Published online Oct 16, 2021. doi: 10.12998/wjcc.v9.i29.8773
Peer-review started: June 26, 2021
First decision: July 16, 2021
Revised: July 20, 2021
Accepted: July 29, 2021
Article in press: July 29, 2021
Published online: October 16, 2021
Processing time: 110 Days and 16.5 Hours
IgG4-related sclerosing cholangitis (IgG4-RSC) is an uncommon benign disease, and its rarer, isolated and mass-forming subtype poses a significant challenge to differential diagnosis from cholangiocarcinoma of the extrahepatic bile duct. We herein report a case of isolated IgG4-RSC with an obstructing bile duct mass, for which extrahepatic bile duct resection was performed under the impression of proximal common bile duct (CBD) cancer.
A 79-year-old male was admitted for jaundice that had developed 1 mo prior. There was no family history for autoimmune diseases or biliary cancer. Computed tomography (CT) and magnetic resonance cholangiopancreaticography revealed a short segmental concentric wall thickening of the proximal CBD with diffuse dilatation of the bile duct to the periphery. The endoscopic biopsy specimen showed no malignant cells. Positron emission tomography-CT showed a focal hypermetabolic lesion (SUVmax 4.2) in and around the proximal CBD area. With the impression of proximal CBD cancer, we performed segmental resection of the extrahepatic bile duct. Histopathology demonstrated marked sclerosis with diffuse lymphoplasmacytic infiltration and some eosinophils. Immunohistochemical staining for IgG4 showed increased positivity in some areas (up to 30/high-power field) and IgG4+/IgG+ cell ratio as 30%-50%. Pathologists’ impression was IgG4-related sclerosing disease. Follow-up serum IgG4 levels were continuously elevated; however, no evidence of relapse or other organ involvement related to IgG4-RSC presented.
Isolated and mass-forming IgG4-RSC displays striking similarity with cholangiocarcinoma. To avoid unnecessary major surgery, high index of suspicion is needed.
Core Tip: IgG4-related sclerosing cholangitis (IgG4-RSC) is an emerging disease and shows frequent other-organ involvement, including of the pancreas. However, when isolation without pancreatic involvement and biliary mass formation are combined, IgG4-RSC becomes extremely rare and brings about a striking confusion in differentiation from cholangiocarcinoma. Avoiding unnecessary surgery and providing proper treatment in patients with isolated and mass-forming IgG4-RSC could be achieved through high index of suspicion according to the amount of information on the five cardinal features (histology, imaging, serology, other-organ involvement, and response to steroid therapy).
- Citation: Song S, Jo S. Isolated mass-forming IgG4-related sclerosing cholangitis masquerading as extrahepatic cholangiocarcinoma: A case report. World J Clin Cases 2021; 9(29): 8773-8781
- URL: https://www.wjgnet.com/2307-8960/full/v9/i29/8773.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i29.8773
Benign bile duct strictures are rare and complicated clinical conditions. Their etiology includes postoperative injury after cholecystectomy, pancreatitis, primary sclerosing cholangitis (PSC), orthotopic liver transplantation, Mirizzi syndrome, radiation, choledochal cysts, recurrent pyogenic cholangitis, fibrosis or inflammatory strictures, stone disease, idiopathic, etc.[1,2]. Of these causes, a subset of inflammatory strictures appear to represent immunoglobulin G4-related sclerosing cholangitis (IgG4-RSC)[2,3]. IgG4-RSC, also designated as IgG4-associated cholangitis[4,5], is an uncommon benign fibroinflammatory stricture of the bile duct. Patients with IgG4-RSC are characterized by increased IgG4 levels in serum and abundant IgG4-positive lymphoplasmacytic infiltrate in the bile ducts[6-8]. IgG4-RSC has been known to be a biliary manifestation of IgG4-related disease (IgG4-RD) and frequently accompanied with autoimmune pancreatitis (AIP)[7,9,10]. However, few cases of IgG4-RSC have been reported to be an isolated form without apparent pancreatic involvement, thus making a challenge to differentiation from cholangiocarcinoma[11-13]. Furthermore, when combined with stricture by mass-forming lesions, isolated IgG4-RSC could be extremely laboring and tricky to be differentiated. We herein report a case of isolated mass-forming IgG4-RSC for which extrahepatic bile duct resection with lymphadenectomy was performed under the impression of proximal common bile duct (CBD) cancer.
A 79-year-old male was admitted to the Department of Gastroenterology of our institution for jaundice that developed 1 mo prior.
The patient had also experienced general weakness and fatigue for a few months before admission, but had shown no change of body weight. The accompanying symptoms were reddish discoloration of urine and pruritus all over the body.
He had taken medicine for hypertension, diabetes mellitus, and benign prostate hypertrophy and had history of tuberculosis pleurisy 18 years before. The patient underwent percutaneous coronary intervention for unstable angina 7 years before. However, the patient had no history of bile duct surgery or intervention.
He was a social drinker and had a 10 pack-year smoking history. There was no family history for autoimmune diseases or biliary cancer.
