Published online Oct 16, 2021. doi: 10.12998/wjcc.v9.i29.8879
Peer-review started: June 7, 2021
First decision: June 25, 2021
Revised: June 30, 2021
Accepted: August 2, 2021
Article in press: August 2, 2021
Published online: October 16, 2021
Processing time: 129 Days and 21.2 Hours
Mycobacterium paragordonae (M. paragordonae), a slow-growing, acid-resistant mycobacterial species, was first isolated from the sputum of a lung infection patient in South Korea in 2014. Infections caused by M. paragordonae are rare.
Herein, we report the case of a 53-year-old patient who presented with fever and low back pain. Lumbar nuclear magnetic resonance imaging revealed the destruction of the lumbar vertebra with peripheral abscess formation. After anti-infective and diagnostic anti-tuberculosis treatment, the patient had no further fever, but the back pain was not relieved. Postoperatively, the necrotic material was sent for pathological examination, and all tests related to tuberculosis were negative, but pus culture suggested nontuberculous mycobacteria. The necrotic tissue specimens were subjected to metagenomic next-generation sequencing, which indicated the presence of M. paragordonae. Finally, the infecting pathogen was identified, and the treatment plan was adjusted. The patient was in good condition during the follow-up period.
M. paragordonae, a rare nontuberculous mycobacterium, can also cause spinal infections. In the clinic, it is necessary to identify nontuberculous mycobacteria for spinal infections similar to Mycobacterium tuberculosis.
Core Tip: Mycobacterium paragordonae (M. paragordonae) is a slow-growing, acid-resistant mycobacterial species. We present herein a 53-year-old patient who presented with fever and low back pain. Lumbar nuclear magnetic resonance imaging revealed the destruction of the lumbar vertebra with peripheral abscess formation. After anti-infective and diagnostic anti-tuberculosis treatment, the patient had no further fever, but the back pain was not relieved. Postoperatively, the necrotic material was sent for pathological examination, and all tests related to tuberculosis were negative. The necrotic tissue specimens were subjected to metagenomic next-generation sequencing, which indicated the presence of M. paragordonae. Finally, the infecting pathogen was identified, and the treatment plan was adjusted. The patient was in good condition during the follow-up period.
- Citation: Tan YZ, Yuan T, Tan L, Tian YQ, Long YZ. Lumbar infection caused by Mycobacterium paragordonae: A case report. World J Clin Cases 2021; 9(29): 8879-8887
- URL: https://www.wjgnet.com/2307-8960/full/v9/i29/8879.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i29.8879
