Published online Oct 26, 2021. doi: 10.12998/wjcc.v9.i30.9276
Peer-review started: June 3, 2021
First decision: June 17, 2021
Revised: April 15, 2021
Accepted: August 23, 2021
Article in press: August 23, 2021
Published online: October 26, 2021
Processing time: 140 Days and 1.9 Hours
β-ketothiolase deficiency (β-KTD) is an inherited disease, and insufficient attention has been paid to imageology due to its lower morbidity. Therefore, few lesions outside the basal ganglia have been found before, and the persistent pathological changes have rarely been reported.
A 10-mo-old Chinese female patient with a free previous medical history but with poor physical and athletic development had received the haemophilus influenzae vaccine and then developed a low fever 2 d prior. She was initially diagnosed with severe brain injury, central respiratory failure, metabolic acidosis com
The case highlights the critical importance of one view that the range of lesions in some patients may be more extensive than previously thought in some β-KTD patients. In addition to biochemical tests, genetic tests and magnetic resonance imaging are not only conducive to quickly diagnosing β-KTD but also to partially evaluating the short- and long-term outcomes. Moreover, more attention should be paid to the two mutations (c.478C>G; c.951C>T) that may be associated with severe β-KTD.
Core Tip: In our report, abnormal signals with a nodular shape were not only found in the bilateral basal ganglia (the lesions were mainly located in the corpus striatum), but they were also found in the bilateral cerebral peduncle and mesencephalon. These severe and extensive lesions caused the irreversible damage to the basal ganglia finally. It is suggested that the range of lesions in some patients may be more extensive than previously thought. In addition, two unusual mutations (c.478C>G; c.951C>T) may associate with this disease. As the clinical symptoms at the first onset were life-threatening with the extensive lesions, it is imperative that clinicians take notice when these two mutations appear in gene sequences.
- Citation: Guo J, Ren D, Guo ZJ, Yu J, Liu F, Zhao RX, Wang Y. Emergence of lesions outside of the basal ganglia and irreversible damage to the basal ganglia with severe β-ketothiolase deficiency: A case report . World J Clin Cases 2021; 9(30): 9276-9284
- URL: https://www.wjgnet.com/2307-8960/full/v9/i30/9276.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i30.9276
β-ketothiolase deficiency (β-KTD), a rare autosomal recessive metabolic disorder, is an inherited disease of isoleucine and ketone body metabolism caused by the ACAT1 gene mutation. The disorder is clinically characterized by intermittent ketoacidotic episodes without glucose utilization disorder, which could cause some fatal complications when serious episodes occur, although the incidence of severe ketoacidotic episodes is apparently lower than that of the normal β-KTD. Intracranial lesions can be found on magnetic resonance imaging (MRI), but few lesions outside the basal ganglia have been found before, and persistent changes have rarely been reported. We presented herein, the first case of lesions found outside of the basal ganglia and irreversible damage to the basal ganglia with severe β-KTD in a female pediatric patient.
A 10-mo-old Chinese female patient with mental retardation, fatigue, dyspnea, and disturbance of consciousness was admitted.
Two weeks ago, the patient had received the haemophilus influenzae vaccine and had a low fever 2 d prior.
The patient had a free previous medical history but with poor physical and athletic development.
The patient’s parents did not have chronic or hereditary diseases, nor did their other relatives.
When admitted, the patient was lethargic, and had lip cyanosis, deep breathing, and moaning. The muscle tone of the arms and legs was low, and the Achilles jerk and tendon reflex were absent.
Glycemia was basically maintained within the normal range. The analysis of arterial blood gas showed metabolic acidosis. The cerebrospinal fluid protein was 0.69 g/L, blood ammonia was 93 μmol/L, and pro-brain natriuretic peptide was higher than normal, which indicated that heart function may be partially damaged.