The patient’s vital signs at admission were within normal limits, with the exception of a body temperature of 38.2 ℃. The skin and sclera showed jaundice. The abdomen was soft and he had no signs of tenderness on the upper abdomen. The gallbladder was not definitely palpable.
Blood analysis revealed no leukocytosis (7560 white blood cells/µL) with normal hematocrit and platelet count. Total bilirubin was markedly increased (17.9 mg/dL; normal range: < 1.2 mg/dL) along with aspartate aminotransferase/alanine aminotransferase (97/52 IU/L; normal range: 4-40/4-41 IU/L) and alkaline phosphatase (339 IU/L; normal range: 35-105 IU/L). Serum amylase/lipase was normal and C-reactive protein was slightly increased (1.48 mg/dL; normal range: < 0.5 mg/dL). Carbohydrate antigen 19-9 was greatly increased (1061 U/mL; normal range < 37 U/mL). Carcinoembryonic antigen was within normal range. Serum IgG/IgG4 levels were not measured. Urine color was dark brown, and bilirubin was 4+ mg/dL.
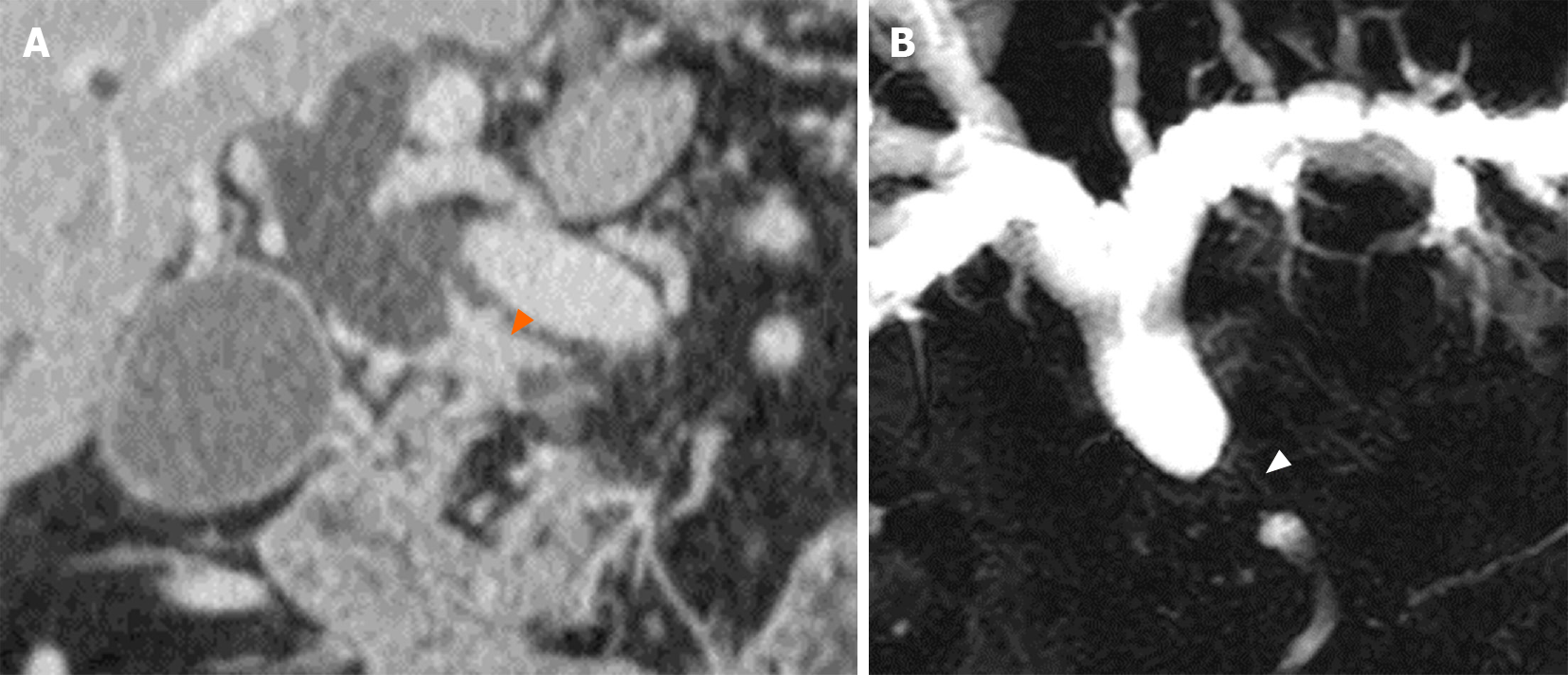
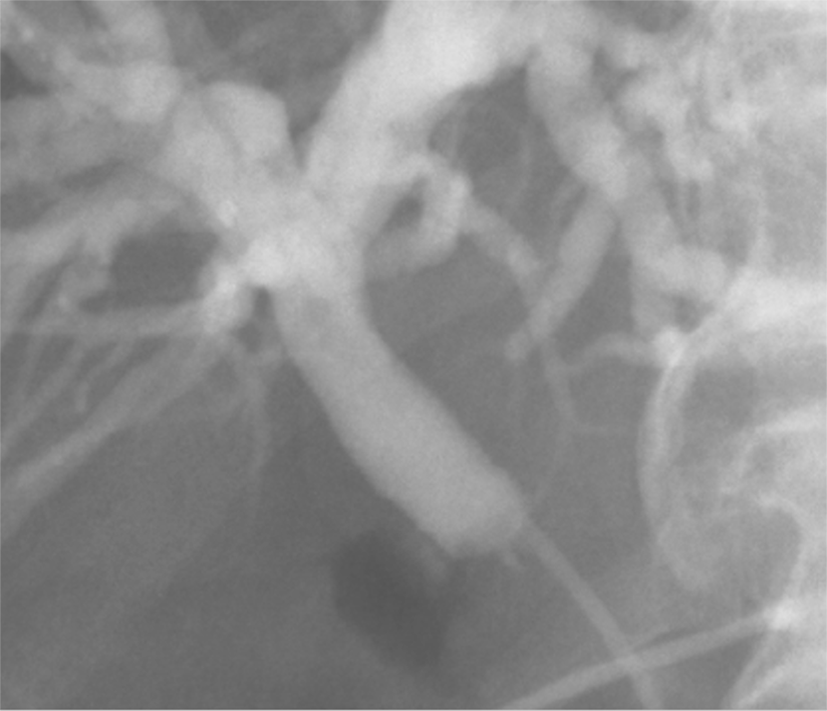
