INTRODUCTION
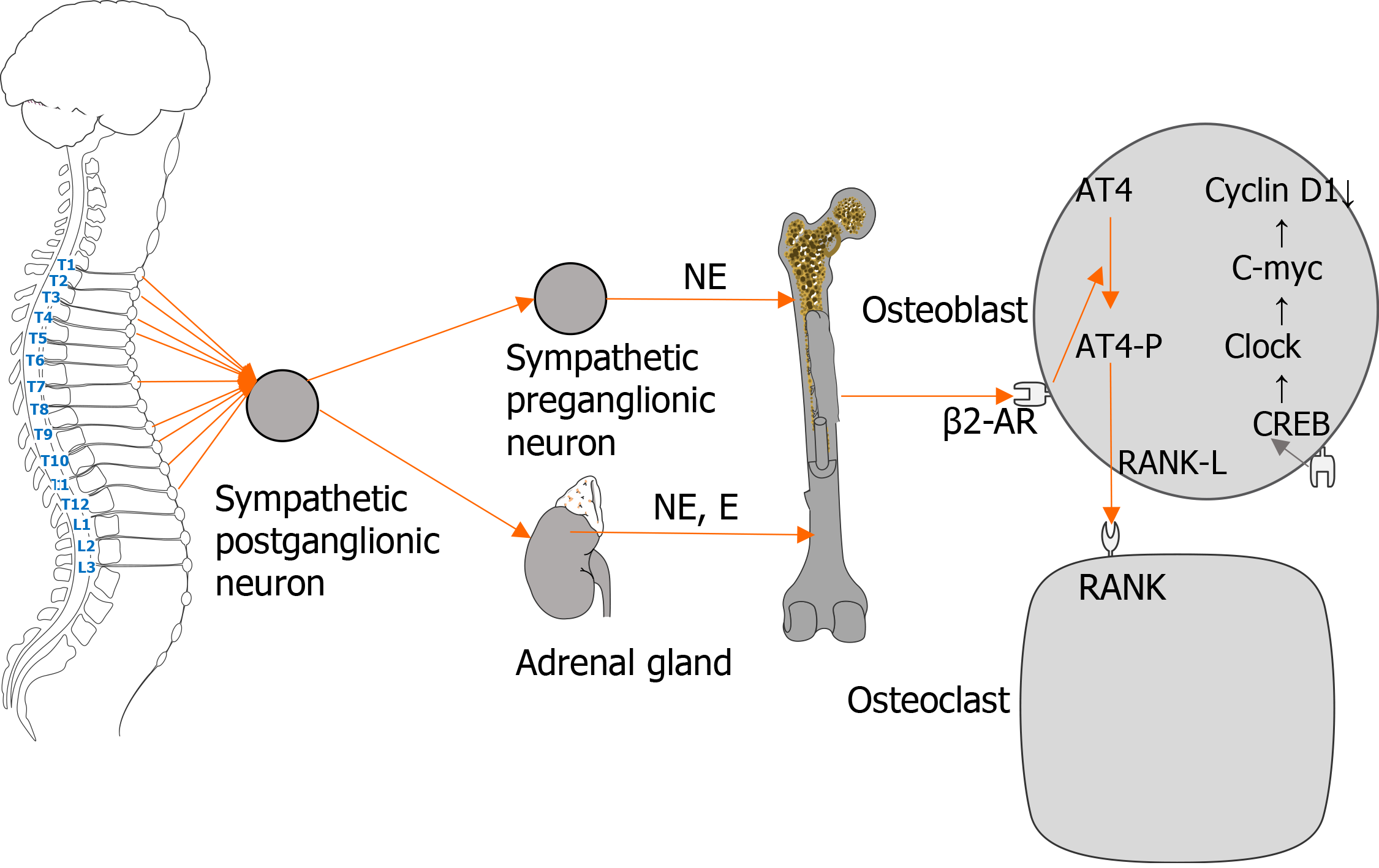
Numerous recent studies have proven that β-adrenergic receptors (β-ARs) have an important role in the metabolism of the skeletal system. As a target organ of the nervous system, bones are innervated by sympathetic nerves. Postsympathetic ganglion neurons release catecholamines that bind to β-ARs on the surface of osteoblasts. After a series of reactions, the activity of osteoclasts is increased, resulting in faster bone resorption compared with bone formation, thereby leading to reduced bone mass and increased risk of fracture. As the increased sympathetic activity has harmful effects on bones, pharmacological destruction of sympathetic signals has become a viable option for improving bone health.