Mycobacterium paragordonae (M. paragordonae), a species of mycobacteria, is a slow-growing, dark colour-producing mycobacterium that has similar growth characteristics and acid resistance to other mycobacteria[1]; this species is usually found in the environment or in colder regions or tissues of the body, such as mucous membranes, and lacks the capacity to infect deeper tissues of the human body[2]. This paper describes a case of lumbar spine infection caused by M. paragordonae, which is a rare nontuberculous mycobacterium with few reported cases.
A 53-year-old male patient presented to a hospital in Zhuzhou, China on February 19, 2021 with a 6-mo history of low back pain that was aggravated for 1 mo. He was a farmer and lived in Zhuzhou, Hunan Province, China.
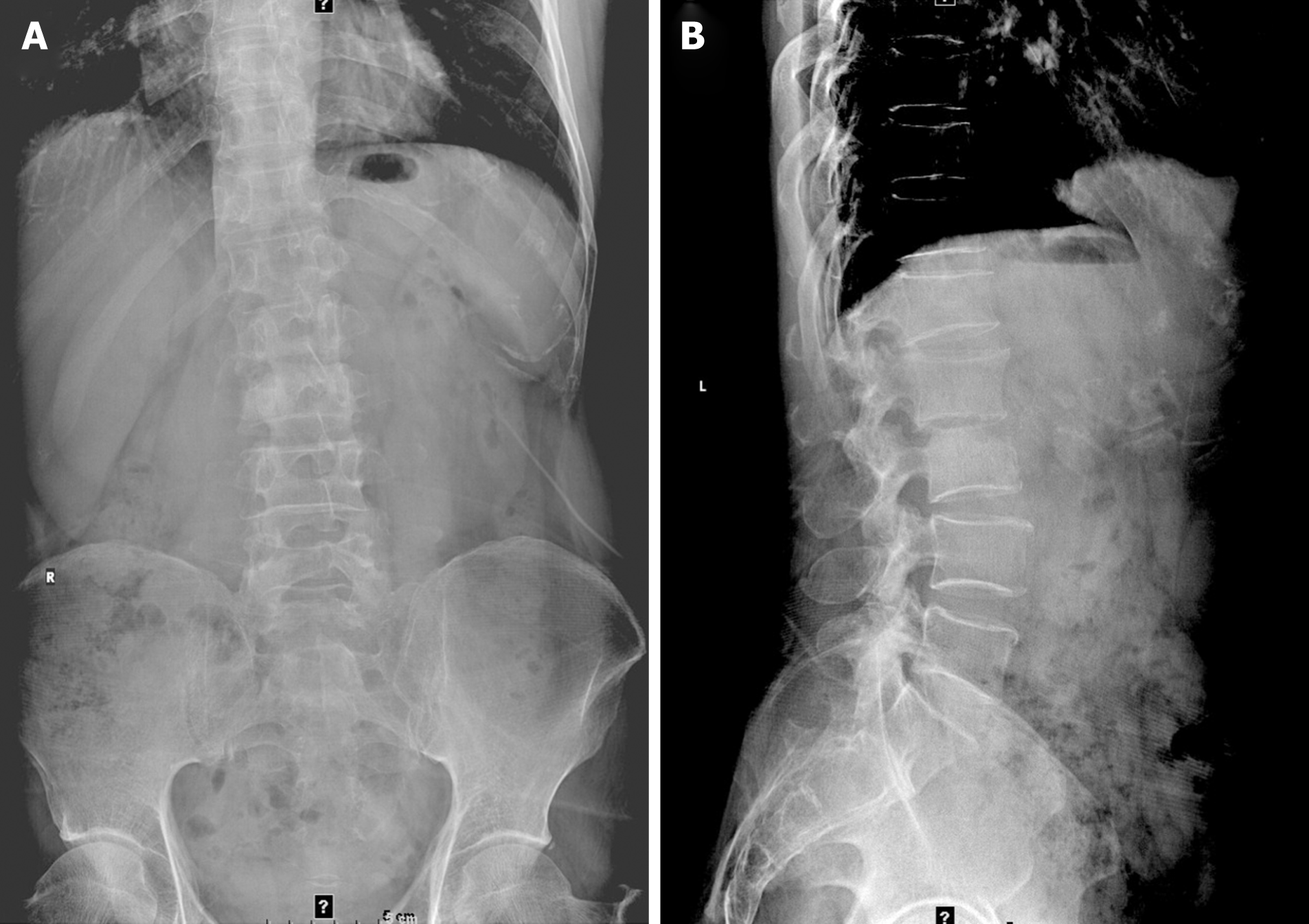
The patient developed low back pain with no obvious cause 6 mo ago, with persistent soreness and swelling to a moderate degree, which was tolerable, but he found no position offering obvious relief. Symptoms were obvious when bending and coughing. There were no symptoms of pain or numbness in either lower limb, and no intermittent claudication. However, there was numbness in the perineum, and he had difficulty walking, urinating, and defecating. He went to a local hospital and was diagnosed with "lumbar disc herniation". He was treated for dehydration and swelling with analgesia and anti-inflammation medicines, along with physiotherapy and nerve nutrition. The symptoms improved slightly but occasionally recurred and improved after a few days of bed rest. In the past month, the pain in the lower back worsened and was accompanied by night sweats, but no numbness in the perineum or difficulty urinating and defecating, so he came to our hospital. Lumbar spine X-ray showed L1 and L2 vertebral body localized bone destruction with a narrowing of this vertebral space, which was indicative of tuberculous lesions. X-ray additionally showed L5 sacralization. No obvious abnormal signs were seen in the pelvis (Figure 1). He had no past medical history, no recent fever, and no trauma. In the past month, he had poor sleep, poor diet, normal bowel movements, and weight loss of approximately 5 kg.
The patient had a free previous medical history.
The patient's personal and family history was not remarkable.
Physical examination revealed significant pressure pain and percussion pain in the spinous process and interspinous process of the upper lumbar spine; significant bilateral paravertebral pressure pain; no bilateral sciatic nerve stroke pressure pain; and significant limitation of lumbar flexion, extension, and rotation activities.
The patient's white blood cell count was 11.26 × 109/L, with 80.6% of neutrophils. His albumin level was 31.7 g/L and globulin was 44.4 g/L. Renal function parameters, cardiac enzymes, electrolytes, blood glucose, pre-transfusion examination parameters, routine urine and stool parameters, calcitoninogen, and tuberculosis antibody tests were normal. His C-reactive protein level was 101.9 mg/L, and erythrocyte sedimentation rate was 104 mm/h. The electrocardiogram was normal.
