon and the biopsy result revealed adenocarcinoma with vascular invasion. Abdominal CT revealed an ileocecal mass with many enlarged lymph nodes, but no distant metastasis. The preoperative stage was evaluated as T3N2M0 and a laparoscopic extended right hemicolectomy was performed. The postoperative pathology results indicated poorly differentiated adenocarcinoma infiltrating the entire layer, particularly the subserosa and muscularis propria (Figure 2A). In addition, the appendix and ileocecal valve were infiltrated by the tumor. The harvested lymph nodes presented the following positivity: Posterior mesenteric lymph nodes (1/6), middle colonic vascular root lymph nodes (4/12), ileocecal vascular root lymph nodes (6/8), right colonic vascular root lymph nodes (4/6), lymph nodes around the cecum (4/4), and lymph nodes around the colon (8/9). The pathological tumor, node, and metastasis stage was then assessed as pT4N2bMo and the patient’s postoperative recovery was uneventful.
After chemotherapy, PET-CT was performed and the result showed multiple lymph nodes metastases around the abdominal aorta, without lung or liver metastases (Figure 1). The patient was recommended for radiotherapy with a total of 50 Gy in five regimens. However, after finishing the second radiotherapy regimen, the patient found a 4 cm × 4 cm mass in his right thigh that caused intolerable pain. Color ultrasound revealed a mass located in the skeletal muscle (Figure 2B). Histological biopsy found poorly differentiated adenocarcinoma. Cytokeratin 20 (CK20) is a member of the CK family. The expression of cytoskeleton proteins depends on the cell type and development and differentiation stage. Abnormal expression of CK has been observed in various forms of tumors and other diseases[4]. CK20 expression is also observed in the majority of colorectal tumors[5]. Based on CK staining on the puncture biopsy tissue of the thigh mass, combined with the morphology of tumor cells and the patient’s medical history, it was suspected to be a distant metastasis of colon cancer. Immunohistochemical examination revealed that the small woven bone component was considered to be ossified (Figure 2C-F). A complete resection was suggested, but was refused by the patient.
The patient in this case was finally diagnosed as a secondary malignant tumor of skeletal muscle (on the right thigh), which is considered to be a metastasis caused by a colon tumor.
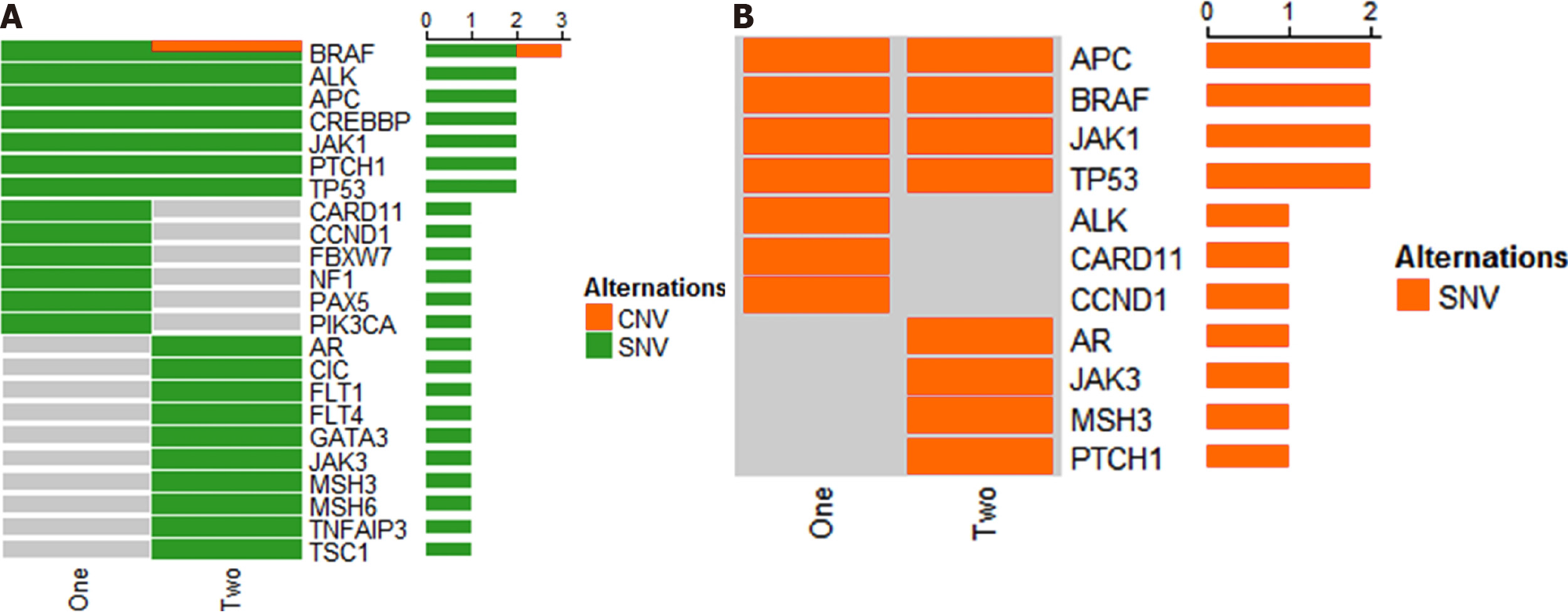
Soon, the patient developed bone metastases to the tibial vertebral bodies and a collagen gel droplet-embedded culture drug sensitivity test (CD-DST) was performed. The result proved that the patient was not sensitive to the chemotherapy regimens of compound tegafur capsules (TS-1), docetaxel, gemcitabine, etoposide (VP-16), or FOLFIRI. Next, the one cycle chemotherapy regimen was changed to bevacizumab, irinotecan, and capecitabine and a gene test was performed. The result showed a mutation of the BRAF gene and wild-type KRAS and NRAS genes (Figure 3). Further