The patient was further evaluated by blood tandem mass spectrometry, which showed that alanine, leucine, and valine were decreased. Urinary gas chromatography–mass spectrometry showed that lactic acid, glycolic acid, 3-hydroxypropionic acid, malonic acid, acetoacetic acid, and other organic acids were slightly increased, and 3-hydroxybutyric acid was significantly increased. The results of genetic testing showed c.478C>G (P160A, chr11: 108009667) (from the patient’s mother) and c.951C>T (D317D, chr11: 108014720) (from the patient’s father). This case was a compound heterozygous mutation.
One day before admission, MRI in another hospital showed that there was no obvious intracranial lesion. Cardiac ultrasound showed no abnormality.
The patient was initially diagnosed with severe brain injury, central respiratory failure, metabolic acidosis complicated with respiratory alkalosis, hyper-IgE, malnutrition, and hyperammonia.
The final diagnosis was β-KTD.
The patient was started on glucose and insulin supplementation, mechanical ventilation, respiratory tract management, stabilization of intracranial pressure, correction of acidosis, supplementation of L-carnitine, and nourishment of neurocytes. During the treatment, the patient was fed a high-carbohydrate, low-protein and low-fat diet.
Five days later, the patients’ consciousness recovered partially, but the response to pain and other stimulation was still poor. Ten days later, the dyspnea was improved evidently and the ventilator was removed, but there were obvious abnormalities on MRI. The lesions mainly invaded the corpus striatum but were not limited to the basal ganglia. The patient got over the illness and was discharged from hospital 1 mo later with partial remission of abnormal lesions on MRI. Since then till June 2020, approximately every 6 mo, the patient was admitted to our hospital complaining of acute gastroenteritis or respiratory infections and diagnosed as metabolic acidosis. However, the nervous and respiratory system symptoms were milder than those at the first onset. Each time after short-term (3-7 d) symptomatic treatment, she was discharged stably. Whilst the irreversible lesions were observed on MRI, the mental and physical development of the patient was obviously retarded, and extra rehabilitation training was needed.
As a whole, the morbidity of β-KTD is unknown. Since the first report in 1971[1], hundreds of patients have been identified worldwide. Most reports are from Europe. In Asia, the largest amount of reports have come from Japan, India, and Vietnam[2-4]. There are rare cases in China, with fewer cases with severe clinical symptoms. Because of the low morbidity, the current research on this disease is not sufficiently in-depth.
There is a consensus being formed on the diagnosis and treatment of β-KTD, although controversy still exists. It can be divided into acute crisis and nonepisodic conditions. During acute attack, suppression of ketogenesis is essential, but the treatment of metabolic acidosis using sodium bicarbonate and hemodialysis remains controversial. L-carnitine supplementation could be included due to low carnitine levels[5]. Whilst the L-carnitine level could not be measured in our institution, and L-carnitine was given empirically to prevent secondary carnitine deficiency. In acute crisis remission, the emphasis is still on carbohydrate supplementation, and the restriction of excess protein and fat intake is a fundamental and reasonable treatment regimen[6]. There has recently been no significant progress in diagnosis and treatment. At the first onset, the patient had severe inhibition of the central nervous system and respiratory symptoms which even had an influence on the cardiovascular system. These effects could be fatal, whilst the patient improved and was discharged. However, the patient improved rapidly. It suggests that early diagnosis and treatment might improve the outcome.
There are few concerns on the imaging manifestations of the disease due to the low morbidity, difficulty in diagnosis, and different medical conditions. Only 10% of patients have abnormal pathological changes on intracranial imaging examination, but there are minor specific reports. The basal ganglia contain the corpus striatum, claustrum nucleus, and amygdaloid nucleus. The corpus striatum contains the caudate nucleus and lenticular nucleus which can be divided into the putamen and globus pallidus. Early studies have shown that in six patients with β-KTD, high T2 intensity changes were seen mainly in the posterolateral putamina[7,8]. A subsequent study showed that abnormal MRI signals can be confined only to the globus pallidus, which is located at the lentiform nucleus[9]. Therefore, the present report illustrates that the lesions were mainly located in the lenticular nucleus. These were shown as abnormal signals of cytotoxicity on MRI. One theory is that the basal ganglia have high energy requirements in childhood, which may make them particularly vulnerable to damage by impaired energy metabolism[10]. It is significant that a speculative view about the mechanism of the phenomenon is related to the neurotoxicity of organic acidemia. Specific neuroimaging is not satisfactory enough in various metabolic diseases of organic acidemia, and the location and extent of brain injury may depend on the nature of the metabolic derangement and individual susceptibility[9]. Therefore, our observation that the lesions were located at the caudate nucleus and even out of the basal ganglia partly supports this conjecture.