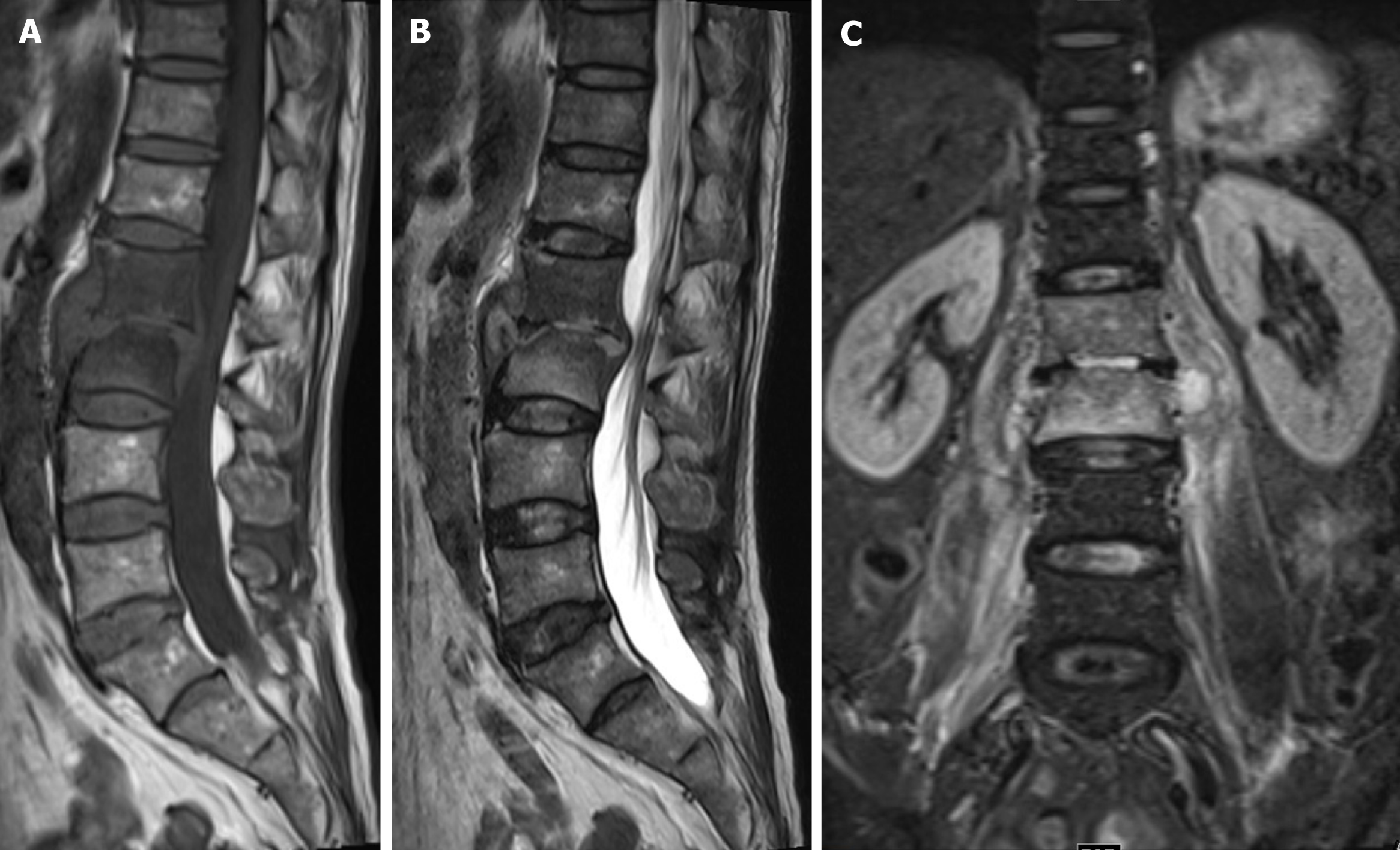
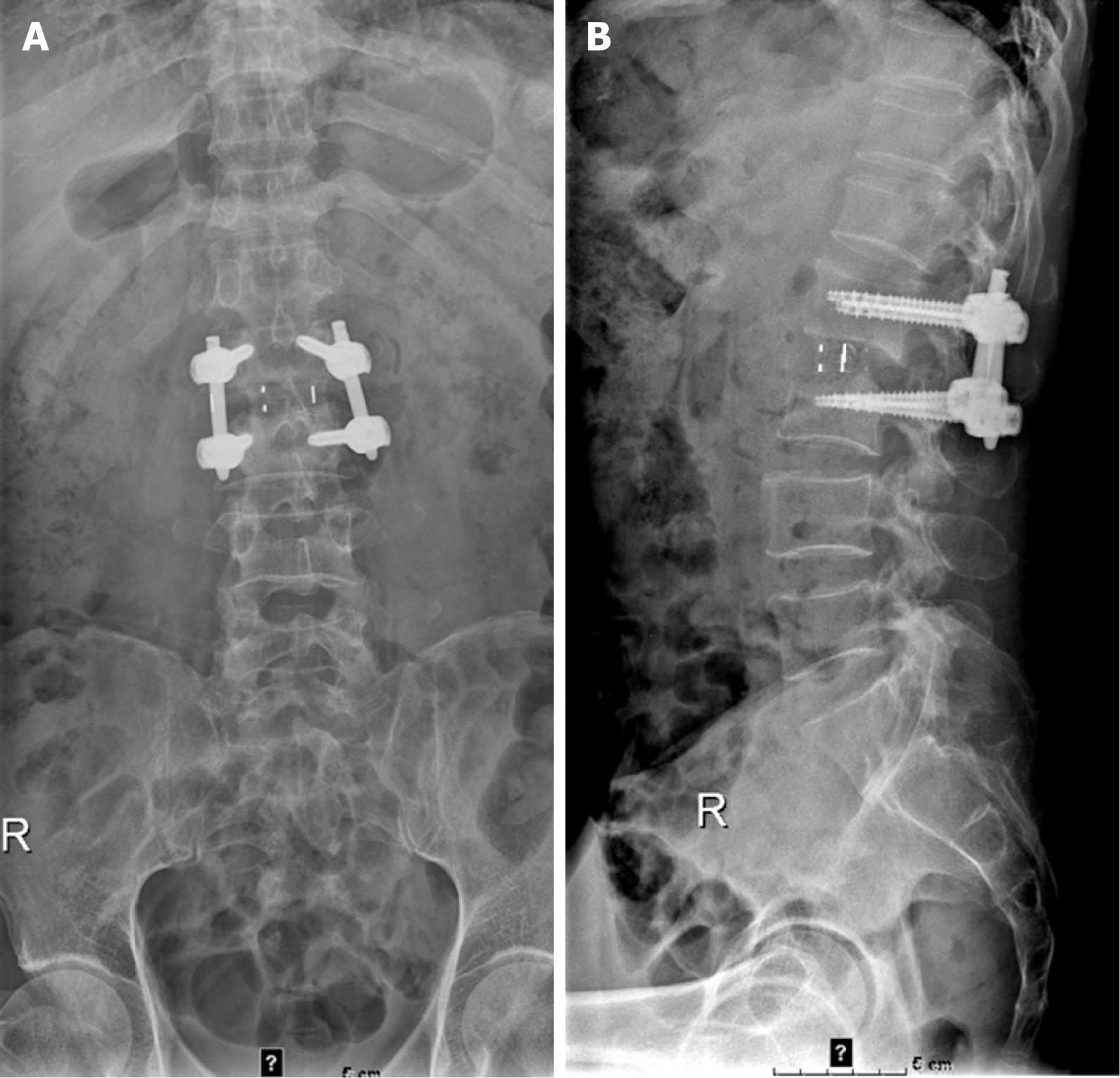
The X-ray on February 19, 2021 showed L1 and L2 vertebral body localized bone destruction with a narrowing of this vertebral space (Figure 1). A computed tomography (CT) scan of the lung shown no abnormalities. On February 20, 2021, magnetic resonance imaging (MRI) of the lumbar spine showed obvious bone destruction of the L1 and L2 vertebrae, a narrowing of the L1/2 intervertebral space, the destruction of the intervertebral disc, and obvious swelling of the surrounding soft tissues; thus, lumbar spine tuberculosis with surrounding cold abscess formation was considered (Figure 2). On March 7, 2021, lumbar spine X-ray showed