Although no liver or lung metastases occurred, the patient had been suffering from thigh pain and the side effects of chemotherapy (such as nausea and vomiting). Performance status (PS) is an indicator of a patient's physical strength to understand his/her general health and tolerance to treatment. According to the Eastern Cooperative Oncology Group scoring standard (ECOG, Zubrod, World Health Organization) designated by the ECOG[6], the patient's activity status is roughly divided into six levels from 0 to 5, and it is considered that patients with PS 3 and 4 are not suitable for chemotherapy[7], and our patient was roughly at level 3. And unfortunately, the patient's overall physical condition was poor. The intractable vomiting caused by chemotherapy led to electrolyte imbalance, which was difficult to correct, and eventually led to the patient's death in October 2018.
The prevalence of SMM ranges from 0.03% to 5.6% in autopsy series of cancer patients[8]. In fact, skeletal muscle comprises about 50% of total body mass. However, metastatic spread to skeletal muscles from colorectal carcinomas is rare, and is usually an indication of systematic spread. Hasegawa et al[9] reported that 0.028% of patients with colorectal cancer (CRC) developed SMM. Meanwhile, SMM implies a poor prognosis, with a mean survival duration from diagnosis to death of 5.4 mo (range: 1–12 mo)[10]. Araki et al[11] reported a patient with SMM in the right teres major muscle who survived for 2 years, but died of carcinoma after a complete resection of metastatic lesions and other therapies. However, patients with SMM mostly develop generalized metastases, which soon results in death. Metastasis to the musculature from colorectal carcinomas is rare, with only 18 cases being reported in the recent English language literature, and among them colon carcinoma was the primary site for only 13 cases. In these case reports, the sites of primary carcinomas and metastatic lesions in SMM were diverse, and the interval from resection of the primary carcinoma to the development of SMM ranged from 5 to 60 mo. However, Laurence et al[12] reported a 51-year male patient who visited the hospital for the painful mass in the right forearm, which proved to be an SMM, after which a transverse carcinoma was found. Although there are few reports of SMM, possibly because of its asymptomatic nature and undetected characteristics, it is possible that the true incidence is underestimated. The possible mechanism of metastatic spread of adenocarcinoma of the colon to skeletal muscles could be via the lymphatics, the hematogenous route, direct extension of primary disease, or from manipulation during surgery[1]. In the case reported by Tunio et al[13], they found two sites of muscular metastasis in the gluteus maximus and rectus abdominis muscles in a 28-year-old man with known colon adenocarcinoma. They hypothesized that the possible mechanism for metastasis in this patient was implantation of tumor cells during surgery.