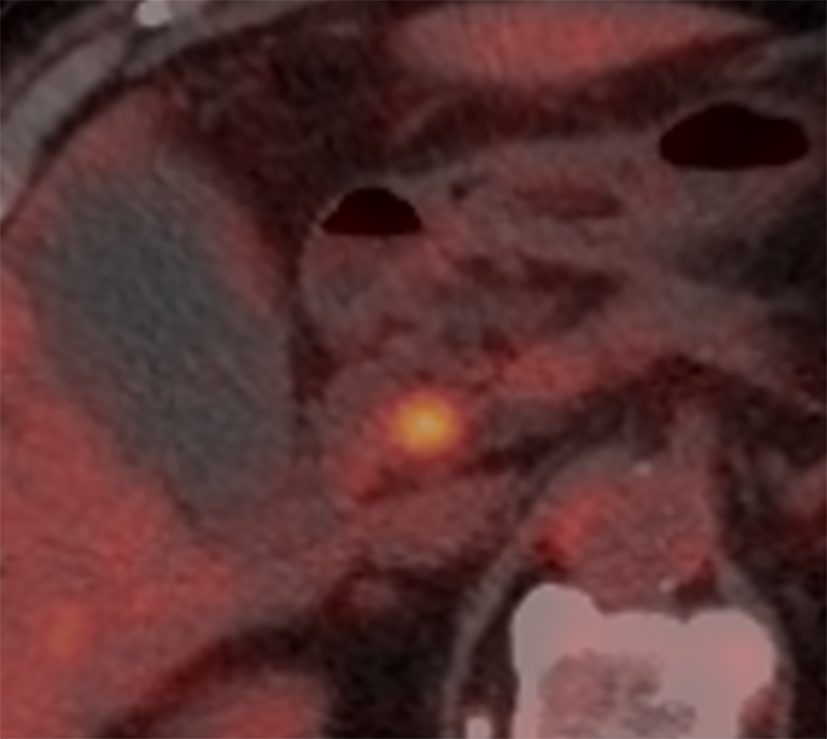
Computed tomography (CT) scan and magnetic resonance cholangiopancreaticography (MRCP) revealed a short segmental concentric wall thickening of the proximal to mid CBD with diffuse dilatation of the bile duct to the periphery and gallbladder distension (Figure 1). Subsequently-performed endoscopic retrograde cholangiopancreaticography (ERCP) demonstrated a 1 cm-long segmental stricture at the proximal CBD level (Figure 2), but no malignant cells or specific histopathological confirmation were proven in biopsy tissues of the bile duct. Positron emission tomography (PET)-CT supported these findings by showing a focal hypermetabolic lesion (SUVmax 4.2) in and around the proximal CBD area (Figure 3). These findings made a straightforward impression of proximal CBD cancer.
Although no biopsy confirmation has been made, the patient should undergo surgical resection because he is strongly suspected of proximal CBD cancer.
CT, MRCP, PET-CT, etc. findings are compatible with proximal CBD cancer.