Many common diseases, such as postmenopausal osteoporosis, chronic stress, depression, and others are related to increased bone loss or increased risk of fracture because of increased sympathetic nerve activity. As one of the ideal drugs for the treatment of cardiovascular diseases in the elderly, it is worth exploring whether BBs have a beneficial effect on bone metabolism. With the advent of an aging society and the emergence of important public health issues for the prevention and treatment of osteoporosis, the relationship of β-blockers, antiosteoporosis, and fracture risk has become a research hotspot, which provides new ideas for the treatment of osteopenia.
β-ARS AND BONE METABOLISM
The innervation of bone cells and bone tissue by the sympathetic nervous system (SNS) is the anatomical basis of bone metabolism. The SNS is an important part of the autonomic nervous system, which is widely distributed in peripheral tissues and organs. It mainly participates in the regulation of bone metabolism through adrenergic receptors (ARs) on tissues and organs. ARs are G protein-coupled receptors, and there are two types: α and β. β-ARs have three subtypes (β1-AR, β2-AR, and β3-AR). The SNS can complete its regulation of osteoblasts and osteoclasts by activating β-AR on the cell surface, thereby affecting the metabolic reconstruction of bone tissue. As the main neurotransmitter of the SNS, catecholamines transmit signals from the cell membrane to cytoplasmic G protein. During G protein signal transduction, activated β2-ARs are phosphorylated and combine with β-arrestin to initiate β2-AR desensitization, endocytosis, and β-arrestin-dependent signal transduction[1]. A previous study reported the existence of parallel pathways for G protein and β-arrestin signaling[2]. In the skeletal system, β2-AR is the main mediator of catecholamine activation in bone remodeling units[3]; β1-AR and β3-AR are also related to the regulation of bone metabolism. Many studies have shown that β-ARs have a significant regulatory role in bone turnover, and their expression has different effects on osteoblasts and osteoclasts.
Regulation of osteoclasts by β-Ars
Osteoblasts are the main cell types involved in molecular events triggered by sympathetic nerves in regulating bone mass[4]. Recent studies have found that β2-AR mainly has an inhibitory role in the regulation of bone formation, and is the main receptor for sympathetic nerves. When the β2-AR pathway on the surface of osteoblasts is activated, it blocks the phosphorylation of cyclic adenosine monophosphate (cAMP)-responsive element binding protein (CREB) by cAMP[5] and promotes the expression of Clock genes, such as PER1 and PER2, which in turn downregulate bone formation by downregulating c-myc and cyclin D1, an important regulator of osteoblast proliferation. Meanwhile, the activation of β2-AR induces an increase of cAMP level and activation of the PKA pathway, which then upregulates the expression of the c-fos gene. Subsequently, c-fos in combination with c-jun form the transcription factor AP-1, which regulates the production of osteocalcin, alkaline phosphatase, and type I collagen, thus affecting bone formation[6].
Animal experiments have also shown similar results. β2-AR gene-deficient mice had high bone mass secondary to increased osteoblast proliferation and bone formation, and decreased bone resorption[7,8]. Similarly, Sato et al[9] found that β-blockers improved bone loss and bone fragility caused by the blocking effect of β2-AR. The above findings demonstrate the inhibitory effect of β2-AR in increasing bone formation. Therefore, selective blocking of β2-AR may be a viable antiosteoporosis option.
Regulation of osteoclasts by β-ARs
β-ARs directly or indirectly act on osteoclasts to participate in bone metabolism, and the effect is mainly realized by β2-AR. The combination of catecholamines with β2-AR on the surface of osteoclast precursor cells induces the maturation of osteoclasts, thereby affecting osteoclast function[10]. Catecholamines activate β2-AR and alter the expression of osteoclast maturation-specific factors such as synthin, carbonic anhydrase II, and protein kinase K, and increase the bone resorption activity of osteoclasts[11]. Meanwhile, β-ARs can indirectly modulate osteoclasts by regulating the secretion of cytokines by osteoblasts. The activation of β2-AR on the surface of osteoblasts inhibits the phosphorylation of CREB and the proliferation of osteoblasts, and subsequently promotes the phosphorylation of activated transcription factor-4, thereby increasing the expression of nuclear factor kappa B receptor activator ligand (RANKL) and stimulating osteoclast differentiation[4]. In addition, animal studies have shown that β-receptor agonists increase the synthesis of prostaglandin E2 and interleukin-6 in the skulls of mice, thereby increasing osteoclasts activity and reducing bone mass. The above studies demonstrated that β-ARs participate in sympathetic nerve-mediated regulation of osteoclasts through various mechanisms that directly or indirectly promote osteoclast activity and reduce bone mass and bone mineral density (BMD, Figure 1). However, excess catecholamines and overstimulation of the SNS may lead to adverse effects, including bone loss, especially trabecular bone, and increased bone resorption[12,13].
Figure 1 Regulation of osteoblasts and osteoclasts by the sympathetic nervous system.