that the L1-L2 interbody internal fixation device was not broken, dislodged, or displaced, and the intervertebral bone graft healed well (Figure 3).
The patient was negative for Mycobacterium tuberculosis (M. tuberculosis) ϒ interferon release test, the M. tuberculosis drug resistance gene, and the M. tuberculosis biochip.
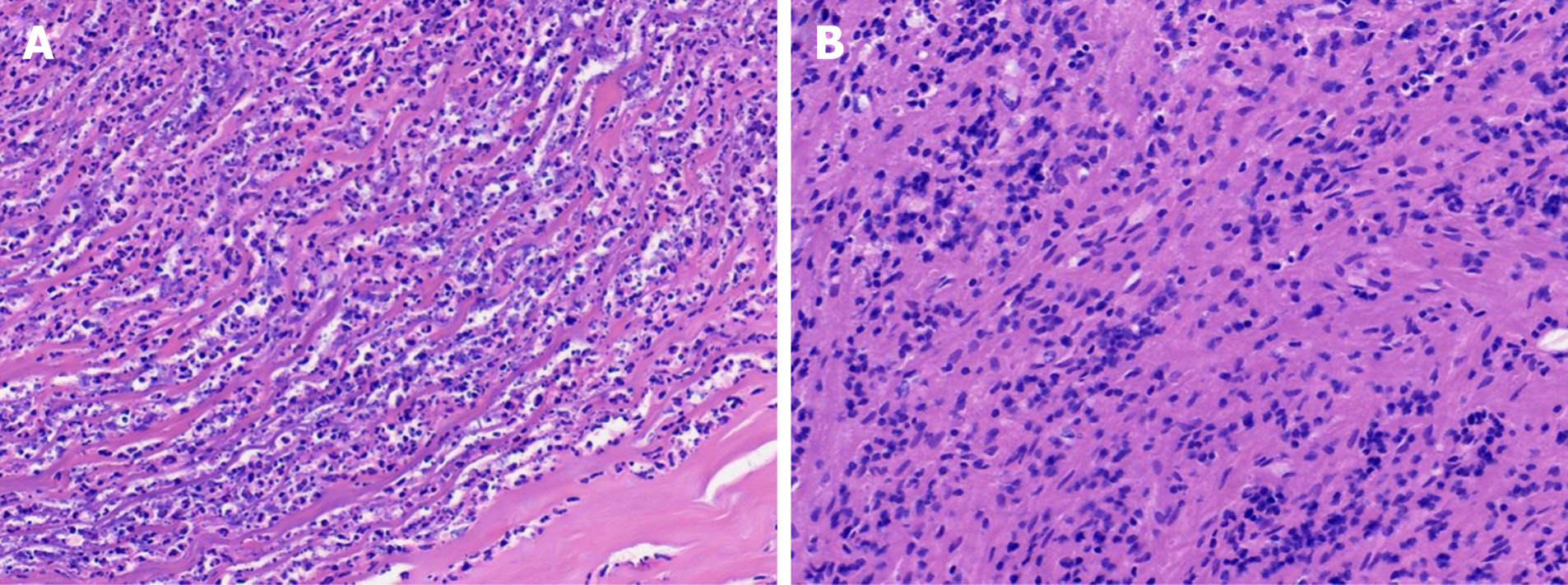
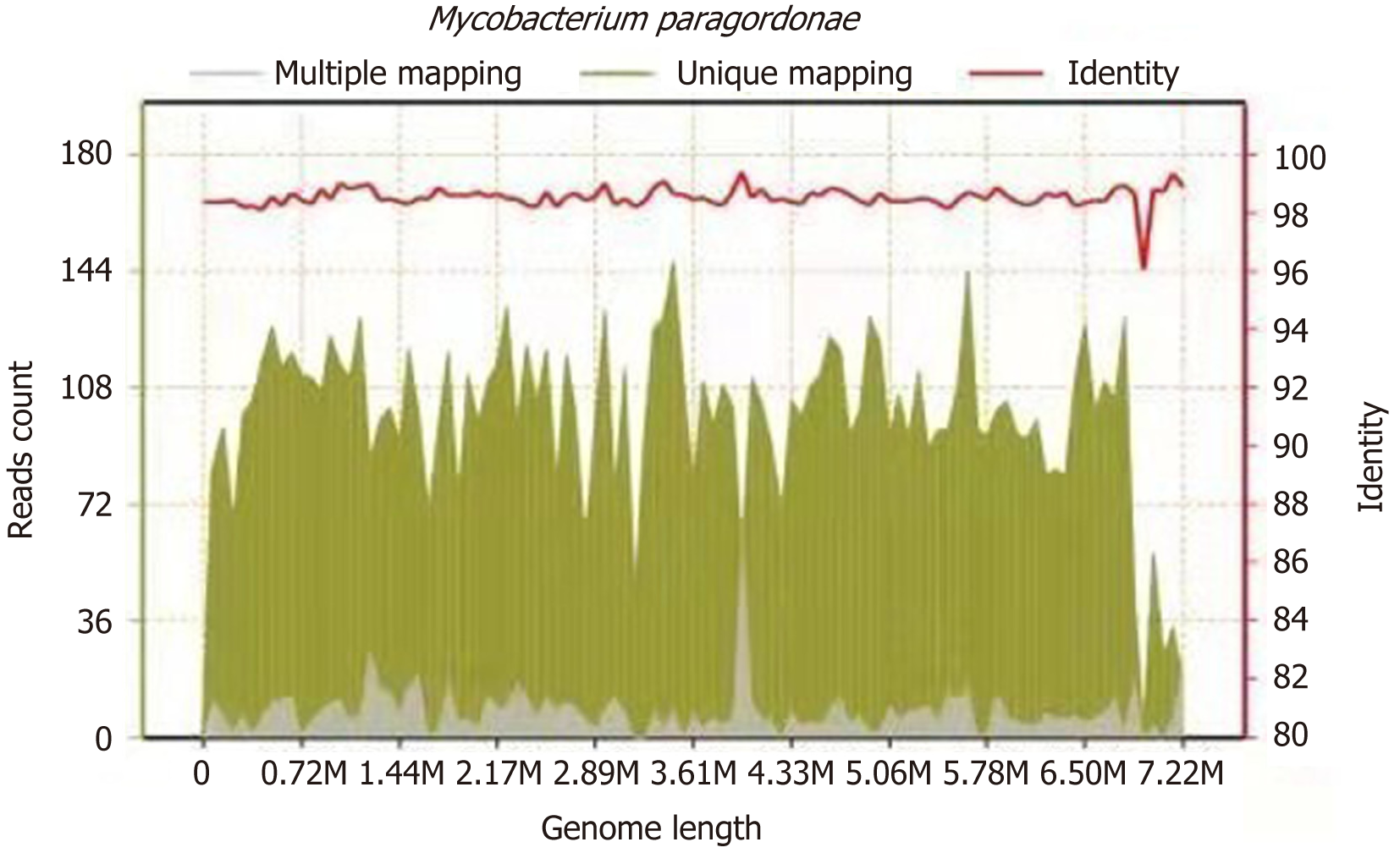
On March 2, 2021, the surgeon performed L1 and L2 vertebral body lesion removal via a left oblique approach + canal decompression + GAGE bone graft + percutaneous nail rod system internal fixation (prepared for iliac bone harvesting) under general anaesthesia, cheese-like necrotic material was seen intraoperatively, and specimens were collected for examination. The results of the pathological examination showed L1 and L2 focal tissue in the examined fibrocartilaginous tissue and hyperplastic fibrous tissue. The area showed acute purulent inflammation and inflammatory necrosis, and a large amount of chronic inflammatory cell infiltration was observed (Figure 4). Negative anti-acid staining of pus was performed. Pus culture indicated the present of nontuberculous mycobacteria. The TB-DNA test was negative. Postoperative necrotic tissue specimens were sent for metagenomic next-generation sequencing, which suggested the presence of M. paragordonae, with 11563 reads detected and a coverage of 8.559% (Figure 5).