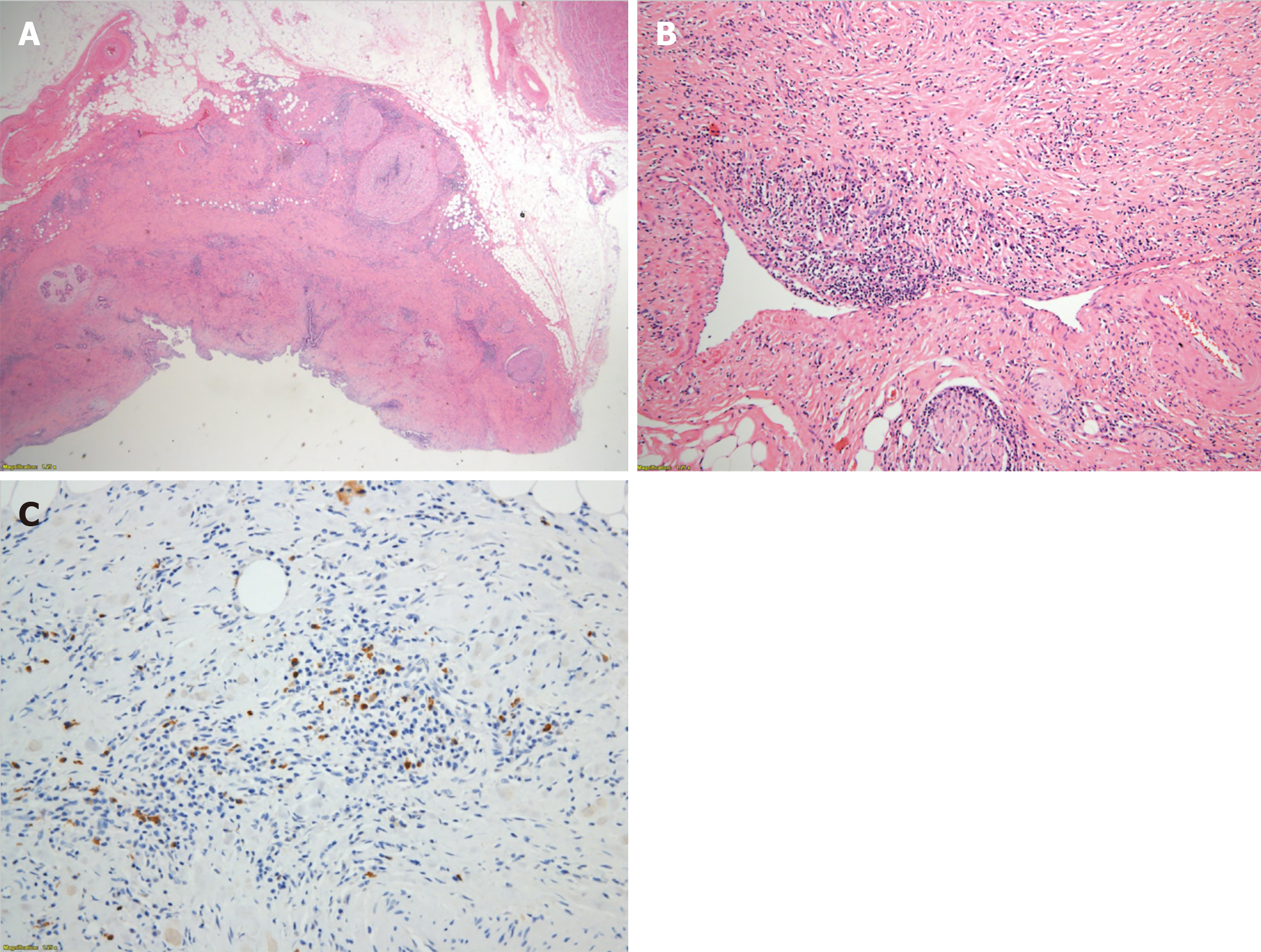
Histopathological examination of the surgical specimen demonstrated a marked sclerosis with diffuse lymphoplasmacytes, some eosinophils’ infiltration (Figure 4A), and obliterative phlebitis (Figure 4B). All lymph nodes were proven as reactive hyperplasia. Immunohistochemical staining for IgG4 showed increased positivity (up to 30/high-power field) in some areas (Figure 4C) and the ratio of IgG4+/IgG+ cells was 30%-50%. Pathologists’ impression was IgG4-related sclerosing disease.