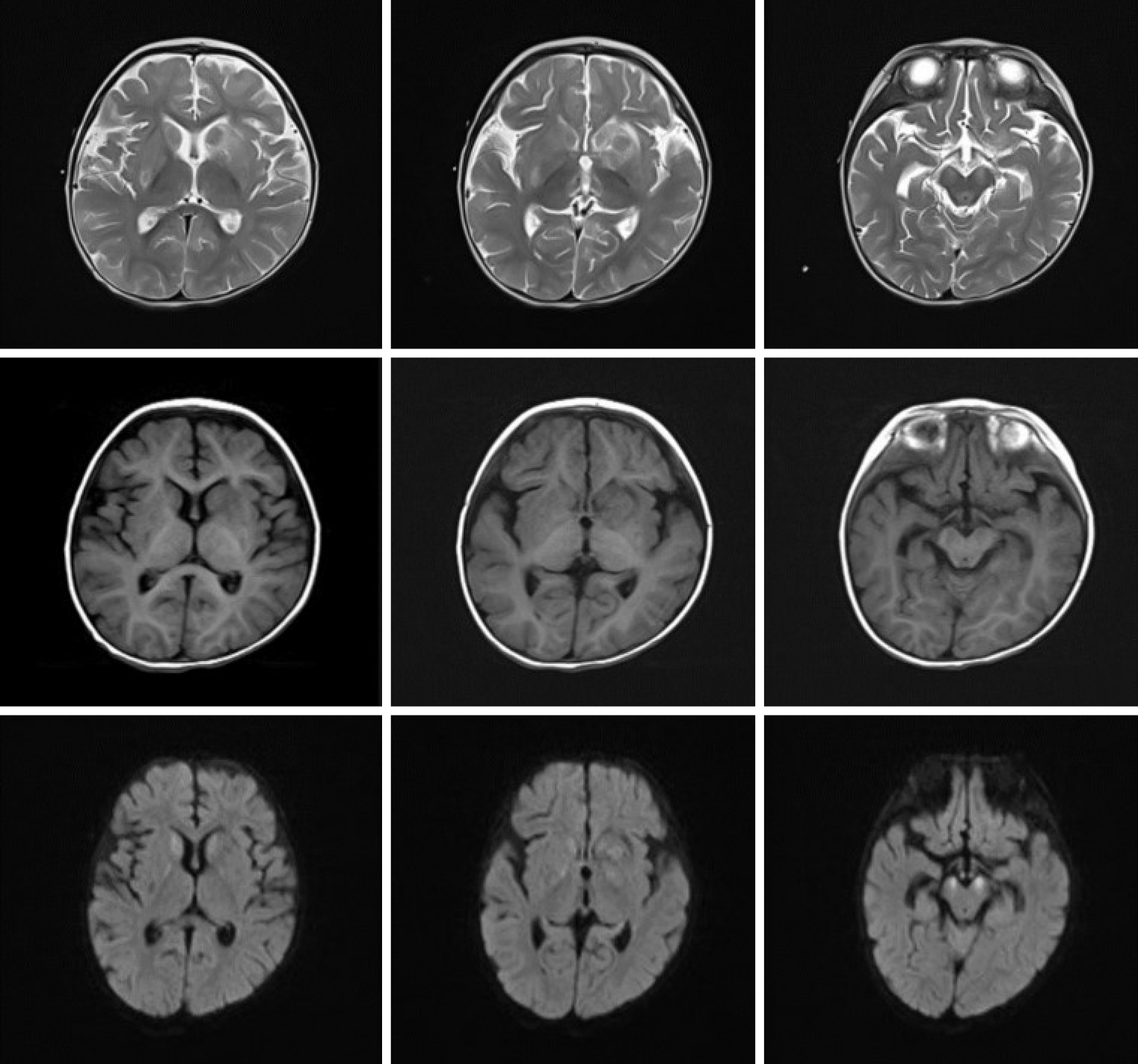
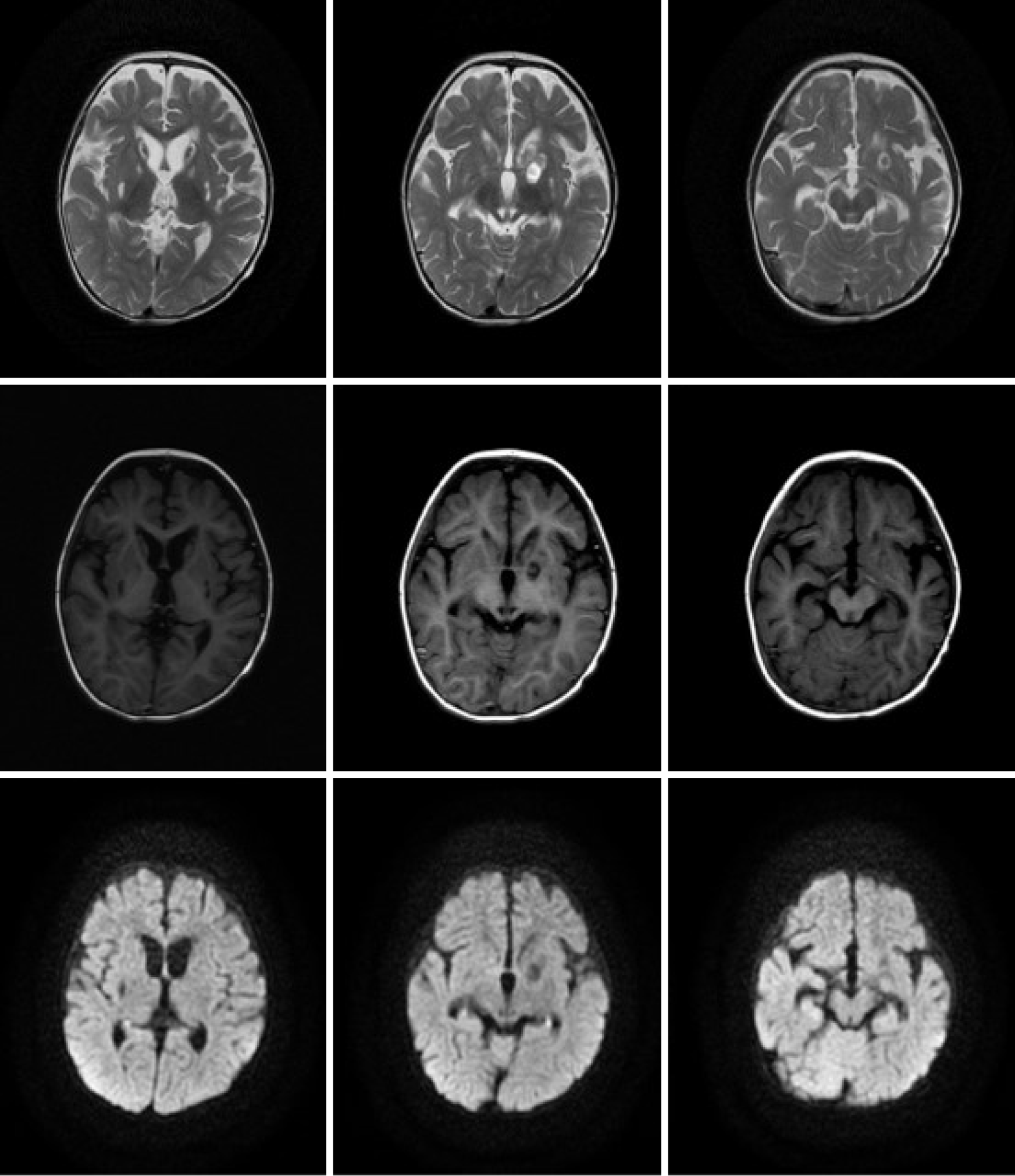
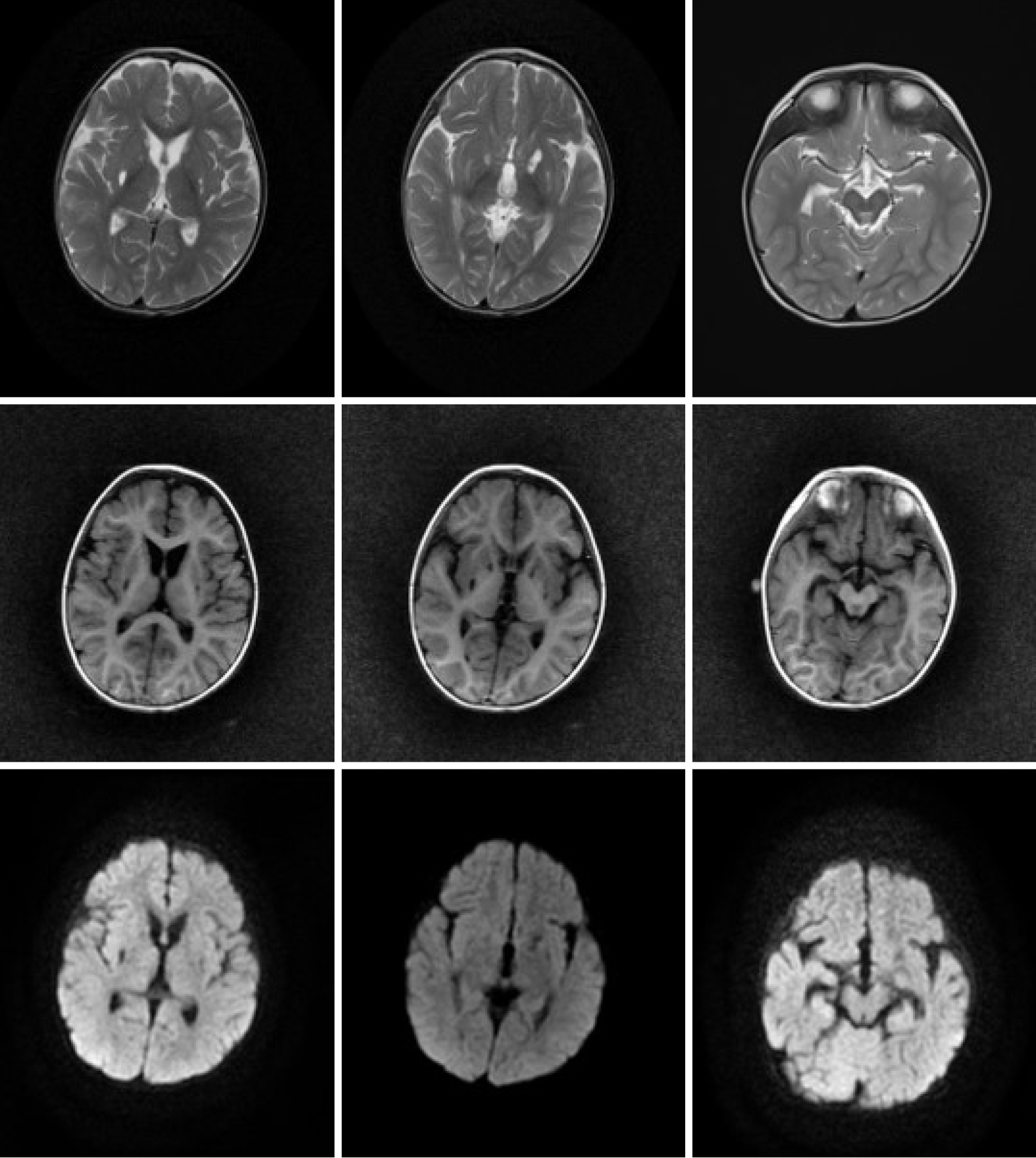
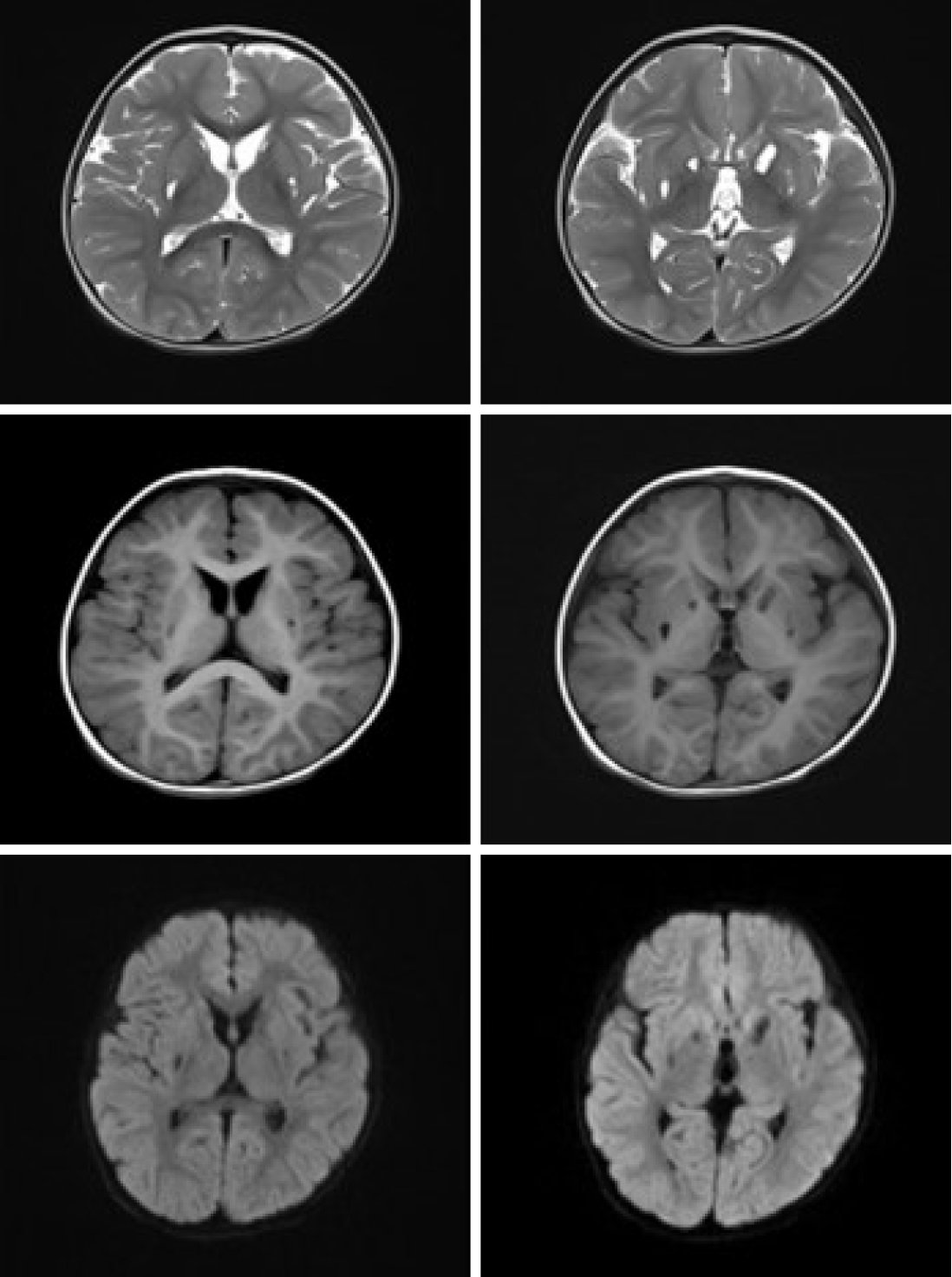
In the present case, abnormal signals with a nodular shape were not only found in the bilateral basal ganglia (the lesions were mainly located in the corpus striatum, and the left was more severe) but they were also found in the bilateral cerebral peduncle and mesencephalon (Figure 1). With a more extensive scope of the lesion, it is demonstrated that the intracranial pathology may be severe, which was consistent with the patient’s clinical manifestation upon the first admission and with the slow recovery of neurological symptoms. Previous studies have not reported finding lesions outside the basal ganglia, and lesions have rarely affected the caudate nucleus. As the mesencephalon is adjacent to the basal ganglia anatomically, the lesion may extend to adjacent tissues when the inflammation is serious enough in