The final diagnosis of the presented case was lumbar spine infection caused by M. paragordonae.
On February 20, the patient developed a fever, with the highest temperature being 39.1 ºC. Based on the symptoms and the MRI results of the lumbar spine, the possibility of lumbar spine tuberculosis was considered, and he was given isoniazid (300 mg qd) + rifampicin (450 mg qd) + etanercept (0.75 g qd) + pyrazinamide (0.5 g tid) for diag
One month after surgery, the patient’s blood count, C-reactive protein, and erythrocyte sedimentation rate returned to normal, and there was no lumbar pain or fever. Lumbar spine X-ray showed that the L1-L2 interbody internal fixation device was not broken, dislodged, or displaced, and the intervertebral bone graft healed well (Figure 3).
Spinal infection can affect both bony tissue, such as the vertebral body, and soft tissue, such as adjacent soft tissue and intervertebral discs[3]. The most common clinical manifestations of spinal infection are fever, back or neck pain, even radiating to the lower limbs, and inguinal hernia, with different manifestations occurring depending on the site of infection[4]; approximately 30% of patients present with neurological symptoms, including sensory abnormalities and muscle weakness[5]. In contrast, 75-95% of patients, during physical examinations, can feel paravertebral muscle spasms, while there is significant pressure pain on percussion over the spinous process of the infected spinal segment[4]. The most frequently involved spinal segments are the lumbar (58%), thoracic (30%), and cervical (11%) spine[6]. The spinal segment involved in this case was the lumbar spine. There are three main routes of spinal infection: (1) Haematogenous infection; (2) direct inoculation infection of adjacent tissues; and (3) postoperative infection[4], which can extend posteriorly and lead to epidural or subdural abscesses and even meningitis, while lateral dissemination can lead to abscesses of the psoas major, retroperitoneum, sub-diaphragm, paravertebral space, retropharynx, and mediastinum[7]. The most common pathogens causing spinal infections are bacteria, fungi and, less commonly, parasites; the most common pathogen was Staphylococcus aureus[8-10], followed by Streptococcus and Enterococcus, while infections caused by M. tuberculosis are significantly less common[11]. In the Mediterranean and the Middle East, Brucella can also lead to spinal infections[12], and in endemic areas, such as countries with warm climates (e.g., South America, Central Asia, and Africa), spinal tapeworm infections can also be seen occasionally[13], but a definite source cannot be identified in nearly 50% of spinal infections[14]. In the present case, the patient was admitted with low back pain, recent night sweats, weight loss, and fever on admission. Lumbar spine MRI suggested lumbar spine tuberculosis with peripheral cold abscess formation, and there was no further fever after anti-tuberculosis treatment was administered. In China, especially in developing regions, there are more cases of M. tuberculosis causing spinal tuberculosis, thus leading to paravertebral abscesses or lumbar major abscesses[15]. Initially, we misdiagnosed this case as lumbar spine infection due to M. tuberculosis, but the tuberculosis antibody test, the smear for the detection of acid-fast bacilli, the pathological molecular biology test, the negative M. tuberculosis ϒ interferon release test, the negative M. tuberculosis drug resistance gene, and the negative M. tuberculosis biochip results did not support the diagnosis of M. tuberculosis infection. Finally, M. paragordonae infection was determined by a macrogenomic test. At present, the diagnosis of spinal infections mainly relies on routine blood tests, infection indicators, the culture of pus secretions, and pathological findings, and imaging modalities mainly include X-rays, MRI, and CT, wherein MRI is the optimal option for the diagnosis of spinal infections and can determine the size and extent of abscesses. Definitive diagnosis still relies on pathological findings and culture of pus secretions[7]. Therefore, it is particularly important to culture specimens from patients with lumbar spine infection combined with paravertebral abscesses in a timely manner, and metagenomic next-generation sequencing plays a role in detecting unknown pathogens of lumbar spine infections. In recent years, mycobacterial infections have been increasing annually both in China and abroad, and the incidence of nontuberculous mycobacteria, types of mycobacteria other than M. tuberculosis, has increased to approximately 39.6 cases per 100000 people per year in the Asian population[16]. Some nontuberculous mycobacteria have a high similarity to M. tuberculosis in lung or extra-pulmonary infections (spine, lymph nodes, intestine, etc.), which can be easily missed or misdiagnosed. Nontuberculous mycobacteria are prevalent in the environment and multiply in soil and water, and it is important to consider nontuberculous mycobacteria when considering M. tuberculosis infections of the lumbar spine. The treatment for spinal infections includes non-surgical and surgical treatments, and non-surgical treatment often involves absolute bed rest, rational and standardized application of antibiotics, and symptomatic supportive treatment, but the treatment period is often long. Commonly used empirical antibiotic regimens include vancomycin and third-generation cephalo