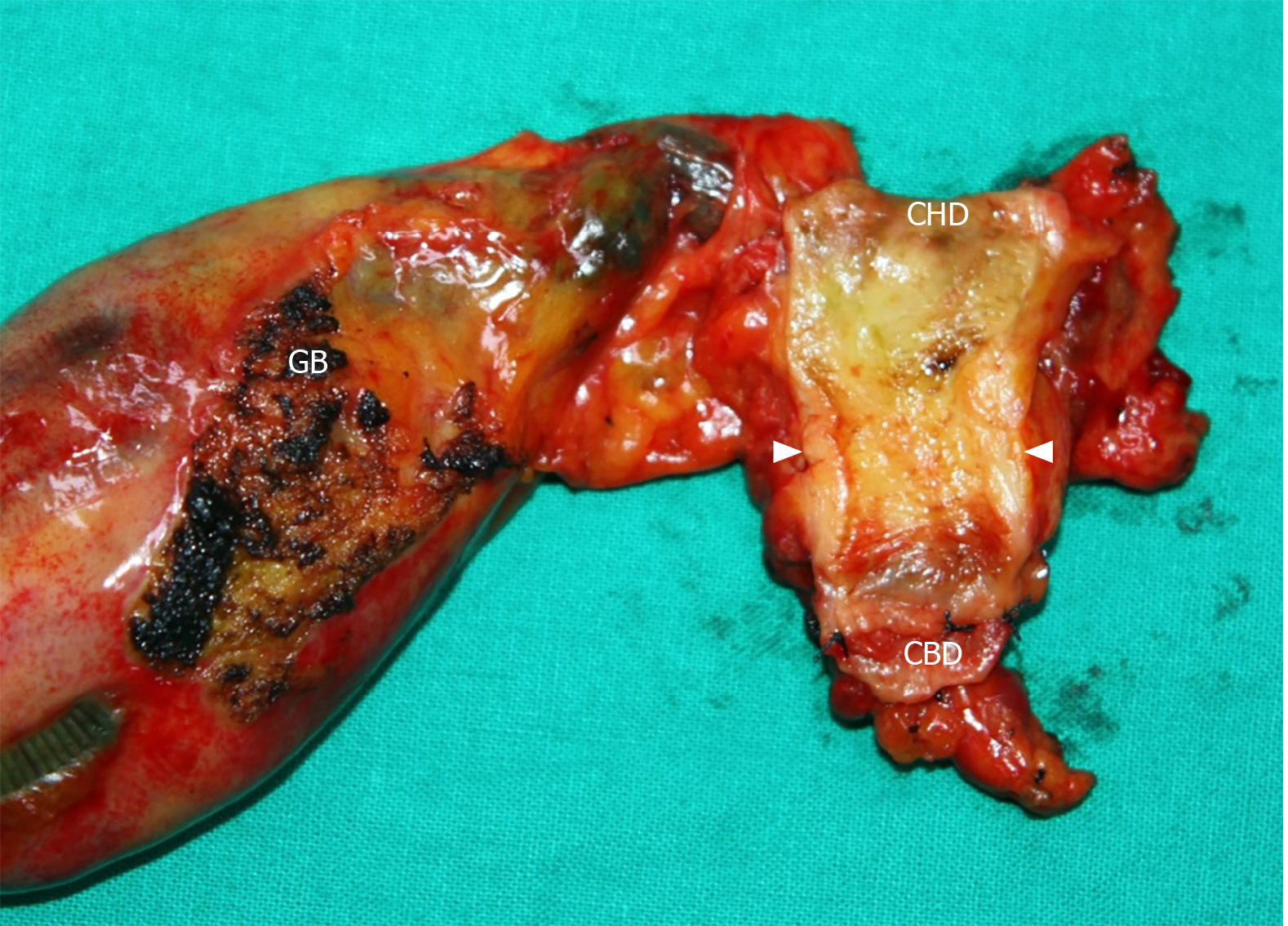
ENBD tube was inserted during the initial ERCP 12 d before operation; however, additional PTBD tubing was placed 1 wk later due to slow reduction in total bilirubin level (17.9 > 13.0 mg/dL). As total bilirubin level went down below 10 mg/dL, we performed segmental resection of the extrahepatic bile duct with en bloc cholecystectomy and regional lymph node dissection. The tumor was located around the cystic duct orifice and was grossly about 2-cm long (Figure 5). The tumor seemed to invade pericholedochal adipose tissue with a depth of about 10 mm, and several enlarged lymph nodes were encountered.
Serum IgG4 level measured 3 wk after surgery was 1050 mg/L (normal range: 30-2010 mg/L), and there was no definite evidence of other-organ involvement associated with IgG4-RD in a retrospective review of the preoperative images. The patient suffered from gastrointestinal bleeding, and successfully recovered through conservative management. He was discharged on the 29th postoperative day, and refused steroids or other immunosuppression therapy due to old age.
Follow-up serum IgG4 levels demonstrated a slight elevation (3060 mg/L) at 4 mo, and was still not normalized at 1 year after surgery (1900 mg/L). The patient showed no symptoms, evidence of relapse, or other organ involvement related to IgG4-RSC by biochemistry and imaging studies, and is under close follow-up without therapy.
An unexpected biliary stricture or mass without relevant history or stone diseases in aged patients, like our case, is presumed to be caused by malignant tumor. In addition, if elevated tumor marker levels and compatible radiologic findings are demonstrated, these make it more difficult to suspect biliary diseases other than cholangiocarcinoma. Unfortunately, not a few cases of benign fibroinflammatory biliary strictures, such as representatively IgG4-RSC, have been steadily reported for patients undergoing surgical resections for presumed cholangiocarcinoma. Therefore, the differentiation between benign and malignant lesions in biliary strictures has been recognized to be a challenging issue.