Usually, most patients with SMM present with painful masses. This might be important to discriminate SMMs from soft tissue sarcoma, which presents as a painless mass. There is no specific diagnostic approach for soft tissue metastases and magnetic resonance imaging and PET-CT have been recommended as the optimal techniques. For example, the CT of the patient in the present case only reported multiple abnormal signals in the lumbar 5, sacrals 1-3, and bilateral iliac bones, and abnormal signals outside of the right iliopsoas muscle, which could only indicate lesions and cannot be used as a basis for diagnosis. Furthermore, PET-CT is not only able to exclude metastatic sites, but also could be used to evaluate the patient’s treatment response[14]. Although there is a high risk of regional seeding or implantation of carcinoma cells, needle aspiration biopsy is still highly recommended as a valuable diagnostic approach, with a low incidence of 0.03% of needle metastases reported by Kline et al[15].
Noticeably, our patient’s pathological outcome of needle biopsy revealed adenocarcinoma with bone metaplasia. Ossification refers to the formation of heterotopic bone, which occurs occasionally in colorectal polyps, Barrett’s esophagus, and mucocele of the appendix[2,13-16], but rarely in metastatic tumor deposits. According to the literature, the bone metaplasia of SMM has only been observed in three case reports of metastatic colonic adenocarcinoma[11,17,18]. The mechanism and pathogenesis of bone metaplasia of SMM remain unclear.
Although the potential malignancy and metastasis mechanism of heterotopic ossification from colon carcinoma are unclear, it indicates high tumor malignancy, because the ossification is commonly induced by tumor progression in a tumor microenvironment[19]. The lack of capsule or pseudo-capsule formation of mass infiltrative borders, makes it hard to achieve complete excision. For most distant soft tissue metastases, Stabler et al[17] recommended that they should be treated with radiotherapy instead of surgery, and SMM accompanied by disseminated metastases should be treated palliatively. In our study, the patient continued to receive radio
Compared with left-sided primary tumors, right-sided primary tumors seem to be associated with a worse survival. Prasanna et al[20] reported that patients with BRAF mutations have a higher incidence of peritoneal metastases, rather than lung and liver limited metastases, leading to poor prognosis. Right-sided colon carcinomas have higher rates of peritoneal metastases (relative risk = 0.6, P < 0.001) than left colon carcinomas. In our study, the patient had multiple lymph nodes metastases around the abdominal aorta and bone metastases were found 2 mo later, which indicated the high malignancy and rapid progression of the tumor. Further study on the association between the BRAF mutations and SMM is warranted.
The metastasis of malignant tumors is usually divided into the following five simple steps: Epithelial-mesenchymal transition (EMT), crossing the vascular barrier, surviving in the blood vessel to obtain the characteristics of stem cells, exuding from the blood vessel, and adapting to the microenvironment.
CRC is a complex disease that involves multiple steps of genetic changes, such as the inactivation of tumor suppressor genes and the activation of tumor genes. It is usually associated with the progression from malignant pre-neoplastic lesions (adenoma) to invasive adenocarcinoma[1]. KRAS mutations have been found in about 35% of colon carcinomas. The mutations mainly occur at codons 12, 13, and 61, which form the constitutively active form of KRAS GTPase[21]. Consequently, multiple RAS effector pathways that regulate fundamental biological processes, such as proliferation, apoptosis, and cell motility, become activated and/or deregulated. More specifically, mutant KRAS disrupts actin cytoskeleton and maintains motility in colon cancer cells[21]. BRAF, a major down-stream effector of KRAS, is also considered an oncogene whose activating mutations appear in 70% of human malignant melanomas and in about 12%-18% of human colon cancers. The most frequent BRAF mutation is at codon 600 that results in elevated kinase activity[22]. Mutant BRAF may also interfere with the organization of the cytoskeleton and affect cell migration and invasion ability.