Regulation of bone marrow mesenchymal stem cells (osteogenic precursor cells) by β-ARs
β2-AR and β3-AR are involved in the process of bone marrow mesenchymal stem cell (BMSC) osteogenic differentiation and regulation. Animal studies have shown that both β2-AR and β3-AR are involved in mouse mesenchymal stem cell (MSC) osteogenesis, in which β2-AR has the dominant role. The underlying mechanism involves activation of β-ARs by the SNS, which inhibits BMSC osteogenic differentiation through the cAMP/PKA signaling pathway. Therefore, β-ARs agonists have a negative effect on BMSC osteogenesis. In contrast, BBs have a positive effect on BMSC osteogenesis[14]. Similarly, another animal experiment found that propranolol (PRO), a nonselective BB, promoted osteogenic differentiation and migration of rat BMSCs in vitro and inhibited osteoclast formation by inhibiting β-AR activation. The above studies suggest that BB might become an alternative antiosteoporosis drug to prevent osteoporosis and increase bone mass[15].
BB AND BONE METABOLISM
Nonselective and selective BBs are commonly used in the clinical treatment of cardiovascular diseases. Recent studies have shown that elderly patients with cardiovascular disease have a reduced risk of osteoporosis and fracture after taking BBs. In contrast, other studies have shown the opposite results. Hence, whether BB can improve osteoporosis remains controversial, and the underlying mechanism remains elusive.
Nonselective BBs
Nonselective BBs simultaneously block β1-AR and β2-AR. Many animal experiments have shown that nonselective BBs are beneficial for the maintenance of bone mass. Sliwiński et al[16] established animal models and found that PRO increased transverse growth of the endosteum and the cross-sectional area of tibia in sham operated and ovariectomized rats, indicating that PRO promoted bone formation. Similarly, another study also demonstrated that nonselective BBs such as PRO promoted bone healing and osseointegration by downregulating the number of osteoclasts, upregulating collagen formation, and promoting mineralization[17]. In addition, low-dose PRO improved bone loss and bone fragility in spontaneously hypertensive rats[9], and inhibit bone resorption by inhibiting RANKL-mediated osteoclastogenesis and inflammatory markers[18]. The above studies have shown that nonselective BBs can reduce bone loss, control bone homeostasis, and may help to prevent diseases such as osteoporosis while treating cardiovascular diseases.
In contrast, some studies have proven that nonselective BBs do not have a beneficial effect on bone metabolism and have negative effect that are correlated with dosage. The results of animal experiments showed that low-dose PRO had no significant positive or negative effects on bone healing and callus strength in a rodent osteotomy model[19]. Another study found that the antiosteoporosis effect of PRO was dose-dependent, showing that 1.0 mg/kg PRO increased the histomorphometric index of bone formation, while 0.1 mg/kg, 1.0 mg/kg, and 10.0 mg/kg decreased the index. In addition, the antiosteoporotic effect of PRO was weakened when 10 mg/kg was given[9]. Moreover, when exploring the effects of PRO on glucocorticoid-induced osteoporosis in male rats, it was found that 10 mg/kg PRO did not improve bone parameters, but had harmful effects on the bone system[20]. Given the differences and limitations in research methods in animal studies, the above results suggest that the antiosteoporotic effect of nonselective BB remains controversial and its mechanism and clinical benefits need further exploration.
Similar to animal studies, there is no consensus on whether nonselective BBs can prevent fractures and increase bone mass in humans in vivo. Some studies have shown that nonselective BB can be used for the clinical treatment of osteoporosis, indicating that they may have an important preventive effect on osteopenia. The results of a case-control study of osteoporosis showed that the hip and forearm BMD of women over 50 years of age who used PRO was higher than that of a control group, and their fracture risk was reduced by 30%, suggesting that nonselective BBs is associated with a decrease in fracture risk and an increase in BMD[21]. Similarly, a cross-sectional study showed that BBs increased the average lumbar BMD in hypertensive postmenopausal women older than 40 years of age[22]. Moreover, PRO improved fracture healing in patients with post-traumatic stress disorder[23]. The above studies have proven the positive effect of nonselective BBs on osteogenesis and increased bone mass.
In contrast, some studies have shown that nonselective BB may not have a positive effect on the skeletal system, and that the effect may be negatively correlated with dosage. A randomized controlled trial (RCT) involving 32 healthy postmenopausal women found no significant differences in serum bone formation marker levels after 12 wk of continuous use of 80 mg/d PRO compared with the control group, indicating that the treatment had no significant regulatory effect on bone metabolism[24]. Other studies have shown that BBs did not stimulate bone formation, and may reduce osteoblast activity. In a placebo-controlled RCT in postmenopausal women, serum osteocalcin decreased by nearly 20% in the first 2 wk of 160 mg/d PRO treatment, and increased over time, suggesting that the nonselective BB had a negative effect on bone formation and bone mass[25]. The above results indicate a lack of consensus on whether nonselective BBs can be safely and effectively used as clinical antiosteoporosis therapy, and that more evidence is needed to support and guide its use.