In order for differentiation of IgG4-RSC, presence of accompanied AIP and the subtype of IgG4-RSC should be taken into consideration. The pancreas is the most common organ involved in patients with IgG4-RSC. Ghazale et al[7] demonstrated in their study, which analyzed 53 cases of IgG4-RSC, that more than 90% of patients had type I AIP, and the rest had isolated cholangitis. Isolated IgG4-RSC in the absence of apparent pancreatic involvement attracts more attention to be discriminated from PSC, cholangiocarcinoma, and pancreatic cancer[6]. On the other hand, IgG4-RSC can be classified into two subtypes, diffuse (or multifocal) and segmental, based on the literature[4,10]; the former mimics PSC and the latter forms stenosis with ductal wall thickening resembling that of cholangiocarcinoma. Thus, segmental subtype can also be designated as mass-forming IgG4-RSC. Like the present case, both isolated and mass-forming IgG4-RSC may be extremely challenging to distinguish from cholangiocarcinoma. In consideration of the different treatment principles of the two diseases, differentiation is crucial.
Differentiating isolated and mass-forming IgG4-RSC from cholangiocarcinoma necessitates a high index of suspicion[4]. In the present study, although no histopathological diagnosis was made through obtained biopsy samples, the patient displayed clinical, biochemical, and radiologic findings compatible with cholangiocarcinoma, including an especially high level of CA 19-9; therefore, we did not suspect the probability of a benign stricture and consequently did not check IgG/IgG4 levels. The lesson from this case is that isolated and mass-forming IgG4-RSC displays a striking similarity with cholangiocarcinoma in clinical, biochemical, and radiological features, and thus may masquerade as cholangiocarcinoma.
Apart from high index of suspicion, several significant parameters for distinguishing IgG4-RSC from cholangiocarcinoma have been proposed. Several years ago, an isolated stricture, a biliary mass, and normal pancreas on CT were reported as statistically significant parameters for discriminating cholangiocarcinoma from IgG4-RSC in analysis of 152 patients with suspicions of cholangiocarcinoma[4]. The present case also had an isolated stricture, a biliary mass, and normal pancreas on CT, but final diagnosis was IgG4-RSC. More recently, increased tumor markers, 6-fold higher levels of serum IgG4, and other-organs’ involvement were suggested to be reference factors for differentiation of IgG4-RSC and cholangiocarcinoma; increased tumor markers were for diagnosis of cholangiocarcinoma, whereas higher levels of serum IgG4 and other-organ’s involvement were for IgG4-RSC[5]. Interestingly, however, our IgG4-RSC case happened to show a highly elevated CA 19-9 level. Li et al[6] clarified that CA 19-9 was not a useful marker to distinguish IgG4-RSC from cholangiocarcinoma, and instead provided key information for differential diagnosis of the two dis
Serum IgG4 level seems to be a cardinal factor for differentiating IgG4-RSC, but its reliability varies across the reports. Some authors reported the sensitivity, specificity, and accuracy of raised serum IgG4 for diagnosing IgG4-RSC to be 50%, 75%, and 60%, respectively[4]; however, these lower values were thought to be resulted from a cutoff level of the upper normal limit, and the analysis was only for 20 patients who underwent serum IgG4 testing. On the contrary, the best cutoff value of serum IgG4 was calculated as 1575 mg/L, 6 times the upper normal limit, with 100% sensitivity and 87.1% specificity for discriminating IgG4-RSC from cholangiocarcinoma[5]. Similarly, Oseini et al[14] emphasized that the minimum level of serum IgG4 for more reliable distinguishment of IgG4-RSC from cholangiocarcinoma with 100% specificity was to be set at approximately four-times the upper normal limit, referring to poor reliability of a 2-fold higher cutoff level of serum IgG4. Accordingly, an elevation of serum IgG4 level alone is not sufficient to differentiate IgG4-RSC, and further investigations are warranted to establish the role of serum IgG4.
Other-organ involvement, especially including the pancreas, is not uncommon in IgG4-RSC, which is recognized as hepatic and biliary involvement of IgG4-RD[15]. The entity of IgG4-RSC is an emerging disorder reported for the first time in the 21st century[8], and thus has not been completely established yet; however, the pathogenesis has recently been recognized to be from autoimmunity. That is why it has been widely accepted that IgG4-RSC is the commonest extrapancreatic manifestation of type-I AIP or IgG4-RD[9,15]. No pancreatic abnormalities or other-organ involvement were demonstrated in our case. This made it more difficult to suspect a benign biliary stricture instead of cholangiocarcinoma.
Importantly, IgG4-RSC shows a striking response to steroid therapy and therefore gives a confirmative clue for discrimination from malignancy[5,6,10,16]. When diagnosed or strongly suspected through mainly histopathological examinations, patients with IgG4-RSC are treated with steroid therapy. However, a short-term steroid trial may be attempted with caution for an equivocal differential diagnosis even after a tissue biopsy, though there has been no international consensus on trial use of steroid thus far.