Key steps in invasion and metastasis are tightly regulated or influenced by the Rho family GTPases, which may include alterations in cell adhesion, cell-matrix, cell-cell interactions, and actin organization, ultimately leading to the acquisition of an invasive phenotype[23]. In order to invade into other tissues, epithelial cancer cells must disrupt the integrity of the epithelium and basement membrane to enter the underlying stroma. This normally requires acquisition of a migratory phenotype, a process frequently referred as EMT. Invasive epithelial cancer cells often show reduced expression of E-cadherin, a cell-cell adhesion protein, and increased expression of mesenchymal markers, such as vimentin and N-cadherin. EMT plays a key role in the invasion and metastasis of malignant tumors. To study the role of the initiating factors of EMT and its downstream pathways in tumor growth, invasion, and metastasis, and to block this process are important for the early diagnosis and early treatment of tumor metastasis.
The BRAF protein is generated from the protooncogene BRAF and is a key regulator in the MAPK/ERK signaling pathway, which has normal downstream effects, including cell division, differentiation, and secretion[24]. In normal signaling, BRAF is activated upon binding the Ras-GTP complex and, subsequently, activates the MEK protein. MEK signaling induces ERK1/2 activation, which has many effectors, one of which is the Snail protein[25].
Snail is a zinc finger transcriptional factor, generated from the protooncogene SNAIL[26]. Snail has significance in embryological development through its contributions to mesoderm formation. Once activated, Snail translocates to the nucleus, where it binds E-box, which is an E-cadherin promoter region[27]. Binding to E-box promotes downregulation of E-cadherin, allowing for the detachment of cells from the epithelium in a process known as EMT[26]. Snail signaling is of particular importance during embryological development as it enables cells to detach from the epithelium and migrate into the developing embryo[28]. In this manner, mesenchymal cells are formed. A number of studies have demonstrated the relevance of Snail function in the progression of various malignancies, including breast carcinoma, osteosarcoma, colorectal carcinoma, lung carcinoma, prostate carcinoma, and clear cell renal carcinoma[29-31].
Besides, TMB is defined as the total number of nonsynonymous mutations per coding area of a tumor genome. The number of mutated genes in the genome will significantly increase in patients with an elevated TMB. As a response, a large number of non-self-antigens will be generated and are more likely to be recognized by the immune system, leading to a strong immune response and higher sensitivity to immunosuppressive agents[32]. Based on a patient’s genomic profile and molecular phenotypes, optimal therapy should be selected.
CD-DST is an in vitro tumor sensitivity testing technique for chemotherapeutic drug sensitivity, which requires a small number of specimens. As a simple, rapid, sensitive, and clinically relevant in vitro sensitivity test, it can help clinicians to select effective drugs scientifically and reasonably, optimize drug combinations, improve clinical efficacy, and reduce toxicity in the practical application of individualized treatment.
Compared with irinotecan with cetuximab, Kopetz et al[33] reported that vemu
Recent advances in radiological examinations and treatment modalities might result in a more frequent diagnosis of SMM. Although it is generally accepted that the prognosis associated with SMM is poor, especially when combined with BRAF mutations, a comprehensive therapy strategy and multidisciplinary treatment might benefit patients.
In summary, we have described a case of skeletal muscle metastasis with bone metaplasia from a colon adenocarcinoma in a patient from China. Although the potential malignancy has not been determined, ossification of SMM might suggest a high tumor malignancy. Examinations, such as PET-CT, CD-DST, and gene testing, are recommended to optimize a comprehensive and individualized treatment modality to prolong the patient’s life expectancy in such intractable cases. It also reminds us that for patients with advanced colorectal tumors, we should be alert to the possibility of unconventional metastasis.