Specimens of necrotic tissue from the lumbar spine of this patient were sent for metagenomic next-generation sequencing, and M. paragordonae was found. This species is a slow-growing, rare non-tuberculous member of the genus Mycobacterium. Its unique 16S rRNA gene sequence is approximately 99% similar to that of Gordon's Mycobacterium. Generally, M. paragordonae is rod-shaped and acid-resistant, with no spores or mycelium under the microscope, and with an optimal growth temperature of 25-30 ºC[1]. M. paragordonae is generally not pathogenic or is weakly pathogenic, and there was concern that the isolated organism may have been contaminated or colonized; however, the specimen from this patient was a lumbar spine surgery specimen, with no contamination or colonization, and the number of reads detected by metagenomic next-generation sequencing was 11563, with a coverage of 8.559%. Given this information, along with the clinical symptoms of the patient, M. paragordonae was considered to be the cause of infection in this patient. Few cases have been reported on the pathogenicity of M. paragordonae. Cheung et al[18] reported the first case of M. paragordonae peritonitis in a 55-year-old man who received continuous ambulatory peritoneal dialysis for 2 years. In contrast, there are no reports on cases of M. paragordonae causing lumbar spine infections. Because of its temperature sensitivity and its ability to produce strong immune responses to M. tuberculosis and Mycobacterium abscesses in inoculated mice, Kim et al reported that M. paragordonae could be used as a potential live vaccine against Mycobacterium[2], and Lee et al reported that this inactivated strain may be used as a potential immunotherapeutic adjuvant to increase the effectiveness of cancer chemotherapy[19]. The diagnosis and treatment of M. paragordonae are not well documented, although Cheung et al[18] reported a treatment plan for cases of peritonitis due to M. paragordonae based mainly on tissue culture drug sensitivity results. Since no pathogen was detected in the pus culture of this patient and M. paragordonae is a nontuberculous mycobacterium, we treated the patient with appropriate medication according to our experience with nontuberculous mycobacteria. Because of the destruction of the vertebral body and paravertebral abscesses, this patient was treated by surgery. The patient achieved a good outcome, but further follow-up is still required for the treatment of similar patients.
Next-generation sequencing technology, such as metagenomic next-generation sequencing, allows thousands to billions of DNA fragments to be sequenced independently and simultaneously and is now a widely used technique in microbial testing. Because almost all pathogens contain DNA or RNA genomes, metagenomic next-generation sequencing of unidentified pathogens is now becoming an important test for definitive diagnosis[20,21]. As demonstrated by this case, such rare and slow-growing pathogens are easily overlooked by ordinary bacterial culture methods due to their slow growth; therefore, metagenomic next-generation sequencing can be an important test for clinical diagnosis in addition to prolonging the culture time.
M. paragordonae is a slow-growing, rare non-tuberculous member of the genus Mycobacterium. There are no reports on cases of M. paragordonae causing lumbar spine infections. Through this case, we need to be alert to the infection with nontuberculous M. paragordonae in patients with spinal infection in future clinical work.
| 1. | Kim BJ, Hong SH, Kook YH, Kim BJ. Mycobacterium paragordonae sp. nov., a slowly growing, scotochromogenic species closely related to Mycobacterium gordonae. Int J Syst Evol Microbiol. 2014;64:39-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 2. | Kim BJ, Kim BR, Kook YH, Kim BJ. A temperature sensitive Mycobacterium paragordonae induces enhanced protective immune responses against mycobacterial infections in the mouse model. Sci Rep. 2017;7:15230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 3. | Lener S, Hartmann S, Barbagallo GMV, Certo F, Thomé C, Tschugg A. Management of spinal infection: a review of the literature. Acta Neurochir (Wien). 2018;160:487-496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 203] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 4. | Babic M, Simpfendorfer CS. Infections of the Spine. Infect Dis Clin North Am. 2017;31:279-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 109] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 5. | Beronius M, Bergman B, Andersson R. Vertebral osteomyelitis in Göteborg, Sweden: a retrospective study of patients during 1990-95. Scand J Infect Dis. 2001;33:527-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 93] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 6. | Solera J, Lozano E, Martínez-Alfaro E, Espinosa A, Castillejos ML, Abad L. Brucellar spondylitis: review of 35 cases and literature survey. Clin Infect Dis. 1999;29:1440-1449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 173] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 7. | Tsantes AG, Papadopoulos DV, Vrioni G, Sioutis S, Sapkas G, Benzakour A, Benzakour T, Angelini A, Ruggieri P, Mavrogenis AF; World Association Against Infection In Orthopedics And Trauma W A I O T Study Group On Bone And Joint Infection Definitions. Spinal Infections: An Update. Microorganisms. 