Diagnosis of IgG4-RSC is tricky, and thus necessitates an interdisciplinary approach. At present, in contrast to AIP, for which a worldwide consensus on diagnostic criteria to differentiate AIP from pancreatic cancer is established, no definite diagnostic criteria to facilitate the distinction of IgG4-RSC from other biliary diseases is at hand; this often causes significant diagnostic delay or unnecessary surgery. In this setting, two diagnostic criteria have been proposed for IgG4-RSC. The first set of criteria is the Histology, Imaging, Serology, Other organ involvement, Response to therapy (HISORt) criteria of the Mayo Clinic, which were originally designed for AIP[17] but later adopted for diagnosis of IgG4-RSC[7]. The more recent set of criteria for IgG4-RSC was proposed by Japanese scholars in 2012, and comprises following components: (1) Characteristic biliary imaging findings; (2) An elevation of serum IgG4 concentrations (> 1350 mg/L); (3) Coexistence of other IgG4-related diseases; and (4) Characteristic histopathological features. Furthermore, the effectiveness of steroid therapy as an optional extra diagnostic criterion to confirm accurate diagnosis of IgG4-RSC[18] was proposed. Although the two criteria have some differences in detail, the overall approach for diagnosis of IgG4-RSC is the same. In order to differentiate IgG4-RSC from cholangiocarcinoma, the five cardinal features need to be taken into consideration. The present case had information on only two of the five cardinal components: imaging and other-organ involvement. This lack of information became a major hurdle to reasonable suspicion and an appropriate diagnosis.
Histopathological diagnosis is of ultimate importance, particularly for patients with suspected isolated and mass-forming IgG4-RSC, given the fact that radiological and biochemical features of IgG4-RSC are not reliable enough for establishing the conclusive diagnosis. Contrary to a key role of histopathological diagnosis, tissue availability and diagnostic accuracy is not high. In the literature, tissue diagnosis during the diagnostic workup was achieved in 68% of patients, and sensitivity and specificity of immunostaining for IgG4-RSC in biopsy specimens were 56% and 89%, respectively[4]. In the same context, Du et al[5] emphasized other clinical manifestations for diagnosis of IgG4-RSC due to difficulty in obtaining a pathological biopsy sample in most cases. We obtained biopsy tissues of the thickened bile duct wall during initial ERCP; however, histopathological examinations revealed no specific diagnostic conclusions. As proximal CBD cancer was strongly suspected, further trials of biopsy and histopathological confirmation were not made.
IgG4-RSC tends to be at high risk of relapse after treatment. According to the previous study, the relapse rates were high and similar in patients with IgG4-RSC after surgery or steroid therapy (44% vs 53%, P = 0.1)[7]. Therefore, in order to avoid relapse, maintenance immunosuppression should be recommended in the majority of patients with IgG4-RSC who underwent surgical resection or achieved remission. The predictive factors for relapse were proposed to include increased serum IgG4 levels and the presence of proximal bile duct strictures[7]. The present case showed increased serum IgG4 level after surgical resection, and therefore should have received maintenance therapy; however, this was refused due to old age.
In conclusion, IgG4-RSC recognized as biliary involvement of IgG4-RD shows frequent other-organ involvement, especially including the pancreas. However, the present case was of isolated IgG4-RSC in the absence of apparent pancreatic involvement but displaying the mass-forming subtype, resulting in a striking similarity with cholangiocarcinoma. In order to reach an appropriate differentiation in patients with isolated mass-forming IgG4-RSC, information on the five cardinal features (histology, imaging, serology, other-organ involvement, and response to steroid therapy) is essential. When this information is lacking, a high index of suspicion is important to lead patients to proper treatment. By failing to suspect a benign IgG4-RSC, our patient underwent unnecessary surgery.
IgG4-RSC is a rare emerging disease and recognized to belong to IgG4-RD. Isolated (without other-organ involvement) and mass-forming IgG4-RSC displays a striking similarity with extrahepatic cholangiocarcinoma in clinical, biochemical, and radiological features, making an extremely challenging issue to be differentiated. To avoid unnecessary major surgery in patients with isolated mass-forming IgG4-RSC, a high index of suspicion on the basis of the diagnostic criteria, HISORt and Japanese ones, is of ultimate importance. Maintenance immunosuppression should be recommended to prevent relapse in the majority of patients with IgG4-RSC who underwent surgical resection or achieved remission.