theory. Twenty-five days later, the high signals in the bilateral corpus striatum and mesencephalon disappeared on diffusion-weighted imaging (DWI), but the lesions in the corpus striatum were still seen on T1WI and T2WI, which diminished with partial softening. The mesencephalon lesions on T1WI and T2WI were less obvious. This may suggest that the cytotoxic reaction and the state of illness were improved (Figure 2). However, due to the persistent destruction of basal ganglia tissue, these changes on MRI may represent a measure of neurological or mental comorbidity. Later observation showed that the patient’s intelligence and physiological development indeed lagged behind. However, it is gratifying that the mesencephalon and bilateral cerebral peduncle lesions were absorbed and did not soften. This may indicate that the lesion recover well so that the possibility of sequelae secondary to mesencephalon damage appears unlikely. The rationale for these diverse outcomes in recovery from various positions has not yet been clarified and may be related to the intensity of inflammation. Six months later, the soft focus at the lentiform nucleus still existed, and the bilateral caudate nuclei were atrophied for at least 1 year after onset (Figures 3 and 4). In particular, the liquefaction lesions of the bilateral lentiform nucleus did not seem to have any changes compared with 1 year prior. This finding suggests that this may be a progressive and irreversible process. Puzzlingly, it is not clear whether this atrophy is due to the disease progression itself or recurrent organic acidemia, although metabolic acidosis during the second and third hospitalizations was milder and rapidly corrected. Another case reported in China showed symmetrical patchy hyperintense shadows of the bilateral lenticular nucleus at the first morbidity, although this patient had severe acidosis and organic acidemia, similar to our patient. One and a half months later, the lesions