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 123] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 8. | Loibl M, Stoyanov L, Doenitz C, Brawanski A, Wiggermann P, Krutsch W, Nerlich M, Oszwald M, Neumann C, Salzberger B, Hanses F. Outcome-related co-factors in 105 cases of vertebral osteomyelitis in a tertiary care hospital. Infection. 2014;42:503-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 9. | Legrand E, Flipo RM, Guggenbuhl P, Masson C, Maillefert JF, Soubrier M, Noël E, Saraux A, Di Fazano CS, Sibilia J, Goupille P, Chevalie X, Cantagrel A, Conrozier T, Ravaud P, Lioté F; Rheumatology Network Organization. Management of nontuberculous infectious discitis. treatments used in 110 patients admitted to 12 teaching hospitals in France. Joint Bone Spine. 2001;68:504-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 96] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 10. | Cebrián Parra JL, Saez-Arenillas Martín A, Urda Martínez-Aedo AL, Soler Ivañez I, Agreda E, Lopez-Duran Stern L. Management of infectious discitis. Outcome in one hundred and eight patients in a university hospital. Int Orthop. 2012;36:239-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 11. | Yee DK, Samartzis D, Wong YW, Luk KD, Cheung KM. Infective spondylitis in Southern Chinese: a descriptive and comparative study of ninety-one cases. Spine (Phila Pa 1976). 2010;35:635-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Ulu-Kilic A, Karakas A, Erdem H, Turker T, Inal AS, Ak O, Turan H, Kazak E, Inan A, Duygu F, Demiraslan H, Kader C, Sener A, Dayan S, Deveci O, Tekin R, Saltoglu N, Aydın M, Horasan ES, Gul HC, Ceylan B, Kadanalı A, Karabay O, Karagoz G, Kayabas U, Turhan V, Engin D, Gulsun S, Elaldı N, Alabay S. Update on treatment options for spinal brucellosis. Clin Microbiol Infect. 2014;20:O75-O82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 13. | Charles RW, Govender S, Naidoo KS. Echinococcal infection of the spine with neural involvement. Spine (Phila Pa 1976). 1988;13:47-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 73] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Jeong SJ, Choi SW, Youm JY, Kim HW, Ha HG, Yi JS. Microbiology and epidemiology of infectious spinal disease. J Korean Neurosurg Soc. 2014;56:21-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | Batirel A, Erdem H, Sengoz G, Pehlivanoglu F, Ramosaco E, Gülsün S, Tekin R, Mete B, Balkan II, Sevgi DY, Giannitsioti E, Fragou A, Kaya S, Cetin B, Oktenoglu T, Celik AD, Karaca B, Horasan ES, Ulug M, Senbayrak S, Arslanalp E, Hasbun R, Ates-Guler S, Willke A, Senol S, Inan D, Güclü E, Ertem GT, Koc MM, Tasbakan M, Ocal G, Kocagoz S, Kusoglu H, Güven T, Baran AI, Dede B, Karadag FY, Yilmaz H, Aslan G, Al-Gallad DA, Cesur S, El-Sokkary R, Sirmatel F, Savasci U, Karaahmetoglu G, Vahaboglu H. The course of spinal tuberculosis (Pott disease): results of the multinational, multicentre Backbone-2 study. Clin Microbiol Infect. 2015;21:1008.e9-1008.e18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 16. | Shteinberg M, Stein N, Adir Y, Ken-Dror S, Shitrit D, Bendayan D, Fuks L, Saliba W. Prevalence, risk factors and prognosis of nontuberculous mycobacterial infection among people with bronchiectasis: a population survey. Eur Respir J. 2018;51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 17. | Saeed K, Esposito S, Ascione T, Bassetti M, Bonnet E, Carnelutti A, Chan M, Lye DC, Cortes N, Dryden M, Fernando S, Gottlieb T, Gould I, Hijazi K, Madonia S, Pagliano P, Pottinger PS, Segreti J, Spera AM; International Society of Antimicrobial Chemotherapy (ISAC) Bone and Skin & Soft Tissue Infections Working Group. Hot topics on vertebral osteomyelitis from the International Society of Antimicrobial Chemotherapy. Int J Antimicrob Agents. 2019;54:125-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 18. | Cheung CY, Cheng NHY, Ting WM, Chak WL. Mycobacterium paragordonae: a rare cause of peritonitis in a peritoneal dialysis patient. Clin Nephrol. 2017;88:371-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Lee SY, Yang SB, Choi YM, Oh SJ, Kim BJ, Kook YH. Heat-killed Mycobacterium paragordonae therapy exerts an anti-cancer immune response via enhanced immune cell mediated oncolytic activity in xenograft mice model. Cancer Lett. 2020;472:142-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Gu W, Miller S, Chiu CY. Clinical Metagenomic Next-Generation Sequencing for Pathogen Detection. Annu Rev Pathol. 2019;14:319-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 968] [Article Influence: 121.0] [Reference Citation Analysis (0)] |
| 21. | Farraj SA, El-Kafrawy SA, Kumosani TA, Yousef JM, Azhar EI. Evaluation of Extraction Methods for Clinical Metagenomic Assay. Microorganisms. 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Fateh A S-Editor: Wang LL L-Editor: Wang TQ P-Editor: Zhang YL