| 1. | Benson AB, Venook AP, Al-Hawary MM, Cederquist L, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D, Engstrom PF, Garrido-Laguna I, Grem JL, Grothey A, Hochster HS, Hoffe S, Hunt S, Kamel A, Kirilcuk N, Krishnamurthi S, Messersmith WA, Meyerhardt J, Miller ED, Mulcahy MF, Murphy JD, Nurkin S, Saltz L, Sharma S, Shibata D, Skibber JM, Sofocleous CT, Stoffel EM, Stotsky-Himelfarb E, Willett CG, Wuthrick E, Gregory KM, Freedman-Cass DA. NCCN Guidelines Insights: Colon Cancer, Version 2.2018. J Natl Compr Canc Netw. 2018;16:359-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 635] [Cited by in RCA: 722] [Article Influence: 90.3] [Reference Citation Analysis (3)] |
| 2. | Damron TA, Heiner J. Distant soft tissue metastases: a series of 30 new patients and 91 cases from the literature. Ann Surg Oncol. 2000;7:526-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 129] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 3. | Surov A, Hainz M, Holzhausen HJ, Arnold D, Katzer M, Schmidt J, Spielmann RP, Behrmann C. Skeletal muscle metastases: primary tumours, prevalence, and radiological features. Eur Radiol. 2010;20:649-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 122] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 4. | Moll R, Löwe A, Laufer J, Franke WW. Cytokeratin 20 in human carcinomas. A new histodiagnostic marker detected by monoclonal antibodies. Am J Pathol. 1992;140:427-447. [PubMed] |
| 5. | Wildi S, Kleeff J, Maruyama H, Maurer CA, Friess H, Büchler MW, Lander AD, Korc M. Characterization of cytokeratin 20 expression in pancreatic and colorectal cancer. Clin Cancer Res. 1999;5:2840-2847. [PubMed] |
| 6. | Young J, Badgery-Parker T, Dobbins T, Jorgensen M, Gibbs P, Faragher I, Jones I, Currow D. Comparison of ECOG/WHO performance status and ASA score as a measure of functional status. J Pain Symptom Manage. 2015;49:258-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 126] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 7. | Prigerson HG, Bao Y, Shah MA, Paulk ME, LeBlanc TW, Schneider BJ, Garrido MM, Reid MC, Berlin DA, Adelson KB, Neugut AI, Maciejewski PK. Chemotherapy Use, Performance Status, and Quality of Life at the End of Life. JAMA Oncol. 2015;1:778-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 425] [Cited by in RCA: 472] [Article Influence: 42.9] [Reference Citation Analysis (0)] |
| 8. | Nocuń A, Chrapko B. Multiple and solitary skeletal muscle metastases on 18F-FDG PET/CT imaging. Nucl Med Commun. 2015;36:1091-1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 9. | Hasegawa S, Sakurai Y, Imazu H, Matsubara T, Ochiai M, Funabiki T, Suzuki K, Mizoguchi Y, Kuroda M, Kasahara M. Metastasis to the forearm skeletal muscle from an adenocarcinoma of the colon: report of a case. Surg Today. 2000;30:1118-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Plaza JA, Perez-Montiel D, Mayerson J, Morrison C, Suster S. Metastases to soft tissue: a review of 118 cases over a 30-year period. Cancer. 2008;112:193-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 135] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 11. | Araki K, Kobayashi M, Ogata T, Takuma K. Colorectal carcinoma metastatic to skeletal muscle. Hepatogastroenterology. 1994;41:405-408. [PubMed] |
| 12. | Laurence AE, Murray AJ. Metastasis in skeletal muscle secondary to carcinoma of the colon--presentation of two cases. Br J Surg. 1970;57:529-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 0.6] [Reference Citation Analysis (1)] |
| 13. | Tunio MA, Alasiri M, Riaz K, Alshakweer W, AlArifi M. Skeletal Muscle Metastasis Secondary to Adenocarcinoma of Colon: A Case Report and Review of Literature. J Gastrointest Dig Syst. 2013;12:1. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Doroudinia A, Mehrian P, Dorudinia A, Kaghazchi F. Rectal adenocarcinoma presenting with thigh muscle metastasis as the only metastatic site. BMJ Case Rep. 2019;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (1)] |
| 15. | Kline TS, Neal HS. Needle aspiration biopsy: a critical appraisal. Eight years and 3,267 specimens later. JAMA. 1978;239:36-39. [PubMed] |