disappeared completely without any liquefaction. There were no sequela changes on MRI for several years, although the patient had recurrent metabolic acidosis.
The critical gene mutation site of β-KTD is still under research. Previous studies have suggested that there may be a few mutations in specific populations: C.622C>T, p.R208* was identified in nearly 80% of Vietnamese subjects[3]. M193R was identified in 45% of mutant alleles in an Indian population[4]. Other common mutations identified are p.G152A, p.N158D, p.N158S, p.G183R, etc. However, studies on East Asian populations suggest that these mutations may lack regularity. Fukao et al[6] have identified more than 80 T2 gene mutations in more than 120 patients. In China, the common mutation patterns have not yet been revealed. A study inferred that c.1124A>G (p.N375S) may be an common important gene mutation in Chinese patients[11]. However, the number of patients in this research was limited, and it lacks support from other evidence. Other new mutations reported are c.370A>C, c.473A>G, c.373G>T (p.V125F), c.419T>G (p.L140R), c.72+1G>A, and c.456C>T. In our report, two heterozygous mutations from the mother and father constituted a compound heterozygous mutation. It seems that a new mutation has been found: c.478C>G (P160A, chr11: 108009667). The effect of this mutation on protein structure and abundance needs further verification in future research. It is speculated that another reported mutation c.951C>T (D317D, chr11: 108014720), which did not cause amino acid changes, is the allele where exon 10 skipping had occurred. Exon 10 skipping could cause a frameshift and produce nonsense-mediated mRNA, which might also cause β-KTD[12]. As the clinical symptoms at the first onset were life-threatening, it is imperative that clinicians take notice when these two mutations appear in gene series, despite it being unclear whether there was an association between the mutations and disease severity.