Selective BBs
Compared with nonselective BBs, selective BBs have a more definite promoting effect on the skeletal system, and also have a certain dose effect. Animal experiments have shown that low-dose selective BBs had a preventive effect on osteoporosis, while high-dose BBs had a certain degree of inhibition. Arai et al[26] treated spontaneously hypertensive rats with various doses of butoxamine, a selective β2-AR blocker. The results showed that low-dose butoxamine improved osteoporosis caused by SNS hyperactivity by blocking β2-AR. Conversely, high-dose β2-AR blocker had an inhibitory effect on osteoblast activity. Similarly, selective β1-AR blockers also promote osteoporosis. A study that established an ovariectomized rat model showed that metoprolol, a selective β1-AR blocker, significantly promoted the proliferation, differentiation, and mineralization of osteoblasts, and reversed the decrease of BMD, microstructure, and biomechanical properties induced by osteoporosis. Metoprolol had a significant antiosteoporotic effect, suggesting that if might become an alternative treatment of postmenopausal osteoporosis in patients with hypertension[27]. These studies have shown that selective BBs are beneficial in reducing bone loss and increasing bone mass, and can be used as an alternative clinical treatment of osteopenia diseases.
Human studies have shown that selective β1-AR blockers promote bone formation. The first RCT conducted in humans to explore the effect of selective β1-AR blockers on bone metabolism found that they decreased bone resorption markers and increased the bone density of the distal radius[28]. The selective β1-AR blocker has an important effect in promoting bone formation and increasing bone density. Similarly, Yavuz Keleş et al[29] found that the use of selective β1-AR blockers was associated with increased BMD at the waist in postmenopausal women. In addition, β1-AR blockers also reduced the risk of human bone fractures. Song et al[30] found that after correcting confounding variables, β-blockers reduced the risk of fracture in people over 65 years of age, and selective β1-blockers had a role in reducing the risk of fracture. Similarly, another study showed that patients treated with selective β1-AR blockers had a 15% lower risk of fracture compared with a control group. The difference was independent of sex, fracture site, and dosage[31]. Therefore, β1-AR blockers can promote osteogenesis, and help to prevent fractures and osteoporosis. Moreover, the use of selective BBs is associated with increased BMD, and may be independently associated with lower fracture risk[32]. In summary, the clinical benefits of nonselective BBs in humans and the underlying mechanism remain unclear. However, selective BBs have antiosteoporosis benefits, and more studies are required to identify the most suitable dosage.
Role of BBs in osteoporosis and fracture
Osteoporosis is a systemic bone disease characterized by reduction of bone mass, destruction of bone trabecular structure, and decrease of BMD. Whether BBs can effectively and safely treat osteoporosis and fracture remains unknown. The reason may be that the majority of clinical studies exploring the relationship between BBs and osteoporosis and fracture were observational studies and there were differences in research methods. There is some evidence that the use of BBs reduces the risk of fracture. Toulis et al[33] found that BBs, especially selective BBs, was associated with a reduced risk of fracture in men and women, which is consistent with the results of Yang et al[31]. A study reported that participants who did not use a BB had a lower bone density than those who did not use it, and the difference was equivalent to about 4.5 years of bone loss[34]. In contrast, some studies have shown that BBs had no significant effect on bone formation and bone density increase. A meta-analysis by Yang et al[35] found that BBs were associated with a reduced the risk of fracture in the elderly, but that the effect may not be significant. Similarly, another study found that the use of BBs did not improve BMD[36]. As the mechanism of β-AR in regulating bone metabolism in the human body and the clinical effects of BB are not fully understood, there can be no definitive conclusion about the effects of BBs on BMD and fracture risk[37]. Moreover, there is not enough evidence to prove that the use of BBs for the treatment of osteoporosis is reasonable[25], or to support BBs as a drug for the treatment of osteoporosis[38]. Therefore, an antiosteoporosis role for BB and reducing the risk of fracture needs additional support from RCTs.
CONCLUSION
The activity of β-ARs is closely related to bone metabolism, inhibiting bone formation, and reducing bone mass and BMD. β1-AR and β3-AR participate in bone metabolism in different ways. Selective BBs promote osteogenesis, increase bone mass, contribute to antiosteoporosis, and prevent fracture, but there is not enough evidence to support nonselective BBs for the clinical treatment of osteoporosis and fracture. More interventional and observational studies are needed to confirm the antiosteoporotic effect of BBs and identify a safe and effective dose range.