| 16. | Liberatore M, Megna V, Patrizi G, Giannotti D, Semproni CP, Rebonato S, Barchetti F, Gallo P, Miscusi G. Multiple metastases of soft tissue visualized by technetium-99m-methylene diphosphonate scintigraphy: a case report. J Med Case Rep. 2014;8:459. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 17. | Stabler J. Case report: ossifying metastases from carcinoma of the large bowel demonstrated by bone scintigraphy. Clin Radiol. 1995;50:730-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Yoshikawa H, Kameyama M, Ueda T, Kudawara I, Nakanishi K. Ossifying intramuscular metastasis from colon cancer: report of a case. Dis Colon Rectum. 1999;42:1225-1227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (2)] |
| 19. | Huang RS, Brown RE, Buryanek J. Heterotopic ossification in metastatic colorectal carcinoma: case report with morphoproteomic insights into the histogenesis. Ann Clin Lab Sci. 2014;44:99-103. [PubMed] |
| 20. | Prasanna T, Karapetis CS, Roder D, Tie J, Padbury R, Price T, Wong R, Shapiro J, Nott L, Lee M, Chua YJ, Craft P, Piantadosi C, Sorich M, Gibbs P, Yip D. The survival outcome of patients with metastatic colorectal cancer based on the site of metastases and the impact of molecular markers and site of primary cancer on metastatic pattern. Acta Oncol. 2018;57:1438-1444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 83] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 21. | Pollock CB, Shirasawa S, Sasazuki T, Kolch W, Dhillon AS. Oncogenic K-RAS is required to maintain changes in cytoskeletal organization, adhesion, and motility in colon cancer cells. Cancer Res. 2005;65:1244-1250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 110] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 22. | Downward J. Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer. 2003;3:11-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2345] [Cited by in RCA: 2418] [Article Influence: 105.1] [Reference Citation Analysis (0)] |
| 23. | Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3598] [Cited by in RCA: 3741] [Article Influence: 155.9] [Reference Citation Analysis (0)] |
| 24. | Daum G, Eisenmann-Tappe I, Fries HW, Troppmair J, Rapp UR. The ins and outs of Raf kinases. Trends Biochem Sci. 1994;19:474-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 377] [Cited by in RCA: 369] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 25. | Haling JR, Sudhamsu J, Yen I, Sideris S, Sandoval W, Phung W, Bravo BJ, Giannetti AM, Peck A, Masselot A, Morales T, Smith D, Brandhuber BJ, Hymowitz SG, Malek S. Structure of the BRAF-MEK complex reveals a kinase activity independent role for BRAF in MAPK signaling. Cancer Cell. 2014;26:402-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 178] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 26. | Venkov CD, Link AJ, Jennings JL, Plieth D, Inoue T, Nagai K, Xu C, Dimitrova YN, Rauscher FJ, Neilson EG. A proximal activator of transcription in epithelial-mesenchymal transition. J Clin Invest. 2007;117:482-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 145] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 27. | Villarejo A, Cortés-Cabrera A, Molina-Ortíz P, Portillo F, Cano A. Differential role of Snail1 and Snail2 zinc fingers in E-cadherin repression and epithelial to mesenchymal transition. J Biol Chem. 2014;289:930-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 128] [Article Influence: 9.8] [Reference Citation Analysis (1)] |