Overall, the clinical manifestations of β-KTD are very different. A few patients tend to have severe symptoms, which could lead to mental retardation, lagging nervous system development, and even death in the short term. Therefore, the diagnosis and treatment as early and precise as possible are very important. In addition to biochemical tests, genetic tests and MRI are not only conducive to quickly diagnosing β-KTD but also to partially evaluating the short- and long-term outcomes. It is inferred in the presented case that β-KTD could lead to lesions scaling out of the basal ganglia and irreversible damage to the basal ganglia. These lesions may be associated with two mutations that contain a new mutation.
We would like to show our appreciation to Dr. Zhou S for her efforts of language style modification.
| 1. | Daum RS, Lamm PH, Mamer OA, Scriver CR. A "new" disorder of isoleucine catabolism. Lancet. 1971;2:1289-1290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 86] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 2. | Fukao T, Scriver CR, Kondo N; t2 Collaborative Working Group. The clinical phenotype and outcome of mitochondrial acetoacetyl-CoA thiolase deficiency (beta-ketothiolase or T2 deficiency) in 26 enzymatically proved and mutation-defined patients. Mol Genet Metab. 2001;72:109-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 64] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Nguyen KN, Abdelkreem E, Colombo R, Hasegawa Y, Can NT, Bui TP, Le HT, Tran MT, Nguyen HT, Trinh HT, Aoyama Y, Sasai H, Yamaguchi S, Fukao T, Vu DC. Characterization and outcome of 41 patients with beta-ketothiolase deficiency: 10 years' experience of a medical center in northern Vietnam. J Inherit Metab Dis. 2017;40:395-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Abdelkreem E, Akella RRD, Dave U, Sane S, Otsuka H, Sasai H, Aoyama Y, Nakama M, Ohnishi H, Mahmoud S, Abd El Aal M, Fukao T. Clinical and Mutational Characterizations of Ten Indian Patients with Beta-Ketothiolase Deficiency. JIMD Rep. 2017;35:59-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Arica V, Arica SG, Dag H, Onur H, Obut O, Gülbayzar S. Beta-ketothiolase deficiency brought with lethargy: case report. Hum Exp Toxicol. 2011;30:1724-1727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 6. | Fukao T, Sasai H, Aoyama Y, Otsuka H, Ago Y, Matsumoto H, Abdelkreem E. Recent advances in understanding beta-ketothiolase (mitochondrial acetoacetyl-CoA thiolase, T2) deficiency. J Hum Genet. 2019;64:99-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Brismar J, Ozand PT. CT and MR of the brain in the diagnosis of organic acidemias. Experiences from 107 patients. Brain Dev. 1994;16 Suppl:104-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 65] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Ozand PT, Rashed M, Gascon GG, al Odaib A, Shums A, Nester M, Brismar J. 3-Ketothiolase deficiency: a review and four new patients with neurologic symptoms. Brain Dev. 1994;16 Suppl:38-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 27] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | O'Neill ML, Kuo F, Saigal G. MRI of pallidal involvement in Beta-ketothiolase deficiency. J Neuroimaging. 2014;24:414-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Akella RR, Aoyama Y, Mori C, Lingappa L, Cariappa R, Fukao T. Metabolic encephalopathy in beta-ketothiolase deficiency: the first report from India. Brain Dev. 2014;36:537-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Xu F, Han L, Qiu W, Zhang H, Ji W, Chen T, Zhan X, Ye J, Gu X. [Retrospective analysis on clinical data and genetic variations of patients with beta-ketothiolase deficiency]. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2019;36:199-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 12. | Fukao T, Horikawa R, Naiki Y, Tanaka T, Takayanagi M, Yamaguchi S, Kondo N. A novel mutation (c.951C>T) in an exonic splicing enhancer results in exon 10 skipping in the human mitochondrial acetoacetyl-CoA thiolase gene. Mol Genet Metab. 2010;100:339-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ravikanth R S-Editor: Yan JP L-Editor: Wang TQ P-Editor: Liu JH