We would like to thank Professor Lee W of Department of Pathology of the Dankook University Hospital for her invaluable assistance in the case.
| 1. | Dadhwal US, Kumar V. Benign bile duct strictures. Med J Armed Forces India. 2012;68:299-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 2. | Corvera CU, Blumgart LH, Darvishian F, Klimstra DS, DeMatteo R, Fong Y, D'Angelica M, Jarnagin WR. Clinical and pathologic features of proximal biliary strictures masquerading as hilar cholangiocarcinoma. J Am Coll Surg. 2005;201:862-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 86] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 3. | Gamblin TC, Krasinskas AM, Slivka AS, Tublin ME, Demetris J, Shue E, Caro S, Marsh JW, James Moser A. Fibroinflammatory biliary stricture: a rare bile duct lesion masquerading as cholangiocarcinoma. J Gastrointest Surg. 2009;13:713-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Lytras D, Kalaitzakis E, Webster GJ, Imber CJ, Amin Z, Rodriguez-Justo M, Pereira SP, Olde Damink SW, Malagoʼ M. Cholangiocarcinoma or IgG4-associated cholangitis: how feasible it is to avoid unnecessary surgical interventions? Ann Surg. 2012;256:1059-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Du S, Liu G, Cheng X, Li Y, Wang Q, Li J, Lu X, Zheng Y, Xu H, Chi T, Zhao H, Xu Y, Sang X, Zhong S, Mao Y. Differential Diagnosis of Immunoglobulin G4-associated Cholangitis From Cholangiocarcinoma. J Clin Gastroenterol. 2016;50:501-505. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Li J, Zhao C, Shen Y. Autoimmune cholangitis and cholangiocarcinoma. J Gastroenterol Hepatol. 2012;27:1783-1789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Ghazale A, Chari ST, Zhang L, Smyrk TC, Takahashi N, Levy MJ, Topazian MD, Clain JE, Pearson RK, Petersen BT, Vege SS, Lindor K, Farnell MB. Immunoglobulin G4-associated cholangitis: clinical profile and response to therapy. Gastroenterology. 2008;134:706-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 667] [Cited by in RCA: 595] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 8. | Zen Y, Harada K, Sasaki M, Sato Y, Tsuneyama K, Haratake J, Kurumaya H, Katayanagi K, Masuda S, Niwa H, Morimoto H, Miwa A, Uchiyama A, Portmann BC, Nakanuma Y. IgG4-related sclerosing cholangitis with and without hepatic inflammatory pseudotumor, and sclerosing pancreatitis-associated sclerosing cholangitis: do they belong to a spectrum of sclerosing pancreatitis? Am J Surg Pathol. 2004;28:1193-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 424] [Cited by in RCA: 391] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 9. | Takuma K, Kamisawa T, Igarashi Y. Autoimmune pancreatitis and IgG4-related sclerosing cholangitis. Curr Opin Rheumatol. 2011;23:80-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Nakazawa T, Naitoh I, Hayashi K, Miyabe K, Simizu S, Joh T. Diagnosis of IgG4-related sclerosing cholangitis. World J Gastroenterol. 2013;19:7661-7670. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 78] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 11. | Graham RP, Smyrk TC, Chari ST, Takahashi N, Zhang L. Isolated IgG4-related sclerosing cholangitis: a report of 9 cases. Hum Pathol. 2014;45:1722-1729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 12. | Rungsakulkij N, Sornmayura P, Tannaphai P. Isolated IgG4-related sclerosing cholangitis misdiagnosed as malignancy in an area with endemic cholangiocarcinoma: a case report. BMC Surg. 2017;17:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Xiao J, Li G, Yang G, Jia C, Li B. Case report: A female case of isolated IgG4-related sclerosing cholangitis mimicking cholangiocarcinoma. Medicine (Baltimore). 2017;96:e6542. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Oseini AM, Chaiteerakij R, Shire AM, Ghazale A, Kaiya J, Moser CD, Aderca I, Mettler TA, Therneau TM, Zhang L, Takahashi N, Chari ST, Roberts LR. Utility of serum immunoglobulin G4 in distinguishing immunoglobulin G4-associated cholangitis from cholangiocarcinoma. Hepatology. 2011;54:940-948. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 136] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 15. | Joshi D, Webster GJ. Biliary and hepatic involvement in IgG4-related disease. Aliment Pharmacol Ther. 2014;40:1251-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 16. | Zen Y, Kawakami H, Kim JH. IgG4-related sclerosing cholangitis: all we need to know. J Gastroenterol. 2016;51:295-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 17. | Chari ST, Smyrk TC, Levy MJ, Topazian MD, Takahashi N, Zhang L, Clain JE, Pearson RK, Petersen BT, Vege SS, Farnell MB. Diagnosis of autoimmune pancreatitis: the Mayo Clinic experience. Clin Gastroenterol Hepatol. 2006;4:1010-6; quiz 934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 722] [Cited by in RCA: 657] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 18. | Ohara H, Okazaki K, Tsubouchi H, Inui K, Kawa S, Kamisawa T, Tazuma S, Uchida K, Hirano K, Yoshida H, Nishino T, Ko SB, Mizuno N, Hamano H, Kanno A, Notohara K, Hasebe O, Nakazawa T, Nakanuma Y, Takikawa H; Research Committee of IgG4-related Diseases; Research Committee of Intractable Diseases of Liver and Biliary Tract; Ministry of Health, Labor and Welfare, Japan; Japan Biliary Association. Clinical diagnostic criteria of IgG4-related sclerosing cholangitis 2012. J Hepatobiliary Pancreat Sci. 2012;19:536-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 254] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gu J, Tovoli F S-Editor: Yan JP L-Editor: A P-Editor: Li X