| 28. | Fleming TP, Papenbrock T, Fesenko I, Hausen P, Sheth B. Assembly of tight junctions during early vertebrate development. Semin Cell Dev Biol. 2000;11:291-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 72] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 29. | De Craene B, Berx G. Snail in the frame of malignant tumor recurrence. Breast Cancer Res. 2006;8:105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 30. | Fan F, Samuel S, Evans KW, Lu J, Xia L, Zhou Y, Sceusi E, Tozzi F, Ye XC, Mani SA, Ellis LM. Overexpression of snail induces epithelial-mesenchymal transition and a cancer stem cell-like phenotype in human colorectal cancer cells. Cancer Med. 2012;1:5-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 160] [Cited by in RCA: 194] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 31. | Yang S, Liu Y, Li MY, Ng CSH, Yang SL, Wang S, Zou C, Dong Y, Du J, Long X, Liu LZ, Wan IYP, Mok T, Underwood MJ, Chen GG. FOXP3 promotes tumor growth and metastasis by activating Wnt/β-catenin signaling pathway and EMT in non-small cell lung cancer. Mol Cancer. 2017;16:124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 340] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 32. | Pai SG, Carneiro BA, Chae YK, Costa RL, Kalyan A, Shah HA, Helenowski I, Rademaker AW, Mahalingam D, Giles FJ. Correlation of tumor mutational burden and treatment outcomes in patients with colorectal cancer. J Gastrointest Oncol. 2017;8:858-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 33. | Kopetz S, Grothey A, Yaeger R, Van Cutsem E, Desai J, Yoshino T, Wasan H, Ciardiello F, Loupakis F, Hong YS, Steeghs N, Guren TK, Arkenau HT, Garcia-Alfonso P, Pfeiffer P, Orlov S, Lonardi S, Elez E, Kim TW, Schellens JHM, Guo C, Krishnan A, Dekervel J, Morris V, Calvo Ferrandiz A, Tarpgaard LS, Braun M, Gollerkeri A, Keir C, Maharry K, Pickard M, Christy-Bittel J, Anderson L, Sandor V, Tabernero J. Encorafenib, Binimetinib, and Cetuximab in BRAF V600E-Mutated Colorectal Cancer. N Engl J Med. 2019;381:1632-1643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 694] [Cited by in RCA: 1060] [Article Influence: 151.4] [Reference Citation Analysis (5)] |
| 34. | Sepulveda AR, Hamilton SR, Allegra CJ, Grody W, Cushman-Vokoun AM, Funkhouser WK, Kopetz SE, Lieu C, Lindor NM, Minsky BD, Monzon FA, Sargent DJ, Singh VM, Willis J, Clark J, Colasacco C, Rumble RB, Temple-Smolkin R, Ventura CB, Nowak JA. Molecular Biomarkers for the Evaluation of Colorectal Cancer: Guideline From the American Society for Clinical Pathology, College of American Pathologists, Association for Molecular Pathology, and the American Society of Clinical Oncology. J Clin Oncol. 2017;35:1453-1486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 256] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 35. | Gong J, Cho M, Sy M, Salgia R, Fakih M. Molecular profiling of metastatic colorectal tumors using next-generation sequencing: a single-institution experience. Oncotarget. 2017;8:42198-42213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 36. | Stulc JP, Petrelli NJ, Herrera L, Lopez CL, Mittelman A. Isolated metachronous metastases to soft tissues of the buttock from a colonic adenocarcinoma. Dis Colon Rectum. 1985;28:117-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 14] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 37. | Torosian MH, Botet JF, Paglia M. Colon carcinoma metastatic to the thigh--an unusual site of metastasis. Report of a case. Dis Colon Rectum. 1987;30:805-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 38. | Caskey CI, Fishman EK. Computed tomography of calcified metastases to skeletal muscle from adenocarcinoma of the colon. J Comput Tomogr. 1988;12:199-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.3] [Reference Citation Analysis (1)] |
| 39. | Prasad A, Avery C, Foley RJ. Abdominal wall metastases following laparoscopy. Br J Surg. 1994;81:1697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 47] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 40. | Naik VR, Jaafar H, Mutum SS. Heterotopic ossification in skeletal muscle metastasis from colonic adenocarcinoma--a case report. Malays J Pathol. 2005;27:119-121. [PubMed] |
| 41. | Takada J, Watanabe K, Kuraya D, Kina M, Hayashi S, Hamada H, Katsuk Y. [An example of metastasis to the iliopsoas muscle from sigmoid colon cancer]. Gan To Kagaku Ryoho. 2011;38:2294-2297. [PubMed] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Surgery
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cai SL S-Editor: Zhang H L-Editor: Wang TQ P-Editor: Liu JH